Probiotics in inflammatory bowel disease: Does it work? (original) (raw)
Review Open Access
Copyright ©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
World J Meta-Anal. Apr 28, 2020; 8(2): 54-66
Published online Apr 28, 2020. doi: 10.13105/wjma.v8.i2.54
Probiotics in inflammatory bowel disease: Does it work?
Natália Oliveira e Silva, Breno Bittencourt de Brito, Filipe Antônio França da Silva, Maria Luísa Cordeiro Santos, Fabrício Freire de Melo, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista 45029-094, Bahia, Brazil
Author contributions: All authors equally contributed to this paper with conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version.
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
http://creativecommons.org/licenses/by-nc/4.0/
Corresponding author: Fabrício Freire de Melo, PhD, Postdoctoral Fellow, Professor, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Rua Hormindo Barros, 58, Quadra 17, Lote 58, Vitória da Conquista 45029-094, Bahia, Brazil. freiremelo@yahoo.com.br
Received: December 29, 2020
Peer-review started: December 29, 2019
First decision: February 29, 2020
Revised: March 26, 2020
Accepted: April 15, 2020
Article in press: April 15, 2020
Published online: April 28, 2020
Processing time: 120 Days and 17.8 Hours
Abstract
The number of patients with inflammatory bowel disease (IBD), a group of diseases mainly represented by Crohn’s disease (CD) and ulcerative colitis (UC), has increased in recent decades. As a consequence, the number of people undergoing any drug treatment against these diseases has expanded. However, IBD conventional therapies present several limitations, which lead researchers to look for better alternatives to improve the quality of life of patients. Moreover, microbiome imbalance seems to play a crucial role in the pathogenesis of IBD, since important alterations in bacterial, viral, protist and fungal populations are observed in the gut microbiota of affected individuals. Given the importance of such life forms in that context, the use of probiotics becomes a plausible alternative for treating affected patients. Trials have been developed aiming the evaluation of probiotics potential to induce and to maintain remission in CD and UC. Regarding the tested microorganisms, various non-pathogenic bacteria and fungi have been assessed. However, consistent results have been obtained only with some of them, including Escherichia coli Nissle 1917, VSL#3, Saccharomyces boulardii, Lactobacillus, and Bifidobacterium. Therefore, this minireview aims to explore the role of microbiota in the genesis of such a disorder and to compile the most concrete data on probiotic-related efficiency in IBD treatment.
Core tip: The clinical management of ulcerative colitis and Crohn’s disease represent a major challenge in the gastroenterology field since conventional therapies present several limitations. Interestingly, changes in gut microbiota are linked to the development of these diseases. In this sense, the use of probiotics becomes a plausible alternative for treating affected individuals. Although several microorganisms have been tested for this purpose, satisfactory results have been obtained only with a portion of them. Therefore, this minireview aims to explore the role of microbiota in the pathogenesis of inflammatory bowel disease and to compile the most concrete data on probiotics efficiency in its treatment.
- Citation: Silva NOE, de Brito BB, da Silva FAF, Santos MLC, de Melo FF. Probiotics in inflammatory bowel disease: Does it work? World J Meta-Anal 2020; 8(2): 54-66
- URL: https://www.wjgnet.com/2308-3840/full/v8/i2/54.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i2.54
INTRODUCTION
Inflammatory bowel disease (IBD) is a group of chronic diseases that significantly affects patients quality of life and is mainly represented by Crohn’s disease (CD) and ulcerative colitis (UC)[1]. Although IBD pathophysiology is widely studied and intestinal microbiota seems to play a crucial role in this process, there are still several unclear points about that[2]. However, it is well known that the existence of positive first-degree relatives for these diseases, as well as environmental exposures including psychological stress, antimicrobial use, and dietary factors, are risk factors for IBD development[3-5].
More than 3.6 million people are estimated to be affected by IBD across the globe, though data scarcity from some regions hinders this calculation[6,7]. In addition, recent studies show that its prevalence has risen in recent decades, with an increase of 75% and 60% in the number of UC and CD patients, respectively, in North America and Europe over the last 20 years[7]. This data becomes even more important if we consider the significant negative impacts caused by these diseases in the quality of life of affected individuals, which include social, professional, sexual, self-esteem and functional prejudices[8,9].
Furthermore, current IBD therapy represents an important economic burden to health systems as it is considered one of the most expensive treatments in the gastroenterology field[10]. Besides that, conventional therapeutic options for IBD also present several limitations regarding the adverse effects associated with their use. Such negative points have motivated researchers to look for better alternatives aiming the clinical control of these diseases, and, in this sense, probiotics emerge as a new option, although there is still limited evidence supporting their use[1,11].
According to the World Health Organization, probiotics are “live organisms which when administered in adequate amounts confer a health benefit on the host”[12]. In this framework, the beneficial effects provided by these agents to IBD patients could arise from various mechanisms that potentially promote attenuation of bowel inflammatory activity, such as antimicrobial properties, immune modulation, and improvement of intestinal barrier integrity[13,14].
Various probiotics have been tested in IBD. However, satisfactory effects were observed only with a portion of them, including Escherichia coli Nissle 1917, VSL#3, Saccharomyces boulardii, Lactobacillus, and _Bifidobacterium_[15-18]. In this context, our study aims to review the main theories about the role of microbiota in IBD pathophysiology and to gather the most consistent results on probiotic-related effectiveness in the treatment of that condition.
NORMAL GUT MICROBIOTA
The current evidence show that the intestinal microbiota is influenced by various factors and can vary between individuals and even be contrasting in different gastrointestinal areas[19]. Although the complete elucidation of the gut microbiota composition is challenging, it is well established that Bacteroidetes and Firmicutes are its main constituents[20]. It is believed that there is a relationship of commensalism between most microorganisms of the gastrointestinal tract (GIT) and host. Whereas the first ones benefit from the nutrients found in GIT environment, the second one takes advantage from important functions performed by the microbes[21].
Among these functions, we highlight the metabolization of nutrients - such as carbohydrates, lipids, and K and B vitamins[22-26], the protection against pathobionts - producing acids, thickening the protective wall and inducing production of immunoglobulins[27], and the immunomodulation of the innate and adaptive systems[28]. Besides that, the relationship between gut microbiota and human health has been widely discussed, not only in the gastroenterology field, but also when the elucidation of pathological manifestations outside GIT, such as allergic processes and neurodegenerative manifestations, is aimed[29,30].
GUT MICROBIOTA IN IBD
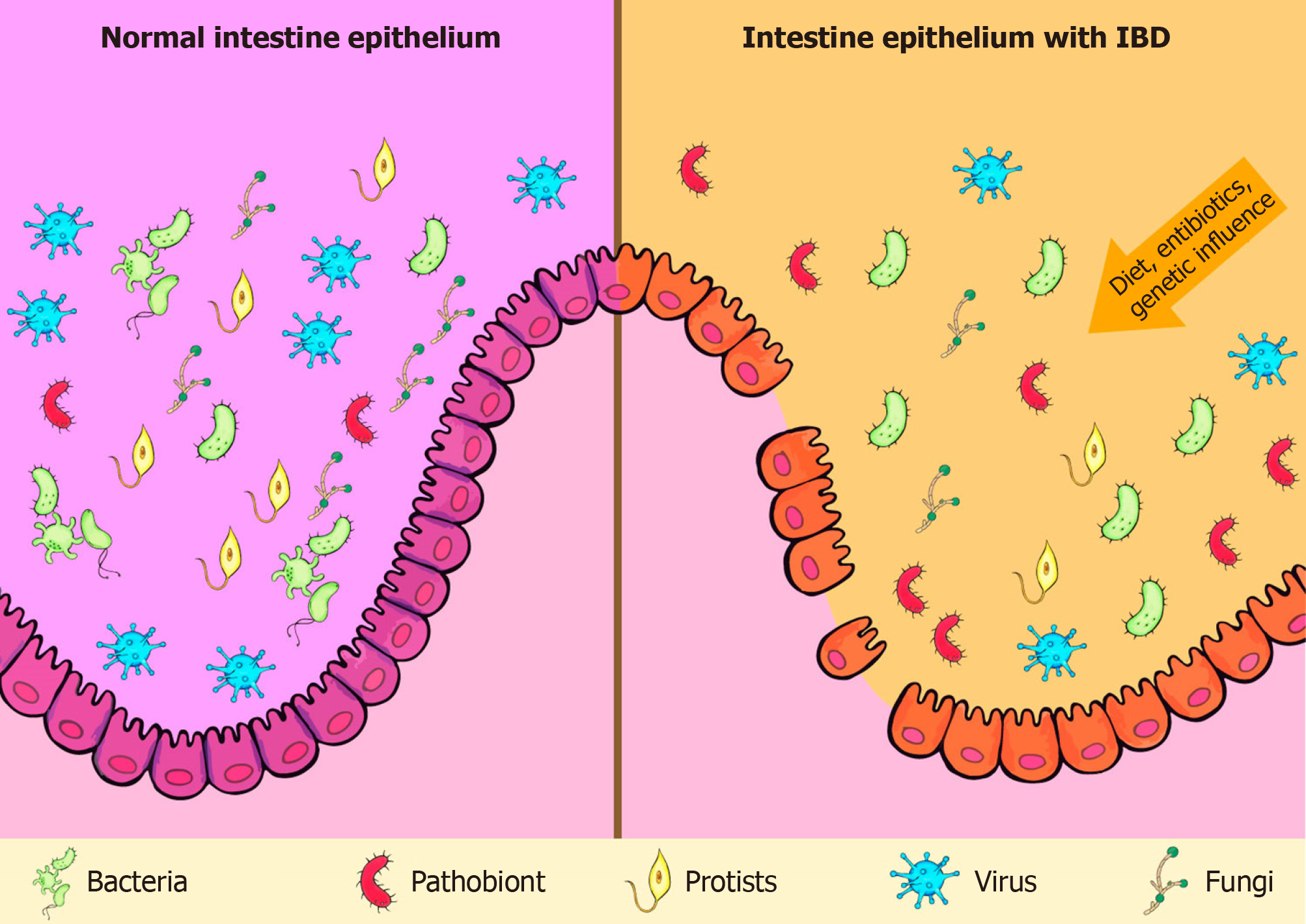
The role of gut microbiota in the pathogenesis of IBD has been extensively discussed. Although intestinal microbiota is mainly represented by bacteria, researches have also highlighted the importance of viruses, fungi, and protists in that process (Figure 1)[31-33]. Moreover, there is no consensus on whether the changes observed in the microbiota of IBD patients are causes or consequences of the disease.
Figure 1 Comparison between normal intestinal microbiota and intestinal microbiota with inflammatory bowel disease. IBD: Inflammatory bowel disease.
Bacterial role
Besides Bacteroides and Firmicutes, Actinobacteria and Proteobacteria phyla make up the group of the most common bacteria in human gut[31,34-38]. However, nowadays, it is being questioned whether this is a pattern among all individuals or whether factors such as genetic susceptibility and inheritance factors can change this profile and facilitate the occurrence of IBD[39].
The decrease of Bacteroides and Firmicutes phyla, as well as the increase of Proteobacteria and Actinobacteria, stand out as the main alterations in the microbiota from feces and intestinal mucosa of affected individuals[40,41]. Furthermore, the abnormal presence of pathogenic microorganisms might also contribute to the above-mentioned imbalance and to IBD emergence, since Mycobacterium avium paratuberculosis, Salmonella, Campylobacter, and Fusobacterium nucleatum have been positively associated with IBD[42-45]. Adherent-invasive E. coli is also supposed to be implicated in IBD pathogenesis, and recent evidences show that its presence not only propitiates the occurrence of IBD but also seems to predispose relapses in affected patients[46]. In addition, studies have correlated IBD relapses to Clostridium difficile infection[47]. Ultimately, Enterobacteriaceae and Streptococcus might also play a role in dysbiosis and further pathogenesis of IBD[48,49], with positive experimental trials for this relation[50].
Few studies evaluated the protective role of gut microbiota against IBD. Presti _et al_[51] suggest a protective role of Akkermansia muciniphila, since IBD patients had a lower presence of this species when compared to control and irritable bowel syndrome groups. Moreover, decreased abundance of Faecalibacterium prausnitzii in IBD has also been reported[51,52]. It is important to be highlighted that there is also a difference in the composition of the microbiome of IBD patients when comparing active and quiescent phases of the disease[53]. In view of the foregoing, it is conclusive in this topic that not only one, nor a few, but many bacteria can be related to IBD manifestations.
Viral role
The gut virobiota, unlike the bacterial microbiota, is not well described, neither in healthy individuals nor in IBD patients. It is known that the human gut virome is composed of eukaryotic viruses (e.g., herpesviruses, adenoviruses) and prokaryotic viruses (e.g., Microviridae and Caudovirales). However, many of them are not yet described, since there is a lack of studies on this area[54,55]. A study from 2016 aimed to identify the components of healthy human gut virome, which were divided into three different groups: the core, the common and the unique. The first group contains viruses found in more than half of the analyzed individuals, the second one is composed of species shared by many of the individuals, and the last one includes those found in a limited number of individuals. Drawing attention to the first group, it was noticed that the 23 bacteriophages that composed it were significantly reduced in IBD patients, bringing up the discussion that these common bacteriophages could have an important role in the pathogenesis of UC and CD when reduced[56].
Furthermore, differences have been observed between the gut virobiota of CD and UC patients. An increase of virobiota abundance in UC patients–mainly of Caudovirales bacteriophages-was reported by Zuo _et al_[57], with a concomitant identification of decreased viral diversity. Among CD patients, Pérez-Brocal _et al_[58] also observed a dysbiosis in virobiota, with abundance of phages that infect Clostridiales, Alteromonadales, and Clostridium. It was also detected a high abundance of the Retroviridae family in individuals with IBD.
Fungal and protist microbiota role
Although mycome represents only 0.1% of the human gut microbiome, a study from 2017 demonstrated that it presents a significant variability between healthy and IBD positive individuals. In the latter, a higher presence of Candida albicans and a lower presence of Saccharomyces, when compared to the control group, was described. Furthermore, the fungal diversity was reduced in the IBD[59]. Tests on animal subjects also corroborates this theory, as mice treated with antifungal drugs presented a higher incidence of acute and chronic colitis when compared to control groups[60].
Regarding the protist microbiota, it is described that the presence of such microorganisms can represent a protective factor against IBD. Comparing the protozoans found in the feces of healthy and IBD positive patients, the latter presented a reduced number of Blastocystis, suggesting that it might play a role in the balance of human healthy gut environment[61].
Microbiota, age and IBD
Concerning individual characteristics that may facilitate the occurrence of IBD, incidence peaks in certain ages (around 25 years old and close to 60 years old) evoke a discussion about what changes in these periods predispose the occurrence of the first episodes of CD and UC. Interestingly, these stages of life are moments in which the microbiome undergoes significant alterations. The first peak is marked by the host adaptation to new microorganisms in intestinal microbiota, while, in the second one, a global decrease in these life forms is observed in the human gut[62].
Immune response in IBD
In the last decades, the scientific community has increasingly investigated the role of host-microbial interactions in the human body immune regulation. Taking into consideration the gastrointestinal scenario, gut microbiome has been described as an integrating system that regulates the intestinal metabolism by means of environmental, genetic and immunological interactions. Therefore, it is expected that disturbances in such an important regulator can lead to complex diseases[63]. Indeed, the normal development of the immune system in the intestine have shown to be directly associated with adequate bacterial colonization during the early life and, in line with that, the result of a study indicated that germ-free mice present deficiencies in their immune functions[64,65]. It is also known that T and B immune cells from the intestinal mucosa play a crucial role in maintaining immune homeostasis, suppressing responses to non-pathogenic antigens and reinforcing the integrity of the intestinal mucosal barrier functions[66]. Among specific mechanisms through which bacteria influence immune response, it has already been observed that segmented filamentous bacteria induce the production of interleukin (IL)-17 and IL-22, which present a pro-inflammatory function[67]. Moreover, a series of 17 bacterial species have shown their potential to stimulate the expression of regulatory T cells and IL-10, which are associated with anti-inflammatory activity[68].
Regarding IBD, recent studies have described that it results from chronic intestinal inflammation which is due to a dysregulation in the expression of pro-inflammatory and anti-inflammatory molecules from the innate and adaptive responses of the intestinal immune system[69]. As an example, a study that evaluated 66 children with early onset IBD found a loss of function in the genes that encode and regulate IL-10 and the IL-10 receptor in those patients, what leads to deficient anti-inflammatory function in the gut environment, favoring the appearance of intestinal diseases[70]. Furthermore, other studies indicate that there is probably an important increase in the expression of pro-inflammatory cytokines in IBD, such as IL-1, IL-6, IL-18, TNF, IL-12, and IL-23, by antigen presenting cells, neutrophils, monocytes, and macrophages[71]. Given the importance of those molecules in IBD, therapeutic alternatives targeting them have been tested. Among which, anti TNF-α agents stand out since they present satisfactory effectiveness in the treatment of ulcerative colitis, being included in the current guidelines for IBD treatment[72]. In summary, the literature has not yet completely understood the role of immunopathogenesis in IBD. In addition, most available data are from association studies and from researches that evaluate molecules expression in patients that already manifested IBD, what impairs the understanding of the immunological phenomenons that occur during the onset of the disease.
CONVENTIONAL IBD TREATMENT
Besides the significant negative impacts caused by the disease on the quality of life of patients, important economic impact is generated by IBD treatment, as it is considered one of the most expensive therapeutics in gastroenterology field[73]. Some guidelines have been published over the years to standardize and to guide IBD treatment[74,75]. The current consensus about this issue aim to improve the symptoms and quality of life of individuals, as well as to reduce the risk of complications and surgical interventions. Moreover, the immediate therapeutic target is the induction of clinical remission of the disease and, subsequently, its maintenance[76,77].
Mesalazine, corticosteroids, immunosuppressive drugs, and monoclonal antibodies targeting TNF-α are some of the IBD therapeutic options, which are arranged along with their main adverse effects in Table 1. Some drug classes are used in both CD and UC management, and the therapeutics of this last condition significantly varies according disease activity and extent[78-80]. Furthermore, new research is being conducted on the incorporation of new corticosteroids, biosimilars, TGF-beta, immunomodulators, anti-TNF agents, and even intestinal microbiota manipulation in the treatment of affected individuals[81].
Table 1 Treatments used in inflammatory bowel disease and their side effects.
| Classes | Adverse effects | Ref. |
|---|---|---|
| Aminosalicylates | Mesalazine-nephrotoxicity and pancreatitis; sulfasalazine-blood dyscrasias | [10,11] |
| Antibiotics | Photosensitivity, tendonitis, tendon rupture, cartilage growth inhibition in fetuses and children oral candidiasis, gastrointestinal disorders, peripheral neuropathy | [14-16] |
| Corticosteroids | Acne, moon face and edema, sleep and mood disorders. Posterior subcapsular cataract, osteoporosis, myopathy, and susceptibility to infection. Acute adrenal insufficiency, arthralgia, increased intracranial pressure and pseudo-rheumatism syndrome | [7,20-22] |
| anti-TNF | Septicemia | [28] |
| Thiopurine | Hepatotoxicity, gastric intolerance and pancreatitis | [32] |
| Methotrexate | Nausea, vomiting and diarrhea | [34] |
It is well established that corticosteroid therapy with prednisone, methylpre-dnisolone or budesonide is indicated in the induction of CD remission[82,83]. However, such therapies present important limitations due to their adverse effects, that include cosmetic effects such as acne and moon face, as well as other multisystemic repercussions associated with a prolonged therapy from which stand out posterior subcapsular cataract, osteoporosis, and a higher susceptibility to infections[77,84,85]. Moreover, the abstinence to these drugs is associated with acute adrenal insufficiency, arthralgia, increased intracranial pressure and pseudo-rheumatism syndrome[86]. Budesonide may have fewer side effects when compared to other corticosteroids, but its use is not recommended in severe CD or exacerbations[87].
Clinical trials with antibiotic therapy generally use ciprofloxacin, metronidazole, rifaximin, clarithromycin, and antituberculosis regimens combined or not with steroids or immunosuppressants[88]. Those therapies are often suitable for infectious complications, especially in perianal disease[89]. Adverse effects of the main antibiotics used include photosensitivity, tendinitis, tendon rupture, cartilage growth inhibition in fetuses and children, oral candidiasis, gastrointestinal disorders and may cause peripheral neuropathy[90-92].
Although used in UC, aminosalicylates were initially considered effective in the treatment of mild CD. However, current meta-analyzes have not observed action in preventing relapse with sulfasalazine and mesalazine[93]. Blood dyscrasias are more frequent in use of the first one, whereas nephrotoxicity and pancreatitis are more common adverse effects when the latter treatment is chosen[94,95].
In active CD, the use of anti-TNF therapeutic strategy is effective. In this sense, adalimumab, infliximab and certolizumab are used in both induction and maintenance protocols of CD and UC[77,96]. Infection represents the worst adverse effect on anti-TNF use and, if it occurs, its use shall be suspended due to the risk of septicemia development[97]. Therefore, any presentation of systemic symptoms suggestive of infection in patients under that therapy demand the exclusion of opportunistic infections[98].
Thiopurines are represented by azathioprine or mercaptopurine and they may be used as an adjunctive treatment[99]. The efficacy of this drug class in inflammatory bowel disease is already evidenced by important studies and it is used for both induction and remission of CD[100]. Its main adverse reactions are hepatotoxicity, gastric intolerance and pancreatitis[101]. Another agent with an interesting immunosuppressive action is methotrexate, which can be also used in the scenarios the thiopurines are indicated[102]. Gastrointestinal changes represented by nausea, vomiting and diarrhea are its main adverse effects[103].
Facing the inconveniences associated with the above-mentioned side effects, IBD patients have gradually searched for alternative therapies[104]. Some potential therapies use plants, including Cannabis sativa, and their active ingredients. However, there is no robust evidence that prove their effectiveness in modifying the course of the disease[105]. Moreover, there is a higher prevalence of psychological disorders among IBD patients, such as stress, anxiety and depression[106,107]. These comorbidities calls attention for non-pharmacological therapies aiming the increase of patients’ quality of life, including cognitive and behavioral therapy, hypnotherapy, psychodynamic therapy, meditation, yoga, acupuncture, and exercise, but all of them present a limited level of evidence[104]. In that context, probiotics still have many conflicting works, however, they emerge as a new perspective for the treatment of these diseases[108].
USE OF PROBIOTICS FOR IBD TREATMENT
Since microbiota plays a crucial role in IBD pathophysiology, efforts have been directed towards the evaluation of the effectiveness of microbial-based therapies for its management, among which the use of probiotics rises as a promising alternative[109]. It is important to be highlighted that fecal microbiota transplantation is also a possibility that have been tried in this scenario, but a recent meta-analysis that included 18 studies did not demonstrate a consistent effectiveness of that method[110]. Regarding probiotics, several studies have been developed in order to evaluate their potential in inducing and maintaining remission in both CD and UC[111-113]. Moreover, encouraging results have been obtained with the use of non-pathogenic bacteria and fungi in the treatment of these patients (Table 2)[15,114,115].
Table 2 Clinical activity of probiotics in inflammatory bowel disease.
| Probiotic | Clinical activity in IBD | Ref. |
|---|---|---|
| Escherichia coli Nissle 1917 | Induction and maintenance of UC remission | [16,57,58,62] |
| VSL#3 | Induction and maintenance of UC remission; prevention of relapses in chronic pouchitis | [65,66,68,69] |
| Saccharomyces boulardii | Clinical remission of UC | [68-70] |
| Bifidobacterium longum | Objective improvements in UC parameters | [71] |
| Lactobacillus acidophilus La-5 + Bifidobacterium BB-12 | Probable improvement of intestinal parameters in IBD | [72] |
In 1997, the Escherichia coli Nissle 1917 (EcN) was tested in a double-blind trial in order to evaluate its efficacy in maintaining UC remission[16]. That study included 120 patients and observed an equivalence between this probiotic and mesalazine in preventing disease relapses, whose rates were 11.3% in mesalazine group and 16.0% in probiotics group, with a relapse-free time of 103 ± 4 d vs 106 ± 5 d, respectively. Since then, other studies on EcN efficacy were performed[116-118], and two meta-analyses reaffirmed the results found in the above-mentioned study[111,112]. The first of them included six trials, embracing 719 patients, and found that EcN induced remission in 61.6% of patients, while in mesalazine that rate was 69.5%[111]. The most recent one, in its turn, comprehended 10 studies, totaling 1049 patients, and observed a related ratio (RR) of 0.94 (95%CI: 0.8-1.03, P = 0.21) in remission rate and of 1.04 (95%CI: 0.82-1.31, P = 0.77) in relapse rate when EcN and Mesalazine groups were compared[114]. Moreover, a current practice position from European Crohn’s and Colitis Organization (ECCO) consider that EcN may be effective in inducing and maintaining remission in UC[109].
Probiotics formulations containing multiple species with different combinations of microorganisms are also commonly applied[115]. The VSL#3 is a widely studied and commercialized combined preparation that contains eight strains of lactic acid-producing bacteria (L. plantarum, L. delbrueckii subsp. bulgaricus, L. casei, L. acidophilus, B. breve, B. longum, B. infantis, and Streptococcus salivarius subsp. thermophilus)[17]. This formulation was firstly tested in 2000 for maintenance of clinical remission in patients with UC and chronic pouchitis in a double-blind placebo-controlled trial. That study included 40 patients during disease remission and its results pointed to the efficacy of this agent in preventing clinical relapses when compared to placebo[119]. Their results showed that only 15% of the patients who received the probiotic therapy presented relapses within 9 months, while all of the individuals from placebo group (100%) experienced such intercurrences (P < 0.01). After that, encouraging results were observed in the use of VSL#3 aiming the remission of acute mild-to-moderate UC[120-122]. Increased regulatory cytokines levels and reduced pro-inflammatory cytokines and toll-like receptors (TLRs) expression are supposed to be induced by this probiotic[123]. According to a new study that used a murine model, the inhibition of NF-κB and TNF-α expression by means of TLR4-NF-κB signal pathway might play an important role in such promising VSL#3 effects on UC[124]. Recently, a meta-analyses concluded that VSL#3 is effective in preventing pouchitis episodes and may have beneficial effects in inducting UC remission (RR = 1.67, 95%CI: 1.06-2.63, P = 0.03) and in avoiding UC relapses (RR = 0.29, 95%CI: 0.10-0.83, P = 0.02) when compared to placebo.
Besides the presence of Lactobacillus and Bifidobacterium in VSL#3 composition, these genera are also evaluated singly or in other combinations, being them the most clinically tested genera in IBD[114]. With regards to Bifidobacterium, a recent double-blind study including 195 patients found that B. breve strain Yakult fermented milk had no effect in maintaining remission in UC patients[18]. On the other hand, a randomized, placebo-controlled, double-blinded trial that included 56 patients demonstrated that B. longum 536 strain promoted a reduction in UC Disease Activity Index (UCDAI) after 8 wk of treatment (3.8 ± 0.4 at baseline vs 2.6 ± 0.4 at week 8, P < 0.01), while no significant improvement in UCDAI was observed among patients that received placebo (4.5 ± 0.5 at baseline vs 3.2 ± 0.6 at week 8, P = 0.88)[125]. Another trial with 305 IBD patients showed that Lactobacillus acidophilus La-5 associated with Bifidobacterium BB-12 probably improves intestinal parameters of affected individuals by means of increasing the prevalence of probiotic bacteria in intestine and colon[126].
The fungus Saccharomyces boulardii, a yeast that induces anti-inflammatory activity, has also been studied in IBD[15]. Some clinical trials observed satisfactory effects when using S. boulardii for the prevention of relapses in CD patients and in clinical remission of UC. A randomized non blinded study with 32 CD patients showed that the clinical relapses rates during six months in S. boulardii plus mesalazine group (6.25%) were lower than in those patients that used mesalazine alone (37.5%)[127], while the other one found improved bowel permeability among patients in whom this probiotic was added to baseline therapy[128]. Regarding UC, a pilot study found an improvement in Rachmilewitz clinical activity index among treated individuals[129]. However, these researches included small populations and were performed using distinct _S. boulardii d_oses.
It is important to be highlighted that the number of randomized controlled trials that evaluate the efficacy of probiotics in IBD remains low. Besides that, the meta-analyses about this therapy modality present potential biases due to the reduced number of included studies[11,111,112]. Furthermore, the lack of standardization of the therapeutic protocols leads to probiotics administration in different doses and frequency in distinct studies. Moreover, a study showed that probiotics composed by identical microorganisms, when underwent to different manufacturing methods, present distinct metabolic characteristics[130]. Complementarily, recent research showed that the effectiveness of a multispecies probiotic formulation depends on microbial metabolic properties, which affect its anti-inflammatory activity[131].
CONCLUSION
Considering the apparent pivotal role of microbiota in IBD genesis and the negative points observed in its conventional treatment, the application of microbial-based therapies seems to be a plausible alternative for affected patients with UC disease. To date, the use of probiotics seems to have no consistent benefit in treating DC. Although more evidence is needed in the evaluation of probiotics efficacy, promising results have been obtained in UC, mainly regarding E. coli Nissle 1917 and VSL#3. Lastly, standardizing therapeutic protocols and probiotics manufacturing methods could improve future studies, minimizing their potential biases.
Footnotes
Manuscript source: Invited Manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Abdolghaffari AH, Marteau P, Mazzarella G S-Editor: Wang J L-Editor: A E-Editor: Qi LL
References
| 7. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3134] [Cited by in F6Publishing: 3431] [Article Influence: 263.9] [Reference Citation Analysis (5)] |
|---|
| 12. | Food and Agriculture Organization and World Health Organization Expert Consultation. Health and nutritional properties of powder milk and live lactic acid bacteria. 2001 Oct 4 [cited 22 December 2019]. In: Probiotics in food - Health and nutritional properties and guidelines for evaluation [Internet]. Cordoba 2001: FAO Food and Nutrition Paper. Available from: ftp://ftp.fao.org/es/esn/food/probio_report_en.pdf. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 14. | Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2568] [Cited by in F6Publishing: 2776] [Article Influence: 185.1] [Reference Citation Analysis (0)] |
|---|
| 25. | Baddini Feitoza A, Fernandes Pereira A, Ferreira da Costa N, Gonçalves Ribeiro B. Conjugated linoleic acid (CLA): effect modulation of body composition and lipid profile. Nutr Hosp. 2009;24:422-428. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 28. | Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 801] [Cited by in F6Publishing: 866] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
|---|
| 31. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7052] [Cited by in F6Publishing: 7560] [Article Influence: 504.0] [Reference Citation Analysis (4)] |
|---|
| 32. | Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z, Klimešová K, Přibylová J, Bártová J, Sanchez D, Fundová P, Borovská D, Srůtková D, Zídek Z, Schwarzer M, Drastich P, Funda DP. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 492] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
|---|
| 40. | Ryan FJ, Ahern AM, Fitzgerald RS, Laserna-Mendieta EJ, Power EM, Clooney AG, O'Donoghue KW, McMurdie PJ, Iwai S, Crits-Christoph A, Sheehan D, Moran C, Flemer B, Zomer AL, Fanning A, O'Callaghan J, Walton J, Temko A, Stack W, Jackson L, Joyce SA, Melgar S, DeSantis TZ, Bell JT, Shanahan F, Claesson MJ. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat Commun. 2020;11:1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 156] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
|---|
| 49. | Rooks MG, Veiga P, Wardwell-Scott LH, Tickle T, Segata N, Michaud M, Gallini CA, Beal C, van Hylckama-Vlieg JE, Ballal SA, Morgan XC, Glickman JN, Gevers D, Huttenhower C, Garrett WS. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014;8:1403-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 311] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
|---|
| 50. | Lo Presti A, Zorzi F, Del Chierico F, Altomare A, Cocca S, Avola A, De Biasio F, Russo A, Cella E, Reddel S, Calabrese E, Biancone L, Monteleone G, Cicala M, Angeletti S, Ciccozzi M, Putignani L, Guarino MPL. Fecal and Mucosal Microbiota Profiling in Irritable Bowel Syndrome and Inflammatory Bowel Disease. Front Microbiol. 2019;10:1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
|---|
| 57. | Zuo T, Lu XJ, Zhang Y, Cheung CP, Lam S, Zhang F, Tang W, Ching JYL, Zhao R, Chan PKS, Sung JJY, Yu J, Chan FKL, Cao Q, Sheng JQ, Ng SC. Gut mucosal virome alterations in ulcerative colitis. Gut. 2019;68:1169-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
|---|
| 59. | Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, Cosnes J, Seksik P, Langella P, Skurnik D, Richard ML, Beaugerie L. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in F6Publishing: 835] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
|---|
| 60. | Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, Arditi M, Underhill DM, Iliev ID. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe. 2016;19:865-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
|---|
| 62. | Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O'Connor M, Harnedy N, O'Connor K, Henry C, O'Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O'Toole PW. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4586-4591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1162] [Cited by in F6Publishing: 1185] [Article Influence: 84.6] [Reference Citation Analysis (2)] |
|---|
| 65. | Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 815] [Cited by in F6Publishing: 851] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
|---|
| 67. | Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3529] [Cited by in F6Publishing: 3415] [Article Influence: 213.4] [Reference Citation Analysis (0)] |
|---|
| 68. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1875] [Cited by in F6Publishing: 2060] [Article Influence: 171.7] [Reference Citation Analysis (2)] |
|---|
| 70. | Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, Pfeifer D, Kreipe H, Pfister ED, Baumann U, Puchalka J, Bohne J, Egritas O, Dalgic B, Kolho KL, Sauerbrey A, Buderus S, Güngör T, Enninger A, Koda YK, Guariso G, Weiss B, Corbacioglu S, Socha P, Uslu N, Metin A, Wahbeh GT, Husain K, Ramadan D, Al-Herz W, Grimbacher B, Sauer M, Sykora KW, Koletzko S, Klein C. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
|---|
| 71. | Ng SC, Benjamin JL, McCarthy NE, Hedin CR, Koutsoumpas A, Plamondon S, Price CL, Hart AL, Kamm MA, Forbes A, Knight SC, Lindsay JO, Whelan K, Stagg AJ. Relationship between human intestinal dendritic cells, gut microbiota, and disease activity in Crohn's disease. Inflamm Bowel Dis. 2011;17:2027-2037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
|---|
| 77. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P. ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1240] [Cited by in F6Publishing: 1370] [Article Influence: 171.3] [Reference Citation Analysis (0)] |
|---|
| 78. | Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 733] [Cited by in F6Publishing: 823] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
|---|
| 80. | Travis SP, Stange EF, Lémann M, Oresland T, Bemelman WA, Chowers Y, Colombel JF, D'Haens G, Ghosh S, Marteau P, Kruis W, Mortensen NJ, Penninckx F, Gassull M; European Crohn's and Colitis Organisation (ECCO). European evidence-based Consensus on the management of ulcerative colitis: Current management. J Crohns Colitis. 2008;2:24-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 402] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
|---|
| 83. | Summers RW, Switz DM, Sessions JT, Becktel JM, Best WR, Kern F, Singleton JW. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology. 1979;77:847-869. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 84. | Schoon EJ, Bollani S, Mills PR, Israeli E, Felsenberg D, Ljunghall S, Persson T, Haptén-White L, Graffner H, Bianchi Porro G, Vatn M, Stockbrügger RW; Matrix Study Group. Bone mineral density in relation to efficacy and side effects of budesonide and prednisolone in Crohn's disease. Clin Gastroenterol Hepatol. 2005;3:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
|---|
| 87. | Malchow H, Ewe K, Brandes JW, Goebell H, Ehms H, Sommer H, Jesdinsky H. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984;86:249-266. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 90. | Park SK, Kim KJ, Lee SO, Yang DH, Jung KW, Duk Ye B, Byeon JS, Myung SJ, Yang SK, Kim JH, Sik Yu C. Ciprofloxacin usage and bacterial resistance patterns in Crohn's disease patients with abscesses. J Clin Gastroenterol. 2014;48:703-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
|---|
| 96. | Hazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, Kuenzig E, Tomlinson G, Siegel CA, Melmed GY, Kaplan GG. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology. 2015;148:344-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
|---|
| 98. | Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, Esteve M, Katsanos K, Lees CW, Macmahon E, Moreels T, Reinisch W, Tilg H, Tremblay L, Veereman-Wauters G, Viget N, Yazdanpanah Y, Eliakim R, Colombel JF; European Crohn's and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 715] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
|---|
| 104. | Torres J, Ellul P, Langhorst J, Mikocka-Walus A, Barreiro-de Acosta M, Basnayake C, Ding NJS, Gilardi D, Katsanos K, Moser G, Opheim R, Palmela C, Pellino G, Van der Marel S, Vavricka SR. European Crohn's and Colitis Organisation Topical Review on Complementary Medicine and Psychotherapy in Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:673-685e. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
|---|
| 105. | Naftali T, Lev LB, Yablecovitch D, Half E, Konikoff FM. Treatment of Crohn's disease with cannabis: an observational study. Isr Med Assoc J. 2011;13:455-458. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 107. | Walker JR, Ediger JP, Graff LA, Greenfeld JM, Clara I, Lix L, Rawsthorne P, Miller N, Rogala L, McPhail CM, Bernstein CN. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103:1989-1997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
|---|
| 108. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1341] [Cited by in F6Publishing: 1475] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
|---|
| 109. | Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am Fam Physician. 2017;96:170-178. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 115. | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 807] [Cited by in F6Publishing: 692] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
|---|
| 117. | Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617-1623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 851] [Cited by in F6Publishing: 793] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
|---|
| 121. | Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, Modeo ME, Rodino' S, D'Amico T, Sebkova L, Sacca' N, Di Giulio E, Luzza F, Imeneo M, Larussa T, Di Rosa S, Annese V, Danese S, Gasbarrini A. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218-2227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
|---|
| 124. | Wang H, Li S, Li H, DU F, Guan J, Wu Y. Mechanism of Probiotic VSL#3 Inhibiting NF-κB and TNF-α on Colitis through TLR4-NF-κB Signal Pathway. Iran J Public Health. 2019;48:1292-1300. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 125. | Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, Kusaka T, Uose S, Hisatsune H, Tojo M, Noda T, Arasawa S, Izuta M, Kubo A, Ogawa C, Matsunaka T, Shibatouge M. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc. 2016;28:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
|---|