HSpin1, a transmembrane protein interacting with Bcl-2/Bcl-xL, induces a caspase-independent autophagic cell death (original) (raw)
Introduction
Important regulators of programmed cell death (PCD) are the Bcl-2 family proteins.1,2 Bcl-2 was first recognized as a modulator of cell death in studies of follicular B-cell lymphoma, in which overexpression of Bcl-2 promoted transformation by rendering cells more resistant to apoptosis. The antiapoptotic activity of Bcl-2 has been observed in numerous cell types in response to various stimuli. Pro- and antiapoptotic members of the Bcl-2 family interact with one another and modulate each other's activities.3 In fact, the relative levels of these proteins in a cell determine whether a cell will live or die.
Apoptotic signal transduction has been extensively studied biochemically and genetically, and it is now known that a family of cysteine proteases with aspartate specificity is the major effector of the process.4 Caspases are synthesized as inactive precursors and are proteolytically activated upon exposure to apoptotic stimuli. Caspases are classified into two groups, initiators and effectors. Once the initiator caspases are activated by their formation of a complex with other molecules, they induce the processing of downstream caspases, which cleave a set of cellular proteins, leading to morphological changes and the degradation of chromosomal DNA.
In contrast to apoptotic cell death, the molecular mechanism for necrosis is not well understood, although several mechanisms, including the release of lysosomal enzymes, the generation of toxic oxygen radicals, and the activation of calcium-dependent phospholipases, have been proposed.5 Necrosis occurs as a result of complement attack, severe hypoxia, hyperthermia, lytic viral infection, or exposure to various toxins and respiratory poisons. Tumor necrosis factor 1 (TNF1) can activate the necrotic death program in some cell lines, such as the mouse L929 cell line.6 The involvement of necrosis in the removal of interdigital cells in the mouse embryo has also been suggested.7 Therefore, both apoptosis and necrosis contribute to PCD.
This study demonstrates that HSpin1, the human ortholog of Drosophila Spin, plays a critical role in necrotic cell death in cultured human cells. The spin gene was originally identified in Drosophila as its mutant forms caused behavioral and developmental defects because of partial loss of PCD in the reproductive and nervous systems.8,9,10 The Spin protein has multiple membrane spanning domains with no significant homology to any of the membrane proteins of known function.9 We present evidence that the HSpin1 protein binds to anticell-death proteins Bcl-2 and apoptosis regulator Bcl-X (Bcl-xL), and induces caspase-independent cell death. The killer effect of HSpin1 is blocked by pyrrolidine dithiocarbamate (PDTC), an inhibitor of caspase-independent necrosis.11 Thus, our study revealed a novel mechanism for necrosis, in which HSpin1 operates as an ON–OFF switch for effector functions.
Results
HSpin1 induces cell death that is inhibited by Bcl-xL
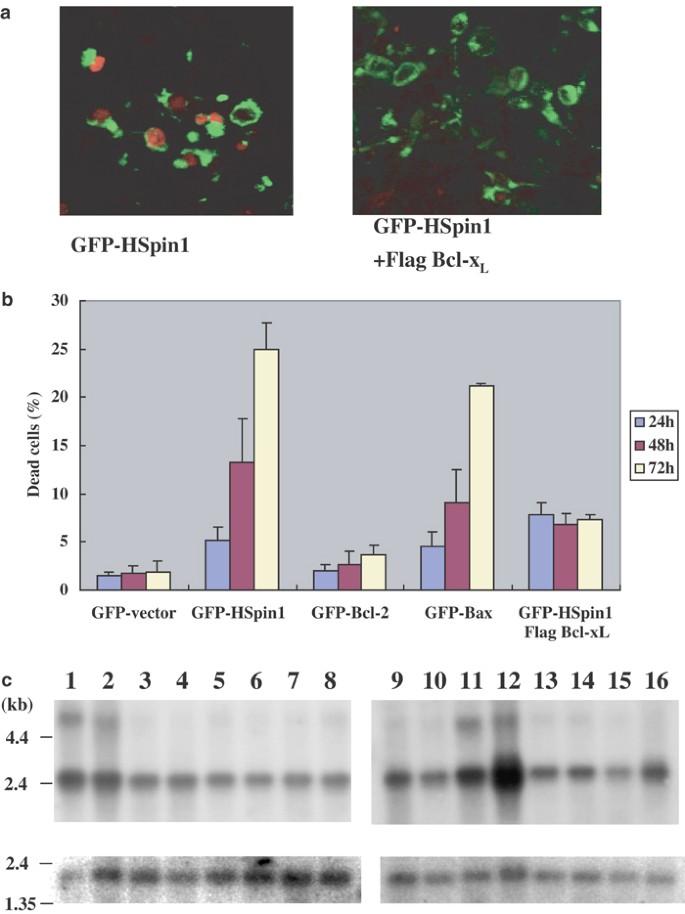
To characterize the cell-death-inducing activity of HSpin1, we transientlyQ2 transfected HEK293 cells with a plasmid that encodes HSpin1 cDNA fused to GFP (GFP–Hspin1). When cell death was assessed by the propidium iodide (PI) exclusion method, HSpin1 was indeed found to induce cell death. As shown in the left panel of Figure 1a, most of the cells expressing GFP–HSpin1 (green) were stained by PI (red), indicating disintegration of the plasma membrane. The level of the cell-death-inducing activity of HSpin1 was comparable with that of Bcl-2-associated X protein (Bax), a proapoptotic Bcl-2 family member (Figure 1b). Since cell death was not induced when the cells were transfected with GFP alone, the cell death observed in HSpin1-transfected cells was specific and not because of the cytotoxic effect of lipofection. Importantly, coexpression of Bcl-xL, an antiapoptotic Bcl-2 family member, markedly inhibited HSpin1-induced cell death (Figure 1a, right panel and Figure 1b). Similar results were obtained when HeLa cells were used for this assay (data not shown). In Drosophila, the spin gene function is required for the normal development of the reproductive and nervous systems9. Interestingly, both HEK293 and HeLa cells have endogenous HSpin1 expression. This suggests that HSpin1 is ubiquitously expressed in human tissues. Our Northern blot analysis indicated that this was indeed the case (Figure 1c). HSpin1 was expressed in all tissues examined, particularly at high levels in prostate, testis, and cerebellum.
Figure 1
HSpin1-induced cell death and its inhibition by Bcl-xL. (a) Cytological detection of the GFP–HSpin1 fusion protein (green) and dead cells (red). Bcl-xL coexpression (right) alleviates the killing effect of HSpin1 (left). HEK293 cells were transiently transfected with 1 _μ_g of the pGFP–HSpin1 construct with (right panel) or without (left panel) 2 _μ_g of pFlag–Bcl-xL. The transfected cells were observed under a confocal laser scanning microscope. The nuclei of dead cells stained with PI are indicated in red and GFP–HSpin1 expression appears in green. (b) Comparison of cell death mediated by various GFP fusion proteins. The number of cells killed as a result of HSpin1 overexpression (second to the left-hand-most graphs) is greatly decreased by Bcl-xL coexpression (the right-hand-most graph). HEK293 cells were transfected with 1 _μ_g each of the plasmids encoding various GFP fusion proteins as indicated. In one series of experiments, 2 _μ_g of the Flag–Bcl-xL plasmid was added to pGFP–HSpin1. At indicated time points after the transfection, the percentage of dead cells (stained by PI) among transfected cells (with green fluorescence) was determined. Data are presented as the means±S.D. obtained from three independent experiments. (c) Northern blot analysis of HSpin1 mRNA. Poly (A)+ mRNA (2 _μ_g) from various tissues of adult human were hybridized with a specific HSpin1 cDNA probe (upper panel). The lanes 1–16 represent blots of RNA from cerebellum (1), cerebral cortex (2), medulla (3), spinal cord (4), occipital pole (5), frontal lobe (6), temporal lobe (7), putamen (8), spleen (9), thymus (10), prostate (11), testis (12), ovary (13), small intestine (14), colon (15) and peripheral blood leukocyte (16), respectively. The lower panel represents the same blot hybridized with a human _β_-actin cDNA to confirm the RNA quality and relative amount of loading. RNA size markers are shown on the left
HSpin1 is expressed localized in mitochondria and is partially colocalized with Bcl-2 and Bcl-xL
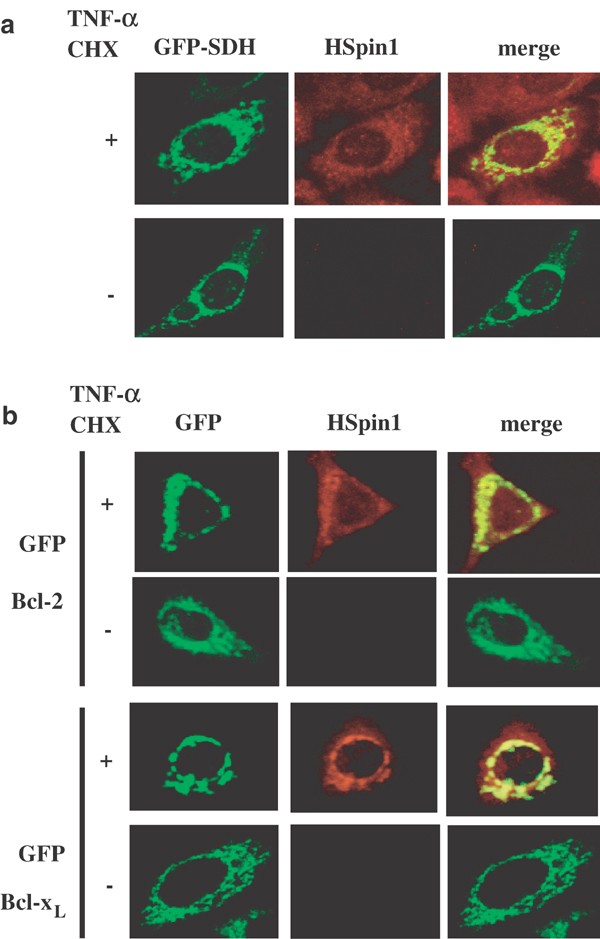
Using our anti-HSpin1 antibody, immunoreactivity was detectable only after the cells were treated with apoptotic stimuli, such as tumor necrosis receptor α (TNF-α) and cycloheximide (CHX), implying that the epitope in HSpin1 was inaccessible to the antibody unless cell-death signaling was initiated (Figure 2a, middle panels). Similar observations have been reported for other proapoptotic molecules such as Bax and Bcl-2 homologous antagonist killer (Bak): some antibodies raised against these proteins are reactive to their respective antigens only in cells undergoing apoptosis.12,13 Following the treatment of cells with TNF-α and CHX, HSpin1 was observed as punctate perinuclear staining that colocalized with the iron–sulfur subunit of succinate dehydrogenase (SDH), an inner-membrane protein of mitochondria,14 although a considerable amount of HSpin1 was also present throughout the cytoplasm (Figure 2a, right panels). This indicates that HSpin1 is present in mitochondria. HSpin1 fused with GFP was also found to be present in mitochondria when cells were stained with an antibody against cytochrome c, another mitochondrial protein (Figure 5b, details will be discussed later). Since Bcl-xL inhibited HSpin1-induced cell death, we next determined whether HSpin1 is colocalized with Bcl-xL and another antiapoptotic protein, Bcl-2, that are also regarded as mitochondrial proteins. As shown in Figure 2b, following the treatment of HeLa cells with TNF-α and CHX, endogenous HSpin1 was observed to colocalize with GFP-fused Bcl-2 and Bcl-xL.
Figure 2
Subcellular localization of HSpin1 in HeLa cells (a) HSpin1 immunoreactivity is colocalized with GFP-succinate dehydrogenase (SDH), a mitochondrial marker. HeLa cells were transiently transfected with pSDH–GFP. After 24 h of cultivation, cells were grown for another 6 h in the presence (+) or absence (−) of TNF-α and CHX. The cells were then stained with the anti-HSpin1 antibody, followed by the Texas red-conjugated secondary antibody and observed under a confocal microscope. Endogenous HSpin1 is detectable only after the cells were treated with TNF-α and CHX. (b) The treatment of cells with TNF-α allowed us to observe colocalization of endogenous HSpin1 with GFP–Bcl-2 or GFP–BclxL. HeLa cells were transiently transfected with pGFP–Bcl-2 or pGFP–Bcl-xL plasmids. Following the transfection, cells were cultured and stained as described in (a)
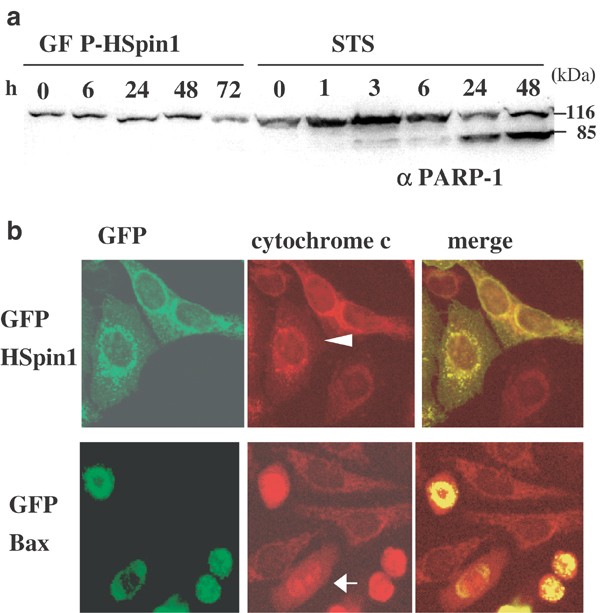
Figure 5
Effects of HSpin1 overexpression on caspase activation and cytochrome c release from mitochondria. (a) The caspase substrate PARP-1 is not cleaved in cells transfected with HSpin1. HEK293 cells were transfected with pGFP–HSpin1 (five left lanes) or treated with STS (six right lanes). At different time points after transfection or after addition of STS, the cells were harvested and the protein extracts were subjected to Western blotting with the anti-PARP-1 antibody. The band at 116 kDa represents full-length PARP-1, whereas that at 85 kDa represents processed PARP-1, a product of caspase-mediated cleavage. (b) Cytochrome c is not released from mitochondria in HSpin1-expressing cells. HeLa cells transiently transfected with pGFP–HSpin1 (upper panels) or pGFP–Bax (lower panels) were immunostained with the anticytochrome c antibody followed by a Texas Red-conjugated secondary antibody. Fluorescence was observed under a confocal laser scanning microscope. Cells successfully transfected with pGFP–HSpin1 and pGFP–Bax are indicated by arrowheads and arrows, respectively
HSpin1 binds to Bcl-2 and Bcl-xL, but not to Bax and Bak
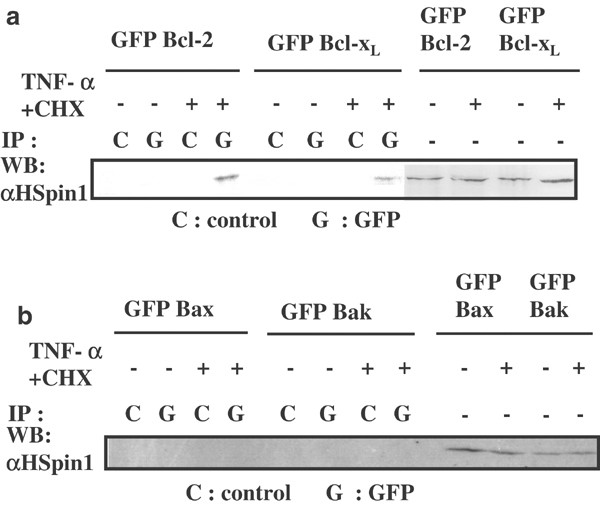
Having found that HSpin1 is colocalized with Bcl-2 and Bcl-xL, an intriguing question arises as to whether HSpin1 physically interacts with these proteins. Proteins were prepared from HEK293 cells transfected with pGFP–Bcl-2 or pGFP–Bcl-xL for immunoprecipitation with an anti-GFP antibody. Endogenous HSpin1 was coimmunoprecipitated with both Bcl-2 and Bcl-xL, interestingly however, only in the presence of TNF-α and CHX (Figure 3a). This indicates that HSpin1 binds to Bcl-2 and Bcl-xL in an apoptotic stimulus-dependent manner. In contrast, HSpin1 did not interact with Bax and Bak, which are proapoptotic members of the Bcl-2 family, even in the presence of TNF-α and CHX (Figure 3b). This is similar to bcl-2 homology 3 (BH3) -only proteins, members of the Bcl-2 family that have only one of the Bcl-2 homology regions: Bad, Bim and Rad9 bind to prosurvival but not to proapoptotic Bcl-2 family members.15,16,17
Figure 3
Detection of HSpin1 binding to Bcl-2 family proteins. (a) The interaction of transgene-derived Bcl-2 or Bcl-xL with endogenous HSpin1. HSpin1 coprecipitates with Bcl-2 or Bcl-xL. HEK293 cells transfected with pGFP–Bcl-2 or pGFP–Bcl-xL were divided into two groups, one of which was treated with TNF-α and CHX (+). The other group of cells was not treated with these agents (−) as in Figure 2. Cell lysates were then obtained and subjected to immunoprecipitation with the anti-GFP monoclonal antibody (G) or an isotype-matched control antibody (C) followed by Western blotting with the anti-HSpin1 polyclonal antibody. To confirm the equivalent expression of HSpin1, each lysate without immunoprecipitation was probed with the anti-HSpin1 antibody (four right lanes). (b) The interaction of transgene-derived Bax or Bak with endogenous HSpin1. HSpin1 did not coprecipitate with Bax or Bak. HEK293 cells were transfected with pGFP–Bax or pGFP–Bak and analyzed as described in (a)
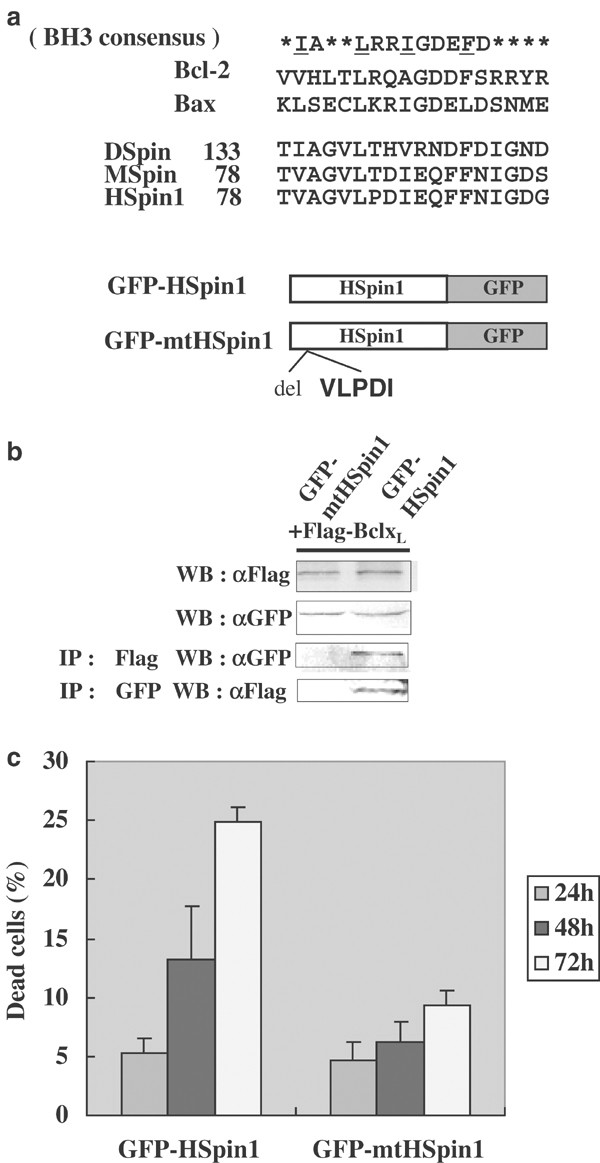
HSpin1 interacts with Bcl-xL via a BH3-like region
Close to its N terminus, HSpin1 has a stretch of amino acids that show low homology with the BH3 domain (Figure 4a). In this BH3-like domain, the hydrophobic residues implicated in the interaction with prosurvival Bcl-2 proteins are conserved from Drosophila to humans (underlined).18 To test whether the BH3-like domain of HSpin1 is essential for the interaction with Bcl-xL, we engineered a deletion mutant of HSpin1 in which the BH3-like domain, including the hydrophobic residues described above (amino-acid residues 82–86), has been eliminated. The expression plasmid producing wild type or mutant HSpin1 fused to GFP and that producing Flag-tagged Bcl-xL were transiently cotransfected into HEK293 cells. Immunoprecipitates were prepared using an anti-Flag antibody, subjected to SDS-PAGE and analyzed by Western blotting with the anti-GFP antibody. As expected, the deletion of residues 82–86 completely eliminated the ability of HSpin1 to interact with Bcl-xL. The result was confirmed by a reciprocal experiment in which cell lysates were immunoprecipitated with the anti-GFP antibody and then analyzed by Western blotting using the anti-Flag antibody as a probe (Figure 4b). Furthermore, this deletion mutant was also deficient in inducing cell death when introduced in HEK293 cells (Figure 4c). Furthermore, the HSpin1 BH3 domain (amino-acid residues 78–93) alone fused to GFP was able to induce cell death when overexpressed in HEK293 cells (data not shown). Therefore, we concluded that the BH3-like region in HSpin1 is essential for both the interaction with Bcl-xL and the ability to mediate cell death.
Figure 4
BH3-like domain of HSpin1 required for cell-death induction. (a) Comparison of the amino-acid sequences in the BH3 regions among Bcl-2 family members and Spin proteins. Hydrophobic amino-acid residues reported to be important for interacting with the antiapoptotic Bcl-2 family members are underlined. DSpin, MSpin, and HSpin1 represent the Drosophila, mouse, and human Spin orthologues, respectively. Schematic presentation of the structures of the wildtype and a deletion mutant of HSpin1 (mtHSpin1) fused to GFP is also shown at the bottom. MtHSpin1 represents the mutant protein, which is devoid of the amino-acid sequence VLPDI that includes two amino-acid residues important for the protein–protein interactions described above. (b) The wildtype but not the mutant HSpin1 protein coimmunoprecipitates with BclxL. HEK293 cells were cotransfected with pGFP–HSpin1 or pGFP–mtHSpin1 and pFlag–Bcl-xL as indicated at the top. Cell lysates (30 _μ_g) was immunoblotted with the anti-Flag (WB: _α_Flag) or anti-GFP antibody (WB: _α_GFP) to confirm the equivalent level of expression of introduced genes. Subsequently, 500 _μ_g of cell lysates was immunoprecipitated with the anti-Flag monoclonal antibody and immunoblotted with the anti-GFP polyclonal antibody (IP: _α_Flag WB: _α_GFP), and vice versa (IP: _α_GFP WB: Flag). (c) The BH3-like domain of HSpin1 is indispensable for cell-death induction. HEK293 cells were transiently transfected with 1 _μ_g each of the plasmid encoding GFP–HSpin1 or GFP–mtHSpin1. The percentages of dead cells (GFP-positive cells) were compared between the cells transfected with the wildtype and those with the mutant HSpin1 at indicated time points after transfection using the PI dye exclusion method described in Figure 1. Data are presented as the means±S.D. obtained from three independent experiments
Cell death induced by HSpin1 is not typical apoptosis
One of the integral events of apoptosis is the activation of caspases, a family of cysteine proteases that cleaves substrates required for cell survival and activates the DNase responsible for chromosomal degradation.19 To test whether caspases are activated in HSpin1-induced cell death, we investigated the cleavage of poly (ADP-ribose) polymerase (PARP)-1, which is a substrate for caspase-3.20 In a control experiment, full-length PARP-1 of 116 kDa was processed when HEK293 cells were treated with staurosporine (STS), a typical inducer of apoptosis, resulting in the production of a cleaved product of 85 kDa. In contrast, transfection of HEK293 cells with the gene encoding HSpin1 did not induce processing of PARP-1 (Figure 5a), demonstrating that caspase is not involved in HSpin1-induced cell death. Another hallmark of apoptosis is the release of cytochrome c from mitochondria into the cytosol.21 To further define the HSpin1-induced cell death, HeLa cells were stained for cytochrome c after transfection with a plasmid expressing HSpin1 or Bax. The cells expressing Bax showed diffuse staining for cytochrome c, indicating that cytochrome c had been released from mitochondria (Figure 5b, arrow). On the other hand, HSpin1-expressing cells retained the perinuclear punctate staining pattern of cytochrome c that colocalized with GFP–HSpin1 (Figure 5b, arrowhead). Therefore, HSpin1-induced cell death is different from typical apoptosis in that cytochrome c is not released from mitochondria during this process.
HSpin1-induced cell death is caspase independent and autophagic
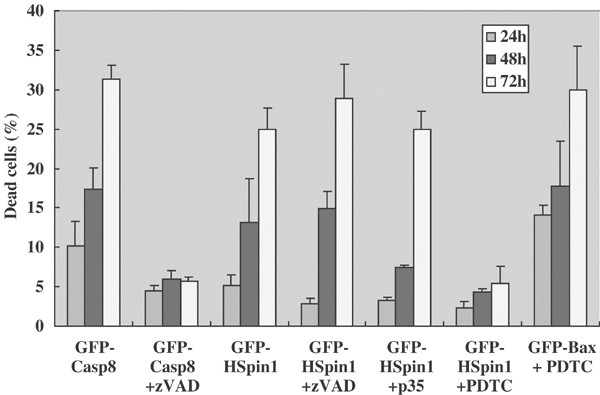
The roles of caspases in HSpin1-induced cell death were evaluated pharmacologically. Cell death caused by the overexpression of caspase-8 was indeed inhibited by carbobenzoxy-VAD-fluoromethyl ketone (zVAD-fmk), a broad-spectrum caspase inhibitor (Figure 6). However, HSpin1-induced cell death was unaffected by zVAD-fmk or by p35, another potent inhibitor of caspases encoded by baculovirus,22 further demonstrating the caspase-independent nature of HSpin1-induced cell death (Figure 6).
Figure 6
Effects of a necrosis inhibitor, PDTC, and a caspase inhibitor, zVAD-fmk, on HSpin1-induced cell death. Cell killing as a result of HSpin1 overexpression (third to the left-hand-most graphs) is effectively blocked by PDTC (sixth graph) but not by zVAD-fmk or p35 (fifth graph). HEK293 cells were transiently transfected with 1 _μ_g each of pGFP–caspase-8, pGFP–HSpin1, or pGFP–Bax in the presence or absence of either zVAD or PDTC as indicated. A measures of 2 _μ_g of the plasmid encoding p35 was included in some transfections. At indicated time points after transfection, the percentages of dead cells among transfected cells (GFP positive) were determined using the PI dye exclusion method described in Figure 1. Data are presented as the means±S.D. obtained from three independent experiments
Compared with apoptosis, the molecular mechanism underlying caspase-independent or necrotic-PCD is less understood. PDTC, a metal chelator and antioxidant, has been reported to inhibit necrotic morphological changes induced by the Fas-associated protein with a death domain (FADD), although the molecular mechanism of its action has not been fully elucidated.11 Interestingly, PDTC potently inhibited cell death caused by HSpin1 overexpression, whereas Bax-induced cell death was not inhibited by PDTC (Figure 6). This suggests that HSpin1-induced cell death is more similar to necrosis than to apoptosis.
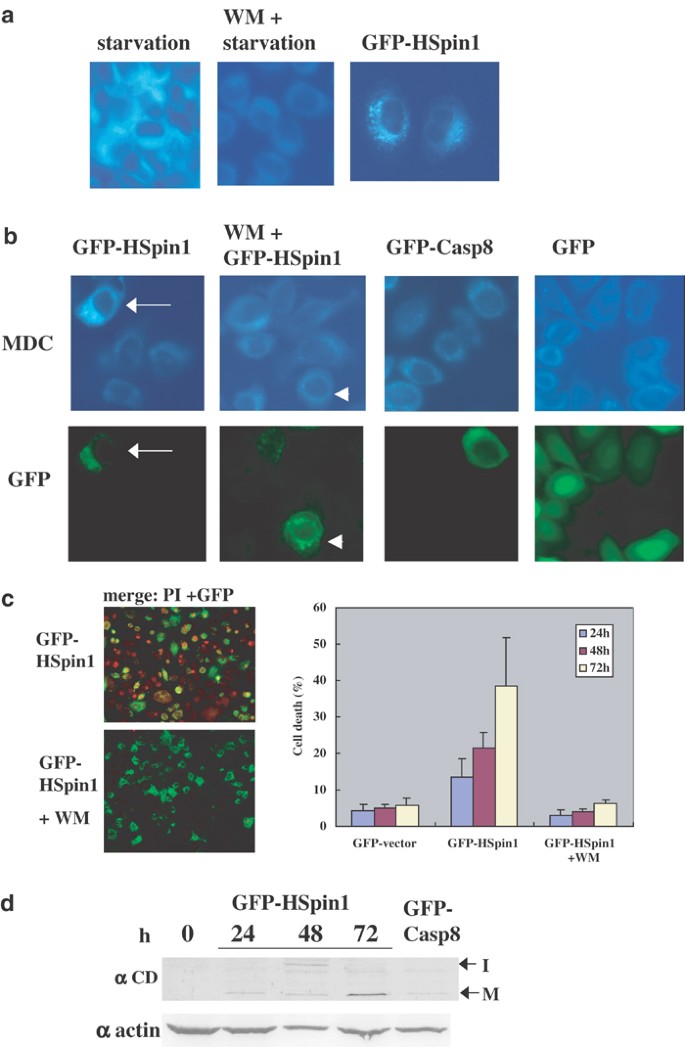
Some types of necrosis are mediated by autophagy.23 Autophagy is a normal degradative mechanism that involves the sequestration of cytoplasm and intracellular organelles into a membrane vacuole called the autophagosome. To test whether HSpin1-induced cell death is associated with the autophagic pathway, we used the autofluorescent drug monodansylcadaverine (MDC), a specific marker for autophagic vacuoles.24 Accumulation of MDC was induced by the overexpression of HSpin1 (Figure 7a) but not by caspase-8-induced apoptosis (Figure 7b). It has been shown that the autophagic pathway is mediated by phosphatidylinositol- 3-kinase (PI3-K).25 As illustrated in Figure 7b, HSpin1-induced accumulation of MDC was blocked by wortmannin (WM), a PI3-K inhibitor. In fact, HSpin1-induced cell death was blocked by WM (Figure 7c).
Figure 7
MDC-labeled autophagic vacuoles induced by HSpin1 overexpression. (a) Autophagy induced by starvation (left-hand-most panel) or by HSpin1 overexpression (right-hand-most panel) is detectable by the autofluorescence emitted by MDC. Autophagy is inhibited by the treatment of cells with WM (middle panel). HeLa cells were incubated in EBSS (starvation) for 2 h in the presence (WM+) or absence of 200 nM WM. They were also incubated with DMEM containing 10% FCS and transfected with pGFP–HSpin1 (GFP–HSpin1). At 48 h post-transfection, cells were then labeled with MDC, fixed and analyzed by fluorescence microscopy. (b) GFP–HSpin1-expressing cells are labeled by MDC in the absence of WM (arrows) but not in the presence of WM (arrowheads). HeLa cells were transiently transfected with pGFP–HSpin1 (GFP–HSpin1), pGFP–caspase-8 (GFP–Casp8) or pGFP–C1 (GFP). After 48 h, cells were labeled with MDC, fixed and analyzed by fluorescence microscopy. WM was added to the culture at 6 h post-transfection where indicated. MDC-labeled vacuoles and the expression of GFP fusion proteins are shown in the upper and lower panels, respectively. (c) Cell death induced by HSpin1 overexpression is effectively blocked by WM. HeLa cells were transiently transfected with 1 _μ_g each of pGFP–HSpin1 in the presence (lower left-hand-side panel) or absence (upper left-hand-side panel) of WM. The transfected cells were observed under a confocal laser scanning microscope. The nuclei of dead cells stained with PI are indicated in red and GFP–HSpin1 expression appears in green. Right-hand-side panel represents quantitative analysis of the effect of WM on cell death induced by Hspin1 overexpression. At indicated time points after transfection, the percentages of dead cells among transfected cells (GFP positive) were determined (right panel) using the PI dye exclusion method. Data are presented as the means±S.D. obtained from three independent experiments. (d) The amount of the mature form of cathepsin D increases with time after transfection of cells with HSpin1. HeLa cells were transiently transfected with GFP–HSpin1. Cells were harvested at the indicated time points after the transfection and subjected to Western blotting with a rabbit anticathepsin D antibody. The intermediate form (I) and mature form (M) of cathepsin D have molecular weights of 48 and 34 kDa, respectively, and are indicated on the right side. To ensure equal protein loading, the same samples were loaded on another gel and subjected to Western blotting with an antiactin monoclonal antibody (lower panel)
In the execution of autophagy, lysosomal proteinases such as cathepsin D play a crucial role.26 We, therefore, assessed the possibility that cathepsin D is activated in cell death induced by HSpin1 overexpression. Activation of cathepsin D occurs after the processing of an inactive proenzyme through the formation of an intermediate form, followed by a final cleavage that creates the mature form.27 The mature form of cathepsin D increased in amount with time (up to 72 h) after transfection of HEK293 cells with the transgene encoding Hspin1 (Figure 7d). Furthermore, HSpin1-induced cell death was blocked by 50 _μ_M pepstatin A, a cathepsin D inhibitor (data not shown).
Taking all of these findings together, we consider that HSpin1 is involved in a necrotic or autophagic cell-death pathway.
Discussion
We have demonstrated that HSpin1-induced cell death is not typical of apoptosis, in that it is not associated with the processing of PARP-1 and release of cytochrome c from mitochondria. Furthermore, we found that HSpin1-induced cell death was blocked by a necrotic inhibitor, PDTC, but not by the caspase inhibitors zVAD-fmk and p35. Nevertheless, HSpin1 binds to Bcl-2 and Bcl-xL, both of which are known to repress typical apoptosis. We hypothesize that HSpin1 binds to and titrates Bcl-2 and/or Bcl-xL, thereby promoting cell death.
Initially, Bcl-2 family proteins were identified as regulators of apoptosis. However, accumulating evidence suggests that they also regulate nonapoptotic caspase-independent PCD. For example, Bax can trigger both caspase-dependent and -independent cell death; it reduces the mitochondrial membrane potential, produces reactive oxygen species, and increases plasma membrane permeability in the presence of zVAD-fmk.28 In addition, cell death triggered by nitric oxide (NO) was enhanced by Bax expression in a caspase-independent manner and was prevented by Bcl-2.29 Recently, Drob-1, a Drosophila member of the proapoptotic Bcl-2 family, has been reported to induce cell death that is resistant to p35, a broad-spectrum caspase inhibitor.30 BNIP3, a member of the Bcl-2 family, has also been reported to induce cell death that is independent of Apaf-1 without caspase activation and cytochrome c release. Instead, BNIP3-induced cell death is characterized by extensive cytoplasmic vacuolation, and mitochondrial autophagy,31 both of which are typical of necrosis. Therefore, the Bcl-2 family proteins are involved in cell death via caspase-independent as well as caspase-dependent mechanisms. In this regard, it is not surprising that HSpin1 binds to certain antiapoptotic Bcl-2 family proteins, and that the ability of HSpin1 to bind to Bcl-2 correlated with its cell-death-inducing ability in the absence of caspase activation.
Interestingly, detection of Hspin1 by the anti-HSpin1 antibody used in our study occurred only after the cells were treated with apoptotic stimuli (Figure 2). When used against proteins denatured by detergents in Western blot analysis, the same antibody detected its antigen regardless of the presence or absence of apoptotic stimuli. The difference in reactivity of the antibody to the HSpin1 protein is probably because of its preference for the HSpin1 conformation in the active state. It can be envisaged that dissociation of an HSpin1-binding protein upon activation exposes the epitope so that the antibody can recognize it. It should be noted that the interaction of HSpin1 with Bcl-2 or Bcl-xL was only observed when the cells received cell-death signals. Therefore, it is intriguing to speculate that after receiving a cell-death signal, HSpin1 undergoes a conformational change, as has been observed for Bax and Bak,12,13 which would enable it to be targeted and integrated into membranes, in particular the mitochondrial outer membrane, where it binds to and inhibits antiapoptotic Bcl-2 family members.
In spin mutants, PCD is inhibited in the ovarian nurse cells that transport cytoplasmic components into the oocyte. Interestingly, the expression of Boo/Diva, a Bcl-2 family member, is highly restricted to the ovary and testis in adult mice,32,33 indicating its important role in the development of these reproductive tissues. In addition, other Bcl-2 family proteins, such as Mcl-1, Bcl-2, and Bcl-x, are expressed in ovarian tissues,34 and Bcl-2-knockout mice have supernumerary primordial follicles.35 Therefore, the interaction of HSpin1 with antiapoptotic Bcl-2 and Bcl-xL may mediate PCD occurring in developing gonads.
Cell death induced by HSpin1 is not only caspase-independent but also characteristic of autophagy. First, cells killed as a result of HSpin1 overexpression stain with MDC, which indicates the occurrence of autophagy. Second, the killing effect of HSpin1 is blocked by WM, an inhibitor of autophagy.24 The reduction in Bcl-2 expression in HL60 cells using antisense technology induced cell death as a result of autophagy, not apoptosis.36 Caspase inhibitors did not rescue _bcl-2_-antisense-mediated autophagy. In addition, Beclin 1, a Bcl-2-interacting coiled-coil protein, promoted autophagy in human MCF7 cells.37 Therefore, these observations are in agreement with the idea that HSpin1 binds to antiapoptotic members of the Bcl-2 family and inhibits their antiautophagic functions.
Autophagy regulates normal cell growth and differentiation through the degradation of cytosolic proteins.38,39 The initial step of autophagy involves surrounding cytoplasmic and organelle portions of the cell with a single isolation membrane. The fusion of the edges of the membrane sac forms a closed double-membrane structure, the so-called autophagosome. Finally, the autophagosome fuses with a lysosome to become the autolysosomes. Within autolysosomes, the sequestered content is degraded by lysosomal hydrolases. Cathepsin D is located in and is processed or activated within acidic lysosomal and endosomal compartments.40 Cathepsin D deficiency induces lysosomal storage of ceroid lipofuscin in mouse CNS neurons.41 Interestingly, the accumulation of lipofuscin-like materials has been observed in the CNS of Drosophila in which the spin gene is mutated.9 More recently, a zebrafish lethal mutant, not really started (nrs), was found to carry a loss-of-function mutation in a spin orthologous gene.42 The nrs mutant embryos accumulate dark materials in the yolk sac prior to death, similar to lipofuscin-like deposits found in spin mutant neurons of Drosophila. This similarity in the phenotype between the zebrafish and Drosophila mutants suggests that the role of spin in cell-death signaling is also conserved in mammals. In this regard, our observation that cathepsin D is activated during HSpin1-induced cell death is intriguing, as it further implies a link between HSpin1 and cathepsin D in autophagic cell death.
Upon autophagic stimulation, depolarized mitochondria are known to move into autophagic vacuoles,43 which ultimately fuse with lysosomes. In this process, GFP–HSpin1 localized in mitochondria (Figure 5a) is likely to be incorporated into MDC-labeled autophagic vacuoles (Figure 7b). Indeed, we found that LAMP-2, a lysosomal marker, was often colocalized with GFP–HSpin1 (H Yanagisawa, unpublished observation; see also Sweeney and Davis53). LAMP-2 is localized in the limiting membrane of the autophagic vacuoles, which can be identified by its cytoplasmic contents (mitochondria). Cathepsin D is found in the space between the limiting membrane and the contents of vacuoles.44 Thus, it is conceivable that HSpin1 interacts with lysosomal components through the activation of cathepsin D.
We consider that the HSpin1 protein contributes to the maintenance of cell number through autophagy. It is conceivable that impaired autophagy because of a disruption of the spin gene results in the accumulation of lipofuscin in lysosomes. Conversely, an increase in the HSpin1 expression level would cause excessive autophagic cell death. Experiments with mice deficient in the spin gene will allow us to substantiate the novel caspase-independent cell-death pathway proposed in this study. Autophagy and the abnormal accumulation of lipofuscin have been reported to occur in many neurodegenerative disorders such as Alzheimer, Huntington, and Parkinson diseases and in the aged brain.45,46,47,48 Therefore, studying the molecular basis of HSpin1 function will help understand the pathogenesis of neurodegeneration and the normal mechanism of aging.
Materials and Methods
Constructs
The human full-length HSpin1 gene was amplified by PCR from an EST cDNA clone 645298 using the primers EcoSpinF (5′-GGAATTCCATGGCCGGGTCCGACACCGCG-3′) and BamSpinR (5′-CG GGATCCCGGATGAGCACACTGGCCAC-3′) (_Eco_RI and _Bam_HI sites are underlined). The amplified product was digested with _Eco_RI and _Bam_HI, and then subcloned into pEGFP-N1 (Clontech) digested with the same enzymes to generate pGFPHSpin1. pGFPmtHSpin1, which encodes a mutant HSpin1 devoid of five amino-acid residues, 82VLPDI86, was generated by deleting the corresponding DNA fragment with _Bsa_HI and _Taq_I. The expression plasmids, pGFP–Bcl-2, pGFP–Bax and pGFP–Casp8, have been described previously.17,49,50 To generate pGFP–Bcl-xL and pSDH–GFP, cDNAs covering the entire coding region of human Bcl-xL and the iron–sulfur subunit of succinate dehydrogenase were obtained by RT-PCR using fetal brain mRNA (Clontech) and primers designed based on the reported sequences. DNA fragments obtained were then subcloned into expression plasmids pEGFP-C1 and pEGFP-N2 (Clontech). pBcl-xL–Flag was kindly provided by John C Reed (The Burnham Institute). pcDNA-p35, generated from pCaspeR–hs-p3551 by digestion with _Eco_RI and _Xba_I, was provided by Masayuki Miura (Brain Science Institute, RIKEN, Japan).
Reagents and antibodies
Human TNF-α was purchased from Clontech. STS, PI, and pepstatin A were purchased from Wako Pure Chemical IndustriesQ3 Ltd, Osaka, Japan. Except where noted, all other reagents were purchased from Sigma. The following antibodies were used for the detection of respective proteins by Western blotting and/or by immunofluorescence: rabbit anti-GFP polyclonal antibody (Clontech), mouse anti-GFP monoclonal antibody (Clontech), mouse anti-Flag M2 monoclonal antibody (Sigma), mouse anti-poly (ADP) ribose polymerase-1 monoclonal antibody (BIOMOL Research Laboratories, Inc.), mouse anticytochrome c monoclonal antibody (Clone: 6H2.B4) (BD PharMingen), rabbit anticathepsin D polyclonal antibody (Oncogene Research Products), mouse antiactin monoclonal antibody (Sigma), mouse anti-LAMP-2 monoclonal antibody (BD PharMingen), goat alkaline phosphatase (AP)-conjugated anti-rabbit and anti-mouse IgG (BIO-RAD Laboratories), donkey Texas Red-conjugated anti-rabbit IgG and anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.). A rabbit anti-HSpin1 antibody was raised against a synthetic peptide corresponding to the N-terminus (amino-acid residues 1–14) of human HSpin1.
Cell culture
HeLa and HEK293 cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, and 100 _μ_g/ml streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Cell death assay
HEK293 cells growing on Lab-Tek chamber slides (Nalge Nunc International) were transfected with 1 _μ_g each of the indicated plasmids using the Effectene reagent (Qiagen) according to the manufacturer's protocol. zVAD-fmk (Peptide Institute, Inc., Osaka, Japan) (50 _μ_M) or PDTC (Sigma) (80 _μ_M) was added in some experiments. At indicated time points after the transfection, cells were stained with 500 _μ_g/ml of PI and the percentage of dead cells (stained by PI) among transfected cells (with green fluorescence) was determined. At least 400 cells were counted for each time point.
Northern blot analysis
‘Multiple human adult tissue Northern blots’ (Clontech, human MNT Blot II and human Brain MNT Blot II) were hybridized with the [_α_-32p]dATP-labeled HSpin1 or human actin cDNA in ExpressHyb hybridization solution (Clontech) at 68°C. The membranes were washed under stringent conditions (0.1 × SSC, 0.1% SDS, 50°C) and exposed to X-ray film (Konica) at −80°C with intensifying screens.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde (PFA) in PBS (pH 7.4) for 30 min at 4°C and permeabilized in 0.5% Triton X-100 in PBS for 30 min. After preblocking with 5% skim milk and 2% BSA for 1 h, cells were incubated with the anti-HSpin1 or anticytochrome c antibody, which was detected with Texas red-conjugated donkey anti-rabbit IgG or anti-mouse IgG. A fluorescent image was obtained using a confocal laser-scanning microscope (Model Radiance 2000, BIO-RAD).
Immunoprecipitation and Western blot analysis
For immunoprecipitation, cells were lysed in lysis buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 1% Triton X-100) containing protease inhibitor tablets (completeTM, Roche Applied Science). Soluble protein (500 _μ_g) was incubated with 1 _μ_g/ml of anti-GFP monoclonal, anti-Flag monoclonal, anti-GFP polyclonal, or control antibody for 6 h at 4°C. Immune complexes were immunoprecipitated with protein A+G-sepharose 4B (Zymed Laboratories Inc.) overnight at 4°C and washed as described previously.52 Immunoprecipitates were subjected to 12% SDS-PAGE and separated products were immunoblotted with the anti-HSpin1 polyclonal, anti-GFP polyclonal, or anti-Flag monoclonal antibody, followed by AP-conjugated goat anti-rabbit or anti-mouse IgG, and then visualized with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue terazolium (Sigma).
Visualization of MDC-labeled vacuoles
Autophagic vacuoles were labeled with 0.05 mM MDC by incubating cells grown on coverslips at 37°C for 10 min. After incubation, cells were washed four times with PBS, immediately fixed with 4% PFA and observed under a fluorescence microscope (Olympus BXFLA) equipped with an appropriate filter system.
Starvation
Autophagy was induced by amino-acid starvation. Cells were washed three times with PBS and incubated with 1 ml Earle's balanced salts solution (EBSS) (Sigma) at 37°C for 2 h. EBSS contained D-glucose, and devoid of amino acids and serum.
Abbreviations
PCD:
programmed cell death
TNF-α:
Tumor necrosis factor-α
CHX:
cycloheximide
Bax:
Bcl-2-associated X protein
Bak:
Bcl-2 homologous antagonist killer
Bcl-xL:
apoptosis regulator Bcl-X
BH3:
bcl-2 homology 3
Bim:
Bcl-2 interacting mediator of cell death
Bad:
Bcl2-antagonist of cell death protein
SDH:
succinate dehydrogenase iron-sulfur protein
FADD:
Fas-associated protein with a death domain
PI:
propidium iodide
z-VAD-fmk:
carbobenzoxy-VAD-fluoromethyl
PARP-1:
poly (ADP-ribose) polymerase-1
PDTC:
pyrrolidine dithiocarbamate
WM:
wortmannin
PI3-K:
phosphatidylinositol-3-kinase
MDC:
monodansylcadaverine
References
- Korsmeyer SJ . (1995) Regulators of cell death. Trends Genet. 11: 101–105
Article CAS Google Scholar - White E (1996) Life, death, and the pursuit of apoptosis. Genes Dev. 10: 1–15
Article CAS Google Scholar - Yin XM, Oltvai ZN and Korsmeyer SJ (1994) BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369: 321–323
Article CAS Google Scholar - Thornberry NA and Lazebnik Y (1998) Caspases: enemies within. Science 281: 1312–1316
Article CAS Google Scholar - Fiers W, Beyaert R, Declercq W and Vandenabeele P (1999) More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene 18: 7719–7730
Article CAS Google Scholar - Vercammen D, Beyaert R, Denecker G, Goossens V, Van LG, Declercq W, Grooten J, Fiers W and Vandenabeele P (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187: 1477–1485
Article CAS Google Scholar - Chautan M, Chazal G, Cecconi F, Gruss P and Golstein P (1999) Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr. Biol. 9: 967–970
Article CAS Google Scholar - Suzuki K, Juni N and Yamamoto D (1997) Enhanced mate refusal in female Drosophila induced by a mutation in the spinster locus. Appl. Entomol. Zool. 32: 235–243
Article Google Scholar - Nakano Y, Fujitani K, Kurihara J, Ragan J, Usui-Aoki K, Shimoda L, Lukacsovich T, Suzuki K, Sezaki M, Sano Y, Ueda R, Awano W, Kaneda M, Umeda M and Yamamoto D (2001) Mutations in the novel membrane protein Spinster interfere with programmed cell death and cause neural degeneration in Drosophila melanogaster. Mol. Cell. Biol. 21: 3775–3788
Article CAS Google Scholar - Usui-Aoki K, Nakano Y and Yamamoto D (2002) Pathology of the adult central nervous system induced by genetic inhibition of programmed cell death in Drosophila pupae. Arch. Insect Biochem. Physiol. 49: 94–101
Article CAS Google Scholar - Matsumura H, Shimizu Y, Ohsawa Y, Kawahara A, Uchiyama Y and Nagata S (2000) Necrotic death pathway in Fas receptor signaling. J. Cell Biol. 151: 1247–1256
Article CAS Google Scholar - Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, Dive C and Hickman JA (1999) Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144: 903–914
Article CAS Google Scholar - Nechushtan A, Smith CL, Hsu YT and Youle RJ (1999) Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 18: 2330–2341
Article CAS Google Scholar - Kita K, Oya H, Gennis RB, Ackrell BA and Kasahara M (1990) Human complex II (succinate–ubiquinone oxidoreductase): cDNA cloning of iron sulfur (Ip) subunit of liver mitochondria. Biochem. Biophys. Res. Commun. 166: 101–108
Article CAS Google Scholar - Yang E, Zha J, Jockel J, Boise LH, Thompson CB and Korsmeyer SJ (1995) Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 80: 285–291
Article CAS Google Scholar - O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S and Huang DC (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17: 384–395
Article CAS Google Scholar - Komatsu K, Miyashita T, Hang H, Hopkins KM, Zheng W, Cuddeback S, Yamada M, Lieberman HB and Wang HG (2000) Human homologue of S. pombe Rad9 interacts with BCL-2/BCL-xL and promotes apoptosis. Nat. Cell Biol. 2: 1–6
Article CAS Google Scholar - Huang DC and Strasser A (2000) BH3-Only proteins-essential initiators of apoptotic cell death. Cell 103: 839–842
Article CAS Google Scholar - Samali A, Zhivotovsky B, Jones D, Nagata S and Orrenius S (1999) Apoptosis: cell death defined by caspase activation. Cell Death Differ. 6: 495–496
Article CAS Google Scholar - Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG and Earnshaw WC (1994) Cleavage of poly (ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371: 346–347
Article CAS Google Scholar - Liu X, Kim CN, Yang J, Jemmerson R and Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157
Article CAS Google Scholar - Bump NJ, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, Licari P, Mankovich J, Shi J, Greenberg AH, Miller LK and Wong WW (1995) Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science 269: 1885–1888
Article CAS Google Scholar - Bursch W (2001) The autophagosomal–lysosomal compartment in programmed cell death. Cell Death Differ. 8: 569–581
Article CAS Google Scholar - Munafo DB and Colombo MI (2001) A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J. Cell Sci. 114: 3619–3629
CAS PubMed Google Scholar - Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H and Meijer AJ (1997) The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 243: 240–246
Article CAS Google Scholar - Uchiyama Y (2001) Autophagic cell death and its execution by lysosomal cathepsins. Arch. Histol. Cytol. 64: 233–246
Article CAS Google Scholar - Hasilik A (1992) The early and late processing of lysosomal enzymes: proteolysis and compartmentation. Experientia 48: 130–151
Article CAS Google Scholar - Xiang J, Chao DT and Korsmeyer SJ (1996) BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA 93: 14559–14563
Article CAS Google Scholar - Okuno S, Shimizu S, Ito T, Nomura M, Hamada E, Tsujimoto Y and Matsuda H (1998) Bcl-2 prevents caspase-independent cell death. J. Biol. Chem. 273: 34272–34277
Article CAS Google Scholar - Igaki T, Kanuka H, Inohara N, Sawamoto K, Nunez G, Okano H and Miura M (2000) Drob-1, a Drosophila member of the Bcl-2/CED-9 family that promotes cell death. Proc. Natl. Acad. Sci. USA 97: 662–667
Article CAS Google Scholar - Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R and Greenberg AH (2000) BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol. Cell. Biol. 20: 5454–5468
Article CAS Google Scholar - Inohara N, Gourley TS, Carrio R, Muniz M, Merino J, Garcia I, Koseki T, Hu Y, Chen S and Nunez G (1998) Diva, a Bcl-2 homologue that binds directly to Apaf-1 and induces BH3-independent cell death. J. Biol. Chem. 273: 32479–32486
Article CAS Google Scholar - Song Q, Kuang Y, Dixit VM and Vincenz C (1999) Boo, a novel negative regulator of cell death interacts with Apaf-1. EMBO J. 18: 167–178
Article CAS Google Scholar - Tilly JL, Tilly KI, Kenton ML and Johnson AL (1995) Expression of members of the bcl-2 gene family in the immature rat ovary: equine chorionic gonadotropin-mediated inhibition of granulosa cell apoptosis is associated with decreased bax and constitutive bcl-2 and bcl-xlong messenger ribonucleic acid levels. Endocrinology 136: 232–241
Article CAS Google Scholar - Ratts VS, Flaws JA, Kolp R, Sorenson CM and Tilly JL (1995) Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the postnatal female mouse gonad. Endocrinology 136: 3665–3668
Article CAS Google Scholar - Saeki K, You A, Okuma E, Yazaki Y, Susin SA, Kroemer G and Takaku F (2000) Bcl-2 down-regulation causes autophagy in a caspase-independent manner in human leukemic HL60 cells. Cell Death Differ. 7: 1263–1269
Article CAS Google Scholar - Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H and Levine B (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402: 672–676
Article CAS Google Scholar - Dunn Jr WA (1990) Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J. Cell Biol. 110: 1923–1933
Article Google Scholar - Hollenbeck PJ (1993) Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J. Cell Biol. 121: 305–315
Article CAS Google Scholar - Dragonetti A, Baldassarre M, Castino R, Demoz M, Luini A, Buccione R and Isidoro C (2000) The lysosomal protease cathepsin D is efficiently sorted to and secreted from regulated secretory compartments in the rat basophilic/mast cell line RBL. J. Cell Sci. 113: 3289–3298
CAS PubMed Google Scholar - Koike M, Nakanishi H, Saftig P, Ezaki J, Isahara K, Ohsawa Y, Schulz-Schaeffer W, Watanabe T, Waguri S, Kametaka S, Shibata M, Yamamoto K, Kominami E, Peters C, von Figura K and Uchiyama Y (2000) Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. J. Neurosci. 20: 6898–6906
Article CAS Google Scholar - Young RM, Marty S, Nakano Y, Wang H, Yamamoto D, Lin S and Allende ML (2002) Zebrafish yolk-specific not really started (nrs) gene is a vertebrate homolog of the Drosophila spinster gene and is essential for embryogenesis. Dev. Dyn. 223: 298–305
Article CAS Google Scholar - Elmore SP, Qian T, Grissom SF, Lemasters JJ (2001) The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 12: 2286–2287.
Article Google Scholar - Eskelinen EL, Illert AL, Tanaka Y, Schwarzmann G, Blanz J, Von Figura K, Saftig P (2002) Role of LAMP-2 in lysosome biogenesis and autophagy. Mol. Biol. Cell. 9: 3355–3368.
Article Google Scholar - Nixon RA, Cataldo AM and Mathews PM (2000) The endosomal–lysosomal system of neurons in Alzheimer's disease pathogenesis: a review. Neurochem. Res. 25: 1161–1172
Article CAS Google Scholar - Kegel KB, Kim M, Sapp E, McIntyre C, Castano JG, Aronin N and DiFiglia M (2000) Huntingtin expression stimulates endosomal–lysosomal activity, endosome tubulation, and autophagy. J. Neurosci. 20: 7268–7278
Article CAS Google Scholar - Stefanis L, Larsen KL, Rideout HJ, Sulzer DS and Greene LA (2001) Expression of A53T mutant, but not wild type, _α_-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J. Neurosci. 21: 9549–9560
Article CAS Google Scholar - Matus A and Green GD (1987) Age-related increase in a cathepsin D-like protease that degrades brain microtubule-associated proteins. Biochemistry 26: 8083–8086
Article CAS Google Scholar - Cuddeback SM, Yamaguchi H, Komatsu K, Miyashita T, Yamada M, Wu C, Singh S and Wang HG (2001) Molecular cloning and characterization of Bif-1. A novel Src homology 3 domain-containing protein that associates with Bax. J. Biol. Chem. 276: 20559–20565
Article CAS Google Scholar - Shikama Y, Shen L, Yonetani M, Miyauchi J, Miyashita T and Yamada M (2002) Death effector domain-only polypeptides of caspase-8 and -10 specifically inhibit death receptor-induced cell death. Biochem. Biophys. Res. Commun. 291: 484–493
Article CAS Google Scholar - Hisahara S, Kanuka H, Shoji S, Yoshikawa S, Okano H and Miura M (1998) Caenorhabditis elegans anti-apoptotic gene ced-9 prevents _ced-3_-induced cell death in Drosophila cells. J. Cell Sci. 111: 667–673
CAS PubMed Google Scholar - Yanagisawa H, Bundo M, Miyashita T, Okamura-Oho Y, Tadokoro K, Tokunaga K and Yamada M (2000) Protein binding of a DRPLA family through arginine–glutamic acid dipeptide repeats is enhanced by extended polyglutamine. Hum. Mol. Genet. 9: 1433–1442
Article CAS Google Scholar - Sweeney ST and Davis GW (2002) Unrestricted synaptic growth in spinster – a late endosomal protein implicated in TGF-_ß_-meditated synaptic growth regulation. Neuron 36: 403–416
Article CAS Google Scholar
Acknowledgements
We thank Takashi Sugiyama (Olympus Optical Co. Ltd) for help with the use of the fluorescence microscope and for the gift of HEK293 cells. We are grateful to Hiroyuki Iwahana (Jichi Medical School) for the gift of HeLa cells, and Masayuki Miura and John C Reed for pcDNA–p35 and pBcl-xL–Flag genes, respectively. We thank the members of the Yamamoto Laboratory for advice and discussion. This work was supported in part by Special Cooperation Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology to Daisuke Yamamoto and by Waseda University Grant No. 2002B-031 to DY and Grant No 2002A-905 to Kazue Usui-Aoki.
Author information
Authors and Affiliations
- School of Human Sciences and Advanced Research Institute for Science and Engineering, Waseda University, 2-579-15 Mikajima, Tokorozawa, Saitama, 359-1192, Japan
H Yanagisawa & D Yamamoto - Department of Genetics, National Research Institute for Child Health and Development, 3-35-31 Taishido, Setagayaku, Tokyo, 154-8567, Japan
T Miyashita - Department of Genetics, Hyogo Medical Collage, 1 Mukogawa-cho, Nishinomiya, 663-8501, Japan
Y Nakano
Authors
- H Yanagisawa
- T Miyashita
- Y Nakano
- D Yamamoto
Corresponding author
Correspondence toD Yamamoto.
Additional information
Edited by S. Roy
Rights and permissions
About this article
Cite this article
Yanagisawa, H., Miyashita, T., Nakano, Y. et al. HSpin1, a transmembrane protein interacting with Bcl-2/Bcl-xL, induces a caspase-independent autophagic cell death.Cell Death Differ 10, 798–807 (2003). https://doi.org/10.1038/sj.cdd.4401246
- Received: 14 November 2002
- Revised: 07 February 2003
- Accepted: 28 February 2003
- Published: 18 June 2003
- Issue Date: 01 July 2003
- DOI: https://doi.org/10.1038/sj.cdd.4401246