Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes (original) (raw)
Introduction
Attention-deficit/hyperactivity disorder (ADHD), the most common neuropsychiatric disorder in children, with a 5.2% worldwide pooled prevalence rate,1 causes significant academic, behavioral and social impairment throughout the life span.2, 3 The phenotype consists of extreme manifestations of continuous traits, including hyperactivity, impulsivity, and dysfunction in executive and self-regulatory skills that involve working memory, temporal organization, planning, organizing, maintaining focus, effort and motivation.4, 5
Brain studies of patients with ADHD have shown abnormalities in frontal-striatal and cerebellar circuitry.6, 7 Although dopaminergic and noradrenergic medications are clinically effective,8 animal models provide support for the involvement of additional molecular pathways.9 Twin studies estimate a heritability of up to 90%,10 indicating a substantial genetic contribution. However, genetic association studies thus far have not identified any genes definitively conferring major risk.10, 11 ADHD comorbidity occurs in many neuropsychiatric disorders, including autism, Tourette syndrome and in children with schizophrenic parents.12, 13
Recently, investigations of autism14, 15, 16 and schizophrenia17, 18, 19, 20, 21 have implicated de novo and/or rare copy number variations (CNVs) as being potentially pathogenic in these disorders. These findings are consistent with a genetic model where any of a large number of individually rare mutations of recent origin, and affecting a substantial number of genes involved in neurodevelopment, can contribute to disease predisposition. Given the highly heritable and variable nature of ADHD, we therefore hypothesized that individually rare and inherited structural variants might contribute to disease risk in ADHD, and that such variants are likely enriched within genes involved in neurological processes and neuropsychiatric disease. To our knowledge, this is the first report investigating the role of structural variants in ADHD.
Materials and methods
ADHD population
Attention-deficit/hyperactivity disorder families were recruited from pediatric and behavioral health clinics in the Philadelphia area. Inclusion criteria included families of European descent with an ADHD proband (age 6–18). Exclusionary criteria included prematurity (<36 weeks), mental retardation, major medical and neurological disorders, pervasive developmental disorder, psychoses and major mood disorders. Blood collected from ADHD families was sent to the Rutgers University Cell and DNA Repository in New Jersey where lymphoblastoid cell lines were developed. Subsequently, DNA was extracted and returned to our laboratory for genotyping.
Healthy control population
Healthy control individuals were recruited from the Philadelphia region through the Hospital's Health Care Network, including four primary care clinics and several group practices and outpatient practices that performed well child visits. Eligibility criteria for this study included both of the following: disease-free children (age range: 6–18 years) and parents of these children who had high-quality, genome-wide genotyping data from blood samples with no serious underlying medical disorder, including, but not limited to neurodevelopmental disorders, cancer, chromosomal abnormalities and known metabolic or genetic disorders. DNA samples from a small set of parents of the participating children were also genotyped and used to assess CNV heritability patterns. A total of 2026 individuals passed all quality control measures and qualified for the study. All participants and/or their parents signed an informed consent permitting the use of their genotypes and health-care records for the study. Ancestry informative markers available on the HumanHap550 BeadChip22 were used to evaluate these 2026 participants to confirm ethnicity.
Measures
A child psychiatrist assessed diagnostic status of ADHD probands by administering the Schedule for Affective Disorders and Schizophrenia for School Age Children -IV Revised (K-SADS P-IVR).23 Intraclass correlations between raters for the diagnostic symptoms of the major disorders were assessed through videotape reviews. Parental ADHD was assessed using the ADHD Self-Report Scale.24, 25 Parents provided consent and children assent.
Genotyping
All samples were uniformly genotyped at the Center for Applied Genomics at the Children's Hospital of Philadelphia. DNA samples were surveyed for quality both by optical density spectrophotometry and the PicoGreen assay (Molecular Probes, Eugene, OR, USA).26 Samples judged to be of sufficient quality for genotyping were assayed on the Illumina Infinium II HumanHap550 BeadChip (Illumina, San Diego, CA, USA) as previously described.27 As BeadChip images were collected, intensity values were determined for all instances of each bead type, and data files were created that summarized intensity values for each bead type. A bead pool manifest was loaded into BeadStudio along with intensity data for the samples. BeadStudio uses a normalization algorithm to minimize BeadChip to BeadChip variability. Once the normalization was complete, a clustering algorithm was used to evaluate cluster positions for each locus and assign individual genotypes. logR ratios (LRRs) and B allele frequencies were calculated for every single nucleotide polymorphism (SNP) based on these clusters and the genotype of each SNP.
CNV detection and analysis
The Illumina BeadStudio 3.0 software package was used for initial CNV detection analysis. LRRs and B allele frequencies were first exported from BeadStudio. LRR values were used as an additional sample-wide genotype quality control measure, and LRRs with a standard deviation above 0.35 were excluded from the study. Chromosomes were segmented on the basis of LRRs, using the circular binary segmentation algorithm implemented in the R statistical package module DNAcopy 1.7 (http://www.bioconductor.org/). We used the reference clusters for each SNP that were supplied by Illumina from a set of HapMap samples. As these samples are a mixture of males and females, the Log R Ratio distribution for the X chromosome differs from that of the autosomal chromosomes. Accordingly, differing thresholds were chosen based on known samples with X chromosome deletions and duplications. For autosomal chromosomes, we used threshold of acceptance values of −2, −0.3 and 0.25 for the mean LRRs of potential homozygous deletions, hemizygous deletions and duplications, respectively. For males, X chromosome thresholds of −2 and 0.1 were used for hemizygous deletions and duplications, respectively. For females, X chromosome thresholds of −1.5, −0.1 and 0.6 were used for homozygous deletions, hemizygous deletions and duplications, respectively. Initial CNV calls were then further filtered by their B allele frequency distribution patterns. Autosomal and female chromosome X B allele frequency patterns for duplications and homozygous deletions were required to fit a three-copy mode more closely than a two-copy mode. In addition, paucity of AB alleles in the segment was a requirement for calling the segment a hemizygous deletion. For comparative purposes, two CNVs were considered the same or non-unique if their overlapping region contained at least 80% of the SNPs comprising each CNV. We limited the CNV set for consideration to those spanning ⩾10 consecutive SNPs after conducting a trial of >200 experimentally validated CNVs that showed >99% accuracy for our CNV prediction method using this threshold. In addition, all CNVs meeting quality and experimental design metrics were independently assessed by inspection of each BeadStudio visualization.
Literature analysis
Literature analysis used the set of 175 non-olfactory receptor genes overlapping CNVs unique to ADHD probands relative to controls. Literature records associated with these genes were assessed by an expert reviewer to determine whether genes had been previously suggested as candidate genes in neuropsychiatric or neural functioning disorders. Accepted evidence was restricted to significant or suggestive findings in genetic association or structural variation studies performed either as gene-specific surveys or genome-wide. To confirm the literature review process, we determined whether biomedical literature mentioning the ADHD CNV gene set preferentially referred to other neuropsychiatric disorders. As the ADHD gene set was acquired using an independent genetic technique that had no memory of prior studies, a null hypothesis would predict that MEDLINE abstracts mentioning the 175 ADHD genes would not be enriched for terms related to autism or schizophrenia, the two disorders comorbid with ADHD with sufficient literature sampling sizes. For this process, sets of genes co-mentioned with terms related to autism or schizophrenia in MEDLINE abstracts and abstract metadata were identified, using the literature mining tool FABLE (http://fable.chop.edu), the Phenopedia tool of the gene resource HuGE Navigator (http://hugenavigator.net/), and for autism queries, the autism gene database AutDB (http://www.mindspec.org/autdb.html). FABLE query terms used were ‘autism’ and/or ‘schizophrenia’, with or without terms relating to structural variation (see Table 1). HuGE Navigator concepts used were Autistic Disorder, Schizophrenia, and for controls, Meningioma, Astrocytoma, Parkinson Disease, and Alzheimer Disease. For AutDB, we used all genes listed as candidates as the test group. For all three resources, the sets of genes implicated in the queried diseases were tested for significantly enriched representation of ADHD CNV genes using the single-sample proportions test.
Table 1 Enrichment of ADHD CNV genes in autism and schizophrenia candidate genes
In silico functional analysis
Functional and pathway analyses were carried out using the Ingenuity Pathway Analysis software package (http://www.ingenuity.com). Gene Ontology (GO) analysis was carried out using the Explain software from Biobase, as part of the Biobase Knowledgebase (http://bkl.biobase.de/cgi-bin/bkl/idb/1.0/login.cgi?previous_url=searchengine/start.cgi). For both sets of analyses, RefSeq genes overlapping the rare and inherited ADHD CNVs were extracted and input into the requisite pathway analysis models.
Validation
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) validation of the GRM5 deletion was performed on metaphase spreads prepared from the participant-derived lymphoblastoid cell lines using standard methodology. Chromosomes were visualized by counter staining with 4,6-diamidino-2-phenylindole. FISH was performed using fosmid W12-2219g4 and BAC RP11-697e14. FISH analysis was carried out as previously described.28 Bacterial artificial chromosomes and fosmids were isolated using the UltraClean plasmid kit (MoBio Labs Inc., Carlsbad, CA, USA) and probes were labeled with Spectrum Red or Green (Abbott Molecular Inc., IL, USA) by nick translation. FISH images were captured using MacProbe software (Applied Imaging, San Jose, CA, USA).
Real-time quantitative PCR
Primers and probes used to detect PTPRD CNVs were designed using Primer Express software (Applied Biosystems, Foster City CA, USA). Primers were purchased from Integrated DNA Technologies (Coralville, IA, USA). Primer sequences are as follows: F primer: 5′-ACATTTCAGAATATCCATCCTTTGG-3′; R primer: 5′-TGCTAATTCGTCCCAGAACGA-3′. Probes were purchased from Operon Biotechnologies, Inc. (Huntsville, AL, USA). The probe 5′-TGGCAGCCAAGCTAAAGCAAATCCTTG-3′ was labeled with FAM (6-carboxy-fluorescine) along with a quencher (BHQ 1; Biosearch Technologies, Novato, CA, USA). Dilutions of control gDNA of 40, 20, 10, 5 and 2.5 ng μl–1 were used as comparators to test DNA samples diluted to ∼10 ng μl–1. All DNAs, probes and primers were diluted in low EDTA TE buffer. Reactions were run on the ABI 7500 Fast Real-time PCR system (Applied Biosystems) using the following cycling parameters: one cycle at 95 °C for 20 s, followed by 40 cycles at 95 °C for 3 s and 60 °C for 30 s. Each reaction was carried out in a total volume of 20 μl containing 1 × Taqman Fast Universal PCR Master mix, 900 nM of each primer and 250 nM probe. A total of 2.5 μl of DNA was added to each reaction. Reactions were carried out in triplicate. Quantitative PCR analysis software supplied by the manufacturer (Applied Biosystems) was used to calculate standard curves and quantify samples. A control probe set within the albumin gene was used as a reference to determine copy number. A ratio of 1 for assay/albumin was viewed as normal copy number.
Results
The ADHD cohort comprised of 335 complete parent–child trios of European descent recruited from pediatric and behavioral clinics that passed genotype and CNV analysis quality metrics (see Methods). A set of 2026 ethnically matched, disease-free children was used as a healthy control group. All samples were genotyped using the Illumina HumanHap550K BeadChip and a single consistent protocol. Genotype data were uniformly analyzed for CNVs using Illumina's BeadStudio software in combination with a customized adaptation of the circular binary segmentation algorithm.29
Comparison of the CNV sets identified in the 335 probands and the healthy controls showed comparable overall frequencies. ADHD patients had a mean of 27.4 CNVs vs 26.9 for healthy controls (_P_=0.28). No significant differences were seen for deletions (_P_=0.67) or duplications (_P_=0.33). Moreover, no difference was seen in CNV size between the patient and control groups, either overall (_P_=0.91), or for duplications (_P_=0.43) or deletions (_P_=0.12).
We focused on rare inherited variants matching the following criteria: size spanning ⩾10 consecutive SNPs for deletions and ⩾20 SNPs for duplications, and present in at least one parent along with one or more related probands but not in the healthy control set. A total of 222 CNVs (158 deletions and 64 duplications) from 154 probands met these criteria (Supplementary Table S1). The median variant size was 102 kb (range from 7.75 to 1775 kb) with 116 of the CNVs (52%) overlapping one or more genes. The CNVs encompassed or overlapped 229 distinct genes, 23 of which were associated with CNVs from multiple individuals. The largest family of genes affected was the olfactory receptor superfamily (54 genes, including 35 genes associated with a single duplication). Numerous recent studies have shown that olfactory receptors are rapidly evolving, mainly because of an unusually high rate of structural variation and close association of many family members with segmental duplications.30, 31, 32 As these genes are overrepresented in our CNV set and present a skewed distribution that might bias our ability to detect pathway enrichment, these genes were removed from subsequent analyses. This reduced our ADHD CNV gene set to 175 genes.
To determine whether the ADHD gene set preferentially contained genes of neurological significance, we first looked for evidence in the literature for prior implication of these genes in neuropsychiatric disorders. An independent, systematic assessment of MEDLINE abstracts, HUGE Navigator, the autism gene database AutsDB and the candidate gene database SchizophreniaGene showed that genes implicated in autism and/or schizophrenia in any of these resources were significantly enriched in ADHD CNV genes (Table 1), but not for other brain-related disorders (Supplementary Table S2). Manual review of literature associated with the 175 genes determined that at least 22 (28 total CNVs) of these genes were previously implicated by genetic studies in various neurological and neuropsychiatric disorders (Tables 2 and 3), including Tourette syndrome (two genes), autism (four genes) and schizophrenia (15 genes). Furthermore, eight of these 22 genes (11 total CNVs) have been very recently identified in structural variation studies carried out on autism and schizophrenia cohorts: A2BP1, PARK2, NKAIN2, DPP6, CNTNAP2, IMMP2L, AUTS2 and GRM7.
Table 2 Genes in ADHD CNVs previously associated with neuropsychiatric and neurological disorders
Table 3 ADHD CNVs within or overlapping genes previously implicated in neuropsychiatric and neurological disorders
We next determined if our ADHD gene set was enriched for particular biological and disease processes. Using Ingenuity Pathway Analysis, the process categories neurological disease, psychological disorders, nervous system development and function and behavior were among the most significantly enriched categories in the ADHD gene set, even after application of a multiple test correction (MTC) (Table 4). Four of the six most highly enriched GO Biological Process categories were learning, cell adhesion, central nervous system development and hindbrain development (Supplementary Table S3), with learning and cell adhesion trending towards statistical significance after MTC application. When GO Biological Process analysis was restricted to genes that overlapped only CNV deletions, the classes representing learning and hindbrain development retained MTC-applied significance (Table 5). ADHD CNV genes associated with learning and cognition included ATM, CTNND2, GRM5, GRM7, PARK2, PTPRD and UNC13C. As an additional control to show the specificity and independence of the inclusion variables, the same CNV inclusion criteria and analysis strategies were reapplied using a large set of parent–child trios for congenital heart disease. The enriched pathway spectrum was almost entirely dissimilar in the cardiovascular cohort, and no neurological function classes were found significant, even without MTC application.
Table 4 Gene functional categories significantly enriched in ADHD CNVs using ingenuity pathway analysis
Table 5 Gene functional categories significantly enriched in ADHD CNV deletions using Gene Ontology (GO) analysis
Several of the most highly enriched GO cellular component terms involved neural functioning, although no terms were sufficiently enriched to pass significance testing after MTC application. Three genes (GRM5, GRM7 and PARK2) were associated with the GO term postsynaptic membrane, four genes (GRM5, GRM7, PARK2 and UNC13C) with synapse, two genes (CHN2 and UNC13C) with synaptosome and two genes (CPLX2, GOSR1) with the SNARE complex, members of which mediate synaptic vesicle fusion.
Two genes, PTPRD and GRM5, were especially intriguing as putative candidate genes for ADHD. Hemizygous deletions involving the protein tyrosine phosphatase gene, PTPRD, were detected in four unrelated ADHD probands, spanning exon 2, exons 3–9, exon 9 and intron 2 of the gene, respectively (Supplementary Figure S1). PTPRD CNVs were experimentally validated by quantitative PCR in two probands (Supplementary Table S4). The deletion was confirmed by q-PCR in both probands as well as in a transmitting parent for the one case where DNA was available (130-386-1). Two of the four ADHD probands with PTPRD deletions reported symptoms consistent with restless legs syndrome (RLS), which shares high rates of bidirectional comorbidity as well as dopaminergic dysfunction with ADHD.33, 34 Intriguingly, a recent genome-wide association study has implicated PTPRD as a locus for RLS.35 Moreover, genetic variants of PTPRD associate with bronchial asthma,36 which is reported to occur at higher rates in children with ADHD,37 and RLS patients have been shown to have increased use of asthma medications.38 Studies of the PTPRD gene in mice show high expression in the hippocampus, the only brain region reported to be enlarged in ADHD,39 along with involvement in spatial learning, long-term potentiation and axonal guidance of motor neurons.40, 41, 42
Catecholaminergic neurotransmission is widely studied in ADHD and has yielded several candidate genes conferring a small amount of risk, suggesting a potential role for additional neurotransmitter systems. We found a maternally derived GRM5 CNV in three siblings. Both GRM5 and GRM7 (above) belong to the glutamatergic receptor gene family hypothesized to play a role in ADHD.43, 44, 45 The GRM5 variant, a hemizygous deletion of 82 kb (11 SNPs; Supplementary Figure S2), showed uniparental inheritance and was experimentally validated by FISH (Figure 1). Neuropsychiatric assessment indicated that all three children in this family, one 18-year-old male and two females with ages 15 and 11, met the criteria for ADHD with significant impairment since early childhood. All three children showed some improvement in symptoms with medications but persistent overall dysfunction in academic, social and behavioral spheres in spite of superior IQ levels. Assessment of the mother using the ADHD Self-Report Scale indicated a likelihood of ADHD (scores of 20 for part A and 12 for part B).46 The mother, and to a lesser extent, all three children, display problems with spatial orientation, a characteristic also observed in the GRM5 null mouse.47 Intriguingly, GRM5 null mice also have deficiencies in long-term potentiation47 as well as an increase in anxiety-like behavior that can be reduced with GRM5 antagonists.48 ADHD frequently occurs in Fragile X syndrome,49 and GRM5 antagonists have recently been shown to de-repress startle inhibition in Fragile X (FMR1) null mice.
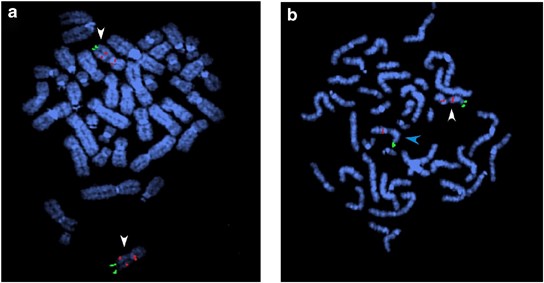
Figure 1
FISH validation of the GRM5 CNV. Metaphase spreads from (a) the non-affected father and (b) an ADHD patient with the deletion hybridized with probes labeled with Spectrum red (red signal) or Spectrum green (green signal). FISH was performed using fosmid W12-2219g4 (red signal) specific for the GRM5 deletion and BAC RP11-697e14, a control probe for the subtelomeric region of chromosome 11q (green signal). A portion of the GRM5 gene, including the 82kb region deleted in this family, is part of a 325kb segmental duplication present in two copies on chromosome 11, one in 11q and the other in 11p. This results in the observation of two red signals for the GRM5 probe in the non-deleted father (a), on both homologs of chromosome 11 indicated by white arrowheads. The annotated GRM5 gene in the reference genome is present on 11q. In the deleted patient (b), the red signal on 11q is missing on one of the two chromosome 11 homologs, because of the deletion within the GRM5 gene, (indicated by a blue arrowhead).
In addition to the parent with the GRM5 variant, we were able to obtain ADHD Self-Report Scales for an additional 20 parents with CNVs overlapping any of the 22 top candidate genes from Table 2 (presented in Supplementary Table S5). Two parents (for genes IMMP2L and A2BP1) met definitive criteria for adulthood ADHD (score of 24 and above on either section A or section B of the ASRS) and three parents (for genes A2BP1, DPP6 and PTPRD) were likely to meet criteria (ASRS score 17–23 on either section). However, interpretation of this data is complex owing to our limited knowledge of adult ADHD and its relationship to the childhood form.
Discussion
The CNV-associated gene set identified in our ADHD cohort did not have an excess of deletions or insertions compared with the controls. It was however significantly enriched for genes reported as candidates in other neuropsychiatric disorders and neurodevelopmental pathways.
In addition to PTPRD and GRM5, several genes in our ADHD CNV gene set are notable, particularly those eight previously reported to have CNVs in another neuropsychiatric disorder (Tables 2 and 3). The A2BP1 gene, whose protein product binds to the ataxin-2 gene responsible for spinocerebellar ataxia-2, was found overlapping with three rare inherited copy number changes in our ADHD probands, and it was also seen in rare de novo copy number changes involving only this gene in a schizophrenic and an autistic proband. Translocations and CNVs disrupting A2BP1 have been reported in patients with autism, schizophrenia, epilepsy and mental retardation. The PARK2, NKAIN2 and DPP6 genes were found overlapping with separate rare inherited copy number changes in our study as well as in CNVs overlapping only the same respective genes in a schizophrenia study. Genomic deletions of the neurexin superfamily gene, CNTNAP2, were also identified in three individuals with schizophrenia and epilepsy. Allelic variants of the CNTNAP2 gene have been found to be associated with autism in three recent studies. Chromosomal rearrangements of both the CNTNAP2 and IMMP2L genes have been reported in patients with Tourette syndrome, and the IMMP2L gene is present within the 7q22 AUTS1 locus identified by several autism association studies. Similarly, chromosomal rearrangements disrupting the AUTS2 gene have been reported in five autism patients. Finally, a rare copy number variant in a schizophrenic patient was found to overlap with the glutamatergic receptor GRM7. We observed an ADHD proband with a CNV in GRM7 who also presented with anxiety. The extinction of conditioned fear, blocked with downregulation of GRM7, suggests that some of these CNVs may potentially explain certain comorbidities frequently associated with ADHD.50, 51
Recent investigations of structural variation in autism and schizophrenia have found increased de novo and/or overall variation in diseased individuals, leading to the speculation that these diseases may have a sizable number of possible molecular etiologies.15, 19, 21, 52, 53 To date, this theory is consistent with the general lack of success of genetic association studies in identifying common causative variants in neuropsychiatric disorders, including ADHD.54 Our results further support this hypothesis, as we found a wide distribution of genes specifically affected by CNVs in our ADHD probands relative to controls, many of which were associated with neurodevelopmental pathways and disorders.
Although our CNV detection platform is of high resolution, common CNV regions are underrepresented; it remains to be determined whether use of a higher resolution platform or a sequencing approach would alter the distribution curve of implicated genes. Nevertheless, our approach has identified a number of intriguing candidates that support a potential role of CNVs in ADHD, and it also suggests a number of candidate genes that may help to explain the spectrum of phenotypic heterogeneity observed in ADHD and other neuropsychiatric disorders.
References
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA . The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007; 164: 942–948.
Article Google Scholar - Barkley RA, Fischer M, Smallish L, Fletcher K . Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry 2006; 45: 192–202.
Article Google Scholar - Kessler RC, Adler L, Ames M, Barkley RA, Birnbaum H, Greenberg P et al. The prevalence and effects of adult attention deficit/hyperactivity disorder on work performance in a nationally representative sample of workers. J Occup Environ Med 2005; 47: 565–572.
Article Google Scholar - Seidman LJ . Neuropsychological functioning in people with ADHD across the lifespan. Clin Psychol Rev 2006; 26: 466–485.
Article Google Scholar - Bidwell LC, Willcutt EG, Defries JC, Pennington BF . Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 62: 991–998.
Article Google Scholar - Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent III TF et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry 2007; 164: 647–655.
Article Google Scholar - Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage 2007; 34: 1182–1190.
Article Google Scholar - Solanto MV . Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res 1998; 94: 127–152.
Article CAS Google Scholar - Gainetdinov RR, Caron MG . Genetics of childhood disorders: XXIV. ADHD, part 8: hyperdopaminergic mice as an animal model of ADHD. J Am Acad Child Adolesc Psychiatry 2001; 40: 380–382.
Article CAS Google Scholar - Mick E, Faraone SV . Genetics of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin NAm 2008; 17: 261–284, vii–viii.
Article Google Scholar - Neale BM, Faraone SV . Perspective on the genetics of Attention Deficit/Hyperactivity Disorder. Am J Med Genet B Neuropsychiatr Genet 2008; 147B: 1334–1336.
Article CAS Google Scholar - Erenberg G . The relationship between Tourette syndrome, attention deficit hyperactivity disorder, and stimulant medication: a critical review. Semin Pediatr Neurol 2005; 12: 217–221.
Article Google Scholar - Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA . Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res 2008; 103: 114–120.
Article Google Scholar - Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 2008; 82: 477–488.
Article CAS Google Scholar - Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T et al. Strong association of de novo copy number mutations with autism. Science 2007; 316: 445–449.
Article CAS Google Scholar - Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet 2007; 39: 319–328.
Article CAS Google Scholar - Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, Faas BH et al. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry 2008; 13: 261–266.
Article CAS Google Scholar - Kirov G, Gumus D, Chen W, Norton N, Georgieva L, Sari M et al. Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Hum Mol Genet 2008; 17: 458–465.
Article CAS Google Scholar - Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 2008; 320: 539–543.
Article CAS Google Scholar - Wilson GM, Flibotte S, Chopra V, Melnyk BL, Honer WG, Holt RA . DNA copy-number analysis in bipolar disorder and schizophrenia reveals aberrations in genes involved in glutamate signaling. Hum Mol Genet 2006; 15: 743–749.
Article CAS Google Scholar - Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M . Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet 2008; 40: 880–885.
Article CAS Google Scholar - Yang N, Li H, Criswell LA, Gregersen PK, Alarcon-Riquelme ME, Kittles R et al. Examination of ancestry and ethnic affiliation using highly informative diallelic DNA markers: application to diverse and admixed populations and implications for clinical epidemiology and forensic medicine. Hum Genet 2005; 118: 382–392.
Article Google Scholar - Ambrosini PJ . Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS). J Am Acad Child Adolesc Psychiatry 2000; 39: 49–58.
Article CAS Google Scholar - Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005; 35: 245–256.
Article Google Scholar - Kessler RC, Adler LA, Gruber MJ, Sarawate CA, Spencer T, Van Brunt DL . Validity of the World Health Organization Adult ADHD Self-Report Scale (ASRS) Screener in a representative sample of health plan members. Int J Methods Psychiatr Res 2007; 16: 52–65.
Article Google Scholar - Ahn SJ, Costa J, Emanuel JR . PicoGreen quantitation of DNA: effective evaluation of samples pre- or post-PCR. Nucleic Acids Res 1996; 24: 2623–2625.
Article CAS Google Scholar - Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature 2007; 448: 591–594.
Article CAS Google Scholar - Shaikh TH, Kurahashi H, Saitta SC, O’Hare AM, Hu P, Roe BA et al. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet 2000; 9: 489–501.
Article CAS Google Scholar - Venkatraman ES, Olshen AB . A faster circular binary segmentation algorithm for the analysis of array CGH data. Bioinformatics 2007; 23: 657–663.
Article CAS Google Scholar - Hasin Y, Olender T, Khen M, Gonzaga-Jauregui C, Kim PM, Urban AE et al. High-resolution copy-number variation map reflects human olfactory receptor diversity and evolution. PLoS Genet 2008; 4: e1000249.
Article Google Scholar - Nozawa M, Kawahara Y, Nei M . Genomic drift and copy number variation of sensory receptor genes in humans. Proc Natl Acad Sci USA 2007; 104: 20421–20426.
Article CAS Google Scholar - Young JM, Endicott RM, Parghi SS, Walker M, Kidd JM, Trask BJ . Extensive copy-number variation of the human olfactory receptor gene family. Am J Hum Genet 2008; 83: 228–242.
Article CAS Google Scholar - Cortese S, Konofal E, Lecendreux M, Arnulf I, Mouren MC, Darra F et al. Restless legs syndrome and attention-deficit/hyperactivity disorder: a review of the literature. Sleep 2005; 28: 1007–1013.
Article Google Scholar - Walters JR, Ruskin DN, Allers KA, Bergstrom DA . Pre- and postsynaptic aspects of dopamine-mediated transmission. Trends Neurosci 2000; 23 (10 Suppl): S41–S47.
Article CAS Google Scholar - Schormair B, Kemlink D, Roeske D, Eckstein G, Xiong L, Lichtner P et al. PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nat Genet 2008; 40: 946–948.
Article CAS Google Scholar - Shyur SD, Wang JY, Lin CG, Hsiao YH, Liou YH, Wu YJ et al. The polymorphisms of protein-tyrosine phosphatase receptor-type delta gene and its association with pediatric asthma in the Taiwanese population. Eur J Hum Genet 2008; 16: 1283–1288.
Article CAS Google Scholar - Blackman JA, Gurka MJ . Developmental and behavioral comorbidities of asthma in children. J Dev Behav Pediatr 2007; 28: 92–99.
Article Google Scholar - Pearson VE, Gamaldo CE, Allen RP, Lesage S, Hening WA, Earley CJ . Medication use in patients with restless legs syndrome compared with a control population. Eur J Neurol 2008; 15: 16–21.
Article CAS Google Scholar - Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2006; 63: 795–807.
Article Google Scholar - Mizuno K, Hasegawa K, Katagiri T, Ogimoto M, Ichikawa T, Yakura H . MPTP delta, a putative murine homolog of HPTP delta, is expressed in specialized regions of the brain and in the B-cell lineage. Mol Cell Biol 1993; 13: 5513–5523.
Article CAS Google Scholar - Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML . Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci 2006; 26: 5872–5880.
Article CAS Google Scholar - Uetani N, Kato K, Ogura H, Mizuno K, Kawano K, Mikoshiba K et al. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J 2000; 19: 2775–2785.
Article CAS Google Scholar - Dorval KM, Wigg KG, Crosbie J, Tannock R, Kennedy JL, Ickowicz A et al. Association of the glutamate receptor subunit gene GRIN2B with attention-deficit/hyperactivity disorder. Genes Brain Behav 2007; 6: 444–452.
Article CAS Google Scholar - Howells FM, Russell VA . Glutamate-stimulated release of norepinephrine in hippocampal slices of animal models of attention-deficit/hyperactivity disorder (spontaneously hypertensive rat) and depression/anxiety-like behaviours (Wistar-Kyoto rat). Brain Res 2008; 1200: 107–115.
Article CAS Google Scholar - Pattij T, Vanderschuren LJ . The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 2008; 29: 192–199.
Article CAS Google Scholar - Kessler RC, Adler LA, Barkley R, Biederman J, Conners CK, Faraone SV et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: results from the national comorbidity survey replication. Biol Psychiatry 2005; 57: 1442–1451.
Article Google Scholar - Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM et al. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci 1997; 17: 5196–5205.
Article CAS Google Scholar - Wu LJ, Ko SW, Toyoda H, Zhao MG, Xu H, Vadakkan KI et al. Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS ONE 2007; 2: e167.
Article Google Scholar - Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P et al. ADHD symptoms in children with FXS. Am J Med Genet A 2006; 140: 2275–2288.
Article Google Scholar - Fendt M, Schmid S, Thakker DR, Jacobson LH, Yamamoto R, Mitsukawa K et al. mGluR7 facilitates extinction of aversive memories and controls amygdala plasticity. Mol Psychiatry 2008; 13: 970–979.
Article CAS Google Scholar - Masugi M, Yokoi M, Shigemoto R, Muguruma K, Watanabe Y, Sansig G et al. Metabotropic glutamate receptor subtype 7 ablation causes deficit in fear response and conditioned taste aversion. J Neurosci 1999; 19: 955–963.
Article CAS Google Scholar - International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 2008; 455: 237–241.
Article Google Scholar - Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S et al. Large recurrent microdeletions associated with schizophrenia. Nature 2008; 455: 232–236.
Article CAS Google Scholar - Elia J, Devoto M . ADHD genetics: 2007 update. Curr Psychiatry Rep 2007; 9: 434–439.
Article Google Scholar
Acknowledgements
The Children's Hospital of Philadelphia Institutional Review Board has approved this study. This work was supported in part by National Institutes of Health Grants K23MH066275 (JE), GM081519 (THS), and P30HD026979 (MD and XG); University of Pennsylvania Grant UL1-RR-024134 (JE); Pennsylvania Department of Health Grant SAP 4100037707 (PSW); and a Developmental Research Award from the Cotswold Foundation (SG and HH). All genome-wide genotyping was funded by an Institutional Development Award from the Children's Hospital of Philadelphia (HH). We thank all the participating individuals and families for making this study possible.
Author information
Author notes
- J Elia and X Gai: These authors contributed equally to this work.
Authors and Affiliations
- Department of Child and Adolescent Psychiatry, The Children's Hospital of Philadelphia, Philadelphia, PA, USA
J Elia, R deBerardinis & T Takeda - Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
J Elia & W Berrettini - Center for Biomedical Informatics, The Children's Hospital of Philadelphia, Philadelphia, PA, USA
X Gai, H M Xie, J C Perin, M D'arcy, B M Muganga, L Wang & P S White - Division of Genetics, The Children's Hospital of Philadelphia, Philadelphia, PA, USA
E Geiger, F Lantieri, S F A Grant, M Devoto & T H Shaikh - Center for Applied Genomics, The Children's Hospital of Philadelphia, Philadelphia, PA, USA
J T Glessner, E Frackelton, C Kim, S F A Grant & H Hakonarson - Joseph Stokes Jr Research Institute,
E F Rappaport - , The Children's Hospital of Philadelphia, Philadelphia, PA, USA
E F Rappaport - Department of Pediatrics, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
S F A Grant, M Devoto, T H Shaikh, H Hakonarson & P S White - Department of Biostatistics and Epidemiology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
M Devoto - Dipartimento di Medicina Sperimentale, University La Sapienza, Rome, Italy
M Devoto - Division of Pulmonary Medicine, The Children's Hospital of Philadelphia, Philadelphia, PA, USA
H Hakonarson - Division of Oncology, The Children's Hospital of Philadelphia, Philadelphia, PA, USA
P S White
Authors
- J Elia
You can also search for this author inPubMed Google Scholar - X Gai
You can also search for this author inPubMed Google Scholar - H M Xie
You can also search for this author inPubMed Google Scholar - J C Perin
You can also search for this author inPubMed Google Scholar - E Geiger
You can also search for this author inPubMed Google Scholar - J T Glessner
You can also search for this author inPubMed Google Scholar - M D'arcy
You can also search for this author inPubMed Google Scholar - R deBerardinis
You can also search for this author inPubMed Google Scholar - E Frackelton
You can also search for this author inPubMed Google Scholar - C Kim
You can also search for this author inPubMed Google Scholar - F Lantieri
You can also search for this author inPubMed Google Scholar - B M Muganga
You can also search for this author inPubMed Google Scholar - L Wang
You can also search for this author inPubMed Google Scholar - T Takeda
You can also search for this author inPubMed Google Scholar - E F Rappaport
You can also search for this author inPubMed Google Scholar - S F A Grant
You can also search for this author inPubMed Google Scholar - W Berrettini
You can also search for this author inPubMed Google Scholar - M Devoto
You can also search for this author inPubMed Google Scholar - T H Shaikh
You can also search for this author inPubMed Google Scholar - H Hakonarson
You can also search for this author inPubMed Google Scholar - P S White
You can also search for this author inPubMed Google Scholar
Corresponding authors
Correspondence toH Hakonarson or P S White.
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Gene-specific references displayed in the text and Table 2 are listed in the Supplementary Materials. Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Elia, J., Gai, X., Xie, H. et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes.Mol Psychiatry 15, 637–646 (2010). https://doi.org/10.1038/mp.2009.57
- Received: 04 December 2008
- Revised: 08 May 2009
- Accepted: 26 May 2009
- Published: 23 June 2009
- Issue Date: June 2010
- DOI: https://doi.org/10.1038/mp.2009.57