Cardiovascular complications in chronic kidney disease: a review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association (original) (raw)
Journal Article
Renal Research Institute, 315 E, 62nd St.
,
New York, NY 10065
,
USA
Associazione Ipertensione Nefrologia e Trapianto Renale (IPNET) c/o Nefrologia e CNR, Grande Ospedale Metropolitano
,
Contrada Camporeale, 83031 Ariano Irpino Avellino
,
Italy
Search for other works by this author on:
Nephrology and Transplantation Unit, Grande Ospedale Metropolitano Reggio Cal and CNR-IFC
,
Via Giuseppe Melacrino 21, 89124 Reggio Calabria
,
Italy
Search for other works by this author on:
Department of Nephrology, Transplantation and Internal Medicine, Medical University of Silesia in Katowice
,
Francuska 20-24 St. 40-027 Katowice
,
Poland
Search for other works by this author on:
Rodrigo Bueno de Oliveira
Department of Internal Medicine (Nephrology), School of Medical Sciences, University of Campinas (Unicamp)
,
Campinas
,
Brazil
Search for other works by this author on:
Ambroise Paré University Hospital, APHP, Boulogne Billancourt/Paris, and INSERM U-1018, Centre de recherche en épidémiologie et santé des populations (CESP), Equipe 5, Paris-Saclay University (PSU) and University of Paris Ouest-Versailles-Saint-Quentin-en-Yvelines (UVSQ), FCRIN INI-CRCT
,
Villejuif
,
France
Search for other works by this author on:
Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki
,
Thessaloniki
,
Greece
Search for other works by this author on:
Indiana University School of Medicine and Richard L. Roudebush VA Medical Center
,
1481 W 10th St, Indianapolis, IN 46202
,
USA
Search for other works by this author on:
School of Cardiovascular and Metabolic Health, University of Glasgow
,
Glasgow
,
UK
Search for other works by this author on:
Renal Research Institute, LLC Icahn School of Medicine at Mount Sinai
,
315 East 62nd Street, 3rd Floor, New York, NY 10065
,
USA
Search for other works by this author on:
Department of Renal Medicine, University Hospitals Birmingham
,
Birmingham
,
UK
Search for other works by this author on:
Received:
07 September 2022
Revision received:
29 December 2022
Accepted:
09 January 2023
Corrected and typeset:
09 June 2023
Cite
Carmine Zoccali, Francesca Mallamaci, Marcin Adamczak, Rodrigo Bueno de Oliveira, Ziad A Massy, Pantelis Sarafidis, Rajiv Agarwal, Patrick B Mark, Peter Kotanko, Charles J Ferro, Christoph Wanner, Michel Burnier, Raymond Vanholder, Andrzej Wiecek, Cardiovascular complications in chronic kidney disease: a review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association, Cardiovascular Research, Volume 119, Issue 11, August 2023, Pages 2017–2032, https://doi.org/10.1093/cvr/cvad083
Close
Navbar Search Filter Mobile Enter search term Search
Abstract
Chronic kidney disease (CKD) is classified into five stages with kidney failure being the most severe stage (stage G5). CKD conveys a high risk for coronary artery disease, heart failure, arrhythmias, and sudden cardiac death. Cardiovascular complications are the most common causes of death in patients with kidney failure (stage G5) who are maintained on regular dialysis treatment. Because of the high death rate attributable to cardiovascular (CV) disease, most patients with progressive CKD die before reaching kidney failure. Classical risk factors implicated in CV disease are involved in the early stages of CKD. In intermediate and late stages, non-traditional risk factors, including iso-osmotic and non-osmotic sodium retention, volume expansion, anaemia, inflammation, malnutrition, sympathetic overactivity, mineral bone disorders, accumulation of a class of endogenous compounds called ‘uremic toxins’, and a variety of hormonal disorders are the main factors that accelerate the progression of CV disease in these patients. Arterial disease in CKD patients is characterized by an almost unique propensity to calcification and vascular stiffness. Left ventricular hypertrophy, a major risk factor for heart failure, occurs early in CKD and reaches a prevalence of 70–80% in patients with kidney failure. Recent clinical trials have shown the potential benefits of hypoxia-inducible factor prolyl hydroxylase inhibitors, especially as an oral agent in CKD patients. Likewise, the value of proactively administered intravenous iron for safely treating anaemia in dialysis patients has been shown. Sodium/glucose cotransporter-2 inhibitors are now fully emerged as a class of drugs that substantially reduces the risk for CV complications in patients who are already being treated with adequate doses of inhibitors of the renin-angiotensin system. Concerted efforts are being made by major scientific societies to advance basic and clinical research on CV disease in patients with CKD, a research area that remains insufficiently explored.
1. Introduction
Kidney diseases are now recognized as a global public health priority.1 On a world scale, about 861 million individuals are affected by kidney diseases, and the vast majority of these presents with chronic kidney disease (CKD).2 This non-communicable disease imposes a greater health burden in low and middle-income countries than in affluent countries.3 The classification of CKD and the risk of cardiovascular (CV) mortality across this spectrum are illustrated in Figure 1. CKD is a key condition in the years of transitional epidemiology, an epochal change characterized by the decline of communicable diseases and the rise of non-communicable conditions.4 Projections made by the global health burden of disease epidemiologists forecast that in 2040, CKD will be the 5th disease in rank responsible for death in the world.5 The CV death in patients with CKD prevents these patients from reaching kidney failure (stage G5, i.e. the stage where dialysis and renal transplantation are needed).6
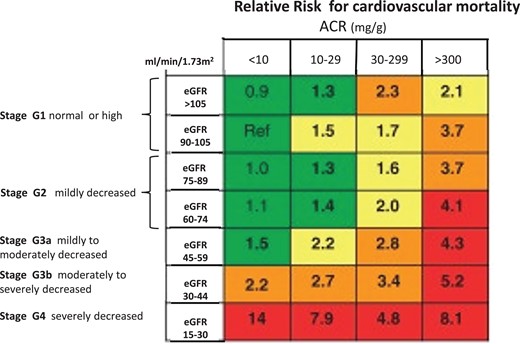
Figure 1
Cardiovascular prognosis in CKD. CKD is defined as abnormalities for kidney function (eGFR) or damage (albuminuria) lasting >3 months. The figure shows the prognosis for cardiovascular disease and CKD progression according to ACR and eGFR and ACR categories. Green, low risk; yellow, moderate risk; orange, high risk; red, very high risk. Adapted by permission by the KDIGO 2012 Guideline on CKD, Kidney International Supplement 1, volume 3, pages 1–150, 2013.
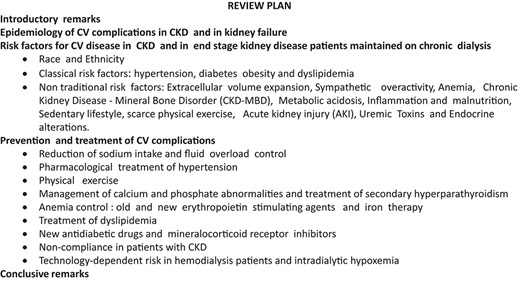
This narrative review is based on a selection of the most cited articles focusing on CV disease in CKD published up to 31 July 2022. Each author made a separate PubMed search in a specific area (see Authors’s contributions of the authors). The selected articles by each author were circulated among all the co-authors and integrated to compose the final list. Whenever possible, we aimed at including systematic reviews and original studies. We included large studies and good-quality articles published recently. We aimed at covering all areas of the problem, from epidemiology to therapy. The plan of this review is shown in Box 1. The allotment of the various themes to contributing authors was made based on individual expertise.
Box 1
2. Epidemiology of CV complications in CKD and kidney failure
In a systematic review and meta-analysis that included over 1 million individuals,7 which considered the estimated glomerular filtration rate (eGFR) of 95 mL/min/1.73 m² as a reference point, the independent risk for death was 1.18 [95% confidence interval (CI) 1.05–1.32] for eGFR 60 mL/min/1.73 m², 1.57 (1.39–1.78) for 45 mL/min/1.73 m², and 3.14 (2.39–4.13) for 15 mL/min/1.73 m². In the same meta-analysis, CV mortality paralleled the risk of death (Figure 2). Independently of the eGFR, the urinary albumin creatinine ratio (ACR) was associated with the risk of all-cause and CV death in a linear fashion without threshold effects.7
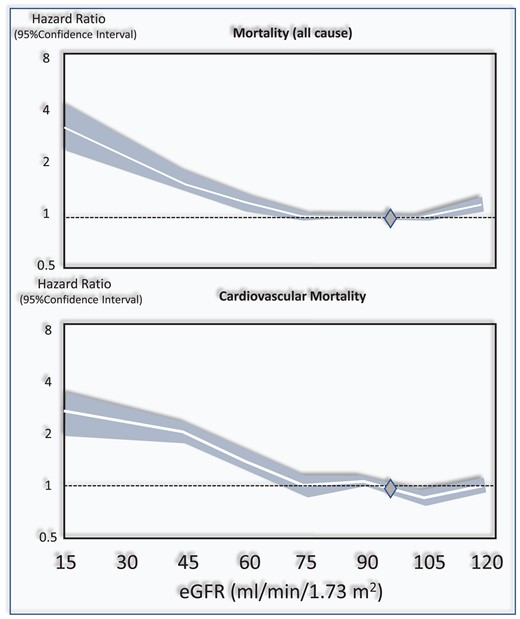
Figure 2
Relationship between the eGFR and the risk of all-cause and cardiovascular mortality in the CKD Epi consortium meta-analysis.7. The Figure has been drawn on the basis of data reported in this study and on Figure 1 data of the same study. The diamond at 95 mL/min/1.73 m2 is the reference point (i.e. the eGFR level assumed as normal).
The prevalence of left ventricular hypertrophy (LVH), a major forerunner of congestive heart failure (HF), is inversely related to the severity of renal dysfunction. The prevalence of LVH is only modestly elevated in stage G2 CKD patients, whereas it reaches 70–80% in patients with kidney failure on dialysis.8 In stage G2-5 CKD patients with HF, the prevalence of preserved ejection fraction (EF) is 60% and that of reduced EF 40%; the risk of death in these two types of HF is inversely associated with eGFR.9 In patients on regular dialysis, the preserved EF type (about 80%) is more common than the reduced EF type and both forms of HF predict a high risk of death.10
Similar to the risk of the aforementioned CV complications, eGFR11 and proteinuria12,13 are related to the incident risk for stroke in a stepwise fashion. Furthermore, CKD and albuminuria are associated with an increased risk of peripheral vascular disease and aortic aneurysms across all stages14 and progressively higher levels of albuminuria.15 Among patients maintained on chronic dialysis, the prevalence of this complication ranges from 23 to 46% depending on the method of assessment and this disease has an almost unique severity with a 77% independent risk excess for mortality.16
3. Risk factors for CV disease in CKD and end stage kidney disease patients maintained on chronic dialysis
3.1 Race and ethnicity
Black individuals are almost four times as likely as Whites to develop kidney failure. In the USA, while black individuals make up about 13% of the population, they account for 35% of the people with kidney failure in this country.17 Diabetes and hypertension are the leading causes of kidney failure among Black individuals. Furthermore, in the USA, Hispanic individuals are almost 1.3 times more likely to be diagnosed with kidney failure compared to non-Hispanic individuals.17
In the Chronic Renal Insufficiency (CRIC) cohort, a cohort representative of the CKD population in the USA, CV risk factors, including mean systolic blood pressure (BP), body mass index, albuminuria, and LDL cholesterol, were higher in young adult Black and Hispanic individuals, a population stratum where early prevention is fundamental. The Black and Hispanic populations had higher incidence rates of HF (17.5 vs. 5.1/1000 person-years), all-cause mortality (15.2 vs. 7.1/1000 person-years), and CKD progression (125 vs. 59/1000 person-years) compared to the white population.18
3.2 Classical risk factors: hypertension, diabetes obesity, and dyslipidaemia
Hypertension is a hallmark of CKD.19 Hypertensive mechanisms in CKD are schematized and commented on in Figure 3. Sodium retention and the attendant volume expansion attributable to the reduced number of functioning nephrons and stimulation of the renin-angiotensin-aldosterone pathway trigger various pro-hypertensive mechanisms and activate the inflammatory-immune system. Sodium in CKD also accumulates non-osmotically in the muscles and the skin, i.e. without parallel water retention, which is associated with the degree of renal dysfunction.20 In experimental models, non-osmotic sodium retention activates inflammatory mechanisms in macrophages in the skin.21 Secondary to volume expansion, the production of endogenous cardiotonic steroids, including ouabain and ouabain-like steroids, is increased in CKD patients. High levels of these steroid compounds contribute to raising BP by impairing vasodilatory mechanisms.22 Vascular stiffness, which is related to inflammation23 and vascular calcification secondary to hyperphosphataemia and secondary hyperparathyroidism, are major contributors to elevated systolic BP in this population.24 Impaired nitrous oxide production due to the accumulation of endogenous inhibitors of nitric oxide synthase, like asymmetric dimethyl arginine (ADMA) and endothelial dysfunction parallels the decline in renal function in CKD patients.25 Progressive accumulation of these substances and a parallel rise in endothelin levels contribute to hypertension and trigger inflammation and oxidative stress n CKD.25 Sympathetic overactivity has a major role in hypertension in CKD patients26,27 and dialysis patients.28–30
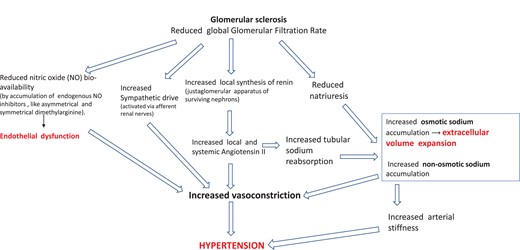
Figure 3
Main pathophysiological alterations leading to hypertension in CKD. High renin and aldosterone levels are common among CKD patients. Angiotensin II, a direct vasoconstrictor, increases vascular resistance and arterial pressure. Angiotensin II also enhances, in a direct manner, sodium reabsorption in the proximal tubule and stimulates via aldosterone hypersecretion sodium reabsorption in the collecting duct. Furthermore, renal function loss per se reduces sodium excretion, which amplifies sodium retention. Non-osmotic sodium accumulation activates pro-hypertensive mechanisms via the inflammatory-immune system (see text). Due to sodium retention and volume expansion secondary to reduced GFR, endogenous cardiotonic steroids (ouabain and other ouabain-like steroids) are increased in CKD patients. High levels of these steroid compounds contribute to raise BP by impairing vasodilatory mechanisms.
Being overweight and obese are the strongest risk factors for Type 2 diabetes.31 In England in 2009–10, 90% of patients with Type 2 diabetes were overweight or obese.32 Both obesity and Type 2 diabetes are dominant risk factors for CKD in economically developed and developing countries.33 Inflammation is the key to the pathogenesis of diabetic kidney disease and its CV consequences.34 Hyperglycaemia induces micro- and macrovascular complications by various mechanisms including enhanced glycoxidation, intracellular generation of reactive oxygen species, and accumulation of glycosylated proteins, which activate and sustain, mainly via epigenetic changes, the pro-inflammatory pathways.35 The imbalance in the synthesis of pro-inflammatory [leptin, resistin, tumour necrosis factor alpha, interleukin 1 beta (IL1β) and interleukin 6 (IL6), and other cytokines] and anti-inflammatory (adiponectin and interleukin 1036) adipose tissue cytokines (adipokines)37 is associated with CV and renal damage in CKD patients.37 Inflammatory mediators are of relevance in diabetes and CKD.38 Randomized trials testing monoclonal antibodies targeting IL1β and IL-6 documented the relevance of these cytokines in atherosclerosis39 and other conditions.40 The gut microbiota is a key inducer of metabolic inflammation in obesity and Type 2 diabetes41 as well as in CKD.42 Risk factors for obesity, Type 2 diabetes, and CKD-related risk factors for CV disease overlap in patients with diabetic kidney disease (Figure 4).
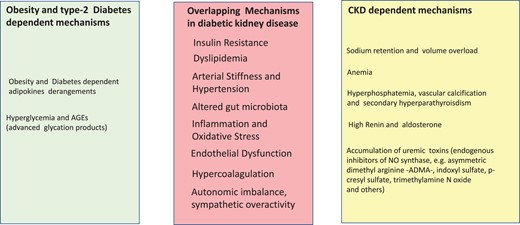
Figure 4
Separate and overlapping risk factors for cardiovascular disease in overweight and obesity and in CKD. Risk factors triggered by obesity and Type 2 diabetes are listed in the light green panel and those by CKD in the yellow panel. Overlapping risk factors by obesity/Type 2 diabetes and CKD are listed in the light red panel, at the centre of the figure.
Dyslipidaemia in CKD patients is characterized by hyper-triglyceridaemia, low HDL cholesterol, variable levels of LDL cholesterol (mostly normal levels), and high lipoprotein(a) [Lp(a)].43 Metabolism of triglyceride-rich LDL (VLDL), intermediate-density lipoprotein (IDL), and LDL (atherogenic small dense LDL particles) is altered in CKD. Furthermore, reverse cholesterol transport—a process mediated by HDL cholesterol—is impaired in this condition. Both HDL and LDL are modified in CKD patients, which enhance their atherogenic potential.43 A description of the key, major steps in lipid metabolism and alterations in lipoprotein metabolism in CKD patients is depicted in Figure 5.
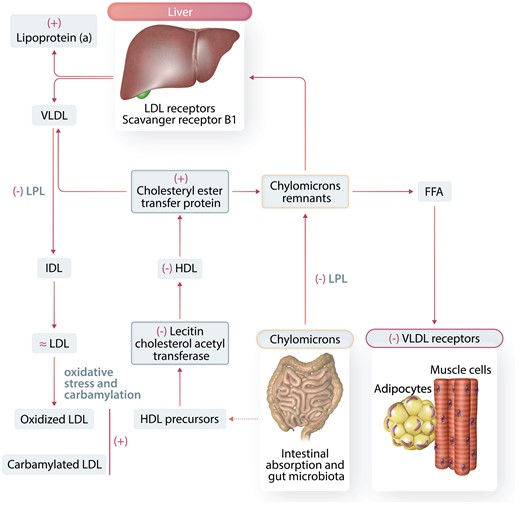
Figure 5
Alterations in lipoprotein metabolism in CKD. The liver generates triglyceride-rich VLDL. Triglycerides are hydrolyzed by lipoprotein lipase (LPL), and the VLDL particles decrease to form IDL particles and finally LDL-cholesterol particles. The LDL particles carry cholesterol to the liver and peripheral tissues. The LDL receptor (LDLR) and scavenger receptors (scavenger receptor B1, SR-B1) are keys to LDL particles clearance. Triglyceride-rich chylomicrons are carriers of lipids from the gut to the liver. Hydrolysis of chylomicrons by LPL produces free fatty acids (FFAs), and chylomicrons in the process become smaller (chylomicron remnants) to be eventually captured by the liver via the LDLR. HDL particles are fundamental for the control of the reverse cholesterol transport (from macrophages and endothelial cells to the liver). Comorbidities like diabetes mellitus and nephrotic syndrome have obvious influences in these alterations. Key alterations in lipoprotein metabolism in CKD patients are clearly identified as increased (+) or decreased (−) or unchanged (≂).
3.3 Non-traditional risk factors
3.3.1 Extracellular volume expansion
Volume overload44 and its main adverse effects, LVH, hypertension and HF, are increasingly common from stage 3 to stage 5 CKD.8,45 In the dialysis population, independent of hypertension and other risk factors, volume overload per se doubles the death risk.45 In CKD patients, high sodium intake46 and volume expansion47 are directly and independently related to the incident risk of CV disease and death.
3.3.2 Sympathetic overactivity
Directly measured sympathetic activity (sympathetic microneurography) is markedly increased in CKD patients.26,27 It is extremely high in patients maintained on regular dialysis treatment.28–30 Increased sympathetic activity does not regress after renal transplantation48 but is abolished after bilateral nephrectomy.28 The causes of sympathetic overactivity in CKD and in kidney failure patients are multiple and include enhanced central sympathetic drive activated by afferent renal nerves in diseased kidneys49 and comorbidities, including sleep apnoea,50 HF and obesity, the latter is now the most common alteration of nutritional status in CKD and in dialysis patients.51 High sympathetic activity in non-dialysis CKD patients and in dialysis patients is associated with concentric LVH52,53 and a high risk of death and CV complications in dialysis patients.54
3.3.3 Anaemia
Inappropriately low synthesis of erythropoietin by failing kidneys, accumulation of uremic toxins, iron deficiency, and inflammation all participate in the pathogenesis of anaemia in patients with CKD.55 Anaemia underlies an increased risk of mortality and CV hospitalization in CKD56 and haemodialysis patients.57–59 By increasing cardiac workload to compensate for reduced oxygen delivery to peripheral tissues, anaemia leads to LVH as well as to arterial remodelling, a process resulting in compensatory intima-media thickening and arteriosclerosis. The association between anaemia and LVH is robust both in non-dialysis CKD patients60 and in dialysis patients.61
3.3.4 CKD-mineral bone disorder
CKD-mineral bone disorder (MBD) is a systemic disorder of mineral and bone metabolism due to CKD manifested by either one or a combination of abnormalities of calcium, phosphate, parathyroid hormone (PTH), or vitamin D metabolism, abnormalities in bone turnover, mineralization, volume, linear growth, or strength and vascular or other soft-tissue calcification.62
In CKD patients, peculiar endocrine alterations are set into motion to increase phosphate excretion in surviving nephrons. Increased plasma fibroblast growth factor-23 (FGF23), produced by osteocytes, is the first factor to be activated to counteract reduced phosphate excretion63 (Figure 6). High FGF23 inhibits 1,25 dihydroxy vitamin D synthesis and via this pathway reduces calcium absorption in the gut leading to hypocalcaemia. In turn, hypocalcaemia reduces the physiological restrain by the calcium receptor on PTH secretion and increases serum PTH, which contributes to enhanced phosphate excretion.64 Furthermore, high PTH tends to suppress FGF23 synthesis in the bone and, in concert with FGF23, suppresses 1,25D synthesis.64,65 Overall, the CKD-BMD is characterized by hyperphosphataemia, hypocalcaemia, high FGF23, and PTH, and low 1,25 VD levels. The prevalence of these abnormalities increase gradually with CKD progression.65 Of note, iron metabolism is strictly connected to FGF23 metabolism, and iron deficiency, a frequent problem in CKD patients, inhibits FGF23 degradation, and raises serum FGF23.66 This bone hormone also functions as a growth factor for the myocardium, and its level is strongly associated with the LV mass index in CKD patients.67 Two large meta-analyses showed that FGF23 is a powerful predictor of the risk of death and cardiovascular (CV) disease in CKD patients68 and in kidney failure patients maintained on chronic dialysis.69 Like FGF23,67 PTH also is related to left ventricular mass, at least in dialysis patients.70 Furthermore, high serum PTH level is associated with a high risk of CV events both in CKD patients71 and in kidney failure patients maintained on regular dialysis treatment.72 A similar association has been observed with a low vitamin D level.73 Calcification of coronary arteries is a strong biomarker of high CV risk in CKD patients74 and particularly in patients on regular dialysis treatment.75 However, evidence that vascular calcification is a modifiable CV risk factor in CKD and dialysis patients is still lacking.76
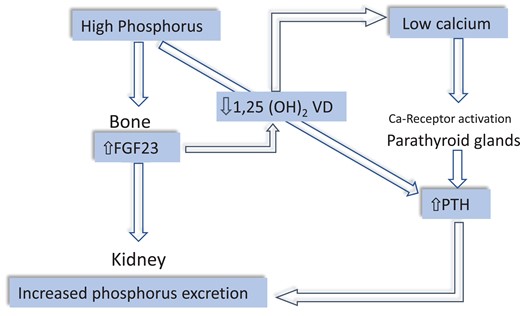
Figure 6
Sequence of CKD-MBD hormones alterations in CKD. Phosphate accumulation and hyperphosphataemia secondary to reduced GFR stimulate FGF23 synthesis in the bone. FGF23 in turn not only augments phosphate excretion but also reduces 1,25 vitamin D levels thereby lowering serum calcium. Reduced renal mass in the course of CKD contributes to lower 1,25 vitamin D, a hormone synthesized in the kidney. Hypocalcaemia stimulates the calcium receptor in the parathyroid glands, which raises circulating PTH. High PTH contributes to increase phosphate excretion.
3.3.5 Metabolic acidosis
Because of reduced ammonia genesis, disturbed secretion of protons in the proximal and distal tubules, and impaired reabsorption of bicarbonate in the kidney tubules, the prevalence of metabolic acidosis (low plasma bicarbonate) is associated with the severity of CKD being 7% for stage 2, 13% for stage 3, and 37% for stage 4 CKD.77,78 Several studies documented that low plasma bicarbonate is associated with a high risk of all-cause death77–79 incident HF, stroke, myocardial infarction, and CV death.80
3.3.6 Inflammation and malnutrition
Persistent, low-grade inflammation is common in CKD81 and almost universal in dialysis patients.82 Increased inflammation is multifactorial and results from oxidative stress,83 a propensity to infection,84 intestinal dysbiosis,85 metabolic acidosis,86 and reduced renal clearance of cytokines.87–89 Chronic inflammation in CKD patients and patients maintained on chronic dialysis portends a high risk for all-cause and CV death.90 In CKD patients, pro-inflammatory cytokines increase resting energy expenditure,91 and suppress the production of growth hormone, insulin growth factor 1, and anabolic hormones.92 It also weakens the stimulatory activity of erythropoietin on erythrocyte generation.93 Inflammation engenders sarcopenia and malnutrition in CKD patients and induces a peculiar condition referred to as the malnutrition/protein energy wasting (PEW) syndrome,94 which manifests as fatigue, nausea, lack of appetite, and depression.95 Importantly, PEW interacts with inflammation to increase mortality risk in this population.96 The causal importance of inflammation for CV disease in CKD patients is emphasized by secondary analyses in the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) trial.97 These analyses showed that canakinumab, a human monoclonal antibody targeting IL-1b, reduces major adverse CV event rates among statin-treated CKD patients (eGFR <60 mL/min/1.73 m2) with a prior history of myocardial infarction and persistently elevated levels of C-reactive protein (CRP), particularly among those with a robust anti-inflammatory response to initial treatment. Furthermore, a very recent secondary analysis of the CANTOS trial showed that in participants without CKD, inflammation biomarkers (CRP and IL-6) and LDL-Cholesterol all predicted major CV events while in CKD patients only inflammatory biomarkers but not LDL cholesterol predicted the same events.98 By the same token, in a double-blind, randomized, placebo-controlled phase 2 trial, IL-6 inhibition with ziltivekimab in CKD patients at high atherosclerotic risk markedly reduced biomarkers of inflammation.99
3.3.7 Sedentary lifestyle, scarce physical exercise
Due to the high comorbidity burden, limited physical activity is pervasive both in CKD patients100 and patients with kidney failure on chronic dialysis treatment.101 Reduced physical activity per se, independently of other risk factors, predicts a high death risk in CKD patients100 and dose-dependently associates with the risk of death and CV disease in the dialysis population.101
3.3.8 Acute kidney injury and CV disease in CKD
In a meta-analysis involving 55 150 patients, acute kidney injury (AKI) was associated with an 86% and a 38% increased risk of CV mortality and major CV events, respectively, as well as with a 38% increased risk for CV death, a 58% increased risk of HF and a 40% increased risk of acute myocardial infarction. Thus, not only CKD but independently of CKD, also AKI generates a high CV risk.102
3.3.9 Uremic toxins and endocrine alterations
Many compounds usually cleared by the kidneys are retained in the body in patients with CKD. A list of these compounds, referred to as uremic toxins, is periodically updated by the European Uremic Toxin Work Group.103 They are classified into three main groups according to their molecular weight, their clearance by dialytic methods, and/or their ability to bind to other molecules.103 High levels of some of these compounds, especially ADMA104,105 beta2 microglobulin,106 indoxyl sulphate, and paracresyl sulphate,107,108 are related to endothelial dysfunction and vascular damage. These compounds are potentially modifiable risk factors, a notion that requires testing in appropriate randomized trials.
CKD is a systemic disease that alters all major endocrine systems.109 Uremic toxins induce systemic inflammation and oxidative stress leading to CV disease and other harmful health effects. The complex inter-relationships between uremic toxins, derangements in endocrine control, inflammation and oxidative stress may all contribute to the high CV risk of CKD (Figure 7). Intervention studies based on clinical endpoints are needed to establish whether uremic toxins and endocrine alterations are causally involved in the high CV risk of CKD.
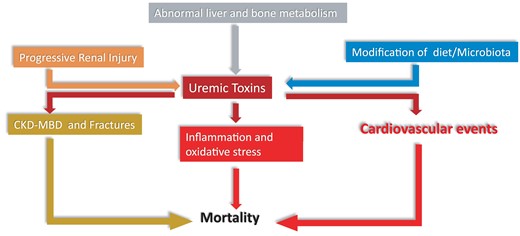
Figure 7
Multiple inter-relationships between uremic toxins, derangements in endocrine control, inflammation and oxidative stress impinging upon cardiovascular risk in CKD. ‘Progressive renal injury, which facilitates accumulation of uremic toxins, and alterations in the gut microbiota, which increase the synthesis of the same compounds, are main factors for the high levels of uremic toxins in CKD patients and alterations in liver and bone metabolism contribute to this process. Uremic toxins incite inflammation and cardiovascular events and contribute to the chronic kidney disease -metabolic bone disorder (CKD-MBD) and the resulting high risk for fractures of CKD patients. Inflammation, the CKD-MBD disorder and the high risk for cardiovascular events all conjure in causing a high death risk in the CKD population’.
4. Prevention and treatment of CV complications
4.1 Reduction of sodium intake and control of fluid overload
Controlling sodium intake and limiting fluid overload are held to be fundamental for the intervention of CV protection in CKD and dialysis patients.110 The Kidney Disease Global Outcomes (KDIGO) guidelines recommend reducing sodium intake to less than 2 g of sodium/day (i.e. approximately 5 g sodium chloride/day) in CKD patients with hypertension.110 However, these recommendations are not effectively applied. In the CRIC cohort, only a minority of patients had urinary sodium <90 mMol/24 (i.e. <2 g of sodium per day).46 The difficulty of educating CKD patients in reducing and maintaining a low sodium intake in the long term fully was shown in two randomized clinical trials that tested educational programmes including devices for self-measurement of urine sodium in stage G3-4 CKD patients.111,112 In both trials very modest reductions in 24 h urine sodium excretion and 24 h systolic ambulatory pressure (about 2 mmHg) were registered. Adherence to a low sodium diet remains an unmet clinical need in non-dialysis CKD patients. The recent Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease trial documented that chlorthalidone added to loop diuretics in treatment-resistant hypertensive CKD patients safely improves 24 h ambulatory BP monitoring values.113 However, both furosemide and thiazides are used less than needed in CKD patients.114 Inadequate use of diuretics in CKD may be due to the need for closer surveillance in patients treated with these drugs, which cannot be warranted in resource-limited contexts. Biomarkers of LV function and the volume status like brain natriuretic peptide (BNP) and pro BNP have not been specifically tested as a guide in the treatment of CKD patients. In HF patients with reduced EF, a strategy of NT-proBNP-guided therapy was not more effective than a usual care strategy in improving outcomes.115 Body impedance spectroscopy (BIS), which detects fluid overload, might be useful in the identification of CKD patients who have fluid overload (about 40%) and those who are volume-depleted patients (about 20%).116,117 Lung ultrasound detects water accumulation in the most critical area of the circulatory system at a pre-clinical stage and may therefore be valuable to guide treatment in CKD patients, a population where LV dysfunction is almost universal.118 The Lung Ultrasound Study trial in haemodialysis patients119 showed that the systematic application of this technique to guide treatment reduced the risk for repeated episodes of decompensated HF and CV events in this population. However, the study did not show a difference in the primary endpoint, which was a composite of all-cause death, non-fatal myocardial infarction and decompensated HF. Studies are needed to assess less ambitious sodium reduction targets in CKD patients. Likewise, studies to assess fluid overload using lung US or BIS in the management of HF in patients with CKD are needed, considering that HF together with acute myocardial infarction are the second CV cause of death in the dialysis population.120
4.2 Pharmacological treatment of hypertension
The BP target in CKD patients is set by the current KDIGO guidelines to less than 120/80 mmHg.110 The KDIGO recommendation specifies that BP should be measured by standardized office BP, the metric adopted in the Systolic Blood Pressure Trial.121 Because standardized office BP is challenging to implement outside specialized clinics and because of the potential risk of adverse events in a frail and multi-morbid population like the CKD population, a more conservative target (<130/80 mmHg) is deemed safer and more appropriate.122,123 Based on systematic reviews and accurate assessment of available literature, KDIGO guidelines recommend starting hypertension treatment with an angiotensin-converting enzyme inhibitor (ACEi) or with an angiotensin receptor blocker (ARB) in stage 1–4 CKD patients with diabetes and albuminuria (≥3 mg/mmol or ≥30 mg/g) and suggest the same drugs for those without diabetes. Furthermore, ACEi and ARB are suggested as initial treatment in patients with diabetes mellitus and CKD, i.e. GFR <60 mL/min/1.73 m2 without albuminuria but not in non-diabetic patients. These guidelines recommend not applying dual blockade of the renin angiotensin system (any combination of ACEi, ARB, and direct renin inhibitors) in patients with CKD with or without diabetes. Of note, KDIGO remarks as a practice point (i.e. a point made on the basis of experts consensus without sufficient supporting evidence), that mineralocorticoid receptor antagonists are effective for the management of refractory hypertension but may cause hyperkalaemia or a decline in kidney function, particularly among patients with low GFR. In renal transplant patients, the BP target is set at <130/80 mmHg but this target is identified as a practice point rather than as a recommendation. ACEi and calcium channel antagonists are recommended as initial treatment in renal transplant patients and this recommendation is also supported by an independent meta-analysis contemporary to the publication of the KDIGO guidelines.124
In patients maintained on chronic dialysis treatment, the number of clinical trials testing antihypertensive drugs is very limited and no formal recommendation for BP targets can be made. The European CV Medicine (EURECAm) working group of the European Renal Association (ERA) points out that the use of ambulatory BP monitoring (ABPM) extended to 48 h is advisable for the diagnosis and monitoring of hypertension in these patients and proposes a BP <130/80 mmHg target by this technique.125 This target may be hazardous in patients with advanced CV comorbidities and in the elderly (>65 years) where a more conservative target (<140/90 mmHg) seems reasonable. Furthermore, EURECAm remarks that pre- and post-dialysis BP measurements are overtly inadequate for the diagnosis and treatment of hypertension in this population. An antecedent consensus document by the American Society of Nephrology and the American Society of Hypertension, while underlying the unreliability of peri-dialysis BP measurements and the usefulness of 44 h ABPM, set a more conservative target for treatment (<140/90 mmHg).126 As to the use of specific antihypertensive agents, a meta-analysis of 11 studies made in 2017 showed that ACE-Is and ARBs do not reduce the risk for CV events in dialysis patients.127 Importantly, a randomized trial comparing an ACEi, lisinopril, with a beta-blocker, atenolol, in haemodialysis patients was stopped because of the clear superiority of atenolol for preventing CV events in this population.128 The results of this trial are in line with the knowledge that high sympathetic activity underlies a high risk for CV events in the haemodialysis population.54
4.3 Physical exercise
A systematic review of observational studies coherently showed that in CKD and dialysis patients both physical activity and physical performance are associated with a reduced risk for all-cause and CV mortality.129 A recent long-term, post-trial observational analysis in 227 dialysis patients130 who participated into a trial investigating the effect of walking exercise on physical performance131 documented that patients randomized to the active arm of the trial had a highly significant 29% risk reduction for hospitalizations, including those for CV complications over a 36-month follow-up. There is still no trial based on clinical outcomes testing physical exercise programmes in CKD and dialysis patients.
4.4 Management of calcium and phosphate abnormalities and treatment of secondary hyperparathyroidism
In CKD patients, treatment decisions are based mainly on time trends in serum phosphate, calcium, and PTH levels rather than on single measurements of individual biomarkers.132 KDIGO guidelines recommend bringing high serum phosphate levels down towards the normal range and maintaining serum calcium levels within the range specific for age and gender. Treatment options for lowering high serum phosphate levels include the use of phosphate binders, limitation of dietary phosphate intake, and, in dialysis patients, increased removal by dialysis if hyperphosphataemia is persistent.
Over the last two decades, several new phosphate binders (sevelamer, colestilan, bixalomer, lanthanum, calcium-magnesium, nicotinamide, and iron compounds) have become available.133 Some of these compounds have pleiotropic effects.133 Sevelamer and ferric citrate lower serum FGF23 levels,134,135 i.e. a putative risk factor implicated in the high CV risk of CKD. However, because of a lack of placebo-controlled trials, the hypothesis that these drugs may have a favourable impact on CV disease remains untested.
Available studies of dietary phosphate restriction in CKD patients are of low quality.136 Nonetheless, also taking into account the noxious effect of processed food on human health,137 limiting processed foods containing phosphate-based additives appears justifiable in CKD and dialysis patients, as also recommended by KDIGO guidelines.132
Adequate prescription of dialysis (longer and/or more frequent sessions) helps to maintain serum phosphate levels in the target range.138 Hypercalcaemia, an alteration favouring vascular calcification, can be avoided by reducing the use of calcium-based phosphate binders and vitamin D and using a low dialysate calcium concentration (1.25–1.5 mmol/L) in patients on dialysis.132
Treatment of secondary hyperparathyroidism depends on the CKD stage. The optimal PTH level to target in stage G3-5 CKD patients not on dialysis is still poorly defined. For this reason, the KDIGO guidelines suggest that patients with levels persistently above the upper normal limit for the assay or with progressively rising levels should be evaluated for modifiable factors, including hyperphosphataemia, hypocalcaemia, high phosphate intake, and vitamin D deficiency, rather than treated with calcium receptor agonists, a class of drugs that directly suppresses PTH. For patients on dialysis, the recommended PTH target level recommended by KDIGO is two to nine times the upper normal limit for assay.132 Treatment options in these patients include cinacalcet hydrochloride, active vitamin D compounds, and phosphate-lowering treatments (see above) or a combination of these treatments is frequently used in clinical practice. The primary analysis of a large-scale trial testing cinacalcet in haemodialysis patients did not show a significant reduction in CV outcomes in this population.139 FGF23 suppression by another calcium-receptor agonist, etelcalcetide, inhibited the progression of LVH compared with alfacalcidol in a randomized trial of haemodialysis patients.140 However, LVH is a weak surrogate endpoint for all-cause and CV mortality in the haemodialysis population.141 In patients with treatment-refractory secondary hyperparathyroidism, sub-total parathyroidectomy effectively reduces serum PTH levels and is associated with a reduction of CV events and mortality.142,143
4.5 Anaemia control: old and new erythropoietin stimulating agents and iron therapy
In the late 1990s, several clinical trials were designed to test the hypothesis that more complete correction of anaemia by erythropoiesis-stimulating agents might reduce CV disease (and further improve the quality of life). However, unexpected harm of haemoglobin normalization emerged in three landmark trials published in 2006–09.144–146 Therefore, the range of haemoglobin targets among patients on dialysis was set to 10–11 g/dL in the USA and 10–12 g/dL in Europe.
More recently, hypoxia-inducible factor prolyl hydroxylase inhibitors, a class of oral drugs that increase the levels of endogenous erythropoietin, have been tested in large clinical trials to assess the possibility that they can bring haemoglobin to the desired targets (see above) without increasing harm.147–149 These agents are promising for addressing the unmet need of non-injectable treatments, especially in patients with CKD not on dialysis, as well as those on peritoneal dialysis. However, they do not appear to offer a clear advantage for the management of anaemia in in-centre haemodialysis. In August 2021, the European Medicines Association approved roxadustat for the treatment of symptomatic anaemia in patients with CKD while the Food and Drug Administration committee in December 2021 voted against the approval of this drug.
Iron administered intravenously has been the standard of care among patients on dialysis. An open, randomized trial published in 2019 found that a high-dose intravenous iron regimen administered proactively was associated with fewer CV events compared to a low-dose regimen administered reactively and resulted in lower doses of the erythropoiesis-stimulating agent being administered.150
4.6 Treatment of dyslipidaemia
4.6.1 LDL-Reduction with statin-based therapies
In the general population, the reduction in LDL-cholesterol achieved with statin therapy is directly associated with the proportional reduction in vascular events.151 These relationships appear to be modified in patients with CKD. Indeed, in a meta-analysis by Herrington the effects of LDL cholesterol lowering with statin-based regimens on CV disease events were progressively attenuated across eGFR strata, denoting increasing CKD severity with no risk reduction being demonstrable in patients on dialysis.152
4.6.2 Other LDL-reduction therapies
Alirocumab and evolocumab, two drugs licensed for clinical use, are monoclonal antibodies which act as proprotein convertase subtilisin/kexin type 9 inhibitors. Both antibodies reduce LDL-cholesterol and Lp(a) levels and reduce major CV events in patients already optimized on statin treatment.153,154 Interestingly, the absolute reduction in CV events observed with evolocumab compared to placebo was greater in patients with more severe CKD.154
A hepatocyte-directed antisense oligonucleotide targeting Lp(a) mRNA has recently been tested in Phase 1 and 2 randomized controlled trials. However, patients with a GFR <60 mL/min/1.73 m2 or with a urinary albumin/creatinine ratio ≥100 mg/g were excluded from trial.155
4.7 New antidiabetic drugs and mineralocorticoid receptor inhibitors
In the past 6 years several large-scale CV outcome trials have provided solid evidence for new treatment options of CV and CKD with, or in part without, Type 2 diabetes. The new armamentarium of treatments includes sodium-glucose cotransporter-2 inhibitor (SGLT2i), glucagon-like peptide 1 receptor agonist and the mineralocorticoid receptor antagonist Finerenone.
4.7.1 SGLT2 inhibitors
SGLT2 inhibitors inhibit the reabsorption of sodium and glucose from the proximal tubule, thereby increasing renal glucose and sodium excretion delivery to the loop of Henle as well also inhibit the sodium: proton exchanger. The increase in sodium delivery to the loop of Henle activates a tubuloglomerular feedback response to correct glomerular hyperfiltration, an effect that protects the nephron from hyperfiltration/glomerular hypertension.156 Three major trials, (i) the Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes,157 a sub-study of the Empagliflozin, CV outcomes, and mortality in Type 2 diabetes, (ii) the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation,158 and (iii) the Dapagliflozin in Patients with CKD (DAPA-CKD)159 investigated albuminuric patients with and without Type 2 diabetes. A 30–40% relative risk reduction of the composite outcomes was consistently observed, and both drugs were labelled by regulatory agencies for the treatment of CV disease and/or CKD. The EMPA-KIDNEY study randomized individuals with diabetic and non-diabetic kidney disease and an eGFR ≥ 20 mL/min/1.73 m2 with no albuminuria and was stopped prematurely due to clear positive efficacy in people with CKD. The benefits of these drugs extend to the CV system. Indeed, Empagliflozin in the Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure (reduced)160 and Dapagliflozin in the Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction trial161 produced a substantial reduction in the risk for CV death in patients with HF and reduced EF.
4.7.2 Glucagon-like peptide 1 receptor agonists
CV outcome trials of GLP1RA have included patients with eGFR as low as 15 mL/min per 1.73 m2 with secondary outcomes for kidney disease. Largely driven by albuminuria reduction, GLP1RA, irrespective of structural variants, reduced the risk for all-cause mortality, hospital admission for HF and worsening kidney function in patients with Type 2 diabetes with no increased risk of severe hypoglycaemia, retinopathy, or pancreatic adverse effects.162
4.7.3 Finerenone
More than 13 000 patients with Type 2 diabetes were randomized into the FIDELIO (focusing on composite kidney outcomes)163 and FIGARO (centred on CV outcomes)164 trials. Finerenone significantly reduced the risk of both outcomes and can now be used for the treatment of adult CKD (stages 3 and 4 with albuminuria) patients. Few episodes of hyperkalaemia were reported but usually, hyperkalaemia limits the optimal use of agents that block the renin-angiotensin-aldosterone system, particularly in patients with CKD and HF.
4.7.4 Other emerging options
The currently recommended first-line treatments by the European Society of Cardiology in patients with HF now include next to SGLT2i also sacubitril-valsartan. Sacubitril, an inhibitor of the neutral endopeptidase neprilysin (an enzyme that degrades natriuretic peptides, bradykinin and adrenomedullin), combined with valsartan proved to be superior to enalapril in reducing the rates of death from CV and non-CV causes or hospitalization for HF among patients with HF and reduced EF.165 A meta-analysis by Kang et al., documented that, compared with the renin-angiotensin system inhibitors, sacubitril/valsartan significantly increases eGFR and decreases BP and NT-proBNP, suggesting that this drug might have CV and renal benefits in patients with HF and CKD.166 The currently recommended first line treatments by the European Society of Cardiology in patients with HF now include also SGLT2i and sacubitril-valsartan. Empagliflozin reduces the risk of CV death or hospitalization for HF in patients with HF with preserved EF,167 and the same is true for Dapagliflozin.168 These observations are of obvious relevance for patients with CKD stage 4 and/or Type 2 diabetes, a population with a high prevalence of HF with preserved EF.
4.8 Non-compliance in patients with CKD
Due to the high pill burden prescribed to mitigate CKD progression, hypertension and CV, and other comorbidities, CKD patients represent a population at very high risk of poor medication adherence.169 In a systematic review of 27 studies of CKD patients not on renal replacement therapy, the pooled prevalence of medication non-adherence was 39% (95% CI 30–48%).170 Risk factors for non-adherence include a high pill burden, eventual adverse medication events, cognitive disorders, and often a low health literacy. Low self-reported medication adherence has been associated with an increased risk for CKD progression.171
In clinical practice, the critical issue is the detection of non-adherence. Simple methods (interviews, questionnaires) tend to be relatively unreliable, and methods providing the best information (electronic monitoring, drug measurements) tend to be more expensive and demanding in terms of infrastructure172 (Table 1). The ideal method should prove the drug ingestion and provide a dosing history. So far, none of the easily accessible methods fulfils both criteria. Today chemical adherence testing using liquid chromatography-mass spectrometry (LC-MS) techniques is considered the preferred approach to detect non-adherence, measuring the presence or absence of drugs in urines or plasma.173 The implementation of this approach not only detects non-adherence but also tends to improve drug adherence by providing feedback to the patient. However, LC-MS is expensive and this technique is available only in a limited number of laboratories. Furthermore, a limitation of this approach is the white coat adherence whereby patients improve their adherence during the days preceding medical encounters. Team-based care involving several healthcare partners is increasingly recommended to manage complex drug prescriptions and support long-term drug adherence.174
Table 1
Methods used by physicians to assess adherence in patients with hypertension and their reliability, cost, and frequency of use
| Method used | Reliability | Cost | Frequency of use in practice | ||
|---|---|---|---|---|---|
| Never | Sometimes | Frequently or always | |||
| Ask questions on missed doses | Low | Low | 1% | 16% | 83% |
| Ask questions on reduced doses | Low | Low | 4% | 26% | 70% |
| Ask questions on changes of medication regimen | Low | Low | 1% | 15% | 84% |
| Speak to family, friends, or healthcare providers | Low | Low | 5.5% | 59% | 35.5% |
| Use of questionnaires | Low | Medium | 50.5% | 35% | 14.5% |
| Take blood or urine to measure medications | High | High | 47% | 33.5% | 19.5% |
| Use electronic monitors | High | High | 65% | 17% | 18% |
| Use pill counts | Medium | Medium | 60% | 30% | 10% |
| Use direct observed treatment (DOT) | High | High | 43% | 44% | 13% |
| Use pharmacy data | Medium | Medium | 44% | 35.5% | 20.5% |
| Use Apps data provided by patients | Low | Low | 64% | 31% | 5% |
| Method used | Reliability | Cost | Frequency of use in practice | ||
|---|---|---|---|---|---|
| Never | Sometimes | Frequently or always | |||
| Ask questions on missed doses | Low | Low | 1% | 16% | 83% |
| Ask questions on reduced doses | Low | Low | 4% | 26% | 70% |
| Ask questions on changes of medication regimen | Low | Low | 1% | 15% | 84% |
| Speak to family, friends, or healthcare providers | Low | Low | 5.5% | 59% | 35.5% |
| Use of questionnaires | Low | Medium | 50.5% | 35% | 14.5% |
| Take blood or urine to measure medications | High | High | 47% | 33.5% | 19.5% |
| Use electronic monitors | High | High | 65% | 17% | 18% |
| Use pill counts | Medium | Medium | 60% | 30% | 10% |
| Use direct observed treatment (DOT) | High | High | 43% | 44% | 13% |
| Use pharmacy data | Medium | Medium | 44% | 35.5% | 20.5% |
| Use Apps data provided by patients | Low | Low | 64% | 31% | 5% |
Table 1
Methods used by physicians to assess adherence in patients with hypertension and their reliability, cost, and frequency of use
| Method used | Reliability | Cost | Frequency of use in practice | ||
|---|---|---|---|---|---|
| Never | Sometimes | Frequently or always | |||
| Ask questions on missed doses | Low | Low | 1% | 16% | 83% |
| Ask questions on reduced doses | Low | Low | 4% | 26% | 70% |
| Ask questions on changes of medication regimen | Low | Low | 1% | 15% | 84% |
| Speak to family, friends, or healthcare providers | Low | Low | 5.5% | 59% | 35.5% |
| Use of questionnaires | Low | Medium | 50.5% | 35% | 14.5% |
| Take blood or urine to measure medications | High | High | 47% | 33.5% | 19.5% |
| Use electronic monitors | High | High | 65% | 17% | 18% |
| Use pill counts | Medium | Medium | 60% | 30% | 10% |
| Use direct observed treatment (DOT) | High | High | 43% | 44% | 13% |
| Use pharmacy data | Medium | Medium | 44% | 35.5% | 20.5% |
| Use Apps data provided by patients | Low | Low | 64% | 31% | 5% |
| Method used | Reliability | Cost | Frequency of use in practice | ||
|---|---|---|---|---|---|
| Never | Sometimes | Frequently or always | |||
| Ask questions on missed doses | Low | Low | 1% | 16% | 83% |
| Ask questions on reduced doses | Low | Low | 4% | 26% | 70% |
| Ask questions on changes of medication regimen | Low | Low | 1% | 15% | 84% |
| Speak to family, friends, or healthcare providers | Low | Low | 5.5% | 59% | 35.5% |
| Use of questionnaires | Low | Medium | 50.5% | 35% | 14.5% |
| Take blood or urine to measure medications | High | High | 47% | 33.5% | 19.5% |
| Use electronic monitors | High | High | 65% | 17% | 18% |
| Use pill counts | Medium | Medium | 60% | 30% | 10% |
| Use direct observed treatment (DOT) | High | High | 43% | 44% | 13% |
| Use pharmacy data | Medium | Medium | 44% | 35.5% | 20.5% |
| Use Apps data provided by patients | Low | Low | 64% | 31% | 5% |
4.9 Technology-dependent risk in haemodialysis patients and intradialytic hypoxaemia
Haemodialysis is a life-sustaining yet highly un-physiological treatment for kidney failure. It requires a connection between the patient and the extracorporeal dialysis circuit, creating a biology-technology interface that may adversely affect the patient, especially in the first 30 to 45 min into haemodialysis. In addition, dialysis-induced perturbations of the (bio)chemical milieu and intercompartmental fluid shift during dialysis impact—due to their recurrent nature—the patients’ long-term well-being (Figure 8).174 Arterial oxygen saturation (SaO2) drops in the first 45 min of haemodialysis, possibly related to clinically otherwise silent bio-incompatibility (transient neutrophil sequestration in the lung) (Figure 8) that may affect the pulmonary gas exchange. On the other hand bicarbonate loading by the dialysate, which typically contains 30–35 mMol/L, may attenuate the respiratory drive. Prolonged intradialytic hypoxaemia (i.e. SaO2 < 90% for more than a third of treatment time) is associated with a pro-inflammatory phenotype and increased morbidity and mortality.175
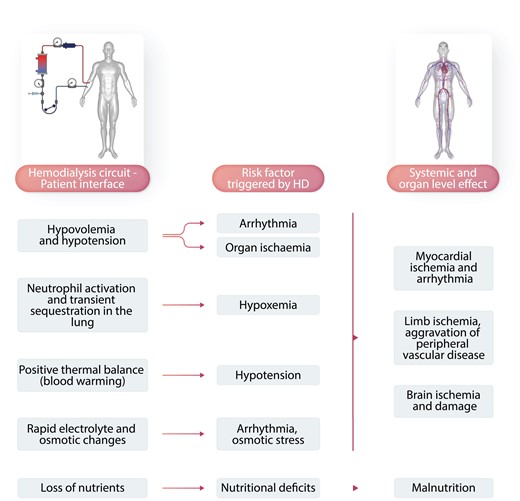
Figure 8
Dialysis-induced systemic stress resulting in a multi-organ injury superimposed on pre-existing comorbidities and affecting outcomes. During haemodialysis, the dialysis apparatus–patient interface triggers a series of risk factors for the cardiovascular system and other organ systems. This may result in myocardial ischaemia and arrhythmia, peripheral vascular disease aggravation, brain ischaemia and damage, and malnutrition.
Without residual kidney function, most patients retain fluid and gain weight between dialysis sessions (interdialytic weight gain). That fluid needs to be removed during haemodialysis by ultrafiltration to prevent fluid overload, a condition associated with increased morbidity and mortality. The decline of blood volume is considered one of the main drivers of haemodialysis-induced circulatory stress. Clinical manifestations are intradialytic hypotension, post-dialysis fatigue, mild to severe neurological symptoms, reduced quality of life, and increased morbidity and mortality.174
5. Conclusive remarks
Over the last two decades, CV problems have emerged as the most important issue to curb the poor prognosis of patients with CKD, particularly in patients with severe renal insufficiency and in patients maintained on chronic dialysis treatment. Advancing research on CV complications in these patients is a public health priority. Several European nephrological organizations have the heart-kidney link and its management as a core focus point.
The ERA has several workgroups. Among these, the European Renal and Cardiovascular Working Group of the European Renal Association (EuReCa-M, https://www.era-online.org/en/eureca-m/) and the European Uremic Toxin Work Group (EUTox, https://www.uremic-toxins.org) are devoted exclusively176 or preferentially to CV problems. The European Kidney Health Alliance (EKHA https://ekha.eu/) defends the case of kidney patients and aims at promoting funding of kidney disease research by the European Commission.177 The European Chronic Disease Alliance (ECDA, https://alliancechronicdiseases.org/), is a large multispecialty alliance including both cardiology and nephrology and other major scientific societies focusing on non-communicable diseases that aims to promote primary and secondary prevention of chronic diseases throughout the European Union. The efforts of these European organizations stressing that CV problems and kidney disease intensify each other underpin the need that nephrological and cardiological communities to join forces in creating awareness about this common problem.
Authors’ contributions
A.W. and C.Z. jointly conceived this review and prepared a writing plan allotting the various knowledge areas covered by the review to contributing authors according to their individual expertise. C.Z.: epidemiology, classical risk factors, extracellular volume expansion, control of sodium intake and fluid overload, pharmacological treatment of hypertension; F.M.: sympathetic activity, physical exercise, extracellular volume expansion; A.W. and M.A.: anaemia, metabolic acidosis; R.B.d.O. and Z.A.M.: CKD-MBD, inflammation and malnutrition, uremic toxins; management of calcium and phosphate metabolism; P.K.: technology dependent risk; R.A.: anaemia control; C.J.F.: treatment of dyslipidaemia; C.W.: new anti-diabetic drugs; M.B.: non-adherence in patients with CKD; R.V.: the European scenario of CV and renal research. C.Z. and A.W. harmonized the various contributions and C.Z. prepared the first draft of the manuscript, which was edited by R.V. and finally approved by all authors.
Acknowledgements
The authors are grateful to Mrs. Jeanne Kluzik who graciously proofread the revised version of this manuscript.
References
1
Cockwell
P
,
Fisher
L-A
.
The global burden of chronic kidney disease
.
Lancet
2020
;
395
:
662
–
664
.
2
Jager
KJ
,
Kovesdy
C
,
Langham
R
,
Rosenberg
M
,
Jha
V
,
Zoccali
C
.
A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases
.
Kidney Int
2019
;
96
:
1048
–
1050
.
3
Xie
Y
,
Bowe
B
,
Mokdad
AH
,
Xian
H
,
Yan
Y
,
Li
T
,
Maddukuri
G
,
Tsai
C-Y
,
Floyd
T
,
Al-Aly
Z
.
Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016
.
Kidney Int
2018
;
94
:
567
–
581
.
4
Omran
AR
.
The epidemiologic transition: a theory of the epidemiology of population change. 1971
.
Milbank Q
2005
;
83
:
731
–
757
.
5
Foreman
KJ
,
Marquez
N
,
Dolgert
A
,
Fukutaki
K
,
Fullman
N
,
McGaughey
M
,
Pletcher
MA
,
Smith
AE
,
Tang
K
,
Yuan
CW
,
Brown
JC
,
Friedman
J
,
He
J
,
Heuton
KR
,
Holmberg
M
,
Patel
DJ
,
Reidy
P
,
Carter
A
,
Cercy
K
,
Chapin
A
,
Douwes-Schultz
D
,
Frank
T
,
Goettsch
F
,
Liu
PY
,
Nandakumar
V
,
Reitsma
MB
,
Reuter
V
,
Sadat
N
,
Sorensen
RJD
,
Srinivasan
V
,
Updike
RL
,
York
H
,
Lopez
AD
,
Lozano
R
,
Lim
SS
,
Mokdad
AH
,
Vollset
SE
,
Murray
CJL
.
Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories
.
Lancet
2018
;
392
:
2052
–
2090
.
6
Keith
DS
,
Nichols
GA
,
Gullion
CM
,
Brown
JB
,
Smith
DH
.
Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization
.
Arch Intern Med
2004
;
164
:
659
–
663
.
7
Matsushita
K
,
van der Velde
M
,
Astor
BC
,
Woodward
M
,
Levey
AS
,
de Jong
PE
,
Coresh
J
,
Gansevoort
RT
,
El-Nahas
M
,
Eckardt
KU
,
Kasiske
BL
,
Tonelli
M
,
Hemmelgarn
B
,
Wang
Y
,
Atkins
RC
,
Polkinghorne
KR
,
Chadban
SJ
,
Shankar
A
,
Klein
R
,
Klein
BEK
,
Wang
H
,
Wang
F
,
Zhang
L
,
Liu
L
,
Shlipak
M
,
Sarnak
MJ
,
Katz
R
,
Fried
LP
,
Jafar
T
,
Islam
M
,
Hatcher
J
,
Poulter
N
,
Chaturvedi
N
,
Rothenbacher
D
,
Brenner
H
,
Raum
E
,
Koenig
W
,
Fox
CS
,
Hwang
SJ
,
Meigs
JB
,
Cirillo
M
,
Hallan
S
,
Lydersen
S
,
Holmen
J
,
Shlipak
M
,
Sarnak
MJ
,
Katz
R
,
Fried
LP
,
Roderick
P
,
Nitsch
D
,
Fletcher
A
,
Bulpitt
C
,
Ohkubo
T
,
Metoki
H
,
Nakayama
M
,
Kikuya
M
,
Imai
Y
,
Jassal
SK
,
Barrett-Connor
E
,
Bergstrom
J
,
Warnock
DG
,
Muntner
P
,
Judd
S
,
McClellan
WM
,
Cushman
M
,
Howard
G
,
McClure
LA
,
Jee
SH
,
Kimm
H
,
Yun
JE
,
Wen
CP
,
Wen
SF
,
Tsao
CK
,
Tsai
MK
,
Ärnlöv
J
,
Auguste
P
,
Veldhuis
K
,
Camarata
L
,
Thomas
B
,
Manley
T
.
Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis
.
Lancet
2010
;
375
:
2073
–
2081
.
9
Smith
DH
,
Thorp
ML
,
Gurwitz
JH
,
McManus
DD
,
Goldberg
RJ
,
Allen
LA
,
Hsu
G
,
Sung
SH
,
Magid
DJ
,
Go
AS
.
Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction
.
Circ Cardiovasc Qual Outcomes
2013
;
6
:
333
–
342
.
10
Antlanger
M
,
Aschauer
S
,
Kopecky
C
,
Hecking
M
,
Kovarik
JJ
,
Werzowa
J
,
Mascherbauer
J
,
Genser
B
,
Säemann
MD
,
Bonderman
D
.
Heart failure with preserved and reduced ejection fraction in hemodialysis patients: prevalence, disease prediction and prognosis
.
Kidney Blood Press Res
2017
;
42
:
165
–
176
.
11
Kelly
DM
,
Ademi
Z
,
Doehner
W
,
Lip
GYH
,
Mark
P
,
Toyoda
K
,
Wong
CX
,
Sarnak
M
,
Cheung
M
,
Herzog
CA
,
Johansen
KL
,
Reinecke
H
,
Sood
MM
.
Chronic kidney disease and cerebrovascular disease
.
Stroke
2021
;
52
:
e328-e346
.
12
Masson
P
,
Webster
AC
,
Hong
M
,
Turner
R
,
Lindley
RI
,
Craig
JC
.
Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis
.
Nephrol Dial Transplant
2015
;
30
:
1162
–
1169
.
13
Lee
M
,
Saver
JL
,
Chang
KH
,
Ovbiagele
B
.
Level of albuminuria and risk of stroke: systematic review and meta-analysis
.
Cerebrovasc Dis
2010
;
30
:
464
–
469
.
14
Lash
JP
,
Go
AS
,
Appel
LJ
,
He
J
,
Ojo
A
,
Rahman
M
,
Townsend
RR
,
Xie
D
,
Cifelli
D
,
Cohan
J
,
Fink
JC
,
Fischer
MJ
,
Gadegbeku
C
,
Hamm
LL
,
Kusek
JW
,
Landis
JR
,
Narva
A
,
Robinson
N
,
Teal
V
,
Feldman
HI
.
Chronic Renal Insufficiency Cohort (CRIC) study: baseline characteristics and associations with kidney function
.
Clin J Am Soc Nephrol
2009
;
4
:
1302
–
1311
.
15
Matsushita
K
,
Kwak
L
,
Ballew
SH
,
Grams
ME
,
Selvin
E
,
Folsom
AR
,
Coresh
J
,
Tang
W
.
Chronic kidney disease measures and the risk of abdominal aortic aneurysm
.
Atherosclerosis
2018
;
279
:
107
–
113
.
16
Garimella
PS
,
Hart
PD
,
O’Hare
A
,
DeLoach
S
,
Herzog
CA
,
Hirsch
AT
.
Peripheral artery disease and CKD: a focus on peripheral artery disease as a critical component of CKD care
.
Am J Kidney Dis
2012
;
60
:
641
–
654
.
17
U.S. Renal Data System
.
USRDS 2016 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD
,
2016
.
18
Fischer
MJ
,
Go
AS
,
Lora
CM
,
Ackerson
L
,
Cohan
J
,
Kusek
JW
,
Mercado
A
,
Ojo
A
,
Ricardo
AC
,
Rosen
LK
,
Tao
K
,
Xie
D
,
Feldman
HI
,
Lash
JP
,
CRIC and H-CRIC Study Groups
.
CKD in hispanics: baseline characteristics from the CRIC (chronic renal insufficiency cohort) and hispanic-CRIC studies
.
Am J Kidney Dis
.
2011
;
58
:
214
–
227
.
19
Hill
NR
,
Fatoba
ST
,
Oke
JL
,
Hirst
JA
,
O’Callaghan
CA
,
Lasserson
DS
,
Hobbs
FDR
.
Global prevalence of chronic kidney disease—a systematic review and meta-analysis
.
PLoS One
2016
;
11
(
7
):
e0158765
.
20
Qirjazi
E
,
Salerno
FR
,
Akbari
A
,
Hur
L
,
Penny
J
,
Scholl
T
,
McIntyre
CW
.
Tissue sodium concentrations in chronic kidney disease and dialysis patients by lower leg sodium-23 magnetic resonance imaging
.
Nephrol Dial Transplant
2021
;
36
:
1234
–
1243
.
21
Machnik
A
,
Neuhofer
W
,
Jantsch
J
,
Dahlmann
A
,
Tammela
T
,
Machura
K
,
Park
J-K
,
Beck
F-X
,
Müller
DN
,
Derer
W
,
Goss
J
,
Ziomber
A
,
Dietsch
P
,
Wagner
H
,
van Rooijen
N
,
Kurtz
A
,
Hilgers
KF
,
Alitalo
K
,
Eckardt
K-U
,
Luft
FC
,
Kerjaschki
D
,
Titze
J
.
Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C–dependent buffering mechanism
.
Nat Med
2009
;
15
:
545
–
552
.
22
Słabiak-Błaż
N
,
Piecha
G
.
Endogenous mammalian cardiotonic steroids—a new cardiovascular risk factor? —a mini-review
.
Life
2021
;
11
(
8
):
727
.
23
Inserra
F
,
Forcada
P
,
Castellaro
A
,
Castellaro
C
.
Chronic kidney disease and arterial stiffness: a two-way path
.
Front Med (Lausanne)
2021
;
8
:
765924
.
24
Chue
CD
,
Townend
JN
,
Steeds
RP
,
Ferro
CJ
.
Arterial stiffness in chronic kidney disease: causes and consequences
.
Heart
2010
;
96
:
817
–
823
.
25
Roumeliotis
S
,
Mallamaci
F
,
Zoccali
C
.
Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: a 2020 update
.
J Clin Med
2020
;
9
(
8
):
2359
.
26
Ligtenberg
G
,
Blankestijn
PJ
,
Oey
PL
,
Klein
IHH
,
Dijkhorst-Oei
L-T
,
Boomsma
F
,
Wieneke
GH
,
van Huffelen
AC
,
Koomans
HA
.
Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure
.
N Engl J Med
1999
;
340
:
1321
–
1328
.
27
Grassi
G
,
Quarti-Trevano
F
,
Seravalle
G
,
Arenare
F
,
Volpe
M
,
Furiani
S
,
Dell’Oro
R
,
Mancia
G
.
Early sympathetic activation in the initial clinical stages of chronic renal failure
.
Hypertension
2011
;
57
:
846
–
851
.
28
Converse
RL
,
Jacobsen
TN
,
Toto
RD
,
Jost
CM
,
Cosentino
F
,
Fouad-Tarazi
F
,
Victor
RG
.
Sympathetic overactivity in patients with chronic renal failure
.
N Engl J Med
1992
;
327
:
1912
–
1918
.
29
Park
J
,
Campese
VM
,
Nobakht
N
,
Middlekauff
HR
.
Differential distribution of muscle and skin sympathetic nerve activity in patients with end-stage renal disease
.
J Appl Physiol
2008
;
105
:
1873
–
1876
.
30
Hausberg
M
,
Kosch
M
,
Harmelink
P
,
Barenbrock
M
,
Hohage
H
,
Kisters
K
,
Dietl
KH
,
Rahn
KH
.
Sympathetic nerve activity in end-stage renal disease
.
Circulation
2002
;
106
:
1974
–
1979
.
31
Must
A
,
Spadano
J
,
Coakley
EH
,
Field
AE
,
Colditz
G
,
Dietz
WH
.
The disease burden associated with overweight and obesity
.
JAMA
1999
;
282
:
1523
–
1529
.
33
Li
H
,
Lu
W
,
Wang
A
,
Jiang
H
,
Lyu
J
.
Changing epidemiology of chronic kidney disease as a result of type 2 diabetes mellitus from 1990 to 2017: estimates from global burden of disease 2017
.
J Diabetes Investig
2021
;
12
:
346
–
356
.
34
Tsalamandris
S
,
Antonopoulos
AS
,
Oikonomou
E
,
Papamikroulis
G-A
,
Vogiatzi
G
,
Papaioannou
S
,
Deftereos
S
,
Tousoulis
D
.
The role of inflammation in diabetes: current concepts and future perspectives
.
Eur Cardiol
2019
;
14
:
50
–
59
.
35
Perrone
A
,
Giovino
A
,
Benny
J
,
Martinelli
F
.
Advanced glycation end products (AGEs): biochemistry, signaling, analytical methods, and epigenetic effects
.
Oxid Med Cell Longev
2020
;
2020
:
3818196
.
36
Juge-Aubry
CE
,
Somm
E
,
Pernin
A
,
Alizadeh
N
,
Giusti
V
,
Dayer
JM
,
Meier
CA
.
Adipose tissue is a regulated source of interleukin-10
.
Cytokine
2005
;
29
:
270
–
274
.
37
Ouchi
N
,
Parker
JL
,
Lugus
JJ
,
Walsh
K
.
Adipokines in inflammation and metabolic disease
.
Nat Rev Immunol
2011
;
11
:
85
–
97
.
38
Kreiner
FF
,
Kraaijenhof
JM
,
von Herrath
M
,
Hovingh
GKK
,
von Scholten
BJ
.
Interleukin 6 in diabetes, chronic kidney disease, and cardiovascular disease: mechanisms and therapeutic perspectives
.
Expert Rev Clin Immunol
2022
;
18
:
377
–
389
.
39
Ridker
PM
,
Everett
BM
,
Thuren
T
,
MacFadyen
JG
,
Chang
WH
,
Ballantyne
C
,
Fonseca
F
,
Nicolau
J
,
Koenig
W
,
Anker
SD
,
Kastelein
JJP
,
Cornel
JH
,
Pais
P
,
Pella
D
,
Genest
J
,
Cifkova
R
,
Lorenzatti
A
,
Forster
T
,
Kobalava
Z
,
Vida-Simiti
L
,
Flather
M
,
Shimokawa
H
,
Ogawa
H
,
Dellborg
M
,
Rossi
PRF
,
Troquay
RPT
,
Libby
P
,
Glynn
RJ
.
Antiinflammatory therapy with canakinumab for atherosclerotic disease
.
N Engl J Med
2017
;
377
:
1119
–
1131
.
40
Kang
S
,
Tanaka
T
,
Narazaki
M
,
Kishimoto
T
.
Targeting interleukin-6 signaling in clinic
.
Immunity
2019
;
50
:
1007
–
1023
.
41
Scheithauer
TPM
,
Rampanelli
E
,
Nieuwdorp
M
,
Vallance
BA
,
Verchere
CB
,
van Raalte
DH
,
Herrema
H
.
Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes
.
Front Immunol
2020
;
11
:
571731
.
42
Koppe
L
,
Soulage
CO
.
The impact of dietary nutrient intake on gut microbiota in the progression and complications of chronic kidney disease
.
Kidney Int
2022
;
102
(
4
):
728
–
739
.
43
Vaziri
ND
.
Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences
.
Am J Physiol Renal Physiol
2006
;
290
:
F262
–
F272
.
44
Tai
R
,
Ohashi
Y
,
Mizuiri
S
,
Aikawa
A
,
Sakai
K
.
Association between ratio of measured extracellular volume to expected body fluid volume and renal outcomes in patients with chronic kidney disease: a retrospective single-center cohort study
.
BMC Nephrol
2014
;
15
(
1
):
189
.
45
Zoccali
C
,
Moissl
U
,
Chazot
C
,
Mallamaci
F
,
Tripepi
G
,
Arkossy
O
,
Wabel
P
,
Stuard
S
.
Chronic fluid overload and mortality in ESRD
.
J Am Soc Nephrol
2017
;
28
:
2491
–
2497
.
46
Mills
KT
,
Chen
J
,
Yang
W
,
Appel
LJ
,
Kusek
JW
,
Alper
A
,
Delafontaine
P
,
Keane
MG
,
Mohler
E
,
Ojo
A
,
Rahman
M
,
Ricardo
AC
,
Soliman
EZ
,
Steigerwalt
S
,
Townsend
R
,
He
J
;
Chronic Renal Insufficiency Cohort (CRIC) Study Investigators
.
Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease
.
JAMA
2016
;
315
:
2200
–
2210
.
47
Tsai
YC
,
Chiu
YW
,
Tsai
JC
,
Kuo
HT
,
Hung
CC
,
Hwang
SJ
,
Chen
TH
,
Kuo
MC
,
Chen
HC
.
Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD
.
Clin J Am Soc Nephrol
2015
;
10
:
39
–
46
.
48
Hausberg
M
,
Lang
D
,
Levers
A
,
Suwelack
B
,
Kisters
K
,
Tokmak
F
,
Barenbrock
M
,
Kosch
M
.
Sympathetic nerve activity in renal transplant patients before and after withdrawal of cyclosporine
.
J Hypertens
2006
;
24
:
957
–
964
.
49
Blankestijn
PJ
,
London
G
,
Fliser
D
,
Jager
KJ
,
Lindholm
B
,
Goldsmith
D
,
Wiecek
A
,
Suleymanlar
G
,
Agarwal
R
,
Ortiz
A
,
Massy
Z
,
Martinez-Castelao
A
,
Covic
A
,
Dekker
FW
,
Zoccali
C
.
Major pathways of the reno–cardiovascular link: the sympathetic and renin–angiotensin systems
.
Kidney Int Suppl
2011
;
1
:
13
–
16
.
50
Zoccali
C
,
Roumeliotis
S
,
Mallamaci
F
.
Sleep apnea as a cardiorenal risk factor in CKD and renal transplant patients
.
Blood Purif
2021
;
50
:
642
–
648
.
51
Kovesdy
CP
,
Furth
SL
,
Zoccali
C
;
World Kidney Day Steering Committee
,
Kam Tao Li
P
,
Garcia-Garcia
G
,
Benghanem-Gharbi
M
,
Bollaert
R
,
Dupuis
S
,
Erk
T
,
Kalantar-Zadeh
K
,
Kovesdy
C
,
Osafo
C
,
Riella
MC
,
Zakharova
E
.
Obesity and kidney disease: hidden consequences of the epidemic
.
Kidney Int
2017
;
91
:
260
–
262
.
52
Siddiqi
L
,
Prakken
NH
,
Velthuis
BK
,
Cramer
MJ
,
Oey
PL
,
Boer
P
,
Bots
ML
,
Blankestijn
PJ
.
Sympathetic activity in chronic kidney disease patients is related to left ventricular mass despite antihypertensive treatment
.
Nephrol Dial Transplant
2010
;
25
:
3272
–
3277
.
53
Zoccali
C
,
Mallamaci
F
,
Tripepi
G
,
Parlongo
S
,
Cutrupi
S
,
Benedetto
FA
,
Cataliotti
A
,
Malatino
LS
.
Norepinephrine and concentric hypertrophy in patients with end-stage renal disease
.
Hypertension
2002
;
40
:
41
–
46
.
54
Zoccali
C
,
Mallamaci
F
,
Parlongo
S
,
Cutrupi
S
,
Benedetto
FA
,
Tripepi
G
,
Bonanno
G
,
Rapisarda
F
,
Fatuzzo
P
,
Seminara
G
,
Cataliotti
A
,
Stancanelli
B
,
Malatino
LS
.
Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease
.
Circulation
2002
;
105
:
1354
–
1359
.
55
Fishbane
S
,
Spinowitz
B
.
Update on anemia in ESRD and earlier stages of CKD: core curriculum 2018
.
Am J Kidney Dis
2018
;
71
:
423
–
435
.
56
Awan
AA
,
Walther
CP
,
Richardson
PA
,
Shah
M
,
Winkelmayer
WC
,
Navaneethan
SD
.
Prevalence, correlates and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease
.
Nephrol Dial Transplant
2021
;
36
:
129
–
136
.
57
Ma
JZ
,
Ebben
J
,
Xia
H
,
Collins
AJ
.
Hematocrit level and associated mortality in hemodialysis patients
.
J Am Soc Nephrol
1999
;
10
:
610
–
619
.
58
Robinson
BM
,
Joffe
MM
,
Berns
JS
,
Pisoni
RL
,
Port
FK
,
Feldman
HI
.
Anemia and mortality in hemodialysis patients: accounting for morbidity and treatment variables updated over time
.
Kidney Int
2005
;
68
:
2323
–
2330
.
59
Brookhart
MA
,
Schneeweiss
S
,
Avorn
J
,
Bradbury
BD
,
Liu
J
,
Winkelmayer
WC
.
Comparative mortality risk of anemia management practices in incident hemodialysis patients
.
JAMA
2010
;
303
:
857
–
864
.
60
Levin
A
,
Thompson
CR
,
Ethier
J
,
Carlisle
EJF
,
Tobe
S
,
Mendelssohn
D
,
Burgess
E
,
Jindal
K
,
Barrett
B
,
Singer
J
,
Djurdjev
O
.
Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin
.
Am J Kidney Dis
1999
;
34
:
125
–
134
.
61
Foley
RN
,
Parfrey
PS
,
Harnett
JD
,
Kent
GM
,
Murray
DC
,
Barre
PE
.
The impact of anemia on cardiomyopathy, morbidity, and mortality in end- stage renal disease
.
Am J Kidney Dis
1996
;
28
:
53
–
61
.
62
Chapter 1: introduction and definition of CKD–MBD and the development of the guideline statements
.
Kidney Int
2009
;
76
:
S3
–
S8
.
63
Isakova
T
,
Wahl
P
,
Vargas
GS
,
Gutiérrez
OM
,
Scialla
J
,
Xie
H
,
Appleby
D
,
Nessel
L
,
Bellovich
K
,
Chen
J
,
Hamm
L
,
Gadegbeku
C
,
Horwitz
E
,
Townsend
RR
,
Anderson
CAM
,
Lash
JP
,
Hsu
CY
,
Leonard
MB
,
Wolf
M
.
Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease
.
Kidney Int
2011
;
79
:
1370
–
1378
.
64
Musgrove
J
,
Wolf
M
.
Regulation and effects of FGF23 in chronic kidney disease
.
Annu Rev Physiol
2020
;
82
:
365
–
390
.
65
Levin
A
,
Bakris
GL
,
Molitch
M
,
Smulders
M
,
Tian
J
,
Williams
LA
,
Andress
DL
.
Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease
.
Kidney Int
2007
;
71
:
31
–
38
.
66
Wolf
M
,
White
KE
.
Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease
.
Curr Opin Nephrol Hypertens
2014
;
23
:
411
–
419
.
67
Faul
C
,
Amaral
AP
,
Oskouei
B
,
Hu
MC
,
Sloan
A
,
Isakova
T
,
Gutiérrez
OM
,
Aguillon-Prada
R
,
Lincoln
J
,
Hare
JM
,
Mundel
P
,
Morales
A
,
Scialla
J
,
Fischer
M
,
Soliman
EZ
,
Chen
J
,
Go
AS
,
Rosas
SE
,
Nessel
L
,
Townsend
RR
,
Feldman
HI
,
Sutton
MSJ
,
Ojo
A
,
Gadegbeku
C
,
di Marco
GS
,
Reuter
S
,
Kentrup
D
,
Tiemann
K
,
Brand
M
,
Hill
JA
,
Moe
OW
,
Kuro-o
M
,
Kusek
JW
,
Keane
MG
,
Wolf
M
.
FGF23 induces left ventricular hypertrophy
.
J Clin Investig
2011
;
121
:
4393
–
4408
.
68
Xue
C
,
Yang
B
,
Zhou
C
,
Dai
B
,
Liu
Y
,
Mao
Z
,
Yu
S
,
Mei
C
.
Fibroblast growth factor 23 predicts all-cause mortality in a dose-response fashion in pre-dialysis patients with chronic kidney disease
.
Am J Nephrol
2017
;
45
:
149
–
159
.
69
Gao
S
,
Xu
J
,
Zhang
S
,
Jin
J
.
Meta-analysis of the association between fibroblast growth factor 23 and mortality and cardiovascular events in hemodialysis patients
.
Blood Purif
2019
;
47
(
Suppl 1
):
24
–
30
.
70
London
GM
,
de Vernejoul
MC
,
Fabiani
F
,
Marchais
SJ
,
Guerin
AP
,
Metivier
F
,
London
AM
,
Llach
F
.
Secondary hyperparathyroidism and cardiac hypertrophy in hemodialysis patients
.
Kidney Int
1987
;
32
:
900
–
907
.
71
Lishmanov
A
,
Dorairajan
S
,
Pak
Y
,
Chaudhary
K
,
Chockalingam
A
.
Elevated serum parathyroid hormone is a cardiovascular risk factor in moderate chronic kidney disease
.
Int Urol Nephrol
2012
;
44
:
541
–
547
.
72
Ganesh
SK
,
Stack
AG
,
Levin
NW
,
Hulbert-Shearon
T
,
Port
FK
.
Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients
.
J Am Soc Nephrol
2001
;
12
:
2131
–
2138
.
73
Drechsler
C
,
Pilz
S
,
Obermayer-Pietsch
B
,
Verduijn
M
,
Tomaschitz
A
,
Krane
V
,
Espe
K
,
Dekker
F
,
Brandenburg
V
,
März
W
,
Ritz
E
,
Wanner
C
.
Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients
.
Eur Heart J
2010
;
31
:
2253
–
2261
.
74
Russo
D
,
Corrao
S
,
Battaglia
Y
,
Andreucci
M
,
Caiazza
A
,
Carlomagno
A
,
Lamberti
M
,
Pezone
N
,
Pota
A
,
Russo
L
,
Sacco
M
,
Scognamiglio
B
.
Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis
.
Kidney Int
2011
;
80
:
112
–
118
.
75
Bashir
A
,
Moody
WE
,
Edwards
NC
,
Ferro
CJ
,
Townend
JN
,
Steeds
RP
.
Coronary artery calcium assessment in CKD: utility in cardiovascular disease risk assessment and treatment?
Am J Kidney Dis
2015
;
65
:
937
–
948
.
76
Vervloet
M
,
Cozzolino
M
.
Vascular calcification in chronic kidney disease: different bricks in the wall?
Kidney Int
2017
;
91
:
808
–
817
.
77
Kovesdy
CP
,
Anderson
JE
,
Kalantar-Zadeh
K
.
Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD
.
Nephrol Dial Transplant
2009
;
24
:
1232
–
1237
.
78
Navaneethan
SD
,
Schold
JD
,
Arrigain
S
,
Jolly
SE
,
Wehbe
E
,
Raina
R
,
Simon
JF
,
Srinivas
TR
,
Jain
A
,
Schreiber
MJ
,
Nally
JV
.
Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease
.
Clin J Am Soc Nephrol
2011
;
26
:
2512
–
2530
.
79
Raphael
KL
,
Zhang
Y
,
Wei
G
,
Greene
T
,
Cheung
AK
,
Beddhu
S
.
Serum bicarbonate and mortality in adults in NHANES III
.
Nephrol Dial Transplant
2013
;
28
:
1207
–
1213
.
80
Collister
D
,
Ferguson
TW
,
Funk
SE
,
Reaven
NL
,
Mathur
V
,
Tangri
N
.
Metabolic acidosis and cardiovascular disease in CKD
.
Kidney Med
2021
;
3
:
753
–
761.e1
.
81
Sarnak
MJ
,
Poindexter
A
,
Wang
SR
,
Beck
GJ
,
Kusek
JW
,
Marcovina
SM
,
Greene
T
,
Levey
AS
.
Serum C-reactive protein and leptin as predictors of kidney disease progression in the modification of diet in renal disease study
.
Kidney Int
2002
;
62
:
2208
–
2215
.
82
Stenvinkel
P
,
Wanner
C
,
Metzger
T
,
Heimbürger
O
,
Mallamaci
F
,
Tripepi
G
,
Malatino
L
,
Zoccali
C
.
Inflammation and outcome in end-stage renal failure: does female gender constitute a survival advantage?
Kidney Int
2002
;
62
:
1791
–
1798
.
83
Himmelfarb
J
.
Linking oxidative stress and inflammation in kidney disease: which is the chicken and which is the egg?
Semin Dial
2004
;
17
:
449
–
454
.
84
Dalrymple
LS
,
Go
AS
.
Epidemiology of acute infections among patients with chronic kidney disease
.
Clin J Am Soc Nephrol
2008
;
3
:
1487
–
1493
.
85
Jazani
NH
,
Savoj
J
,
Lustgarten
M
,
Lau
WL
,
Vaziri
ND
.
Impact of gut dysbiosis on neurohormonal pathways in chronic kidney disease
.
Diseases
2019
;
7
(
1
):
21
.
86
Eustace
JA
,
Astor
B
,
Muntner
PM
,
Ikizler
TA
,
Coresh
J
.
Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease
.
Kidney Int
2004
;
65
:
1031
–
1040
.
87
Poole
S
,
Bird
TA
,
Selkirk
S
,
Gaines-Das
RE
,
Choudry
Y
,
Stephenson
SL
,
John Kenny
A
,
Saklatvaa
J
.
Fate of injected interleukin 1 in rats: sequestration and degradation in the kidney
.
Cytokine
1990
;
2
:
416
–
422
.
88
Bemelmans
MH
,
Gouma
DJ
,
Buurman
WA
.
Influence of nephrectomy on tumor necrosis factor clearance in a murine model
.
J Immunol
1993
;
150
:
2007
–
2017
.
89
Cobo
G
,
Lindholm
B
,
Stenvinkel
P
.
Chronic inflammation in end-stage renal disease and dialysis
.
Nephrol Dial Transplant
2018
;
33
(
suppl 3
):
iii35
–
iii40
.
90
Li
WJ
,
Chen
XM
,
Nie
XY
,
Zhang
J
,
Cheng
YJ
,
Lin
XX
,
Wu
SH
.
Cardiac troponin and C-reactive protein for predicting all-cause and cardiovascular mortality in patients with chronic kidney disease: a meta-analysis
.
Clinics
2015
;
70
:
301
–
311
.
91
Avesani
CM
,
Draibe
SA
,
Kamimura
MA
,
Basile Colugnati
FA
,
Cuppari
L
.
Resting energy expenditure of chronic kidney disease patients: influence of renal function and subclinical inflammation
.
Am J Kidney Dis
2004
;
44
:
1008
–
1016
.
92
Slee
AD
.
Exploring metabolic dysfunction in chronic kidney disease
.
Nutr Metab (Lond)
2012
;
9
:
1
–
16
.
93
Jelkmann
W
.
Proinflammatory cytokines lowering erythropoietin production
.
J Interferon Cytokine Res
1998
;
18
:
555
–
559
.
94
Fouque
D
,
Kalantar-Zadeh
K
,
Kopple
J
,
Cano
N
,
Chauveau
P
,
Cuppari
L
,
Franch
H
,
Guarnieri
G
,
Ikizler
TA
,
Kaysen
G
,
Lindholm
B
,
Massy
Z
,
Mitch
W
,
Pineda
E
,
Stenvinkel
P
,
Trevinho-Becerra
A
,
Wanner
C
.
A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease
.
Kidney Int
2008
;
73
:
391
–
398
.
95
Kalantar-Zadeh
K
,
Ikizler
TA
,
Block
G
,
Avram
MM
,
Kopple
JD
.
Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences
.
Am J Kidney Dis
2003
;
42
:
864
–
881
.
96
Zoccali
C
,
Goldsmith
D
,
Agarwal
R
,
Blankestijn
PJ
,
Fliser
D
,
Wiecek
A
,
Suleymanlar
G
,
Ortiz
A
,
Massy
Z
,
Covic
A
,
Martinez-Castelao
A
,
Jager
KJ
,
Dekker
FW
,
Lindholm
B
,
London
G
.
The complexity of the cardio–renal link: taxonomy, syndromes, and diseases
.
Kidney Int Suppl
2011
;
1
:
2
–
5
.
97
Ridker
PM
,
MacFadyen
JG
,
Glynn
RJ
,
Koenig
W
,
Libby
P
,
Everett
BM
,
Lefkowitz
M
,
Thuren
T
,
Cornel
JH
.
Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease
.
J Am Coll Cardiol
2018
;
71
:
2405
–
2414
.
98
Ridker
PM
,
Tuttle
KR
,
Perkovic
V
,
Libby
P
,
MacFadyen
JG
.
Inflammation drives residual risk in chronic kidney disease: a CANTOS substudy
.
Eur Heart J
2022
;
43
(
46
):
4832
–
4844
.
99
Ridker
PM
,
Devalaraja
M
,
Baeres
FMM
,
Engelmann
MDM
,
Hovingh
GK
,
Ivkovic
M
,
Lo
L
,
Kling
D
,
Pergola
P
,
Raj
D
,
Libby
P
,
Davidson
M
.
IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial
.
Lancet
2021
;
397
:
2060
–
2069
.
100
Beddhu
S
,
Baird
BC
,
Zitterkoph
J
,
Neilson
J
,
Greene
T
.
Physical activity and mortality in chronic kidney disease (NHANES III)
.
Clin J Am Soc Nephrol
2009
;
4
:
1901
–
1906
.
101
Martins
P
,
Marques
EA
,
Leal
DV
,
Ferreira
A
,
Wilund
KR
,
Viana
JL
.
Association between physical activity and mortality in end-stage kidney disease: a systematic review of observational studies
.
BMC Nephrol
2021
;
22
(
1
):
227
.
102
Odutayo
A
,
Wong
CX
,
Farkouh
M
,
Altman
DG
,
Hopewell
S
,
Emdin
CA
,
Hunn
BH
.
AKI and long-term risk for cardiovascular events and mortality
.
J Am Soc Nephrol
2017
;
28
:
377
–
387
.
103
Duranton
F
,
Cohen
G
,
de Smet
R
,
Rodriguez
M
,
Jankowski
J
,
Vanholder
R
,
Argiles
A
.
Normal and pathologic concentrations of uremic toxins
.
J Am Soc Nephrol
2012
;
23
:
1258
–
1270
.
104
Zhang
H
,
Xiang
S
,
Dai
Z
,
Fan
Y
.
Asymmetric dimethylarginine level as biomarkers of cardiovascular or all-cause mortality in patients with chronic kidney disease: a meta-analysis
.
Biomarkers
2021
;
26
:
579
–
585
.
105
Ye
J
,
Dai
Y
,
Mao
H
,
Zheng
W
,
Zhang
J
.
Prognostic value of asymmetric dimethylarginine in patients with coronary artery disease: a meta-analysis
.
Nitric Oxide
2021
;
109-110
:
50
–
56
.
106
Liabeuf
S
,
Lenglet
A
,
Desjardins
L
,
Neirynck
N
,
Glorieux
G
,
Lemke
HD
,
Vanholder
R
,
Diouf
M
,
Choukroun
G
,
Massy
ZA
.
Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients
.
Kidney Int
2012
;
82
:
1297
–
1303
.
107
Nakamura
T
,
Kawagoe
Y
,
Matsuda
T
,
Ueda
Y
,
Shimada
N
,
Ebihara
I
,
Koide
H
.
Oral adsorbent AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure
.
Kidney Blood Press Res
2004
;
27
:
121
–
126
.
108
Vanholder
R
,
Schepers
E
,
Pletinck
A
,
Nagler
EV
,
Glorieux
G
.
The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review
.
J Am Soc Nephrol
2014
;
25
:
1897
–
1907
.
109
Zoccali
C
,
Vanholder
R
,
Massy
ZA
,
Ortiz
A
,
Sarafidis
P
,
Dekker
FW
,
Fliser
D
,
Fouque
D
,
Heine
GH
,
Jager
KJ
,
Kanbay
M
,
Mallamaci
F
,
Parati
G
,
Rossignol
P
,
Wiecek
A
,
London
G
.
The systemic nature of CKD
.
Nat Rev Nephrol
2017
;
13
:
344
–
358
.
110
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group
.
KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease
.
Kidney Int
2021
;
99
:
S1
–
S87
.
111
Meuleman
Y
,
Hoekstra
T
,
Dekker
FW
,
Navis
G
,
Vogt
L
,
van der Boog
PJM
,
Bos
WJW
,
van Montfrans
GA
,
van Dijk
S
,
Boeschoten
EW
,
Verduijn
M
,
ten Brinke
L
,
Spijker
A
,
Kwakernaak
AJ
,
Humalda
JK
,
van Hirtum
T
,
Bokelaar
R
,
Loos
ML
,
Bakker-Edink
A
,
Poot
C
,
Ciere
Y
,
Zwaard
S
,
Veldscholte
G
,
Heuveling
L
,
Storm
M
,
Prantl
K
.
Sodium restriction in patients with CKD: a randomized controlled trial of self-management support
.
Am J Kidney Dis
2017
;
69
:
576
–
586
.
112
Panuccio
V
,
Mallamaci
F
,
Pizzini
P
,
Tripepi
R
,
Garofalo
C
,
Parlongo
G
,
Caridi
G
,
Provenzano
M
,
Mafrica
A
,
Simone
G
,
Cutrupi
S
,
D’Arrigo
G
,
Porto
G
,
Tripepi
G
,
Nardellotto
A
,
Meneghel
G
,
Dattolo
P
,
Pizzarelli
F
,
Rapisarda
F
,
Ricchiuto
A
,
Fatuzzo
P
,
Verdesca
S
,
Gallieni
M
,
Gesualdo
L
,
Conte
G
,
Plebani
M
,
Zoccali
C
.
Reducing salt intake by urine chloride self-measurement in non-compliant patients with chronic kidney disease followed in nephrology clinics: a randomized trial
.
Nephrol Dial Transplant
2021
;
36
:
1192
–
1199
.
113
Agarwal
R
,
Sinha
AD
,
Cramer
AE
,
Balmes-Fenwick
M
,
Dickinson
JH
,
Ouyang
F
,
Tu
W
.
Chlorthalidone for hypertension in advanced chronic kidney disease
.
N Engl J Med
2021
;
385
:
2507
–
2519
.
114
De Nicola
L
,
Minutolo
R
,
Chiodini
P
,
Zoccali
C
,
Castellino
P
,
Donadio
C
,
Strippoli
M
,
Casino
F
,
Giannattasio
M
,
Petrarulo
F
,
Virgilio
M
,
Laraia
E
,
di Iorio
BR
,
Savica
V
,
Conte
G
.
Global approach to cardiovascular risk in chronic kidney disease: reality and opportunities for intervention
.
Kidney Int
2006
;
69
:
538
–
545
.
115
Felker
GM
,
Anstrom
KJ
,
Adams
KF
,
Ezekowitz
JA
,
Fiuzat
M
,
Houston-Miller
N
,
Januzzi
JL
,
Mark
DB
,
Piña
IL
,
Passmore
G
,
Whellan
DJ
,
Yang
H
,
Cooper
LS
,
Leifer
ES
,
Desvigne-Nickens
P
,
O’Connor
CM
.
Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial
.
JAMA
2017
;
318
:
713
–
720
.
116
Khan
YH
,
Sarriff
A
,
Adnan
AS
,
Khan
AH
,
Mallhi
TH
.
Chronic kidney disease, fluid overload and diuretics: a complicated triangle
.
PLoS One
2016
;
11
(
7
):
e0159335
.
117
Verdalles
U
,
de Vinuesa
SG
,
Goicoechea
M
,
Quiroga
B
,
Reque
J
,
Panizo
N
,
Arroyo
D
,
Luño
J
.
Utility of bioimpedance spectroscopy (BIS) in the management of refractory hypertension in patients with chronic kidney disease (CKD)
.
Nephrol Dial Transplant
2012
;
27
(
Suppl 4
):
iv31
–
iv35
.
118
Zoccali
C
,
Mallamaci
F
,
Picano
E
.
Detecting and treating lung congestion with kidney failure
.
Clin J Am Soc Nephrol
2022
;
17
:
757
–
765
.
119
Zoccali
C
,
Torino
C
,
Mallamaci
F
,
Sarafidis
P
,
Papagianni
A
,
Ekart
R
,
Hojs
R
,
Klinger
M
,
Letachowicz
K
,
Fliser
D
,
Seiler-Mußler
S
,
Lizzi
F
,
Wiecek
A
,
Miskiewicz
A
,
Siamopoulos
K
,
Balafa
O
,
Slotki
I
,
Shavit
L
,
Stavroulopoulos
A
,
Covic
A
,
Siriopol
D
,
Massy
ZA
,
Seidowsky
A
,
Battaglia
Y
,
Martinez-Castelao
A
,
Polo-Torcal
C
,
Coudert-Krier
MJ
,
Rossignol
P
,
Fiaccadori
E
,
Regolisti
G
,
Hannedouche
T
,
Bachelet
T
,
Jager
KJ
,
Dekker
FW
,
Tripepi
R
,
Tripepi
G
,
Gargani
L
,
Sicari
R
,
Picano
E
,
London
GM
.
A randomized multicenter trial on a lung ultrasound–guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk
.
Kidney Int
2021
;
100
:
1325
–
1333
.
120
Saravanan
P
,
Davidson
NC
.
Risk assessment for sudden cardiac death in dialysis patients
.
Circ Arrhythm Electrophysiol
2010
;
3
:
553
–
559
.
121
SPRINT Research Group
;
Wright
JT
Jr,
Williamson
JD
,
Whelton
PK
,
Snyder
JK
,
Sink
KM
,
Rocco
MV
,
Reboussin
DM
,
Rahman
M
,
Oparil
S
,
Lewis
CE
,
Kimmel
PL
,
Johnson
KC
,
Goff
DC
Jr,
Fine
LJ
.
A randomized trial of intensive versus standard blood-pressure control
.
N Engl J Med
2015
;
373
:
2103
–
2116
.
122
Dasgupta
I
,
Zoccali
C
.
Is the KDIGO systolic blood pressure target <120 mm Hg for chronic kidney disease appropriate in routine clinical practice?
Hypertension
2022
;
79
:
4
–
11
.
123
Whelton
PK
,
Carey
RM
,
Aronow
WS
,
Casey
DE
Jr,
Collins
KJ
,
Himmelfarb
CD
,
DePalma
SM
,
Gidding
S
,
Jamerson
JA
,
Jones
DW
,
MacLaughlin
EJ
,
Muntner
P
,
Ovbiagele
B
,
Smith
SC
Jr,
Spencer
CC
,
Stafford
RS
,
Taler
SJ
,
Thomas
RJ
,
Williams
KA
Sr,
Williamson
JD
,
Wright
JT
Jr.
ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines
.
Hypertension
.
2017
;
71
:
1269
–
1324
.
124
Pisano
A
,
Bolignano
D
,
Mallamaci
F
,
D’Arrigo
G
,
Halimi
JM
,
Persu
A
,
Wuerzner
G
,
Sarafidis
P
,
Watschinger
B
,
Burnier
M
,
Zoccali
C
.
Comparative effectiveness of different antihypertensive agents in kidney transplantation: a systematic review and meta-analysis
.
Nephrol Dial Transplant
2020
;
35
:
878
–
887
.
125
Sarafidis
PA
,
Persu
A
,
Agarwal
R
,
Burnier
M
,
de Leeuw
P
,
Ferro
CJ
,
Halimi
J-M
,
Heine
GH
,
Jadoul
M
,
Jarraya
F
,
Kanbay
M
,
Mallamaci
F
,
Mark
PB
,
Ortiz
A
,
Parati
G
,
Pontremoli
R
,
Rossignol
P
,
Ruilope
L
,
van der Niepen
P
,
Vanholder
R
,
Verhaar
MC
,
Wiecek
A
,
Wuerzner
G
,
London
GM
,
Zoccali
C
.
Hypertension in dialysis patients: a consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) and the hypertension and the kidney working group of the European Society of Hypertension (ESH)
.
Nephrol Dial Transplant
2017
;
32
:
620
–
640
.
126
Agarwal
R
,
Flynn
J
,
Pogue
V
,
Rahman
M
,
Reisin
E
,
Weir
MR
.
Assessment and management of hypertension in patients on dialysis
.
J Am Soc Nephrol
2014
;
25
:
1630
–
1646
.
127
Liu
Y
,
Ma
X
,
Zheng
J
,
Jia
J
,
Yan
T
.
Effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on cardiovascular events and residual renal function in dialysis patients: a meta-analysis of randomised controlled trials
.
BMC Nephrol
2017
;
18
(
1
):
206
.
128
Agarwal
R
,
Sinha
AD
,
Pappas
MK
,
Abraham
TN
,
Tegegne
GG
.
Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial
.
Nephrol Dial Transplant
2014
;
29
:
672
–
681
.
129
MacKinnon
HJ
,
Wilkinson
TJ
,
Clarke
AL
,
Gould
DW
,
O’Sullivan
TF
,
Xenophontos
S
,
Watson
EL
,
Singh
SJ
,
Smith
AC
.
The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review
.
Ther Adv Chronic Dis
2018
;
9
:
209
–
226
.
130
Mallamaci
F
,
D’Arrigo
G
,
Tripepi
G
,
Lamberti
N
,
Torino
C
,
Manfredini
F
,
Zoccali
C
.
Long-term effect of physical exercise on the risk for hospitalization and death in dialysis patients: a post-trial long-term observational study
.
Clin J Am Soc Nephrol
2022
;
17
:
1176
–
1182
.
131
Manfredini
F
,
Mallamaci
F
,
D’Arrigo
G
,
Baggetta
R
,
Bolignano
D
,
Torino
C
,
Lamberti
N
,
Bertoli
S
,
Ciurlino
D
,
Rocca-Rey
L
,
Barillà
A
,
Battaglia
Y
,
Rapanà
RM
,
Zuccalà
A
,
Bonanno
G
,
Fatuzzo
P
,
Rapisarda
F
,
Rastelli
S
,
Fabrizi
F
,
Messa
P
,
de Paola
L
,
Lombardi
L
,
Cupisti
A
,
Fuiano
G
,
Lucisano
G
,
Summaria
C
,
Felisatti
M
,
Pozzato
E
,
Malagoni
AM
,
Castellino
P
,
Aucella
F
,
Elhafeez
SA
,
Provenzano
PF
,
Tripepi
G
,
Catizone
L
,
Zoccali
C
.
Exercise in patients on dialysis: a multicenter, randomized clinical trial
.
J Am Soc Nephrol
2017
;
28
:
1259
–
1268
.
132
Erratum: Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). kidney int suppl. 2017;7:1-59
.
Kidney Int Suppl
2017
;
7
(
3
):
e1
.
133
Floege
J
.
Phosphate binders in chronic kidney disease: an updated narrative review of recent data
.
J Nephrol
2020
;
33
:
497
–
508
.
134
Oliveira
RB
,
Cancela
ALE
,
Graciolli
FG
,
dos Reis
LM
,
Draibe
SA
,
Cuppari
L
,
Carvalho
AB
,
Jorgetti
V
,
Canziani
ME
,
Moysés
RMA
.
Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy?
Clin J Am Soc Nephrol
2010
;
5
:
286
–
291
.
135
Koiwa
F
,
Kazama
JJ
,
Tokumoto
A
,
Onoda
N
,
Kato
H
,
Okada
T
,
Nii-Kono
T
,
Fukagawa
M
,
Shigematsu
T
.
Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients
.
Ther Apher Dial
2005
;
9
:
336
–
339
.
136
Liu
Z
,
Su
G
,
Guo
X
,
Wu
Y
,
Liu
X
,
Zou
C
,
Zhang
L
,
Yang
Q
,
Xu
Y
,
Ma
W
.
Dietary interventions for mineral and bone disorder in people with chronic kidney disease
.
Cochrane Database Syst Rev
2015
;
2015
(
9
):
CD010350
.
137
Srour
B
,
Fezeu
LK
,
Kesse-Guyot
E
,
Allès
B
,
Méjean
C
,
Andrianasolo
RM
,
Chazelas
E
,
Deschasaux
M
,
Hercberg
S
,
Galan
P
,
Monteiro
CA
,
Julia
C
,
Touvier
M
.
Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé)
.
BMJ
2019
;
365
:
l1451
.
138
Messa
P
,
Cerutti
R
,
Brezzi
B
,
Alfieri
C
,
Cozzolino
M
.
Calcium and phosphate control by dialysis treatments
.
Blood Purif
2009
;
27
:
360
–
368
.
139
EVOLVE Trial Investigators
;
Chertow
GM
,
Block
GA
,
Correa-Rotter
R
,
Drüeke
TB
,
Floege
J
,
Goodman
WG
,
Herzog
CA
,
Kubo
Y
,
London
GM
,
Mahaffey
KW
,
Christian
T
,
Mix
H
,
Moe
SM
,
Trotman
M-L
,
Wheeler
DC
,
Parfrey
PS
.
Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis
.
N Engl J Med
2012
;
367
:
2482
–
2494
.
140
Dörr
K
,
Kammer
M
,
Reindl-Schwaighofer
R
,
Lorenz
M
,
Prikoszovich
T
,
Marculescu
R
,
Beitzke
D
,
Wielandner
A
,
Erben
RG
,
Oberbauer
R
.
Randomized trial of etelcalcetide for cardiac hypertrophy in hemodialysis
.
Circ Res
2021
;
128
:
1616
–
1625
.
141
Badve
SV
,
Palmer
SC
,
Strippoli
GFM
,
Roberts
MA
,
Teixeira-Pinto
A
,
Boudville
N
,
Sci
M
,
Cass
A
,
Hawley
CM
,
Hiremath
SS
,
Pascoe
EM
,
Biostat
M
,
Perkovic
V
,
Whalley
GA
,
Craig
JC
,
Johnson
DW
.
The validity of left ventricular mass as a surrogate end point for all-cause and cardiovascular mortality outcomes in people with CKD: a systematic review and meta-analysis
.
Am J Kidney Dis
2016
;
68
:
554
–
563
.
142
Costa-Hong
V
,
Jorgetti
V
,
Gowdak
LHW
,
Moyses
RMA
,
Krieger
EM
,
de Lima
JJG
.
Parathyroidectomy reduces cardiovascular events and mortality in renal hyperparathyroidism
.
Surgery
2007
;
142
:
699
–
703
.
143
Komaba
H
,
Taniguchi
M
,
Wada
A
,
Iseki
K
,
Tsubakihara
Y
,
Fukagawa
M
.
Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism
.
Kidney Int
2015
;
88
:
350
–
359
.
144
Drüeke
TB
,
Locatelli
F
,
Clyne
N
,
Eckardt
K-U
,
Macdougall
IC
,
Tsakiris
D
,
Burger
H-U
,
Scherhag
A
.
Normalization of hemoglobin level in patients with chronic kidney disease and Anemia
.
N Engl J Med
2006
;
355
:
2071
–
2084
.
145
Pfeffer
MA
,
Burdmann
EA
,
Chen
C-Y
,
Cooper
ME
,
de Zeeuw
D
,
Eckardt
K-U
,
Feyzi
JM
,
Ivanovich
P
,
Kewalramani
R
,
Levey
AS
,
Lewis
EF
,
McGill
JB
,
McMurray
JJV
,
Parfrey
P
,
Parving
H-H
,
Remuzzi
G
,
Singh
AK
,
Solomon
SD
,
Toto
R
.
A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease
.
N Engl J Med
2009
;
361
:
2019
–
2032
.
146
Singh
AK
,
Szczech
L
,
Tang
KL
,
Barnhart
H
,
Sapp
S
,
Wolfson
M
,
Reddan
D
.
Correction of anemia with epoetin Alfa in chronic kidney disease
.
N Engl J Med
2006
;
355
:
2085
–
2098
.
147
Chertow
GM
,
Pergola
PE
,
Farag
YMK
,
Agarwal
R
,
Arnold
S
,
Bako
G
,
Block
GA
,
Burke
S
,
Castillo
FP
,
Jardine
AG
,
Khawaja
Z
,
Koury
MJ
,
Lewis
EF
,
Lin
T
,
Luo
W
,
Maroni
BJ
,
Matsushita
K
,
McCullough
PA
,
Parfrey
PS
,
Roy-Chaudhury
P
,
Sarnak
MJ
,
Sharma
A
,
Spinowitz
B
,
Tseng
C
,
Tumlin
J
,
Vargo
DL
,
Walters
KA
,
Winkelmayer
WC
,
Wittes
J
,
Eckardt
K-U
.
Vadadustat in patients with anemia and non–dialysis-dependent CKD
.
N Engl J Med
2021
;
384
:
1589
–
1600
.
148
Singh
AK
,
Carroll
K
,
McMurray
JJV
,
Solomon
S
,
Jha
V
,
Johansen
KL
,
Lopes
RD
,
Macdougall
IC
,
Obrador
GT
,
Waikar
SS
,
Wanner
C
,
Wheeler
DC
,
Więcek
A
,
Blackorby
A
,
Cizman
B
,
Cobitz
AR
,
Davies
R
,
DiMino
TL
,
Kler
L
,
Meadowcroft
AM
,
Taft
L
,
Perkovic
V
.
Daprodustat for the treatment of anemia in patients not undergoing dialysis
.
N Engl J Med
2021
;
385
:
2313
–
2324
.
149
Chen
N
,
Hao
C
,
Peng
X
,
Lin
H
,
Yin
A
,
Hao
L
,
Tao
Y
,
Liang
X
,
Liu
Z
,
C
,
Chen
J
,
Luo
L
,
Zuo
L
,
Liao
Y
,
Liu
B-C
,
Leong
R
,
Wang
C
,
Liu
C
,
Neff
T
,
Szczech
L
,
Yu
K-HP
.
Roxadustat for anemia in patients with kidney disease not receiving dialysis
.
N Engl J Med
2019
;
381
:
1001
–
1010
.
150
Macdougall
IC
,
White
C
,
Anker
SD
,
Bhandari
S
,
Farrington
K
,
Kalra
PA
,
McMurray
JJV
,
Murray
H
,
Tomson
CRV
,
Wheeler
DC
,
Winearls
CG
,
Ford
I
.
Intravenous iron in patients undergoing maintenance hemodialysis
.
N Engl J Med
2019
;
380
:
447
–
458
.
151
Ferro
CJ
,
Mark
PB
,
Kanbay
M
,
Sarafidis
P
,
Heine
GH
,
Rossignol
P
,
Massy
ZA
,
Mallamaci
F
,
Valdivielso
JM
,
Malyszko
J
,
Verhaar
MC
,
Ekart
R
,
Vanholder
R
,
London
G
,
Ortiz
A
,
Zoccali
C
.
Lipid management in patients with chronic kidney disease
.
Nat Rev Nephrol
2018
;
14
:
727
–
749
.
152
Herrington
WG
,
Emberson
J
,
Mihaylova
B
,
Blackwell
L
,
Reith
C
,
Solbu
MD
,
Mark
PB
,
Fellström
B
,
Jardine
AG
,
Wanner
C
,
Holdaas
H
,
Fulcher
J
,
Haynes
R
,
Landray
MJ
,
Keech
A
,
Simes
J
,
Collins
R
,
Baigent
C
.
Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials
.
Lancet Diabetes Endocrinol
2016
;
4
:
829
–
839
.
153
Guedeney
P
,
Giustino
G
,
Sorrentino
S
,
Claessen
BE
,
Camaj
A
,
Kalkman
DN
,
Vogel
B
,
Sartori
S
,
De Rosa
S
,
Baber
U
.
Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials
.
Eur Heart J
2022
;
43
:
e17
–
e25
.
154
Charytan
DM
,
Sabatine
MS
,
Pedersen
TR
,
Im
KA
,
Park
JG
,
Pineda
AL
,
Wasserman
SM
,
Deedwania
P
,
Olsson
AG
,
Sever
PS
,
Keech
AC
,
Giugliano
RP
.
Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER trial
.
J Am Coll Cardiol
2019
;
73
:
2961
–
2970
.
155
Fernandez-Prado
R
,
Perez-Gomez
MV
,
Ortiz
A
.
Pelacarsen for lowering lipoprotein(a): implications for patients with chronic kidney disease
.
Clin Kidney J
2020
;
13
:
753
–
757
.
156
Wilcox
CS
.
Antihypertensive and renal mechanisms of SGLT2 (sodium-glucose linked transporter 2) inhibitors
.
Hypertension
2020
;
75
:
894
–
901
.
157
Wanner
C
,
Inzucchi
SE
,
Lachin
JM
,
Fitchett
D
,
von Eynatten
M
,
Mattheus
M
,
Johansen
OE
,
Woerle
HJ
,
Broedl
UC
,
Zinman
B
.
Empagliflozin and progression of kidney disease in type 2 diabetes
.
N Engl J Med
2016
;
375
:
323
–
334
.
158
Perkovic
V
,
Jardine
MJ
,
Neal
B
,
Bompoint
S
,
Heerspink
HJL
,
Charytan
DM
,
Edwards
R
,
Agarwal
R
,
Bakris
G
,
Bull
S
,
Cannon
CP
,
Capuano
G
,
Chu
P-L
,
de Zeeuw
D
,
Greene
T
,
Levin
A
,
Pollock
C
,
Wheeler
DC
,
Yavin
Y
,
Zhang
H
,
Zinman
B
,
Meininger
G
,
Brenner
BM
,
Mahaffey
KW
.
Canagliflozin and renal outcomes in type 2 diabetes and nephropathy
.
N Engl J Med
2019
;
380
:
2295
–
2306
.
159
Heerspink
HJL
,
Stefánsson
BV
,
Correa-Rotter
R
,
Chertow
GM
,
Greene
T
,
Hou
F-F
,
Mann
JFE
,
McMurray
JJV
,
Lindberg
M
,
Rossing
P
,
Sjöström
CD
,
Toto
RD
,
Langkilde
A-M
,
Wheeler
DC
.
Dapagliflozin in patients with chronic kidney disease
.
N Engl J Med
2020
;
383
:
1436
–
1446
.
160
Packer
M
,
Anker
SD
,
Butler
J
,
Filippatos
G
,
Pocock
SJ
,
Carson
P
,
Januzzi
J
,
Verma
S
,
Tsutsui
H
,
Brueckmann
M
,
Jamal
W
,
Kimura
K
,
Schnee
J
,
Zeller
C
,
Cotton
D
,
Bocchi
E
,
Böhm
M
,
Choi
D-J
,
Chopra
V
,
Chuquiure
E
,
Giannetti
N
,
Janssens
S
,
Zhang
J
,
Gonzalez Juanatey
JR
,
Kaul
S
,
Brunner-La Rocca
H-P
,
Merkely
B
,
Nicholls
SJ
,
Perrone
S
,
Pina
I
,
Ponikowski
P
,
Sattar
N
,
Senni
M
,
Seronde
M-F
,
Spinar
J
,
Squire
I
,
Taddei
S
,
Wanner
C
,
Zannad
F
.
Cardiovascular and renal outcomes with empagliflozin in heart failure
.
N Engl J Med
2020
;
383
:
1413
–
1424
.
161
McMurray
JJV
,
Solomon
SD
,
Inzucchi
SE
,
Køber
L
,
Kosiborod
MN
,
Martinez
FA
,
Ponikowski
P
,
Sabatine
MS
,
Anand
IS
,
Bělohlávek
J
,
Böhm
M
,
Chiang
C-E
,
Chopra
VK
,
de Boer
RA
,
Desai
AS
,
Diez
M
,
Drozdz
J
,
Dukát
A
,
Ge
J
,
Howlett
JG
,
Katova
T
,
Kitakaze
M
,
Ljungman
CEA
,
Merkely
B
,
Nicolau
JC
,
O’Meara
E
,
Petrie
MC
,
Vinh
PN
,
Schou
M
,
Tereshchenko
S
,
Verma
S
,
Held
C
,
DeMets
DL
,
Docherty
KF
,
Jhund
PS
,
Bengtsson
O
,
Sjöstrand
M
,
Langkilde
A-M
.
Dapagliflozin in patients with heart failure and reduced ejection fraction
.
N Engl J Med
2019
;
381
:
1995
–
2008
.
162
Sattar
N
,
Lee
MMY
,
Kristensen
SL
,
Branch
KRH
,
del Prato
S
,
Khurmi
NS
,
Lam
CSP
,
Lopes
RD
,
McMurray
JJV
,
Pratley
RE
,
Rosenstock
J
,
Gerstein
HC
.
Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials
.
Lancet Diabetes Endocrinol
2021
;
9
:
653
–
662
.
163
Bakris
GL
,
Agarwal
R
,
Anker
SD
,
Pitt
B
,
Ruilope
LM
,
Rossing
P
,
Kolkhof
P
,
Nowack
C
,
Schloemer
P
,
Joseph
A
,
Filippatos
G
.
Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes
.
N Engl J Med
2020
;
383
:
2219
–
2229
.
164
Pitt
B
,
Filippatos
G
,
Agarwal
R
,
Anker
SD
,
Bakris
GL
,
Rossing
P
,
Joseph
A
,
Kolkhof
P
,
Nowack
C
,
Schloemer
P
,
Ruilope
LM
.
Cardiovascular events with finerenone in kidney disease and type 2 diabetes
.
N Engl J Med
2021
;
385
:
2252
–
2263
.
165
Okumura
N
,
Jhund
PS
,
Gong
J
,
Lefkowitz
MP
,
Rizkala
AR
,
Rouleau
JL
,
Shi
VC
,
Swedberg
K
,
Zile
MR
,
Solomon
SD
,
Packer
M
,
Mcmurray
JJV
.
Effects of sacubitril/valsartan in the PARADIGM-HF trial (prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure) according to background therapy
.
Circ Heart Fail
2016
;
9
(
9
):
e003212
.
166
Kang
H
,
Zhang
J
,
Zhang
X
,
Qin
G
,
Wang
K
,
Deng
Z
,
Fang
Y
,
Chen
G
.
Effects of sacubitril/valsartan in patients with heart failure and chronic kidney disease: a meta-analysis
.
Eur J Pharmacol
2020
;
884
:
173444
.
167
Anker
SD
,
Butler
J
,
Filippatos
G
,
Ferreira
JP
,
Bocchi
E
,
Böhm
M
,
Brunner–La Rocca
H-P
,
Choi
D-J
,
Chopra
V
,
Chuquiure-Valenzuela
E
,
Giannetti
N
,
Gomez-Mesa
JE
,
Janssens
S
,
Januzzi
JL
,
Gonzalez-Juanatey
JR
,
Merkely
B
,
Nicholls
SJ
,
Perrone
SV
,
Piña
IL
,
Ponikowski
P
,
Senni
M
,
Sim
D
,
Spinar
J
,
Squire
I
,
Taddei
S
,
Tsutsui
H
,
Verma
S
,
Vinereanu
D
,
Zhang
J
,
Carson
P
,
Lam
CSP
,
Marx
N
,
Zeller
C
,
Sattar
N
,
Jamal
W
,
Schnaidt
S
,
Schnee
JM
,
Brueckmann
M
,
Pocock
SJ
,
Zannad
F
,
Packer
M
.
Empagliflozin in heart failure with a preserved ejection fraction
.
N Engl J Med
2021
;
385
:
1451
–
1461
.
169
Burnier
M
,
Pruijm
M
,
Wuerzner
G
,
Santschi
V
.
Drug adherence in chronic kidney diseases and dialysis
.
Nephrol Dial Transplant
2015
;
30
:
39
–
44
.
170
Tesfaye
WH
,
Erku
D
,
Mekonnen
A
,
Tefera
YG
,
Castelino
R
,
Sud
K
,
Thomas
J
,
Obamiro
K
.
Medication non-adherence in chronic kidney disease: a mixed-methods review and synthesis using the theoretical domains framework and the behavioural change wheel
.
J Nephrol
2021
;
34
:
1091
–
1125
.
171
Cedillo-Couvert
EA
,
Ricardo
AC
,
Chen
J
,
Cohan
J
,
Fischer
MJ
,
Krousel-Wood
M
,
Kusek
JW
,
Lederer
S
,
Lustigova
E
,
Ojo
A
,
Porter
AC
,
Sharp
LK
,
Sondheimer
J
,
Diamantidis
C
,
Wang
X
,
Roy
J
,
Lash
JP
,
Appel
LJ
,
Feldman
HI
,
Go
AS
,
He
J
,
Kusek
JW
,
Lash
JP
,
Rahman
M
,
Rao
PS
,
Townsend
RR
.
Self-reported medication adherence and CKD progression
.
Kidney Int Rep
2018
;
3
:
645
–
651
.
172
Burnier
M
,
Egan
BM
.
Adherence in hypertension
.
Circ Res
2019
;
124
:
1124
–
1140
.
173
Kotsis
F
,
Schultheiss
UT
,
Wuttke
M
,
Schlosser
P
,
Mielke
J
,
Becker
MS
,
Oefner
PJ
,
Karoly
ED
,
Mohney
RP
,
Eckardt
KU
,
Sekula
P
,
Köttgen
A
.
Self-reported medication use and urinary drug metabolites in the German chronic kidney disease (GCKD) study
.
J Am Soc Nephrol
2021
;
32
(
9
):
2315
–
2329
.
174
Canaud
B
,
Kooman
JP
,
Selby
NM
,
Taal
MW
,
Francis
S
,
Maierhofer
A
,
Kopperschmidt
P
,
Collins
A
,
Kotanko
P
.
Dialysis-induced cardiovascular and multiorgan morbidity
.
Kidney Int Rep
2020
;
5
:
1856
–
1869
.
175
Meyring-Wösten
A
,
Zhang
H
,
Ye
X
,
Fuertinger
DH
,
Chan
L
,
Kappel
F
,
Artemyev
M
,
Ginsberg
N
,
Wang
Y
,
Thijssen
S
,
Kotanko
P
.
Intradialytic hypoxemia and clinical outcomes in patients on hemodialysis
.
Clin J Am Soc Nephrol
2016
;
11
:
616
–
625
.
176
Covic
A
,
Fliser
D
,
Goldsmith
D
,
Lindholm
B
,
London
G
,
Martinez
A
,
Suleymanlar
G
,
Wiecek
A
,
Zoccali
C
.
Promoting scientific collaboration and education in cardiovascular-renal medicine: EURECAM: an ERA-EDTA-based working group
.
Clin Kidney J
2009
;
2
:
522
–
525
.
177
Vanholder
R
,
Conway
PT
,
Gallego
D
,
Scheres
E
,
Wieringa
F
.
The European Kidney Health Alliance (EKHA) and the decade of the kidney
.
Nephrol Dial Transplant
2023
;
38
(
5
):
1113
–
1122
.
Author notes
Conflict of interest: None declared.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
Advertisement intended for healthcare professionals
Citations
Views
Altmetric
Email alerts
Related articles in PubMed
Citing articles via
More from Oxford Academic
Advertisement intended for healthcare professionals