Water deficit inhibits cell division and expression of transcripts involved in cell proliferation and endoreduplication in maize endosperm (original) (raw)
Journal Article
Department of Crop and Soil Sciences, Cornell University, Ithaca, NY 14853, USA
Search for other works by this author on:
Department of Crop and Soil Sciences, Cornell University, Ithaca, NY 14853, USA
Search for other works by this author on:
Accepted:
18 February 2001
Cite
Tim L. Setter, Brian A. Flannigan, Water deficit inhibits cell division and expression of transcripts involved in cell proliferation and endoreduplication in maize endosperm, Journal of Experimental Botany, Volume 52, Issue 360, 1 July 2001, Pages 1401–1408, https://doi.org/10.1093/jexbot/52.360.1401
Close
Navbar Search Filter Mobile Enter search term Search
Abstract
Water deficit at the early post‐pollination stage in cereal grains decreases endosperm cell division and, in turn, decreases the capacity for storage material accumulation. Post‐mitotic replication of nuclear DNA (endoreduplication) may also play a role in stress effects. To gain a better understanding of the extent to which cell proliferation and endoreduplication are affected by water deficit, nuclear numbers and size were examined in endosperms of maize (Zea mays L.) by flow cytometry and the transcript levels of genes which have recognized roles in the cell cycle were quantified. Water deficit from 5–13 d after pollination (DAP) decreased the rate of endosperm cell division by 90% and inhibited [3H]‐thymidine incorporation into DNA from 9–13 DAP. The proportion of nuclei engaging in endoreduplication and nuclear DNA content increased steadily from 9–13 DAP in controls, but water deficit initially increased the proportion of endoreduplicating nuclei at 9 DAP, then halted further entry into endoreduplication and S‐phase cycling from 9–13 DAP. Transcript levels of α‐tubulin, and the S‐phase gene products histone H3 and PCNA were not affected by water deficit until 13 DAP, whereas those of ZmCdc2, a cyclin dependent kinase (CDK) with regulatory roles in mitosis, were inhibited substantially from 9–13 DAP. Cell proliferation and associated processes were inhibited at initial stages of the stress episode, whereas endoreduplication and associated S‐phase processes were not inhibited until the stress was more advanced. It was concluded that endosperm mitosis has greater sensitivity than endoreduplication to water deficit.
Introduction
The storage capacity of developing cereal grains is established during the early stage of endosperm development by the processes of cell division, organelle proliferation and cell enlargement, which create the metabolic capacity and final volume of this tissue (Jones et al., 1996; Olsen et al., 1999). Environmental stresses in the initial period following fertilization inhibit these early events and, in turn, decrease final grain yield (Artlip et al., 1995; Commuri and Jones, 1999; Nicolas et al., 1985; Ober et al., 1991). During early endosperm development, the mitotic phase is followed by a developmental transition, after which cells engage in endoreduplication, a process of repeated rounds of S phase in the absence of mitosis (Grafi, 1998; Kowles et al., 1990). Endoreduplication coincides with the period of organelle proliferation and rapid expression of mRNAs for starch pathway enzymes and storage protein (Ober et al., 1991). It is thought to contribute to growth capacity as it is correlated with reproductive‐organ and cell size among contrasting genotypes and plants subjected to various stress treatments (Artlip et al., 1995; Cavallini et al., 1995; Cebolla et al., 1999; Lemontey et al., 2000). However, with respect to kernel growth and yield, the stage in which the number of endosperm cells is established is particularly sensitive to stress; whereas the later phases, when endoreduplication and starch accumulation occur, are relatively tolerant of stress (Ouattar et al., 1987; reviewed in Mambelli and Setter, 1998). Hence, there is a need to understand better the differential response of these developmental events to stress.
Knowledge of cell cycle regulation in eukaryotic cells has advanced considerably in recent years. The eukaryotic cell cycle is controlled by a family of protein kinases, each with a positive regulatory subunit, termed a cyclin, and a catalytic subunit, termed a cyclin dependent kinase (CDK) (reviewed in den Boer and Murray, 2000). There are several distinct cyclin/CDK pairs, each functioning at a specific phase(s) of the cell cycle (Roberts, 1999). The activity of each of these cyclin/CDK complexes oscillates with each turn of the cell cycle to control progress at each step and respond to signals arising from the environment, such as those created by stress. Given the specificity of these regulatory components for particular phases of the cell cycle, it is possible that during a stress episode mitotic cell cycling might be affected differently from cell‐cycle phases specific to endoreduplication.
There is evidence in maize (Zea mays L.) that mitosis and endoreduplication are differentially regulated. Auxin stimulates endoreduplication of maize endosperm while not affecting cell numbers (Lur and Setter, 1993). Among a set of defective kernel mutants most decreased the relative extent to which nuclei undergo endoreduplication, but one mutant maintained DNA endoreduplication at wild‐type levels (Kowles et al., 1992). Studies using maize inbreds differing in endoreduplication and their reciprocal crosses, showed that genotypic effects on the extent of endoreduplication are largely under maternal control (Kowles et al., 1997).
The impact of stress on mitosis and endoreduplication in maize endosperm has been tested with short‐term post‐pollination treatments. In studies where plants were subjected to short‐term water deprivation at two stages of endosperm development, from 1–10 d after pollination (DAP), when mitotic cell cycling predominates, or from 9–15 DAP, when endoreduplication predominates, water deficit drastically inhibited the rate of endosperm cell division whereas the rate of endoreduplication was inhibited to a lesser extent (Artlip et al., 1995). Studies of maize endosperm have also shown that mitosis is more sensitive than endoreduplication to exogenously applied abscisic acid (ABA) applied from 5–11 DAP, coinciding with maximal cell division rates and the onset of endoreduplication (Mambelli and Setter, 1998). The distribution of nuclei among DNA‐content size‐classes indicated that water deficit and ABA inhibited both the rate of transition from mitotic to endoreduplication status, and the rate of S‐phase cycling. Studies of _in vitro_‐cultured maize kernels have indicated that although high temperature stress (35 °C), imposed from 4–8 or 4–10 DAP, inhibited both mitosis and endoreduplication, the impact on endoreduplication was later, during recovery after stress was relieved (Engelen‐Eigles et al., 2000).
Previous studies of stress effects on endosperm cell cycle involved short‐term stress imposition during maximal cell division or endoreduplication (Artlip et al., 1995; Mambelli and Setter, 1998). The objective of the current study was to determine the extent to which mitosis and endoreduplication in maize endosperm are affected by water deficit imposed during a time‐frame bracketing the maximum activity of both of these processes. In addition to flow cytometry, the impact of stress on expression of gene products with roles in the cell cycle was assessed. The results indicate that water deficit inhibited both mitosis and endoreduplication, but mitosis was affected earlier in the stress episode, whereas endoreduplication was affected after stress had advanced further.
Materials and methods
Plant material
Maize (Pioneer Brand 3925) was grown in a glasshouse with hourly irrigation and artificial illumination as previously described (Artlip et al., 1995). When solar photon flux density of photosynthetically active radiation (PAR, 400–700 nm wavelengths) was less than 250 μmol m−2 s−1, it was supplemented between 06.00 h and 20.00 h with illumination from 1000 W metal halide lamps.
Watering treatments
Water deficit stress was imposed as previously described (Artlip et al., 1995), except irrigation water was withheld beginning at 5 DAP. Briefly, the mass of each well‐watered pot, containing plants plus soil, was measured at 5 DAP, pots were transferred to an automatic gravimetric system that allowed water to be depleted until the mass reached 50% of initial wet mass, and then this set‐point was maintained by hourly addition of irrigation solution. At about 7 DAP soil water content was depleted to the set‐point which was sufficient to induce leaf wilt as indicated by leaf rolling and appearance of glaucous leaf surfaces. Tests indicated that during days of high transpiration (high light flux density), this set‐point corresponded to midday leaf water potentials of about −2.2 MPa while well‐watered control leaf water potentials were about −1.2 MPa.
Analysis of nuclei
Endosperms from kernels in the apical zone of each ear, the upper 33% with respect to ear longitudinal length, were dissected free of embryo and nucellus, and endosperms were fixed in 3 : 1 (v/v) ethanol:acetic acid. Nuclei in homogenates were analysed by flow cytometry, as previously described (Artlip et al., 1995). Briefly, fixative was removed by washing endosperms in water, endosperms were then incubated with pectinase, and cell walls were disrupted by gentle passage through successively smaller hypodermic needles to release individual nuclei. RNA was destroyed during the pectinase reaction. Nuclei in homogeneous suspension were stained with propidium iodide (a DNA‐binding fluorochrome), and analysed on a flow cytometer which measured the propidium fluorescence intensity, a measure of DNA content, of each nucleus. For each sample, between 5000 and 19 000 nuclei were analysed. Histograms of frequency (nuclear counts) versus logarithm of fluorescence were then produced, as shown in Fig. 1.
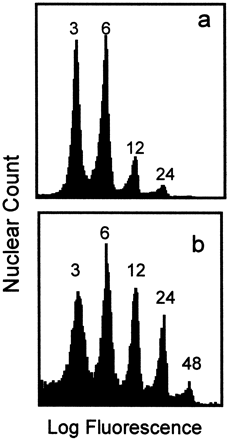
Fig. 1.
Histogram illustrating the distribution of nuclei into DNA‐content size‐classes, as analysed by flow cytometry. Nuclei from control endosperms at 9 DAP (a) and 13 DAP (b) are shown. Endosperms were treated with pectinase to release nuclei into homogeneous suspension, treated with propidium iodide fluorochrome, and analysed by flow cytometry. Each peak is labelled with its DNA copy number, where 1C is the haploid nuclear DNA content.
Tritiated thymidine labelling
Endosperms from the apical ear zone, as described above, were cut in half lengthwise, dissected free of embryo and nucellus, and incubated at 22 °C for 2 h in 0.25 ml of 10 mM Tris‐HCl (pH 7.5), 1% (w/v) glucose, to which 3.7 MBq of [3H‐methyl]‐thymidine (740 MBq mmol−1) was added. DNA was extracted and purified as described earlier (Davis et al., 1986), then radioactivity was determined by liquid scintillation spectrometry.
Analysis of mRNA
Maize endosperms were dissected as above, immediately frozen in liquid N2, then RNA was extracted and purified as previously described (Wadsworth et al., 1988). Briefly, thawing tissue was homogenized in 2.5 vols of RNA extraction buffer (100 mM Tris‐HCl, pH 8.5, 20 mM aurin tricarboxylic acid, 200 mM LiCl, 100 mM EDTA, 100 mM β‐mecaptoethanol). The mixture was centrifuged at 10 000 g for 10 min and the supernatant was extracted with phenol/chloroform. RNA in the aqueous phase was precipitated with 3 M LiCl, redissolved and reprecipitated with ethanol. Poly(A)+ RNA was isolated by oligo(dT) cellulose column chromatography (Sambrook et al., 1989).
RNA was quantified by UV spectroscopy and electrophoresed on 1% agarose/formaldehyde gels. Gels were blotted downward onto charged nylon membranes (Nytran, Schleicher & Schuell) utilizing a neutral solution of 10×SSC (Sambrook et al., 1989), and crosslinked with ultraviolet radiation (Stratalinker, Stratagene Cloning Sys., La Jolla, CA). Radiolabelled probes (indicated in figure legends) were synthesized using the Multiprime DNA labelling system (Amersham) and [32P]dCTP. Membranes were prehybridized for 0.5 h at 65 °C in blocking buffer (Boehringer‐Mannheim), modified to include 10% SDS, and then hybridized with [32P]‐labelled probe at 68 °C for 16–20 h. Membranes were washed twice at room temperature in 2×SSC for 30 s, once at 68 °C in 1×SSC for 10 min, and once at 68 °C in 0.1×SSC for 20 min. The hybridized membranes were exposed to X‐ray film at −80 °C using intensifying screens. Each blot was subsequently washed free of probe and rehybridized with ribosomal RNA probe, pGMR from soybean, to establish that lanes were equally loaded (data not shown).
Results and discussion
Water deficit inhibition of cell division and 3H‐thymidine incorporation into DNA
The rate of cell division in maize endosperm is maximal from about 8–11 d after pollination, preceding rapid starch accumulation (Artlip et al., 1995; Lur and Setter, 1993; Ober et al., 1991). In the current studies, maize plants were subjected to water deficit from 5–13 DAP encompassing the period of maximal cell division activity. Water deficit substantially inhibited endosperm cell division (Fig. 2): in the period from 9–13 DAP the control endosperms had a nuclear doubling time of about 2 d, while cell division was essentially halted in endosperms of water deficit plants. Plants in which water was withheld beginning at 5 DAP depleted soil water and did not reach the gravimetric set point until about 7 DAP, corresponding to the first appearance of leaf rolling and glaucous leaf surface. Nuclear counts are a measure of treatment effects exerted over the time‐frame of treatment imposition, so treatment differences in nuclear counts gradually became apparent between 9 and 13 DAP. But 3H‐thymidine incorporation into DNA, a measure of cell DNA synthesis activity at a specific point in time, revealed that at 9 DAP, as well as later samplings, cell cycling was substantially inhibited by water deficit (Fig. 3). In controls, DNA synthesis activity μg−1 DNA was maximal at 9 DAP and decreased at 11 and 13 DAP, while water deficit inhibited activity 60, 80 and 88% at 9, 11 and 13 DAP, respectively. The decline from 9–11 and 13 DAP of DNA synthesis activity μg−1 DNA in controls is an expected result of gradual exit from mitotic cell cycling of a portion of the cell population.
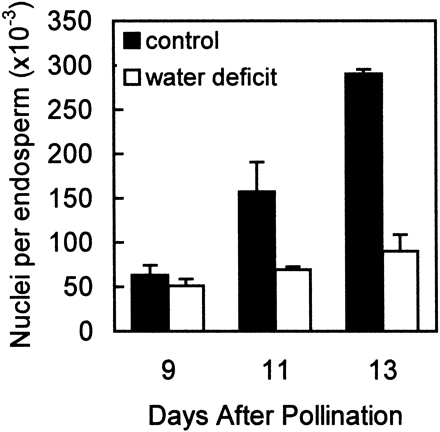
Fig. 2.
Effect of water deficit on the proliferation of endosperm nuclei in maize kernels. Samples were obtained from well‐watered control plants (▪) and from plants subjected to water deficit from 5–13 DAP (□). Nuclear counts were obtained by flow cytometry. Means ±SE of six replicates are indicated.
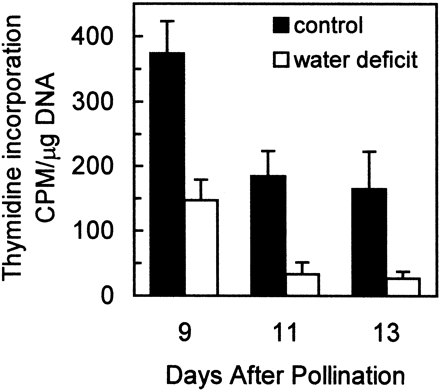
Fig. 3.
Effect of water deficit on DNA synthetic activity, estimated with 3H‐thymidine incorporation into DNA, of endosperms in developing maize kernels. Samples were obtained from well‐watered control plants (▪) and from plants subjected to water deficit from 5–13 DAP (□). Data are expressed as the counts min−1 (CPM) of 3H‐thymidine incorporated μg−1 of total DNA. Means±SE of six replicates are indicated.
Water deficit inhibition of endoreduplication
Although 3H‐thymidine incorporation into DNA is conventionally interpreted as a measure of cell mitotic activity, in maize endosperm, DNA synthesis associated with endoreduplication may also substantially contribute to the observed 3H‐thymidine incorporation. To distinguish treatment effects on mitosis versus those on endoreduplication, DNA content of nuclei was measured by flow cytometry and nuclei were grouped based on DNA‐content. Nuclei with 3C and 6C DNA contents (where 1C is the haploid DNA content) were considered mitotic while those with ≥12C as endoreduplicated. This conservatively estimates the proportion of cells that have advanced to an endoreduplication developmental status, since some of the 6C nuclei might also have advanced to an endoreduplicative state. In controls, the proportion of nuclei with ≥12C DNA content steadily increased from 9–13 DAP (Fig. 4). Water deficit initially (at 9 DAP) increased the proportion of nuclei classified as endoreduplicated, then halted further increase in this proportion so that at 13 DAP it was less than controls. Hence, at 9 DAP, the water deficit inhibition of 3H‐thymidine incorporation (Fig. 3) was apparently not due to a decrease in the rate of endoreduplicative S‐phase cycling (Fig. 4). Instead, it was probably due to an initial inhibition of S phase in mitotic cells, before endoreduplication rate was affected. This allowed endoreduplication to advance the existing pool of nuclei to higher DNA contents at 9 DAP. However, as the duration of stress increased, both cell proliferation (Fig. 2) and endoreduplication (Fig. 4) were inhibited. Thus, the inhibition of 3H‐thymidine incorporation later, at 11 and 13 DAP (Fig. 3), was from both decreased mitotic cell cycling, as well as decreased endoreduplication.
Another possible effect of water deficit on endoreduplication is the rate of progressive cycling between successive rounds of S phase. To assess this, the proportion of nuclei in each of the DNA‐content size classes of endoreduplicating (≥12C) nuclei were examined. Controls had a steady, progressive increase in the proportion of nuclei in larger size classes (24C, 48C, and 96C), reflecting S‐phase cycling of the nuclei in endoreduplication. If water deficit inhibited S‐phase cycling of endoreduplicating nuclei, the proportion of nuclei in the large size classes (24C, 48C, 96C) would decrease relative to controls, whereas that in the smallest class (12C) would increase relative to controls. The relative proportions (Fig. 5) indicate that initially (at 9 DAP) water deficit did not inhibit S‐phase cycling, permitting the average size of the existing endoreduplicated nuclei to increase. As the stress became progressively more severe from 9–13 DAP, stress inhibited further S‐phase cycling so the proportion of large nuclei remained about the same.
The current study is consistent with a previous study of developing maize endosperm where water deficit was timed to coincide with either an early phase where the majority of cells are mitotically cycling (1–10 DAP) or a later phase where endoreduplication predominates (9–15 DAP) (Artlip et al., 1995). In that work, water stress at both the early and late phases substantially decreased cell division in apical‐kernel endosperms, whereas these stresses decreased endoreduplication to a lesser extent, and only in the stress from 1–10 DAP (Artlip et al., 1995). Indeed, in that study, water stress from 9–15 DAP increased the proportion of endoreduplicated nuclei relative to well‐watered controls due to a larger inhibition of mitotic cycling than endoreduplication. This is similar to the transient increase in the proportion of endoreduplicated nuclei at 9 DAP in the current study (Fig. 4).
Other stresses also appear to have effects that change temporally. Studies of _in vitro_‐cultured maize kernels indicated that although high temperature stress (35 °C from 4–8 or 4–10 DAP) decreased the extent of both endosperm cell division and endoreduplication in the recovery phase; on the final date of stress imposition, only cell division was inhibited (Engelen‐Eigles et al., 2000).
The present data are also consistent with studies involving exogenous application of ABA to maize endosperm from 5–11 DAP (Mambelli and Setter, 1998). In that work, mitotic cycling was inhibited 50% by 100 μM ABA whereas transition to endoreduplication and endoreduplicative cycling were not inhibited until ABA concentrations were ≥300 μM. Such differential sensitivity to ABA may play a role in the water deficit effects observed in the present study. Water deficit increases ABA levels in maize endosperm, particularly in kernels from apical zones of the ear (Ober et al., 1991). Also, although only apical‐zone kernels were used in the present work, in studies where apical and basal kernels were compared, inhibition of cell division and endoreduplication was greatest in the apical zone (Artlip et al., 1995).
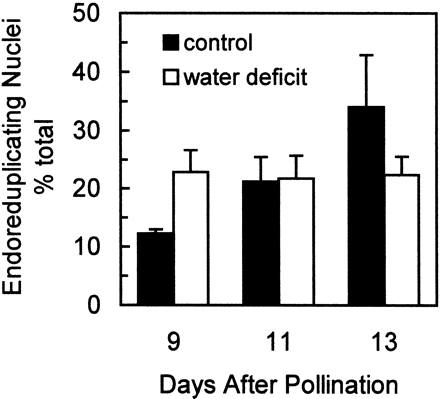
Fig. 4.
Effect of water deficit treatments on the proportion of nuclei that have undergone endoreduplication. Nuclei with DNA contents ≥12C were summed to calculate the percent of nuclei in endoreduplication. Samples were obtained from well‐watered control plants (▪) and from plants subjected to water deficit from 5–13 DAP (□). The number of nuclei was determined using flow cytometry and nuclear counts were summed for each DNA‐content size class, where 1C is the haploid nuclear DNA content (approximately 2.7 pg for this maize genotype). Means±SEM of six replicates are indicated.
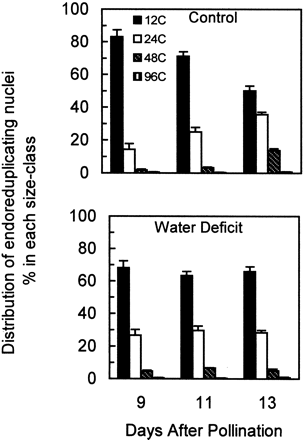
Fig. 5.
Effect of water deficit on the proportional distribution of endoreduplicated nuclei among each of the DNA‐content size‐classes ≥12C, representing various stages of endoreduplication S‐phase cycling. Nuclei were analysed as in Fig. 4. Data are expressed relative to the total number of endoreduplicating nuclei (≥12C). Samples were obtained from well‐watered control plants (top panel) and plants subjected to water deficit from 5–13 DAP (bottom panel). Means±SE of six replicates are indicated.
Water deficit inhibition of cell‐cycle gene expression
To gain insight into the component processes of the cell cycle that are affected by water deficit, the expression of genes which have recognized roles in various phases of the cell cycle was examined. Four cDNAs were used as hybridization probes in RNA gel blots of endosperm samples: (1) maize α‐tubulin (Montoliu et al., 1989), (2) maize histone H3 (Chaubet et al., 1986), (3) a maize CDK, ZmCdc2 (Colasanti et al., 1991), and (4) rice (Oryza sativa L.) PCNA, a highly conserved component of DNA polymerase complexes (Suzuka et al., 1991). As shown in Fig. 6, at the final sampling date (13 DAP) water deficit decreased the abundance, relative to 9 DAP controls, of all four of the probed mRNAs. However, at the earlier sampling dates (9 and 11 DAP), when cell division was first affected in this material, only ZmCdc2 abundance was significantly (_P_≤0.05) decreased by water deficit. The extent to which stress decreased ZmCdc2 mRNA abundance was similar to that for cell division (Fig. 2). Furthermore, in controls, even though cell division rates declined during the period from 9–13 DAP, the mRNA abundance of α‐tubulin, histone H3 and PCNA remained high whereas ZmCdc2 decreased substantially at 13 DAP, as expected for a gene product whose expression is specific for cells engaged in cell division.
Given that α‐ and β‐tubulin subunits assemble to form microtubules and that a large quantity of microtubules are needed to form spindle fibres and phragmoplasts in dividing cells, the observed high level of α‐tubulin expression at 9 DAP (Fig. 6), when cells were predominantly mitotic (Fig. 4), was expected (Montoliu et al., 1990). However, microtubules are also used in a variety of other processes, such as in directing the deposition of cellulose. This lack of specificity to mitosis was reflected in the continued high expression of α‐tubulin in controls from 9–13 DAP and the relative insensitivity of its expression in response to stress at 9 and 11 DAP (Fig. 5).
Histone H3 is a structural component of chromatin and PCNA has multiple functions in S phase as an enhancer of DNA polymerase processivity and as a component of regulatory complexes with cyclin and S‐phase CDK (Laquel et al., 1993; Tsurimoto, 1999). Hence, these gene products are good markers for cells actively synthesizing DNA (Fobert et al., 1994; Shimizu and Mori, 1998). In well‐watered endosperms, histone H3 and PCNA expression remained high throughout the observed period (Fig. 6), and, in agreement with flow cytometry (Figs 2, 4, 5), water deficit did not affect their expression until later stages of stress when S phase of both mitosis and endoreduplication were inhibited (Fig. 6).
ZmCdc2 is a member of the family of CDKs that contain the conserved PSTAIRE motif. It is homologous to Arabidopsis (Arabidopsis thaliana L.) CDC2aAt and alfalfa (Medicago sativa L.) Cdc2MsA, and to rice Cdc2Os1, with which it shares 94% amino acid sequence identity (Dudits et al., 1998; Umeda et al., 1999). Studies of the expression of various CDKs with respect to cell cycle phases have indicated that PSTAIRE‐containing CDKs in rice (Cdc2Os1), Arabidopsis (CDC2aAt) and alfalfa (Cdc2MsA) are predominantly expressed in actively dividing cells, but their expression is not specific to a particular phase of the cell cycle (Magyar et al., 1997; Segers et al., 1996; Umeda et al., 1999). Nevertheless, in situ hybridization and flow cytometric analyses of vegetative shoot apices in Arabidopsis (Jacqmard et al., 1999) and young tomato (Lycopersicum esculentum L.) fruit (Joubès et al., 1999) have shown that PSTAIRE‐containing CDKs are restricted to mitotically dividing cells and are not expressed in endoreduplicating cells. The current data are consistent with this developmental pattern of expression. In controls, ZmCdc2 expression progressively decreased from 9–13 DAP (Fig. 6) while an increasing proportion of cells became engaged in endoreduplication (Fig. 4). Expression of histone H3 and PCNA, which are expressed in S phase of endoreduplicating cells, remained at high levels (Fig. 6). Furthermore, at 9 DAP, water deficit decreased ZmCdc2 expression while the percentage of endoreduplicating cells increased.
As discussed above, water deficit inhibited mitotic cell cycling beginning at 9 DAP, and concomitantly inhibited ZmCdc2 expression from 9–13 DAP (Fig. 6). However, such inhibition was only partial, whereas cell division was nearly halted (Fig. 2). This indicates that decreases in ZmCdc2 transcript levels were not solely responsible for decreased rates of mitotic cell cycling during water deficit. Additional regulation may be due to post‐transcriptional inhibition of CDK activity in response to water deficit. Studies have shown that decreases in wheat leaf cell division in response to water deficit are associated with decreased activation state of mitotic CDK, which is correlated with an increased extent of its tyrosine phosphorylation (Schuppler et al., 1998), a recognized mode of post‐transcriptional CDK regulation (Sun et al., 1999a).
Another possible mode of post‐transcriptional regulation of CDK is ABA‐induced expression of an inhibitor of CDK, ICK1, whose interaction with Cdc2aAt and cyclin‐D3 decreases CDK activity (Wang et al., 1998). ABA levels in maize endosperm increase substantially in response to water deficit (Ober et al., 1991), consistent with the possibility such an inhibitor might play a role in the current system.
The observed temporal separation of water deficit influence on mitosis and endoreduplication suggests that water deficit down‐regulates mitotic cell cycling and endoreduplication via different mechanisms. In maize endosperm, the transition of mitotic cells to an endoreduplicating status is accompanied by several changes that might play a role in the developmental transition. Endoreduplication is accompanied by (1) increases in S‐phase‐related CDK activity (Grafi and Larkins, 1995) associated with an increased phosphorylation of the G1/S regulatory protein retinoblastoma (ZmRb) (Grafi et al., 1996), (2) down‐regulation of the G2/M cyclin transcript CycZme1 (Sun et al., 1999b), (3) an increase in the level of a mitotic CDK inhibitor (Grafi and Larkins, 1995), and (4) an increased level of ZmWee1, a protein kinase responsible for inhibitory tyrosine phosphorylation of mitotic CDK (Sun et al., 1999a). Thus, the mechanisms cited above as possible contributors to cell cycle arrest during water deficit and concomitant increase in ABA levels (Schuppler et al., 1998; Wang et al., 1998), overlap with those associated with endoreduplication. Further studies are needed to elucidate the interplay of these and other regulatory factors responsible for developmental down‐regulation of G2/M during the transition to endoreduplication and to distinguish them from those involved in stress‐mediated inhibition of mitosis and endoreduplication.
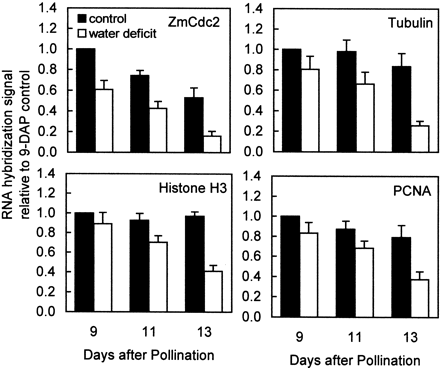
Fig. 6.
Effect of water deficit on relative abundance of transcripts encoding gene products involved in cell proliferation and endoreduplication. Samples were obtained from well‐watered control plants (▪) and from plants subjected to water deficit from 5–13 DAP (□). Levels of RNA encoding ZmCdc2 (Colasanti et al., 1991), α‐tubulin (Montoliu et al., 1989), histone H3 (Chaubet et al., 1986), and PCNA (Suzuka et al., 1991) in 20 μg of total RNA were determined by hybridization of RNA gel blots with 32P‐labelled cDNA probes. Each blot was subsequently washed free of probe and rehybridized with ribosomal RNA probe, pGMR from soybean, to establish that lanes were equally loaded (data not shown). Signals on autoradiograms were quantified by laser densitometry and normalized with respect to the 9 DAP control sample for each probe. Means±SEM of four replicates are indicated.
1
To whom correspondence should be addressed. Fax: +1 607 255 2644. E‐mail: [email protected]
References
Artlip TS, Madison JT, Setter TL.
1995
. Water deficit in developing endosperm of maize: cell division and nuclear DNA endoreduplication.
Plant, Cell and Environment
18,
1034
–1040.
Cavallini A, Natali L, Balconi C, Rizzi E, Motto M, Cionini G, D'Amato D.
1995
. Chromosome endoreduplication in endosperm cells of two maize genotypes and their progenies.
Protoplasma
189,
156
–162.
Cebolla A, Vinardell JM, Kiss E, Oláh B, Roudier F, Kondorosi A, Kondorosi E.
1999
. The mitotic inhibitor ccs52 is required for endoreduplication and ploidy‐dependent cell enlargement in plants.
The EMBO Journal
18,
4476
–4484.
Chaubet N, Phillipps G, Chaboute ME, Ehling M, Gigot C.
1986
. Nucleotide sequences of two corn (Zea mays) histone H‐3 genes. Genomic organization of the corn histone H‐3 and H‐4 genes.
Plant Molecular Biology
6,
253
–264.
Colasanti J, Tyers M, Sundaresan V.
1991
. Isolation and characterization of complementary DNA clones encoding a functional p34‐cdc‐2 homologue from Zea mays.
Proceedings of the National Academy of Sciences, USA
88,
3377
–3381.
Commuri PD, Jones RJ.
1999
. Ultrastructural characterization of maize (Zea mays L.) kernels exposed to high temperature during endosperm cell division.
Plant, Cell and Environment
22,
375
–385.
Davis LG, Dibner MD, Battey JD.
1986
. Rapid DNA preparation. In:
Basic methods in molecular biology
. Amsterdam: Elsevier Science Publishing,
42
–43.
den Boer BGW, Murray JAH.
2000
. Triggering the cell cycle in plants.
Trends in Cell Biology
10,
245
–250.
Dudits D, Magyar Z, Deák M, Mészáros T, Miskolczi P, Fehér A, Brown S, Kondorosi É, Athanasiadis A, Pongor S, Bakó L, Koncz Cs, Györgyey J.
1998
. Cyclin‐dependent and calcium‐dependent kinase families: response of cell division cycle to hormone and stress signals. In: Francis D, Dudits D, Inzé D, eds.
Plant cell division
. London: Portland Press Research Monograph,
21
–45.
Engelen‐Eigles G, Jones RJ, Phillips RL.
2000
. DNA endoreduplication in maize endosperm cells: the effect of exposure to short‐term high temperature.
Plant, Cell and Environment
23,
657
–663.
Fobert PR, Coen ES, Murphy GJP, Doonan JH.
1994
. Patterns of cell division revealed by transcriptional regulation of genes during the cell cycle in plants.
The EMBO Journal
13,
616
–624.
Grafi G.
1998
. Cell cycle regulation of DNA replication: the endoreduplication perspective.
Experimental Cell Research
244,
372
–378.
Grafi G, Burnett RJ, Helentjaris T, Larkins BA, DeCaprio JA, Sellers WR, Kaelin Jr WG.
1996
. A maize cDNA encoding a member of the retinoblastoma protein family: Involvement in endoreduplication.
Proceedings of the National Academy of Sciences, USA
93,
8962
–8967.
Grafi G, Larkins BA.
1995
. Endoreduplication in maize endosperm: involvement of M phase‐promoting factor inhibition and induction of S phase‐related kinases.
Science
269,
1262
–1264.
Jacqmard A, De Veylder L, Segers G, de Almeida Engler J, Bernier G, Van Montagu M, Inzé D.
1999
. Expression of CKS1At in Arabidopsis thaliana indicates a role for the protein in both the mitotic and the endoreduplication cycle.
Planta
207,
496
–504.
Jones RJ, Schreiber BMN, Roessler JA.
1996
. Kernel sink capacity in maize: genotypic and maternal regulation.
Crop Science
36,
301
–306.
Joubès J, Phan T‐H, Just D, Rothan C, Bergounioux C, Raymond P, Chevalier C.
1999
. Molecular and biochemical characterization of the involvement of cyclin‐dependent kinase A during the early development of tomato fruit.
Plant Physiology
121,
857
–869.
Kowles RV, Srienc F, Phillips RL.
1990
. Endoreduplication of nuclear DNA in the developing maize endosperm.
Developmental Genetics
11,
125
–132.
Kowles RV, McMullen MD, Yerk G, Phillips RL, Kraemer S, Srienc F.
1992
. Endosperm mitotic activity and endoreduplication in maize affected by defective kernel mutations.
Genome
35,
68
–77.
Kowles RV, Yerk GL, Haas KM, Phillips RL.
1997
. Maternal effects influencing DNA endoreduplication in developing endosperm of Zea mays.
Genome
40,
798
–805.
Laquel P, Litrak S, Castroviejo M.
1993
. Mammalian proliferating cell nuclear antigen stimulates the processivity of two wheat embryo DNA polymerases.
Plant Physiology
102,
107
–114.
Lemontey C, Mousset‐Déclas C, Munier‐Jolain N, Boutin J‐P.
2000
. Maternal genotype influences pea seed size by controlling both mitotic activity during early embryogenesis and final endoreduplication level/cotyledon cell size in mature seed.
Journal of Experimental Botany
51,
167
–175.
Lur HS, Setter TL.
1993
. Role of auxin in maize endosperm development. Timing of nuclear DNA endoreduplication, zein expression, and cytokinin.
Plant Physiology
103,
273
–280.
Magyar Z, Mészáros T, Miskolczi P, Deák M, Fehér A, Brown S, Kondorosi É, Athanasiadis A, Pongor S, Bilgin M, Bakó L, Koncz C, Dudits D.
1997
. Cell cycle phase specificity of putative cyclin‐dependent kinase variants in synchronized alfalfa cells.
The Plant Cell
9
,
223
–235.
Mambelli S, Setter TL.
1998
. Inhibition of maize endosperm cell division and endoreduplication by exogenously applied abscisic acid.
Physiologia Plantarum
104,
266
–277.
Montoliu L, Puigdomènech P, Rigau J.
1990
. The Tub_α_3 gene from Zea mays: structure and expression in dividing plant tissues.
Gene
94,
201
–207.
Montoliu L, Rigau J, Puigdomènech P.
1989
. A tandem of α‐tubulin genes preferentially expressed in radicular tissues from Zea mays.
Plant Molecular Biology
14,
1
–15.
Nicolas ME, Gleadow RM, Dalling MJ.
1985
. Effect of post‐anthesis drought on cell division and starch accumulation in developing wheat grains.
Annals of Botany
55,
433
–444.
Ober ES, Setter TL, Madison JT, Thompson JF, Shapiro PS.
1991
. Influence of water deficit on maize endosperm development. Enzyme activities and RNA transcripts of starch and zein synthesis, abscisic acid, and cell division.
Plant Physiology
97,
154
–164.
Olsen O‐A, Linnestad C, Nichols SE.
1999
. Developmental biology of the cereal endosperm.
Trends in Plant Science
4,
253
–257.
Ouattar S, Jones RJ, Crookston RK
1987
. Effects of moisture stress during grain filling on the pattern maize kernel growth and development.
Crop Science
27,
726
–730.
Roberts JM.
1999
. Evolving ideas about cyclins.
Cell
98,
129
–132.
Sambrook J, Fritsch EF, Maniatis T.
1989
.
Molecular cloning: a laboratory manual
, 2nd edn. Plainview, New York: Cold Spring Harbor Laboratory Press.
Schuppler U, He P‐H, John PCL, Munns R.
1998
. Effect of water stress on cell division and cell‐division‐cycle 2‐like cell‐cycle kinase activity in wheat leaves.
Plant Physiology
117,
667
–678.
Segers G, Gadisseur I, Bergounioux C, de Almeida Engler J, Jacqmard A, Van Montagu M, Inzé D.
1996
. The Arabidopsis cyclin‐dependent kinase gene cdc2bAt is preferentially expressed during S and G2 phases of the cell cycle.
The Plant Journal
10,
601
–612.
Shimizu S, Mori H.
1998
. Analysis of cycles of dormancy and growth in pea axillary buds based on mRNA accumulation patterns of cell cycle‐related genes.
Plant Cell Physiology
39,
255
–262.
Sun Y, Dilkes BP, Zhang C, Dante RA, Carneiro NP, Lowe KS, Jung R, Gordon‐Kamm WJ, Larkins BA.
1999
a. Characterization of maize (Zea mays L.) Wee1 and its activity in developing endosperm.
Proceedings of the National Academy of Sciences, USA
96,
4180
–4185.
Sun Y, Flannigan BA, Setter TL.
1999
b. Regulation of endoreduplication in maize (Zea mays L.) endosperm. Isolation of a novel B1‐type cyclin and its quantitative analysis.
Plant Molecular Biology
41,
245
–258.
Suzuka I, Hata S, Matsuoka M, Kosugi S, Hashimoto J.
1991
. Highly conserved structure of proliferating cell nuclear antigen DNA polymerase delta auxiliary protein gene in plants.
European Journal of Biochemistry
195,
571
–576.
Tsurimoto T.
1999
. PCNA binding proteins.
Frontiers in Bioscience
4,
849
–858.
Umeda M, Umeda‐Hara C, Yamaguchi M, Hashimoto J, Uchimiya H.
1999
. Differential expression of genes for cyclin‐dependent protein kinases in rice plants.
Plant Physiology
119,
31
–40.
Wadsworth GJ, Redinbaugh MG, Scandalios JG.
1988
. A procedure for the small‐scale isolation of plant RNA suitable for RNA blot analysis.
Analytical Biochemistry
172,
279
–283.
Wang H, Qi Q, Schorr P, Cutler AJ, Crosby WL, Fowke LC.
1998
. ICK1, a cyclin‐dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid.
The Plant Journal
15,
501
–510.
© Society for Experimental Biology
I agree to the terms and conditions. You must accept the terms and conditions.
Submit a comment
Name
Affiliations
Comment title
Comment
You have entered an invalid code
Thank you for submitting a comment on this article. Your comment will be reviewed and published at the journal's discretion. Please check for further notifications by email.