Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells (original) (raw)
Main
Immunological memory plays a major role in host defense against infection. Expansion of antigen-specific T cells and their differentiation into memory cells occurs during immune responses1,2,3. Memory T cells can also be generated when mature T cells are placed in a lymphopenic environment. Thus, mature T cells have the potential to undergo extensive and spontaneous proliferation when small numbers of T cells are adoptively transferred into syngeneic nude, recombination-activating gene 1 (RAG-1)–deficient or sublethally irradiated syngeneic hosts4,5,6,7,8,9,10,11,12. This lymphopenia-induced expansion of naïve T cells requires specific T cell receptor (TCR) interactions with self–major histocompatibility complex (MHC) molecules loaded with self-peptides5,6,7,8 and can induce the surface phenotype of memory T cells on the mature T cells9,10,11. This is not accompanied by an up-regulation of early activation markers (CD25, CD69), but does result in the expression of several memory markers on the T cells, which increase progressively with each successive cell division6,7,8,12. However, the molecular mechanisms involved in memory T cell development are currently unclear.
Naïve B cells also proliferate and differentiate into memory cells in response to T cell–dependent antigens. Antigen-activated B cells interact with CD4+ helper T cells in periarteriolar lymphoid sheaths in the spleen13 and migrate into the primary follicles to form germinal centers (GCs). GC B cells proliferate extensively and somatically mutate their immunoglobulin genes14,15. Affinity-matured GC B cells are then selected to differentiate into memory B cells16,17. These GC B cells show high Bcl-6 expression18,19. Bcl-6 contains a bric-a-brac, tramtrack, broad complex (BTB), also called the poxvirus and zinc finger (POZ), domain and Krüppel-type zinc-finger motifs20,21,22. Because the BTB domain of Bcl-6 recruits the silencing mediator of retinoid and thyroid receptor–histone deacetylase complex23, Bcl-6 can function as a sequence-specific transcriptional repressor. To test the functions of Bcl-6, its gene was disrupted in the mouse germ line24,25,26. Although all hematopoietic lineages, including mature lymphocytes, developed in Bcl-6–deficient mice, GC formation was impaired in these mice due to an abnormality of B cells25. Thus, Bcl-6 is essential in B cells for memory B cell development in GCs.
The relationship between the processes involved in T and B cell memory generation is unknown. These two distinct lymphocyte populations could potentially share certain molecules required for the generation of memory cells. Because Bcl-6 is ubiquitously expressed in various tissues, including mature T cells22, we studied the role of Bcl-6 in memory T cell development with Bcl-6−/− and Bcl-6–transgenic mice. We show here that Bcl-6 is required for the maintenance of activated CD8+ T cells at the memory cell stage during both lymphopenia- and antigen-induced activation procedures. Thus, Bcl-6 is a key molecule for the establishment of immunological memory for both T and B cells.
Results
Bcl-6 in memory phenotype CD8+ T cells
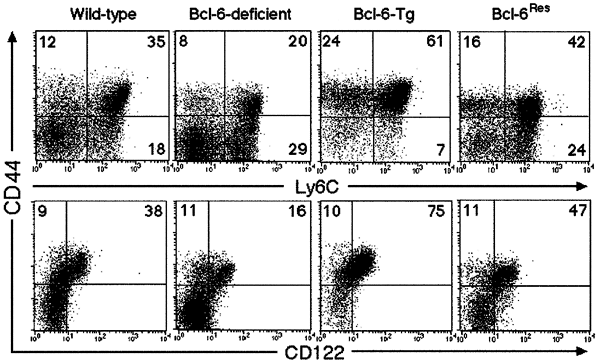
Because Bcl-6 is expressed in CD8+ T cells22, we analyzed the percentage of CD8+ T cells with a memory phenotype (CD44+Ly6C+CD122+) in the spleens of Bcl-6−/− mice27. At 4 weeks of age, the percentage of T cells with this phenotype in Bcl-6−/− mice was lower than that in wild-type mice (Fig. 1). Less than 1% of the CD8+ T cells in both wild-type and Bcl-6–deficient spleens expressed CD25 and CD69 (data not shown), confirming that the population represents memory, but not activated, T cells. If the lack of memory phenotype CD8+ T cells in Bcl-6−/− mice was due to Bcl-6, then forced expression of this protein in T cells should increase the numbers of memory phenotype cells. Indeed, we observed a marked increase in the percentage of memory phenotype CD8+ T cells in the spleens of 4-week-old transgenic mice that were expressing murine Bcl-6 driven by the lck proximal promoter28 (referred to hereafter as Bcl-6–transgenic mice) (Fig. 1). In addition, the Bcl-6 transgene—which was expressed specifically in T lineage cells—rescued the defective accumulation of memory phenotype CD8+ T cells in the spleens of Bcl-6−/− mice (referred to hereafter as Bcl-6Res mice) (Fig. 1). This indicated that the presence of Bcl-6 in CD8+ T cells per se is key to the generation of memory phenotype CD8+ T cells.
Figure 1: Memory phenotype CD8+ T cells in the spleen of very young mice.
Expression of CD44 and Ly6C or CD44 and CD122 on CD8+ T cells in the spleens of Bcl-6–deficient, Bcl-6–transgenic, Bcl-6Res and wild-type mice at 4 weeks of age. The numbers indicate the percentage of CD8+ T cells in each quadrant.
Properties of memory phenotype CD8+ T cells
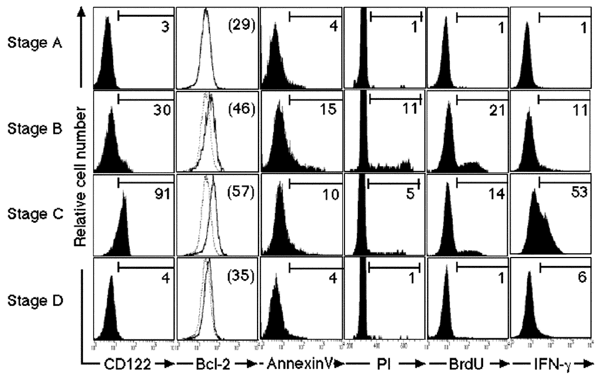
To examine the properties of the memory phenotype CD8+ T cells in the spleens of normal 4-week-old mice, we sorted splenic CD8+ T cells into four subsets based on expression of the surface markers CD44 and Ly6C. These were stages A (CD44−Ly6C−), B (CD44+Ly6C−), C (CD44+Ly6C+) and D (CD44−Ly6C+). CD8+ T cells in stage C (CD44+Ly6C+) should include memory T cells29. Memory T cells also express CD122 and Bcl-210,11,30. Ninety-one percent of stage C T cells expressed CD122; they also expressed more Bcl-2 compared to T cells in other stages (Fig. 2). More cells in stages B and C were apoptotic, as detected by annexin V staining, compared to cells from the other stages. Cell cycle analysis with propidium iodide (PI) staining and in vivo 5-bromodeoxyuridine (BrdU) incorporation revealed that cell proliferation occurred mainly at stage B. Fewer, but still large numbers of, stage C T cells were still in cell cycle, which was consistent with the observed slow proliferation of memory T cells12,31,32. In addition, a large population of stage C T cells produced interferon-γ (IFN-γ) as early as 8 h after stimulation with anti-CD3 in vitro, whereas IFN-γ production was less prominent among anti-CD3–stimulated CD8+ T cells from the other stages. After antigenic stimulation, memory T cells differentiate into effector cells more rapidly than naïve T cells33,34. Thus, these results indicated that some, if not all, of stage C T cells in the spleens of young mice were in fact functional memory T cells. In addition, the stage C T cells of both Bcl-6–deficient and Bcl-6–transgenic mice secreted similar amounts of IFN-γ (on a per cell basis) to wild-type memory T cells after stimulation with anti-CD3 (data not shown). Thus, the changes in the memory phenotype CD8+ T cell subset seen in Bcl-6−/− and Bcl-6–transgenic mice were quantitative rather than qualitative.
Figure 2: Phenotype of CD8+ T cells in each stage.
CD8+ T cells from the spleens of wild-type 4-week-old mice were sorted into four subsets (stages A–D) by their expression of surface markers as follows: stage A (CD44−Ly6C−), B (CD44+Ly6C−), C (CD44+Ly6C+) and D (CD44−Ly6C+). Their expression of CD122, Bcl-2 and annexin V as well as cell proliferation were analyzed. In addition, those cells were stimulated with plate-bound anti-CD3 for 8 h, and intracellular IFN-γ production was determined. The numbers in parentheses and the dotted lines indicate mean fluorescent intensity (MFI) and the profile of stage A T cells of Bcl-2 staining, respectively. The numbers indicate the percentage of CD8+ T cells that stained positive for the indicated markers.
Transient memory phenotype CD8+ T cell expansion
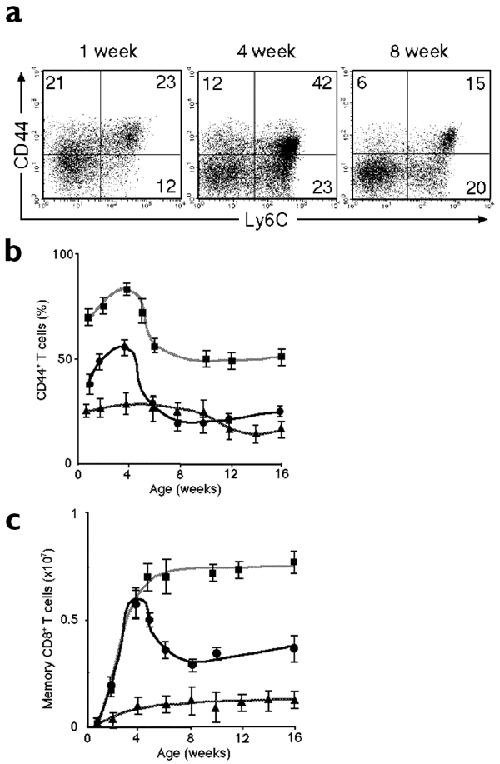
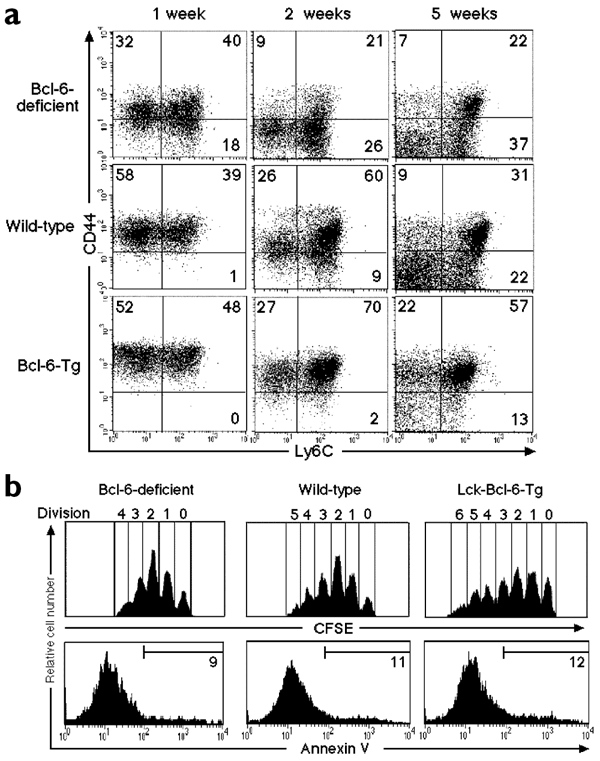
Approximately 25% of CD8+ T cells in the spleen of normal 1-week-old mice had a memory phenotype (Fig. 3a). Eight-week-old mice had a lower percentage of stage C T cells compared to 4-week-old mice (Fig. 3a). The percentage of wild-type splenic CD8+ T cells in stages B and C (CD44+CD25−) increased progressively to a peak of 50–60% at 4 weeks of age, and then decreased to 20–30% at around 8 weeks of age (Fig. 3b). Likewise, the number of splenic CD8+ stage C T cells increased and reached a peak at around 4 weeks after birth and decreased thereafter (Fig. 3c). The percentage of CD8+ T cells in stages B and C in the spleens of Bcl-6−/− mice did not, however, show a peak at around 4 weeks after birth and remained more or less constant at 25–30% until 9–10 weeks of age. The absolute number of stage C splenic T cells in Bcl-6−/− mice also remained low. Unlike wild-type mice, the numbers of stage C CD8+ T cells in Bcl-6–transgenic mice did not fall after 4 weeks of age but rather were maintained in high numbers until the mice were at least 25 weeks of age (data not shown). These results suggested that Bcl-6 plays a key role in the generation and maintenance of memory CD8+ T cells in the spleens of very young mice.
Figure 3: Transient expansion of memory phenotype CD8+ T cells in the spleen of very young mice.
(a) Expression of CD44 and Ly6C on CD8+ T cells in the spleens of normal mice. The numbers indicate the percentages of CD8+ T cells in each quadrant. The percentages of CD44+CD25− T cells (b) and numbers of stage C (CD44+Ly6C+) T cells (c) within CD8+ T cells in the spleens of Bcl-6−/− (triangles), Bcl-6–transgenic (squares) and wild-type (circles) mice at various ages are shown. Data are mean ± s.e.m. of three to six mice.
Lymphopenia-induced CD8+ T cell proliferation
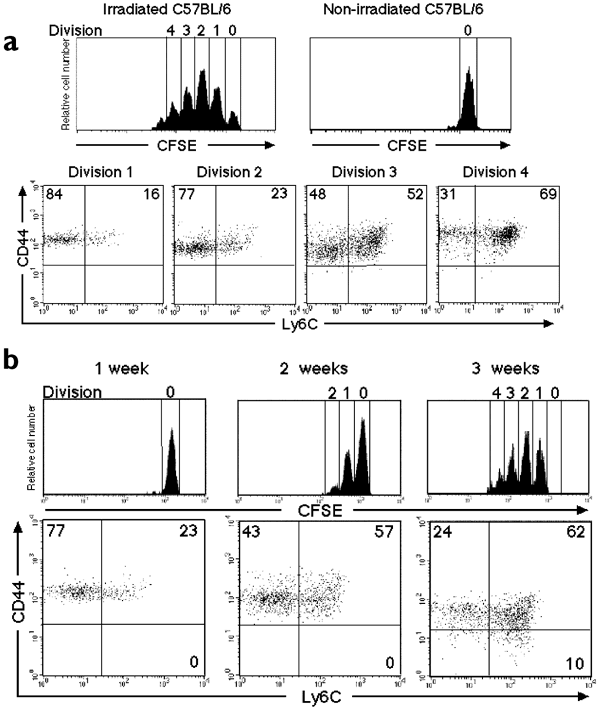
The above observations led us to consider the idea that the memory T cells in very young mice might be generated by a mechanism similar to that of lymphopenia-induced proliferation, termed homeostatic proliferation4,5,6,7,8,9,10,11,12. Thus, we examined the proliferation of naïve CD8+ T cells in the spleens of normal 3-week-old mice. Carboxyfluorescein diacetate-succinimidyl ester (CFSE)-labeled naïve CD8+ T cells (stage A) of 3-week-old mice barely divided in nonirradiated adult (12-week-old) mice, whereas they proliferated vigorously in sublethally irradiated adult mice (Fig. 4a). In the latter case, the transferred naïve CD8+ T cells progressively altered their surface phenotype from that of stages A through B to C as they proliferated (Fig. 4a, lower panels), in a manner similar to homeostatically proliferating naïve CD8+ T cells from normal 12-week-old mice (data not shown). Thus, splenic naïve CD8+ T cells from 3-week-old mice show a similar potential for lymphopenia-induced proliferation as adult splenic naïve T cells.
Figure 4: Homeostatic proliferation of CD8+ T cells in the spleens of very young mice.
CFSE-labeled naïve (CD44−Ly6C−) CD8+ T cells were transferred into normal syngeneic mice. (a) Cell division and expression of CD44 and Ly6C on CFSE+CD8+ T cells from wild-type 3-week-old mice were analyzed 1 week after transfer into nonirradiated and irradiated 12-week-old mice. Dot plots show the expression of CD44 and Ly6C on CFSE+CD8+ T cells that had undergone the indicated numbers of divisions. (b) Cell division and expression of CD44 and Ly6C on CFSE+CD8+ T cells of normal 12-week-old mice were analyzed 1, 2 and 3 weeks after transfer into wild-type neonatal mice. Dot plots show the expression of CD44 and Ly6C on CFSE+CD8+ T cells in spleens. The numbers indicate the percentages of CFSE+CD8+ T cells in each quadrant. These results are representative of four independent experiments.
We then examined the splenic environment of very young mice by transferring CFSE-labeled stage A T cells from wild-type adult (12-week-old) mice into neonatal mice. When we transferred CFSE-labeled stage A T cells into nonirradiated wild-type neonatal mice, stage A T cells proliferated and differentiated into stage B, C and D T cells within 3 weeks after transfer (Fig. 4b); this indicated that the spleens of very young mice—even without irradiation—allowed naïve CD8+ T cells to proliferate and differentiate into memory CD8+ T cells. Those CFSE+CD8+ T cells did not express CD25 and CD69 1 week after transfer (data not shown). Similar results were obtained when CFSE-labeled stage A T cells from 12-week-old wild-type mice were transferred to nonirradiated wild-type mice at 3 weeks of age (data not shown). In contrast, the proliferation of transferred naïve T cells was barely detectable in the spleens of nonirradiated hosts at 4 weeks of age or older (data not shown), which is consistent with our current knowledge of homeostatic proliferation4,5,6,7,8,9,10,11,12. Thus, the spleens of very young animals, unlike adult ones, appear to have a special environment in which homeostatic proliferation can occur. We also observed that homeostatic proliferation of CD8+ T cells was detected in the inguinal lymph nodes of very young mice (data not shown). These results suggested that the generation of memory CD8+ T cells through homeostatic proliferation occurs physiologically in very young mice.
Maintenance of memory CD8+ T cells
From our previous data, one might predict that Bcl-6 controls the homeostatic proliferation of CD8+ T cells. We tested this possibility by transferring CFSE-labeled naïve CD8+ T cells (stage A) from Bcl-6−/− or Bcl-6–transgenic mice into sublethally irradiated normal adult mice. The percentages of stage B and C T cells generated from transferred naïve Bcl-6−/− T cells were lower than those derived from transferred Bcl-6–transgenic or wild-type cells, even 1 week after transfer; and the numbers of stage B and stage C T cells generated from naïve Bcl-6−/− T cells decreased markedly thereafter (Fig. 5a). In contrast, ∼80% of transferred Bcl-6–transgenic CD8+ T cells stayed in stages B and C, even 5 weeks after transfer. By this time, considerable numbers of the wild-type–transferred CD8+ T cells had down-regulated CD44 expression. These phenotypic alterations were reflected in the reduction of total numbers of transferred CD8+ T cells in the spleens of irradiated hosts. (Bcl-6−/−, 3.0 × 106 ± 0.8 × 106; wild-type, 4.1 × 106 ± 0.5 × 106; Bcl-6–transgenic 5.3 × 106 ± 0.9 × 106). These results suggested that Bcl-6 is required for both the proliferation and maintenance of memory T cells.
Figure 5: A role for Bcl-6 in homeostatic proliferation of CD8+ T cells.
CFSE-labeled naïve (CD44−Ly6C−) CD8+ splenic T cells from Bcl-6–deficient, Bcl-6–transgenic or wild-type mice with the Ly5.2 phenotype were transferred into irradiated normal syngeneic adult hosts with the Ly5.1 phenotype. (a) Expression of CD44 and Ly6C on Ly5.2+CD8+ T cells was analyzed 1, 2 and 5 weeks after transfer. The numbers indicate the percentages of Ly5.2+CD8+ T cells in each quadrant. The numbers of Ly5.2+CD8+ T cells in the spleens were counted 5 weeks after transfer. Data are mean ± s.e.m. of three to six mice. (b) Cell division and expression of annexin V on CFSE+CD8+ T cells in the spleen were analyzed 1 week after transfer. The numbers indicate the percentages of CFSE+CD8+ T cells stained with each marker. These results are representative of three independent experiments.
One might claim that the altered Bcl-6 expression in CD8+ T cells affected their proliferation and/or cell death. Thus, we transferred CFSE-labeled stage A T cells from Bcl-6−/− and Bcl-6–transgenic mice into irradiated normal adult mice and analyzed their proliferation and death. Bcl-6−/− T cells proliferated (up to four divisions) within 1 week after transfer, albeit slightly more slowly than wild-type cells (up to five divisions) (Fig. 5b). Likewise, Bcl-6–transgenic T cells proliferated (up to six divisions) slightly better than control T cells, indicating that the initial proliferation of CD8+ T cells in a lymphopenic environment is not markedly affected by the amount of Bcl-6 expressed by the T cells. In addition, apoptotic T cells in the transferred population were detected by annexin V staining. The percentages of apoptotic cells were similar among the transferred Bcl-6−/−, Bcl-6–transgenic and wild-type cells. Thus, the inability of Bcl-6−/− CD8+ T cells to maintain memory cells in vivo is probably due to the phenotypic changes, namely rapid conversion of stage C to stage DT cells, rather than the death of memory CD8+ T cells.
Bcl-6 in antigen-induced memory CD8+ T cells
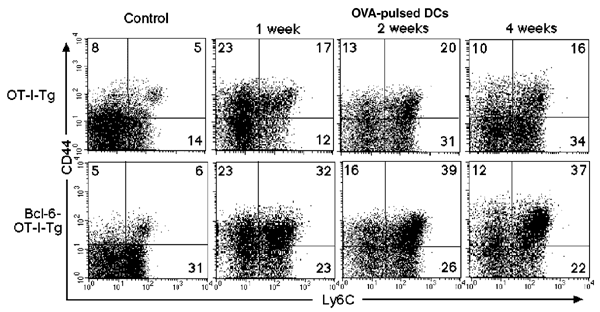
Altered Bcl-6 expression in CD8+ T cells may also affect the generation and maintenance of memory CD8+ T cells induced during immune responses to foreign antigens. To examine this, OT-I–transgenic35 and Bcl-6–OT-I double-transgenic mice that expressed a TCR (Vα2,Vβ5) specific for the H-2Kb–restricted ovalbumin peptide (SIINFEKL)—which consists of amino acids 257–264 and is referred to hereafter as OVA(257–264)—were intravenously injected with OVA(257–264)-pulsed dendritic cells. The generation of memory CD8+ T cells in the spleen was then analyzed. One week after stimulation, >98% of Vα2+CD8+ T cells were Vβ5+, and <1% of the CD8+ T cells expressed CD25 and CD69 (data not shown). The percentage of stage B and C Vα2+ T cells in spleens increased 1 week after stimulation and remained more or less constant in both OT-I– and Bcl-6–OT-I–transgenic mice for at least 4 weeks (Fig. 6). The percentage of splenic stage C T cells in Bcl-6–OT-I–transgenic mice was about twice as high as that in OT-I–transgenic mice. Accordingly, the absolute number of stage C T cells was higher in Bcl-6–OT-I–transgenic mice (7.2 × 106 ± 0.9 × 106) than in the OT-I–transgenic mice (3.5 × 106 ± 0.6 × 106). These results strongly suggested that Bcl-6 plays a role in the generation and maintenance of memory CD8+ T cells induced in response to antigenic stimulation in the spleen.
Figure 6: A role for Bcl-6 in the generation of antigen-induced memory CD8+ T cells.
OT-I–transgenic and Bcl-6–OT-I doubly transgenic mice were immunized with OVA(257–264)-pulsed dendritic cells. Expression of CD44 and Ly6C on Vα2+CD8+ T cells was analyzed in the spleens of naïve mice (control) and immunized mice 1, 2 and 4 weeks after stimulation. The numbers indicate the percentages of Vα2+CD8+ T cells in each quadrant. These results are representative of three independent experiments.
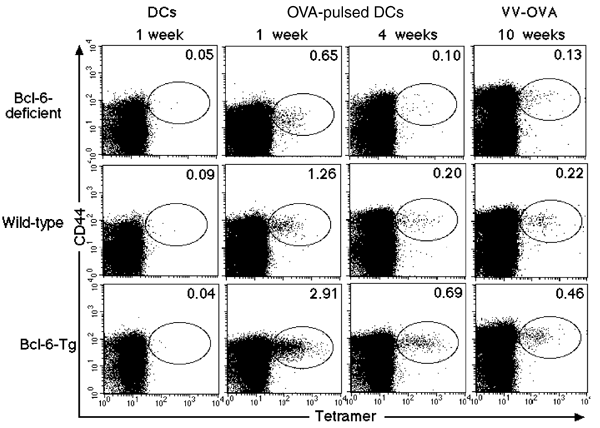
The effect of Bcl-6 on the generation and maintenance of antigen-induced memory CD8+ T cells in the spleen was further confirmed with Bcl-6−/− and Bcl-6–transgenic mice that did not carry a TCR transgene. These mice were immunized with OVA(257–264)-pulsed dendritic cells (DCs), and antigen-specific CD8+ T cells in the spleen were detected by staining with OVA–major histocompatibility complex (MHC) class I tetramers (OVA(257–264) tetramers). The percentage of OVA-specific CD44+CD8+ T cells in wild-type mice immunized with OVA(257–264)-pulsed dendritic cells, but not with nonpulsed dendritic cells, increased 1 week after stimulation and were detectable 4 weeks after stimulation (Fig. 7). Although OVA-specific CD44+CD8+ T cells were generated in Bcl-6−/− mice 1 week after stimulation, the percentage was lower than that in wild-type mice and decreased to an amount that was virtually indistinguishable from the background amounts (<0.1%) within 4 weeks of stimulation. In contrast, the percentage of the same population in Bcl-6–transgenic mice was initially high and was maintained for at least 4 weeks. When we immunized wild-type mice with vaccinia virus encoding OVA (VV-OVA)36, OVA-specific CD44+CD8+ T cells were detected in the spleen 10 weeks after stimulation. Again, the percentage and absolute numbers of OVA-specific CD44+CD8+ T cells were highest in Bcl-6–transgenic (7.9 × 104 ± 1.8 × 104) and lowest in Bcl-6−/− (0.5 × 104) mice; wild-type mice had 3.4 × 104 ± 1.1 × 104 cells. These OVA-specific CD44+CD8+ T cells bore stage C surface markers, and thus included memory T cells (data not shown).
Figure 7: A role for Bcl-6 in the generation and maintenance of antigen-induced memory CD8+ T cells.
Bcl-6–deficient, Bcl-6–transgenic and wild-type mice were immunized with OVA(257–264)-pulsed dendritic cells, nonpulsed DCs or VV-OVA. OVA-specific CD44+CD8+ T cells in the spleen were detected by staining with OVA-tetramers 1, 4 and 10 weeks after stimulation. The numbers indicate the percentages of CD8+ T cells in each oval. These results are representative of three independent experiments.
In summary, the percentage of antigen-induced memory CD8+ T cells in spleens correlated well with the amount of Bcl-6 expressed by T cells, which further supports the role played by Bcl-6 in the generation and maintenance of antigen-induced memory CD8+ T cells in the spleens of mice.
Discussion
We developed here a method for monitoring the differentiation pathway of memory CD8+ T cells generated through homeostatic proliferation by cataloging the cells in stages A–D, according to the surface markers they expressed. Naïve CD8+ T cells in stage A expressed CD44 and moved into stage B after transfer into lymphopenic mice. Because 30% of T cells in stage B expressed CD122—a component of interleukin 15 receptor (IL-15R)—and ∼20% of them were in cell cycle, IL-1532,37 might stimulate T cells in stage B to proliferate. Stage B T cells expressed Ly6C and moved into stage C. Some of CD8+ T cells in stage C were still in cell cycle, hence memory T cells can also undergo homeostatic proliferation12,31,32. In addition, the stage C T cells moved into stage D. However, it is not certain whether after homeostatic proliferation, CD8+ T cells in stages B, C and D return to stage A. CD8+ T (OT-I) cells transferred into irradiated syngeneic adult hosts closely resemble memory cells during the first few weeks after transfer but after 2 months they become naïve in terms of both phenotype and function11. Naïve T cells detected in the spleens of irradiated hosts after homeostatic proliferation result from de novo thymic T cell development of hematopoietic stem cells present in the donor spleen38. Further work is required to clarify whether memory phenotype CD8+ T cells can revert to the naïve T cells in stage A. Nevertheless, we have shown here that Bcl-6 is required for CD8+ T cells activated through homeostatic proliferation to stay at stages B and C.
The differentiation pathway of splenic memory CD8+ T cells during homeostatic expansion may be similar to that of memory CD8+ T cells generated by antigenic stimulation. Bcl-6 plays a role in keeping antigen-activated CD8+ T cells in stage C. These results suggest that Bcl-6 is critical for the homeostasis of memory CD8+ T cells generated both physiologically through homeostatic proliferation and in response to immunization. Bcl-6 is also essential for memory B cell development in GCs24,25,26. In addition, the generation and maintenance of memory phenotype CD4+ T cells is well correlated with the amount of Bcl-6 in the T cells (unpublished observation). Thus, Bcl-6 functions in the generation and maintenance of immunological memory cells irrespective of the lymphocyte lineage. Because Bcl-6 is required for activated CD8+ T cells to stay at the stages that correlate with both functional memory cells and proliferating cells, one may consider—by analogy—that Bcl-6 is important for activated B cells to stay at the GC stage, when B cells are proliferating extensively and differentiating into memory cells. However, the role played by Bcl-6 in activated CD8+ T cells may not be identical to that in GC B cells. Although reduced, but significant numbers, of memory CD8+ T cells can be generated in immunized Bcl-6−/− mice, no GC B cells are detected in Bcl-6−/− mice. Indeed, Bcl-6 is thought to play a role in survival of GC B cells26. In contrast the amount of Bcl-6 expression does not affect the cell death of activated CD8+ T cells under homeostatic proliferation. Whether the roles for Bcl-6 in B and T cells are identical or not, unraveling the functions of Bcl-6 at the molecular level (that is, the target genes of Bcl-6 action) will shed new light on the mechanism of immunological memory generation and maintenance and would help in the development of efficient vaccines.
The use of Bcl-6−/− and Bcl-6–transgenic mice enabled us to show that homeostatic proliferation may have physiological relevance. This was suggested by the finding that mature T cells proliferate and differentiated into stage C memory phenotype cells in very young mice. The peripheral T cell population expands after neonatal thymectomy of mice39,40, and naïve T cells proliferate strongly in neonatal mice41. Together, these results suggest homeostatic proliferation may have important implications for the ontogeny of the immune system. Because homeostatic proliferation of T cells normally requires a lymphopenic environment, the spleens of very young mice may constitute a relatively lymphopenic environment, suggesting that the supply from the thymus in very young mice is not enough to fill all the available “space” in the spleen. In this regard, one could speculate that homeostatic proliferation in very young mice has evolved to allow a rapid increase in the number of peripheral CD8+ T cells, compensating for the presumably inefficient production of T cells by the thymus. Notably, the increase in the T cell population is accompanied by the generation of large numbers of functional memory T cells. The abundance of memory T cells—which respond more rapidly to antigens than naïve T cells33,34,42—would be beneficial to very young mice in protecting them from pathogens. The magnitude of this type of proliferation and memory T cell generation is Bcl-6–dependent, as we have shown here. Thus, Bcl-6 is a versatile molecule in terms of host defense. It plays roles in the efficient establishment of T cell immunity through homeostatic proliferation during infancy, as well as in the generation and maintenance of memory T and B cells during immune responses.
Methods
Animals.
C57BL/6 mice were from Japan SLC (Hamamatsu, Japan). Bcl-6–deficient mice27 were backcrossed to C57BL/6 mice five times. C57BL/6 Bcl-6–transgenic mice were generated as described28. Thymocytes and splenic T cells from the Bcl-6–transgenic mice produced two- to threefold larger amounts of Bcl-6 protein than those from control littermates, as estimated by immunoblotting (data not shown). One (number 83) of two independent lines (numbers 73 and 83) of Bcl-6–transgenic mice was crossed to Bcl-6–deficient mice to generate Bcl-6Res mice. C57BL/6 (B6-Ly5.1) mice were from Charles River Japan (Tsukuba, Japan). OT-I–transgenic mice35, which were originally established by W. R. Heath (The Walter and Eliza Hall Institute, Victoria, Australia) were provided by H. Kosaka (Osaka University, Osaka, Japan). All these mice were maintained under specific pathogen–free conditions in the animal center of Chiba University School of Medicine.
Flow cytometric analysis of CD8+ T cells.
Spleen cells from C57BL/6 mice were stained with various monoclonal antibodies as follows. Spleen cells (106) were first blocked with unconjugated anti–CD32/16 (2.4G2, Pharmingen, San Diego, CA), followed by incubation with biotinylated antibodies, and then incubation with directly conjugated antibodies and peridinine chlorophyll protein (PerCP)-streptavidin (Pharmingen). The following antibodies (Pharmingen) were used for staining: allophycocyanin-CD8 (53-6.7), fluorescein isothiocyanate (FITC)-CD8, phycoerythrin (PE)-CD44 (IM7), allophycocyanin-CD44, FITC-CD122 (IL-2Rβ, TM-β1), FITC-Ly6C (AL-21), biotin-Ly6C, FITC-CD25 (IL-2Rα, 7D4), FITC-CD69 (H1.2F3), FITC-Ly5.2 (104). H-2Kb tetramers bearing OVA(257–264) were provided by P. Marrack (National Jewish Medical and Research Center, Denver, CO). Spleen cells (2 × 106) were incubated with 5–10 μg/ml of tetramers at 37 °C for 2 h. The remaining antibodies were then added and incubated for 20–40 min before analysis on a FACSCalibur machine (Becton Dickinson, San Jose, CA).
Intracellular staining of Bcl-2 and analysis for apoptosis.
Spleen cells from C57BL/6 mice were stained with antibodies (allophycocyanin-CD8, PE-CD44 and biotin-Ly6C), and then subjected to intracellular staining for Bcl-2 with the CytoFix-Cytoperm kit, according to the manufacturer's instructions (Pharmingen). For intracellular Bcl-2 staining, FITC-conjugated mouse anti–human Bcl-2 (124, Dako, Carpinteria, CA) or its isotype control antibody (mouse IgG, Dako) was used. Apoptotic cells were detected by the FITC–annexin V kit following the manufacturer's instructions (Bender MesSystem, Vienna, Austria).
Cell cycle analysis.
Cell cycle analysis was done as described44. Briefly, CD8+ T cells from each stage were sorted from spleen cells with a FACSVantage, and purified CD8+ T cell subpopulations were incubated in hypotonic lysis buffer that contained PI (0.1% sodium citrate, 0.01% Triton X, 0.1 mg/ml of RNase A and 0.1 mg/ml of PI). The DNA content in each nucleus was analyzed on a FACSCalibur (Becton Dickinson) with CellQuest software. BrdU (1 mg) was injected intraperitoneally into 4-week-old mice. After 3 h, spleen cells were isolated and stained with allophycocyanin-CD8, PE-CD44 and biotin-Ly6C. After surface staining, these cells were further stained with anti-BrdU with the BrdU Flow kit, according to the manufacturer's instructions (Pharmingen).
IFN-γ production by CD8+ T cells.
Purified CD8+ T cells were incubated in the presence or absence of immobilized anti-CD3 (145-2C11) for 3 h. These cells were then cultured with Monensin (2 μM) for another 5 h and stained with antibodies (allophycocyanin-CD8, PE-CD44 and biotin-Ly6C). These stained cells were further fixed with 4% paraformaldehyde and made permeable with 0.5% Triton X-100. The cells were incubated with anti–IFN-γ (XMG1.2-FITC, Pharmingen) to detect intracellular IFN-γ on a FACSCalibur.
Homeostatic proliferation.
Spleen cells from C57BL/6 mice were incubated with a mixture of antibodies to CD4, Mac-1, NK1.1, CD44, Ly6C and B220, followed by antibody-coated microbeads (Miltenyi Biotec, Auburn, CA) with 2 beads/cell, then purified on a SuperMACS cell sorter (Miltenyi Biotec). Magnetically purified naïve CD8+ T cells (>90% pure) were labeled with CFSE. These CD8+ T cells were injected intravenously into C57BL/6 mice that had been irradiated (7 Gy) 2 days before transfer or intraperitoneally into nonirradiated mice.
Induction of antigen-specific memory CD8+ T cells.
DCs were prepared from bone marrow cells of C57BL/6 mice by culturing in 1000 U/ml of GM-CSF (produced from the B78Hi/GMCSF45 cell line, which was provided by H. Levisky, Johns Hopkins, Baltimore, MD) and IL-4 in complete RPMI 1640 medium. On day 6 of culture, those cells were stimulated with LPS (1 μg/ml) for 24 h and incubated with 2 μg/ml of OVA(257–264) for 3 h. OVA(257–264)-pulsed DCs (5 × 106) were injected intravenously into mice. In other experiments, VV-OVA46 was propagated as described36 and 2 × 106 plaque-forming units (PFU) of VV-OVA were used to challenge mice intravenously.
References
- Freitas, A.A. & Rocha, B.B. Lymphocyte lifespans: homeostasis, selection and competition. Immunol. Today 14, 25–29 (1993).
Article CAS Google Scholar - Goldrath, A.W. & Bevan, M.J. Selecting and maintaining a diverse T-cell repertoire. Nature 402, 255–262 (1999).
Article CAS Google Scholar - Marrack, P. et al. Homeostasis of αβTCR+ T cells. Nature Immunol. 1, 107–111 (2000).
Article CAS Google Scholar - Surh, C.D. & Sprent, J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J. Exp. Med. 192, F9–14 (2000).
Article CAS Google Scholar - Viret, C., Wong, F.S. & Janeway, C.A. Jr., Designing and maintaining the mature TCR repertoire: the continuum of self-peptide: self-MHC complex recognition. Immunity 10, 559–568 (1999).
Article CAS Google Scholar - Ernst, B., Lee, D.S., Chang, J.M., Sprent, J. & Surh, C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999).
Article CAS Google Scholar - Kieper, W.C. & Jameson, S.C. Homeostatic expansion and phenotypic conversion of naïve T cells in response to self-peptide/MHC ligands. Proc. Natl. Acad. Sci. USA 96, 13306–13311 (1999).
Article CAS Google Scholar - Goldrath, A.W. & Bevan, M.J. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11, 183–190 (1999).
Article CAS Google Scholar - Cho, B.K., Rao, V.P., Ge, Q., Eisen, H.N. & Chen, J. Homeostasis-stimulated proliferation drives naïve T cells to differentiate directly into memory T cells. J. Exp. Med. 192, 549–556 (2000).
Article CAS Google Scholar - Murali-Krishna, K. & Ahmed, R. Naïve T cells masquerading as memory cells. J. Immunol. 165, 1733–1737 (2000).
Article CAS Google Scholar - Goldrath, A.W., Bogatzki, L.Y. & Bevan, M.J. Naïve T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192, 557–564 (2000).
Article CAS Google Scholar - Tanchot, C., Lemonnier, F.A., Perarnau, B., Freitas, A.A.B. & Rocha, B. Differential requirements for survival and proliferation of CD8 naïve or memory T cells. Science 276, 2057–2062 (1997).
Article CAS Google Scholar - Hanna, M.G. An autoradiographic study of the germinal center in spleen white pulp during early intervals of the immune response. Lab. Invest. 13, 95–104 (1964).
PubMed Google Scholar - Berek, C., Berger, A. & Apel, M. Maturation of the immune response in germinal centers. Cell 67, 1121–1129 (1991).
Article CAS Google Scholar - Jacob, J., Kelsoe, G., Rajewsky, K. & Weiss, U. Intraclonal generation of antibody mutants in germinal centers. Nature 354, 389–392 (1991).
Article CAS Google Scholar - Schittek, B. & Rajewsky, K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature 346, 749–751 (1990).
Article CAS Google Scholar - Gray, D., Dullforce, P. & Jainandunsing, S. Memory B cell development but not germinal center formation is impaired by in vivo blockade of CD40-CD40 ligand interaction. J. Exp. Med. 180, 141–155 (1994).
Article CAS Google Scholar - Cattoretti, G. et al. BCL-6 protein is expressed in germinal-center B cells. Blood 86, 45–53 (1995).
CAS PubMed Google Scholar - Onizuka, T. et al. BCL-6 gene product, a 92- to 98-kD nuclear phosphoprotein, is highly expressed in germinal center B cells and their neoplastic counterparts. Blood 86, 28–37 (1995).
CAS PubMed Google Scholar - Kerckaert, J.P. et al. LAZ3, a novel zinc-finger encoding gene, is disrupted by recurring chromosome 3q27 translocations in human lymphomas. Nature Genet. 5, 66–70 (1993).
Article CAS Google Scholar - Ye, B.H. et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science 262, 747–750 (1993).
Article CAS Google Scholar - Fukuda, T. et al. The murine BCL6 gene is induced in activated lymphocytes as an immediate early gene. Oncogene 11, 1657–1663 (1995).
CAS PubMed Google Scholar - Dhordain, P. et al. Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein. Proc. Natl. Acad. Sci. USA 94, 10762–10767 (1997).
Article CAS Google Scholar - Dent, A.L., Shaffer, A.L., Yu, X., Allman, D. & Staudt, L.M. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276, 589–592 (1997).
Article CAS Google Scholar - Fukuda, T. et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J. Exp. Med. 186, 439–448 (1997).
Article CAS Google Scholar - Ye, B.H. et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nature Genet. 16, 161–170 (1997).
Article CAS Google Scholar - Yoshida, T. et al. The role of Bcl6 in mature cardiac myocytes. Cardiovasc. Res. 42, 670–679 (1999).
Article CAS Google Scholar - Chaffin, K.E. et al. Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. EMBO J. 9, 3821–3829 (1990).
Article CAS Google Scholar - Walunas, T.L., Bruce, D.S., Dustin, L., Loh, D.Y. & Bluestone, J.A. Ly-6C is a marker of memory CD8+ T cells. J. Immunol. 155, 1873–1883 (1995).
CAS PubMed Google Scholar - Grayson, J.M., Zajac, A.J., Altman, J.D. & Ahmed, R. Increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 164, 3950–3954 (2000).
Article CAS Google Scholar - Murali-Krishna, K. et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286, 1377–1381 (1999).
Article CAS Google Scholar - Ku, C.C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678 (2000).
Article CAS Google Scholar - Cho, B.K., Wang, C., Sugawa, S., Eisen, H.N. & Chen, J. Functional differences between memory and naïve CD8 T cells. Proc. Natl. Acad. Sci. USA 96, 2976–2981 (1999).
Article CAS Google Scholar - Veiga-Fernandes, H., Walter, U., Bourgeois, C., McLean, A. & Rocha, B. Response of naïve and memory CD8+ T cells to antigenic stimulation in vivo. Nature Immunol. 1, 47–53 (2000).
Article CAS Google Scholar - Hogquist, K. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Article CAS Google Scholar - Mitchell, T., Kappler, J. & Marrack, P. Bystander virus infection prolongs activated T cell survival. J. Immunol. 162, 4527–4535 (1999).
CAS PubMed Google Scholar - Zhang, X., Sun, S., Hwang, I., Tough, D.F. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Article CAS Google Scholar - Ge, Q., Hu, H., Eisen, H.N. & Chen, J. Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naïve and memory T cell compartments. Proc. Natl. Acad. Sci. USA 99, 2989–2994 (2002).
Article CAS Google Scholar - Piguet, P.F., Irle, C., Kollatte, E. & Vassalli, P. Post-thymic T lymphocyte maturation during ontogenesis. J. Exp. Med. 154, 581–593 (1981).
Article CAS Google Scholar - Gruta, N.L., Driel, I.R. & Gleeson, P.A. Peripheral T cell expansion in lymphopenic mice results in a restricted T cell repertoire. Eur. J. Immunol. 30, 3380–3386 (2000).
Article Google Scholar - Le Campion, A. et al. Naïve T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes. Proc. Natl. Acad. Sci. USA 99, 4538–4543 (2002).
Article CAS Google Scholar - Veiga-Fernandes, H., Walter, U., Bourgeois, C., McLean, A. & Rocha, B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nature Immunol. 1, 47–53 (2000).
Article CAS Google Scholar - White, J., Crawford, F., Fremont, D., Marrack, P., & Kappler, J. Soluble class I MHC with β2-microglobulin covalently linked peptides; specific binding to a T cell hybridoma. J. Immunol. 162, 2671–2676 (1999).
CAS PubMed Google Scholar - Nicoletti, I., Migliorati, G., Pagliacci, M.C., Grignani, F. & Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Meth. 139, 271–279 (1991).
Article CAS Google Scholar - Levisky, H.I., Lazenby, A., Hayashi, R.J. & Pardoll, D.M. In vivo priming of two distinct antitumor effector populations: the role of MHC class I complexes using a monoclonal antibody. Immunity 6, 715–726 (1994).
Google Scholar - Restifo, N.P. et al. Antigen processing in vivo and the elicitation of primary CTL responses. J. Immunol. 154, 4414–4422 (1995).
CAS PubMed PubMed Central Google Scholar
Acknowledgements
We thank P. Marrack, K. Takatsu, T. Nakayama, K. Yamashita and M. Yamashita for discussions; H. Levitsky for the GM-CSF–producing cell line; H. Satake for skillful technical assistance; K. Ujiie for secretarial services; and M. Ohara for language assistance. Supported in part by Grants-in-Aid from the Ministry of Education, Science, Technology, Sports and Culture of Japan and the Yamanouchi Foundation for Research on Metabolic Disorders.
Author information
Author notes
- Shinsuke Taki
Present address: Department of Transplantation Immunology, Shinshu University Graduate School of Medicine, Matsumoto, 390-8621, Japan
Authors and Affiliations
- Department of Developmental Genetics (H2), Graduate School of Medicine, Chiba University, Chiba, 260-8670, Japan
Hirohito Ichii, Akemi Sakamoto, Masahiko Hatano, Seiji Okada, Hirochika Toyama, Masafumi Arima & Takeshi Tokuhisa - First Department of Surgery, Kobe University School of Medicine, Kobe, 650-0017, Japan
Hirohito Ichii, Hirochika Toyama & Yoshikazu Kuroda - Department of Molecular Genetics (H1), Graduate School of Medicine, Chiba University, Chiba, 260-8670, Japan
Shinsuke Taki
Authors
- Hirohito Ichii
You can also search for this author inPubMed Google Scholar - Akemi Sakamoto
You can also search for this author inPubMed Google Scholar - Masahiko Hatano
You can also search for this author inPubMed Google Scholar - Seiji Okada
You can also search for this author inPubMed Google Scholar - Hirochika Toyama
You can also search for this author inPubMed Google Scholar - Shinsuke Taki
You can also search for this author inPubMed Google Scholar - Masafumi Arima
You can also search for this author inPubMed Google Scholar - Yoshikazu Kuroda
You can also search for this author inPubMed Google Scholar - Takeshi Tokuhisa
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toTakeshi Tokuhisa.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ichii, H., Sakamoto, A., Hatano, M. et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells.Nat Immunol 3, 558–563 (2002). https://doi.org/10.1038/ni802
- Received: 22 April 2002
- Accepted: 06 May 2002
- Published: 20 May 2002
- Issue Date: 01 June 2002
- DOI: https://doi.org/10.1038/ni802