Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children (original) (raw)
A new multisystem inflammatory syndrome apparently related to infection with SARS-CoV-2 has recently been reported in older children (known as MIS-C), manifested by severe abdominal pain, cardiac dysfunction and shock. Here, I discuss the similarities and differences between MIS-C and Kawasaki disease, focusing on their epidemiology, aetiology and pathophysiological mechanisms.
When the COVID-19 pandemic was first reported in Asia and initially spread throughout the globe, paediatricians were grateful that children seemed to be only mildly symptomatic with the infection in most cases. Then, an alarming warning came from the National Health Service in England in April 2020 about cases of older school-aged children and adolescents presenting with fever, hypotension, severe abdominal pain and cardiac dysfunction who tested positive for SARS-CoV-2 infection either by nasopharyngeal RT-PCR assay or by antibody testing. These children had laboratory findings of cytokine storm, including high serum IL-6 levels, and generally required inotropic support to increase cardiac output with rare need for extracorporeal membrane oxygenation. Almost all of these children no longer required intensive care after only a few days and completely recovered, although rare deaths resulted from complications of extracorporeal membrane oxygenation. Case series of children presenting with this condition have now been reported from the UK1, Italy2, Spain[3](/articles/s41577-020-0367-5#ref-CR3 "Cabrero-Hernández, M. et al. Severe SARS-CoV-2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain. Pediatr. Infect. Dis. J. https://doi.org/10.1097/INF.0000000000002777
(2020)."), France and Switzerland[4](/articles/s41577-020-0367-5#ref-CR4 "Belhadjer, Z. et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.048360
(2020)."), and the United States[5](/articles/s41577-020-0367-5#ref-CR5 "Chiotos, K. et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J. Pediatr. Infect. Dis. Soc. https://doi.org/10.1093/jpids/piaa069
(2020)."). The Centers for Disease Control and Prevention (CDC) has developed a case definition for use in the United States and has termed the condition multisystem inflammatory syndrome in children (MIS-C).
Physicians have noted some clinical similarities between MIS-C and Kawasaki disease (KD), a febrile illness of young childhood involving inflammation of the blood vessels that can result in coronary artery aneurysms. KD is presently of unknown aetiology, although substantial recent progress supports a presently unidentified ubiquitous virus as the cause[6](/articles/s41577-020-0367-5#ref-CR6 "Rowley, A. H. et al. A protein epitope targeted by the antibody response to Kawasaki disease. J. Infect. Dis. https://doi.org/10.1093/infdis/jiaa066
(2020)."). Patients with MIS-C may have some of the clinical features of KD, including fever, dilation of conjunctival blood vessels, rash and redness of the oropharynx. However, these clinical signs can be observed in many infectious diseases in childhood and are not specific for any one diagnosis. The question has therefore arisen as to whether MIS-C and KD are the same entity.
The epidemiology of KD has been virtually identical in all countries in the world for the past 50 years or more, with 80% of cases occurring in children <5 years of age and with a peak incidence at ~10 months of age. This is in marked contrast to the epidemiology of MIS-C, which affects older children and adolescents. Various characteristic laboratory findings in MIS-C, such as leukopenia and extremely high levels of ventricular natriuretic peptide (a marker of heart failure), are not features of KD. Asian children have the highest rate of KD in the world, whereas children of African descent seem to be at particular risk of developing MIS-C1. No cases of MIS-C have been reported in China and Japan7. It is clear that the epidemiology of the two conditions is quite dissimilar and, therefore, it is important to avoid jumping to conclusions regarding a similar aetiology.
Because of the overlapping clinical features and the lack of a diagnostic test for either KD or MIS-C, distinguishing the two conditions in an individual patient can be difficult. Several groups have reported the rare occurrence of coronary aneurysms in children with MIS-C1,2, but it is unclear whether MIS-C can result in this complication, or whether these children actually had KD. If SARS-CoV-2 infection can result in coronary aneurysms in childhood, it would be the first virus to be proven to do so. More often, mild transient dilation of the coronary arteries is reported in MIS-C, as occurs in another paediatric condition that is also associated with high serum IL-6 levels, systemic onset juvenile idiopathic arthritis.
Although SARS-CoV-2 has not been definitively proven as the cause of MIS-C, the fact that MIS-C appeared during outbreaks of COVID-19 in Europe and the United States is highly suggestive. If the condition becomes less common as the pandemic ceases, it will further support an association. However, many questions remain. Why was this condition not observed in China, where the virus was first reported? Is it such a rare condition that it is observed only in nations with a very large number of cases of COVID-19 (such as the United States, Spain, Italy, France and the UK) but not in nations with fewer cases (such as Japan and China)? Or has the virus changed in some way over time that has resulted in a change of its pathogenicity? Or has some other policy in individual countries affected the prevalence of MIS-C (for example, childhood administration of BCG vaccine)? Data are presently lacking to answer these important questions.
If MIS-C is indeed related to infection with SARS-CoV-2, the pathophysiological mechanism of disease is unclear. Some have proposed that the condition is not the result of acute viral infection but is a post-infectious phenomenon related to IgG antibody-mediated enhancement of disease. This hypothesis seems to have emerged for two main reasons. First, MIS-C cases have lagged in time compared with the peak of SARS-CoV-2 infection in at least some countries. However, as children likely acquire the virus from their parents because of stay-at-home restrictions, a lag from the peak of cases in adults could be expected. Second, children with MIS-C more often test positive for antibody to SARS-CoV-2 than for virus using nasopharyngeal RT-PCR assay. However, children with MIS-C have a predominantly gastrointestinal presentation of their illness with few, if any, respiratory symptoms in most cases. Therefore, the virus may be primarily replicating in the gastrointestinal tract; enterocytes have been shown to be readily infected by SARS-CoV-2 (REF.[8](/articles/s41577-020-0367-5#ref-CR8 "Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science https://doi.org/10.1126/science.abc1669
(2020).")), and patients with MIS-C who have undergone exploratory laparotomy have been found to have mesenteric adenitis, supporting gastrointestinal infection[4](/articles/s41577-020-0367-5#ref-CR4 "Belhadjer, Z. et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.048360
(2020)."). Stool RT-PCR assays for the virus are not widely clinically available and have not been reported for children with MIS-C. Moreover, the presence of antibody to SARS-CoV-2 does not itself imply a post-infectious process, because antibodies may arise during the second week of infection. Furthermore, there is a lack of information regarding the specificity of the antibody assays carried out in patients with MIS-C, which can be widely variable. As SARS-CoV-2 infection spreads through a community, resulting in asymptomatic or mildly symptomatic infection in the majority of children, positive antibody tests will become increasingly common, and childhood controls will be necessary to establish an association between SARS-CoV-2 and a particular disease. Of interest, worsening of illness has so far not been an apparent clinical problem in patients with COVID-19 who are treated with convalescent plasma, as one might expect if antibody-mediated enhancement is an important mechanism for the development of severe COVID-19 complications.
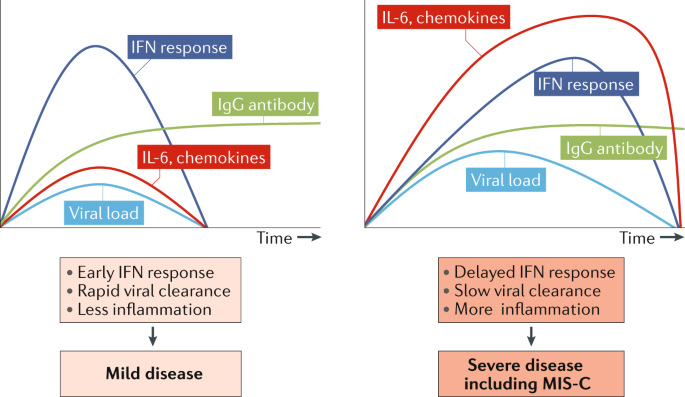
One compelling alternative hypothesis for the marked cytokine storm experienced by children with MIS-C derives from the well-known ability of coronaviruses to block type I and type III interferon responses9, with the potential outcome of delayed cytokine storm in patients with immune responses that cannot control viral replication well or in those with initially high SARS-CoV-2 viral load9,10 (Fig. 1).
Fig. 1: Pathogenesis of multisystem inflammatory syndrome in children: a hypothesis.
The timing of the interferon (IFN) response to SARS-CoV-2 infection can vary with viral load and genetic differences in host response. When viral load is low, IFN responses are engaged and contribute to viral clearance, resulting in mild infection. When viral load is high and/or genetic factors slow antiviral responses, virus replication can delay the IFN response and cytokine storm can result before adaptive responses clear the virus, resulting in severe disease including multisystem inflammatory syndrome in children (MIS-C). Adapted with permission from REF.9, Elsevier.
The CDC case definition of MIS-C is extremely broad and would be met in many children with acute COVID-19, KD, other viral infection, systemic onset juvenile idiopathic arthritis, and many other infectious and inflammatory conditions of childhood. Such a broad case definition will likely complicate the identification of the true spectrum and potential complications of MIS-C. It is likely that focusing on patients with the initially described presentations of shock, severe abdominal pain and myocardial dysfunction will be most informative in urgently needed research studies to understand the pathophysiology and clinical outcomes of MIS-C.
References
- Riphagen, S., Gomez, X., Gonzalez-Martinez, C., Wilkinson, N. & Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395, 1607–1608 (2020).
Article CAS Google Scholar - Verdoni, L. et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395, 1771–1778 (2020).
Article CAS Google Scholar - Cabrero-Hernández, M. et al. Severe SARS-CoV-2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain. Pediatr. Infect. Dis. J. https://doi.org/10.1097/INF.0000000000002777 (2020).
Article PubMed Google Scholar - Belhadjer, Z. et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.048360 (2020).
Article PubMed Google Scholar - Chiotos, K. et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J. Pediatr. Infect. Dis. Soc. https://doi.org/10.1093/jpids/piaa069 (2020).
Article Google Scholar - Rowley, A. H. et al. A protein epitope targeted by the antibody response to Kawasaki disease. J. Infect. Dis. https://doi.org/10.1093/infdis/jiaa066 (2020).
Article PubMed Google Scholar - Xu, S., Chen, M. & Weng, J. COVID-19 and Kawasaki Disease in Children. Pharmacol. Res. 159, 104951 (2020).
Article CAS Google Scholar - Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science https://doi.org/10.1126/science.abc1669 (2020).
Article PubMed PubMed Central Google Scholar - Park, A. & Iwasaki, A. Type I and type III interferons — induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 27, 870–878 (2020).
Article CAS Google Scholar - Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045.e9 (2020).
Article CAS Google Scholar
Author information
Authors and Affiliations
- Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Anne H. Rowley - Department of Microbiology-Immunology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Anne H. Rowley - Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA
Anne H. Rowley
Authors
- Anne H. Rowley
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toAnne H. Rowley.
Ethics declarations
Competing interests
A.H.R. is a named investigator on National Institutes of Health R21AI140029 and Provisional Patent 62/811,930 on Antibodies and Antigens of Kawasaki disease.
Rights and permissions
About this article
Cite this article
Rowley, A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children.Nat Rev Immunol 20, 453–454 (2020). https://doi.org/10.1038/s41577-020-0367-5
- Published: 16 June 2020
- Issue Date: August 2020
- DOI: https://doi.org/10.1038/s41577-020-0367-5