Increase in colonic PRopionate as a method of prEVENTing weight gain in adults aged 20–40 years (iPREVENT): protocol of a multi-centre, double-blind, randomised, parallel-group trial to investigate the efficacy of inulin-propionate ester versus inulin (control) in the prevention of weight gain over 12 months (original) (raw)
Pugh JE, Anjum A, Petropoulou K et al. Increase in colonic PRopionate as a method of prEVENTing weight gain in adults aged 20–40 years (iPREVENT): protocol of a multi-centre, double-blind, randomised, parallel-group trial to investigate the efficacy of inulin-propionate ester versus inulin (control) in the prevention of weight gain over 12 months [version 1; peer review: 1 approved]. F1000Research 2022, 11:1157 (https://doi.org/10.12688/f1000research.125950.1)
Study Protocol
[version 1; peer review: 1 approved]
https://orcid.org/0000-0002-5310-2882
1*, Aisha Anjum2*, Katerina Petropoulou1, [...] George Thom3, Louise Mccombie3, Martina Tashkova1, Sumayya Alaraj-Alshehhi1, Daphne Babalis2, Christina Prechtl
https://orcid.org/0000-0003-4122-8899
2, Mike J Lean3, A. Toby Prevost4, Joana C. Vasconcelos
https://orcid.org/0000-0001-7709-4058
4, Tom Preston5, Douglas Morrison5*, Gary Frost
https://orcid.org/0000-0003-0529-6325
1*
Jennifer E Pugh
https://orcid.org/0000-0002-5310-2882
1*, Aisha Anjum2*, [...] Katerina Petropoulou1, George Thom3, Louise Mccombie3, Martina Tashkova1, Sumayya Alaraj-Alshehhi1, Daphne Babalis2, Christina Prechtl
https://orcid.org/0000-0003-4122-8899
2, Mike J Lean3, A. Toby Prevost4, Joana C. Vasconcelos
https://orcid.org/0000-0001-7709-4058
4, Tom Preston5, Douglas Morrison5*, Gary Frost
https://orcid.org/0000-0003-0529-6325
1*
1 Section of Nutrition Research, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, W12 0NN, UK
2 Imperial Clinical Trials Unit, School of Public Health, Faculty of Medicine, Imperial College London, London, UK
3 School of Medicine, Dentistry and Nursing, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 8QQ, UK
4 Nightingale-Saunders Clinical Trials & Epidemiology Unit, King's Clinical Trials Unit, King's College London, London, SE5 8AF, UK
5 Scottish Universities Environmental Research Centre, College of Science and Engineering, University of Glasgow, Glasgow, G75 0QF, UK
Jennifer E Pugh
Roles: Investigation, Writing – Original Draft Preparation, Writing – Review & Editing
Aisha Anjum
Roles: Project Administration, Validation, Writing – Original Draft Preparation, Writing – Review & Editing
Katerina Petropoulou
Roles: Investigation, Writing – Review & Editing
George Thom
Roles: Investigation, Writing – Review & Editing
Louise Mccombie
Roles: Investigation, Writing – Review & Editing
Martina Tashkova
Roles: Investigation, Writing – Review & Editing
Sumayya Alaraj-Alshehhi
Roles: Investigation, Writing – Review & Editing
Daphne Babalis
Roles: Methodology, Project Administration, Writing – Review & Editing
Christina Prechtl
Roles: Project Administration, Writing – Review & Editing
Mike J Lean
Roles: Methodology, Writing – Review & Editing
A. Toby Prevost
Roles: Methodology, Writing – Review & Editing
Joana C. Vasconcelos
Roles: Formal Analysis, Methodology, Writing – Review & Editing
Tom Preston
Roles: Methodology, Writing – Review & Editing
Douglas Morrison
Roles: Conceptualization, Funding Acquisition, Methodology, Writing – Original Draft Preparation, Writing – Review & Editing
Gary Frost
Roles: Conceptualization, Funding Acquisition, Methodology, Supervision, Writing – Original Draft Preparation, Writing – Review & Editing
OPEN PEER REVIEW
REVIEWER STATUS
Abstract
Introduction: Overweight and obesity affects over 70% of the UK population and is a major risk factor for the development of co-morbidities, including type 2 diabetes and cardiovascular disease. There now exists a considerable evidence base for the management of obesity. However, this is not the case for the prevention of obesity. Preventing weight gain in periods of life where there is an elevated risk of fat mass expansion could be beneficial to preventing associated diseases in later life. This protocol investigates the impact of novel food ingredient inulin propionate ester (IPE) in the prevention of weight gain. This trial aims to investigate the primary hypothesis that IPE has a superior effect on preventing body weight gain, compared with inulin, in young (<40 years old) adults over 12 months, whilst also investigating several complementary mechanisms that may explain the prevention of weight gain and improved long-term energy balance from consuming IPE.
Methods: In this multi-centre, double-blind, randomised, parallel-group study, eligible participants will be randomly assigned to consume 10g IPE or 10g inulin (control) daily for 12 months. Study visits will be conducted at baseline, two-month, six-month and 12-month time points. The primary outcome is weight gain from baseline to 12 months. Secondary outcomes will examine changes in metabolic and cardiovascular health biomarkers, body composition and appetite. A mechanistic sub-group will explore causal mechanisms around energy balance, body composition, appetite regulation and the gut microbiota. Based on the power calculation, the sample size required is 270 participants or 135 per study group.
Ethics and dissemination: The trial protocol and participant-facing documents have been reviewed and approved, by the London Hampstead Ethics Committee (REC Reference 19/LO/0095, 29th January 2019). Upon completion, the trial results will be published in peer-reviewed journals and presented at scientific conferences.
Trial registration number: ISRCTN16299902, 1st March 2018.
Keywords
obesity, prevention, short-chain fatty acids, propionate, gut microbiota
Corresponding authors: Douglas Morrison, Gary Frost Competing interests: GF, DM, and TP are named inventors on the patent Compounds and their effects on appetite control and insulin sensitivity WO2014020344A1 and are founding directors of a Spinout company aimed at commercialising IPE production.
Grant information: This work is funded by National Institute for Health Research (NIHR) under the Efficacy and Mechanism Evaluation Programme (EME) [15/185/16].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright: © 2022 Pugh JE et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. How to cite: Pugh JE, Anjum A, Petropoulou K et al. Increase in colonic PRopionate as a method of prEVENTing weight gain in adults aged 20–40 years (iPREVENT): protocol of a multi-centre, double-blind, randomised, parallel-group trial to investigate the efficacy of inulin-propionate ester versus inulin (control) in the prevention of weight gain over 12 months [version 1; peer review: 1 approved]. F1000Research 2022, 11:1157 (https://doi.org/10.12688/f1000research.125950.1) First published: 10 Oct 2022, 11:1157 (https://doi.org/10.12688/f1000research.125950.1) Latest published: 10 Oct 2022, 11:1157 (https://doi.org/10.12688/f1000research.125950.1)
Trial Registration
ISRCTN: 16299902, 1st March 2018, https://doi.org/10.1186/ISRCTN16299902
Protocol version
V5.0, 15th February 2021
Introduction
Overweight and obesity affect over 68.2% of men, and 60.4% of women in the UK and drive the prevalence of several common co-morbidities, including type 2 diabetes, cardiovascular disease and cancer. Although significant advances have been achieved in the management of obesity, there has not been the same level of research activity in obesity prevention. Once an individual becomes obese the probability of returning to normal body weight is extremely low (1 in 210 for men and 1 in 124 for women).1
Weight gain occurs commonly throughout adulthood and younger adults are at the greatest risk of substantial gains in body weight, according to a National Health and Examination Survey (NHANES) of adults aged 25–74. Major weight gain over 10 years is categorised as a gain in Body Mass Index (BMI) ≥5 kg/m2, this weight gain was highest in those aged 25–35 years.2 Whilst a relatively modest weight gain of 1 kg over a single year would present a very low risk to health in young adults, the accumulated weight gain over a decade or longer leads to a clear deterioration of cardiovascular and diabetes risk factors. For example, the 10-year Coronary Artery Risk Development in Young Adults (CARDIA) study demonstrated that weight gain during early adulthood produced measurable adverse changes in blood lipids, fasting insulin, and blood pressure, irrespective of race or gender.3
Epidemiological and experimental studies have demonstrated an inverse association between dietary fibre intake and body weight gain.4,5 Current fibre intake is approximately 17–20g/d,6 well below the 30g recommendation.7 The daily intake of dietary fibre in the UK has not increased for 10 years.6 Although the mechanisms surrounding how elevated dietary fibre intake affects energy balance are not fully understood there is evidence that the fermentation of dietary fibre in the colon by the microbiota produces short chain fatty acids (SCFA) that stimulate the release of anorectic gastrointestinal hormones PYY and GLP-1.8,9 In previous research, a methodology for delivering the SCFA propionate to the colon was developed by esterifying it to a non-digestible fructooligosaccharide. It has been demonstrated that this novel food ingredient, inulin propionate ester (IPE), stimulated the release of PYY and GLP-1 and lowered energy intake.10 The proof of principle study demonstrated that over six months, the consumption of 10 g of IPE daily significantly prevented weight gain.10
The current study aims to investigate the impact of IPE on the prevention of weight gain in young adults aged 20 to 40 years over a period of 12 months.
Protocol
Trial design
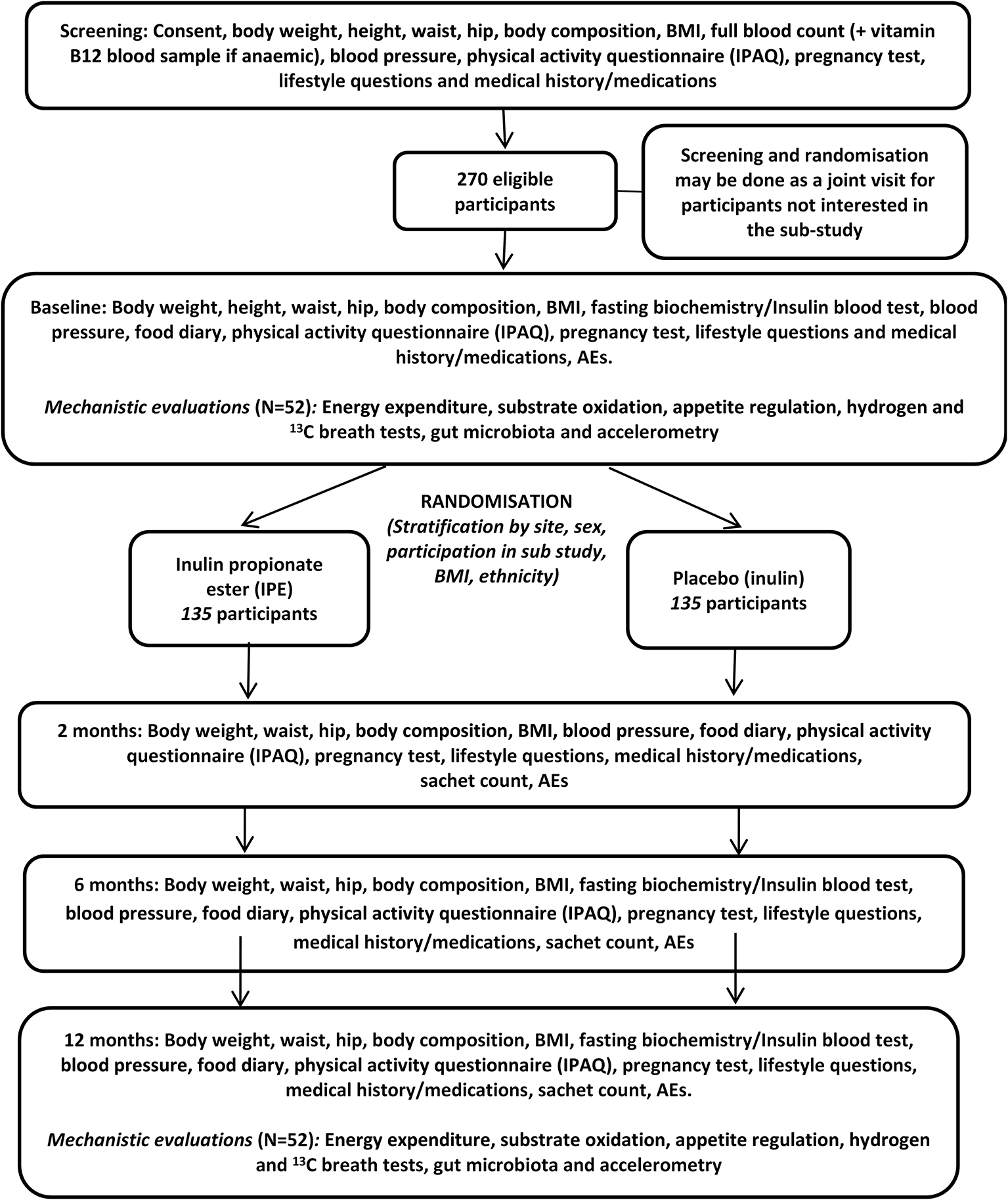
This clinical trial is a randomised, placebo-controlled, double-blind trial to investigate the efficacy and safety of inulin propionate ester (IPE) versus the inulin (fermentable oligosaccharide) control upon weight gain prevention. Participants will be randomised to either IPE or inulin for 12 months. This trial will be performed at two UK sites: Imperial Clinical Research Facility (CRF) in London - Imperial College Healthcare NHS Trust and Glasgow CRF - NHS Research Scotland. This trial was registered with International Standard Randomised Controlled Trial Number (ISRCTN) registry (ISRCTN16299902) on 1st March 2018 (https://doi.org/10.1186/ISRCTN16299902), before recruitment commenced. Participants will be invited to attend a screening, baseline/randomisation and all subsequent trial visits at two, six and 12 months will be conducted at the CRF of each participating site. See participant timeline, Figure 1. Participants recruited at Imperial will also be invited to participate in a mechanistic sub-study and if they agree these assessments will take place at baseline and 12 months at the Imperial CRF.
Figure 1. Clinical trial flow chart.
Inclusion and exclusion criteria
Inclusion criteria
- • Males and females aged 20–40 years
- • Body Mass Index (BMI) of 24.0–27.0 kg/m2 if of South-Asian ethnicity or 25.0–30.0 kg/m2 if non-South-Asian
- • At least one of the following:
- ○ A self-reported weight gain of 2 kg or more over the last 12 months
- ○ Low self-reported physical activity (‘low’ activity as per International Physical Activity Questionnaire)
- ○ <2 servings of fruit and vegetables consumed per day
- ○ >1 serving of sugar-sweetened beverages per day
- • On same medication for >3 months at point of screening
- • Written informed consent
Exclusion criteria
- • Diagnosed chronic disease Type 1 and 2 diabetes, cancer, renal failure, heart disease, organic acidaemia (propionic acidaemia, methylmalonic acidaemia)
- • Diagnosed gastrointestinal condition (coeliac disease, inflammatory bowel disease and irritable bowel syndrome)
- • Previous bowel reconstruction surgery
- • Pregnancy or lactation
- • Use of antibiotics in the past three months
- • Vitamin B12 deficiency (<160 ng/L)
- • Taking part in a weight loss program or consuming a weight loss product
- • Have lost 3 kg or more in the last three months
- • Gastrointestinal upset in the last two weeks
Recruitment
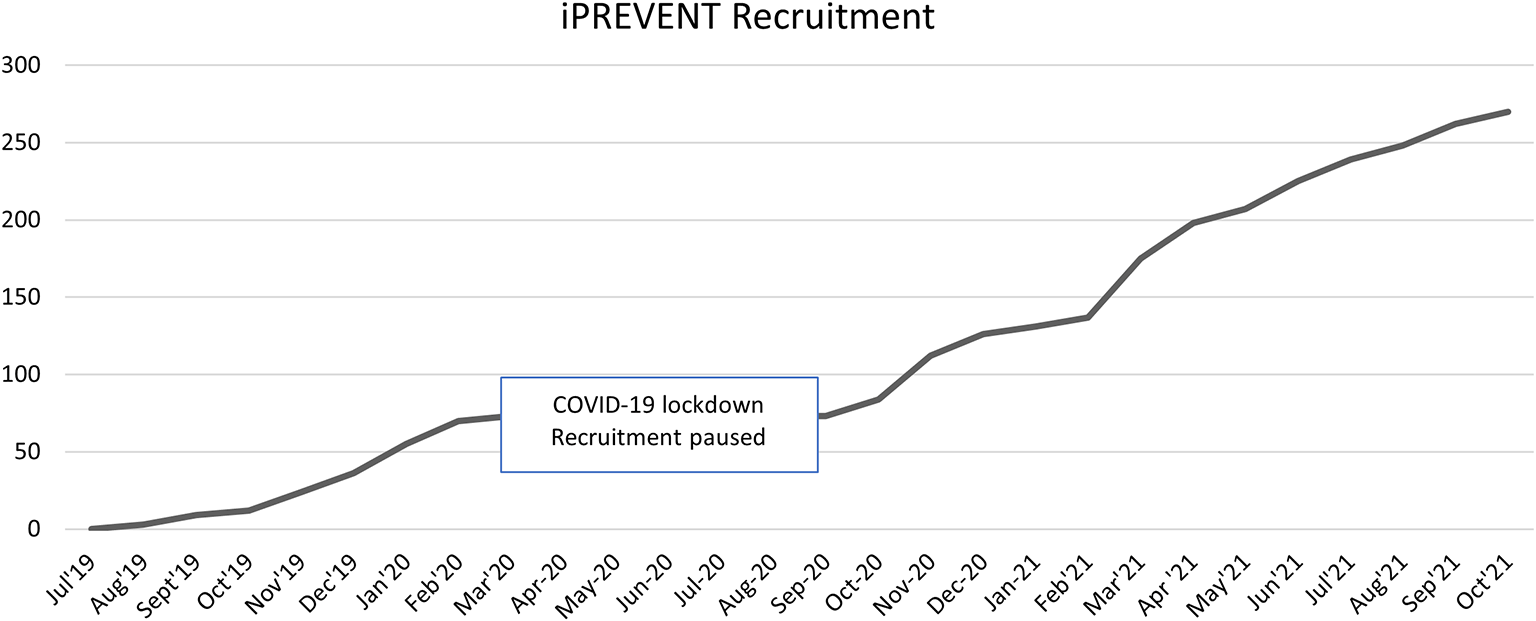
A wide variety of recruitment methods were used for this clinical trial, including recruitment via GP practices and within NHS trusts, newspaper adverts, pop-up events and posters, amongst other methods. Recruitment was completed in October 2021 (Figure 2).
Figure 2. Recruitment timeline.
Allocation and randomisation
Randomisation will be undertaken using minimisation with a random element to balance the arms by research centre, sex, and BMI within ethnicity (South Asians: 24.00–25.49 kg/m2 and 25.50–27.00 kg/m2/non-South Asians: 25.00–27.49 kg/m2 and 27.50–30.00 kg/m2) and whether they take part in the mechanistic sub-study. Minimisation will be conducted by researchers using sealed envelope software (Open-source software, www.sealedenvelope.com).
Blinding and unblinding
Participants will receive blinded and identical-looking trial intervention of either IPE or inulin control. Both IPE and inulin present as white powders and will be delivered in pre-packed plain foil-backed sachets. Participants will be identified with a unique trial identifier and each IPE or control sachet will be identified with a unique treatment code linked to the allocation and trial identification (ID). The treatment code will not be broken except in medical emergencies or if expedited reporting to the Research Ethics Committee (REC) of an unexpected and related Serious Adverse Event (SAE) is required.
Study procedures
At each trial visit, trial researchers will conduct all necessary measurements and sample collections as described in Tables 1 and 2. In the main study, written informed consent is given at the screening visit, blood samples are taken (excluding two-month visits), blood pressure, body weight, body composition and compliance are measured and occurrence of Adverse Events (AEs) and SAEs are documented at all study visits. In the sub-study breath, stool, urine and blood samples are taken and breath hydrogen, energy expenditure, subjective appetite and energy intake are measured.
Table 1. Main study visit schedule.
| Assessment | Screening | Baseline/randomisation | Two months (± two weeks) | Six months (± four weeks) | 12 months (± four weeks) |
|---|---|---|---|---|---|
| Joint screening/randomisation | |||||
| Consent | X | ||||
| Demographics | X | ||||
| Randomisation | X | ||||
| Medical History | X | X | X | X | X |
| Concomitant medications | X | X | X | X | X |
| Pregnancy test - females only | X | X | X | X | X |
| Vital signs: Diastolic Blood Pressure (DBP) Systolic Blood Pressure (SBP) Heart rate (HR) | X | X | X | X | X |
| Trial intervention (IPE or inulin control) | X | X | X | ||
| Height, body weight, waist/hip measurements, BMI, body composition | X | X | X | X | X |
| Fasting blood test (glucose, insulin, lipid profile) | X | X | X | ||
| Full Blood Count (FBC) and vitamin B12 (if required) | X | ||||
| Food diary | X | X | X | X | |
| International Physical Activity Questionnaire (IPAQ) | X | X | X | X | X |
| Lifestyle questions | X | X | X | X | X |
| Sachet count (compliance) | X | X | X | ||
| Adverse event tracking | X | X | X | X |
Table 2. Mechanistic sub-study visit schedule.
| Assessment | Screening | Baseline/randomisation | 12 months (± four weeks) |
|---|---|---|---|
| Blood sample to measure the natural abundance of deuterated water | X | ||
| Energy expenditure (indirect calorimetry) | X | X | |
| Appetite regulation (VAS, food diary, ad libitum test meal, and blood tests for anorectic gut hormones) | X | X | |
| Substrate oxidation/DNL (via stable isotope tracers in water consumption) – 13C breath, urine and blood samples | X | X | |
| Gut microbiota (stool sample and hydrogen breath test) | X | X | |
| Neuroendocrine cell number (stool sample) | X | X | |
| Accelerometry | X | X |
Outcomes
Primary outcome
The primary outcome is weight gain from baseline to 12 months.
Secondary outcomes
- • Occurrence of AEs and SAEs
- • Changes in fasting biochemistry:
- - Glucose
- - Insulin
- - Triglycerides
- - Total cholesterol
- - Low-Density Lipoprotein (LDL) cholesterol
- - High-Density Lipoprotein (HDL) cholesterol
- • Changes in blood pressure
- • Changes in body weight
- • Changes in waist/hip/BMI/body composition measurements
- • Changes in compliance
Mechanistic outcomes
- • Gut microbiota: 16S rRNA profiles from stool samples
- • Impact on neuroendocrine cell number: Proliferation in intestinal organoids using the level of SCFA and other metabolites identified from nuclear magnetic resonance spectroscopic analyses of stool
- • Appetite regulation: Measured by visual analogue scales (VAS), food diaries, ad libitum intake, and appetite-regulating gut hormones PYY, GLP-1, Gastrin and CCK
- • Energy expenditure: Open-loop indirect calorimetry
- • Hepatic lipid metabolism: Stable isotope tracers of fat oxidation (13C palmitate) and De Novo Lipogenesis (DNL)
- • Total body water through dilution analysis of 2H2O as already applied.
Protocol amendments
Proposed amendments to the protocol and aforementioned documents will be submitted to the REC for approval as instructed by the Sponsor. Amendments requiring REC approval may be implemented only after a copy of the REC’s approval letter has been obtained. The regulatory authorities and REC will be sent annual progress reports and informed about the end of the trial, within the required timelines. Table 3 details the amendments that have been submitted/approved by the REC so far.
Table 3. Protocol amendments.
The following amendments have been submitted/approved by the REC so far.
| Amendment No. | Type | Approval date by Ethics/HRA | Changes |
|---|---|---|---|
| Amendment 1 | Substantial | Aug-19 | • Revised protocol (admin changes, updated sampling details, NHS digital follow-up details, updated packaging company details).• PIS/CF changes to reflect the above.• Study templates include newspaper advertisements, participant diaries, questionnaires and instructions. |
| Amendment 2 | Substantial | Jan-20 | • Age in inclusion criteria extended from '20-35 years' to '20-40 years'.• PIS/CF changes to reflect the above.• Updated advertising details including template text message for GPs and study webpage.• Study templates including poster ad and updated pre-screening questionnaire. |
| Amendment 3 | Non-substantial | Apr-20 | • Notification of pause in recruitment due to initial COVID-19 lockdown.• Guideline document created for remote follow-up visits/changes to the trial during this period. |
| Amendment 4 | Substantial | Aug-20 | • The title of the project was amended as requested by NIHR, following the previous change in inclusion criteria (age).• Clarification of recruitment strategies being used.• Addition of PIC site.• Script and topic guide drafted for animation video and information videos to be created, for social media. |
| Amendment 5 | Non-substantial | Nov-20 | • Study clinic visits resuming at all sites, following the pause in March 2020 due to COVID-19 restrictions.• Additional data collection was required as remote visits did not capture accurate primary outcome data, and blood samples (for secondary outcomes) were missed.• Wider visit windows for flexibility.• Additional NHS boards were added for Scotland, for a wider search of research volunteers via the SHARE database.• Addition of the PIC site. |
| Amendment 6 | Substantial | Feb-21 | • Removal of ECG from screening procedures.• Exclusion criteria for the main study were amended from 'vitamin B12 deficiency' to 'untreated vitamin B12 deficiency.• ‘Anaemia’ specified as an exclusion for sub-study only (previously for main study too).• Joint screening/randomisation visit for those not taking part in the sub-study and addition of remote consent as a 7-day food diary required to be completed before the joint visit.• PIS/CF changes to reflect all the above. |
| Amendment 7 | Non-substantial | Sep-21 | • Costed extension from NIHR - new grant end date. |
Withdrawals
Participants may discontinue trial intervention for the following reasons:
- • At the request of the participant
- • Due to an Adverse Event/Serious Adverse Event
- • If the investigator considers that a participant's health will be compromised due to AEs or concomitant illnesses that develop after entering the trial.
If a participant permanently discontinues the trial intervention, they will be invited to continue to attend trial visits, if possible, to allow for the collection of key outcome and safety data.
Discontinuation of trial intervention and procedures can occur for the following reasons:
- • Participant decision (withdrawal of consent)
- • Loss to follow-up
If a participant withdraws from trial procedures, an attempt will be made to obtain self-reported body weight (primary endpoint) at the point of withdrawal and at the final study visit time point. If the participant does not agree for data and samples collected to be retained, the samples must be destroyed and data excluded from the analyses.
If the participant withdraws consent to further be contacted at all for the study purposes, no attempts to obtain a self-reported body weight for the primary endpoint will be made.
Adverse events and serious adverse events
All serious and non-serious AEs will be reported on the trial database, from the point of screening until the end of the study, except for elective medical procedures. All SAEs will be reviewed by both the local investigator and chief investigator or a designated medically qualified representative to confirm ‘expectedness’ and ‘causality’. SAEs are assessed on whether they are possibly, probably or definitely related to the trial protocol/intervention. An unexpected event would be a type of event that is not listed in the trial protocol or document associated with the intervention, as an expected occurrence. Expected AEs for this trial are gastrointestinal effects. If the investigator becomes aware of safety information that appears to be related to the trial, this should be reported to the trial coordination centre and sponsor.
Patient and public involvement (PPI)
PPI panels were held during the design and management and recruitment phases of this clinical trial.
Study status
Recruitment for this study was completed in October, the study is currently in follow-up phase and 12-month visits will be completed by the end of October 2022.
Statistical analysis
Sample size calculations
In the randomised proof of concept trial, the difference between arms in the change in body weight over 24 weeks was 1.4 kg (95% CI: -0.3 to 3.1), p = 0.099. Using a Bayesian method recommended for preliminary trials in which evidence in the 95% CI is translated into probabilities,11 there was a 95% posterior probability of an underlying positive between-arm difference favouring the intervention. The posterior probability of intervention-favouring differences greater than 1 kg, 1.5 kg, and 2 kg were respectively 69%, 47% and 25% based on 24-week intervention. The difference increased in magnitude through successive eight-week, 16-week, and 24-week time points. By 24 weeks there were significant reductions in the proportion of intervention participants gaining 3%, and 5% of body weight from a mean baseline of 90 kg. A 2 kg between-arm 12-month effect size was therefore chosen. This agreed with a weight gain prevention trial over nine months in young adults12 which aimed to detect a 2 kg effect and achieved 4.3 kg, with a pooled standard deviation (SD) for body weight change of 4.35 kg, and 81% retention.
On this basis a sample size of 270 randomised participants (135 per arm) was chosen to provide 90% power to detect a 2 kg difference between arms in mean body weight change over 12 months using a two-sided 5% level significance test, assuming a 4.35 kg SD and with 25% dropout allowance (68 participants). The sample size was calculated using R Project for Statistical Computing (RRID: SCR_001905).
For the mechanistic study, 34 volunteers (17 per group) would provide sufficient statistical power to detect a 15 pmol/L effect size in PYY and GLP-1 concentrations between groups, with 90% power, 5% significance level, SD 13 pmol/L. These differences are based on previously published findings that report enhanced gut hormone release following IPE supplementation.9,13 A subsample of 52 volunteers (26 per group), was chosen, to allow a 70% retention rate.
Data analysis plan
This trial has both a pragmatic element, to answer the question of whether the policy of prescribing and uptake of inulin propionate ester as specified in the trial will reduce further weight gain compared with control, and an explanatory element to understand the mechanisms of the causal pathway of such body weight change and any limitations from compliance. Therefore, analyses will be primarily on an ‘Intention to Treat’ basis. Secondarily, analyses will incorporate mechanistic sub-study data, and use this to understand the ‘Intention to Treat’ effect estimated in the trial.
Statistical methods for the analysis of the study endpoints are described below. IBM SPSS Statistics (RRID: SCR_019096), version 28.0.1, will be used for all statistical analyses. A separate detailed Statistical Analysis Plan (SAP) has been prepared and approved by the Trial Steering Committee.14 This contains the rationale for the methods chosen and the assessment of their assumptions. It includes pre-specifying the handling of covariates and missing data, compliance analyses, and the primary estimand. Changes to the plan can be revised and re-approved by trial oversight committees prior to database lock.
Any deviations from the SAP during analysis after the data lock will be documented and signed off by the statisticians and CI and filed in the statistics section of the Trial Master File (TMF) which will be merged with the main TMF at the end of the study. The final approved SAP, being in accordance with the protocol, takes precedence for undertaking the analysis of the main trial paper.
Primary endpoint analysis
The analysis of the primary endpoint will incorporate the earlier correlated interim measurements of body weight in a linear mixed effects model and will adjust for baseline continuous body weight and other categorical randomisation stratifiers with further specification of the role of timepoint, and correlation structure, detailed in the SAP. The implicit ‘missing at random’ assumption will be challenged through a set of sensitivity analyses.15 As these involve all randomised participants, this is therefore an Intention to Treat Strategy.16
Secondary endpoints analysis
Where possible, continuous secondary endpoints will be adjusted for their baseline to improve the precision of estimated intervention effects. Repeated measures will be analysed using linear mixed-effects models adjusting also for randomisation stratifiers. Comparisons between arms for binary outcomes will be summarised as differences in proportions. Confidence intervals of 95% will be used to make inferences from estimated effect sizes.
Ethics and dissemination
Ethical and safety considerations
The investigator(s) will ensure that this trial is conducted in full conformity with the 7th revision of the 1964 Declaration of Helsinki. The trial will be conducted following the guidelines laid down by the International Conference on Harmonisation for Good Clinical Practice (ICH GCP E6 guidelines and Addendum ICH GCP E6 (R2)). The trial protocol and participant-facing documents have been reviewed and approved by the London Hampstead REC Reference 14/LO/2004). Clinical trial authorisation from the Medicines and Healthcare products Regulatory Agency (MHRA) is not required as the study intervention is a dietary supplement.
Data management
All personal identifiable data, including screened patients, will be kept securely in the local site files and will not be uploaded to the main trial database. The InForm™ trial database will be used for all visits data entries. All data recorded in the eCRFs will be signed off by the site investigator. The central coordinating site will visit local recruiting sites to ensure compliance with the protocol, good clinical practice and local regulatory compliance.
Dissemination policy
All publications and presentations relating to the study will be authorised by the Trial Management Group. Authorship will be determined according to the internationally agreed criteria for authorship (www.icmje.org). Authorship of parallel studies initiated outside of the Trial Management Group will be according to the individuals involved in the project but must acknowledge the contribution of the Trial Management Group and the Study Coordination Centre.
Trial organisation and committees
A fully independent Data Monitoring and Ethics Committee (DMEC) has been set up to monitor progress, participant safety and any ethical issues involved in this trial. They review trial progress, recruitment rates and safety data. Meetings are approximately six-monthly. Furthermore, a Study Advisory Group consisting of public representatives was also set up. The committees were set up and coordinated in accordance with the funder guidelines. Upon completion, the trial results will be published in peer-reviewed journals and presented at national and international scientific meetings. A Trial Steering Committee (TSC) has been convened to provide overall supervision of trial conduct and progress. The TSC meet approximately six-monthly throughout the trial. DMEC reports include details of recruitment, randomisation balance and stratification effectiveness, baseline characteristics, unblinding, withdrawals, compliance, concomitant medications, efficacy, mediators, and Aes.
Discussion
To date, few randomised controlled trials focus on the prevention of obesity, especially in those most at risk of weight gain.2 High intakes of dietary fibre are associated with lower body weight.13 Evidence suggests that this is due to the generation of SCFA from colonic fermentation of dietary fibre by the gut microbiota.8 Furthermore, there is prior evidence to suggest that IPE could have a substantial impact on lowering individuals’ weight gain trajectory leading to a lowering of their long-term risk of obesity-related co-morbidities.9,12 This trial will explore all aspects of the novel food ingredient IPE; assessing efficacy via the primary endpoint of weight change, whilst attempting to identify potential mechanisms via the sub-study and accounting for compliance, tolerability and safety. The latter is an aspect that often goes unreported in similar trials; however, it is required to build a multifaceted evaluation of any intervention. This study methodology is not without limitations, primarily, narrowing the age range of the study sample may mean that results cannot be translated to the wider population. Additionally, the aforementioned scarcity of studies exploring the effects of SCFA on human populations meant that researchers had few studies to refer to when calculating population sample size, which may lead to a difference in predicted versus actual study power. However, it should be acknowledged that the study duration and sample size are greater than similar previously conducted studies.9,17–20 This randomised controlled trial aims to build upon previous literature to support evidence for the delivery of propionate to the colon for the prevention of weight gain.21
Conclusions
Obesity research has limited emphasis on prevention. Therefore, this randomised controlled double-blinded trial was designed to investigate whether inulin-propionate ester prevents further weight gain in adults most at risk of substantial increases in their BMI. Furthermore, the study adopts novel approaches to determine the underlying mechanisms influencing these beneficial effects.
Data availability
Underlying data
No data are associated with this article.
Extended data
Mendeley Data: Extended data for ‘Increase in colonic Propionate as a method of prEVENTing weight gain in adults aged 20-40 years (iPREVENT): A multi-centre, double-blind, randomised, parallel-group study to investigate the efficacy of inulin-propionate ester versus inulin (control) in the prevention of weight gain over 12 months’ statistical analysis plan’.
- • Data file 1: iPREVENT Statistical Analysis Plan. pdf. https://doi.org/10.17632/n33kky5dww.214
- • Data file 2: iPREVENT Protocol v5.0 15Feb2021.pdf. https://doi.org/10.17632/5n7h4xfz2n.1.21
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)
Acknowledgements
Infrastructure support for this research was provided by the NIHR Imperial Biomedical Research Centre (BRC) and the NIHR Imperial Clinical Research Facility.
References
- 1. Fildes A, Charlton J, Rudisill C, et al.: Probability of an Obese Person Attaining Normal Body Weight: Cohort Study Using Electronic Health Records. Am. J. Public Health. 2015 Sep; 105(9): e54–e59. PubMed Abstract | Publisher Full Text
- 2. Williamson DF, Kahn HS, Remington PL, et al.: The 10-year incidence of overweight and major weight gain in US adults. Arch. Intern. Med. 1990 Mar; 150(3): 665–672. PubMed Abstract | Publisher Full Text
- 3. Norman JE, Bild D, Lewis CE, et al.: The impact of weight change on cardiovascular disease risk factors in young black and white adults: the CARDIA study. Int. J. Obes. Relat. Metab. Disord. 2003 Mar; 27(3): 369–376. PubMed Abstract | Publisher Full Text
- 4. Ludwig DS, Pereira MA, Kroenke CH, et al.: Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA. 1999 Oct 27; 282(16): 1539–1546. PubMed Abstract | Publisher Full Text
- 5. Du H, van der AD, Boshuizen HC, et al.: Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am. J. Clin. Nutr. 2010 Feb; 91(2): 329–336. PubMed Abstract | Publisher Full Text
- 6. Whitton C, Nicholson SK, Roberts C, et al.: National Diet and Nutrition Survey: UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br. J. Nutr. 2011 Dec; 106(12): 1899–1914. PubMed Abstract | Publisher Full Text
- 7. Eaton SB: The ancestral human diet: what was it and should it be a paradigm for contemporary nutrition? Proc. Nutr. Soc. 2006 Feb; 65(1): 1–6. Publisher Full Text
- 8. Cummings JH, Pomare EW, Branch WJ, et al.: Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct; 28(10): 1221–1227. PubMed Abstract | Publisher Full Text | Free Full Text
- 9. Chambers ES, Viardot A, Psichas A, et al.: Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015 Nov; 64(11): 1744–1754. PubMed Abstract | Publisher Full Text
- 10. Burton PR, Gurrin LC, Campbell MJ: Clinical significance not statistical significance: a simple Bayesian alternative to p values. J. Epidemiol. Community Health. 1998 May; 52(5): 318–323. PubMed Abstract | Publisher Full Text | Free Full Text
- 11. Allman-Farinelli M, Partridge SR, McGeechan K, et al.: A Mobile Health Lifestyle Program for Prevention of Weight Gain in Young Adults (TXT2BFiT): Nine-Month Outcomes of a Randomized Controlled Trial. JMIR Mhealth Uhealth. 2016 Jun 22; 4(2): e78. PubMed Abstract | Publisher Full Text
- 12. Polyviou T, MacDougall K, Chambers ES, et al.: Randomised clinical study: inulin short-chain fatty acid esters for targeted delivery of short-chain fatty acids to the human colon. Aliment. Pharmacol. Ther. 2016 Oct; 44(7): 662–672. PubMed Abstract | Publisher Full Text | Free Full Text
- 13. Reynolds A, Mann J, Cummings J, et al.: Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019 Feb 2; 393(10170): 434–434, 445. PubMed Abstract | Publisher Full Text
- 14. Vasconcelos J, Toby P, Morrison D, et al.: Increase in colonic PRopionate as a method of prEVENTing weight gain in young adults: IPREVENT Clinical Trial Statistical Analysis Plan. Mendeley Data, V2, [Dataset]. 2022. Publisher Full Text
- 15. Sivaprasad S, Prevost AT, Vasconcelos JC, et al.: Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017 Jun; 389(10095): 2193–2203.
- 16. White IR, Carpenter J, Horton NJ: Including all individuals is not enough: lessons for intention-to-treat analysis. Clin. Trials. 2012; 9(4): 396–407. PubMed Abstract | Publisher Full Text
- 17. Chambers ES, Byrne CS, Rugyendo A, et al.: The effects of dietary supplementation with inulin and inulin-propionate ester on hepatic steatosis in adults with non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019 Feb; 21(2): 372–376. PubMed Abstract | Publisher Full Text
- 18. Chambers ES, Byrne CS, Morrison DJ, et al.: Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut. 2019 Aug 1; 68(8): 1430–1438. Publisher Full Text
- 19. Byrne CS, Chambers ES, Preston T, et al.: Effects of Inulin Propionate Ester Incorporated into Palatable Food Products on Appetite and Resting Energy Expenditure: A Randomised Crossover Study. Nutrients. 2019 Apr 16; 11(4): E861. Publisher Full Text
- 20. Polyviou T, MacDougall K, Chambers ES, et al.: Randomised clinical study: inulin short-chain fatty acid esters for targeted delivery of short-chain fatty acids to the human colon. Aliment. Pharmacol. Ther. 2016 Oct; 44(7): 662–672. PubMed Abstract | Publisher Full Text | Free Full Text
- 21. Frost G, Morrison D, Anjum A, et al.: “Protocol for the IPREVENT Trial”, Mendeley Data, V1, [Dataset].2022. Publisher Full Text
Comments on this article Comments (0)
Version 1
VERSION 1 PUBLISHED 10 Oct 2022
GF, DM, and TP are named inventors on the patent Compounds and their effects on appetite control and insulin sensitivity WO2014020344A1 and are founding directors of a Spinout company aimed at commercialising IPE production.
This work is funded by National Institute for Health Research (NIHR) under the Efficacy and Mechanism Evaluation Programme (EME) [15/185/16].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© 2022 Pugh JE et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Open Peer Review
Current Reviewer Status: ?
Key to Reviewer Statuses VIEW HIDE
ApprovedThe paper is scientifically sound in its current form and only minor, if any, improvements are suggested
Approved with reservations A number of small changes, sometimes more significant revisions are required to address specific details and improve the papers academic merit.
Not approvedFundamental flaws in the paper seriously undermine the findings and conclusions
Version 1
VERSION 1
PUBLISHED 10 Oct 2022
Reviewer Report 06 May 2024
Hoi Leong Xavier Wong, Hong Kong Baptist University, Hong Kong, China
Approved
VIEWS 0
- Is the rationale for, and objectives of, the study clearly described?
Yes - Is the study design appropriate for the research question?
Yes - Are sufficient details of the methods provided to allow replication by others?
Yes - Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Metabolic disorder, Microbiome, Metalloproteinase
Close
Comments on this article Comments (0)
Version 1
VERSION 1 PUBLISHED 10 Oct 2022
Open Peer Review
Reviewer Status
Alongside their report, reviewers assign a status to the article:
Approved
The paper is scientifically sound in its current form and only minor, if any, improvements are suggested
Approved with reservations
A number of small changes, sometimes more significant revisions are required to address specific details and improve the papers academic merit.
Not approved
Fundamental flaws in the paper seriously undermine the findings and conclusions
Reviewer Reports
| Invited Reviewers | |
|---|---|
| 1 | |
| Version 1 10 Oct 22 | read |
- Hoi Leong Xavier Wong, Hong Kong Baptist University, Hong Kong, China
Comments on this article
Sign up for content alerts