Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex (original) (raw)
Main
Metastasis is the main cause for failure of therapy in colorectal cancer (CRC; Christofori, 2006; Watson and Collins, 2011). Many cancers share the common mechanisms required for metastasis including enhancement of motility, change of adhesion ability, secretion of proteolytic enzyme to degrade extracellular matrix, and others (Fidler, 2003). However, the molecular mechanism causing tumour initiation and metastasis in CRC remains elusive (Fearon and Vogelstein, 1990; Bernards and Weinberg, 2002; Takayama et al, 2006). It has generally been assumed that cancer may only be regulated by protein-coding genes. With thousands of non-coding RNA transcripts were identified over the past several years, the function of non-coding RNAs (ncRNA) in cancer development has become an attractive research area (Eddy, 2001; Kapranov et al, 2007; Rossi et al, 2008; Guttman et al, 2009). To date, a significant number of small ncRNAs (sncRNA), such as microRNAs have been reportedly associated with tumorigenesis by acting as tumour suppressor genes or oncogenes (Bartels and Tsongalis, 2009; Carthew and Sontheimer, 2009; Gandellini et al, 2009). However, little is known in terms of long ncRNAs (lncRNA) and their impact on carcinogenesis and tumour metastasis.
Metastasis associated with lung adenocarcinoma transcript-1 (MALAT1) is an evolutionarily highly conserved lncRNA, which lacks open reading frames, and cannot be translated into protein in vivo. MALAT1, however, has been broadly expressed in normal human as well as mouse tissues (Ji et al, 2003; Hutchinson et al, 2007; Tseng et al, 2009), and especially overexpressed in various carcinomas including lung, cervical, liver, and bladder (Lin et al, 2007; Guo et al, 2010; Schmidt et al, 2011; Ying et al, 2012; Gutschner et al, 2013). MALAT1 is specifically retained in nuclear speckles, associated with modification or storage of the pre-mRNA processing machinery, potentially effecting gene function regulation (Wilusz et al, 2008; Tripathi et al, 2010; Xu et al, 2011; Lai et al, 2012; Gutschner et al, 2013). Our previous study has shown that MALAT1 could promote the proliferation and migration of LoVo and HCT116 cells (Ji et al, 2013). This implied that MALAT1 may play a key role in regulating human cancer development.
Studies have shown that the PTBP2 (Patton et al, 1991) is highly expressed in cancer cells and can promote the growth of cancer cells (He et al, 2007). SFPQ, also known as PSF (PTB-associated splicing factor), regulates the tumour-promoting effects of PTBP2 by the combination of PTBP2 and SFPQ (Patton et al, 1993; Gozani et al, 1994; Meissner et al, 2000). Since SFPQ contains two RNA-binding domains (RBDs), the combination between RNA and SFPQ may affect the regulatory role of SFPQ on PTBP2 (Wang et al, 2009; Li et al, 2009). We hypothesised that MALAT1, an important regulatory RNA, could interact with SFPQ to partially inhibit the combination of SFPQ and PTBP2 proteins, thereby enabling the release of SFPQ-detached PTBP2 from the SFPQ/PTBP2 complex and leading to the promotion of tumour growth and metastasis. Thus, this study aimed to investigate the effect of MALAT1 on the proliferation and migration of CRC cells in vitro and in vivo, and to detect the expression of MALAT1, SFPQ, and PTBP2 in tumour tissues from patients with CRC. Moreover, the potential underlying mechanism for MALAT1’s biological effects on the development of CRC, in respect to the interaction among MALAT1, SFPQ and PTBP2, was also investigated.
Materials and methods
Cell culture
LoVo (human colon, Dukes’ type C, grade IV, colorectal adenocarcinoma) and HCT116 (human colon, CRC) cells from ATCC were grown in F12K medium and in 1640 RIPM, respectively. Both mediums were supplemented with 10% fetal calf serum (FCS), 100 U ml−1 penicillin, and 100 g ml−1 streptomycin. Cells were cultured at 37°C, high humidity, and 5% CO2. Other CRC cells including HCT8, LS174T, SW480, and SW640 cells were cultured as HCT116 cells. Human HEK 293T cells were cultured in DMEM medium supplemented with 10% FCS, 100 U ml−1 penicillin, and 100 g ml−1 streptomycin.
Overexpression and knockdown of MALAT1
Three human MALAT1 (Gene ID: 378938) fragments (MALAT1/1nt-3200nt, MALAT1/3175nt-6100nt, MALAT1/6074nt-8708nt) were amplified by RT–PCR from the RNA extracted from LoVo cells, using the primers in Supplementary Table S1, sequenced by the Sangon Biotech company (Shanghai, China), and the right MALAT1 fragments were sub-cloned into the expression vector pLV4, named pLV4-MALAT1/F1, pLV4-MALAT1/F2, and pLV4-MALAT1/F3. The full length of MALAT1 gene was spliced from above three fragments by series of PCR, following by gene sequencing, and the right full fragments with no SNP (single-nucleotide polymorphism) was cloned into pLV4 expression vector, named pLV4-over/MALAT1. In addition, human MALAT1 gene was searched for suitable siRNA target sequences, which were listed in Supplementary Table S2. Lentiviral particles were produced by co-transfecting expression vector pLV4-over/MALAT1 or pLV4-shRNA/MALAT1 with viral particle packaging helper vector into 293T cells. Titres of viral particles containing pLV4-over/MALAT1, pLV4-shRNA/MALAT1, pLV4-shRNA/NT, and pLV4-vector were determined by limited serial dilution. LoVo or HCT116 cells were infected with above packaged lentivirus. The efficiencies of overexpression or knockdown were determined by real-time PCR.
Overexpression of SFPQ and knockdown of SFPQ and PTBP2
Human SFPQ (Gene ID: 6421) gene was amplified by RT–PCR from the RNA extracted from LoVo cells, using the primers in Supplementary Table S1, sequenced by the Sangon Biotech company, and the right full-length SFPQ fragments were sub-cloned into the expression vector pLV4, named pLV4-over/SFPQ. Additionally, SFPQ and PTBP2 (Gene ID: 58155) were searched for suitable shRNA target sequences, respectively, and listed in Supplementary Table S2. The shRNA vector for stable knockdown was pGFU6/GFP/Neo, and the final shRNA vector of SFPQ and PTBP2 were named pGFU6-shRNA/SFPQ and pGFU6-shRNA/PTBP2, respectively. LoVo cells were transfected with the above two shRNA vectors for 48 h using Lipofectamine 2000 transfection reagent according to manufacturer’s instruction, and the transfected cells were selected with neomycin (G418, 1 mg ml−1, Sigma, St Louis, MO, USA). The efficiency of knockdown was determined by quantitative real-time PCR and western blot.
Quantitative mRNA analysis
For RT–PCR of short fragments of MALAT1 (189nt), the primers were designed as the sequences in Supplementary Table S1, and the product were confirmed with agarose electrophoresis. For real-time PCR of MALAT1, SFPQ and PTBP2, the primers and TaqMan probe were synthesised as the sequences in Supplementary Table S3, with the control of GAPDH gene. All assays were performed in triplicate and independently repeated three times.
Tumour mouse model and in vivo optical imaging
Cells were harvested from culture flasks and transferred to serum-free PBS. Single-cell suspensions (2 × 106 in 100 _μ_l) were injected into the subcutaneous area of female BALB/c nude mice (4–6 weeks old) obtained from SLAC (SLAC Laboratory Lab, Shanghai, China). Tumour size was evaluated using a standard caliper measuring tumour length and width in a blinded manner and the tumour volume was calculated using the formula: length × width2 × 0.52. After 42 days, animals were killed by cervical dislocation in deep CO2 anaesthesia, primary tumours were surgically removed and weighted (g), and the expression levels of human MALAT1 in the tumours were evaluated by real-time PCR. Experimental lung metastases were induced by injections of a single-cell suspension of each of four kinds of cells containing green fluorescent protein (GFP): LoVo-shRNA/NT, LoVo-shRNA/MALAT1, LoVo-vector, and LoVo-over/MALAT1 into the lateral tail vein. To prevent pulmonary embolism caused by the injection of tumour cells, all cells were administered in a total volume of 500 _μ_l PBS containing 0.1% BSA (bovine serum albumin) over a 60-s duration. Seven weeks later, prior to in vivo imaging, the mice were anaesthetised with phenobarbital sodium, and established lungs metastases images were observed by LB983 NIGHTOWL II system (Berthold Technologies GmbH, Calmbacher, Germany). Then, the organs of lung were excised, and the metastatic lesions were determined by haematoxylin and eosin (H&E) staining. All experimental protocols were reviewed and approved by the Committee on Animal Experimentation.
RNA-binding protein immunoprecipitation
RNA-binding protein immunoprecipitation (RIP) was made according to the manufacturer’s protocol from the EZ-Magna RIP Kit (EMD Millipore, Billerica, MA, USA). Briefly, LoVo cells were rinsed with ice-cold PBS, lysed using RIP lysis buffer, and then lysates were prepared. Magnetic beads binding antibody of interest for immunoprecipitation were prepared. Mouse SFPQ antibody (sc-101137, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit PTBP2 antibody (sc-98491) and nonspecific mouse IgG antibody were used. Immunoprecipitation of RNA-binding protein-RNA complex started by incubating the RIP lysates and magnetic beads binding antibody of interest together and rotating overnight at 4°C. Each immunoprecipitate was digested with proteinase K and 10% SDS at 55°C for 30 min after washing with RIP washing buffer. After incubation, centrifuge was used to obtain the supernatant, followed by washing and adding phenol : chloroform : isoamyl alcohol (125 : 24 : 1) to separate the phases. The aqueous phase was separated by adding chloroform, and RNA was precipitated from the aqueous phase using 80% ethanol. Isolated RNA was treated with DNase I to remove any genomic DNA contamination. Each sample was reverse-transcribed using the PrimeScript RT reagent Kit (Takara, DaLian, China), followed by quantitative mRNA analysis. All assays were performed in triplicate and independently repeated three times.
Immunofluorescence staining and confocal microscopy detection
Cells were fixed with methanol, blocked with 5% BSA. The cells were first stained with SFPQ mouse antibody followed by Cy3-conjugated goat anti-mouse IgG (Millipore). After the cells were washed four times with PBS, the PTBP2 rabbit antibody was added, followed by FITC-conjugated goat anti-rabbit IgG (Millipore). Nuclear staining was done with DAPI (4′,6-diamidino-2-phenylindole). Cells were imaged with a TCS SP2 spectral confocal system (Leica, Ernst-Leitz, Wetzlar, Germany). All experiments were conducted according to instructions from the antibody manufacturer.
Protein immunoprecipitation
Cells were lysed in lysis buffer containing 100 mM Tris-HCl (Sangon, Shanghai, China) (pH 7.4), 150 mM NaCl, 10% v/v glycerol, 0.5% Triton X-100 (Sangon), and protease inhibitor cocktail. Cell lysates were centrifuged and supernatants obtained. Protein A/G sepharose beads were added to the supernatant to preclear nonspecific binding. SFPQ antibody was then added and incubated with precleared lysates at 4°C. After overnight incubation, protein A/G sepharose beads were added for 1 h. The pellets were washed four times with lysis buffer and eluted with SDS–PAGE sample buffer, which was analysed by western blot using either SFPQ (sc-101137) antibody or PTBP2 (sc-98491) antibody (Santa Cruz Biotechnology).
Cell proliferation assay
Cells were cultured at a density of 2.5 × 103 cells per well in a flat-bottomed 96-well plate. MTT [3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide] was applied to measure the cell viability by measuring the absorbance at 490 nm. All assays were performed in triplicate and independently repeated three times.
Soft agar colony formation assay
A total of 2 × 103 cells were added to 3 ml F12K medium supplemented with 10% FCS, and the assay was performed in 35-mm dishes that contained two layers of soft agar. The top and bottom layers were 0.33% and 0.8% agarose (low melt, Bio-Rad, Hercules, CA, USA) in 5% F12K medium, respectively. The cells were cultured at 37°C, high humidity, and 5% CO2. Colonies were counted after 14 days growth by two investigators (LZ and QJ).
Migration assay
A total of 2.5 × 105 cells (in 100 _μ_l F12K with 0.5% FCS) were seeded into the upper part of a transwell chamber (Transwell filter inserts in 6.5 mm diameter, pore size of 8 _μ_m, Corning Incorporated, One Riverfront Plaza Corning, NY, USA). In the lower part of the chamber, 600 _μ_l F12K with 15% FCS and 10 _μ_g ml−1 fibronectin was added and the assay was performed for 24 h at 37°C and 5% CO2. Migrated cells were analysed by crystal violet staining, followed by observation under a DMI3000 B inverted microscope (Leica). All assays were performed in triplicate and independently repeated three times.
Wound healing
Cells (4.5 × 105) were seeded on a six-well plate to form a confluent monolayer in 10% FCS-containing medium. The monolayer cells were scratched by a plastic tip and washed with PBS to remove cell debris; 0.5% FCS-containing F12K were then added to each well, and the scratched monolayer was incubated in a 37°C incubator with 5% CO2 for 24 h. Wound closure was measured in five random fields at × 200 magnification using Image-Pro Express software and a DMI3000 B inverted microscope (Media Cybernetics, Inc., Rockville, MD, USA). Percentage of wound healing was calculated as follows: migrated cell surface area / total surface area × 100, in which, migrated cell surface area=length of cell migration (mm) × 2 × length of defined areas, total surface area=beginning width × length of defined areas.
Fluorescence in situ hybridisation
Paraffin-embedded tumour and adjacent normal tissue samples from 60 CRC patients (30–80 years of age) who underwent tumour resection at Pu Tuo Hospital, Shanghai University of Traditional Chinese Medicine, between 2010 and 2011 were selected for hybridisation with FITC-labeled MALAT1 DNA probe (Shinegene Molecular Biotechnology, Shanghai, China). The tissues were fixed in formalin, routinely processed, embedded in paraffin wax, and cut into 5 _μ_m unstained tissue for FISH and IHC staining. Unstained tissue sections were baked for 24 h at 56°C. The slides were immersed in 1 M sodium thiocyanate at 80°C for 10 min, and in protease solution for 10 min, washed with purified water, air-dried, and dehydrated in ascending grades of alcohol; 10 _μ_l of the MALAT1 probe mixture was applied on the air-dried slides. Denaturation and hybridisation was performed using the Thermobrite system (Abbott Molecular Inc., Des Plaines, IL, USA), which was programmed to 75°C for 5 min. The slides were then washed with 0.4 × saline sodium citrate (SSC) solution at 70°C for 2 min, and 2 × SSC at room temperature for another 5 min. Lastly, 10 _μ_l of DAPI (4′,6-diamidino-2-phenylindole) was applied on the slides, and the slides were examined using a fluorescent microscope. The experiment was carried out according to the method described by Chaumeil et al (2008); Takayama et al, 2006. The result was evaluated and blindly classified by two investigators (LZ and JS). The experimental protocol was reviewed and approved by the Ethics Committee on Human Experimentation.
Immunohistochemical staining
Above paraffin-embedded tumour and adjacent normal tissue samples were applied for immunohistochemical staining of SFPQ and PTBP2. The experiments were performed using mouse SFPQ antibody or rabbit PTBP2 antibody, diluted 1 : 50, and HRP-conjugated anti-mouse or anti-rabbit IgG (AP124P, AP132P, Millipore), and detected by DAB (diaminobenzidine). DAPI was used as a nuclear counterstaining. The DMI3000 B microscope connected to a digital imaging system was used for observation and analysis. The result was evaluated and classified blindly by two investigators (LZ and JS).
Statistical analysis
All data were presented as means with standard deviation (SD) or median with 95% confidence interval (95% CI). Statistic comparison was performed using the Student’s _t_-test, one-way ANOVA analysis, Mann–Whitney test, or Kruskal–Wallis test, as appropriate, with the significance level at P<0.05. All statistical analyses were completed with SPSS (version 18) software (IBM, Armonk, NY, USA).
Results
MALAT1 promoted the proliferation and migration of CRC cell lines in vitro
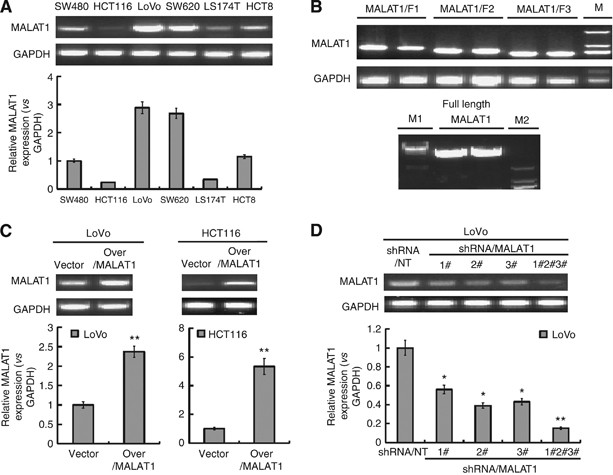
MALAT1 expression in several CRC cells was found to be quite strong in LoVo and SW620 in contrast to SW480, HCT116, LS174T and HCT8 (Figure 1A). LoVo was chosen for use in our experiments due to the strong migration ability, and HCT116 with lower MALAT1 expression and relative weak migration ability was also investigated in parallel experiments.
Figure 1
Upregulation and downregulation of MALAT1 expression. (A) Six established human CRC cell lines SW480, HCT116, LoVo, SW620, LS174T, and HCT8 were analysed for the MALAT1 expression by RT–PCR (up) and real-time PCR (down), GAPDH was chosen as a control. (B) RT–PCR was used to amplify three fragments of MALAT1 gene (upper panel), and the full length of MALAT1 gene was spliced by series of PCR (lower panel). (C) LoVo and HCT116 cells were infected with lentiviral particles containing pLV4-over/MALAT1 and pLV4-vector. The MALAT1 transcript was evaluated by RT–PCR (up) and real-time PCR (down). **P<0.01, compared with LoVo-vector or HCT116-vector cells. (D) LoVo cells were infected with lentiviral particles containing pLV4-shRNA/MALAT1 or pLV4-shRNA/NT, and the knockdown efficiency was evaluated by RT–PCR (up) and real-time PCR (down). *P<0.05; **P<0.01, compared with LoVo-shRNA/NT cells.
In order to determine the biological function of MALAT1 on CRC cells, we firstly upregulated and downregulated the expression of MALAT1. To obtain the full-length gene of MALAT1, we divided the MALAT1 gene into three fragments: MALAT1/F1, MALAT1/F2 and MALAT1/F3, and the right amplified fragments by RT–PCR were shown in upper panel of Figure 1B. Then, the full length of MALAT1 was spliced from above three fragments by series of PCR, and finally we got the full-length MALAT1 shown in lower panel of Figure 1B. We characterised the full-length MALAT1 by gene sequencing, and the right full fragments with no SNP was cloned into pLV4 expression vector, named pLV4-over/MALAT1. Above three fragments of MALAT1 were also sub-cloned into pLV4-vector for following vital experiment, and named pLV4-MALAT1/F1, pLV4-MALAT1/F2, and pLV4-MALAT1/F3.
Additionally, we synthesised three candidate siRNAs targeted MALAT1 gene to knockdown the MALAT1 expression, and cloned into pLV4-vector. Lentiviral particles containing pLV4-vector, pLV4-over/MALAT1, pLV4-shRNA/NT, and pLV4-shRNA/MALAT1 were packaged. LoVo or HCT116 cells were infected with above packaged lentivirus containing pLV4-vector, pLV4-over/MALAT1, and as expected, significant upregulation of MALAT1 was achieved in LoVo and HCT116 cells (Figure 1C). Similarly, we obtained the downregulation of MALAT1 in LoVo cells by infection with packaged lentivirus containing pLV4-shRNA/MALAT1, and from Figure 1D we knew, LoVo cells infected together with three packaged lentivirus achieved the best efficiency of knockdown.
In our previous study (Ji et al, 2013), by MTT proliferation assay and transwell migration assay, we have shown that, MALAT1 could promote the proliferation and migration of LoVo and HCT116 cells in vitro. Here, in Supplementary Figure S1, by soft agar colony formation assay and wound healing assay, we also demonstrated that MALAT1 could promote colony formation and horizontal migration ability of LoVo and HCT116 cells.
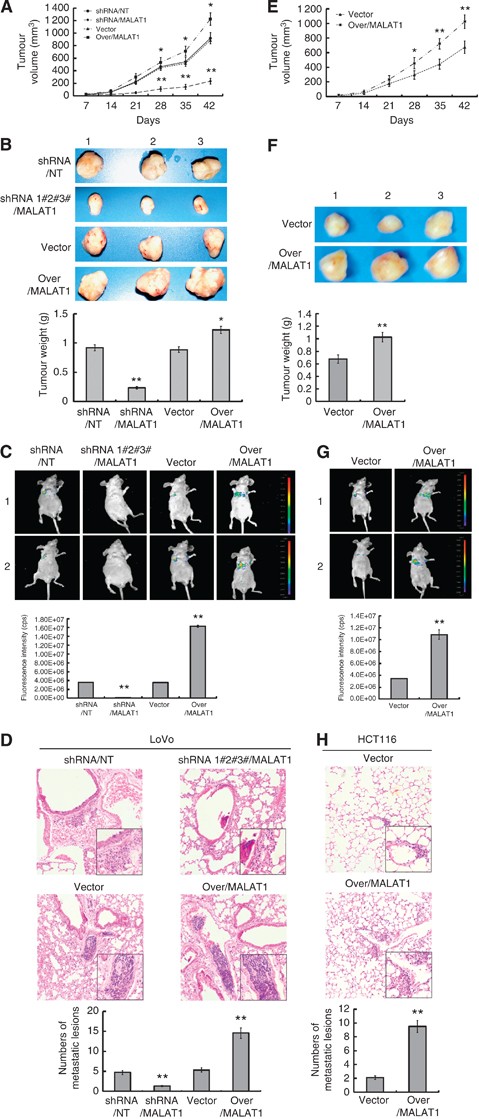
MALAT1 promoted the transplanted tumour growth and metastasis in nude mice
In order to observe the effect of MALAT1 in vivo, LoVo-shRNA/NT, LoVo-shRNA/MALAT1, LoVo-vector, and LoVo-over/MALAT1 cells were subcutaneously transplanted into nude mice and the tumour growth was calculated based on tumour size. As shown in Figure 2A, tumour development was observed every 7 days after inoculation. Compared to tumours of LoVo-shRNA/NT cells or LoVo-vector cells, tumours of LoVo-shRNA/MALAT1 cells grew significantly slower (volume), while tumours of LoVo-over/MALAT1 grew significantly faster. Furthermore, LoVo-shRNA/MALAT1 tumours weighed significantly less, while LoVo-over/MALAT1 tumours weighed significantly more at 42 days after inoculation, compared to LoVo-shRNA/NT and LoVo-vector tumours (Figure 2B). Quantitative evaluation of MALAT1 in tumours showed that MALAT1 expression was lower in the LoVo-shRNA/MALAT1 group, but higher in LoVo-over/MALAT1 group, compared with the LoVo-shRNA/NT and LoVo-vector groups (Supplementary Figure S2A).
Figure 2
MALAT1 promoted growth and metastasis of LoVo cells in vivo. (A) LoVo-shRNA/NT, LoVo-shRNA/MALAT1, LoVo-vector, and LoVo-over/MALAT1 cells were respectively injected subcutaneously into nude mice (_n_=8). Length and width of the tumours were measured every 7 days. (B) After 42 days, mice were killed for determination of tumour weights. (C) LoVo-shRNA/NT, LoVo-shRNA/MALAT1, LoVo-vector, and LoVo-over/MALAT1 cells were respectively injected into the lateral tail vein. Seven weeks later, the established lungs metastases images were observed by LB983 NIGHTOWL II system. (D) The organs of lung were excised, the metastases originated from i.v. injections were checked by haematoxylin and eosin (H&E) staining, and the numbers of metastatic lesions were counted. The magnification of the microscopic pictures was × 100, and the selected metastatic lesions in the lower right corner were magnified by × 200. **P<0.01, compared with LoVo-shRNA/NT or LoVo-vector group. (E–H) HCT116-vector and HCT116-over/MALAT1 cells were analysed for the influence of MALAT1 on tumour growth, tumour weights, and metastasis ability in vivo as LoVo cells. *P<0.05; **P<0.01, compared with HCT116-vector group.
After investigating the effect of MALAT1 on metastasis ability, we found metastatic tumour lesions in the lung areas of nearly all mice 7 weeks after injection of the four cell types (LoVo-shRNA/NT, LoVo-shRNA/MALAT1, LoVo-vector, and LoVo-over/MALAT1). Fewer lung metastatic lesions were seen in the mice injected with LoVo-shRNA/MALAT1 cells, whereas more lung metastatic lesions were seen in those injected with the LoVo-over/MALAT1, in contrast to those injected with LoVo-shRNA/NT or LoVo-vector cells (Figure 2C). And, the H&E staining showed in the lung that, in contrast to LoVo-shRNA/NT or LoVo-vector group, the numbers of metastatic lesions were decreased largely in LoVo-shRNA/MALAT1 group, but increased greatly in LoVo-over/MALAT1group (Figure 2D).
Regarding HCT116 cells, our results also showed elevated tumour growth (Figure 2E and F), higher MALAT1 expression (Supplementary Figure S2B), and more lung metastatic lesions (Figure 2G and H) in HCT116-over/MALAT1 cells injected mice vs those injected with HCT116-vector cells.
Interaction between MALAT1 and SFPQ but no interaction between MALAT1 and PTBP2, and co-localisation of SFPQ and PTBP2
As the results have demonstrated, MALAT1 may promote cell proliferation and migration in vitro, and enhance tumour growth and metastasis in vivo in CRC. We further explored any possible underlying mechanisms of these effects. First, we investigated the role of MALAT1 on the expressions of SFPQ and PTBP2, but found no obvious variation of SFPQ and PTBP2 at the mRNA and protein level regardless of whether the expression of MALAT1 was downregulated or upregulated (Supplementary Figure S2C and S2D). This excluded the possibility that MALAT1 may regulate the function of SFPQ and PTBP2 by changing their mRNA or protein expression, thereby indicating other mechanisms may be involved.
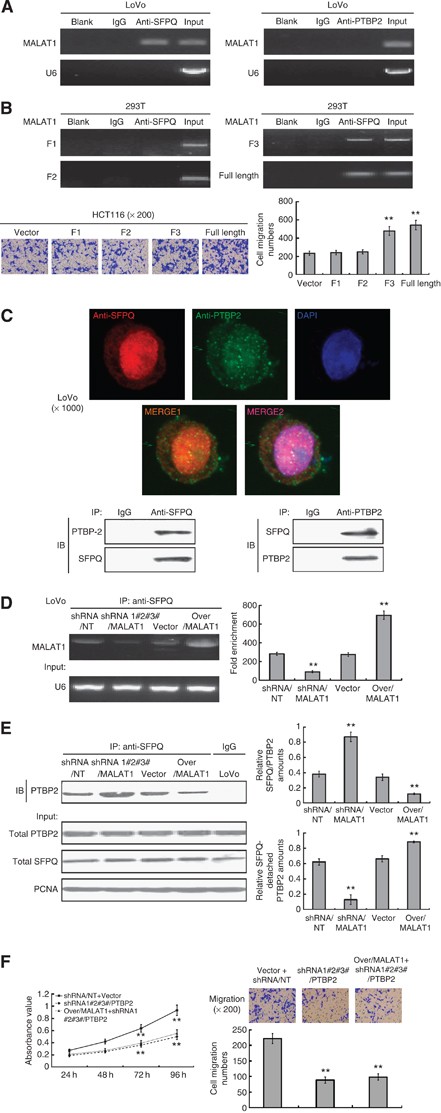
In research on tumorigenesis, several non-coding RNAs binding to the SFPQ protein was found, which mediated pro-oncogene transcription (Gozani et al, 1994; Wang et al, 2009). MALAT1 was just one among several important SFPQ-binding RNAs. Based on our RIP assay, using the SFPQ antibody and MALAT1 special primers, we found that MALAT1 could bind to the SFPQ protein in CRC LoVo cells. However, there was no direct interaction between MALAT1 and PTBP2 (Figure 3A). According to Xu et al (2011), MALAT1 has an important biological motif located at the 3′ end of MALAT1, so we used above constructed expression vector: pLV4-MALAT1/F1, pLV4-MALAT1/F2, pLV4-MALAT1/F3, and pLV4-over/MALAT1 to investigate what’s the region of interaction between MALAT1 and SFPQ. Above four vectors were transfected into 293T cells, together with the pLV4-over/SFPQ vector, following by the RIP analysis. As demonstrated in Figure 3B, the interaction between MALAT1/F3 and SFPQ was found, so was the interaction between full-length MALAT1 and SFPQ. Nevertheless, no interaction between SFPQ and MALAT1/F1 or MALAT1/F2 was found. We next settled to overexpress MALAT1-F1, F2, F3 and full-length individually in HCT116, and investigate the migration ability of the transfected cells. As expected, the results showed that F3 fragment could increase the migration of HCT116 cells, while F1 and F2 fragments by themselves cannot do so. These results implied that the 3′ end of MALAT1 indeed played vital biological function in promoting the invasion and migration of CRC cells.
Figure 3
MALAT1 competitively bound to SFPQ and released SFPQ from the SFPQ/PTBP2 complex. (A) RIP analysis between MALAT1 and SFPQ. As a control, mouse monoclonal IgG was used. The blank group was the PCR results with no cDNA, and the input group was the cDNA from cell lysates without RIP procedure. U6, a abundant nuclear RNA, was used as a negative control for enrichment calculation. RIP analysis between MALAT1 and PTBP2 was performed as SFPQ. (B) Four constructed expression vector: pLV4-MALAT1/F1, pLV4-MALAT1/F2, pLV4-MALAT1/F3, and pLV4-over/MALAT1, respectively, together with the pLV4-over/SFPQ vector, were co-transfected into 293T cells, following by RIP analysis, to verify the region of interaction between MALAT1 and SFPQ. Above four expression vectors, together with pLV4-vector, were also transfected into HCT116 cells, and the cell migration was analysed by transwell method. **P<0.01, compared with pLV4-vector group. (C) Immunofluorescence and immunoprecipitation analysis for co-localisation of SFPQ and PTBP2. Protein SFPQ and PTBP2 were respectively detected by Cy3-conjugated secondary antibody (red colour) and FITC-conjugated secondary antibody (green colour), and the nucleus was stained with DAPI (blue colour). The images were taken with a TCS SP2 spectral confocal system. Image MERGE1 was merged with red and green colours, and image MERGE2 was merged with red, green and blue colours. The magnification of the microscopic pictures was × 1000. Immunoprecipitation was applied to verify the results of immunofluorescence. (D) RIP analysis was used to detect the effect of MALAT1 expression on the interaction between MALAT1 and SFPQ in LoVo-shRNA/NT, LoVo-shRNA/MALAT1, LoVo-vector and LoVo-over/MALAT1, and the quantities of the binding were determined by density analysis. **P<0.01, compared with LoVo-shRNA/NT or LoVo-vector group. (E) Extracted proteins from above four cells were immunoprecipitated with anti-SFPQ antibody or mouse IgG control. The precipitates were subjected to western blot with anti-PTBP2 antibody. **P<0.01, compared with LoVo-shRNA/NT or LoVo-vector group. (F) MALAT1 was overexpressed and a simultaneous knockdown of PTBP2 was performed in LoVo cells, and the growth and metastasis were detected and evaluated. **P<0.01, compared with LoVo-shRNA/NT-vector group. The full colour version of this figure is available at British Journal of Cancer online.
Several findings have indicated a specific interaction between SFPQ and PTBP2, such as co-localisation of SFPQ and PTBP2 in protein binding assays. In order to test whether SFPQ/PTBP2 complexes exist in the nucleus of LoVo, we preformed double immunofluorescence staining in LoVo cells and analysed the staining under confocal microscopy. We found both SFPQ and PTBP2 proteins in the nucleus, and a fraction of SFPQ and PTBP2 co-localised (Figure 3C, upper panel). Immunoprecipitation has also confirmed that protein SFPQ and PTBP2 could bind to each other in LoVo cells (Figure 3C, lower panel).
MALAT1 competitively bound to SFPQ and released SFPQ from the SFPQ/PTBP2 complex
As noted above, there was interaction between MALAT1 and SFPQ. In studying the impact of MALAT1 expression on the binding of MALAT1 and SFPQ, we found that MALAT1 binding to SFPQ decreased in LoVo-shRNA/MALAT1 cells, while MALAT1 binding to SFPQ increased in LoVo-over/MALAT1 cells (Figure 3D). This indicated that with upregulation of MALAT1 expression, the MALAT1 binding to SFPQ increased, implying that overexpression of MALAT1 might affect the function of SFPQ.
In order to understand the functional relevance of the association among PTBP2, SFPQ, and MALAT1, we performed immunoprecipitation tests on LoVo-shRNA/NT, LoVo-shRNA/MALAT1, LoVo-vector, and LoVo-over/MALAT1 cells. The results showed that, in contrast to the LoVo-shRNA/NT and LoVo-vector group, the LoVo-shRNA/MALAT1 group had a stronger binding of PTBP2 to the SFPQ, and the LoVo-over/MALAT1 group had a weaker binding of PTBP2 to the SFPQ, indicating that SFPQ-detached PTBP2 in the nucleus might be accordingly increased in the LoVo-over/MALAT1 cells but decreased in LoVo-shRNA/MALAT1cells (Figure 3E). Regarding the interaction between MALAT1 and SFPQ, we propose that MALAT1 might competitively bind to SFPQ and release SFPQ from the SFPQ/PTBP2 complex; this in turn increases the SFPQ-detached PTBP2. We next overexpressed MALAT1 (that should squench SFPQ), and performed a simultaneous knockdown of PTBP2, and the results showed a decrease in tumour growth and migration (Figure 3F). This implied that SFPQ-detached PTBP2 could enhance tumour growth and migration.
SFPQ, not PTBP2, played a critical role in the regulatory effect of MALAT1 on cell proliferation and migration
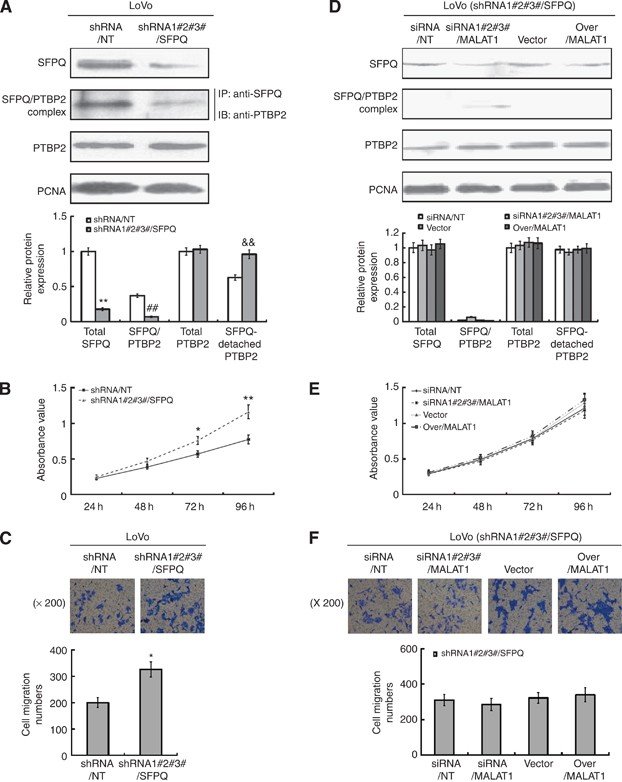
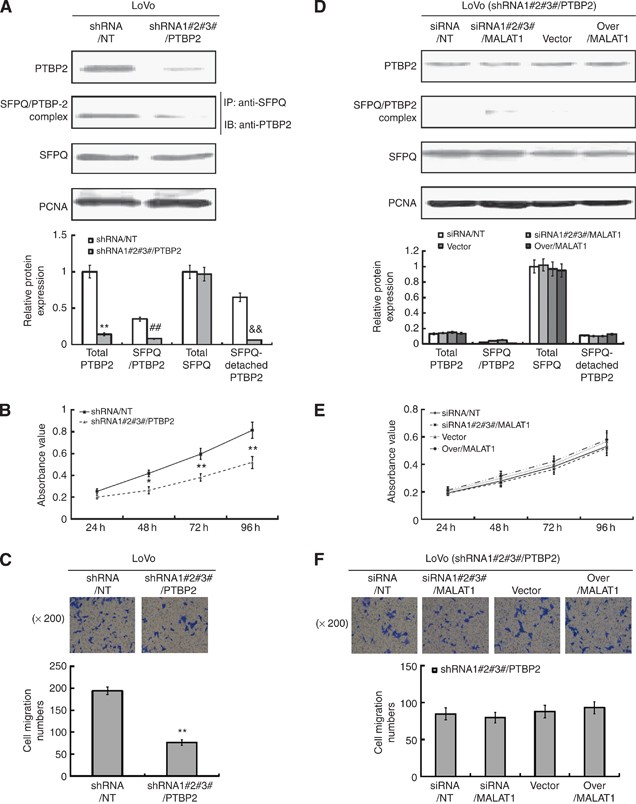
To further clarify the role of SFPQ in the regulatory effect of MALAT1 in cell proliferation and migration, we downregulated the SFPQ expression in LoVo cells with stable transfection of shRNA/SFPQ (Supplementary Figure S2E provided the silent efficacy of three shRNAs, and in our experiment we performed the transfection with three shRNAs together). We found that, in LoVo-shRNA/SFPQ cells, the SFPQ expression downregulated obviously, and the SFPQ/PTBP2 complex decreased accordingly, which in turn led to the increased SFPQ-detached PTBP2 protein to a certain extent (Figure 4A). Naturally, the cell proliferation and migration elevated because of the increased SFPQ-detached PTBP2 (Figure 4B and C). However, being knockdown of SFPQ in LoVo cells, MALAT1 had a very limited effect on the SFPQ/PTBP2 complex and the quantity of SFPQ-detached PTBP2 (Figure 4D). As a result, the proliferation and migration of LoVo cells remained unchanged (Figure 4E and F), implying that SFPQ plays a key role in the regulatory effect of MALAT1 on SFPQ/PTBP2 complex and the downstream cell proliferation and migration.
Figure 4
SFPQ played a key role in the regulatory effect of MALAT1 on cell proliferation and migration. (A) LoVo cells were transfected with shRNA/NT or shRNA/SFPQ, and the stable knockdown cells were selected with neomycin. The silencing efficiency of SFPQ was evaluated by western blot. Total quantities of SFPQ/PTBP2 complex were evaluated by immunoprecipitation. Total PTBP2 and control PCNA proteins were detected by western blot. **P<0.01; ##P<0.01; &&P<0.01, compared with LoVo-shRNA/NT cells. (B and C) MTT and transwell assay were performed to evaluate the proliferation and migration ability of LoVo-shRNA/NT and LoVo-shRNA/SFPQ cells. *P<0.05, compared with LoVo-shRNA/NT cells. (D) LoVo-shRNA/SFPQ cells were transiently transfected with siRNA/NT, siRNA/MALAT1, pLV4-vector, and pLV4-over/MALAT1 for 48 h. Total quantities of SFPQ/PTBP2 complex were evaluated by immunoprecipitation. Total SFPQ, PTBP2 and control PCNA proteins were detected by western blot. (E and F) MTT and transwell assay were performed to evaluate the proliferation and migration ability of four kinds of LoVo-shRNA/SFPQ cells transiently transfected with siRNA/NT, siRNA/MALAT1, pLV4-vector, and pLV4-over/MALAT1, respectively.
We also observed the role of PTBP2 on the regulatory effect of MALAT1 on cell proliferation and migration in LoVo cells with downregulated PTBP2 expression by shRNA/PTBP2 (Supplementary Figure S2F provided the silent efficacy of three shRNAs, and in our experiment we performed the transfection with three shRNAs together). Our results showed that, after downregulation of PTBP2, the total PTBP2 protein decreased largely, and the SFPQ/PTBP2 complex was little detected (Figure 5A). So the SFPQ-detached PTBP2 protein should be very little, which made the cell proliferation and migration of LoVo-shRNAPTBP2 cells decreased to a large extent (Figure 5B and C). Being present with the unchanged SFPQ protein, however, MALAT1 could hardly regulate the SFPQ/PTBP2 complex by binding to SFPQ and releasing more SFPQ-detached PTBP2 protein (Figure 5D), for the total PTBP2 was so little that the effect of MALAT1 was nearly neglected. As a result, the proliferation and migration of LoVo-shRNAPTBP2 cells changed little (Figure 5E and F), this demonstrated that, although the SFPQ protein was present, MALAT1 could not regulate the biological function by binding to SFPQ and releasing more SFPQ-detached PTBP2 from the SFPQ/PTBP2 complex, because of quite a few decrease of total SFPQ/PTBP2 complex resulted from the knockdown of total PTBP2 protein.
Figure 5
Role of PTBP2 in the regulation of MALAT1 on the proliferation and migration. (A) LoVo cells were transfected with shRNA/NT or shRNA/PTBP2, and the cells of stable knockdown were selected with neomycin. The silencing efficiency of PTBP2 was evaluated by western blot. Total quantities of SFPQ/PTBP2 complex were evaluated by immunoprecipitation. Total SFPQ and control PCNA proteins were detected by western blot. **P<0.01; ##P<0.01; &&P<0.01, compared with LoVo-shRNA/NT cells. (B and C) MTT and transwell assay were executed to evaluate the proliferation and migration ability of LoVo-shRNA/NT and LoVo-shRNA/PTBP2 cells. **P<0.05, compared with LoVo-shRNA/NT cells. (D) LoVo-shRNA/PTBP2 cells were transiently transfected with siRNA/NT, siRNA/MALAT1, pLV4-vector, and pLV4-over/MALAT1 for 48 h. Total quantities of SFPQ/PTBP2 complex were evaluated by immunoprecipitation. Total SFPQ, PTBP2 and control PCNA proteins were detected by western blot. (E and F) MTT and transwell assay were executed to evaluate the proliferation and migration ability of four kinds of LoVo-shRNA/PTBP2 cells transiently transfected with siRNA/NT, siRNA/MALAT1, pLV4-vector, and pLV4-over/MALAT1, respectively.
Expression of MALAT1, SFPQ and PTBP2 in human CRC tissues
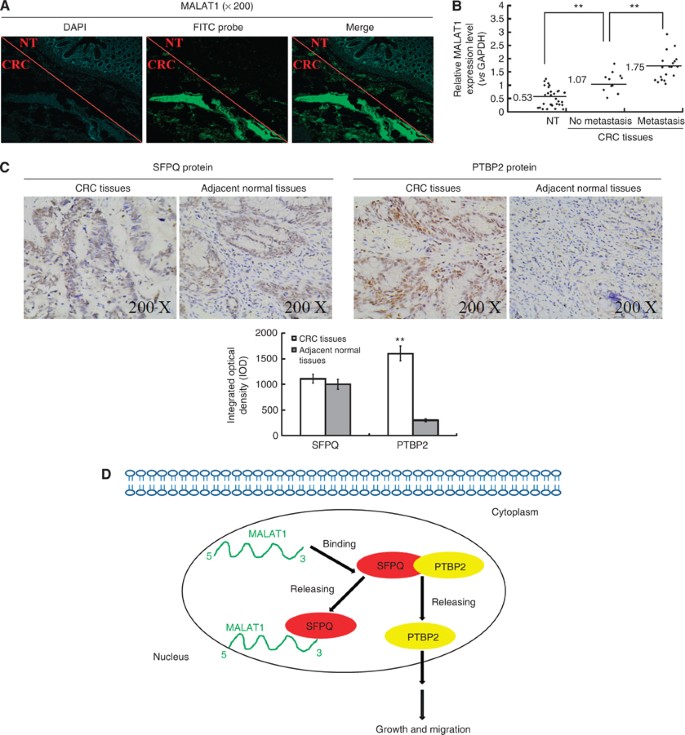
Finally, we investigated MALAT1 expression in tumour tissues and adjacent normal tissues collected from patients with CRC by fluorescence in situ hybridisation (FISH) and real-time PCR. From several typical tissues specially selected, with both tumour section and normal section in one tissue, we observed high MALAT1 expression in tumour section, but low MALAT1 expression in normal section by FISH (Figure 6A). The real-time PCR results also showed that, the expression of MALAT1 was higher in tumour tissues than in adjacent normal tissues, and patients with metastasis had higher MALAT1 expression in tumour tissues, compared to those without metastasis (Figure 6B). The results presented here were consistent with our previous research by another detection method (Ji et al, 2013).
Figure 6
Expression of MALAT1 transcripts, SFPQ and PTBP2 protein in human colorectal cancer, and a hypothetical illustration for the role and interaction of MALAT1, SFPQ and PTBP2. (A) Fluorescence in situ hybridisation was applied to investigate the MALAT1 expression in tissues using both tumour section and normal section. (B) Real-time PCR detected the relative MALAT1 expression level in adjacent normal tissues (_n_=60), CRC tissues with no metastasis (_n_=20, **P<0.01, compared with adjacent normal tissues), CRC tissues with metastasis (_n_=40, **P<0.01, compared with CRC tissues with no metastasis). (C) Immunohistochemistry was performed to detect SFPQ and PTBP2 protein expression in CRC tissues and adjacent normal tissues. Integrated optical densities of each protein expression were determined. **P<0.01, compared with adjacent normal tissues. (D) A hypothetical illustration for the role and interaction of MALAT1, SFPQ and PTBP2.
We also detected the expression of SFPQ and PTBP2 in the tumour tissues of patients with CRC, as SFPQ and PTBP2 were shown to be involved in the regulatory effect of MALAT1 both in in vitro experiments in this study and in previous reports (Patton et al, 1993; Gozani et al, 1994). Immunohistochemical staining showed strong expressions of SFPQ in both malignant and nonmalignant colorectal tissues, with no differences seen between the two. In contrast, PTBP2 staining was higher in human malignant compared to nonmalignant tissues (Figure 6C and Table 1). Correlation analysis between PTBP2 expression in CRC tissues and clinicopathological characteristics of CRC patients was conducted, and a statistically significant association was observed between PTBP2 expression and characteristics of metastasis and invasion (Table 2). These data suited to our previous results about the association between MALAT1 expression and clinicopathological characteristics of CRC patients (Ji et al, 2013), which strengthened the importance of the biological function of PTBP2 and MALAT1.
Table 1 Immunohistochemical staining of PTBP2 in human CRC tissues
Table 2 PTBP2 expression and clinicopathological parameters in human CRC tissues
Discussion
Long non-coding RNA MALAT1 is overexpressed in numerous human cancers, such as non-small cell lung cancer (Ji et al, 2003), hepatocellular carcinoma (Lin et al, 2007), bladder cancer (Ying et al, 2012), CRC (Xu et al, 2011), and so on. MALAT1 is predominantly localised in nuclear speckles (Tripathi et al, 2010). Its abundance and aberrant expression in many cancers suggests that it plays an important role in the development of cancer. Gutschner et al (2013) showed that MALAT1-deficient lung cancer cells show impaired migration and form fewer tumours in mice. Wilusz et al (2008) found that MALAT1 functions as a precursor for the production of small RNAs and indentified a highly conserved small RNA of 61 nucleotides originating from the MALAT1 locus, which is broadly expressed in human tissues. In our previous research (Ji et al, 2013), our data have shown the promoted effect of MALAT1 on proliferation and migration in LoVo and HCT116 cells by MTT and transwell assay. In the presented paper, we further demonstrated that MALAT1 could promote the colony formation ability and the horizontal migration ability of LoVo and HCT116 cells by soft agar colony formation assay and wound healing assay. Xu et al (2011) found that MALAT1 has an important biological motif located at the 3′ end of MALAT1 (6918nt-8441nt), which plays a pivotal role in the biological processes of cell proliferation, migration and invasion. To this point, we verified the importance of 3′ end of MALAT1 by transfecting MALAT1 fragments expression vectors into 293T cells, together with the pLV4-over/SFPQ vector, following by the RIP analysis. The 3′ end of MALAT1 (MALAT1/F3) showed strong interaction with SFPQ protein, just like the full-length MALAT1. However, the detail binding location still needed further investigation in the future.
Our results also suggested that MALAT1 could promote tumour growth and metastasis in CRC in vivo. However, metastasis was not found in the lymph nodes, lungs, or adrenal glands (data not shown), causing concern that the subcutaneously transplanted tumour model was not an established metastasis model. However, experimental lung metastasis and in vivo optical imaging indicated that MALAT1 could promote the metastasis of CRC cells in vivo.
Although previous research has demonstrated the biological function of MALAT1 (Ji et al, 2003; Schmidt et al, 2011), the effective mechanism of MALAT1 still needs further investigation. Guo et al (2010) showed that MALAT1 was involved in cervical cancer cell growth, cell cycle progression, and invasion through the regulation of gene expression, such as caspase-3, -8, Bax, Bcl-2, and Bcl-xL. Our data demonstrated that MALAT1 could affect the function of SFPQ and PTBP2 protein. SFPQ is a tumour suppressor protein inhibiting oncogene expression (Patton et al, 1993; Gozani et al, 1994), containing a DNA-binding domain, and thus could form the SFPQ and DNA-binding synergies in a number of human cell lines. SFPQ also contains two RBDs. Proto-oncogene PTBP2 is highly expressed in cancer cells and can promote their growth (He et al, 2007). MALAT1, however, regulated the function of SFPQ and PTBP2 but did not change their mRNA or protein expression, indicating other mechanisms may be involved.
Furthermore, our results showed that MALAT1 could promote growth and migration in CRC cells by competitively binding to tumour suppressor gene SFPQ and releasing SFPQ from the SFPQ/PTBP2 complex, which then leads to increased SFPQ-detached PTBP2. Li et al (2009) found that MALAT1 could interact with SFPQ and thereby inhibit the combination of SFPQ protein with proto-oncogene GAGE6 transcriptional regulatory regions, in order to promote a large number of transcription GAGE6 and induce tumours. Meissner et al (2000) studied the positioning of SFPQ and PTBP2, and found that SFPQ can combine with PTBP2, thereby affect the function of PTBP2. Downregulation of SFPQ leads to the large decrease of MALAT1 regulation effect on PTBP2 and downstream proliferation and migration. SFPQ could naturally bind not only to MALAT1, but also to other DNA such as GAGE6 (De Backer et al, 1999), or to RNA such as L1PA16, HN, MER11C (Li et al, 2009; Wang et al, 2009), or to proteins such as TDP-43 (Sephton et al, 2011), RAD51D (Rajesh et al, 2011). This implied that SFPQ might mediate the effects of other factors and play different regulatory roles. Thus, targeting MALAT1 with antisense oligonucleotides and/or upregulating the expression of SFPQ provides a potential therapeutic approach to prevent CRC metastasis.
Our experiment also indicated that PTBP2 could directly promote the proliferation and migration of CRC cells, similar to the results in ovarian cancer (He et al, 2007). But the PTBP2 downstream targets still need extensive research. Galbán et al (2008) found that, PTBP2 and HUR jointly promoted the translation of hypoxia-inducible factor 1 α (HIF-1_α_), and HIF-1_α_ regulated many cancer-related genes, such as vascular endothelial growth factor, _β_-catenin, and many other genes implicated in cellular functions, such as proliferation, angiogenesis, and survival (Wenger et al, 2005). In our previous study (Ji et al, 2013), we have found that MALAT1 gene could indirectly increase the nuclear translocation of _β_-catenin from cytoplasm, which implied the correlation between MALAT1 and Wnt/_β_-catenin signalling pathway. Ying et al (2012) also revealed that, in bladder cancer, MALAT1 promoted EMT by activating Wnt signalling in vitro. It provides our clues that we could continue further investigation of PTBP2 downstream targets through the PTBP2–HIF-1_α_–_β_-catenin signalling pathway.
Fluorescence in situ hybridisation and real-time PCR both suggested that MALAT1 had a higher expression in CRC tissues than in adjacent normal tissues, and that the MALAT1 transcript was predominantly localised in the cell nuclei. Our data are in agreement with what has been previously reported including our own (Meissner et al, 2000; Xu et al, 2011), and similar to other cancer tissues (Ji et al, 2003; Lin et al, 2007; Ying et al, 2012). This indicates that MALAT1 transcript is associated with the development of CRC, and might be involved in the regulation of metastasis. Immunohistochemical staining showed strong expressions of SFPQ in both adjacent normal and malignant colorectal tissues, with no obvious difference between the two. In contrast, PTBP2 staining was higher in human malignant tissues than in adjacent normal tissues. A statistically significant association was observed between PTBP2 expression and characteristics of metastasis and invasion, and these data suited to our previous results about the association between MALAT1 expression and clinicopathological characteristics of CRC patient (Ji et al, 2013). It suggested that, the joint detection of PTBP2 and MALAT1 may serve as both predictive marker and therapeutic target in CRC.
In conclusion, we have demonstrated that SFPQ plays a vital role in the entire regulation process, and that PTBP2 was the relative final effector in our research. In details, MALAT1 could promote tumour growth and migration in CRC cells by competitively binding to tumour suppressor gene SFPQ and releasing SFPQ from the SFPQ/PTBP2 complex, which leads to increased SFPQ-detached proto-oncogene PTBP2 (Figure 6D). Our findings imply that the long non-coding RNA MALAT1 might serve as a potential therapeutic target in CRC.
Change history
12 August 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Bartels CL, Tsongalis GJ (2009) MicroRNAs: novel biomarkers for human cancer. Clin Chem 55 (4): 623–631.
Article CAS PubMed Google Scholar - Bernards R, Weinberg RA (2002) A progression puzzle. Nature 418 (6900): 823.
Article CAS PubMed Google Scholar - Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136 (4): 642–655.
Article CAS PubMed PubMed Central Google Scholar - Chaumeil J, Augui S, Chow JC, Heard E (2008) Combined immunofluorescence, RNA fluorescent in situ hybridization, and DNA fluorescent in situ hybridization to study chromatin changes, transcriptional activity, nuclear organization, and X-chromosome inactivation. Methods Mol Biol 463: 297–308.
Article CAS PubMed Google Scholar - Christofori G (2006) New signals from the invasive front. Nature 441 (7092): 444–450.
Article CAS PubMed Google Scholar - De Backer O, Arden KC, Boretti M, Vantomme V, De Smet C, Czekay S, Viars CS, De Plaen E, Brasseur F, Chomez P, Van den Eynde B, Boon T, van der Bruggen P (1999) Characterization of the GAGE genes that are expressed in various human cancers and in normal testis. Cancer Res 59 (13): 3157–3165.
CAS PubMed Google Scholar - Eddy SR (2001) Non-coding RNA genes and the modern RNA world. Nat Rev Genet 2 (12): 919–929.
Article CAS PubMed Google Scholar - Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61 (5): 759–767.
Article CAS PubMed Google Scholar - Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3 (6): 453–458.
Article CAS PubMed Google Scholar - Galbán S, Kuwano Y, Pullmann R Jr, Martindale JL, Kim HH, Lal A, Abdelmohsen K, Yang X, Dang Y, Liu JO, Lewis SM, Holcik M, Gorospe M (2008) RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1. Mol Cell Biol 28 (1): 93–107.
Article PubMed Google Scholar - Gandellini P, Folini M, Longoni N, Pennati M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta P, Valdagni R, Daidone MG, Zaffaroni N (2009) miR-205 exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cepsilon. Cancer Res 69 (6): 2287–2295.
Article CAS PubMed Google Scholar - Gozani O, Patton JG, Reed R (1994) A novel set of spliceosome-associated proteins and the essential splicing factor PSF bind stably to pre-mRNA prior to catalytic step II of the splicing reaction. EMBO J 13 (14): 3356–3367.
Article CAS PubMed PubMed Central Google Scholar - Guo FJ, Li YL, Liu Y, Wang JJ, Li YH, Li GC (2010) Inhibition of metastasis-associated lung adenocarcinoma transcript 1 in CaSki human cervical cancer cells suppresses cell proliferation and invasion. Acta Biochim Biophys Sin (Shanghai) 42 (3): 224–229.
Article CAS Google Scholar - Gutschner T, Hämmerle M, Diederichs S (2013) MALAT1—a paradigm for long noncoding RNA function in cancer. J Mol Med (Berl) 91 (7): 791–801.
Article CAS Google Scholar - Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, Zörnig M, MacLeod AR, Spector DL, Diederichs S (2013) The non-coding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res 73 (3): 1180–1189.
Article CAS PubMed Google Scholar - Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES (2009) Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458 (7235): 223–227.
Article CAS PubMed PubMed Central Google Scholar - He X, Pool M, Darcy KM, Lim SB, Auersperg N, Coon JS, Beck WT (2007) Knockdown of polypyrimidine tract-binding protein suppresses ovarian tumor cell growth and invasiveness in vitro. Oncogene 26 (34): 4961–4968.
Article CAS PubMed PubMed Central Google Scholar - Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A (2007) A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 8: 39.
Article PubMed PubMed Central Google Scholar - Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Müller-Tidow C (2003) MALAT-1, a novel noncoding RNA, and thymosin b4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22 (39): 8031–8041.
Article PubMed Google Scholar - Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, Sun J, Cai J, Qin J, Ren J, Li Q (2013) Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/_β_-catenin signal pathway. PLoS One 8 (11): e78700.
Article CAS PubMed PubMed Central Google Scholar - Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR (2007) RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316 (5830): 1484–1488.
Article CAS PubMed Google Scholar - Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY, Zhang F, Wu LM, Chen LM, Zheng SS (2012) Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol 29 (3): 1810–1816.
Article CAS PubMed Google Scholar - Li L, Feng TT, Lian YY, Zhang GF, Garen A, Song X (2009) Role of human noncoding RNAs in the control of tumorigenesis. Proc Natl Acad Sci USA 106 (31): 12956–12961.
Article CAS PubMed PubMed Central Google Scholar - Lin R, Maeda S, Liu C, Karin M, Edgington TS (2007) A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene 26 (6): 851–858.
Article CAS PubMed Google Scholar - Meissner M, Dechat T, Gerner C, Grimm R, Foisner R, Sauermann G (2000) Differential nuclear localization and nuclear matrix association of the splicing factors PSF and PTB. J Cell Biochem 76 (4): 559–566.
Article CAS PubMed Google Scholar - Patton JG, Mayer SA, Tempst P, Nadal-Ginard B (1991) Characterization and molecular cloning of polypyrimdine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Genes Dev 5 (7): 1237–1251.
Article CAS PubMed Google Scholar - Patton JG, Porto EB, Galceran J, Temps P, Nadal-Ginard Bl (1993) Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev 7 (3): 393–406.
Article CAS PubMed Google Scholar - Rajesh C, Baker DK, Pierce AJ, Pittman DL (2011) The splicing-factor related protein SFPQ/PSF interacts with RAD51D and is necessary for homology-directed repair and sister chromatid cohesion. Nucleic Acids Res 39 (1): 132–145.
Article CAS PubMed Google Scholar - Rossi S, Sevignani C, Nnadi SC, Siracusa LD, Calin GA (2008) Cancer-associated genomic regions (CAGRs) and noncoding RNAs: bioinformatics and therapeutic implications. Mamm Genome 19 (7-8): 526–540.
Article CAS PubMed Google Scholar - Schmidt LH, Spieker T, Koschmieder S, Schäffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D, Marra A, Hillejan L, Wiebe K, Berdel WE, Wiewrodt R, Muller-Tidow C (2011) The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol 6 (12): 1984–1992.
Article PubMed Google Scholar - Sephton CF, Cenik C, Kucukural A, Dammer EB, Cenik B, Han Y, Dewey CM, Roth FP, Herz J, Peng J, Moore MJ, Yu G (2011) Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J Biol Chem 286 (2): 1204–1215.
Article CAS PubMed Google Scholar - Takayama T, Miyanishi K, Hayashi T, Sato Y, Niitsu Y (2006) Colorectal cancer: genetics of development and metastasis. J Gastroenterol 41 (3): 185–192.
Article CAS PubMed Google Scholar - Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV (2010) The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 39 (6): 925–938.
Article CAS PubMed PubMed Central Google Scholar - Tseng JJ, Hsieh YT, Hsu SL, Chou MM (2009) Metastasis associated lung adenocarcinoma transcript 1 is up-regulated in placenta previa increta/percreta and strongly associated with trophoblast-like cell invasion in vitro. Mol Hum Reprod 15 (11): 725–731.
Article CAS PubMed Google Scholar - Wang G, Cui Y, Zhang GF, Garen A, Song X (2009) Regulation of proto-oncogene transcription, cell proliferation, and tumorigenesis in mice by PSF protein and a VL30 noncoding RNA. Proc Natl Acad Sci USA 106 (39): 16794–16798.
Article CAS PubMed PubMed Central Google Scholar - Watson AJ, Collins PD (2011) Colon cancer: a civilization disorder. Dig Dis 29 (2): 222–228.
Article PubMed Google Scholar - Wenger RH, Stiehl DP, Camenisch G (2005) Integration of oxygen signaling at the consensus HRE. Sci STKE 2005 (306): re12.
PubMed Google Scholar - Wilusz JE, Freier SM, Spector DL (2008) 3′-end processing of a long nuclear retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 135 (5): 919–932.
Article CAS PubMed PubMed Central Google Scholar - Xu CA, Yang MH, Tian J, Wang XY, Li ZG (2011) MALAT-1: a long non-coding RNA and its important 3′ end functional motif in colorectal cancer metastasis. Int J Oncol 39 (1): 169–175.
PubMed Google Scholar - Ying L, Chen Q, Wang YW, Zhou ZH, Huang YR, Qiu F (2012) Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst 8 (9): 2289–2294.
Article CAS PubMed Google Scholar
Acknowledgements
We thank Elektra McDermott (Developmental Editor, Springer) for helping with the critical reading of the manuscript. This work was supported by National Natural Science Foundation of China (81303102, 81303103, 81202812, 81273958), Program of Shanghai Municipal Education Commission (12YZ058), Shanghai Municipal Health Bureau (2011ZJ030, 20114Y013, 20114Y001, 20114037).
Author information
Author notes
- Q Ji and L Zhang: These authors contributed equally to this work.
Authors and Affiliations
- Department of Medical Oncology, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Q Ji, X Liu, L Zhou, W Wang, Z Han, H Sui, Y Tang, N Liu & Q Li - Shanghai Key Laboratory of Regulatory Biology, Institute of Biomedical Sciences and School of Life Sciences, East China Normal University, Shanghai, China
L Zhang - Cancer Institute & Longhua Hospital, Shanghai University of Chinese Medicine, Shanghai, China
Y Wang - Department of Oncology, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai, China
J Ren & F Hou
Authors
- Q Ji
You can also search for this author inPubMed Google Scholar - L Zhang
You can also search for this author inPubMed Google Scholar - X Liu
You can also search for this author inPubMed Google Scholar - L Zhou
You can also search for this author inPubMed Google Scholar - W Wang
You can also search for this author inPubMed Google Scholar - Z Han
You can also search for this author inPubMed Google Scholar - H Sui
You can also search for this author inPubMed Google Scholar - Y Tang
You can also search for this author inPubMed Google Scholar - Y Wang
You can also search for this author inPubMed Google Scholar - N Liu
You can also search for this author inPubMed Google Scholar - J Ren
You can also search for this author inPubMed Google Scholar - F Hou
You can also search for this author inPubMed Google Scholar - Q Li
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toQ Li.
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ji, Q., Zhang, L., Liu, X. et al. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex.Br J Cancer 111, 736–748 (2014). https://doi.org/10.1038/bjc.2014.383
- Received: 16 February 2014
- Revised: 10 June 2014
- Accepted: 17 June 2014
- Published: 15 July 2014
- Issue Date: 12 August 2014
- DOI: https://doi.org/10.1038/bjc.2014.383