Time-course analysis of DNA damage response-related genes after in vitro radiation in H460 and H1229 lung cancer cell lines (original) (raw)
Introduction
Radiation therapy has been regarded as an effective treatment for various cancers. It causes DNA double strand breaks, base damage and DNA-protein crosslink to increase genomic instability. Subsequently, it leads to cell cycle arrest and cell death. To compensate for these cytotoxic events, cells recognize DNA damage and activate DNA repair system, in which DNA damage response (DDR) signaling plays a pivotal role. Upon DNA damage by ionizing radiation, DDR signaling promptly activates sensor kinases, ATM and ATR. The kinases firstly phosphorylate downstream kinases, Chk1 and Chk2 (Falck et al., 2005). Then, these ultimately activate the numerous cellular responses involved in cell cycle arrest and DNA repair (Durocher and Jackson, 2001; Bartek and Lukas, 2003; Shiloh, 2003; Stracker et al., 2009).
Aberrantly and constitutively activated DDR signaling is often observed in cancers (Bartkova et al., 2006; Bartkova et al., 2007). For example, γH2AX and the activation of ATM-Chk2-p53 pathway revealed that DDR machinery is constitutively activated in gliomas (Bartkova et al., 2010). Possibly, aberrantly activated DDR signaling could be associated with radioresistance since the activation of DDR signaling can delay cell death and can repair damaged DNA. Overexpression of cyclin D1 was reported to have activated DDR signaling which was further activated by positive feedback through AKT/GSK3β/cyclin D1/Cdk4 pathway. The radioresistance of human tumor cells can be achieved via aberrantly activated DDR signaling (Shimura et al., 2010).
In spite of the possible correlation between aberrantly activated DDR signaling and radioresistance, it remains to be elucidated whether radioresistant cancer cells differentially modulate the expressions of DDR-related genes after ionizing radiation compared to radiosensitive cells. Additionally, because radiosensitive cancer cells also promptly attempt to arrest cell cycle progression, to recover damaged DNA, and/or to induce cell death after radiation, the temporal changes in DDR-related genes would be very important for understanding the underlying mechanisms of cellular response to radiation.
In this study, we used two non-small cell lung cancer (NSCLC) cell lines, which have different radiosensitivity (radiosensitive H460 and radioresistnat H1299). With these cell lines, we compared gene expression patterns of DDR-related genes after in vitro radiation. Further, the changes in the DDR-related gene expressions were confirmed via Western blot analysis. This result indicated that different radioresistance of H460 and H1299 might be affected by the different response of DDR signaling to radiation.
Results
In vitro radio-resistance of NSCLC cell lines, H460 and H1299
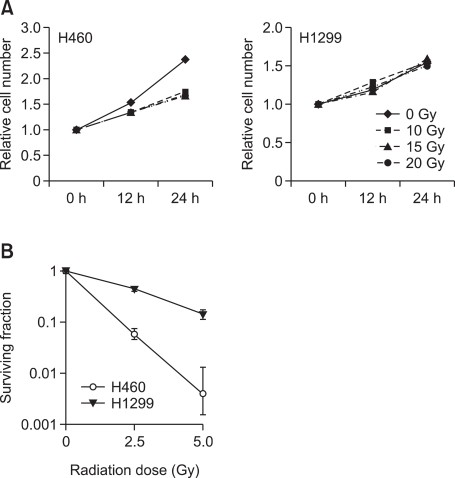
As mentioned earlier, two kinds of NSCLC cell lines were used in this study, H460 (wild-type p53) and H1299 (p53-null) (Jeong et al., 2009). Before comparing the expressional changes of the DDR-related genes, we first confirmed that they showed different radiosensitivity through short-term survival and clonogenic assay. In the short-term survival analysis, viability of H460 was significantly reduced 24 h after 10-, 15- and 20 Gy in vitro radiation (Figure 1A). In contrast, that of H1299 was not affected even when 20 Gy in vitro radiation was applied (Figure 1A). Concomitantly, the clonogenic potential of H460 was reduced by 2.5 and 5 Gy in vitro radiation significantly more than that of H1299 (Figure 1B), indicating that H1299 is more radioresistant than H460.
Figure 1
Radiosensitivity of NSCLC cell lines. The radiosensitivity of H460 and H1299 cells were compared via short-term survival and clonogenic assay. (A) For the short-term survival assay, the cells were irradiated with 10, 15, 20 Gy and harvested after 0, 12, 24 and 48 h. (B) The cells were treated with 2.5 and 5 Gy radiation, and the surviving fraction was calculated based on the number of colonies in the soft agar clonogenic assay.
Temporal changes in the expression of DNA damage checkpoint signaling genes in response to in vitro radiation
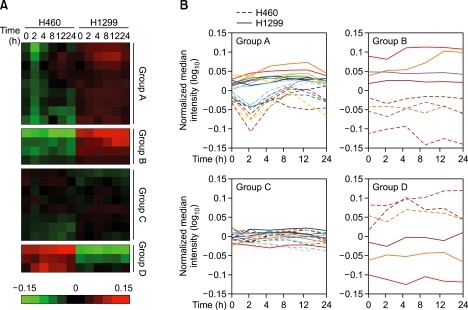
Although hundreds of genes are involved in the transduction of DDR signaling (Yoo et al., 2004; Zhou and Bartek, 2004; Kumagai et al., 2006), we select 24 DDR signaling genes that are primarily and directly associated with DDR signal transduction and that act as sensors, mediators, effectors and transducers (Supplemental Data Figure S1). To determine the differences in DDR signaling between radiosensitive H460 and radioresistant H1299 cell line, expressional changes of the 24 DDR signaling genes (Table 1) were compared via RNA expression arrays after 0, 2, 4, 8, 12 and 24 h of 10 Gy in vitro radiation. Generally, it is interesting that the temporal changes in several DDR signaling genes were clearly observed only in the radiosensitive H460 cell, while the expression level of the radioresistant H1299 cell was mostly unchanged after in vitro radiation (Figure 2A).
Table 1 List of DNA damage response-related genes whose expressions were analyzed
Figure 2
Expressional alteration patterns of the DNA damage checkpoint signaling genes in response to in vitro radiation. The expressional changes of the 24 DNA damage checkpoint signaling genes were analyzed via microarray expression analysis 0, 2, 4, 8, 12 and 24 h after 10 Gy radiation and were categorized into four subgroups according to the temporal expression patterns. Each level was normalized to the average value of both the H460 and H1299 controls and was visualized with ClusterTM /Treeview analysis program (A) or log-scale graph (B).
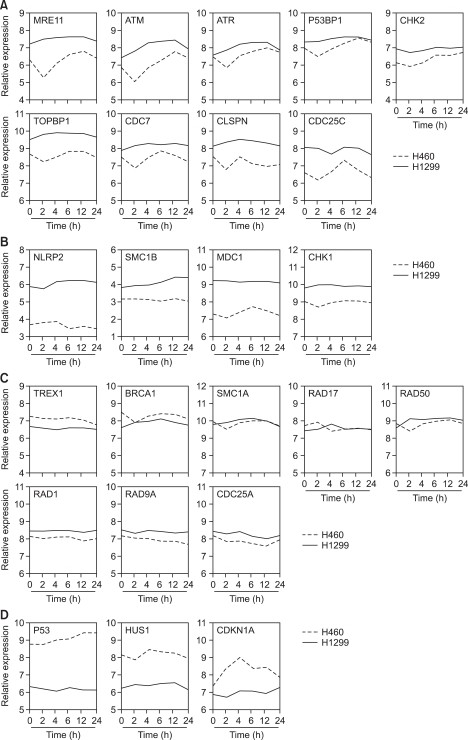
Further, we normalized each expression level to the average value of 0 h time point in both H460 and H1299, then, the genes were classified into four subgroups according to two criteria; (i) the difference in basal expression level between H460 and H1299 and (ii) the pattern of the temporal changes after in vitro radiation. In group A, the expression levels of MRE11A, ATM, ATR, p53BP1, CHK2, TOPBP1, CDC7, Claspin and CDC25C were relatively high and were generally unchanged in the radioresistant H1299 cells, but these genes were transiently decreased and restored after 4-6 h only in the radiosensitive H460 cells (Figures 2B and 3A). In other cases, NLRP2, SMC1B, MDC1 and CHK1 genes showed higher expression levels in the radioresistant H1299 cells than in the radiosensitive H460 cells and the expression patterns in both cell lines remained unchanged (group B; Figures 2B and 3B). On the contrary, p53, HUS1 and CDKN1A genes showed higher expression levels in radiosensitive H460 cells (group D; Figures 2B and 3D). Additionally, other gene sets such as TREX1, BRCA1, SMC1A, RAD17, RAD50, RAD1, RAD9A, and CDC25A were evenly expressed in both H460 and H1299 cells (group C; Figures 2B and 3C).
Figure 3
Changes in the DDR-related genes after in vitro radiation. According to our classification, the time-course changes in each gene in H460 and H1299 were plotted and visualized [(A), group A; (B), group B; (C), group C; (D), group D].
Difference in the activation of DNA damage checkpoint signaling in response to in vitro radiation
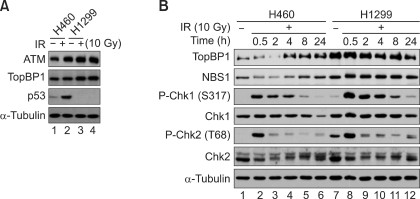
To confirm the microarray results, we further investigated if the expression patterns of certain DDR signaling genes reflect the microarray analysis results. The p53 levels of H460 and H1299 were first addressed to validate the cell line models. As shown in Figure 4A, it is evident that p53 protein was highly expressed in the H460 cells, whereas it was barely detected in the H1299 cells. When the protein levels of several DDR-related genes were tested (Figure 4B), TopBP1 and Chk2, which were assigned to group A, were transiently reduced at 1 h and were recovered after 8 h following in vitro radiation treatments. Furthermore, one of the group B genes, Chk1, was shown to be constitutively higher in the radioresistant H1299 cells, validating the microarray data and pattern classification. Interestingly, it is notable that DDR signaling was strengthened in the radioresistant H1299 cells. For example, the phosphorylation levels of Chk1 and Chk2 in H1299 were stronger than those of H460 and were slightly prolonged. Taken together, the temporal changes in the DDR-signaling genes such as TopBP1, Chk1 and Chk2 supported the microarray results. It was also found that both H460 and H1299 rapidly activated DDR signaling in response to in vitro radiation but DDR signaling seems to be higher in the H1299 cells.
Figure 4
Differential activation of DDR signaling after in vitro radiation. The activation of DDR signaling was analyzed via Western blotting. (A) The protein levels of certain DDR-related genes in H460 and H1299 were addressed after in vitro radiation (10 Gy). (B) The H460 and H1299 cells were treated with in vitro radiation (10 Gy) and were harvested after 0.5, 2, 4, 8, and 24 h. Then, the temporal changes in the DDR-related genes such as TopBP1, NBS1, Chk1 and Chk2 were visualized via Western blotting.
Discussion
In this study, two NSCLC cell lines were applied to explore radiosensitivity as well as time-dependent alteration in the gene expression profile after in vitro radiation. H460 cells were found to be relatively sensitive to radiation, showing a decreased cellular proliferation rate and clonogenic potential, whereas the H1299 cells seemed to be radioresistant (Figure 1). When the gene expression patterns of H460 and H1299 were compared, it is of interest that only H460 changed the expressions of the DDR-related genes after in vitro radiation, and that H1299 did not (Figures 2 and 3). Regarding the fact that the temporal regulation of the target genes might be critical for deciding whether to resume cell proliferation, to enter senescence, or to induce apoptosis (Snyder and Morgan, 2004), the transient suppression of several DDR-related genes in H460 might be important for modulating DDR signaling and radiosensitivity.
Although H1299 is relatively radiosensitive, some cells ultimately survived and made visible colonies in the clonogenic assay (Figure 1). Many previous studies have reported that radioresistant subclones could be artificially derived from radiosensitive cancer cells (Tyrsina et al., 2005; Ogawa et al., 2006; Nojiri et al., 2009). According to our observation, it is likely that most of the genes in group A was associated with the initial step of signal transduction, which contributed as signal sensors and transducers (ATM, ATR, MRE11, 53BP1, TopBP1, Claspin and Chk2). This can be explained by the fact that the radiosensitive H460 cells temporally undergo DDR signaling attenuation (group A, within 2 h), resulting in the impairment of the proper response, such as the DNA repair process. Rather, they promptly respond to radiation by suppressing cell proliferation through the induction of cell cycle arrest genes in group D (p53 and CDKN1A). This might initially lead to the cell cycle arrest and cell death of radiosensitive H460 cells. After several hours, these cells actively restored the expressions of the group A genes to sustain intact DDR signaling, which might have been responsible for the recovery from the radiation-induced DNA damages and acquired radioresistance. This provides a clue for targeting DDR signaling and for developing anti-cancer therapeutics based on the underlying mechanisms in response to radiation, but much remains to be elucidated.
It has been well-known that H460 harbors wildtype p53, whereas H1299 does not express p53 (Choi et al., 2000; Kataoka et al., 2000; Lai et al., 2000). In H460 cells, it is evident that ionizing radiation normally activates p53 and induces p21 mRNA expression through DDR signaling (Figures 3D and 4A). This tendency was consistently observed in other studies (Amundson et al., 1999; Tusher et al., 2001; Sak et al., 2003). Conversely, although H1299 cells can initiate DDR signaling at the level of phospho-Chk1/2 (Figure 4B), the absence of master regulator p53 enables H1299 cells to ignore the radiation-induced cell cycle arrest and the subsequent apoptosis (Figures 1, 2 and 4A). It has been also observed that p53-null H1299 cells are more resistant to anti-tumor therapy than H460 cells (Nishizaki et al., 2001). Similarly, the restoration of p53 signaling through the ectopic expression of wild-type p53 or MDM2-targeting antisense oligonucleotide causes human cancer cells to become radiosensitive (Zhang et al., 2004; Mazzatti et al., 2005), suggesting that the lack of p53 function allows cell proliferation rather than apoptosis-mediated elimination. Thus, it is plausible that a different p53 status may be primarily devoted to the radiosensitivity of H460 and H1299. However, it has to be elucidated how subpopulation of p53-expressing cancer cells such as H460 could acquire radioresistance after in vitro radiation.
It is also worthwhile to investigate the direct relationship between p53 and different DDR-related gene expressions. As the temporal reduction of the group A genes was restrictively observed in the H460 cells, p53 possibly contributes to the expressions of DDR-related genes. Indeed, several reports have demonstrated that p53-mediated transcriptional suppression affects the expression of several DNA repair and cell survival genes, such as survivin, deoxyuridine triphosphate nucleotidohydrolase (dUTPase), manganese superoxide dismutase (Mn-SOD), apurinic/apyrimidinic (AP) endonuclease and myeloid cell leukemia sequence 1 (MCL-1) (Mirza et al., 2002; Lohr et al., 2003; Dhar et al., 2006; Zaky et al., 2008; Wilson et al., 2009). Thus, it is possible that the temporal reduction of the group A genes is caused by radiation-induced p53 activation.
In conclusion, the present study on the temporal changes in the DDR-related gene expressions after in vitro radiation provides a novel insight for the short-term response of NSCLC cell lines. By comparing two NSCLC cell lines that have different radiosensitivity levels and p53 statuses, it was revealed that the transient reduction of certain DDR genes can be observed only in radiosensitive H460 cells, which was confirmed via Western blotting. Furthermore, the possibility that p53 is involved in these events was discussed. Collectively, this serves as a basis for the transcriptional control of DDR-related genes, which highlights the first step to uncovering the molecular responses of lung cancer cells to radiation.
Methods
Cell culture
H460 and H1299 cells were maintained in Roswell Park Memorial Institute medium 1640 (RPMI1640) supplemented with 10% FBS (Hyclone, Logan, UT), 1% penicillin/streptomycin (Gibco-BRL, Carlsbad, CA), and 2 mM L-glutamine (Gibco-BRL, Carlsbad, CA).
Survival and clonogenic assay
In the survival assay, H460 or H1299 cells in 96-well plates were irradiated (0, 10, 15 and 20 Gy) using a blood irradiator (IBL-437C, CIS-US, Inc., Bedford, MA). The cell viability was determined via colorimetric assay (Cell Counting Kit-8, Dojindo Molecular Technologies, Gaithersburg, MD) 0, 12, 24 and 48 h after radiation (n = 3 for each group).
For the clonogenic assay, the cells were seeded into 6-well plates at a density of 200-500 cells/well and were incubated overnight. After exposure to ionizing radiation from a blood irradiator (0, 2.5 and 5 Gy), the cells were cultured for 10-15 days. The colonies were fixed with 100% chilled methanol and were stained with crystal violet (Sigma, St. Louis, MO). The colonies with over 50 cells were scored and the cell survival was determined after correcting for plating efficiency.
RNA expression array and data analysis
Total RNAs were isolated from 10 Gy irradiated H460 or H1299 cells at 0, 2, 4, 8, 12, and 24 h after radiation (n = 3 for each group) using TRIZOL reagent (Invitrogen, Carlsbad, CA) and were further purified using RNeasy Mini Kit (Qiagen, Hilden, Germany). The samples were processed and hybridized to Affymetrix U-133 plus2 GeneChip Arrays according to the Affymetrix GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA). All the arrays were confirmed to be within the acceptable minimal quality control parameters. The gene expression CEL files were normalized using the Robust Multichip Averaging procedure and PM-MM difference model was used to obtain the expression values. Statistical analysis comparing the expression of the 24 DNA damage checkpoint signaling genes was performed using R Package Software 2.6.0. The normalized value was visualized by using the Cluster™ and Treeview programs according to the developer's instructions (Eisen Lab, http://www.eisenlab.org).
Western blot analysis
The cells were lysed and prepared for immunoblotting analysis as previously described (Yoo et al., 2009). The cells were processed at the indicated time after irradiation (10 Gy), were washed (PBS), and were harvested. Anti-ATM, anti-Chk1, anti-Chk2, anti-Rad17, anti-p21, and anti-p53 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Chk1 phospho-Ser317 and anti-Chk2 phospho-Thr68 antibodies were obtained from Cell Signaling Technology (Danvers, MA). Anti-TopBP1, anti-Nbs1, and anti-human α-tubulin antibodies were obtained from Bethyl Laboratoratoies, Inc (Montgomery, TX), Calbiochem (La Jolla, CA) and Oncogene Research Products (Cambridge, MA), respectively.
Abbreviations
DDR:
DNA-damage response
NSCLC:
non small cell lung cancer
References
- Amundson SA, Bittner M, Chen Y, Trent J, Meltzer P, Fornace AJ . Fluorescent cDNA microarray hybridization reveals complexity and heterogeneity of cellular genotoxic stress responses . Oncogene 1999 ; 18 : 3666 - 3672
Article CAS PubMed Google Scholar - Bartek J, Lukas J . Chk1 and Chk2 kinases in checkpoint control and cancer . Cancer Cell 2003 ; 3 : 421 - 429
Article CAS PubMed Google Scholar - Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG . Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints . Nature 2006 ; 444 : 633 - 637
Article CAS PubMed Google Scholar - Bartkova J, Horejsi Z, Sehested M, Nesland JM, Rajpert-De Meyts E, Skakkebaek NE, Stucki M, Jackson S, Lukas J, Bartek J . DNA damage response mediators MDC1 and 53BP1: constitutive activation and aberrant loss in breast and lung cancer, but not in testicular germ cell tumours . Oncogene 2007 ; 26 : 7414 - 7422
Article CAS PubMed Google Scholar - Bartkova J, Hamerlik P, Stockhausen MT, Ehrmann J, Hlobilkova A, Laursen H, Kalita O, Kolar Z, Poulsen HS, Broholm H, Lukas J, Bartek J . Replication stress and oxidative damage contribute to aberrant constitutive activation of DNA damage signalling in human gliomas . Oncogene 2010 ; 29 : 5095 - 5102
Article CAS PubMed Google Scholar - Choi JH, Ahn KS, Kim J, Hong YS . Enhanced induction of Bax gene expression in H460 and H1299 cells with the combined treatment of cisplatin and adenovirus mediated wt-p53 gene transfer . Exp Mol Med 2000 ; 32 : 23 - 28
Article CAS PubMed Google Scholar - Dhar SK, Xu Y, Chen Y, St Clair DK . Specificity protein 1-dependent p53-mediated suppression of human manganese superoxide dismutase gene expression . J Biol Chem 2006 ; 281 : 21698 - 21709
Article CAS PubMed Google Scholar - Durocher D, Jackson SP . DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme ? Curr Opin Cell Biol 2001 ; 13 : 225 - 231
Article CAS PubMed Google Scholar - Falck J, Coates J, Jackson SP . Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage . Nature 2005 ; 434 : 605 - 611
Article CAS PubMed Google Scholar - Jeong SH, Wu HG, Park WY . LIN28B confers radio-resistance through the posttranscriptional control of KRAS . Exp Mol Med 2009 ; 41 : 912 - 918
Article CAS PubMed PubMed Central Google Scholar - Kataoka M, Wiehle S, Spitz F, Schumacher G, Roth JA, Cristiano RJ . Down-regulation of bcl-2 is associated with p16INK4-mediated apoptosis in non-small cell lung cancer cells . Oncogene 2000 ; 19 : 1589 - 1595
Article CAS PubMed Google Scholar - Kumagai A, Lee J, Yoo HY, Dunphy WG . TopBP1 activates the ATR-ATRIP complex . Cell 2006 ; 124 : 943 - 955
Article CAS PubMed Google Scholar - Lai SL, Perng RP, Hwang J . p53 gene status modulates the chemosensitivity of non-small cell lung cancer cells . J Biomed Sci 2000 ; 7 : 64 - 70
Article CAS PubMed Google Scholar - Lohr K, Moritz C, Contente A, Dobbelstein M . p21/CDKN1A mediates negative regulation of transcription by p53 . J Biol Chem 2003 ; 278 : 32507 - 32516
Article PubMed Google Scholar - Mazzatti DJ, Lee YJ, Helt CE, O'Reilly MA, Keng PC . p53 modulates radiation sensitivity independent of p21 transcriptional activation . Am J Clin Oncol 2005 ; 28 : 43 - 50
Article PubMed Google Scholar - Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, Nielsen LL, Pickett CB, Liu S . Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway . Oncogene 2002 ; 21 : 2613 - 2622
Article CAS PubMed Google Scholar - Nishizaki M, Meyn RE, Levy LB, Atkinson EN, White RA, Roth JA, Ji L . Synergistic inhibition of human lung cancer cell growth by adenovirus-mediated wild-type p53 gene transfer in combination with docetaxel and radiation therapeutics in vitro and in vivo . Clin Cancer Res 2001 ; 7 : 2887 - 2897
CAS PubMed Google Scholar - Nojiri K, Iwakawa M, Ichikawa Y, Imadome K, Sakai M, Nakawatari M, Ishikawa K, Ishikawa A, Togo S, Tsujii H, Shimada H, Imai T . The proangiogenic factor ephrin-A1 is up-regulated in radioresistant murine tumor by irradiation . Exp Biol Med (Maywood) 2009 ; 234 : 112 - 122
Article CAS Google Scholar - Ogawa K, Utsunomiya T, Mimori K, Tanaka F, Haraguchi N, Inoue H, Murayama S, Mori M . Differential gene expression profiles of radioresistant pancreatic cancer cell lines established by fractionated irradiation . Int J Oncol 2006 ; 28 : 705 - 713
CAS PubMed Google Scholar - Sak A, Wurm R, Elo B, Grehl S, Pottgen C, Stuben G, Sinn B, Wolf G, Budach V, Stuschke M . Increased radiation-induced apoptosis and altered cell cycle progression of human lung cancer cell lines by antisense oligodeoxy-nucleotides targeting p53 and p21(WAF1/CIP1) . Cancer Gene Ther 2003 ; 10 : 926 - 934
Article CAS PubMed Google Scholar - Shiloh Y . ATM and related protein kinases: safeguarding genome integrity . Nat Rev Cancer 2003 ; 3 : 155 - 168
Article CAS PubMed Google Scholar - Shimura T, Kakuda S, Ochiai Y, Nakagawa H, Kuwahara Y, Takai Y, Kobayashi J, Komatsu K, Fukumoto M . Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression . Oncogene 2010 ; 29 : 4826 - 4837
Article CAS PubMed Google Scholar - Snyder AR, Morgan WF . Gene expression profiling after irradiation: clues to understanding acute and persistent responses ? Cancer Metastasis Rev 2004 ; 23 : 259 - 268
Article CAS PubMed Google Scholar - Stracker TH, Usui T, Petrini JH . Taking the time to make important decisions: the checkpoint effector kinases Chk1 and Chk2 and the DNA damage response . DNA Repair (Amst) 2009 ; 8 : 1047 - 1054
Article CAS Google Scholar - Tusher VG, Tibshirani R, Chu G . Significance analysis of microarrays applied to the ionizing radiation response . Proc Natl Acad Sci U S A 2001 ; 98 : 5116 - 5121
Article CAS PubMed PubMed Central Google Scholar - Tyrsina EG, Slanina SV, Kakpakova ES, Kalendo GS, Kan NG, Tyrsin OY, Ryskov AP . Isolation and characterization of highly radioresistant malignant hamster fibroblasts that survive acute gamma irradiation with 20 Gy . Radiat Res 2005 ; 164 : 745 - 754
Article CAS PubMed Google Scholar - Wilson PM, Fazzone W, LaBonte MJ, Lenz HJ, Ladner RD . Regulation of human dUTPase gene expression and p53-mediated transcriptional repression in response to oxaliplatin-induced DNA damage . Nucleic Acids Res 2009 ; 37 : 78 - 95
Article CAS PubMed Google Scholar - Yoo HY, Kumagai A, Shevchenko A, Dunphy WG . Adaptation of a DNA replication checkpoint response depends upon inactivation of Claspin by the Polo-like kinase . Cell 2004 ; 117 : 575 - 588
Article CAS PubMed Google Scholar - Yoo HY, Kumagai A, Shevchenko A, Dunphy WG . The Mre11-Rad50-Nbs1 complex mediates activation of TopBP1 by ATM . Mol Biol Cell 2009 ; 20 : 2351 - 2360
Article CAS PubMed PubMed Central Google Scholar - Zaky A, Busso C, Izumi T, Chattopadhyay R, Bassiouny A, Mitra S, Bhakat KK . Regulation of the human AP-endonuclease (APE1/Ref-1) expression by the tumor suppressor p53 in response to DNA damage . Nucleic Acids Res 2008 ; 36 : 1555 - 1566
Article CAS PubMed PubMed Central Google Scholar - Zhang Z, Wang H, Prasad G, Li M, Yu D, Bonner JA, Agrawal S, Zhang R . Radiosensitization by antisense anti-MDM2 mixed-backbone oligonucleotide in in vitro and in vivo human cancer models . Clin Cancer Res 2004 ; 10 : 1263 - 1273
Article CAS PubMed Google Scholar - Zhou BB, Bartek J . Targeting the checkpoint kinases: chemosensitization versus chemoprotection . Nat Rev Cancer 2004 ; 4 : 216 - 225
Article CAS PubMed Google Scholar
Acknowledgements
This study was supported by a grant of The Korea Healthcare Technology R&D Project, Ministry for Health & Welfare Affairs, Republic of Korea (A090253).
Author information
Author notes
- Kang Ho Kim, Hae Yong Yoo and Kyeung Min Joo: These authors contributed equally to this work.
Authors and Affiliations
- Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.,
Kang Ho Kim, Yong Jung, Juyoun Jin, Yonghyun Kim, Ho Jun Seol & Do-Hyun Nam - Samsung Biomedical Research Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.,
Kang Ho Kim, Hae Yong Yoo, Yong Jung, Juyoun Jin, Yonghyun Kim, Su Jin Yoon, Seung Ho Choi, Ho Jun Seol & Do-Hyun Nam - Cancer Stem Cell Research Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.,
Kang Ho Kim, Kyeung Min Joo, Yong Jung, Juyoun Jin, Yonghyun Kim, Ho Jun Seol & Do-Hyun Nam - Department of Anatomy, Seoul National University College of Medicine, Seoul 110-799, Korea.,
Kyeung Min Joo - Department of Biomedical Sciences, Biochemistry and Molecular Biology, Seoul National University College of Medicine, Seoul 110-799, Korea.,
Woong-Yang Park
Authors
- Kang Ho Kim
You can also search for this author inPubMed Google Scholar - Hae Yong Yoo
You can also search for this author inPubMed Google Scholar - Kyeung Min Joo
You can also search for this author inPubMed Google Scholar - Yong Jung
You can also search for this author inPubMed Google Scholar - Juyoun Jin
You can also search for this author inPubMed Google Scholar - Yonghyun Kim
You can also search for this author inPubMed Google Scholar - Su Jin Yoon
You can also search for this author inPubMed Google Scholar - Seung Ho Choi
You can also search for this author inPubMed Google Scholar - Ho Jun Seol
You can also search for this author inPubMed Google Scholar - Woong-Yang Park
You can also search for this author inPubMed Google Scholar - Do-Hyun Nam
You can also search for this author inPubMed Google Scholar
Corresponding authors
Correspondence toWoong-Yang Park or Do-Hyun Nam.
Additional information
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kim, K., Yoo, H., Joo, K. et al. Time-course analysis of DNA damage response-related genes after in vitro radiation in H460 and H1229 lung cancer cell lines.Exp Mol Med 43, 419–426 (2011). https://doi.org/10.3858/emm.2011.43.7.046
- Accepted: 30 May 2011
- Published: 02 June 2011
- Issue Date: July 2011
- DOI: https://doi.org/10.3858/emm.2011.43.7.046