Clinical approaches to non-alcoholic fatty liver disease (original) (raw)
Topic Highlight Open Access
Copyright ©2014 Baishideng Publishing Group Co., Limited. All rights reserved.
World J Gastroenterol. Feb 21, 2014; 20(7): 1712-1723
Published online Feb 21, 2014. doi: 10.3748/wjg.v20.i7.1712
Clinical approaches to non-alcoholic fatty liver disease
Katherine JP Schwenger, Institute of Medical Science, University of Toronto, 1 King’s Circle, Toronto M5S 1A8, Canada
Johane P Allard, Department of Medicine, Toronto General Hospital, University Health Network, Toronto M5G 2C4, Canada
ORCID number: $[AuthorORCIDs]
Author contributions: Schwenger KJP performed and wrote the literature review; Allard JP supervised and edited.
Correspondence to: Johane P Allard, MD, FRCPC, Department of Medicine, Toronto General Hospital, University Health Network, 585 University Avenue, Suite 9-N-973, Toronto M5G 2C4, Canada. johane.allard@uhn.on.ca
Telephone: +1-416-3405159 Fax: +1-416-3480065
Received: October 29, 2013
Revised: December 5, 2013
Accepted: January 3, 2014
Published online: February 21, 2014
Processing time: 134 Days and 0.2 Hours
Abstract
Non-alcoholic fatty liver disease (NAFLD) ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), leading to fibrosis and potentially cirrhosis, and it is one of the most common causes of liver disease worldwide. NAFLD is associated with other medical conditions such as metabolic syndrome, obesity, cardiovascular disease and diabetes. NASH can only be diagnosed through liver biopsy, but noninvasive techniques have been developed to identify patients who are most likely to have NASH or fibrosis, reducing the need for liver biopsy and risk to patients. Disease progression varies between individuals and is linked to a number of risk factors. Mechanisms involved in the pathogenesis are associated with diet and lifestyle, influx of free fatty acids to the liver from adipose tissue due to insulin resistance, hepatic oxidative stress, cytokines production, reduced very low-density lipoprotein secretion and intestinal microbiome. Weight loss through improved diet and increased physical activity has been the cornerstone therapy of NAFLD. Recent therapies such as pioglitazone and vitamin E have been shown to be beneficial. Omega 3 polyunsaturated fatty acids and statins may offer additional benefits. Bariatric surgery should be considered in morbidly obese patients. More research is needed to assess the impact of these treatments on a long-term basis. The objective of this article is to briefly review the diagnosis, management and treatment of this disease in order to aid clinicians in managing these patients.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is associated with the metabolic syndrome and patients who present with nonalcoholic steatohepatitis can progress to cirrhosis and liver failure requiring transplantation. NAFLD is becoming a public health issue due to its increased prevalence. It is important to recognize the disease early to prevent its progression. Proper management is required in order to reduce associated with it. This review discusses what current practices are and provides suggestions for future research.
- Citation: Schwenger KJ, Allard JP. Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol 2014; 20(7): 1712-1723
- URL: https://www.wjgnet.com/1007-9327/full/v20/i7/1712.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i7.1712
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of liver disease worldwide, with a prevalence of 15%-30% in Western populations[1-4]. The prevalence increases to 58% in overweight individuals and can be as high as 98% in non-diabetic obese individuals[5]. NAFLD ranges from simple steatosis to nonalcoholic steatohepatitis (NASH) and potentially cirrhosis[6]. NASH is the picture of hepatocellular injury and inflammation of the liver[7]. Cirrhosis, which occurs in 25% of patients with NASH, can result in liver failure, portal hypertension, and hepatocellular carcinoma and patients with cirrhosis are at a high risk for developing cardiovascular disease[8]. Not only the presence of excess weight and obesity, but also the location of fat storage plays a role in NAFLD pathogenesis. Visceral fat stores increase the risk for NAFLD in both obese and non-obese individuals[9]. NAFLD is associated with metabolic syndrome and obesity[10]. The diagnosis of NAFLD is made when there is evidence of liver steatosis on imaging modalities and this is associated with features of the metabolic syndrome in the context of a patient who does not have other causes of liver disease[11] and where alcohol consumption is less than 21 drinks and 14 drinks per week for men and women, respectively[12]. Diagnosis for NASH is confirmed when a liver biopsy shows the presence of perilobular inflammation, or the presence of hepatocyte ballooning, Mallory hyaline and acidophil bodies with or without fibrosis. Non-invasive tests such as liver enzymes, medical imaging, Fatty Liver Index, NAFLD fibrosis score, FibroMeter and Fibroscan[13] may suggest the presence of NASH by detecting fibrosis and research is on-going to assess surrogate markers for NASH such as CK18, but this remains experimental[14]. Therefore, for a definite diagnosis of NASH, patients still need a liver biopsy.
PATHOGENESIS
In NAFLD, the accumulation of fat in the liver[15] is a result of increased delivery of free fatty acid (FFA) to the liver, increased synthesis, decreased triglyceride export through very-low density lipoprotein (VLDL) and reduced beta-oxidation[16]. Universally, patients with NAFLD have insulin resistance (IR) which increases lipolysis from the adipose tissue[17]. The resulting FFA will be taken up by the liver and can cause lipid peroxidation[17]. Lipid peroxidation can increase the production of pro-inflammatory cytokines[17]. The increase in FFA can also exceed mitochondrial beta-oxidation further increasing the oxidative stress[18] and inflammation[18].
Liver de novo lipogenesis (DNL)[19] also contributes to the steatosis. De novo lipogenesis is due to the hyperinsulinemia associated with IR, which stimulates the enzymes in the DNL pathway, increasing the production and storage of triglycerides. In NAFLD, DNL contributes to 26% of hepatic triglyceride accumulation where it accounts for < 5% in healthy individuals[19]. Hyperinsulinemia can also cause a reduction in VLDL secretion[20], leading to triglyceride accumulation in the liver.
The presence of inflammation or steatohepatitis depends on a number of factors such as the presence of free fatty acids (FFA), inflammatory cytokines and adipokines, oxidative stress and mitochondrial dysfunction[16].
Proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) are elevated and generally produced by the liver and adipose tissue, from NF-κB activation[21] (e.g., from lipid peroxidation or activation of Toll-like receptors). Increased TNF-α and IL-6 are also associated with IR by interfering with insulin signaling[7,22]. On the other hand, adiponectin[7,22] is produced by the adipose tissue and is an anti-inflammatory adipokine that can increase insulin sensitivity[23]. In NAFLD adiponectin is reduced which decreases fatty acid oxidation and hepatic gluconeogenesis[24]. The role of other adipokines, like visfatin, leptin and resistin are still controversial[25-27]. Potential pathways are promotion of IR, oxidative stress and inflammation as well as fibrogenesis[28].
Diet is an important contributor to NAFLD, mainly because excessive energy intake leads to obesity, which in turn increases the risk for NAFLD. However, not only the amount of energy but also the quality of the diet could play an important role for the development and progression of NAFLD. Diets rich in saturated fat, cholesterol, and low in polyunsaturated fat, fiber and antioxidant vitamins C and E[29] have been associated with NASH. High saturated fat diets are associated with IR and hepatic inflammation[29]. Other research has also demonstrated a relationship between increased dietary fat consumption and NAFLD[30,31]. Conversely, a study investigating pre-surgical bariatric patients in the United States reported that increased carbohydrate intake was associated with hepatic inflammation[32]. Among carbohydrates, specifically fructose might contribute to NAFLD progression. Fructose intake has been linked to increasing hepatic fat, inflammation and possibly fibrosis[33]. Fructose has also been associated with both an increase in visceral adipose tissue[34] and plasma triglycerides[35].
Recently, new evidence has linked intestinal microbiota to NALFD pathogenesis. Intestinal microbiota (IM) may play a role in the development of NAFLD, however very few human studies have been conducted and most were cross-sectional[36-39]. One study suggested an association between low percentage of fecal bacteroidetes and the presence of NASH, independent of diet and body mass index (BMI)[36]. Other studies showed an increased abundance of E. coli associated with higher blood alcohol levels[39] or differences in IM associated with differences in volatile organic compounds[38]. Development of fatty liver on a choline deficient diet was also associated with IM at baseline and single nucleotide polymorphism in the phosphatidylethanolamine methyl transferase gene region[37]. IM can be altered by the type of diet consumed and may contribute to NAFLD through several mechanisms. These include salvaging energy from food, contributing to inflammation via cytokines by increasing intestinal permeability leading to endotoxemia, modulating the innate immune system such as activation of Toll-like receptors and inflammasomes, regulating bile acid, metabolizing dietary choline and increasing endogenous ethanol by bacteria[37,40].
DIAGNOSIS NAFLD
NAFLD should be suspected in individuals who are either obese, diabetic or have metabolic syndrome[40]. However, the majority of patients with NAFLD are asymptomatic and the disease may be detected via routine blood tests showing elevated liver enzymes or when an ultrasound is performed for various reasons and detects liver steatosis (Figure 1). However, secondary causes of hepatic steatosis or elevated liver enzymes, such as excess alcohol consumption, medications, toxins, lipodystrophy, autoimmune and inflammatory diseases, nutrition (malnutrition, total parenteral nutrition, severe weight loss, and refeeding syndrome), viral hepatitis and metabolic liver disease should be excluded by reviewing patient’s history and proper investigation[40,41].
Figure 1 Diagnosis and staging of non-alcoholic fatty liver disease. NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
Although it is still not possible to diagnose NAFLD based solely on blood work, elevated transaminases can be used as a first step[42]. Elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in the absence of other liver diseases may support NAFLD[41,43], and have been found in up to approximately 50% of simple steatosis patients and 80% of NASH patients[42]. An AST:ALT ratio less than 1 is also seen in NAFLD[44] and supports NASH. However, it is important to note that patients with normal transaminases and liver steatosis on imaging may also have NASH[45].
Ultrasonography is a non-invasive tool that is used in the detection of liver steatosis[40,46]. Other imaging techniques such as computed tomography and nuclear magnetic resonance imaging can also detect liver steatosis, but neither of these more expensive techniques provide more information then ultrasonography[46,47] except for fat quantification[48]. A review conducted by Festi _et al_[46] concluded that ultrasonography should be used as a first-line diagnostic tool because of its evaluation of liver steatosis and other abdominal organs.
The Fatty Liver Index is an algorithm based on four markers; BMI, waist circumference, triglyceride and γ-glutamyltransferase (GGT)[26], which is confirmed to accurately identify NAFLD[49,50]. This index has been used in population studies[40] and has achieved an accuracy of 0.84 in detecting fatty liver[51]. The Fatty Liver Index provides a score out of 100, indicating that a score < 30 can rule out and a score ≥ 60 to rule in hepatic steatosis[51]. The formula for the Fatty Liver Index is [e0.953× loge(triglycerides) + 0.139 × BMI + 0.718 × loge(GGT) + 0.053 × waist circumference - 15.745]/[1 + e0.953× loge(triglycerides) + 0.139 × BMI + 0.718 × loge(GGT) + 0.053 × waist circumference - 15.745] × 100[51].
Liver biopsy is currently the gold standard for diagnosing NASH[41], as it also establishes the stage of NASH[52]. This invasive procedure is used to analyze the degree of hepatocyte injury and level of fibrosis and inflammation[46]. However, it is used after imaging techniques, laboratory abnormalities and/or non-invasive methods suggest the presence and severity of NASH[46,52].
STAGING OF NAFLD
Recent advances have allowed for non-invasive techniques to be used to diagnose the level of inflammation/fibrosis (Figure 1)[40].
The NAFLD fibrosis score (NFS) evaluates six variables; age, hyperglycemia, BMI, platelet count, albumin and AST/ALT ratio[40,53]. The NAFLD fibrosis score formula is = -1.675 + 0.037 × age (year) + 0.094 × BMI (kg/m2) + 1.13 × IFG/diabetes (yes = 1; no = 0) + 0.99 × AST/ALT ratio - 0.013 × platelets (× 109/L) - 0.66 × albumin (g/dL)[53]. This equation is used to classify the probability of fibrosis as < -1.5 for low probability and > -1.5 to < 0.67 for intermediate probability and > 0.67 for high probability[54]. Angulo _et al_[53] validated NFS to a liver biopsy and found that this accurately classifies NAFLD patients with and without advanced fibrosis. Furthermore, a study investigating NAFLD in a morbidly obese population undergoing bariatric surgery found that the NFS is accurate at excluding advanced fibrosis within this population[55]. Overall, this tool is widely used in practice to exclude advanced fibrosis.
Another tool used in clinical practice is the FibroMeter. This tool uses age, weight, fasting glucose, AST, ALT, ferritin and platelet count to diagnose significant fibrosis[46,56,57]. The formula for the FibroMeter is -0.007 × platelets (× 109/L) -0.049 × prothrombin time (%) + 0.012 × AST (U/L) + 0.005 ×α2 macroglobulin (g/L) + 0.021 × hyaluronate (ng/mL) -0.270 × urea (mmol/L) + 0.270 × age (year) + 3.718[58]. The FibroMeter provides the probability of significant fibrosis and the percentage of hepatic fibrosis[58]. Calès _et al_[56] compared the FibroMeter to NFS and found that the FibroMeter provides a more reliable diagnosis for significant fibrosis then the NFS. This tool can be used to confirm or disconfirm advanced fibrosis in NAFLD patients[57].
FibroScan, also known as transient elastography, is another noninvasive method to assess liver fibrosis[59]. This method measures liver stiffness, which was originally designed for the hepatitis C population[60], but is now being used in the NAFLD population[61]. The FibroScan sends a pulse through the skin, which is circulated through the liver. The velocity of the wave, which is correlated with liver stiffness, is measured by ultrasound. The stiffer the liver the greater the degree of fibrosis[62]. The liver stiffness measurement (LSM) is used to assess the current stage of liver fibrosis. The cutoffs are 4.85, 7.38, 9.28, 13.33 and 25.34 kPa which represent stages, 0 (no steatosis), 1 (perivenular and/or perisinusoidal fibrosis), 2 (combined pericellular portal fibrosis), 3 (septal fibrosis) and 4 (cirrhosis)[59]. Yoneda _et al_[59] investigated the usefulness of the transient elastography in NAFLD patients. They found that there is a significant correlation between liver stiffness and fibrosis stage, which was confirmed by liver biopsy[59]. Therefore, this measurement can be used in the NAFLD population to determine the stage of fibrosis. However, special consideration is needed for overweight and obese patients. Studies have shown that obesity, (BMI > 30 kg/m2) provides inaccurate LSMs. The use of a FibroScan XL probe has been shown to provide reliable LSM[63,64].
Overall, these noninvasive measurements to assess NAFLD/NASH should be used prior to a liver biopsy as they pose minimal risk to the patient. However, liver biopsy should be considered in patients when these noninvasive tests suggesting fibrosis are inconclusive[40], or the patients have risk factors associated with advanced fibrosis, such as age > 50 years, presence of diabetes, morbid obesity or metabolic syndrome[65].
MANAGEMENT OF NAFLD
The goal of managing NAFLD is to improve steatosis and prevent fibrosis. No standard treatment currently exists, however, treating risk factors such as obesity and IR, remains the focus of managing NAFLD. Currently, lifestyle interventions, medical treatments, alternative therapies and surgery are being used to treat risk factors associated with NAFLD.
Lifestyle interventions
As previously mentioned IR and obesity increase the risk of developing NAFLD and are instrumental in NAFLD progression. In today’s society obesity and IR have been linked to poor diet choices, as well as sedentary lifestyles. Weight management through improvements in diet and increased physical activity can help to improve liver histology as well as delay disease progression (Table 1)[66].
Table 1 Summary of lifestyle intervention studies: diet and/or physical activity.
| Ref. | Population, Study Design | Intervention | Results |
|---|---|---|---|
| [71] | n = 96, 12-mo intervention on adults with hepatic steatosis and type 2 diabetes | Combination of moderate caloric restriction (1200-1800 kcal/d) and increased moderate physical activity (175 min per week) | Significant decreases in BMI, weight, waist circumference, percent body fat and A1C |
| [72] | n = 50, longitudinal study with lifestyle intervention in NAFLD adults | 10 concealing sessions with a dietitian, and moderate intensity activity 3 h/wk | Significantly decreased body fat and liver fat and increased fitness. NAFLD at baseline resolved in 20 participants |
| [68] | n = 28, randomized control trial adults with elevated ALT or AST, BMI of 25-40 | Combination of diet (1000-1500/d), exercise (10000 steps per day and 200 min/wk of moderate physical activity) and behavior modification | Weight in intervention group decreased by 9.3%, significant improvement of NASH. > 7% weight loss significantly improved steatosis |
| [73] | n = 152, randomized intervention of adults with elevated liver enzymes, central obesity and metabolic risk factors | Randomized to moderate (6 sessions/10 wk) or low-intensity (3 sessions/4 wk) or control. Physical activity 150 min/wk and low saturated fat and process food diet (1700-2400 kcal/d) | Moderate intensity – improvement in all risk factors, greater reduction in liver enzymes and weight loss than low-intensity |
| [74] | n = 19, 8 wk exercise intervention in NAFLD adults | 8 wk (3 × wk) of resistance exercise (n = 11) vs control (n = 8) | 13% reduction in liver lipid. Lipid oxidation, glucose and IR improved. No effect on weight or body fat |
NAFLD patients have been found to have an increased energy intake when compared to healthy individuals[67]. Several studies have shown that weight loss is successful in improving liver enzymes, insulin sensitivity, reducing inflammation and liver histology[68-72]. Recent studies use diet, physical activity and behavior modification to help promote weight loss in NAFLD patients[69]. A randomized controlled trial conducted by Promrat _et al_[68] used a combination of diet, physical activity and behavior modification to trigger 7%-10% weight loss in obese NASH patients. Those who achieved a minimum of 7% weight loss had improvements in their liver histology[68]. A similar study used NAFLD patients with elevated liver enzymes and central obesity to assess the effectiveness of lifestyle interventions. Patients were randomly assigned to either low (3 sessions/4 wk) or moderate (6 sessions/10 wk) physical activity intensity groups and were compared to a control group. The lifestyle interventions included physical activity and dietary guidance as well as behavior modification[73]. St George _et al_[73] found that there was a reduction in liver enzymes, which was greater in the moderate-intensity lifestyle intervention group in comparison to the control.
Physical activity alone has been found to reduce hepatic steatosis, independent of weight loss. A study on sedentary NAFLD patients examined the effects of resistant exercises on liver lipid levels[74]. Hallsworth _et al_[74] found that after 8 wk (3 times per week lasting 45-60 min) of resistance based exercise resulted in a reduction of liver lipids, and improvements of lipid oxidation, glucose control and insulin resistance. A review conducted by Thoma _et al_[69] analyzed 23 studies using diet modification, physical activity, or a combination of both. Thoma _et al_[69] found that lifestyle modifications that led to weight reduction and/or increased physical activity greatly reduced liver fat and improved insulin sensitivity.
Overall, lifestyle modification (diet and exercise) resulting in weight loss or increased physical activity can reduce liver enzymes and inflammation and improve liver histology, glucose control, and insulin sensitivity and lipid oxidation. Therefore, when developing a treatment plan for NAFLD patients, lifestyle modification should be used as a first step in clinical settings.
Medical treatment
Lifestyle interventions may not be effective in certain cases and thus other approaches must be considered in the management of NAFLD (Table 2). Pharmacological treatment has been studied in this population, specifically insulin-sensitizing agents. Two insulin-sensitizing agents, metformin and thiazolidinediones (TZD), have been investigated in this population, however there are conflicting results. In addition, vitamin E therapy has been used in the treatment of NAFLD, as it inhibits oxidative stress and reduces the promotion of hepatic fibrosis[75].
Table 2 Summary of medication intervention studies.
| Ref. | Population, Study Design | Intervention | Results |
|---|---|---|---|
| [79] | n = 15, open label study with histologically confirmed NAFLD adults | All patients received 20 mg/kg per day of metformin for 48 wk | In the initial 3 mo there was improvement in ALT and AST levels and insulin sensitivity, after 3 mo no further improvement noted |
| [80] | n = 57 24-mo observational study with NAFLD or NASH overweight and obese children | Metformin was progressively titrated from 250-500 mg tid at weekly intervals and patients were given a hypocaloric or isocaloric diet and recommended to engage in 45 min/d of physical activity (n = 57) compared to control group (n = 30) with the same diet and physical activity recommendations | ALT significantly improved with decreasing body weight. NAS score decreased in both groups, no significant changes in fibrosis |
| [85] | n = 63, randomized, double-blind placebo – controlled in NASH adults | 32 patients were given rosiglitazone (4 mg/d for 1 mo then 8 mg/d for 11 mo) vs placebo (n = 31) | Improved steatosis and normalized transaminase, only ½ responded. Improvement of insulin sensitivity |
| [86] | n = 47, randomized control study in adults with impaired glucose tolerance or type 2 diabetes with NASH | 6 mo of hypocaloric diet and 45 mg (n = 26) of pioglitazone vs 6 mo of hypocaloric diet (n = 21) | Diet and pioglitazone improved glucose tolerance and normalized ALT. Histologic features of NASH improved, no significant reduction in fibrosis |
| [87] | n = 13 patient cohort with NASH adults | All were treated with 30 mg/d of pioglitazone for 48 wk, than followed up 48 wk after stopping pioglitazone. | Stopping pioglitazone increased ALT, decreased adiponectin, worsened insulin sensitivity and increased hepatic fat, no change in fibrosis |
| [89] | n = 247, randomization of adults with NASH without diabetes | 96 wk of either 30 mg pioglitazone (n = 80), vitamin E (800 IU/d) (n = 84) or placebo (n = 83) | Vitamin E significantly improved NASH. AST and ALT significantly improved in vitamin E and pioglitazone groups, and reduction in hepatic steatosis with no improvement in fibrosis score. |
| [90] | n = 45 prospective, double-blind randomized, placebo controlled trial in NASH adults | Received vitamin E and C (1000 IU and 1000 mg) (n = 23) or placebo for 6 mo (n = 22) additionally patients received weight loss counselling and encouraged to follow a low fat diet | Vitamin treatment significantly improved fibrosis score |
Metformin is used in the treatment for type 2 diabetes, as it lowers blood glucose by decreasing gluconeogenesis in the liver as well as decreasing intestinal glucose absorption which stimulates glucose uptake in muscle, and increases fatty acid oxidation[76,77], resulting in improved insulin sensitivity[78]. Clinical studies have investigated the use of metformin in the treatment of NAFLD, specifically looking at liver histology and aminotransferases. Nair _et al_[79] conducted a pilot study to investigate the efficiency and safety of metformin in NAFLD patients. Patients were prescribed 20 mg/kg per day of metformin for one year, comparing liver histology pre and post treatment regimen[79]. Three months into the treatment, aminotransferase decreased, which was related to an improvement in insulin sensitivity[79]. However, this improvement was not sustained for the duration of the treatment; therefore Nair _et al_[79] concluded that metformin should not be used for the treatment of NAFLD. More recently, a study conducted on children with NAFLD used lifestyle interventions and metformin (1.5 g/d for 24 mo) to determine the effect on liver enzymes[80]. Nobili _et al_[80] found that metformin was no more effective than lifestyle interventions in improving liver enzymes or histology. Additionally, other studies have also failed to prove benefits of using metformin to improve liver histology[4,81]. In conclusion, metformin should not be used in the treatment of NAFLD, as research has shown that it is ineffective in the management of NAFLD.
TZD are peroxisomal proliferator activated receptor-γ (PPAR-γ) agonists that are used primarily in the type 2 diabetes population to help improve insulin sensitivity within the liver, muscle and adipose tissue, promote hepatic fatty acid oxidation and decrease hepatic lipogenesis[82,83]. TZD use in NAFLD patients, specifically the effects of pioglitazone and rosiglitazone, have shown to decrease hepatic fat and decrease cellular injury, however these medications have also shown to cause weight gain[84]. Ratziu _et al_[85] studied the treatment and safety of rosiglitazone in NASH patients. The treatment group received 4 mg/d for the first month, and then 8 mg/d for 11 mo[85]. They found that rosiglitazone only improved steatosis and transaminase levels, and resulted in weight gain (mean gain of 1.5 kg)[85]. Belfort _et al_[86] studied the effects of a hypocaloric diet (500 kcal reduction) and 45 mg of pioglitazone per day on 55 NASH patients with impaired glucose tolerance or type 2 diabetes. The results indicated that the diet and pioglitazone improved glycemic control, glucose tolerance, improved liver enzymes and increased hepatic sensitivity[86]. Conversely, Lutchman _et al_[87] found that discontinuing TZD therapy resulted in NASH recurrence, indicating that long-term use is necessary for successful treatment. In addition, long-term use of TZDs can result in medical complications such as edema, congestive heart failure, osteoporosis and weight gain[87,88]. Overall, pioglitazone is used in the medical community as a treatment for NASH, however, careful consideration is needed when prescribing this pharmacological treatment to patients.
Vitamin E is an antioxidant used to treat NAFLD, due to its ability to inhibit oxidative stress. Several studies have been conducted to further analyze the benefits of administering high doses of vitamin E to NASH patients. One notable study is the PIVENS clinical trial, which administered high doses of vitamin E (800 IU/d for 96 d) in non-diabetic patients[89]. Sanyal _et al_[89] found a reduction in hepatocellular inflammation, hepatic steatosis and improvements in liver function tests were noted. They concluded that vitamin E is an effective treatment for NASH patients without diabetes[89]. Harrison _et al_[90] also investigated the effects of a combination of vitamin E (1000 IU/d) and vitamin C (1000 mg/d) on liver histology in 45 diagnosed NASH patients over a 6-mo period. Their findings were that vitamins E and C were effective in improving fibrosis scores, though no improvements in inflammation or liver function tests were noted[90]. Caution needs to be taken when prescribing vitamin E as studies have shown that there is a potential harm for patients. A meta-analysis of 135967 people taking 400 IU/d of vitamin E found that there is an increase of all-cause mortality and therefore its use should be avoided[91]. In addition, a study conducted by Klein _et al_[92] studied the long-term effects of vitamin E (400 IU/d)[92]. The study found that vitamin E supplementation significantly increases the risk of developing prostate cancer in healthy men[92]. Overall, caution needs to be taken when prescribing vitamin E, especially to diabetic NASH patients, as there is no research to support vitamin E at this time for this population.
TZDs and vitamin E medical treatment need to be carefully considered when developing a treatment plan for NAFLD/NASH patients.
Other therapies
Due to the rise in NAFLD cases, as well as other compounding diseases, additional therapies have been investigated and used in clinical practice, such as ursodeoxycholic acid (UDCA), omega-3 polyunsaturated fatty acids (_N_-3 PUFA), statins and pre and probiotics. These therapies target risk factors of NAFLD, such as obesity, dyslipidemia, cardiovascular disease, insulin resistance and IM.
UDCA has been studied in clinical trials to determine its effectiveness on the NAFLD population. UDCA is a naturally occurring secondary bile acid that has been used in clinical trials to determine its effectiveness for treatment of patients with NAFLD/NASH[93]. A randomized double blind study investigated using UDCA (10 mg/kg per day) in obese NAFLD patients over a 3 mo period[94]. The results showed that UDCA was able to reduce liver enzymes, though there was no effect on liver fat content[94]. Lindor _et al_[93] conducted a large randomized trial using UDCA (receiving between 13-15 mg/kg per day) on NASH diagnosed patients and found that there was no significant differences between the placebo and UDCA groups. Therefore, UDCA is not recommend for the treatment of NAFLD.
_N_-3 PUFAs have been used in the treatment of hyperlipidemia and cardiovascular disease, and more recently in the treatment of NAFLD[95]. Studies have highlighted the correlation between insulin resistance and changes in fatty acids, specifically a deficiency in n-3 PUFA[96]. As a result Capanni _et al_[96] investigated the effects of _N_-3 PUFA supplementation (1 g/d for 12 mo) in 56 NAFLD patients. Their results indicated that n-3 PUFA improves biochemical aspects of NAFLD as well as liver steatosis[96]. Similarly, a literature review conducted by Masterton _et al_[95], found that in animal studies _N_-3 PUFA reduced hepatic steatosis, improved insulin sensitivity and biochemical markers of inflammation; human studies yielded similar results. Masterton _et al_[95] and Capanni _et al_[96] concluded that _N_-3 PUFA is a promising therapeutic approach to the treatment of NAFLD.
Statins are used in the medical field to manage dyslipidemia; and are typically used in patients with cardiovascular disease. NAFLD patients often have dyslipidemia along with other features of metabolic syndrome[97]. Several studies have shown that statin use in NAFLD patients with dyslipidemia can improve liver function tests[97-99] as well as steatosis[100]. In addition, these studies have determined that the use of statins producing liver injury is rare[97], and that statins are safe to use in NAFLD/NASH patients with dyslipidemia[98,101]. However, there is a lack of evidence to use statins to treat NASH patients without dyslipidemia[12]. Therefore statin use should be considered for NAFLD/NASH patients with dyslipidemia, but at this time, should not be used for the specific treatment of NAFLD/NASH.
IM has been shown to be beneficial to human health. Research has found that IM regulates energy homeostasis and ectopic fat deposition[102], which has been related to metabolic diseases. NAFLD is associated with metabolic syndrome, and therefore has been the focus of recent pre-probiotic research.
Prebiotics are non-digestible carbohydrates that stimulate growth and activity on bacteria in the colon[103]. The majority of research has been conducted using mice-models; however there have been a limited number of human clinical trials. The majority of studies have investigated risk factors associated with NAFLD. Parnell _et al_[103] conducted a randomized double-blind, placebo-controlled trial to examine the effects of oligofructose (21 g/d for 12 wk) in 48 overweight and obese adults. The results found that oligofructose promoted weight loss and improved glucose regulation[103]. Daubioul _et al_[104] also used oligofructose (16 g/d for 8 wk) in a randomized double-blind crossover study and investigated the effects of oligofructose on glucose and lipid metabolism in 7 NASH patients. Compared to the placebo, AST and ALT decreased after 8 wk and insulin levels after 4 wk, supporting the use of prebiotics in management of liver disease[104]. There is a need for studies to specifically evaluate the use of prebiotics in NAFLD patients with histological end points.
Probiotics (live microorganisms) have been found to improve liver enzymes and liver histology in NAFLD patients[105]. An open pilot study conducted by Loguercio _et al_[106] used probiotic VSL#3 (containing 450 billion bacteria in different strains) for 3 mo. This study had 78 participants, 22 that were biopsy proven NAFLD. In the NAFLD group, plasma levels and lipid peroxidation markers (malondialdehyde and 4-hydroxynonenal) improved[106]. Another study using the same probiotic found that VSL#3 had no beneficial effect on liver disease[107]. Solga _et al_[107] studied the effect of VSL#3 on 4 NAFLD adult subjects in an open pilot study over a 4-mo period. All 4 subjects had a significant increase in liver fat, and no significant differences in biochemical or clinical parameters[107]. As the researchers highlighted, the small sample size is an important limitation to consider[107]. Another study using a randomized double blind clinical trial evaluated the effects of a different probiotic[108]. This study evaluated the effects of Lactobacillus bulgarius and Streptococcus thermophilus (1 tablet/d) in 28 NAFLD patients over a 3-mo period[108]. The results were that ALT, AST and gamma-glutamyl transferase levels decreased[108].
Pre and probiotics have been proven to be useful in the NAFLD population. However, there is a need for larger longitudinal clinical trials to be able to determine the optimal dose and pre and probiotic composition.
Bariatric surgery
Obesity is on the rise in today’s society, which is taking a toll on today’s healthcare system. Obesity is associated with metabolic syndrome, cardiovascular disease, insulin resistance and type 2 diabetes resulting in an increased risk of individuals developing NAFLD. NAFLD is very common in the morbidly obese population; in fact the prevalence of NAFLD in this population is between 75%-100%[109]. Bariatric surgery induces weight loss by reducing the size of a patient’s stomach by either removing a portion of the stomach, using a gastric band, or by gastric bypass[110], and is considered in patients who have a BMI greater than 40 kg/m2 or with a BMI of 35 kg/m2 who have obesity related comorbidities[111]. Prospective and retrospective studies have found that bariatric surgery improved insulin resistance, steatosis and inflammation[112]. Moschen _et al_[113] prospectively found that weight loss after bariatric surgery improved insulin resistance, liver function tests and histology in 18 NAFLD patients. Similarly, the prospective study by Furuya _et al_[114] found that significant weight loss two years post-bariatric surgery significantly improved steatosis and fibrosis in 18 patients with NAFLD. However, a recent Cochrane review concluded that there is insufficient data due to a lack of well-designed randomized control study trials to determine if bariatric surgery is an effective treatment for NAFLD[115]. Overall, the usefulness of bariatric surgery as a treatment for NAFLD, particularly for inflammation and fibrosis, is not clear and future well designed studies need to be conducted.
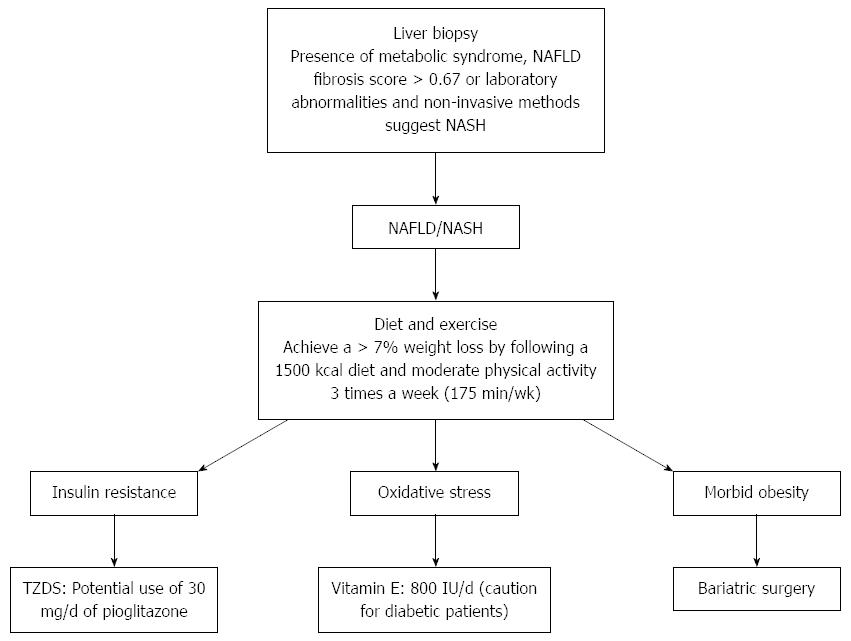
CONCLUSION
The increase in NAFLD has and will continue to burden the health care system, especially because of its ties to obesity, IR and metabolic syndrome. Currently, the understanding of its epidemiology and pathogenesis are well understood, guidelines for proper care are constantly changing as new information emerges, but still NAFLD remains a complex multifaceted issue (Figure 2). The development of non-invasive measures to assess inflammation and fibrosis are commonly used in practice, with liver biopsy being used only in specific cases or within research protocol. Addressing the risk factors associated with NAFLD, such as IR, weight loss and lipid levels remain the primary way to improve NAFLD. However, bariatric surgery, insulin sensitizing agents, antioxidants and fish oil, may also be considered although further research is necessary to clearly document the effect.
Figure 2 Suggested diagnosing and treatment of non-alcoholic fatty liver disease. NAFLD: Non-alcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis; TZDS: Thiazolidinediones.
Currently, weight loss and lifestyle modification should be used as first line therapy. In addition, cardiovascular disease needs to be investigated and treated as this increases in NAFLD. Future studies need to have larger high-quality clinical trials with rigorous methodology in order to establish standards of care.
Footnotes
P- Reviewers: Chiu KW, Das UN, Williams GM S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
| 1. | Clark JM, Diehl AM. Hepatic steatosis and type 2 diabetes mellitus. Curr Diab Rep. 2002;2:210-215. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 8. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 10. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1907] [Cited by in F6Publishing: 1870] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
|---|
| 18. | Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474-2486. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 23. | Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84-89. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 24. | Widhalm K, Ghods E. Nonalcoholic fatty liver disease: a challenge for pediatricians. Int J Obes (Lond). 2010;34:1451-1467. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 32. | Solga S, Alkhuraishe AR, Clark JM, Torbenson M, Greenwald A, Diehl AM, Magnuson T. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004;49:1578-1583. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 35. | Lê KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr. 2006;84:1374-1379. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 42. | Yan E, Durazo F, Tong M, Hong K. Nonalcoholic fatty liver disease: pathogenesis, identification, progression, and management. Nutr Rev. 2007;65:376-384. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 67. | Capristo E, Miele L, Forgione A, Vero V, Farnetti S, Mingrone G, Greco AV, Gasbarrini G, Grieco A. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 84. | Caldwell SH, Argo CK, Al-Osaimi AM. Therapy of NAFLD: insulin sensitizing agents. J Clin Gastroenterol. 2006;40 Suppl 1:S61-S66. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 88. | Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 522] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
|---|
| 89. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2215] [Cited by in F6Publishing: 2369] [Article Influence: 157.9] [Reference Citation Analysis (2)] |
|---|
| 91. | Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 94. | Santos VN, Lanzoni VP, Szejnfeld J, Shigueoka D, Parise ER. A randomized double-blind study of the short-time treatment of obese patients with nonalcoholic fatty liver disease with ursodeoxycholic acid. Braz J Med Biol Res. 2003;36:723-729. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 106. | Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540-543. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 108. | Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090-1095. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 109. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 111. | NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956-961. [PubMed] [DOI] [Cited in This Article: ] |
|---|