Allele-specific regulation of primary cilia function by the von Hippel–Lindau tumor suppressor (original) (raw)
Introduction
Heterozygous mutations in the von Hippel–Lindau gene (VHL, OMIM 608537) predispose patients to a variety of tumors and cysts.1, 2 Similarly, kidney-specific inactivation of VHL in conditional knockout mice results in cyst development.3 The role of VHL as a global gatekeeper of cellular growth in the kidney is further supported by the occurrence of biallelic somatic VHL mutations in 70% of conventional kidney cancer.2, 4
Many lines of research have linked the VHL gene product to regulation of the hypoxia inducible factor (HIF).5 We and others have also described new functions for VHL in microtubule dynamics and regulation of the primary cilium.6, 7, 8, 9, 10 Here, we provide the first evidence that cilia function in renal carcinoma cells is dependent on two domains in VHL: residues 1–53, constituting an acidic domain, and residues 95–123, previously implicated in microtubule binding and tumor suppression.11, 12
Materials and methods
Cell culture
Primary mouse kidney cells were isolated from 4-day-old mice and cultured in DMEM supplemented with antibiotics and 20% fetal calf serum; all other cell lines were cultured in 5% fetal calf serum. KC12TR and KC12TR/VHL-TO cell lines were generated and induced with 1 _μ_g/ml doxycycline (Sigma-Aldrich, St Louis, MO, USA) as previously described.10 Transfections were performed using Fugene-6 (Boehringer Mannheim, Ingelheim, Germany) (HEK293T) or by electroporation (KC12 cells: 270 V, 1.0 mF).
Cilia detection
Cells were cultured to confluency, then received serum-free medium for an additional three days. Immunofluorescence was performed with: _α_-VHL (Ig32, 1:500; BD-Pharmingen, San Diego, CA, USA), _α_-acetylated tubulin (1:20 000, Sigma-Aldrich), _α_–_γ_-tubulin (1:500, Sigma-Aldrich), or _α_-Vsv (1:400, Abcam, Cambridge, UK). Secondary antibody was goat-anti-mouse Alexa568, goat anti-rabbit Alexa633 (1:400, Molecular Probes, Eugene, OR, USA) or goat anti-rabbit 488, (1:400, DAKO, Glostrup, Denmark). Secondary antibody controls revealed no aspecific staining. As indicated, VHL constructs were cloned into pEGFP-C1/2 (Clontech, Palo Alto, CA, USA) and transfected into KC12 cells (VHL−/−) before being seeded onto coverslips. One thousand interphase nuclei (as determined by 4'-6-diamidino-2-phenylindole (DAPI)) were scored for the presence of cilia without knowledge of sample identity. Z-stack images of every 20th cell validated the presence of cilia. Experiments were performed on at least two different days and the data combined. Staining was visualized on a Zeiss LSM510 confocal imaging unit (Jena, Germany). Cilia length was measured with the ruler function of LSM Image Browser (Zeiss).
Western blots
Standard western blots13 were immunostained either with _α_-VHL (1:500, Ig32, BD-Pharmingen), or with _α_–_β_-actin (1:10 000; Sigma Aldrich) followed by rabbit _α_-mouse HRP (1:20 000; Pierce, Rockford, IL, USA).
Ca2+ microfluorimetry
Cells were grown to full confluence and then cultured an additional three days in serum-free medium on uncoated 24 × 60 mm glass coverslips. KC12TR/VHL-TO cells were treated with doxycycline for 24 h prior to some experiments. As indicated, four million KC12 cells were transfected by electroporation with 10 _μ_g appropriate plasmid and 1 _μ_g pBABE-puro before seeding. Twenty-four hours after transfection, cells were selected on puromycin. The experimental setup for flow has previously been described in detail.14 Briefly, we incubated cells for 30 min with the Ca2+ sensitive probe Fura-2/AM (5 μ M) at 37°C. We then washed cells three times to remove excess Fura-2/AM and placed them in a perfusion chamber in 20 mM HEPES buffer (pH 7.4) containing 132 mM NaCl, 4.2 mM NaHCO3, 6 mM KCl, 1 mM MgSO4, 1.2 mM KH2PO4, and supplemented with 5 mM glucose, 1 mM CaCl2, and 0.5% human serum albumin. We captured images every 4 s at excitation wavelengths of 340 nm and 380 nm, and detected the signal emission wavelengths at 512 nm. After equilibration of the cells in media for at least 5 min, we applied a fluid shear stress of 0.75 dyn/cm2 to the cells. Freshly diluted thrombin (1 μ M) was used to validate the potential for [Ca2+]i response after each flow experiment.
Results
VHL-mediated cilia function
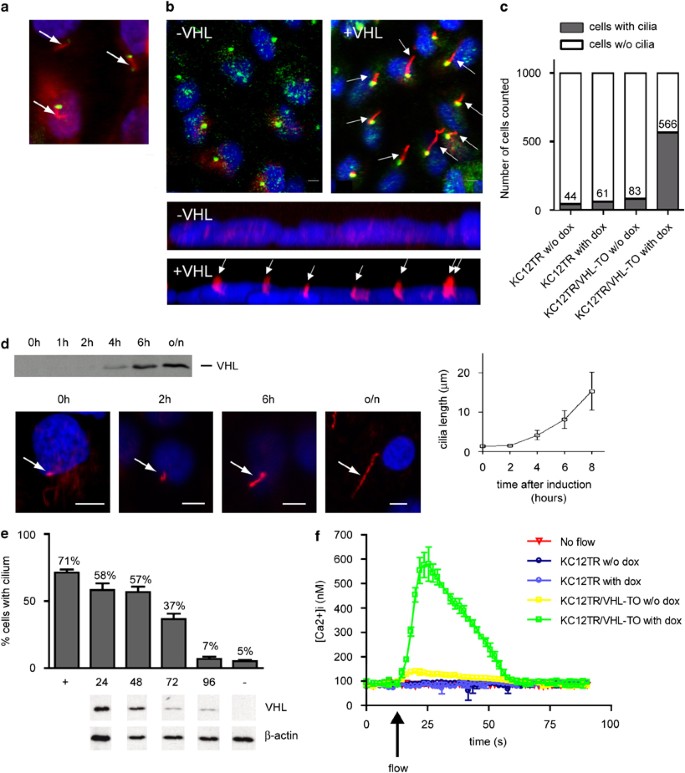
We performed immunofluorescence and confocal microscopy to confirm endogenous VHL localization to ciliary structures in primary cells derived from murine kidney tissue. In addition to diffuse cytoplasmic staining, VHL clearly localized to cilia extending form basal bodies stained with _γ_-tubulin (Figure 1a). To confirm the effect of VHL on ciliogenesis in our preferred cell system, we used a stable cell line derived from a VHL patient engineered to re-express empty vector (KC12TR) or VHL (KC12TR/VHL-TO) upon induction by doxycycline10 and observed rapid assembly of cilia (Figures 1b–c). Because the kinetics of VHL-dependent cilia growth is unknown, ciliary assembly/extension in induced KC12TR/VHL-TO cells was measured over time. We observed an 80-min doubling time (Figure 1d), consistent with published growth rates.15 We next asked what the effect might be of doxycycline withdrawal on ciliated KC12TR/VHL-TO. Cellular levels of VHL dropped significantly by 72 h after the removal of doxycycline, which was followed by a drop in ciliated cell frequency (Figure 1e).
Figure 1
von Hippel–Lindau (VHL) regulates renal cilia maintenance and function. (a) Endogenous VHL localizes to the primary cilium of primary mouse kidney cells. Immunofluorescent _γ_-tubulin staining (green) indicates the position of the basal body at the base of the cilium. VHL (red) is seen to be diffusely cytoplasmic with brighter staining along hair-like structures (arrows) extending from the basal bodies. Size bar, 5 _μ_m. The nucleus is stained with 4'-6-Diamidino-2-phenylindole (DAPI) (blue). (b) Re-expression of VHL in KC12 renal cell carcinoma cells derived from a VHL patient restores cilia. KC12TR/VHL-TO cells were untreated (−VHL) or induced to express VHL with doxycycline (+VHL), then immunostained for acetylated tubulin (red) and for _γ_-tubulin (green, upper panels only). Nuclei are DAPI stained (blue). Lower panels depict a _z_-axis 3D projection generated from a confocal z-stack of a representative cell field at 63 × . Arrows indicate cilia; size bar indicates 5 _μ_m. (c) Frequency of cilia in 1000 KC12TR cells and KC12TR/VHL-TO cells untreated (w/o dox) or treated with doxycycline for 24 h. _Y_-axis shows absolute number of ciliated cells. (d) Dynamics of VHL-induced ciliogenesis. KC12TR/VHL-TO cells were grown to confluency and VHL expression was induced with doxycycline. The upper panel shows a western blot analysis of whole cell lysates analyzed for VHL-protein expression after 0, 2, 4, 6 h, and overnight (o/n) induction of VHL expression by doxycycline. The lower four panels show representative figures of cilia length at each time point. Arrows indicate cilia stained for acetylated tubulin (red); size bar, 5 _μ_m. (e) VHL regulates cilia maintenance. Withdrawal of doxycycline from ciliated KC12TR/VHL-TO cells for 0 (+), 24, 48, 72, 96 h and uninduced control (−) were scored for ciliated cell frequency (>500 cells scored), after doxycycline removal. Error bars represent SDs. Below each time point are western blots of VHL and _β_-actin protein levels. (f) Re-expression of VHL in _VHL_−/− cells restores ciliary mechanotransduction. KC12TR cells and KC12TR/VHL-TO cells were treated with or without (w/o) doxycycline (dox) and used to measure intracellular calcium ([Ca2+]i) concentrations in response to flow (turned on at arrow). Data shown are experiments performed in triplicate or quadruplicate from a single representative day; however, all elements of these data were repeated on multiple days with similar results. Error bars represent SDs.
When cultured kidney cells are subjected to flow, they rapidly demonstrate a cilia-related calcium increase.16 To explore whether the mechanotransduction events triggered by flow are sensitive to levels of VHL, confluent cultures of KC12TR/VHL-TO cells treated with or without doxycycline for 30 h were incubated with fluorescent Fura-2/AM and placed in a perfusion chamber under flow as previously described.14 We observed that only KC12 cells re-expressing VHL were capable of responding to flow by mounting a rapid increase in intracellular Ca2+ concentrations (max. 539–612 nM) as compared to the same cells stably transfected with empty plasmids (KC12TR) and induced with doxycycline (max. 78–90 nM) or uninduced cells (max. 123–133 nM; 56 cells) (Figure 1f), demonstrating significant changes in the area under the curve (AUC, _P_=0.004). These data are the first to demonstrate that VHL-induced cilia function normally in the mechanotransduction characteristic of primary cilia.17
Allelic variants of VHL do not localize to the renal cilium
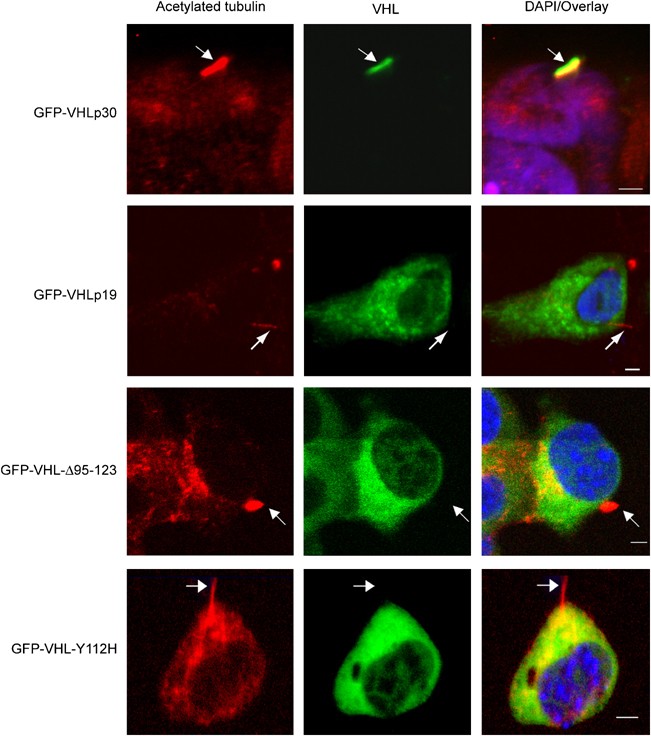
No one has yet determined whether VHL alleles unable to initiate ciliogenesis are capable of ciliary localization. To this end, we transfected GFP-tagged VHL variants into HEK293T cells and then immunostained for cilia with anti-acetylated tubulin. Like endogenous VHL, GFP-VHLp30 clearly localized to cilia. By contrast, GFP-VHLp19, a naturally occurring VHL isoform lacking the acidic domain, was not observed to be present in the cilium. Similarly, VHL variants such as VHL disease type 2A GFP-VHL-Y112H, and GFP-VHL-Δ95–123, both of which affect the microtubule-binding domain showed diffuse cytoplasmic staining and no particular overlap with anti-acetylated tubulin (Figure 2).
Figure 2
Variant alleles of von Hippel–Lindau (VHL) do not localize to the cilium (arrows). Confocal image projections of ciliated kidney cells expressing GFP-VHLp30 (green), GFP-VHLp19, GFP-VHL-Δ95–123, and GFP-VHL-Y112H, and counterstained for acetylated tubulin (red). Nuclei are stained with DAPI (blue). Cilia are indicated by arrows. Size bars in the overlay/DAPI column indicate 5 _μ_m.
Natural and patient-associated alleles fail to form functional cilia
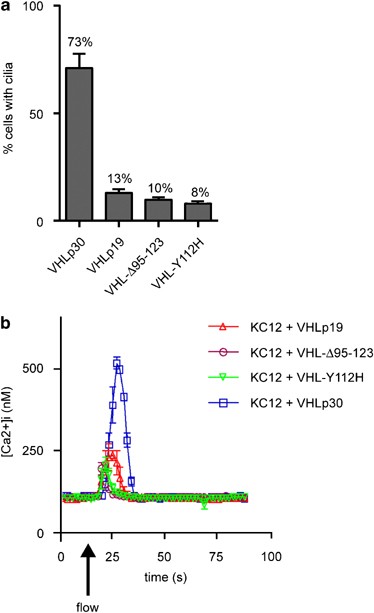
Whether these _VHL_-allelic variants could reconstitute cilia in VHL-deficient KC12 cells was addressed by transfecting GFP-VHLp30, GFP-VHLp19, GFP-VHL-Y112H or GFP-VHL-Δ95–123 into KC12 cells (Figure 3a). Most of the KC12 cells transfected with GFP-VHLp30 produced cilia; however, GFP-VHLp19, GFP-VHL-Δ95–123, and GFP-VHL-Y112H were unable to recapitulate the efficacy of full-length VHL (Figure 3a). To analyze whether VHL variants could stimulate cellular calcium influx characteristic of cilia bending, we reconstituted KC12 cells with pBABE-puro and Vsv-tagged _VHL_-allelic variants before measuring the intracellular calcium response of these cells to fluid flow. Vsv-VHLp30 recapitulated 85% of the response observed in Figure 1f (41 cells). Measuring the AUCs, we observed that the level of response was significantly decreased in cells expressing Vsv-VHLp19 (_P_=0.004, 58 cells), Vsv-VHL-Δ95–123 (P<0.001, 77 cells) or Vsv-VHL-Y112H (_P_<0.001, 69 cells). After the experiment, we confirmed >90% transfection efficiency by _α_-Vsv immunostaining. Similarly, _α_-acetylated tubulin/_γ_-tubulin staining confirmed that the cilia frequency correlated with Figure 3a. We, therefore, conclude that the reduction of calcium influx in VHL variants is probably a result of reduced cilia frequency.
Figure 3
von Hippel–Lindau (VHL)-mutant alleles fail to reconstitute functional cilia. (a) One thousand interphase nuclei were scored in three experiments to determine the frequency of cilia in GFP-expressing cells. Variant alleles of VHL all show significantly decreased cilia frequency compared to GFP-VHLp30: GFP-VHLp19 (_P_=0.007), GFP-VHL-Y112H (_P_=0.006), and GFP-VHL-Δ95–123 (_P_=0.005). (b) Variant alleles of VHL reduce calcium influx in response to flow. Intracellular calcium concentrations ([Ca2+]i) were determined by influx assays on KC12 cells reconstituted with Vsv-VHLp30, Vsv-VHLp19, Vsv-VHL-Δ95–123, or Vsv-VHL-Y112H variants. Experiments were performed in quadruplicate on at least two independent days. Error bars represent SD.
Discussion
Our data support and expand upon recent findings establishing a role for VHL in cilia regulation.6, 7, 8, 9 However, discrepancies between the five studies, including ours, are evident. For example, Esteban et al6 claim HIF responsible for the development of cilia, whereas we and others fail to find this connection.7, 8, 9 The KC12 cells we use here are devoid of HIF1/2_α_10 and subsequently do not show upregulated mRNA levels of HIF targets VEGF and GLUT1 in RT-PCR experiments (unpublished data), arguing that cilia regulation is indeed a HIF-independent function of VHL. Furthermore, our data support the notion put forward by the Krek group8 that regulating primary cilia requires residues 95–123, previously implicated in microtubule stability.12 The Burk and Benzing labs do not observe this connection in their cell systems.7, 9 In different cells, Lutz et al7 report cilia regulation by the murine-short isoform of VHL, Vhlh-p18; however, we observe no human VHL-p19 localization in the cilium, and significantly reduced functioning of this organelle in our calcium-flow experiments. Thus, our data suggest that cilia function in renal carcinoma cells is dependent on two domains in VHL, residues 1–53, known as the acidic domain, and residues 95–123, previously implicated in microtubule binding and tumor suppression.
Note added in proof
Since the acceptance of this manuscript, we have published that VHL binds microtubules through the kinesin-2 motor complex; VHL residues 1–53 and 95–123 mediate this interaction (Lolkema et al: The von Hippel-Lindau tumour suppressor interacts with microtubules through kinesin-2. FEBS Letters 2007).
References
- Latif F, Tory K, Gnarra J et al: Identification of the von Hippel–Lindau disease tumor suppressor gene. Science 1993; 260: 1317–1320.
Article CAS Google Scholar - Lonser RR, Glenn GM, Walther M et al: von Hippel–Lindau disease. Lancet 2003; 361: 2059–2067.
Article CAS Google Scholar - Rankin EB, Tomaszewski JE, Haase VH : Renal cyst development in mice with conditional inactivation of the von Hippel–Lindau tumor suppressor. Cancer Res 2006; 66: 2576–2583.
Article CAS Google Scholar - Kim WY, Kaelin WG : Role of VHL gene mutation in human cancer. J Clin Oncol 2004; 22: 4991–5004.
Article CAS Google Scholar - Pugh CW, Ratcliffe PJ : The von Hippel–Lindau tumor suppressor, hypoxia-inducible factor-1 (HIF-1) degradation, and cancer pathogenesis. Semin Cancer Biol 2003; 13: 83–89.
Article CAS Google Scholar - Esteban MA, Harten SK, Tran MG, Maxwell PH : Formation of primary cilia in the renal epithelium is regulated by the von Hippel–Lindau tumor suppressor protein. J Am Soc Nephrol 2006; 17: 1801–1806.
Article CAS Google Scholar - Lutz MS, Burk RD : Primary cilium formation requires von hippel–Lindau gene function in renal-derived cells. Cancer Res 2006; 66: 6903–6907.
Article CAS Google Scholar - Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W : pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol 2007; 9: 588–595.
Article CAS Google Scholar - Schermer B, Ghenoiu C, Bartram M et al: The von Hippel–Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol 2006; 175: 547–554.
Article CAS Google Scholar - Lolkema MP, Mehra N, Jorna AS, van Beest M, Giles RH, Voest EE : The von Hippel–Lindau tumor suppressor protein influences microtubule dynamics at the cell periphery. Exp Cell Res 2004; 301: 139–146.
Article CAS Google Scholar - Datta K, Sundberg C, Karumanchi SA, Mukhopadhyay D : The 104–123 amino acid sequence of the beta-domain of von Hippel–Lindau gene product is sufficient to inhibit renal tumor growth and invasion. Cancer Res 2001; 61: 1768–1775.
CAS Google Scholar - Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W : Regulation of microtubule stability by the von Hippel–Lindau tumour suppressor protein pVHL. Nat Cell Biol 2003; 5: 64–70.
Article CAS Google Scholar - Lolkema MP, Gervais ML, Snijckers CM et al: Tumor suppression by the von Hippel–Lindau protein requires phosphorylation of the acidic domain. J Biol Chem 2005; 280: 22205–22211.
Article CAS Google Scholar - Ulfman LH, Joosten DP, van der Linden JA, Lammers JW, Zwaginga JJ, Koenderman L : IL-8 induces a transient arrest of rolling eosinophils on human endothelial cells. J Immunol 2001; 166: 588–595.
Article CAS Google Scholar - Brown JM, Marsala C, Kosoy R, Gaertig J : Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in tetrahymena. Mol Biol Cell 1999; 10: 3081–3096.
Article CAS Google Scholar - Praetorius HA, Spring KR : Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 2001; 184: 71–79.
Article CAS Google Scholar - Nauli SM, Alenghat FJ, Luo Y et al: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33: 129–137.
Article CAS Google Scholar
Acknowledgements
This work is supported by the Dutch Cancer Society (Grants UU 1999-1879/2114 to EV) and the Netherlands Organization for Scientific Research (NWO Grants: AGIKO grant 920-03-179 to ML and VIDI Award 016.066.354 to RG).
Author information
Author notes
- Stefano Volpi
Present address: 4Current address: 2nd Division of Pediatrics, G. Gaslini Institute for Children, University of Genoa, Italy., - Martijn P Lolkema, Dorus A Mans, Emile E Voest and Rachel H Giles: These authors contributed equally.
Authors and Affiliations
- Department of Medical Oncology, University Medical Center Utrecht, Heidelberglaan 100, Utrecht, The Netherlands
Martijn P Lolkema, Dorus A Mans, Stefano Volpi, Emile E Voest & Rachel H Giles - Department of Pulmonary Diseases, University Medical Center Utrecht, Heidelberglaan 100, Utrecht, The Netherlands
Laurien H Ulfman
Authors
- Martijn P Lolkema
You can also search for this author inPubMed Google Scholar - Dorus A Mans
You can also search for this author inPubMed Google Scholar - Laurien H Ulfman
You can also search for this author inPubMed Google Scholar - Stefano Volpi
You can also search for this author inPubMed Google Scholar - Emile E Voest
You can also search for this author inPubMed Google Scholar - Rachel H Giles
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toRachel H Giles.
Rights and permissions
About this article
Cite this article
Lolkema, M., Mans, D., Ulfman, L. et al. Allele-specific regulation of primary cilia function by the von Hippel–Lindau tumor suppressor.Eur J Hum Genet 16, 73–78 (2008). https://doi.org/10.1038/sj.ejhg.5201930
- Received: 30 May 2007
- Revised: 22 August 2007
- Accepted: 28 August 2007
- Published: 03 October 2007
- Issue Date: January 2008
- DOI: https://doi.org/10.1038/sj.ejhg.5201930