Familial Adenomatous Polyposis: Background, Pathophysiology, Etiology (original) (raw)
Background
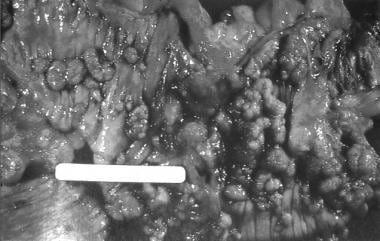
Familial adenomatous polyposis (FAP) is the most common adenomatous polyposis syndrome. It is an autosomal dominant inherited disorder characterized by the early onset of hundreds to thousands of adenomatous polyps throughout the colon. If left untreated, all patients with this syndrome will develop colon cancer by age 35-40 years. In addition, an increased risk exists for the development of other malignancies. See the image below.
Colectomy specimen obtained from a patient with familial adenomatous polyposis. Note the presence of numerous synchronous adenomatous polyps lining the luminal surface.
See Benign or Malignant: Can You Identify These Colonic Lesions?, a Critical Images slideshow, to help identify the features of benign lesions as well as those with malignant potential.
The genetic defect in FAP is a germline mutation in the adenomatous polyposis coli (APC) gene. Syndromes once thought to be distinct from FAP are now recognized to be, in reality, part of the phenotypic spectrum of FAP. [1]
Syndromes with a germline mutation in the APC gene include FAP, Gardner syndrome, some families with Turcot syndrome, and attenuated adenomatous polyposis coli (AAPC). Gardner syndrome is characterized by colonic polyposis typical of FAP, along with osteomas (bony growth most commonly on the skull and the mandible), dental abnormalities, and soft tissue tumors. Turcot syndrome is characterized by the colonic polyposis that is typical of FAP, along with central nervous system tumors (medulloblastoma). AAPC is characterized by fewer colonic polyps (average number of polyps, 30-35) as compared with the classic FAP. The polyps also tend to develop at a later age (average age, 36 y), and they tend to involve the proximal colonic area. [2]
In considering the spectrum of polyposis syndromes, patients with multiple adenomatous polyps most likely have FAP (or one of its variants), AAPC, or MYH-associated polyposis (MAP). If a patient with a suspected polyposis syndrome undergoes genetic testing and does not have an APC gene mutation, MYH gene testing should be performed to assess for MAP, as 10%-20% of patients who do not have an APC gene mutation have biallelic MYH gene mutations. [3]
The phenotype of MAP is often indistinguishable from FAP or AAPC, with patients having usually 10-100 polyps but sometimes more than 100. The age of onset of MAP is usually in patients older than 45 years, and patients often present symptomatically, with colorectal carcinoma commonly found at the time of the diagnosis. This is in part because there is usually no family history given the autosomal recessive inheritance pattern of MAP. Duodenal polyps can be found in up to one fifth of patients. [4] There is no increased risk of other types of cancers associated with this syndrome.

Pathophysiology
The APC gene is a tumor suppressor gene that is located on band 5q21. [5] Its function is not completely understood but has been shown to play a part in metaphase chromosome alignment. [6] Normal APC protein promotes apoptosis in colonic cells. Its most important function may be to sequester the growth stimulatory effects of b-catenin, a protein that transcriptionally activates growth-associated genes in conjunction with tissue-coding factors. Mutations of the APC gene result in a truncated/nonfunctional protein.
The resultant loss of APC function prevents apoptosis and allows b-catenin to accumulate intracellularly and to stimulate cell growth with the consequent development of adenomas. As the clonal expansion of cells that lack APC function occurs, their rapid growth increases the possibility for other growth-advantageous genetic events to occur. This causes alterations in the expression of a variety of genes, thereby affecting the proliferation, differentiation, migration, and apoptosis of cells.
Ultimately, enough genetic events transpire that allow the adenomatous polyps to become malignant in patients with FAP. This process is similar to that which occurs in sporadic adenomas. As a result, APC is considered the gatekeeper of colonic neoplasia. Its mutation/inactivation is the initial step in the development of colorectal cancer in patients with FAP.
Germline (ie, inherited) mutations of the APC gene, as is the case with FAP, result in cells containing one mutated copy and one normal copy of the gene. Patients inherit one mutated APC allele from an affected parent, and adenomas develop as the second allele from the unaffected parent becomes mutated or lost. Consequently, every colonic epithelial cell in patients with FAP has one mutated APC allele. Inactivation of the remaining normal copy of the APC gene, by deletion or mutation, completely removes the tumor suppressive function of APC, thus initiating the growth of adenomatous polyps. Inactivation of the second APC allele occurs frequently in the colon, resulting in the development of numerous adenomas.
A retrospective study of outcomes in 492 patients with polyposis found that the age at polyposis onset and years of survival differed significantly by genotype, although the age of onset of colorectal cancer did not. [7] Patients with a mutation in APC 0-178 or 312-412 developed polyposis later and survived longer, whereas patients with mutations in APC 1249-1549 developed polyposis earlier and did not survive as long.

Etiology
FAP is caused by a germline mutation of the APC tumor suppressor gene, located on band 5q21. Most mutations of the APC gene are nonsense or frameshift mutations, leading to truncation of the APC protein (nonfunctional protein).
More virulent forms of FAP are associated with a mutation in exon 15 between codons 1250 and 1464, the middle portion of the gene. [8]
In patients with AAPC, mutations of the APC gene occur at the extreme amino terminus of the protein.

Epidemiology
United States data
Estimates vary from 1 case in 6,850 persons to 1 case in 31,250 persons.
International data
The frequency is constant worldwide.
Race-, sex-, and age-related demographics
FAP has been described in all races, and males and females are equally affected (1:1).
The average age of onset of polyposis in FAP is 16 years, whereas the average age of onset for colorectal cancer is 39 years.
The average age of onset for polyps in AAPC is 36 years, and the average age of onset for cancer in AAPC is 54 years. These patients have fewer polyps (approximately 30 polyps) compared to patients with FAP.

Prognosis
Patients with untreated FAP have a median life expectancy of 42 years. Life expectancy is extended greatly in those treated with colectomy.
Upper gastrointestinal cancers and desmoid tumors are the most common causes of death in patients who have undergone colectomy. This is why surveillance programs, especially after colectomy, are essential. Colectomy only addresses the risk of colon cancer development.
The cumulative probability of developing any type of noncolorectal cancer, mostly periampullary tumors, is 11% by age 50 years and 52% by age 75 years.
Morbidity/mortality
The principal cause of mortality is colorectal cancer, which develops in all patients unless they are treated. The mean age at which colorectal cancer develops in patients with classic FAP is 39 years. Patients with adenomatous polyposis itself often are asymptomatic.
The second reported lethal complication of FAP is diffuse mesenteric fibromatosis and is referred to as a desmoid tumor. It involves intra-abdominal organs and vessels, causing gastrointestinal obstruction and constriction of veins, arteries, and ureters. Desmoid tumors are reported in 4%-32% of patients. Even after the appropriate surgical treatment of FAP, 20% of patients may develop desmoid tumors after colectomy. Studies have not found a correlation between specific APC mutation sites and desmoid tumor development. [9] Risk factors include a positive family history. The mortality from these tumors is 10%-50%. The second most common malignancy in patients with FAP is adenocarcinoma of the duodenum and the papilla of Vater. It affects as many as 12% of patients.
Rarer cancers associated with FAP include medulloblastomas (Turcot syndrome), hepatoblastoma, thyroid cancer, gastric cancer, pancreatic cancer, and adrenal cancer. [10]
Complications
Complications of FAP include the following:
- Colorectal cancer (100% in untreated patients)
- Duodenal or periampullary adenocarcinoma (4%-12%)
- Desmoid formation (as many as 20%, typically postcolectomy)
- Development of rectal cancer in patients with a retained rectum

- Schulmann K, Pox C, Tannapfel A, Schmiegel W. The patient with multiple intestinal polyps. Best Pract Res Clin Gastroenterol. 2007. 21(3):409-26. [QxMD MEDLINE Link].
- Dekker E, Boparai KS, Poley JW, et al. High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy. 2009 Aug. 41(8):666-9. [QxMD MEDLINE Link].
- Zhang J, Ahmad S, Mao Y. BubR1 and APC/EB1 cooperate to maintain metaphase chromosome alignment. J Cell Biol. 2007 Aug 27. 178(5):773-84. [QxMD MEDLINE Link]. [Full Text].
- Newton KF, Mallinson EK, Bowen J, et al. Genotype-phenotype correlation in colorectal polyposis. Clin Genet. 2012 Jun. 81(6):521-31. [QxMD MEDLINE Link].
- Waller A, Findeis S, Lee MJ. Familial adenomatous polyposis. J Pediatr Genet. 2016 Jun. 5(2):78-83. [QxMD MEDLINE Link].
- Nieuwenhuis MH, De Vos Tot Nederveen Cappel W, Botma A, et al. Desmoid tumors in a dutch cohort of patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008 Feb. 6(2):215-9. [QxMD MEDLINE Link].
- Will OC, Hansmann A, Phillips RK, et al. Adrenal incidentaloma in familial adenomatous polyposis: a long-term follow-up study and schema for management. Dis Colon Rectum. 2009 Sep. 52(9):1637-44. [QxMD MEDLINE Link].
- American Gastroenterological Association. American Gastroenterological Association medical position statement: hereditary colorectal cancer and genetic testing. Gastroenterology. 2001 Jul. 121(1):195-7. [QxMD MEDLINE Link].
- Ponti G, Losi L, Pellacani G, et al. Wnt pathway, angiogenetic and hormonal markers in sporadic and familial adenomatous polyposis-associated juvenile nasopharyngeal angiofibromas (JNA). Appl Immunohistochem Mol Morphol. 2008 Mar. 16(2):173-8. [QxMD MEDLINE Link].
- Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008 Feb. 6(2):180-5. [QxMD MEDLINE Link].
- Tajika M, Nakamura T, Nakahara O, et al. Prevalence of adenomas and carcinomas in the ileal pouch after proctocolectomy in patients with familial adenomatous polyposis. J Gastrointest Surg. 2009 Jul. 13(7):1266-73. [QxMD MEDLINE Link].
- Wachsmannova-Matelova L, Stevurkova V, Adamcikova Z, Holec V, Zajac V. Different phenotype manifestation of familial adenomatous polyposis in families with APC mutation at codon 1309. Neoplasma. 2009. 56(6):486-9. [QxMD MEDLINE Link].
- Duncan RE, Gillam L, Savulescu J, Williamson R, Rogers JG, Delatycki MB. The challenge of developmentally appropriate care: predictive genetic testing in young people for familial adenomatous polyposis. Fam Cancer. 2010 Mar. 9(1):27-35. [QxMD MEDLINE Link].
- Syngal S, Brand RE, Church JM, et al, for the American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb. 110(2):223-62; quiz 263. [QxMD MEDLINE Link]. [Full Text].
- Lynch PM, Morris JS, Wen S, et al. A proposed staging system and stage-specific interventions for familial adenomatous polyposis. Gastrointest Endosc. 2016 Jul. 84(1):115-125.e4. [QxMD MEDLINE Link].
- Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001 Jul. 121(1):198-213. [QxMD MEDLINE Link].
- Rex DK, Johnson DA, Anderson JC, et al, for the American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009 Mar. 104(3):739-50. [QxMD MEDLINE Link].
- Burt RW, Barthel JS, Dunn KB, et al. NCCN clinical practice guidelines in oncology. Colorectal cancer screening. J Natl Compr Canc Netw. 2010 Jan. 8(1):8-61. [QxMD MEDLINE Link].
- Lynch PM. Jurisprudential considerations in the evaluation and screening of high-risk patients. Rozen P, Winawer SJ, eds. In: Secondary Prevention of Colorectal Cancer. An International Perspective. Basel, Switzerland: Karger; 1986. Vol 10: 55-63.
- McEwen JE, McCarty K, Reilly PR. A survey of medical directors of life insurance companies concerning use of genetic information. Am J Hum Genet. 1993 Jul. 53(1):33-45. [QxMD MEDLINE Link].
- Friederich P, van Heumen BW, Nagtegaal ID, et al. Increased epithelial cell proliferation in the ileal pouch mucosa of patients with familial adenomatous polyposis. Virchows Arch. 2007 Sep. 451(3):659-67. [QxMD MEDLINE Link]. [Full Text].
- Durno CA, Wong J, Berk T, Alingary N, Cohen Z, Esplen MJ. Quality of life and functional outcome for individuals who underwent very early colectomy for familial adenomatous polyposis. Dis Colon Rectum. 2012 Apr. 55(4):436-43. [QxMD MEDLINE Link].
- Rodriguez-Bigas MA, Vasen HF, O'Malley L, et al. Health, life, and disability insurance and hereditary nonpolyposis colorectal cancer. Am J Hum Genet. 1998 Mar. 62(3):736-7. [QxMD MEDLINE Link]. [Full Text].
- Iaquinto G, Fornasarig M, Quaia M, et al. Capsule endoscopy is useful and safe for small-bowel surveillance in familial adenomatous polyposis. Gastrointest Endosc. 2008 Jan. 67(1):61-7. [QxMD MEDLINE Link].
- Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg. 2010 Feb. 14(2):229-35. [QxMD MEDLINE Link].
- Ishikawa H, Wakabayashi K, Suzuki S, et al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial. Cancer Med. 2013 Feb. 2(1):50-6. [QxMD MEDLINE Link]. [Full Text].
- Jacoby RF, Cole CE, Hawk ET, Lubet RA. Ursodeoxycholate/Sulindac combination treatment effectively prevents intestinal adenomas in a mouse model of polyposis. Gastroenterology. 2004 Sep. 127(3):838-44. [QxMD MEDLINE Link].
- Parc Y, Desaint B, Flejou JF, et al. The effect of ursodesoxycholic acid on duodenal adenomas in familial adenomatous polyposis: a prospective randomized placebo-control trial. Colorectal Dis. 2012 Jul. 14(7):854-60. [QxMD MEDLINE Link].
- Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009 Oct 12. 4:22. [QxMD MEDLINE Link]. [Full Text].
- Bresalier RS. Malignant neoplasms of the large intestine. Feldman M, Friedman LS, Brandt LJ, eds. In: Sleisenger & Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 8th ed. Philadelphia: Saunders Elsevier; 2006. 2759-810.
- Quinn KP, Lightner AL, Pendegraft RS, Enders FT, Boardman LA, Raffals LE. Pouchitis is a common complication in patients with familial adenomatous polyposis following ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2016 Sep. 14(9):1296-301. [QxMD MEDLINE Link].
- Tonelli F, Ficari F, Bargellini T, Valanzano R. Ileal pouch adenomas and carcinomas after restorative proctocolectomy for familial adenomatous polyposis. Dis Colon Rectum. 2012 Mar. 55(3):322-9. [QxMD MEDLINE Link].
- Nieuwenhuis MH, Douma KF, Bleiker EM, Aaronson NK, Clevers H, Vasen HF. Clinical evidence for an association between familial adenomatous polyposis and type II diabetes. Int J Cancer. 2012 Sep 15. 131(6):1488-9. [QxMD MEDLINE Link]. [Full Text].
- Brosens LA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005 Jul. 54(7):1034-43. [QxMD MEDLINE Link]. [Full Text].
- Burt R, Neklason DW. Genetic testing for inherited colon cancer. Gastroenterology. 2005 May. 128(6):1696-716. [QxMD MEDLINE Link].
- Doxey BW, Kuwada SK, Burt RW. Inherited polyposis syndromes: molecular mechanisms, clinicopathology, and genetic testing. Clin Gastroenterol Hepatol. 2005 Jul. 3(7):633-41. [QxMD MEDLINE Link].
- Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol. 2006 Feb. 101(2):385-98. [QxMD MEDLINE Link].
- Valle L. Recent discoveries in the genetics of familial colorectal cancer and polyposis. Clin Gastroenterol Hepatol. 2017 Jun. 15(6):809-19. [QxMD MEDLINE Link].
- Lynch PM. Chemoprevention of familial adenomatous polyposis. Fam Cancer. 2016 Jul. 15(3):467-75. [QxMD MEDLINE Link].
- Short E, Sampson J. The role of inherited genetic variants in colorectal polyposis syndromes. Adv Genet. 2019. 103:183-217. [QxMD MEDLINE Link].
- de Oliveira JC, Viana DV, Zanardo C, Santos EMM, de Paula AE, Palmero EI, et al. Genotype-phenotype correlation in 99 familial adenomatous polyposis patients: A prospective prevention protocol. Cancer Med. 2019 Mar 21. [QxMD MEDLINE Link]. [Full Text].
- Solomon I, Rybak C, Van Tongeren L, et al. Experience gained from the development and execution of a multidisciplinary multi-syndrome hereditary colon cancer family conference. J Cancer Educ. 2018 Sep 27. [QxMD MEDLINE Link].
Author
Coauthor(s)
Jae W Nam, MD Fellow in Gastroenterology, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine; Consulting Staff, Department of Critical Care, Decatur Hospital
Jae W Nam, MD is a member of the following medical societies: American College of Gastroenterology
Disclosure: Nothing to disclose.
Vincent W Yang, MD, PhD Simons Chair of Medicine, Professor, Departments of Medicine and Physiology and Biophysics, Renaissance School of Medicine at Stony Brook University
Vincent W Yang, MD, PhD is a member of the following medical societies: Alpha Omega Alpha, American Gastroenterological Association, American Society for Clinical Investigation, Association of American Physicians
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Chief Editor
Additional Contributors
Acknowledgements
Simmy Bank, MD Chair, Professor, Department of Internal Medicine, Division of Gastroenterology, Long Island Jewish Hospital, Albert Einstein College of Medicine
Disclosure: Nothing to disclose.
Nicole M Griglione, MD Fellow in Gastroenterology, Department of Medicine, Emory University School of Medicine
Nicole M Griglione, MD is a member of the following medical societies: American Medical Association and Illinois State Medical Society
Disclosure: Nothing to disclose.