Postprandial glycaemic dips predict appetite and energy intake in healthy individuals (original) (raw)
- Article
- Published: 12 April 2021
- Sarah E. Berry2,
- Graham Finlayson3,
- Ruairi O’Driscoll ORCID: orcid.org/0000-0003-3995-00733,
- George Hadjigeorgiou1,
- David A. Drew4,
- Haya Al Khatib1,2,
- Long H. Nguyen ORCID: orcid.org/0000-0002-5436-42194,
- Inbar Linenberg1,
- Andrew T. Chan4,
- Tim D. Spector ORCID: orcid.org/0000-0002-9795-03655,
- Paul W. Franks ORCID: orcid.org/0000-0002-0520-76046,
- Jonathan Wolf ORCID: orcid.org/0000-0002-0530-22571,
- John Blundell ORCID: orcid.org/0000-0002-7085-95963 &
- …
- Ana M. Valdes ORCID: orcid.org/0000-0003-1141-44715,7,8
Nature Metabolism volume 3, pages 523–529 (2021)Cite this article
- 6684 Accesses
- 60 Citations
- 990 Altmetric
- Metrics details
Subjects
This article has been updated
Abstract
Understanding how to modulate appetite in humans is key to developing successful weight loss interventions. Here, we showed that postprandial glucose dips 2–3 h after a meal are a better predictor of postprandial self-reported hunger and subsequent energy intake than peak glucose at 0–2 h and glucose incremental area under the blood glucose curve at 0–2 h. We explore the links among postprandial glucose, appetite and subsequent energy intake in 1,070 participants from a UK exploratory and US validation cohort, who consumed 8,624 standardized meals followed by 71,715 ad libitum meals, using continuous glucose monitors to record postprandial glycaemia. For participants eating each of the standardized meals, the average postprandial glucose dip at 2–3 h relative to baseline level predicted an increase in hunger at 2–3 h (r = 0.16, P < 0.001), shorter time until next meal (r = −0.14, P < 0.001), greater energy intake at 3–4 h (r = 0.19, P < 0.001) and greater energy intake at 24 h (r = 0.27, P < 0.001). Results were directionally consistent in the US validation cohort. These data provide a quantitative assessment of the relevance of postprandial glycaemia in appetite and energy intake modulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Fig. 1: Average glycaemic responses to standardized breakfasts illustrating the key measures used in the study (n = 1,070, m = 8,624).
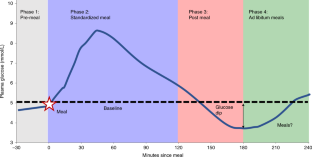
Fig. 2: Postprandial measures according to the top and bottom quartiles of the 2–3 h glucose dip (n = 763, m = 5,667; UK n = 685, m = 5,667; US validation cohort n = 78, m = 602).

Fig. 3: Differences in postprandial measures across repeated meals within individuals (n = 1,053, m = 6,428; UK n = 958, m = 5,928; US validation cohort n = 95, m = 500).

Similar content being viewed by others
Data availability
The data used for analysis in this study are held by the Department of Twin Research at King’s College London. The data can be released to bona fide researchers using our normal procedures overseen by the Wellcome Trust and its guidelines as part of our core funding. We receive around 100 requests per year for our datasets and have a meeting three times a month with independent members to assess proposals. Application is via https://twinsuk.ac.uk/resources-for-researchers/access-our-data/. Data must be anonymized and conform to General Data Protection Regulation standards. Source data are provided with this paper.
Change history
13 July 2021
A Correction to this paper has been published: https://doi.org/10.1038/s42255-021-00436-1
References
- Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Article PubMed PubMed Central Google Scholar - Young-Hyman, D. Introduction to special issue: self-regulation of appetite—it’s complicated. Obesity (Silver Spring) 25, S5–S7 (2017).
Article Google Scholar - Montesi, L. et al. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab. Syndr. Obes. 9, 37–46 (2016).
PubMed PubMed Central Google Scholar - El Ghoch, M., Calugi, S. & Dalle Grave, R. The effects of low-carbohydrate diets on psychosocial outcomes in obesity/overweight: a systematic review of randomized, controlled studies. Nutrients 8, 402 (2016).
Article PubMed Central Google Scholar - Sumithran, P. & Proietto, J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin. Sci. (Lond.) 124, 231–241 (2013).
Article Google Scholar - Gilbert, J.-A. et al. Milk supplementation facilitates appetite control in obese women during weight loss: a randomised, single-blind, placebo-controlled trial. Br. J. Nutr. 105, 133–143 (2011).
Article CAS PubMed Google Scholar - Hintze, L. J., Mahmoodianfard, S., Auguste, C. B. & Doucet, É. Weight loss and appetite control in women. Curr. Obes. Rep. 6, 334–351 (2017).
Article PubMed Google Scholar - Tremblay, A., Lepage, C., Panahi, S., Couture, C. & Drapeau, V. Adaptations to a diet-based weight-reducing programme in obese women resistant to weight loss. Clin. Obes. 5, 145–153 (2015).
Article CAS PubMed Google Scholar - Melby, C. L., Paris, H. L., Foright, R. M. & Peth, J. Attenuating the biologic drive for weight regain following weight loss: must what goes down always go back up? Nutrients 9, 468 (2017).
Article PubMed Central Google Scholar - Lam, Y. Y. & Ravussin, E. Variations in energy intake: it is more complicated than we think. Am. J. Clin. Nutr. 106, 1169–1170 (2017).
Article CAS PubMed Google Scholar - Blundell, J. E. et al. The drive to eat in Homo sapiens: energy expenditure drives energy intake. Physiol. Behav. 219, 112846 (2020).
Article CAS PubMed Google Scholar - Kleinridders, A., Ferris, H. A., Cai, W. & Kahn, C. R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63, 2232–2243 (2014).
Article PubMed PubMed Central Google Scholar - Gonzalez-Anton, C. et al. Glycemic responses, appetite ratings and gastrointestinal hormone responses of most common breads consumed in Spain. A randomized control trial in healthy humans. Nutrients 7, 4033–4053 (2015).
Article CAS PubMed PubMed Central Google Scholar - Bonnema, A. L., Altschwager, D. K., Thomas, W. & Slavin, J. L. The effects of the combination of egg and fiber on appetite, glycemic response and food intake in normal weight adults: a randomized, controlled, crossover trial. Int. J. Food Sci. Nutr. 67, 723–731 (2016).
Article CAS PubMed Google Scholar - Ludwig, D. S. et al. High glycemic index foods, overeating, and obesity. Pediatrics 103, E26 (1999).
Article CAS PubMed Google Scholar - Mayer, J. Glucostatic mechanism of regulation of food intake. N. Engl. J. Med. 249, 13–16 (1953).
Article CAS PubMed Google Scholar - Smith, F. J. & Campfield, L. A. Meal initiation occurs after experimental induction of transient declines in blood glucose. Am. J. Physiol. 265, R1423–R1429 (1993).
CAS PubMed Google Scholar - Campfield, L. A. & Smith, F. J. Blood glucose dynamics and control of meal initiation: a pattern detection and recognition theory. Physiol. Rev. 83, 25–58 (2003).
Article CAS PubMed Google Scholar - Kovacs, E. M. R. et al. Associations between spontaneous meal initiations and blood glucose dynamics in overweight men in negative energy balance. Br. J. Nutr. 87, 39–45 (2002).
Article CAS PubMed Google Scholar - Woods, S. C. & D’Alessio, D. A. Central control of body weight and appetite. J. Clin. Endocrinol. Metab. 93, S37–S50 (2008).
Article CAS PubMed PubMed Central Google Scholar - Morton, G. J., Meek, T. H. & Schwartz, M. W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 15, 367–378 (2014).
Article CAS PubMed PubMed Central Google Scholar - Dryden, S., Pickavance, L., Henderson, L. & Williams, G. Hyperphagia induced by hypoglycemia in rats is independent of leptin and hypothalamic neuropeptide Y (NPY). Peptides 19, 1549–1555 (1998).
Article CAS PubMed Google Scholar - Sprague, J. E. & Arbeláez, A. M. Glucose counterregulatory responses to hypoglycemia. Pediatr. Endocrinol. Rev. 9, 463–473 (2011).
PubMed PubMed Central Google Scholar - Kim, J. et al. In a free-living setting, obesity is associated with greater food intake in response to a similar premeal glucose nadir. J. Clin. Endocrinol. Metab. 104, 3911–3919 (2019).
Article PubMed Central Google Scholar - Te Morenga, L., Mallard, S. & Mann, J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 346, e7492 (2012).
Article PubMed Google Scholar - Mozaffarian, D., Hao, T., Rimm, E. B., Willett, W. C. & Hu, F. B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 364, 2392–2404 (2011).
Article CAS PubMed PubMed Central Google Scholar - Bao, J., Atkinson, F., Petocz, P., Willett, W. C. & Brand-Miller, J. C. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: glycemic load compared with carbohydrate content alone. Am. J. Clin. Nutr. 93, 984–996 (2011).
Article CAS PubMed Google Scholar - Pek, J., Wong, O. & Wong, A. C. M. How to address non-normality: a taxonomy of approaches, reviewed, and illustrated. Front. Psychol. 9, 2104 (2018).
Article PubMed PubMed Central Google Scholar - Berry, S. et al. Personalised REsponses to DIetary Composition Trial (PREDICT): an intervention study to determine inter-individual differences in postprandial response to foods. Preprint at Protocol Exchange https://doi.org/10.21203/rs.2.20798/v1 (2020).
- Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26, 964–973 (2020).
Article CAS PubMed PubMed Central Google Scholar - Flint, A., Raben, A., Blundell, J. E. & Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 24, 38–48 (2000).
Article CAS PubMed Google Scholar
Acknowledgements
We thank the participants of the PREDICT 1 study. We thank the staff of Zoe Global, the Department of Twin Research and Massachusetts General Hospital for their tireless work in contributing to the running of the study and data collection. We thank Abbott for their support with using their CGMs. This work was supported by Zoe Global Ltd and Massachusetts General Hospital and the Translational and Clinical Research Center. It also received support from grants from the Wellcome Trust (no. 212904/Z/18/Z) and Medical Research Council (MRC)/British Heart Foundation Ancestry and Biological Informative Markers for Stratification of Hypertension (no. MR/M016560/1). P.W.F. was supported in part by grants from the European Research Council (no. CoG-2015_681742_NASCENT), Swedish Research Council, Novo Nordisk Foundation and the Swedish Foundation for Strategic Research (IRC award). A.M.V. was supported by the National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre. TwinsUK is funded by the Wellcome Trust, MRC, European Union, Chronic Disease Research Foundation, Zoe Global Ltd and the NIHR-funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London. The sponsor, Zoe Global Ltd, was directly involved in study design, data collection and analysis for this manuscript. Zoe Global Ltd, the Wellcome Trust and NIHR funded this study.
Author information
Authors and Affiliations
- Zoe Global Ltd, London, UK
Patrick Wyatt, George Hadjigeorgiou, Haya Al Khatib, Inbar Linenberg & Jonathan Wolf - Department of Nutritional Sciences, King’s College London, London, UK
Sarah E. Berry & Haya Al Khatib - Appetite and Energy Balance Research Group, School of Psychology, Faculty of Medicine and Health, University of Leeds, Leeds, UK
Graham Finlayson, Ruairi O’Driscoll & John Blundell - Clinical & Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
David A. Drew, Long H. Nguyen & Andrew T. Chan - Department of Twin Research and Genetic Epidemiology, King’s College London, London, UK
Tim D. Spector & Ana M. Valdes - Genetic & Molecular Epidemiology Unit, Department of Clinical Science, Lund University, Malmö, Sweden
Paul W. Franks - School of Medicine, University of Nottingham, Nottingham City Hospital, Nottingham, UK
Ana M. Valdes - Nottingham National Institute for Health Research Biomedical Research Centre, Queens Medical Centre, Nottingham, UK
Ana M. Valdes
Authors
- Patrick Wyatt
You can also search for this author inPubMed Google Scholar - Sarah E. Berry
You can also search for this author inPubMed Google Scholar - Graham Finlayson
You can also search for this author inPubMed Google Scholar - Ruairi O’Driscoll
You can also search for this author inPubMed Google Scholar - George Hadjigeorgiou
You can also search for this author inPubMed Google Scholar - David A. Drew
You can also search for this author inPubMed Google Scholar - Haya Al Khatib
You can also search for this author inPubMed Google Scholar - Long H. Nguyen
You can also search for this author inPubMed Google Scholar - Inbar Linenberg
You can also search for this author inPubMed Google Scholar - Andrew T. Chan
You can also search for this author inPubMed Google Scholar - Tim D. Spector
You can also search for this author inPubMed Google Scholar - Paul W. Franks
You can also search for this author inPubMed Google Scholar - Jonathan Wolf
You can also search for this author inPubMed Google Scholar - John Blundell
You can also search for this author inPubMed Google Scholar - Ana M. Valdes
You can also search for this author inPubMed Google Scholar
Contributions
J.W., G.H. and T.D.S. obtained the funding. P.W. and A.M.V. designed the study and developed its concept. S.E.B., P.W.F., D.A.D., H.A.K., L.H.N., A.T.C., R.O.D. and G.F. collected the data. P.W. and A.M.V. analysed the data. S.E.B., P.W.F., H.A.K., D.A.D., G.H., J.W. and I.L. coordinated the study. P.W., J.B. and A.M.V. wrote the manuscript. All authors reviewed and revised the final manuscript.
Corresponding author
Correspondence toAna M. Valdes.
Ethics declarations
Competing interests
T.D.S., S.E.B., A.M.V., P.W.F. and A.T.C. are consultants to Zoe Global. J.W., G.H., H.A.K, P.W. and I.L. are or have been employees of Zoe Global. J.B. is a member of the Zoe Global scientific advisory board. The other authors declare no conflicts of interest.
Additional information
Peer review information Nature Metabolism thanks Jennie C Brand-Miller, Lisa Chow and Hao Wang for their contribution to the peer review of this work. Primary Handling Editors: Pooja Jha; Isabella Samuelson.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Wyatt, P., Berry, S.E., Finlayson, G. et al. Postprandial glycaemic dips predict appetite and energy intake in healthy individuals.Nat Metab 3, 523–529 (2021). https://doi.org/10.1038/s42255-021-00383-x
- Received: 27 August 2020
- Accepted: 09 March 2021
- Published: 12 April 2021
- Issue Date: April 2021
- DOI: https://doi.org/10.1038/s42255-021-00383-x