Shengjiang Powder alleviates oxidative stress damage and fibrosis in mice with atherosclerosis concurrent with non-alcoholic fatty liver disease (original) (raw)
Introduction
“Non-alcoholic fatty liver disease (NAFLD) and atherosclerosis (AS) are both chronic metabolic diseases characterized by high clinical incidence, difficult prevention and treatment, and long-term significant harm. NAFLD is the most prevalent chronic liver disease [1], while AS represents the leading cause of death from cardiovascular diseases [2]. Both conditions share similar risk factors, pathological bases, and developmental inducements, often occurring concurrently. Numerous meta-analyses and epidemiological evidence have demonstrated a significant association between their pathogenesis [3,4,5,6,7]. Given the notably increasing comorbidity ratio, the clinical field has consistently sought a safe, reliable, effective, and affordable treatment regimen that can simultaneously benefit the prevention and treatment of both diseases. Although the primary drugs currently used to treat AS, such as statins, have shown some efficacy in managing NAFLD [8], they are not without issues, including a single target mechanism and potential side effects, particularly liver injury. In our clinical practice, we have observed that the “simultaneous treatment of liver and heart” formula can effectively lower blood lipids and ameliorate the progression of fatty liver in patients with AS.
“Co-morbidity of liver and heart diseases” is an extension and expansion of the traditional Chinese medicine (TCM) principle of “treating different diseases with the same method,” embodying the holistic dialectical perspective and treatment strategies of TCM [9, 10]. Liver diseases can affect the heart vessels, leading to vascular endothelial damage. Although they involve different organs, they are physiologically closely connected through meridians and blood vessels, and their pathologies mutually influence each other. The theory of “simultaneous treatment of liver and heart” is proposed under the guidance of the TCM principle of treating different diseases with the same method, based on the close physiological relationship between the heart and liver and the shared pathological foundation of their diseases [11, 12]. The theory of combined liver and heart diseases represents an innovation and development of the holistic thinking in TCM. Ancient TCM texts do not specifically record the disease names of metabolic dysfunction-associated fatty liver disease (NAFLD) combined with atherosclerosis (AS). Our research has found that it can be categorized under the TCM concepts of “liver stagnation” and “chest obstruction”. Based on the principle from “Xue’s Medical Cases” that “when liver qi is smooth, heart qi is harmonious; when liver qi is stagnant, heart qi is deficient,” and combined with years of clinical experience, our team proposes that according to the physiological characteristics of “liver-blood-heart-vessel” and “harmony between liver, heart, and qi” in TCM, it falls within the category of “co-morbidity of liver and heart diseases,” and the treatment principle is “simultaneous treatment of liver and heart”. We believe that “qi mechanism dysfunction” is the key to the development of NAFLD combined with AS. Treatment should focus on soothing the liver and strengthening the spleen in the early stage, protecting the liver and kidney in the middle stage, and addressing phlegm-dispelling and blood-stasis removal throughout the process, providing ideas and methods for the clinical prevention and treatment of NAFLD combined with AS. Therefore, this study intends to construct a mouse model of AS combined with NAFLD, administer different doses of Shengjiang Powder through gavage to obtain in vivo evidence and possible mechanisms of the effective intervention of such drugs on the progression of NAFLD-related inflammation and fibrosis associated with AS, and compare its efficacy with the currently mainstream drug for treating AS, atorvastatin, to explore the advantages of TCM treatment.
Materials and methods
Network pharmacology analysis
Chemical compound data was sourced from the HERB database, a high-throughput platform rooted in experiments and references for traditional Chinese medicine (TCM), which includes all four medicinal herbs examined in this research (http://herb.ac.cn/Contact/). Target genes related to TCM components, herbs, and formulations were pinpointed by comparing the chemical fingerprint similarity between TCM ingredients and known drugs. Recognized fatty liver-associated targets were collected through a search of the GeneCards database. Cytoscape (version 3.10.0) was used to build a network that depicts the interaction between herbs and the fatty liver-related targets of Shengjiang Powder. To clarify the biological processes (BP) through which potential Shengjiang Powder targets participate in fatty liver, gene ontology (GO) enrichment analysis was performed. Moreover, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was conducted to gain a thorough understanding of the mechanisms behind Shengjiang Powder’s impact on fatty liver. AutodockVina 1.2.2, an in silico protein-ligand docking tool, was utilized to assess the binding affinities and interaction patterns between candidate compounds and their targets. The molecular structure of emodin, danthron, curcumin was retrieved from PubChem Compound (https://pubchem.ncbi.nlm.nih.gov/), and the 3D coordinates of AMPK (PDB ID: 3AQV) were obtained from the Protein Data Bank (PDB) (https://www.rcsb.org/). In preparation for docking simulations, all protein and ligand files were converted to PDBQT format; this involved removing water molecules and adding polar hydrogen atoms. The grid box was positioned to cover each protein’s domain, enabling unrestricted molecular movement during docking. Autodock Vina 1.2.2 (http://autodock.scripps.edu/) was employed to carry out the molecular docking studies.
Reagents
Hematoxylin-Eosin Dye (Servicebio, Cat#: G1004-500ML); Oil Red O Dye (Servicebio, Cat#: G1015-100ML); Masson’s Trichrome Dye (Servicebio, Cat#:G1006-100ML); SOD Assay Kit (Solarbio, Cat#:BC0170); MDA Assay Kit (Solarbio, Cat#:BC0025); GSH Assay Kit (Solarbio, Cat#:BC1175); Primary Antibody Rb pAb to LYVE1 (Abcam, Cat#:ab10278); Goat Anti-Rabbit IgG H&L (Alexa Fluor 488) (Bioswamp, Cat#:SAB51361); DAPI (4’, 6-diaminyl-2-phenylindoline dihydrochloride)(Bioswamp, Cat#:DW019); O.C.T. Embedding Medium (Sakura Finetek Japan Co., Ltd., Cat#: 4583); 4% Paraformaldehyde (Servicebio, Cat#: G1101-3 M).
Animal and disease model preparation
Seven-week-old ApoE−/−/C57/B6J mice (specific pathogen-free, SPF grade) were purchased from Beijing SPF Biotechnology Co., Ltd. (Animal Qualification Certificate No.: 202413455). The mice, with a body weight range of 20–30 g, were given one week of adaptive feeding (free access to regular feed and water) before the commencement of modeling. To establish a model of atherosclerosis (AS) combined with non-alcoholic fatty liver disease (NAFLD), the mice were fed a Western high-fat diet purchased from Ruidi Biotechnology (Shenzhen) Co., Ltd. (78.85% regular feed + 21% lard + 0.15% cholesterol). The animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of China Medical University (Shenyang, China; Approval No. CMUXN2023078).
Ten wild-type male C57/B6J mice were assigned to the control group, and 40 ApoE−/− mouse were randomly divided into the model group, atorvastatin group, and traditional Chinese medicine (TCM) treatment groups (low-dose and high-dose Shengjiang Powder groups).
Subsequently, the control group continued to receive a normal diet, while the remaining groups were given a Western high-fat diet for 16 weeks. Starting from the 8th week of the Western high-fat diet administration, each mouse was given a daily intragastric gavage of 0.2 mL per 20 g of body weight. The gavage volume was adjusted weekly based on the average body weight changes of each group. After 8 weeks of continuous gavage, the samples were collected.
Drug treatment
Composition of Shengjiang Powder: Bombyx Batryticatus (Jiangcan) 10 g, Periostracum Cicadae (Chantui) 5 g, Rhei Radix et Rhizoma (Dahuang) 10 g, Curcumae Rhizoma (Jianghuang) 10 g. All Chinese herbal medicines were purchased from the Pharmacy Department of the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine. Each herbal component was weighed according to the ratio, soaked for 1 h, boiled vigorously, simmered for 20 min, filtered, and decocted three times. The filtrates were combined to make a decoction, which was stored at 4℃ for later use. The daily dosage of raw herbs contained in the Shengjiang Powder decoction for adults was 35 g. According to the “Pharmacological Experiment Methods,” the dosage for mice was calculated based on the equivalent dose ratio converted from the body surface area between humans and animals: the raw herb dosage for mice = 35 g ÷ 70 kg × 70 kg × 0.018 ÷ 0.2 kg = 3.15 g/kg of body weight·d. The calculated daily dosage of raw herbs per kilogram of body weight was 3.15 g/kg of body weight·d, which was used as the medium dose for intragastric gavage of Shengjiang Powder in mice. The dosages for the high-dose and low-dose groups were respectively double and half of this medium dose, with the high-dose group receiving 6.3 g/kg of body weight·d and the low-dose group receiving 1.58 g/kg of body weight·d.
Body weight and serum biochemical analysis
Starting from the initiation of modeling, the body weight of mice in each group was recorded weekly until the end of drug intervention, which was at the 24th week. At this point, blood was collected from the eyeballs of mice under isoflurane inhalation anesthesia. The whole blood was centrifuged at 4 °C and 3000 rpm for 20 min, and the serum was carefully collected. After diluting with phosphate-buffered saline (PBS) in a 1:2 ratio, the levels of low-density lipoprotein (LDL), triglycerides (TRIG), total cholesterol (TC), glutamic pyruvic transaminase (ALT), millet straw transaminase (AST), and high-density lipoprotein (HDL) were measured. All these measurements were performed using the Beckman Coulter automated biochemical analysis system in the Laboratory Department of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine.
Histomorphological and pathological examinations
Two liver samples (approximately 0.5 cm × 0.5 cm × 0.3 cm in size, taken from the central part of the largest liver lobe) were immediately placed in 4% paraformaldehyde solution upon extraction. After fixation at 4 °C for 48 h, one liver sample was sequentially dehydrated in 10%, 20%, and 30% sucrose-PBS solutions for 24 h each. The sample was then slightly air-dried and embedded in OCT frozen embedding medium. The maximum cross-section of the aortic sinus was selected, and 8 μm frozen sections of the liver were prepared, taking the largest intact cross-section. The other liver sample was dehydrated and embedded using an automated paraffin dehydration machine, followed by preparation of 5 μm paraffin sections, taking the largest intact cross-section.
Hematoxylin-eosin staining for detection of liver fatty degeneration
Liver paraffin sections were baked at 60℃ overnight, dewaxed with xylene and graded ethanol to water, stained with HE, washed thoroughly, differentiated with 4% hydrochloric acid ethanol, dehydrated with graded ethanol, cleared with xylene, mounted with neutral balsam, and observed under a microscope.
Oil red O staining for detection of atherosclerotic plaque size in the aorta and hepatic lipid accumulation
Frozen sections of the aorta and liver were dried at room temperature and fixed with 4% paraformaldehyde. After washing, the sections were stained with Oil Red O working solution, incubated at 37 °C for 2 h, washed, differentiated with 75% ethanol, stained with hematoxylin solution, differentiated with 4% hydrochloric acid ethanol, washed, mounted with gelatin glycerol, and observed under a microscope.
Detection of oxidative stress damage factors in the liver
Mouse liver samples were frozen in liquid nitrogen. After accurate weighing and thorough homogenization, a 10% liver tissue homogenate was prepared. The supernatant was collected, and the levels of oxidative stress markers (SOD, MDA, GSH) in the liver tissue were measured step by step according to the kit instructions. The OD values were determined using a microplate reader, and the results were calculated based on the formulas provided in the instructions.
Western blot analysis
Total protein was isolated from liver tissue using radioimmunoprecipitation assay (RIPA) and quantified using the bicinchoninic acid assay (BCA) protein detection kit. The isolated protein was diluted in loading buffer, boiled for 5 min, and then separated by 12.5% SDS-PAGE using 20 µg of protein sample. The separated proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane. The membrane was probed with primary antibodies against TNF-ɑ (dilution 1:1000), IL-6 (dilution 1:1000), AMPK (dilution 1:1000), mTOR (dilution 1:200), p-AMPK (dilution 1:1000), p-mTOR (dilution 1:200) at 4 °C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. The protein bands were detected using an ECL advanced system (Millipore) and quantified using Photoshop software.
Statistical methods
Statistical analysis was performed using SPSS 26.0. Data are presented as mean ± standard deviation. For multiple groups with normally distributed data, one-way ANOVA was used for intergroup comparisons; otherwise, nonparametric tests were employed. A _P_-value < 0.05 was considered statistically significant.
Results
Network pharmacology predicts potential pharmacological mechanisms for Shengjiang Powder treatment of fatty liver
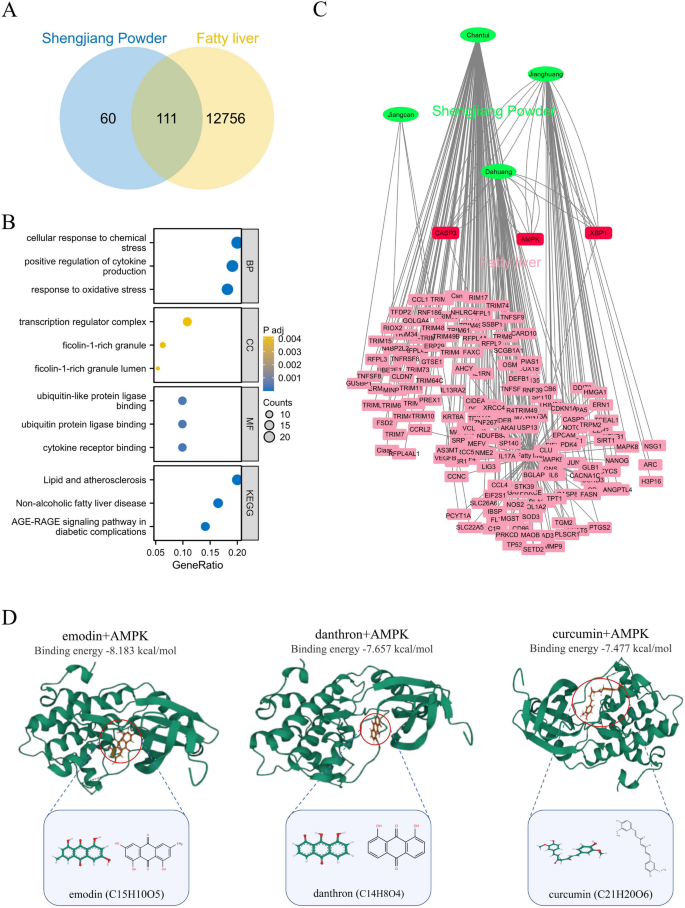
We retrieved 171 target genes of Shengjiang Powder through predictions using the HERB database. To find target genes shared between Shengjiang Powder and fatty liver, we used a Venn diagram for screening, which resulted in 111 common targets (Fig. 1A). Next, these 111 shared target genes underwent GO and KEGG enrichment analyses. In the biological process (BP) category, the most prominently enriched items were “cellular response to chemical stress” and “positive regulation of cytokine production”. For the cellular component (CC) category, the top enriched terms were “transcription regulator complex” and “ficolin-1-rich granule”. When it comes to the molecular function (MF) category, the most significantly enriched items were “ubiquitin-like protein ligase binding” and “ubiquitin protein ligase binding”. In the KEGG pathway analysis, the top enriched terms were “Lipid and atherosclerosis” and “Non-alcoholic fatty liver disease” (Fig. 1B).
Fig. 1
Network pharmacological prediction for Shengjiang Powder treatment of fatty liver. A: Venn diagram of the corresponding targets of the effective components of Shengjiang Powder and fatty liver. B: GO and KEGG pathway enrichment analysis. C: herbs-disease-target networks of Shengjiang Powder against fatty liver. The Green oval nodes represent herbs of Shengjiang Powder, the Red rectangle nodes represent fatty liver genes (≥ 3 effective components targeting the same gene together), and the pink rectangle nodes represent fatty liver genes (≤ 2 effective components targeting the same gene together). D: Molecular docking reveals that the three compounds of Shengjiang Powder target AMPK
To make the target prediction results easier to visualize and interpret further, we built a compound-target (C-T) network. AMPK, a key molecule that plays a role in both regulating lipid metabolism and adjusting inflammatory responses related to fatty liver, stood out in this network. The C-T network showed that three compounds from Shengjiang Powder target AMPK: emodin, danthron, and curcumin (Fig. 1C). Emodin and danthron come from emodin, while curcumin is obtained from curcumin.
We carried out molecular docking analysis to test the binding affinity between these three Shengjiang Powder compounds and AMPK. Using AutoDock Vina v.1.2.2, we evaluated the binding poses and interactions of these compounds with AMPK, and also calculated the binding energy for each interaction (Fig. 1D). The results showed that all three compounds from Shengjiang Powder formed bonds with AMPK via visible hydrogen bonds and strong electrostatic interactions. What’s more, these compounds successfully filled the hydrophobic pockets of AMPK. In terms of KDR, the binding energies of emodin, danthron, and curcumin were − 8.183, −7.657, and − 7.477 kcal/mol respectively, which points to highly stable binding interactions.
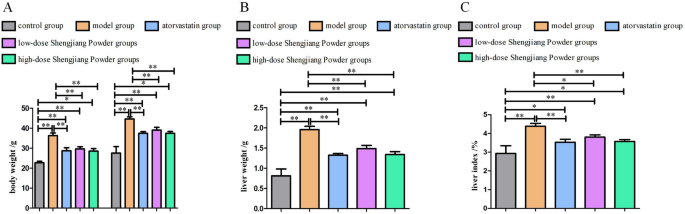
Effect of Shengjiang Powder on reducing liver weight, liver index, and body weight gain in mice
Mice were fed a high-fat western diet starting at 8 weeks of age. Drug treatment was initiated at 16 weeks of age and continued for 8 weeks until 24 weeks of age. After the 8-week drug treatment, the body weight of mice in the model group increased significantly from 12 weeks to 24 weeks (P < 0.01, P < 0.05) (Fig. 2A). The liver weight in the model group increased significantly, while the high-dose traditional Chinese medicine group showed a significant reduction in liver weight (P < 0.01, P < 0.05) (Fig. 2B). The liver index (liver weight/body weight × 100%) was higher in the model group compared to the control group, and the high-dose traditional Chinese medicine group showed significant improvement compared to the model group. However, there was no significant difference in liver weight or liver index between the low-dose traditional Chinese medicine group and the model group (Fig. 2C).
Fig. 2
Changes in body weight, liver weight, and liver index in mice. A: Changes in body weight before and after drug treatment (16 weeks vs. 24 weeks). Compared to body weight at 16 weeks. B-C: Comparison of liver weight and liver index (liver weight/body weight × 100%) after drug treatment
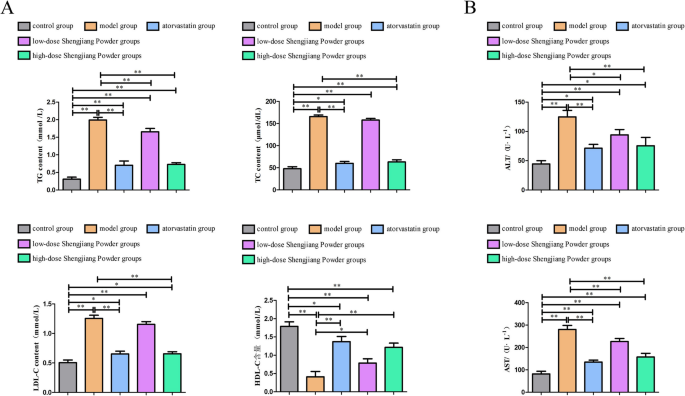
Effect of Shengjiang Powder on improving serum lipid parameters in mice
All four serum lipid parameters were significantly higher in the model group compared to the control group (P < 0.01). After intervention with high-dose traditional Chinese medicine and atorvastatin, there was a significant reduction in TRIG levels (P < 0.01, P < 0.05). Atorvastatin showed a better effect in reducing TC and LDL levels (P < 0.01), while the high-dose traditional Chinese medicine group also demonstrated a significant effect (P < 0.05). However, the low-dose traditional Chinese medicine did not show any lipid-lowering effects. There were no statistically significant differences in HDL levels among the various interventions. Additionally, high-dose traditional Chinese medicine showed AST-lowering and ALT-lowering effects (P < 0.05), while the atorvastatin group showed AST-elevating and ALT-elevating effects (P < 0.05). The remaining comparisons between groups were not statistically significant (Fig. 3).
Fig. 3
Serum lipids, liver, and kidney function in mice. A: Comparison of serum lipid parameters among groups. Compared to the model group. B: Comparison of serum liver and kidney function among groups
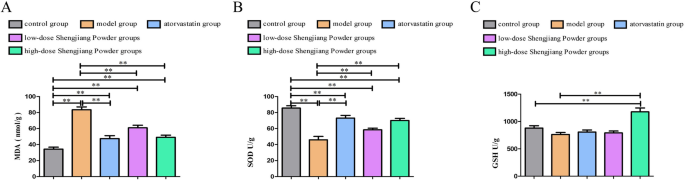
Effect of Shengjiang Powder on MDA, SOD, and GSH levels in the liver of mice
As shown in Fig. 4A, all drug-treated groups significantly decreased MDA levels (P < 0.01). Figure 3B demonstrates that the high-dose Shengjiang Powder group significantly increased SOD levels (P < 0.05). Although the reduction in GSH activity in the model group compared to the control group was not statistically significant, the Shengjiang Powder intervention treatment group still showed a significant increase in GSH levels in the liver tissue of the model group (P < 0.05, P < 0.01), as illustrated in Fig. 4C.
Fig. 4
MDA, SOD, and GSH levels in the liver of mice. A: MDA levels in liver tissue. B: SOD levels in liver tissue. C: GSH levels in liver tissue.”
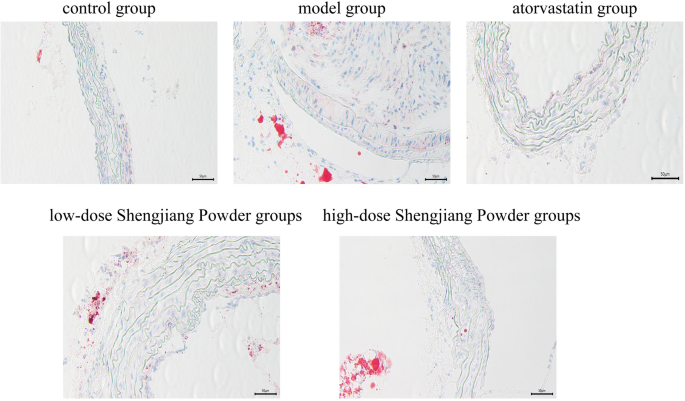
Shengjiang Powder reduces the area of aortic sinus plaques in mice
AS lipid areas were stained red, nuclei were stained blue-purple, and the interstitium was colorless. The control group showed no significant lipid deposition. The model group exhibited extensive and typical lipid plaques with significant luminal stenosis. The high and low-dose Shengjiang Powder groups improved the plaque area, and the efficacy was positively correlated with the dose. Atorvastatin significantly reduced the lipid extent of the plaques (Fig. 5).
Fig. 5
Microscopic changes in aortic sinus of mice with oil red O staining (40× magnification)
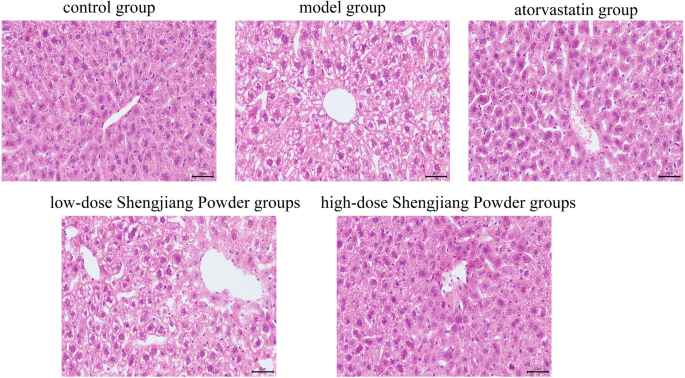
Shengjiang Powder improves hepatic steatosis and inflammation in mice
Microscopic images of liver HE staining in mice from various groups are presented. The model group exhibited extensive and severe hepatic steatosis (ballooning degeneration) accompanied by increased inflammatory cell infiltration. In the Chinese herbal treatment groups, the extent and severity of hepatic steatosis were reduced in a dose-dependent manner. The atorvastatin group showed a reduction in steatosis, but more inflammatory cell infiltration was observed in the portal area (Fig. 6).
Fig. 6
Microscopic changes in mouse liver with HE staining (200× magnification)
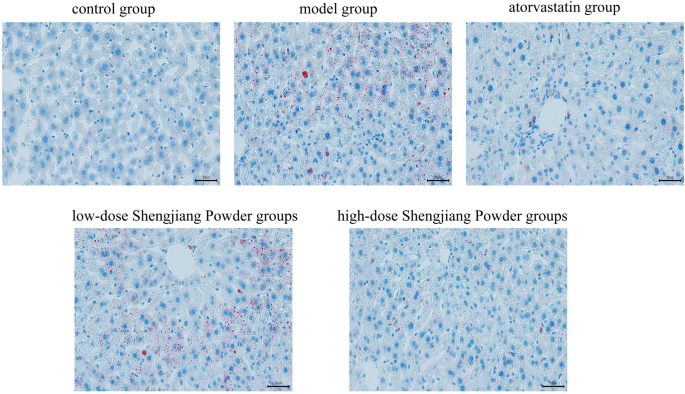
Shengjiang Powder ameliorates lipid deposition in mouse liver
In order to assess the pathological effects of Shengjiang powder on fatty liver disease tissues, oil red O staining was used to observe the lesions of liver fat (Fig. 7), the results showed that the livers of control mouse were normal and almost oil droplet free while the livers of the model group showed a high number of oil droplets and a large size with the presence of lipid accumulation, and the number and size of the droplets in the atorvastatin group and the Shengjiang powder group were significantly reduced, and the Shengjiang powder showed dose effect, the higher the concentration, the better the effect.
Fig. 7
Microscopic changes in mouse liver with oil red O staining (40× magnification)
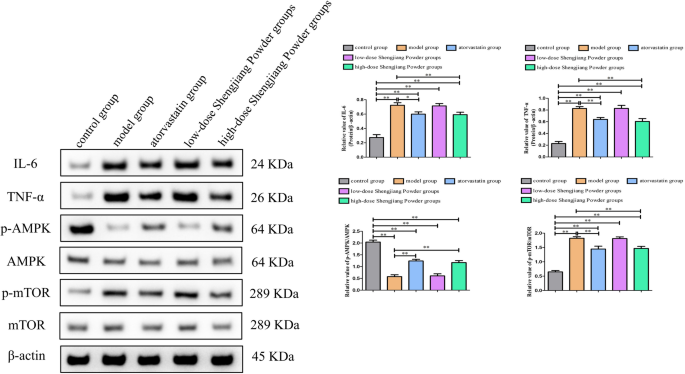
Shengjiang Powder inhibited the expression of inflammatory factors and activated the AMPK/mTOR signaling pathway
Compared to the model group, the protein expression levels of IL-6 and TNF-α were both suppressed in the high-dose Shengjiang Powder group(both p < 0.01) and atorvastatin group (IL-6 _p_ < 0.05;TNF-α _p_ < 0.01), but there was no difference compared in the low-dose Shengjiang Powder group(both _p_ > 0.05). Compared to the model group, we found that high-concentration Shengjiang San and atorvastatin treatment both significantly increased the p-AMPK/AMPK ratio (both p < 0.01) in the liver, decreased the p-mTOR/mTOR ratio (both _p_ < 0.01), but there was also no difference compared in the low-dose Shengjiang Powder group(both _p_ > 0.05) (Fig. 8).
Fig. 8
Shengjiang Powder inhibited the expression of inflammatory factors and activated the AMPK/mTOR signaling pathway. Western blot analysis revealed that high-concentration Shengjiang Powder downregulated the protein expression level of IL-6 and TNF-α, increased the _p_-AMPK/AMPK ratio, decreasing the _p_-mTOR/mTOR ratio. Statistical significance was determined as *p < 0.05, **p < 0.01
Discussion
NAFLD is one of the most prevalent chronic liver diseases worldwide, with a global adult incidence rate reaching 25% [13], and an even higher rate of 29.2% among adults in China [14, 15]. Its progression encompasses simple non-alcoholic fatty liver and non-alcoholic steatohepatitis, which can advance to cirrhosis and ultimately hepatocellular carcinoma [16]. Numerous studies have demonstrated that NAFLD and various metabolic disorders, including atherosclerosis (AS), share a reciprocal causal relationship and mutually exacerbate each other’s development [17].
The traditional Chinese medicine formula “Shengjiang San,” highly esteemed by Chinese medicine practitioners, originates from the “Shanghan Wenyi Tiaobian.” Initially comprising six ingredients—Bombyx Batryticatus, Cicadae Periostracum, Curcumae Longae Rhizoma, Rhei Radix et Rhizoma, yellow rice wine, and honey—modern practice often utilizes the first four for medicinal purposes, hence the current composition of Shengjiang San refers to Bombyx Batryticatus, Cicadae Periostracum, Curcumae Longae Rhizoma, and Rhei Radix et Rhizoma. In this formula, Bombyx Batryticatus and Cicadae Periostracum detoxify and elevate the clear, while Curcumae Longae Rhizoma and Rhei Radix et Rhizoma dispel epidemic qi and descend the turbid. The combination employs both cold and warm properties, facilitating the interdependent ascending and descending movements, thereby achieving the effect of elevating the clear and descending the turbid. Modern pharmacological studies have demonstrated that Cicadae Periostracum, Curcumae Longae Rhizoma, and Rhei Radix et Rhizoma all possess lipid-lowering effects. Specifically, curcumin activates autophagy to regulate oxidative stress and inflammatory responses, improving fatty degeneration in mice with NAFLD and AS [18]. Modified Shengjiang San or its combination with other medications exhibits therapeutic effects on NAFLD complicated by AS. However, such research is mostly confined to simple observations of clinical efficacy, with a notable lack of studies investigating the application of the original Shengjiang San formula and its underlying mechanisms [19, 20]. This significantly constrains the exploratory development of Shengjiang San for the treatment of NAFLD complicated by AS. In summary, based on the theories of “simultaneous liver and heart disease” and “qi mechanism dysregulation,” and integrating the holistic perspective of Chinese medicine, we innovatively propose the “autophagy-qi mechanism dysregulation-simultaneous liver and heart disease” theory. From the angle of “treating the liver and heart together, and dispelling stasis to dredge collaterals,” we have selected the traditional Chinese medicine formula Shengjiang San for the treatment of NAFLD complicated by AS. Experimental validation is conducted to explore the therapeutic effects of Shengjiang San and its potential mechanisms, which is of great significance for seeking effective medications for the prevention and treatment of NAFLD complicated by AS.
In the treatment of atherosclerosis, statins are commonly used as anti-cholesterol ester medications, and clinical studies have reported their beneficial effects on non-alcoholic fatty liver disease (NAFLD) [21]. However, with the prolongation of treatment duration, the issue of drug-induced hepatorenal dysfunction caused by statins cannot be overlooked [22]. In this study, two mice in the atorvastatin group exhibited marked lethargy; one showed a significant decrease in lower limb mobility, and both serum and pathological data indicated evident liver function impairment. The other mouse had a notable increase in serum creatinine, suggesting renal dysfunction, potentially related to rhabdomyolysis. Such adverse effects were not observed in the Shengjiang San treatment group. Based on the experimental results, statins demonstrated overall better direct improvement in atherosclerosis (AS) compared to the Chinese herbal medicine group. However, the high-dose Shengjiang San group exhibited superior efficacy in treating AS-associated NAFLD compared to the atorvastatin group, without apparent abnormalities in liver and kidney function. Moreover, liver antioxidant stress capacity was significantly enhanced, leading to higher overall treatment benefits.
Inflammation and abnormal lipid metabolism are pivotal throughout the entire course of atherosclerosis (AS) and non-alcoholic fatty liver disease (NAFLD). Under the pathological stimulation of various inflammatory factors, pathways related to lymphangiogenesis are activated, leading to lymphatic hyperplasia [23,24,25]. However, excessive pathological lymphatic hyperplasia and remodeling may become the pathophysiological drivers of impaired lipid efflux and inflammatory accumulation [26]. The permeability of intercellular junctions in hepatic lymphatic endothelial cells is crucial for the outward transport of lipoproteins and lipid peroxides from the liver. Pathological remodeling, characterized by thickened lymphatic walls, impairs this clearance function, exacerbating intrahepatic lipid metabolism disorders. Traditional Chinese medicine (TCM), particularly those that promote blood circulation and dredge collaterals or strengthen the spleen and dispel turbidity, plays a positive role in regulating lymphangiogenesis by inhibiting its excessive proliferation and reducing inflammation progression [27,28,29]. We observed a significant increase in the number of hepatic lymphatic vessels, irregular growth morphology, and thickened edges in the model group. Compared to the model group, the TCM treatment group showed marked improvement, suggesting that Shengjiang San also exerts a certain inhibitory effect on the pathological generation of lymphatic vessels.
Upon activation, AMPK promotes mitochondrial biogenesis and autophagy, thereby clearing damaged mitochondria and oxidative products, and alleviating oxidative stress [30]. Additionally, AMPK enhances cellular antioxidant capacity by upregulating the expression of antioxidant enzymes such as SOD and CAT [31, 32]. Excessive activation of mTOR inhibits the autophagy process, leading to the accumulation of damaged mitochondria and oxidative products within cells, thereby exacerbating oxidative stress [33]. Furthermore, mTOR promotes the expression of inflammatory factors by regulating the activity of transcription factors such as nuclear factor-κB (NF-κB), further intensifying oxidative stress and inflammatory responses [34]. The AMPK/mTOR pathway plays a crucial role in the development and progression of atherosclerosis and fatty liver by modulating oxidative stress and inflammatory processes [35, 36]. We found that high-concentration Shengjiang San treatment significantly increased the p-AMPK/AMPK ratio in the liver, while decreasing the p-mTOR/mTOR ratio and the expression levels of inflammatory factors (IL-6, TNF-α), indicating that Shengjiang San inhibits oxidative stress and inflammation through the AMPK/mTOR pathway, thereby exerting a beneficial effect on fatty liver.
In conclusion, our experimental evidence preliminarily demonstrates that Shengjiang San is safe and effective in the treatment of AS combined with NAFLD, with comprehensive efficacy superior to statin therapy. Its underlying mechanism involves inhibiting oxidative stress and inflammation through the AMPK/mTOR pathway and regulating the number of locally generated lymphatic vessels, thereby exerting a therapeutic effect on fatty liver.
References
- Abosheaishaa H, Nassar M, Abdelhalim O, et al. Relation between non-alcoholic fatty liver disease and carotid artery intimal media thickness as a surrogate for atherosclerosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2024;36(5):592–607.
Article CAS PubMed Google Scholar - Młynarska E, Hajdys J, Czarnik W, et al. The role of antioxidants in the therapy of cardiovascular Diseases-A. Literature Rev Nutrients. 2024;16(16):2587.
Google Scholar - Tretyakova VA, Zhernakova NI, Arisheva OS, et al. Meta-Analysis of Non-Alcoholic fatty liver disease and electromechanical reconstruction of myocardium. Arch Razi Inst. 2022;77(1):135–40.
CAS PubMed PubMed Central Google Scholar - Tang ASP, Chan KE, Quek J, et al. Non-alcoholic fatty liver disease increases risk of carotid atherosclerosis and ischemic stroke: an updated meta-analysis with 135,602 individuals. Clin Mol Hepatol. 2022;28(3):483–96.
Article PubMed PubMed Central Google Scholar - Wong MYZ, Yap JJL, Sultana R, et al. Association between non-alcoholic fatty liver disease and subclinical atherosclerosis in Western and Asian cohorts: an updated meta-analysis. Open Heart. 2021;8(2):e001850.
Article PubMed PubMed Central Google Scholar - Yuan W, Ran Y, Wang J, et al. Mediating effect of diabetes on the relationship between nonalcoholic fatty liver disease and atherosclerotic cardiovascular disease: a prospective cohort study. Eur J Gastroenterol Hepatol. 2024;36(9):1133–40.
Article CAS PubMed Google Scholar - Wang W, Guo XL, Qiu XP, et al. Systemic immune-inflammation index mediates the association between metabolic dysfunction-associated fatty liver disease and sub-clinical carotid atherosclerosis: a mediation analysis. Front Endocrinol. 2024;15:1406793.
Article Google Scholar - Ho A, Kiener T, Nguyen QN, et al. Effect of statin use on liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease (NAFLD). J Clin Lipidol. 2024;18(4):e501–8.
Article Google Scholar - Liu M, Shi W, Huang Y, et al. Intestinal flora: a new target for traditional Chinese medicine to improve lipid metabolism disorders. Front Pharmacol. 2023;14:1134430.
Article CAS PubMed PubMed Central Google Scholar - Hung TC, Zhao N, Huang C, et al. Exploring the mechanism of PingTang 5 capsule on nonalcoholic fatty liver disease through network pharmacology and experimental validation.Biomed Pharmacother. Biomed Pharmacother. 2021;138:111408.
Article CAS PubMed Google Scholar - Li B, Fan S, Hu J, et al. Phytochemical analysis using UPLC-MS/MS combined with network pharmacology methods to explore the biomarkers for the quality control of Lingguizhugan decoction. Evid Based Complement Alternat Med. 2021;2021:7849032.
Article PubMed PubMed Central Google Scholar - Liu X, Zhong F, Tang XL, et al. Cordyceps sinensis protects against liver and heart injuries in a rat model of chronic kidney disease: a metabolomic analysis. Acta Pharmacol Sin. 2014;35(5):697–706.
Article CAS PubMed PubMed Central Google Scholar - Kang CC, Wang TE, Liu CY, et al. Update on imaging-based noninvasive methods for assessing hepatic steatosis in nonalcoholic fatty liver disease. J Med Ultrasound. 2024;32(2):116–20.
Article PubMed PubMed Central Google Scholar - Huang XJ, Yin M, Zhou BQ, et al. Impact renaming non-alcoholic fatty liver disease to metabolic associated fatty liver disease in prevalence, characteristics and risk factors. World J Hepatol. 2023;15(8):985–1000.
Article PubMed PubMed Central Google Scholar - Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–52.
Article PubMed Google Scholar - Safaei A, Arefi Oskouie A, Mohebbi SR, et al. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol Hepatol Bed Bench. 2016;9(3):158–73.
PubMed PubMed Central Google Scholar - Torres-Peña JD, Arenas-de Larriva AP, Alcala-Diaz JF, et al. Different dietary Approaches, Non-Alcoholic fatty liver disease and cardiovascular disease. Literature Rev Nutrients. 2023;15(6):1483.
Google Scholar - Jabczyk M, Nowak J, Hudzik B, et al. Curcumin in metabolic health and disease. Nutrients. 2021;13(12):4440.
Article CAS PubMed PubMed Central Google Scholar - Han JH, Su Hs. Observation on the curative effect of Jiadingsan combined with Metformin in the treatment of nonalcoholic fatty liver with metabolic syndrome. Shaanxi Tradit Chin Med. 2013;34(08):989–91.
CAS Google Scholar - Zhang JJ, Yao KW, Li C, et al. Yao Kuiwu uses the method of elevation and elevation in the treatment of cardiovascular diseases. Liaoning J Traditional Chin Med. 2022;49(04):24–7.
CAS Google Scholar - Shah R, Kong A, De Melo S, et al. Association of statins with nonalcoholic fatty liver disease in patients with diabetes. J Investig Med. 2024;72(6):497–510.
Article PubMed Google Scholar - Liu HQ, Ryu D, Hwang S, et al. Therapies for cirrhotic cardiomyopathy: current perspectives and future possibilities. Int J Mol Sci. 2024;25(11):5849.
Article CAS PubMed PubMed Central Google Scholar - Xu X, Lu H, Lin H, et al. Lymphangiogenesis promotes inflammation and neointimal hyperplasia after adventitia removal in the rat carotid artery. Int J Cardiol. 2009;134(3):426–7.
Article PubMed Google Scholar - Huggenberger R, Ullmann S, Proulx ST, et al. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med. 2010;207(10):2255–69.
Article CAS PubMed PubMed Central Google Scholar - Výbohová D, Adamicová K, Mellová Y, et al. Microvascular changes in relation to inflammation and epidermal hyperplasia in chronic cutaneous lesions of psoriasis vulgaris. Histol Histopathol. 2017;32(5):461–70.
PubMed Google Scholar - Burchill MA, Goldberg AR, Tamburini BAJ. Emerging roles for lymphatics in chronic liver disease. Front Physiol. 2020;10:1579.
Article PubMed PubMed Central Google Scholar - Guo Y, Gao W, Ding X, et al. Assessment of the renal function and fibrosis indexes of conventional Western medicine with Chinese medicine for dredging collaterals on treating renal fibrosis: a systematic review and meta-analysis. Open Med. 2024;19(1):20230815.
Article CAS Google Scholar - Shi Y, Wang S, Deng D, et al. Taohong siwu decoction: a classical Chinese prescription for treatment of orthopedic diseases. Chin J Nat Med. 2024;22(8):711–23.
CAS PubMed Google Scholar - Liu P, Xu Y, Ye J, et al. Qingre Huazhuo Jiangsuan Decoction promotes autophagy by inhibiting PI3K/AKT/mTOR signaling pathway to relieve acute gouty arthritis. J Ethnopharmacol. 2023;302Pt A:115875.
Article Google Scholar - Zhou L, Mo Y, Zhang H, et al. Role of AMPK-regulated autophagy in retinal pigment epithelial cell homeostasis: a review. Medicine. 2024;103(28):e38908.
Article CAS PubMed PubMed Central Google Scholar - Tian J, Lu Y, Zhao QL, et al. DHA-enriched phosphatidylserine alleviates bisphenol A-induced liver injury through regulating glycerophospholipid metabolism and the SIRT1-AMPK pathway. Heliyon. 2024;10(14):e34835.
Article CAS PubMed PubMed Central Google Scholar - Guo JT, Li HY, Cheng C, et al. Lead-induced liver fibrosis and inflammation in mice by the AMPK/MAPKs/NF-κB and STAT3/TGF-β1/Smad2/3 pathways: the role of isochlorogenic acid a. Toxicol Res. 2024;13(3):tfae072.
Article CAS Google Scholar - Xie Y, Zhao G, Lei X, et al. Advances in the regulatory mechanisms of mTOR in necroptosis. Front Immunol. 2023;14:1297408.
Article CAS PubMed PubMed Central Google Scholar - Sahan-Firat S, Temiz-Resitoglu M, Sinem Guden D, et al. Protection by mTOR inhibition on zymosan-induced systemic inflammatory response and oxidative/nitrosative stress: contribution of mTOR/MEK1/ERK1/2/IKKβ/IκB-α/NF-κB signalling pathway. Inflammation. 2018;41(1):276–98.
Article CAS PubMed Google Scholar - Lashgari NA, Roudsari NM, Momtaz S, et al. Regulatory mechanisms of vanillic acid in cardiovascular diseases: a review. Curr Med Chem. 2023;30(22):2562–76.
Article CAS PubMed Google Scholar - Sunjaya AP, Sunjaya AF. Targeting ageing and preventing organ degeneration with metformin. Diabetes Metab. 2021;47(1):101203.
Article CAS PubMed Google Scholar