A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids (original) (raw)
4 figures, 2 videos and 2 tables
Figures
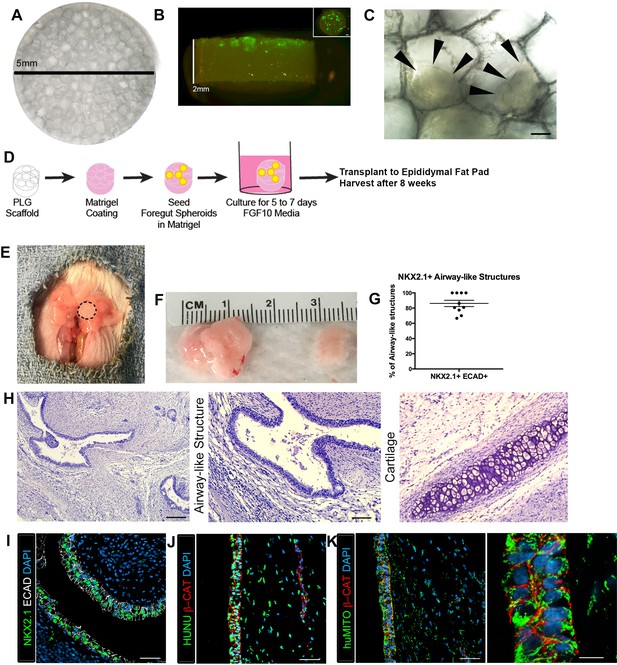
Transplanted HLO-scaffold constructs engrafted, grew and possessed airway-like structures.
(A) PLG scaffold are 5 mm in diameter with honeycomb-patterned architecture. (B) The majority of Di-O labeled 1d HLOs (green) remained at the surface of the scaffold with a few organoids descending toward the middle of the scaffold. Inset shows aerial view of the scaffold with 1d HLOs (green) scattered throughout. (C) 1d HLOs settled within the pores of the scaffold. Scale bar represents 100 µm. (D) PLG scaffolds were seeded with 1d HLOs and cultured for 5 to 7 days in vitro in media supplemented with FGF10. The HLO-laden scaffolds were then transplanted into the mouse epididymal fat pad and harvested at 8 weeks. (E) HLO-scaffold (dotted line) was placed in mouse epididymal fat pad. (F) Transplanted HLOs (tHLOs) ranged from 0.5 cm to 1.5 cm in length. (G) The average number airway-like structures that were NKX2.1+ ECAD+ out of all ECAD+ structures was 86.19% +/- 4.14% (N = 10, error bars represent SEM). (H) H&E of tHLOs showed airway-like structures (right two panels, low and high mag) and pockets of cartilage (left panel). Scale bar at low mag represents 200 µm and high mag 100 µm. (I) Airway-like structures outlined by ECAD (white) expressed the lung marker NKX2.1 (green). Scale bar represents 50 µm. (J–K) Both the epithelium (β-CAT, red) and mesenchyme expressed the human nuclear marker, HUNU (J, green) and the human mitochondrial marker huMITO (K, green). Scale bars represent 50 µm in J–K and 10 µm in high mag image in K.
https://doi.org/10.7554/eLife.19732.003
Figure 1—source data 1
Summary of NKX2.1+ epithelial structures in individual tHLOs.
The number of ECAD+ structures were counted and scored as positive or negative for NKX2.1 expression. The percent NKX2.1+ epithelial structures were calculated (NKX2.1+ECAD+/total ECAD+) for each tHLO (conditions listed). The averages are listed in the bottom row.
https://doi.org/10.7554/eLife.19732.004
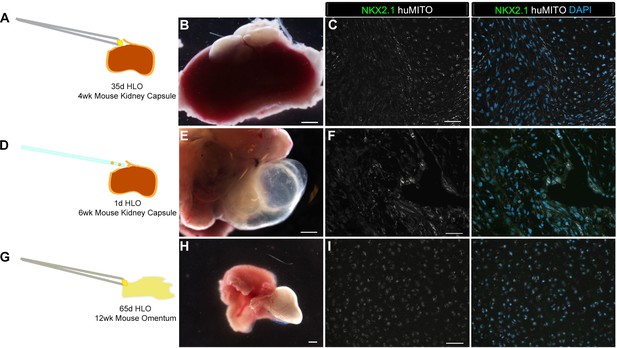
Highly vascular engraftment sites did not maintain lung epithelium.
(A) 35d HLOs were transplanted under the mouse kidney capsule and retrieved after 4 weeks. (B, E, H) Whole-mount of the retrieved tissue within the kidney capsule. Transplanted tissue (white) was situated adjacent to the kidney (pink). (C) Tissue retrieved from the kidney capsule did not express the lung marker NKX2.1 (green), but expressed the human mitochondrial marker (huMITO, white) n = 6. (D) 1d HLOs were placed under the kidney capsule and retrieved after 6 weeks. (E) 1d HLOs transplanted under the kidney capsule consisted of transparent cysts and dense tissue. (F) Tissue retrieved from the kidney capsule did not express NKX2.1 (green), but expressed huMITO (white) n = 1. (G) 65d HLOs were sewn into the omentum surrounding the stomach and proximal intestine. The tissue was retrieved after 12 weeks. (H) The outgrowth (white) is the transplant after 12 weeks. (I) The majority of the tissue did not express NKX2.1 (green), but all the retrieved tissue expressed huMITO (white). n = 13 Scale bars in B,E,H represent 250 µm and scale bars in C,F,I represent 50 µm.
https://doi.org/10.7554/eLife.19732.005
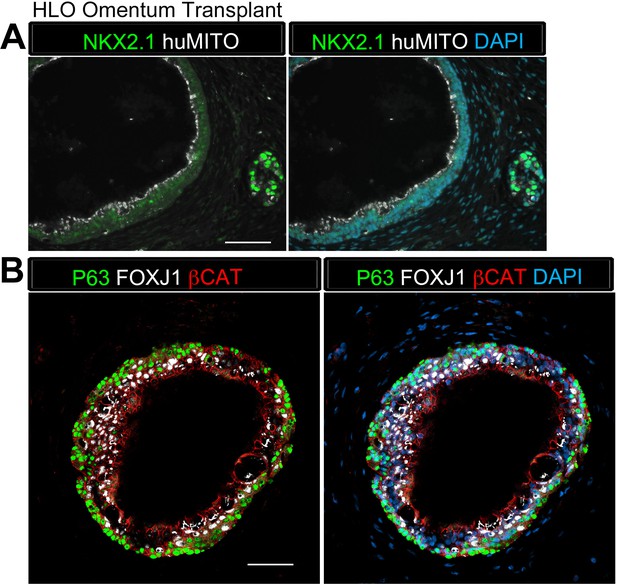
HLO omentum transplants maintained lung epithelium poorly.
(A) 2 out of 13 HLO omentum transplants expressed the lung marker NKX2.1 (green) and all the transplant tissue expressed the human mitochondria marker (huMITO, white). (B) 2 out 13 transplants possessed airway-like structures that expressed the basal cell marker P63 (green) and the ciliated cell marker FOXJ1 (white). Scale bars represent in A–B 50 µm.
https://doi.org/10.7554/eLife.19732.006
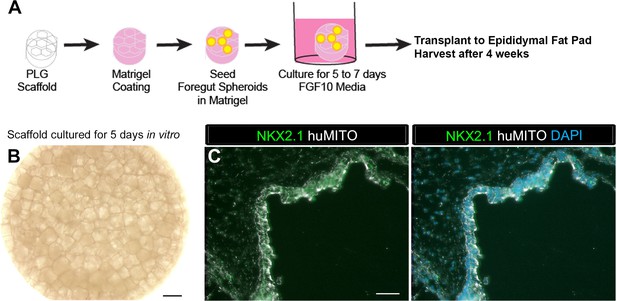
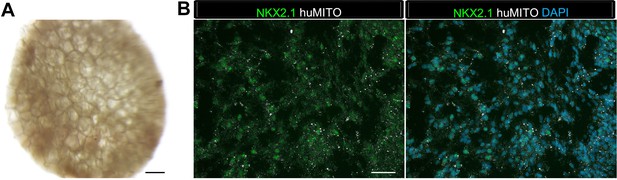
1d HLOs grown on a scaffold and transplanted into the mouse epididymal fat pad expressed lung markers when harvested at 4 weeks.
(A) PLG scaffolds were coated with a mix of Matrigel and DMEM/F12. 1d HLOs were then seeded onto the scaffold in 100% Matrigel. The scaffolds were cultured for 5 to 7 days in vitro in media supplemented with FGF10. The scaffolds were retrieved after 4 weeks. (B) Whole-mount image of 1d HLOs seeded on the scaffold and cultured for 5 days in vitro. (C) Tissue retrieved after 4 weeks expressed the lung marker NKX2.1 (green) and huMITO (white) within the airway-like structures n = 4. Scale bars in B represent 500 µm and in C represent 50 µm.
https://doi.org/10.7554/eLife.19732.007
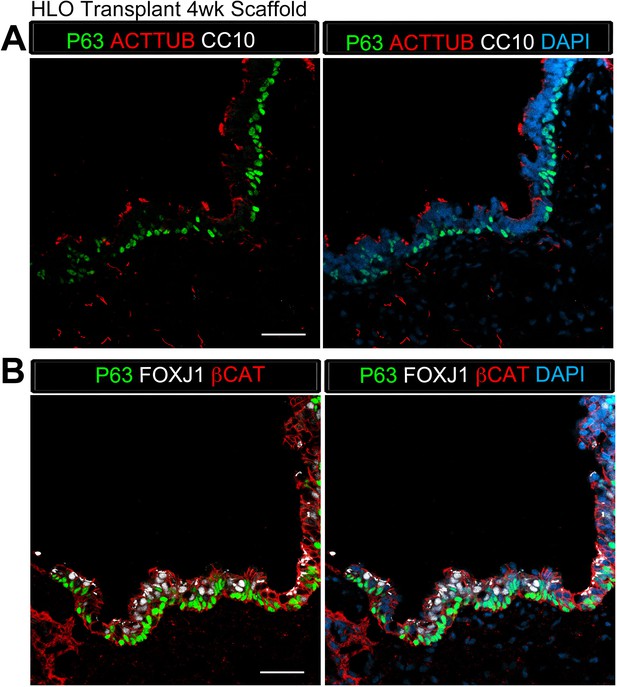
Transplanted HLO-scaffold constructs retrieved 4 weeks post-transplantation possessed airway-like structures that expressed basal and ciliated cell markers.
(A) 4 week HLO-scaffold transplants had airway-like structures that expressed the basal cell marker P63 (green) and the ciliated cell marker ACTTUB (red), but did not express the club cell marker CC10 (white). (B) Airway-like structures expressed P63 (green) and the ciliated cell marker FOXJ1 (white). β-Catenin (βCAT, red) outlines the epithelium n = 4. Scale bars represent in A–B 50 µm.
https://doi.org/10.7554/eLife.19732.008
1d HLOs grown on PLG scaffolds in vitro maintained NKX2.1 expression but did not generate airway-like structures after 8 weeks.
(A) Whole mount image of scaffold grown for 8 weeks in vitro. Organoid tissue remained localized to the scaffold. Scale bar represents 500 µm. (B) HLOs within the scaffold expressed the markers NKX2.1 (green) and huMITO (white), but did not form organized airway-like structures. Scale bars represent 50 µm.
https://doi.org/10.7554/eLife.19732.009
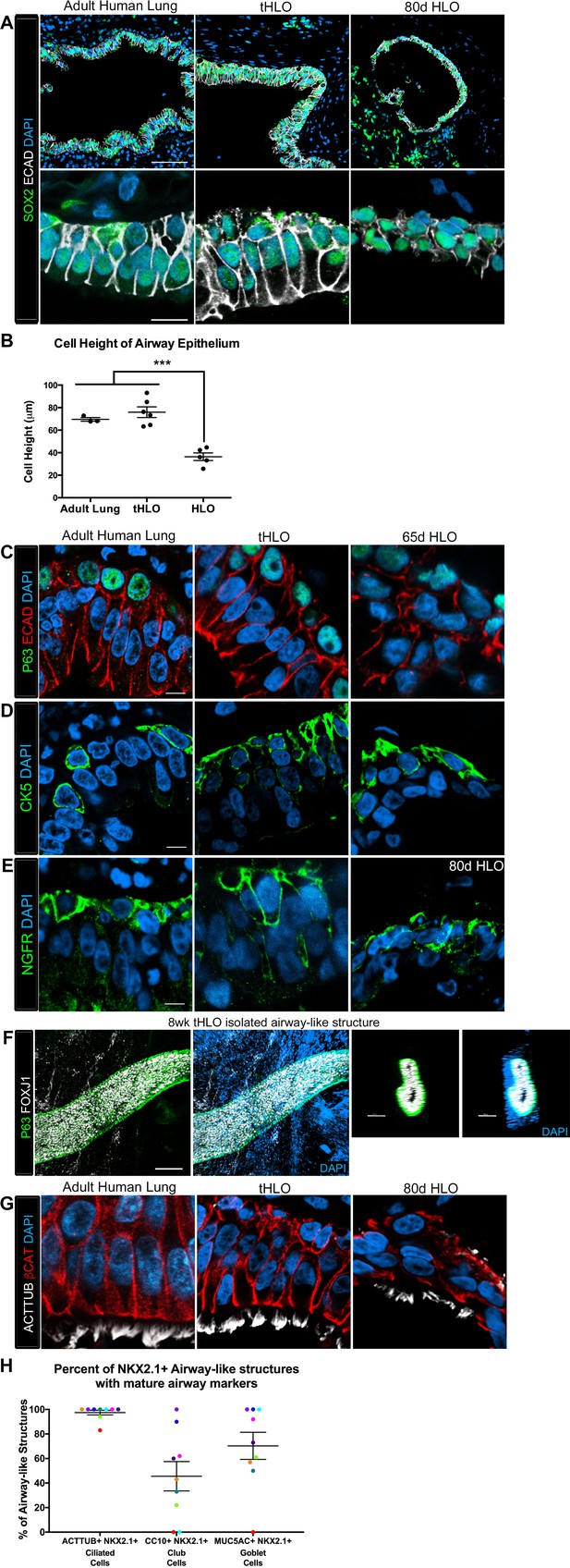
Transplanted HLO-scaffold constructs harvested at 8 weeks possessed mature airway-like structures and had an enhanced epithelial structure.
(A) Adult human lung, tHLOs and 80d HLOs possess SOX2+ (green) epithelium marked by ECAD (white). Only adult lung airways and tHLO airway-like structures possessed a pseudostratified epithelium (ECAD, white). Scale bars represent 50 µm in low mag images and 10 µm in high mag. (B) Measurements of cell height were taken from adult human lung (n = 3), tHLO (n = 5), and HLO (n = 6) airways of cells facing toward the lumen. Averages were adult: 69.59 µm ± 1.65, tHLO: 75.69 µm ± 4.74, HLO = 36.39 µm ± 3.39. *** represents p<.0005 and error bars represent SEM. All HLOs were derived from hESC line UM63-1. (C–E) Adult human lung, tHLOs, and HLOs (65d, 80d) expressed the basal cell markers P63 (C, green), cytokeratin5 (CK5, D, green), and NGFR (E, green) Scale bars represent 10 µm. (F) 3D rendering of z-stack images revealed tube-like structures with cells lining the tube expressing the basal cell marker P63 (green) and cells within the tube cells expressing the ciliated cell marker FOXJ1 (white). A cross section of the z-stack images through the tube revealed that P63 (green) lines the tube while FOXJ1+ cells (white) are within the tube. Scale bars represent 100 µm. (G) Adult human lung, tHLO, and 80d HLO possessed ciliated cells labeled by ACTTUB (white) with the cilia facing in toward the lumen. Scale bars represent 10 µm. (H) NKX2.1+ airway-like structures within each tHLO (n = 9) that contained NKX2.1+ACTTUB+(ciliated cells), NKX2.1+CC10+ (club cells), or NKX2.1+MUC5AC+ (goblet cells) in each tHLO. Data was quantified from UM63-1 hESC-derived tHLOs transplanted for 8 weeks. Each independent tHLO counted is represented by a different color. (n = 9, error bars represent SEM).
https://doi.org/10.7554/eLife.19732.010
Figure 2—source data 1
Summary of NKX2.1+ airway-like structures that contain ACTTUB+ cells, CC10+ cells, or MUC5AC+ cells in individual UM63-1 hESC-derived tHLOs.
The number of NKX2.1+ airway-like structures were counted that also possessed ACTTUB+ multiciliated cells, CC10+ secretory cells, or MUC5AC+ secretory cells. The percent of airways possessing each cell type was calculated (ACTTUB+NKX2.1+/Total NKX2.1+ epithelial structures) for each tHLO (conditions listed). This same equation was applied for CC10+ and MUC5AC+ secretory cells. The averages are listed in the bottom row.
https://doi.org/10.7554/eLife.19732.011
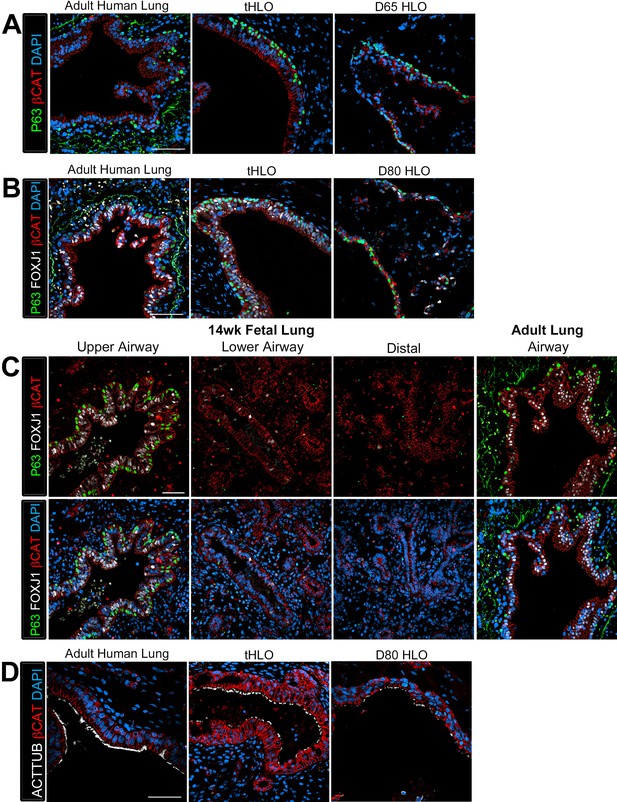
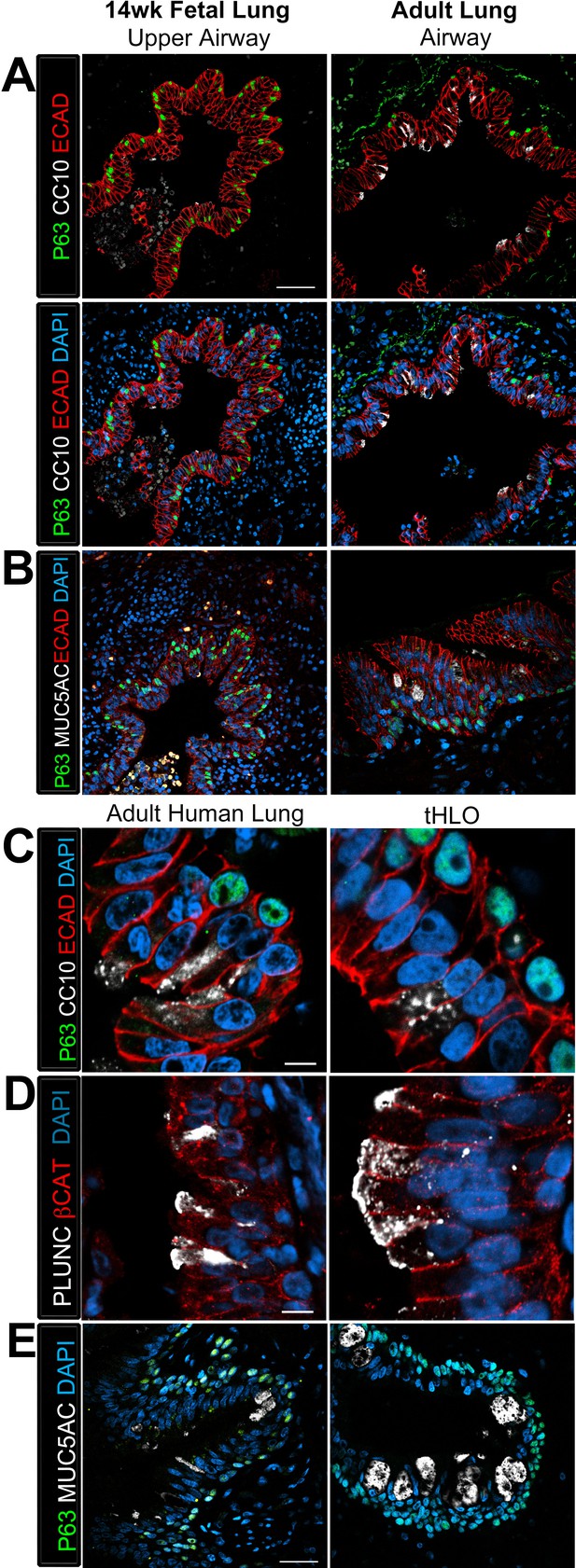
Human airways, in vitro grown HLOs, and transplanted HLOs express airway markers.
(A–B) Adult human lung, tHLOs, and 80d HLOs express the basal cell marker P63 (green) and the ciliated cell marker FOXJ1 (white, B). (C) 14 wk fetal lung upper airway and adult lung airway express P63 (green) and FOXJ1 (white) whereas little to no P63 and FOXJ1 is observed in the fetal lower airways and fetal distal bud tips. (D) Cells facing in toward the lumen were multiciliated and stained positive for acetylated tubulin (ACTTUB, white) . Scale bars in A–D represent 50 µm.
https://doi.org/10.7554/eLife.19732.012
Transplanted HLO-scaffolds possessed lung secretory cells.
(A–B) 14 week fetal upper airway possessed P63+ basal cells (green), but did not possess CC10+ club cells (A, white) or MUC5AC+ goblet cells (B, white) while the adult airway expressed both, CC10 (A) and MUC5AC (B). Scale bars in A–B represent 50 µm. (C–D) Airway-like structures in tHLOs were lined with the basal cell marker P63 (green). Some luminal cells expressed the club cell markers CC10 (C, white), or PLUNC (D, white), and the goblet cell marker MUC5AC (E, white) . Scale bars in C–E represent 10 µm.
https://doi.org/10.7554/eLife.19732.013
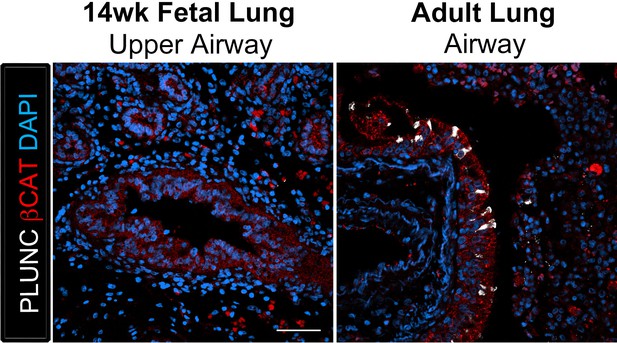
The club cell marker PLUNC is not detected in 14 wk fetal lungs, but is expressed in the adult lung airway epithelium.
The adult airway epithelium expressed βCAT (red) and contained cells expressing the club cell marker PLUNC (white) while no expression was detected in the 14 wk fetal lung upper airways. Scale bar represents 50 µm.
https://doi.org/10.7554/eLife.19732.014
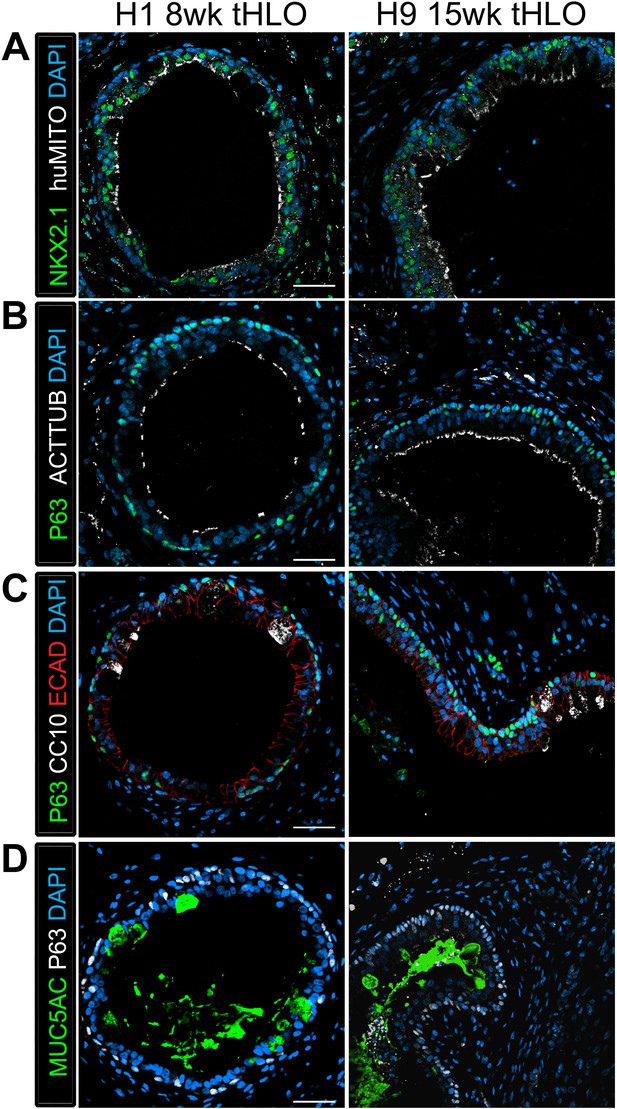
tHLOs derived from H1 and H9 hESC lines contain airway-like structures that express mature markers.
(A) H1 8 wk tHLO and H9 15 wk tHLO express the lung marker NKX2.1 (green) and the human mitochondria marker (huMITO, white) within airway-like structures. (B) H1 8 wk tHLO and H9 15 wk tHLO possess multiciliated cells labelled by ACTTUB (white) that line the lumen of the airway-like structures and P63+ (green) basal cells l. (C) Cells within H1 8 wk tHLO and H9 15 wk tHLO airway-like structures express CC10 (white)and P63+ (green). The epithelium is labelled by ECAD (red). (D) H1 8 wk and H9 15 wk tHLOs have cells facing in toward the lumen of the airway structures that express MUC5AC (green), with P63+ (white) cells lining the basal side of the epithelium. All scale bars represent 50 µm.
https://doi.org/10.7554/eLife.19732.015
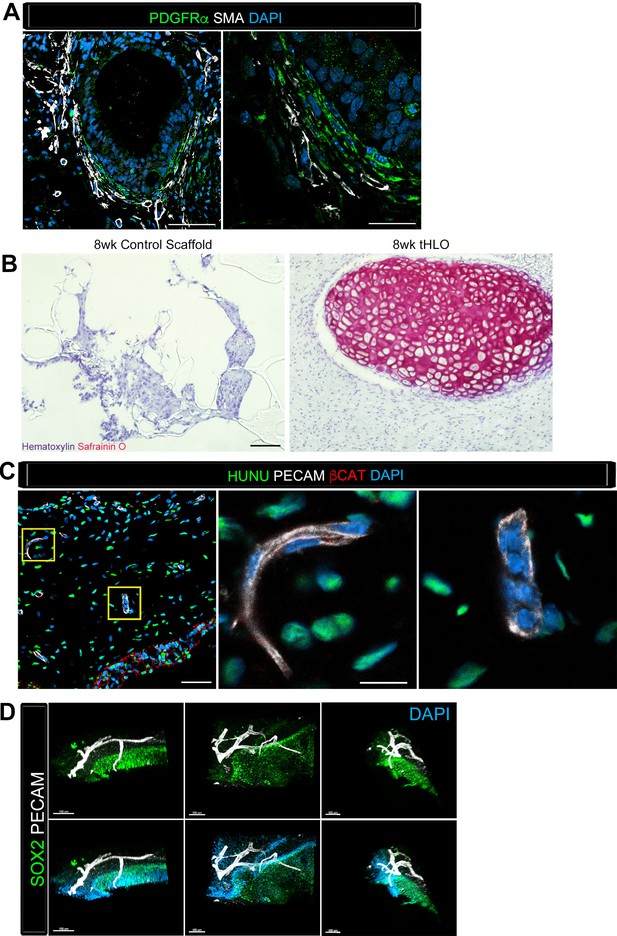
Transplanted HLO-scaffolds consisted of mesenchymal cells and vasculature.
(A) Airway-like structures were surrounded by myofibroblasts, PDGFRα+ (green) and SMA+ (white) as well as smooth muscle, PDGFRα-/SMA+ (white only). Scale bars represent 50 µm in the lower mag image (left panel) and 25 µm in the lower mag image (right panel). (B) SafraninO staining showed clusters of cartilage in the 8 wk tHLO (right panel) but not in the scaffold grown in vitro for 8 weeks (left panel) Scale bar represents 100 µm. (C) Some βCAT+ (red) cells surrounding the airway-like structures expressed vasculature marker PECAM (white), but did not express human nuclear marker (HUNU, green) indicating that the vasculature is of host origin. Scale bars in A represent 50 µm lower mag image (left panel) 25 µm in bottom panel in the lower mag image (right panel). The low mag image scale bar represents 50 µm and high mag represents 10 µm. (D) 3D rendering of z-stack images on thick 12 wk tHLO sections (derived from H9 hESC) revealed the PECAM+ vascular network (white) around the SOX2+ airway epithelium (green). Scale bar represents 100 µm.
https://doi.org/10.7554/eLife.19732.017
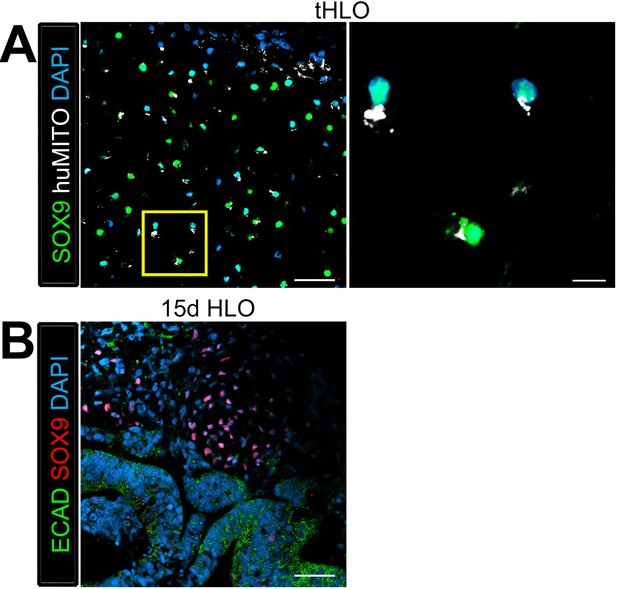
Cartilage observed in transplanted HLOs is of human origin.
The cartilage in the tHLO expressed SOX9 (green) and huMITO (white). Scale bar represents 50 µm in the low mag image and 10 µm in the high mag image. (B) 15d HLOs grown in vitro only possess clusters of SOX9+ cells in the mesenchyme, suggesting HLOs possess cartilage precursor cells. Scale bar represents 50 µm.
https://doi.org/10.7554/eLife.19732.018
Videos
Tables
Overview of Organoid transplants. Transplant site refers to where the tissue was placed in the mouse. HLOs grown in vitro from 1 to 65 days (d) were transplanted and tissues were harvested at various time points ranging from 4 to 15 weeks (wks). Three hESC lines were used including UM63-1, H9, and H1. The most successful transplants that contained mature airway-like structures were 1d HLOs seeded onto the PLG scaffolds with or without Matrigel and FGF10 after 8 to 15 weeks.
https://doi.org/10.7554/eLife.19732.002
| Transplant site | Transplanted tissue | Time | N | Treatment | Procedure | Outcome | Cell line |
|---|---|---|---|---|---|---|---|
| Kidney Capsule | 35d HLOs | 4 weeks | 6 | - | Placed with forceps | 6/6 huMITO+ NKX2.1- | UM63-1 |
| Kidney Capsule | 1d HLOs | 6 weeks | 3 | Mixed with 100% Matrigel | Injected into capsule | 3/3 huMITO+ NKX2.1- | UM63-1 |
| Omentum | 65d HLOs | 12 weeks | 13 | - | Sutured into greater omentum | 11/13 huMITO+ NKX2.1- 2/13 huMITO+ NKX2.1- Immature airway-like structures | UM63-1 |
| Fat Pad | 1d HLOs | 8 weeks | 5 | 100% Matrigel plug filled with spheroids | Enveloped by epididymal fat pad | No tissue retrieved | H9 |
| Fat Pad | 1d HLOs | 4 weeks | 4 | Seeded on Scaffold mixed with 100% Matrigel and FGF10 | Enveloped by epididymal fat pad | 4/4 huMITO+ NKX2.1+ immature airway-like structures | UM63-1 |
| Fat Pad | 1d HLOs | 8 weeks | 8 | Seeded on scaffold, mixed with 100% Matrigel and FGF10 | Enveloped by epididymal fat pad | 8/8 huMITO+ NKX2.1+ mature airway-like structures | UM63-1 |
| Fat Pad | 1d HLOs | 8 weeks | 4 | Seeded on scaffold (without Matrigel, FGF10) | Enveloped by epididymal fat pad | 4/4 huMITO+ NKX2.1+ mature airway-like structures | UM63-1 |
| Fat Pad | 1d HLOs | 15 weeks | 3 | Seeded on scaffold (without Matrigel, FGF10) | Enveloped by epididymal fat pad | 3/3 huMITO+ NKX2.1+ mature airway-like structures | H9 |
| Fat Pad | 1d HLOs | 8 weeks | 4 | Seeded on Scaffold (without Matrigel, FGF10) | Enveloped by epididymal fat pad | 4/4 huMITO+ NKX2.1_ mature airway-like structures | H1 |
Primary and secondary antibody information.
https://doi.org/10.7554/eLife.19732.020
| Primary antibody | Source | Catalog # | Dilution | Clone |
|---|---|---|---|---|
| Goat anti-β-Catenin (βCAT) | Santa Cruz Biotechnology | sc-1496 | 1:200 | C-18 |
| Goat anti-CC10 | Santa Cruz Biotechnology | sc-9770 | 1:200 | C-20 |
| Goat anti-SOX9 | R&D Systems | AF3075 | 1:500 | polyclonal |
| Goat anti-VIMENTIN (VIM) | Santa Cruz Biotechnology | sc-7558 | 1:100 | S-20 |
| Mouse anti-Acetylated Tubulin (ACTTUB) | Sigma-Aldrich | T7451 | 1:1000 | 6-11B-1 |
| Mouse anti-E-Cadherin (ECAD) | BD Transduction Laboratories | 610181 | 1:500 | 36/E-Cadherin |
| Mouse anti-FOXJ1 | eBioscience | 14-9965-82 | 1:500 | 2A5 |
| Moues anti- Human Nuclear Antigen** | Millipore | MAB1281 | 1:200 | monoclonal |
| Mouse anti- Human Mitochondria (huMITO) | Millipore | MAB1273 | 1:500 | 113-1 |
| Mouse anti-PLUNC | R&D Systems | MAP1897 | 1:200 | monoclonal |
| Rabbit anti-Cytokeratin5 (CK5) | Abcam | ab53121 | 1:500 | polyclonal |
| Rabbit anti-NKX2.1 | Abcam | ab76013 | 1:200 | EP1584Y |
| Rabbit anti-P63 | Santa Cruz Biotechnology | sc-8344 | 1:200 | H-129 |
| Rabbit anti-PDGFRα | Santa Cruz Biotechnology | sc-338 | 1:100 | C-20 |
| Rabbit anti-SOX2 | Seven Hills Bioreagents | WRAB-SOX2 | 1:500 | polyclonal |
| Rat anti-PECAM-1 | BD Biosciences | 557355 | 1:200 | monoclonal |
| Biotin-Mouse anti MUC5AC* | Abcam | ab79082 | 1:100 | monoclonal |
| Cy3- Mouse anti Actin-alpha smooth muscle (SMA)* | Sigma | C6198 | 1:400 | monoclonal |
| Secondary antibody | Source | Catalog # | Dilution |
|---|---|---|---|
| Donkey anti-goat 488 | Jackson Immuno | 705-545-147 | 1:500 |
| Donkey anti-goat 647 | Jackson Immuno | 705-605-147 | 1:500 |
| Donkey anti-goat Cy3 | Jackson Immuno | 705-165-147 | 1:500 |
| Donkey anti-mouse 488 | Jackson Immuno | 715-545-150 | 1:500 |
| Donkey anti-mouse 647 | Jackson Immuno | 415-605-350 | 1:500 |
| Donkey anti-mouse Cy3 | Jackson Immuno | 715-165-150 | 1:500 |
| Donkey anti-rabbit 488 | Jackson Immuno | 711-545-152 | 1:500 |
| Donkey anti-rabbit 647 | Jackson Immuno | 711-605-152 | 1:500 |
| Donkey anti-rabbit Cy3 | Jackson Immuno | 711-165-102 | 1:500 |
| Donkey anti-goat 488 | Jackson Immuno | 705-545-147 | 1:500 |
| Donkey anti-goat 647 | Jackson Immuno | 705-605-147 | 1:500 |
| Donkey anti-goat Cy3 | Jackson Immuno | 705-165-147 | 1:500 |
| Donkey anti-mouse 488 | Jackson Immuno | 715-545-150 | 1:500 |
| Donkey anti-mouse 647 | Jackson Immuno | 415-605-350 | 1:500 |
| Donkey anti-mouse Cy3 | Jackson Immuno | 715-165-150 | 1:500 |
| Donkey anti-rabbit 488 | Jackson Immuno | 711-545-152 | 1:500 |
| Donkey anti-rabbit 647 | Jackson Immuno | 711-605-152 | 1:500 |
| Donkey anti-rabbit Cy3 | Jackson Immuno | 711-165-102 | 1:500 |
Download links
A two-part list of links to download the article, or parts of the article, in various formats.
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
- Briana R Dye
- Priya H Dedhia
- Alyssa J Miller
- Melinda S Nagy
- Eric S White
- Lonnie D Shea
- Jason R Spence
(2016)
A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids
eLife 5:e19732.