The E2F1–3 transcription factors are essential for cellular proliferation (original) (raw)
- Letter
- Published: 22 November 2001
- Cynthia Timmers1,
- Baidehi Maiti1,
- Harold I. Saavedra1,
- Ling Sang1,
- Gabriel T. Chong1,
- Faison Nuckolls3,
- Paloma Giangrande3,
- Fred A. Wright1,
- Seth J. Field4,
- Michael E. Greenberg4,
- Stuart Orkin5,
- Joseph R. Nevins3,
- Michael L. Robinson2 &
- …
- Gustavo Leone1
Nature volume 414, pages 457–462 (2001)Cite this article
- 5135 Accesses
- 547 Citations
- 14 Altmetric
- Metrics details
Abstract
The retinoblastoma tumour suppressor (Rb) pathway is believed to have a critical role in the control of cellular proliferation by regulating E2F activities1,2. E2F1, E2F2 and E2F3 belong to a subclass of E2F factors thought to act as transcriptional activators important for progression through the G1/S transition3. Here we show, by taking a conditional gene targeting approach, that the combined loss of these three E2F factors severely affects E2F target expression and completely abolishes the ability of mouse embryonic fibroblasts to enter S phase, progress through mitosis and proliferate. Loss of E2F function results in an elevation of p21Cip1 protein, leading to a decrease in cyclin-dependent kinase activity and Rb phosphorylation. These findings suggest a function for this subclass of E2F transcriptional activators in a positive feedback loop, through down-modulation of p21Cip1, that leads to the inactivation of Rb-dependent repression and S phase entry. By targeting the entire subclass of E2F transcriptional activators we provide direct genetic evidence for their essential role in cell cycle progression, proliferation and development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Similar content being viewed by others
References
- Dyson, N. The regulation of E2F by pRB-family proteins. Genes Dev. 12, 2245–2262 (1998).
Article CAS Google Scholar - Nevins, J. R. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 9, 585–593 (1998).
CAS PubMed Google Scholar - DeGregori, J., Leone, G., Miron, A., Jakoi, L. & Nevins, J. R. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl Acad. Sci. USA 94, 7245–7250 (1997).
Article ADS CAS Google Scholar - Duronio, R. J., O'Farrell, P. H., Xie, J E., Brook, A. & Dyson, N. The transcription factor E2F is required for S phase during Drosophila embryogenesis. Genes Dev. 9, 1445–1455 (1995).
Article CAS Google Scholar - Royzman, I., Whittaker, A. J. & Orr-Weaver, T. L. Mutations in Drosophila DP and E2F distinguish G1–S progression from an associated transcriptional program. Genes Dev. 11, 1999–2011 (1997).
Article CAS Google Scholar - Field, S. J. et al. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell 85, 549–561 (1996).
Article CAS Google Scholar - Humbert, P. O. et al. E2F4 is essential for normal erythrocyte maturation and neonatal viability. Mol. Cell 6, 281–291 (2000).
Article CAS Google Scholar - Humbert, P. O. et al. E2f3 is critical for normal cellular proliferation. Genes Dev. 14, 690–703 (2000).
Article CAS Google Scholar - Leone, G. et al. Myc requires distinct E2F activities to induce S phase and apoptosis. Mol. Cell 8, 105–113 (2001).
Article CAS Google Scholar - Lindeman, G. J. et al. A specific, nonproliferative role for E2F-5 in choroid plexus function revealed by gene targeting. Genes Dev. 12, 1092–1098 (1998).
Article CAS Google Scholar - Rempel, R. E. et al. Loss of E2F4 activity leads to abnormal development of multiple cellular lineages. Mol. Cell. 6, 293–306 (2000).
Article CAS Google Scholar - Yamasaki, L. et al. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 85, 537–548 (1996).
Article CAS Google Scholar - Lees, J. A. et al. The retinoblastoma protein binds to a family of E2F transcription factors. Mol. Cell. Biol. 13, 7813–7825 (1993).
CAS PubMed PubMed Central Google Scholar - Leone, G. et al. E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev. 12, 2120–2130 (1998).
Article CAS Google Scholar - Leone, G. et al. Identification of a novel E2F3 product suggests a mechanism for determining specificity of repression by Rb proteins. Mol. Cell. Biol. 20, 3626–3632 (2000).
Article CAS Google Scholar - Lukas, C. et al. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 401, 815–881 (1999).
Article ADS CAS Google Scholar - Ishida, S. et al. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol. Cell. Biol. 21, 4684–4699 (2001).
Article CAS Google Scholar - Muller, H. et al. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15, 267–285 (2001).
Article CAS Google Scholar - Brehm, A. et al. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 391, 597–601 (1998).
Article ADS CAS Google Scholar - Harbour, J. W. & Dean, D. C. Chromatin remodeling and Rb activity. Curr. Opin. Cell Biol. 12, 685–689 (2000).
Article CAS Google Scholar - Luo, R. X., Postigo, A. A. & Dean, D. C. Rb interacts with histone deacetylase to repress transcription. Cell 92, 463–473 (1998).
Article CAS Google Scholar - Magnaghi-Jaulin, L. et al. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature 391, 601–605 (1998).
Article ADS CAS Google Scholar - Weintraub, S. J., Prater, C. A. & Dean, D. C. Retinoblastoma protein switches the E2F site from positive to negative element. Nature 358, 259–261 (1992).
Article ADS CAS Google Scholar - Weintraub, S. J. et al. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature 375, 812–815 (1995).
Article ADS CAS Google Scholar - Zhang, H. S., Postigo, A. A. & Dean, D. C. Active transcriptional repression by the Rb–E2F complex mediates G1 arrest triggered by p16INK4a, TGFβ, and contact inhibition. Cell 97, 53–61 (1999).
Article CAS Google Scholar - Tsai, K. Y. et al. Mutation of E2f-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol. Cell 2, 293–304 (1998).
Article CAS Google Scholar - Yamasaki, L. et al. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1(+/-) mice. Nature Genet. 18, 360–364 (1998).
Article CAS Google Scholar - Ziebold, U., Reza, T., Caron, A. & Lees, J. A. E2F3 contributes both the inappropriate proliferation and to the apoptosis arising in Rb mutant embryos. Genes Dev. 15, 386–391 (2001).
Article CAS Google Scholar - Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl Acad. Sci. USA 90, 8392–8396 (1993).
Article ADS CAS Google Scholar - Nevins, J. R., DeGregori, J., Jakoi, L. & Leone, G. in Methods in Enzymology (ed. Dunphy, W. G.) 678 (Academic, San Diego, 1997).
Google Scholar
Acknowledgements
We are grateful to R. Premont for providing the loxP vectors. We thank C. Bock for assistance in generating the E2F3 chimaeric mice; C. Brown and L. Jakoi for various technical assistance; and M. Weinstein for critical comments on the manuscript. This work was supported by grants from the National Institutes of Health (NIH) (G.L.). L.W. was supported by an NIH award, C.T. was supported by the Up on the Roof Human Cancer Genetics Posdoctoral Fellowship, J.R.N. is an investigator of the Howard Hughes Medical Institute, and G.L. is a V-Foundation and Pew Charitable Trust Scholar.
Author information
Authors and Affiliations
- Division of Human Cancer Genetics, Department of Molecular Virology, and Department of Molecular Genetics, Immunology and Medical Genetics, The Ohio State University, Columbus, Ohio, 43210, USA
Lizhao Wu, Cynthia Timmers, Baidehi Maiti, Harold I. Saavedra, Ling Sang, Gabriel T. Chong, Fred A. Wright & Gustavo Leone - Division of Molecular and Human Genetics, Children's Research Institute, The Ohio State University, Columbus, 43210, Ohio, USA
Michael L. Robinson - Department of Genetics, Howard Hughes Medical Institute, Duke University Medical Center, Durham, 27710, North Carolina, USA
Faison Nuckolls, Paloma Giangrande & Joseph R. Nevins - Department of Neuroscience, Harvard Medical School, Boston, Massachusetts, 02115
Seth J. Field & Michael E. Greenberg - Howard Hughes Medical Institute, Children's Hospital, Harvard Medical School, Boston, 02115, Massachusetts, USA
Stuart Orkin
Authors
- Lizhao Wu
- Cynthia Timmers
- Baidehi Maiti
- Harold I. Saavedra
- Ling Sang
- Gabriel T. Chong
- Faison Nuckolls
- Paloma Giangrande
- Fred A. Wright
- Seth J. Field
- Michael E. Greenberg
- Stuart Orkin
- Joseph R. Nevins
- Michael L. Robinson
- Gustavo Leone
Corresponding author
Correspondence toGustavo Leone.
Supplementary information

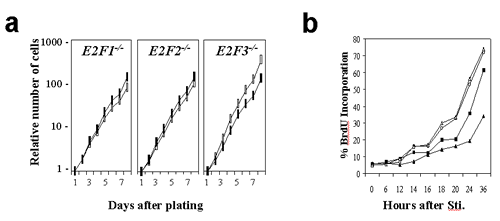
Supplementary Figure 1
(GIF 11.6 KB)
Loss of E2F3 leads to defects in cellular proliferation and S-phase entry. Growth curves (a) and BrdU incorporation assays (b) of _E2F_-deficient primary MEFs (closed symbols) in comparison with their wild-type counterparts (open symbols).
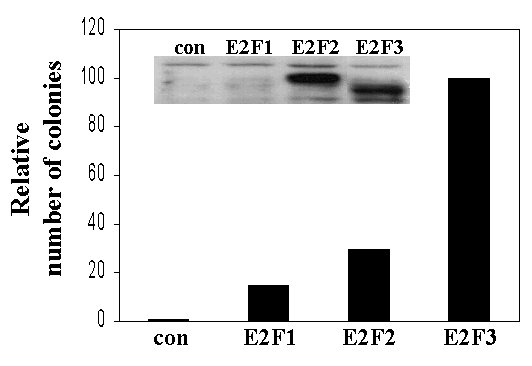
Supplementary Figure 2
(GIF 8.03 KB)
Ectopic expression of E2F1, E2F2, or E2F3 can rescue the proliferation defects of TKO cells. Colony formation assays of E2F1 -/- E2F2 -/- E2F3 f/f MEFs infected with the indicated retrovirus as well as pB_puro_-Cre. (con): pB_hygro_; (E2F1, E2F2, E2F3): pB_hygro-HA-_tagged E2F1, E2F2, and E2F3a, respectively. Similar results were obtained in four independent experiments; a representative experiment is shown. (inset) Western blot using an antibody against HA-epitope.

Supplementary Figure 3
(JPG 23 KB)
Northern blot analysis of various E2F-responsive genes in E2F3 f/f (a) or E2F2 -/- E2F3 f/ (b) MEFs that were infected with a control or a Cre-retrovirus. The experiment was performed as described in Figure 3a of the main text. (con): pB_hygro_; (E2F3a): pB_hygro-_myc-tagged E2F3a. c, Deletion of E2F1, E2F2, and E2F3 does not affect early mitogenic events. Western blot of retrovirus-infected E2F1 -/- E2F2 -/- E2F3 f/f MEFs using antibodies against phosphorylated forms of Erk1/2 or Akt; the same blots were re-probed with antibodies against total Erk1/2 and Akt to verify equal loading (data not shown).
Rights and permissions
About this article
Cite this article
Wu, L., Timmers, C., Maiti, B. et al. The E2F1–3 transcription factors are essential for cellular proliferation.Nature 414, 457–462 (2001). https://doi.org/10.1038/35106593
- Received: 30 July 2001
- Accepted: 25 September 2001
- Issue Date: 22 November 2001
- DOI: https://doi.org/10.1038/35106593