Anemia of Chronic Disease and Kidney Failure: Overview, Mechanism of Anemia of Chronic Disease, Prevalence of Anemia of Chronic Disease and CKD (original) (raw)
Overview
Overview
Anemia may arise as a complication of several chronic diseases, and chronic kidney disease (CKD) in particular. By definition, anemia refers to an absolute reduction of the total number of circulating red blood cells (RBCs). For practical purposes, anemia is considered when one or more of the following are decreased: hemoglobin concentration, hematocrit, or RBC count. This condition is a laboratory finding that signifies the presence of illness or disease; anemia itself should not be considered a diagnosis.
Anemia usually is grouped into three etiologic categories: decreased RBC production, increased RBC destruction, and blood loss. Anemia of chronic illness and anemia of CKD [1] both fall under the category of decreased RBC production. When the classification of anemia is based on the morphology of the RBCs, both anemia of chronic illness and anemia of CKD usually fall under the classification of normochromic, normocytic anemia.
See Anemia and Chronic Kidney Disease for more complete information on those topics. For patient education information, see Anemia and Chronic Kidney Disease, as well as the National Kidney Foundation's Anemia and Chronic Kidney Disease.

Mechanism of Anemia of Chronic Disease
Anemia of chronic illness traditionally encompassed any inflammatory, infectious, or malignant disease of a long-standing nature. The modern definition includes rheumatoid arthritis, severe trauma, heart disease, diabetes mellitus, and inflammatory bowel disease. [2] Anemia of chronic disease is characterized primarily by the following [3] :
- Decreased availability of iron
- Relatively decreased levels of erythropoietin
- Mild decrease in the lifespan of RBCs to 70-80 days (normally 120 days)
Hepcidin, an endogenous antimicrobial peptide secreted by the liver, has been identified as controlling the level of plasma iron by regulating the intestinal absorption of dietary iron, as well as the release of iron from macrophages and the transfer of iron stored in the hepatocytes. Increase in hepcidin level in the course of inflammatory disease may be a significant mediator of the accompanying anemia. [4, 5, 6]
Another proposed mechanism for anemia of chronic illness involves cytokines, such as interleukins (IL-1 and IL-6) and tumor necrosis factor (TNF-alpha). These are believed to cause the destruction of RBC precursors and decrease the number of erythropoietin receptors on progenitor cells. [7, 8, 9]
The severity of anemia of CKD is directly related to the degree of loss of kidney function, as the kidneys are responsible for approximately 90% of erythropoietin production. [10] Whereas hypoxia in an individual with normally functioning kidneys leads to erythropoietin gene transcription, and hence increased RBC production, CKD results in primary deficiency of erythropoietin production by the interstitial fibroblasts, also known as type I interstitial cells.
In individuals with advanced stages of CKD, the etiology of anemia tends to be multifactorial and include the following [11] :
- Decreased RBC production due to lack of erythropoietin and iron deficiency
- Increased RBC destruction due to hemolysis (intravascular or extravascular)
- Increased blood loss due to multiple venipunctures for an array of indications

Prevalence of Anemia of Chronic Disease and CKD
In general, anemia is more common in women, in particular, those in their childbearing years. In the latter decades of life, anemia tends to occur without any particular sex predilection. However, in patients with chronic kidney disease (CKD), the risk of developing anemia is 30% higher in males than in females. Although males have higher hemoglobin values, they also have higher rates of advanced CKD. The prevalence of anemia is lower in current smokers, which has been attributed to secondary erythrocytosis.
Anemia is common in patients with CKD. The landmark study by Obrador et al showed that among predialysis patients, 68% of those with advanced CKD who required renal replacement therapy had a hematocrit of less than 30%; of those, 51% had a hematocrit less than 28%. [12] A review of National Health and Nutrtion Examination Survey (NHANES) data determined that the prevalence of anemia increased with stage of CKD, as follows [13] :
- Stage 1: 8.4%
- Stage 2: 12.2%
- Stage 3: 17.4%
- Stage 4: 50.3%
- Stage 5: 53.4%
The prevalence of anemia of CKD is also greater in those older than 60 years, as compared with those age 46 to 60 years (see Anemia in Elderly Persons). This is probably secondary to the greater rate of CKD in older individuals, as well as the lower estimated glomerular filtration rates (GFRs) that are associated with aging.
Blacks/African Americans make up about 13% of the population but account for 35% of the people with kidney failure in the United States. [14] In addition, anemia is more prevalent and severe in Blacks than in Whites at each stage of CKD. [15]

Evaluation of Anemia and CKD
Symptoms and physical findings
Although the diseases that lead to anemia, such as malignancy or chronic kidney disease (CKD), may cause obvious symptoms, the anemia itself tends to cause quite nonspecific symptoms. Clinicians must be wary of the tendency to dismiss these symptoms as insignificant—for example, as being due to old age—when in fact they should serve as alarming signals of disease or pathology.
Patients with anemia of chronic disease or CKD may present with the following symptoms:
- Generalized weakness or malaise, easy fatigability
- Generalized body aches, or myalgias
- Orthostatic symptoms (eg, lightheadedness, dizziness)
- Syncope or near-syncope
- Decreased exercise tolerance
- Chest discomfort
- Palpitations
- Cold intolerance
- Sleep disturbances
- Inability to concentrate
- Loss of appetite
The following physical findings may be noted:
- Skin - Pallor
- Neurovascular - Decreased cognitive ability
- Eyes - Pale conjunctivae
- Cardiovascular - Orthostatic hypotension, tachyarrhythmias
- Pulmonary - Tachypnea
- Abdomen - Ascites, hepatosplenomegaly

Differential Diagnosis
Other causes of normochromic, normocytic anemia and decreased RBC production (hypoproliferative) should be noted, and conditions involving the bone marrow and secondary conditions involving the liver (eg, cirrhosis) and endocrine system should be assessed.
The following diseases, which primarily involve the bone marrow, should be included in the differential diagnosis:
- Myelophthisic anemia (see the image below)
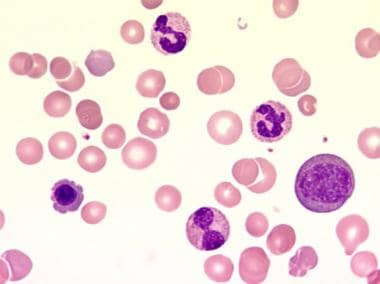
This blood film at 1000X magnification demonstrates a leukoerythroblastic blood picture with the presence of precursor cells of the myeloid and erythroid lineage. In addition, anisocytosis, poikilocytosis, and polychromasia can be seen. Courtesy of U. Woermann, MD, Division of Instructional Media, Institute for Medical Education, University of Bern, Switzerland. - Myeloid metaplasia (see the image below)
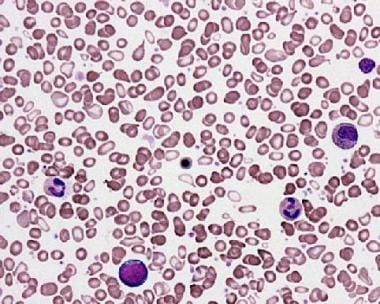
Peripheral smear from a patient with agnogenic myeloid metaplasia. This image shows the presence of teardrop red blood cells (RBCs) and a leukoerythroblastic picture with the presence of nucleated RBC precursors and immature myeloid cells. Courtesy of Wei Wang, MD, and John Lazarchick, MD; Department of Pathology, Medical University of South Carolina.
Bone marrow biopsy can be useful in establishing the etiology of anemia in patients with decreased production of RBCs. The results can confirm whether the marrow is aplastic or hypoplastic; hyperplastic; or infiltrated with nonhematopoietic elements.
The following endocrine disorders should be included in the differential diagnosis:
- Hypoadrenalism

Laboratory Tests
The following laboratory tests are vital in the evaluation of anemia of chronic illness or chronic kidney disease:
- RBC indices
- Peripheral blood smear
- Reticulocyte count
Laboratory tests that may help eliminate other common causes of anemia include the following:
- Iron panel – serum iron, ferritin, total iron-binding capacity (TIBC), iron saturation (see Role of iron under Management of Anemia of CKD)
- Serum vitamin B12 and folic acid
- Serum bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT)
- Thyroid-stimulating hormone (TSH)
- Electrophoretic studies of serum and urine
- Serum levels of heavy metals (eg, lead, arsenic)
Measurement of serum erythropoietin levels is of no current diagnostic utility in patients with chronic kidney disease, as it is expected to be low. Neither does it influence the starting dose or any adjustment in dosing of erythropoiesis-stimulating agents (ESAs) in such patients.
Reticulocyte count
A low reticulocyte count usually points to decreased RBC production as the primary mechanism responsible for anemia, whereas an elevated reticulocyte count points to increased RBC destruction or hemolysis as the most likely cause.
Although decreased RBC production is the main mechanism in both anemia of chronic illness and anemia of chronic kidney disease, oftentimes the anemia is due to a combination of events, including concomitant blood loss. Therefore, a reticulocyte count should always be interpreted with caution.

Treatment Considerations
In general, patients with anemia of chronic illness or of chronic kidney disease can be treated on an outpatient basis. Confounding factors that need to be addressed in both diseases include concomitant blood loss, iron deficiency, or deficiencies of vitamin B12 and/or folic acid.
The preferred initial form of therapy for anemia of chronic illness is treatment of the underlying disease. Erythropoiesis-stimulating agents (ESAs) and blood transfusions are reserved for severe and symptomatic cases. Administration of ESAs is usually best done under the auspices of a hematologist or nephrologist, who may be more informed regarding the latest guidelines on the uses of such agents, as well as for insurance policy coverage.

Management of Anemia of CKD
Erythropoiesis-stimulating agents
The preferred initial therapy for anemia of chronic kidney disease (CKD) is the use of erythropoiesis-stimulating agents (ESAs). ESAs available in the United States include epoetin alfa and darbepoetin alfa (Aranesp).
The US Food and Drug Administration (FDA) advises clinicians to consider starting ESA treatment for patients with CKD when the hemoglobin level is less than 10 g/dL, but does not define how far below 10 g/dL would be an appropriate threshold for initiating ESA treatment in an individual patient. [16] Kidney Disease: Improving Global Outcomes (KDOGI) guidelines suggest basing the decision whether to initiate ESA therapy in non–dialysis-dependent CKD patients with a hemoglobin concentration < 10.0 g/dL on the following:
- Rate of fall of the hemoglobin concentration
- Prior response to iron therapy
- Risk of needing a transfusion
- Risks related to ESA therapy
- Presence of symptoms attributable to anemia
To evaluate response to ESA treatment, the KDIGO guidelines recommend measuring hemoglobin at least monthly during the initiation phase. During the maintenance phase, measurement is recommended at least every 3 months in patients with non–dialysis-dependent CKD patients, and at least monthly in CKD 5D patients. [17]
In patients with CKD who are receiving ESA treatment, raising hemoglobin levels to normal may worsen cardiovascular outcomes. The optimal level of hemoglobin correction with ESA therapy in patients with anemia of CKD was addressed in two landmark trials published in 2006: the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta (CREATE) study [18] and the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) study. [19] Both provided evidence against full correction of the hemoglobin concentration (ie, to at least 13 g/dL).
As a result of the CREATE and CHOIR studies, in March 2007, the FDA added a black box warning to the labeling of epoetin alfa and darbepoetin alfa to emphasize that use of these ESAs may increase the risk of serious cardiovascular events and death when they are dosed to achieve a target hemoglobin of greater than 12 g/dL. In November 2007, the FDA stated that "ESAs should be used to maintain a hemoglobin level between 10 g/dL to 12 g/dL. Maintaining higher hemoglobin levels in patients with chronic kidney failure increases the risk for death and for serious cardiovascular reactions such as stroke, heart attack or heart failure." [20]
In 2011, the FDA abandoned the concept of a target range for the hemoglobin level in ESA treatment. Instead, the FDA recommended using the lowest dose of ESA sufficient to reduce the need for red blood cell transfusions for each patient, and adjusting the dose as appropriate. [16]
The 2012 KDIGO guidelines recommend that in general, the hemoglobin level in adult patients with CKD should not be maintained above 11.5 g/dL; some patients may have improvements in quality of life at hemoglobin concentrations above 11.5 g/dL (115 g/l) and will be prepared to accept the risks, but hemoglobin should not exceed 13 g/dL. [17] These goals are associated with lower mortality and less frequent hospitalization rates.
In pediatric CKD patients receiving ESA therapy, the KDIGO guidelines suggest a hemoglobin concentration in the range of 11.0 to 12.0 g/dL. However, National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) commentary on the guidelines recommends a range of 11-13 g/dL, to avoid the need for multiple dosing adjustments. [17]
A post-hoc analysis of the CHOIR study showed that a high hemoglobin target may be associated with a greater risk of progression of CKD, which is apparently augmented by concurrent smoking. [21] The Trial to Reduce Cardiovascular Events With Aranesp® Therapy (TREAT) resulted in two reports raising concern about the use of target-based strategies in managing anemia of chronic kidney disease. The initial report, conducted in patients with type 2 diabetes melitus, CKD, and moderate anemia, indicated an increased risk of stroke and no reduction in the risk of death or a cardiovascular or renal event with darbepoetin alfa. [22]
The second report noted that when doses of darbepoetin alfa were increased to meet target hemoglobin levels in patients with a poor initial hematopoietic response, the subsequent risk of death or cardiovascular events increased. [23] (See Cardiovascular disease under Complications of Anemia of Chronic Disease and CKD.)
A systematic review and meta-analysis published in 2016 found that ESA treatment of anemia to obtain higher hemoglobin targets did not result in important differences in health-related quality of life in patients with CKD. [24]
The NEPHRODIAB2 Prospective Randomized Controlled Open-Labelled Trial Comparing Effect of Two Haemoglobin levels, which was conducted in patients with type 2 diabetes mellitus and stage 3-4 CKD, found that raising hemoglobin to the normal range (13-14 g/dL) with ESA treatment was safe but did not significantly slow renal function decline and increased treatment cost. [25]
Methoxy polyethylene glycol-epoetin beta (Mircera) is a third-generation, pegylated epoetin (PEG-EPO) beta under the new category of a continuous erythropoietin receptor activator (CERA) that was approved by the FDA in 2007 for the treatment of anemia of CKD. In phase 3 trials, methoxy polyethylene glycol-epoetin beta was given every 2 or 4 weeks, with both regimens achieving hemoglobin targets. Disadvantages of this agent include concerns about possibility of pure red cell aplasia (PRCA). In addition, in 2009, the FDA upheld a 2008 injunction against the marketing of methoxy polyethylene glycol-epoetin beta by Roche due to infringement of several Amgen patents.
In the MIRcerA CLinical Evidence on Renal Survival in CKD patients with anemia (MIRACLE-CKD) study, a multicenter prospective observational study in 2851 non–dialysis-dependent Japanese patients treated with a CERA, kidney outcome was superior in patients who achieved a hemoglobin concentration of ≥11 g/dL by week 12 of therapy. The kidney survival rate in patients in the ≥11 g/dL group was 51.47%, significantly higher than the 37.57% rate in the < 11 g/dL group (P < 0.0001). [26]
Peginesatide (Hematide) is a pegylated, peptidic ESA (also called an erythropoietin mimetic [EPO mimetic]) that binds to the erythropoietin receptor, thereby activating intracellular signaling pathways. [27] This agent was approved in the United States in March 2012 for treatment of anemia of CKD, but was discontinued in February 2013 following postmarketing reports of severe hypersensitivity, including fatalities. [28]
In kidney transplant recipients, the Correction of Anaemia and Progression of Renal Failure on Transplanted Patients found that correction of hemoglobin values to 13 g/dL or higher reduces progression of chronic allograft nephropathy. No increase in cardiovascular events was noted. [29]
The studies on ESA use in anemia of CKD and the FDA actions have been followed by a change in clinical practice. Between 2006 and 2015, CKD patients in the US were increasingly less likely to be treated with ESAs and more likely to receive intravenous iron supplementation and blood transfusions. [30]
Adverse effects of ESAs
Long-term treatment with ESAs has been associated with increased systemic blood pressure and occurrence of seizures; hypertension has been documented to be a common side effect of intravenous use of ESAs. For this reason, blood pressure should always be closely monitored in patients administered with such agents. The postulated mechanism is believed to be an imbalance between endothelin and proendothelin that leads to hyperresponsiveness to the effects of norepinephrine (vasoconstriction) and hyporesponsiveness to the effects of nitric oxide (vasodilatation).
Reports of neutralizing "anti-epoetin antibodies" have been linked to the unusual occurrence of PRCA in European cohorts, but this finding has been attributed to the difference in immunogenicity of the ESAs marketed between the US and Europe.
ESA resistance
The working definition of ESA resistance is the requirement for greater than 150 units/kg of ESA at least 3 times per week or the sudden response refractoriness to a previous stable maintenance dose, such that hemoglobin levels fall below target levels.
The most common cause of ESA resistance is iron deficiency. Therefore, it is imperative that iron stores are adequate during ESA treatment. The second most common cause of ESA resistance is a chronic infection/inflammatory state, and such resistance is attributed to inflammatory cytokines (eg, IL-1).
Other less common causes of ESA resistance include hyperparathyroidism (the mechanism appears to be related to bone marrow fibrosis), as well as severe malnutrition.
Role of iron
As noted above, iron deficiency is the most common identifiable cause of ESA resistance. The 2 most important tests to order to assess iron deficiency are transferrin saturation (TSAT) and serum ferritin.
The importance of these tests lies in the fact that the diagnosis of iron deficiency anemia is not truly straightforward, as the possible etiologies include both insufficient iron stores (absolute iron deficiency) and insufficient release of stored iron by the reticuloendothelial tissues, so that too little iron is available for erythropoiesis (functional iron deficiency). [11]
Anemia of CKD tends to primarily involve functional iron deficiency. Traditionally, this is characterized by a TSAT less than 20% and a ferritin level less than 100 ng/mL; however, there is evidence that those cutoffs may not be sensitive to detect iron deficiency. In a study by Stancu et al in 100 patients with CKD (stages 3–5), those indices identified 17% of patients as iron deficient but bone marrow iron staining showed that 48% were iron deficient. [31] Consequently, iron therapy should be considered in patients with CKD whose TSAT is ≤30%, as iron therapy has the potential to increase the hemoglobin concentration or permit a decrease in the ESA dose. [17]
Clinicians must also be aware that although a low ferritin level has high specificity for absolute iron deficiency, ferritin is an acute-phase reactant that can be elevated in states of chronic infection or inflammation. [31] Therefore, an elevated ferritin does not necessarily imply iron store adequacy or overload. KDIGO guidelines recommend a trial of iron repletion if the serum ferritin is ≤500 ng/ml, in CKD patients with a TSAT ≤30%. [17] Current guidelines recommend against use of iron products when ferritin is 500 ng/mL or greater.
At present, various new potential markers of iron status are being developed and experiments are under way to identify every component that may be involved in the mobilization of iron throughout the body. [11] One of these markers is an endogenous antimicrobial peptide, hepcidin. The possible central role of hepcidin in the pathogenesis of anemia of chronic disease has been the subject of numerous publications. [4, 5, 6]
There are some newer agents that hold great promise in the treatment of anemia in chronic illness or CKD. [32] Some of these have been approved by the FDA, while others are undergoing clinical trials.
In 2015, the FDA approved ferric pyrophosphate DIALYSATE (Triferic), a soluble iron replacement therapy that is added to the hemodialysate solution. Approval was based on the PRIME study, which showed that soluble ferric pyrophosphate was able to maintain hemoglobin and not increase ferritin, while significantly reducing the use of ESAs by 37.1% compared with regular dialysate. [33]
In 2020, the FDA approved ferric pyrophosphate IV (Triferic AVNU), which is designed for direct intravenous (IV) infusion. This IV formulation is indicated for iron replacement to maintain hemoglobin in adults with hemodialysis-dependent CKD (HDD-CKD). Its efficacy in patients with HDD-CKD was established in two randomized, single-blind, placebo-controlled clinical trials (CRUISE 1 and 2). In both trials, hemoglobin concentration was maintained from baseline to end of treatment in the treatment group but decreased by 0.4 g/dL in the placebo group. Placebo treatment resulted in significantly larger mean decreases from baseline in reticulocyte hemoglobin content (0.9 pg versus -0.4 pg), serum ferritin (-133.1 µg/L versus -69.7 µg/L) than ferric pyrophosphate treatment. [34]
For CKD patients who require iron supplementation, IV iron is generally recommended, as IV iron consistently achieves higher TSAT and ferritin values than oral iron. [17] Examples of IV iron replacement therapies include the following [35] :
- Iron dextran complex (Dexferrum, INFeD): 500-1000 mg with variable frequency
- Iron sucrose (Venofer): 200 mg given in 5 doses over 2 weeks
- Ferric carboxymaltose (Injectafer): 750 mg given in 2 doses 1 week apart
- Ferric gluconate (Ferrlecit): 250 mg given in 4 doses weekly
- Ferumoxytol (Feraheme): 510 mg given in 2 doses, 3-8 days apart
- Iron isomaltoside: 1000 mg in a single dose
HIF-PH inhibitors
An alternative to the administration of exogenous ESAs is the use of agents that stimulate endogenous erythropoietin production in the kidney and nonrenal tissues. One such class of agents works to stabilize hypoxia-inducible factor (HIF) by inhibiting prolyl hydroxylase (PH) enzymes. [36] HIF is a key regulator of erythropoietic gene expression, iron absorption, energy metabolism, pH, and angiogenesis; as its name indicates, HIF is induced by hypoxia.
HIF-PH inhibitors improve iron mobilization to the bone marrow and induce considerably lower but more consistent blood erythropoietin levels than ESAs. They also promote erythroferrone production by erythroblasts, which reduces hepcidin interference, allowing for greater utilization of iron. As well, these agents have the advantage of being administered orally.
Daprodustat (Jesduvroq) is the first HIF-PH inhibitor to be approved by the FDA. [37] It is indicated for treatment of anemia due to CKD in adults on dialysis for at least 4 months. Approval was based on the phase III ASCEND clinical trials. In the ASCEND-D trial (patients on dialysis), daprodustat was found to be noninferior to ESAs. [38] The ASCEND-ND (patients not on dialysis) showed noninferiority of daprodustat compared with darbepoetin alfa with respect to change in hemoglobin level from baseline and first occurrence of a major adverse cardiovascular event. [39]
Other HIF-PH inhibitors (eg, roxadustat, vadadustat, molidustat) are currently under development. [36] Roxadustat and vadadustat were submitted to the FDA, however, as of early 2023, they have not been approved. A phase II trial found that roxadustat was well tolerated and effective in maintaining target hemoglobin levels in CKD patients on peritoneal dialysis, both those who had been previously treated with an ESA and those with no previous ESA treatment. [40] In a phase IIa trial, vadadustat increased hemoglobin levels and improved biomarkers of iron mobilization and utilization in patients with anemia secondary to stage 3 or 4 CKD. Phase III trials of vadadustat in non–dialysis-dependent patients are ongoing. [41]
In 2019, the phase II DIALOGUE 1,2 & 4 trials of molidustat were released. DIALOGUE 1 and 2 showed that over a period of 16 weeks, hemoglobin increased in patients that received molidustat compared with those who received darbepoetin or placebo. DIALOGUE 4 did not show significant improvement verses epoetin, but the cohort was small and hemoglobin levels were maintained within the treatment goals. Further phase III trials are planned with larger cohorts. [42]
Oral iron products
Oral iron products may also be helpful for management of iron deficient anemia in patients with CKD who are not on dialysis. Ferrous sulfate is inexpensive, but other oral iron products are also available (eg, carbonyl iron, ferric citrate). Ferric citrate is an oral phosphate binder for the control of serum phosphorus levels in patients with CKD on dialysis. It also approved by the FDA for adults with iron deficiency anemia who have CKD and are not on dialysis. [43]
Iron polysaccharide complex, as an oral iron trivalent supplement, offers a high iron content (46%) and lower gastrointestinal irritation. Clinical trials are in progress to evaluate its effectiveness and safety in dialysis patients with CKD-related anemia. [44]

Complications of Anemia of Chronic Disease and CKD
Hypoxia
Hypoxia is the most potent stimulus to the production of erythropoietin by the kidneys. In the healthy individual, erythropoietin exerts its effects in the bone marrow to help in the production of RBCs, thereby improving oxygen concentration in the blood, relieving the hypoxia.
Another complication that commonly occurs in those with chronic kidney disease is that of secondary hyperparathyroidism and the development of renal osteodystrophy. In these patients, the bone marrow tends to be fibrotic and, hence, less responsive to the effects of erythropoietin.
Cardiorenal anemia syndrome
Silverberg et al described the "cardiorenal syndrome," which refers to a vicious cycle whereby decreased kidney function, as seen in chronic kidney disease, leads to decreased erythropoietin production and, thence, anemia. [45] Severe anemia leads to a compensatory left ventricular hypertrophy (LVH). Such compensatory LVH eventually precipitates chronic heart failure (CHF), which causes a decline in blood perfusion to the kidneys, resulting in further kidney damage.
Levin et al estimated that for every 1-g decrease in hemoglobin concentration, there is an increased 6% risk of LVH in patients with chronic kidney disease. [46] Foley et al estimated that such a 1-g decrease in hemoglobin concentration also translated into a 42% increase in left ventricular dilatation in patients with stage 5 chronic kidney disease. [43] Regression of LVH is a known benefit of treatment with ESAs.
Cardiovascular disease
The risk of death from cardiovascular disease also increases with advancing age, and the impact of anemia on cardiovascular disease and chronic kidney disease (CKD) in this elderly population cannot be understated. Cardiovascular disease remains the most common cause of mortality in these patients, much higher than in the general population. [47] Anemia has been shown to be an independent risk factor for increased cardiovascular morbidity and mortality.
The Dialysis Outcomes Practice Pattern Study (DOPPS), which involved several countries, showed that as hemoglobin concentrations decreased to less than 11 g/dL, there was a corresponding increase in the rates of hospitalization and mortality in patients with CKD. [48] Ofsthun et al analyzed the databases from Fresenius Medical Care of North America (FMCNA) (selection restricted to patients in the census for 6 consecutive months from July 1, 1998, through June 30, 2000) and showed that the longer it took for these patients with stage 5 CKD resolve their hemoglobin concentrations from less than 11 g/dL, the more dramatic an increase in their mortality hazard ratio. [49] The investigators further added that lower hemoglobin concentrations clearly correlated positively with adverse events in these patients.
In summary, one can derive that maintenance of hemoglobin levels at the recommended target goals translates into decreased LVH, decreased hospitalizations related to cardiovascular disease, and decreased mortality from cardiovascular disease. Moreover, quality of life tends to improve, in the following ways:
- Less easy fatigability and fatigue
- Improved physical well-being and exercise tolerance
- Improved functional well-being

Questions & Answers
- Shaikh H, Hashmi MF, Aeddula NR. Anemia Of Chronic Renal Disease. 2022 Jan. [QxMD MEDLINE Link]. [Full Text].
- Mücke V, Mücke MM, Raine T, Bettenworth D. Diagnosis and treatment of anemia in patients with inflammatory bowel disease. Ann Gastroenterol. 2017. 30 (1):15-22. [QxMD MEDLINE Link]. [Full Text].
- Besarab A, Levin A. Defining a renal anemia management period. Am J Kidney Dis. 2000 Dec. 36(6 suppl 3):S13-23. [QxMD MEDLINE Link].
- Ueda N, Takasawa K. Role of Hepcidin-25 in Chronic Kidney Disease: Anemia and Beyond. Curr Med Chem. 2017 Mar 16. 36(5):301-9. [QxMD MEDLINE Link].
- Sangkhae V, Nemeth E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv Nutr. 2017 Jan. 8 (1):126-136. [QxMD MEDLINE Link].
- Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, et al. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009 Jun. 4(6):1051-6. [QxMD MEDLINE Link]. [Full Text].
- Wang CQ, Udupa KB, Lipschitz DA. Interferon-gamma exerts its negative regulatory effect primarily on the earliest stages of murine erythroid progenitor cell development. J Cell Physiol. 1995 Jan. 162(1):134-8. [QxMD MEDLINE Link].
- Taniguchi S, Dai CH, Price JO, Krantz SB. Interferon gamma downregulates stem cell factor and erythropoietin receptors but not insulin-like growth factor-I receptors in human erythroid colony-forming cells. Blood. 1997 Sep 15. 90(6):2244-52. [QxMD MEDLINE Link]. [Full Text].
- Fraenkel PG. Understanding anemia of chronic disease. Hematology Am Soc Hematol Educ Program. 2015. 2015:14-8. [QxMD MEDLINE Link]. [Full Text].
- McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004 Sep. 20(9):1501-10. [QxMD MEDLINE Link].
- Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy CP, Jalal DI. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J Am Soc Nephrol. 2020 Mar. 31 (3):456-468. [QxMD MEDLINE Link].
- Obrador GT, Ruthazer R, Arora P, Kausz AT, Pereira BJ. Prevalence of and factors associated with suboptimal care before initiation of dialysis in the United States. J Am Soc Nephrol. 1999 Aug. 10(8):1793-800. [QxMD MEDLINE Link]. [Full Text].
- Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014. 9 (1):e84943. [QxMD MEDLINE Link]. [Full Text].
- Social Determinants of Kidney Disease. National Kidney Foundation. Available at https://www.kidney.org/news/newsroom/factsheets/African-Americans-and-CKD. June 29, 2021; Accessed: February 7, 2023.
- Lea JP, Norris K, Agodoa L. The role of anemia management in improving outcomes for African-Americans with chronic kidney disease. Am J Nephrol. 2008. 28 (5):732-43. [QxMD MEDLINE Link]. [Full Text].
- FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. U.S. Food & Drug Administration. Available at https://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. June 24, 2011; Accessed: February 7, 2023.
- [Guideline] Kliger AS, Foley RN, Goldfarb DS, Goldstein SL, Johansen K, Singh A, et al. KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am J Kidney Dis. 2013 Nov. 62 (5):849-59. [QxMD MEDLINE Link]. [Full Text].
- Drueke TB, Locatelli F, Clyne N, et al, for the CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006 Nov 16. 355(20):2071-84. [QxMD MEDLINE Link].
- Singh AK, Szczech L, Tang KL, et al, for the CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006 Nov 16. 355(20):2085-98. [QxMD MEDLINE Link].
- US Food and Drug Administration. FDA strengthens boxed warnings, approves other safety labeling changes for erythropoiesis-stimulating agents (ESAs). November 8, 2007. [news release]. Available at https://wayback.archive-it.org/7993/20170112002707/https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109024.htm. November 8, 2007; Accessed: May 14, 2020.
- Inrig JK, Barnhart HX, Reddan D, Patel UD, Sapp S, Califf RM, et al. Effect of Hemoglobin Target on Progression of Kidney Disease: A Secondary Analysis of the CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) Trial. Am J Kidney Dis. 2012 Apr 24. [QxMD MEDLINE Link].
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009 Nov 19. 361(21):2019-32. [QxMD MEDLINE Link].
- Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010 Sep 16. 363(12):1146-55. [QxMD MEDLINE Link].
- Collister D, Komenda P, Hiebert B, Gunasekara R, Xu Y, Eng F, et al. The Effect of Erythropoietin-Stimulating Agents on Health-Related Quality of Life in Anemia of Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann Intern Med. 2016 Apr 5. 164 (7):472-8. [QxMD MEDLINE Link].
- Villar E, Lièvre M, Kessler M, Lemaître V, Alamartine E, Rodier M, et al. Anemia normalization in patients with type 2 diabetes and chronic kidney disease: results of the NEPHRODIAB2 randomized trial. J Diabetes Complications. 2011 Jul-Aug. 25(4):237-43. [QxMD MEDLINE Link].
- Hayashi T, Uemura Y, Kumagai M, Kimpara M, Kanno H, Ohashi Y, et al. Effect of achieved hemoglobin level on renal outcome in non-dialysis chronic kidney disease (CKD) patients receiving epoetin beta pegol: MIRcerA CLinical Evidence on Renal Survival in CKD patients with renal anemia (MIRACLE-CKD Study). Clin Exp Nephrol. 2018 Oct 5. [QxMD MEDLINE Link].
- Macdougall IC, Provenzano R, Sharma A, Spinowitz BS, Schmidt RJ, Pergola PE, et al. Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. N Engl J Med. 2013 Jan 24. 368(4):320-32. [QxMD MEDLINE Link].
- US Food and Drug Administration. Affymax and Takeda Announce a Nationwide Voluntary Recall of All Lots of OMONTYS® (peginesatide) Injection. February 23, 2013. Available at https://wayback.archive-it.org/7993/20170406104941/https://www.fda.gov/Safety/Recalls/ArchiveRecalls/2013/ucm340893.htm.
- Choukroun G, Kamar N, Dussol B, Etienne I, Cassuto-Viguier E, Toupance O, et al. Correction of postkidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol. 2012 Feb. 23(2):360-8. [QxMD MEDLINE Link]. [Full Text].
- Park H, Liu X, Henry L, Harman J, Ross EA. Trends in anemia care in non-dialysis-dependent chronic kidney disease (CKD) patients in the United States (2006-2015). BMC Nephrol. 2018 Nov 9. 19 (1):318. [QxMD MEDLINE Link]. [Full Text].
- Stancu S, Stanciu A, Zugravu A, Bârsan L, Dumitru D, Lipan M, et al. Bone marrow iron, iron indices, and the response to intravenous iron in patients with non-dialysis-dependent CKD. Am J Kidney Dis. 2010 Apr. 55 (4):639-47. [QxMD MEDLINE Link].
- Grabe DW, Manley HJ, Barton Pai A. Therapeutic advances in chronic kidney disease: management of anemia and the role of pharmacy (slides with transcript) (CME/CE). Medscape. June 29, 2007. [Full Text].
- Gupta A. Continuous Soluble Ferric Pyrophosphate (SFP) Iron Delivery Via Dialysate in Hemodialysis Patients (PRIME). Presented at the 34th Annual Dialysis Conference in Atlanta, GA. February 8-11, 2014.
- Triferic AVNU (ferric pyrophosphate citrate) [package insert]. Wixom, MI: Rockwell Medical, Inc. March 2020. Available at [Full Text].
- Gutiérrez OM. Treatment of Iron Deficiency Anemia in CKD and End-Stage Kidney Disease. Kidney Int Rep. 2021 Sep. 6 (9):2261-2269. [QxMD MEDLINE Link]. [Full Text].
- Gupta N, Wish JB. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am J Kidney Dis. 2017 Jun. 69 (6):815-826. [QxMD MEDLINE Link]. [Full Text].
- FDA Approves First Oral Treatment for Anemia Caused by Chronic Kidney Disease for Adults on Dialysis. U.S. Food & Drug Administration. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-anemia-caused-chronic-kidney-disease-adults-dialysis. February 1, 2023; Accessed: February 7, 2023.
- Singh AK, Carroll K, Perkovic V, and the, ASCEND-D Study Group. Daprodustat for the Treatment of Anemia in Patients Undergoing Dialysis. N Engl J Med. 2021 Dec 16. 385 (25):2325-2335. [QxMD MEDLINE Link]. [Full Text].
- Singh AK, Carroll K, McMurray JJV, and the, ASCEND-ND Study Group. Daprodustat for the Treatment of Anemia in Patients Not Undergoing Dialysis. N Engl J Med. 2021 Dec 16. 385 (25):2313-2324. [QxMD MEDLINE Link]. [Full Text].
- Akizawa T, Otsuka T, Reusch M, Ueno M. Intermittent Oral Dosing of Roxadustat in Peritoneal Dialysis Chronic Kidney Disease Patients with Anemia: A Randomized, Phase 3, Multicenter, Open-Label Study. Ther Apher Dial. 2020 Apr. 24 (2):115-125. [QxMD MEDLINE Link]. [Full Text].
- Martin ER, Smith MT, Maroni BJ, Zuraw QC, deGoma EM. Clinical Trial of Vadadustat in Patients with Anemia Secondary to Stage 3 or 4 Chronic Kidney Disease. Am J Nephrol. 2017. 45 (5):380-388. [QxMD MEDLINE Link]. [Full Text].
- Macdougall IC, Akizawa T, Berns JS, Bernhardt T, Krueger T. Effects of Molidustat in the Treatment of Anemia in CKD. Clin J Am Soc Nephrol. 2019 Jan 7. 14 (1):28-39. [QxMD MEDLINE Link].
- Fishbane S, Block GA, Loram L, Neylan J, Pergola PE, Uhlig K, et al. Effects of Ferric Citrate in Patients with Nondialysis-Dependent CKD and Iron Deficiency Anemia. J Am Soc Nephrol. 2017 Jun. 28 (6):1851-1858. [QxMD MEDLINE Link]. [Full Text].
- Lu R, Zhang X, Cai X, Wang X, Li H, Wang L, et al. Efficacy and safety of polysaccharide iron complex capsules compared with iron sucrose in hemodialysis patients: study protocol for a randomized, open-label, positive control, multicenter trial (IHOPE). Trials. 2021 Oct 10. 22 (1):691. [QxMD MEDLINE Link]. [Full Text].
- Silverberg DS, Wexler D, Blum M, et al. The correction of anemia in severe resistant heart failure with erythropoietin and intravenous iron prevents the progression of both the heart and the renal failure and markedly reduces hospitalization. Clin Nephrol. 2002 Jul. 58 suppl 1:S37-45. [QxMD MEDLINE Link].
- Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996 Mar. 27(3):347-54. [QxMD MEDLINE Link].
- Sarnak MJ, Levey AS. Epidemiology of cardiac disease in dialysis patients: uremia-related risk factors. Semin Dial. 1999. 12:69-76.
- Pisoni RL, Bragg-Gresham JL, Young EW, et al. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004 Jul. 44(1):94-111. [QxMD MEDLINE Link].
- Ofsthun NJ, LaBrecque J, Keen M, et al. The association of mortality and hospitalization with hemoglobin (Hb) and missed dialysis treatments in stage 5 chronic kidney disease (CKD) patients with and without cardiac comorbidities [abstract]. Nephrol Dial Transplant. 2005. 20(suppl 5):v268.
- This blood film at 1000X magnification demonstrates a leukoerythroblastic blood picture with the presence of precursor cells of the myeloid and erythroid lineage. In addition, anisocytosis, poikilocytosis, and polychromasia can be seen. Courtesy of U. Woermann, MD, Division of Instructional Media, Institute for Medical Education, University of Bern, Switzerland.
- Peripheral smear from a patient with agnogenic myeloid metaplasia. This image shows the presence of teardrop red blood cells (RBCs) and a leukoerythroblastic picture with the presence of nucleated RBC precursors and immature myeloid cells. Courtesy of Wei Wang, MD, and John Lazarchick, MD; Department of Pathology, Medical University of South Carolina.


Author
Gates B Colbert, MD, FASN Clinical Assistant Professor, Texas A&M University College of Medicine; Teaching Faculty in Internal Medicine (Nephrology) and Surgical Critical Care, Baylor University Medical Center; Nephrologist, Kidney and Hypertension Associates of Dallas, Baylor University Hospital, and Parkland Health and Hospital System
Gates B Colbert, MD, FASN is a member of the following medical societies: American Medical Association, American Society of Hypertension, American Society of Nephrology, Cardio Renal Society of America, Texas Medical Association
Disclosure: Serve(d) as a speaker or a member of a speakers bureau for: Relypsa
Received income in an amount equal to or greater than $250 from: AstraZeneca.
Coauthor(s)
Edgar V Lerma, MD, FACP, FASN, FAHA, FASH, FNLA, FNKF Clinical Professor of Medicine, Section of Nephrology, Department of Medicine, University of Illinois at Chicago College of Medicine; Research Director, Internal Medicine Training Program, Advocate Christ Medical Center; Consulting Staff, Associates in Nephrology, SC
Edgar V Lerma, MD, FACP, FASN, FAHA, FASH, FNLA, FNKF is a member of the following medical societies: American Heart Association, American Medical Association, American Society of Hypertension, American Society of Nephrology, Chicago Medical Society, Illinois State Medical Society, National Kidney Foundation, Society of General Internal Medicine
Disclosure: Serve(d) as a speaker or a member of a speakers bureau for: Astra Zeneca
Author for: UpToDate, ACP Smart Medicine, Elsevier, McGraw-Hill, Wolters Kluwer.
Robert Stein, MD Chief, Section of Hematology, Department of Internal Medicine, Vice President, Medical Management, Advocate Christ Medical Center
Robert Stein, MD is a member of the following medical societies: American Medical Association, American Society of Hematology, Chicago Medical Society, Eastern Cooperative Oncology Group
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Chief Editor
Emmanuel C Besa, MD Professor Emeritus, Department of Medicine, Division of Hematologic Malignancies and Hematopoietic Stem Cell Transplantation, Kimmel Cancer Center, Jefferson Medical College of Thomas Jefferson University
Emmanuel C Besa, MD is a member of the following medical societies: American Association for Cancer Education, American Society of Clinical Oncology, American College of Clinical Pharmacology, American Federation for Medical Research, American Society of Hematology, New York Academy of Sciences
Disclosure: Nothing to disclose.
Additional Contributors
Pradyumna D Phatak, MBBS, MD Chair, Division of Hematology and Medical Oncology, Rochester General Hospital; Clinical Professor of Oncology, Roswell Park Cancer Institute
Pradyumna D Phatak, MBBS, MD is a member of the following medical societies: American Society of Hematology
Disclosure: Received honoraria from Novartis for speaking and teaching.