Colon Cancer: Practice Essentials, Background, Pathophysiology (original) (raw)
Practice Essentials
Colon cancer is the most common type of gastrointestinal cancer. It is a multifactorial disease process, with etiology encompassing genetic factors, environmental exposures (including diet), and inflammatory conditions of the digestive tract.
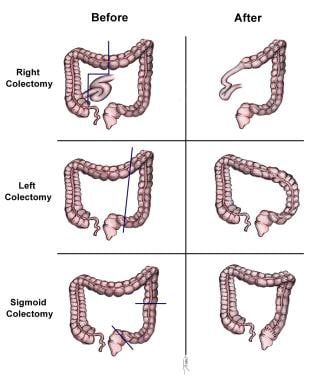
Surgery currently is the definitive treatment modality. [1] The image below depicts standard colectomies for adenocarcinoma of the colon.
Standard colectomies for adenocarcinoma of the colon.
Signs and symptoms
Colon cancer is now often detected during screening procedures. Common clinical presentations include the following:
- Iron-deficiency anemia
- Rectal bleeding
- Abdominal pain
- Change in bowel habits
- Intestinal obstruction or perforation
Physical findings may include the following:
- Early disease: Nonspecific findings (fatigue, weight loss) or none at all
- More advanced disease: Abdominal tenderness, macroscopic rectal bleeding, palpable abdominal mass, hepatomegaly, ascites
See Presentation for more detail.
Diagnosis
Laboratory studies that may be helpful include the following:
- Complete blood count
- Chemistries and liver function tests
- Serum carcinoembryonic antigen
Imaging studies that may facilitate staging include the following:
- Chest radiography
- Chest computed tomography
- Abdominal barium study
- Abdominal/pelvic CT
- Contrast ultrasonography of the abdomen and liver
- Abdominal/pelvic MRI
- Positron emission tomography, including fusion PET-CT scan
Other procedures that may be warranted include the following:
- Colonoscopy
- Sigmoidoscopy
- Biopsy of suspicious lesions
- Double-contrast barium enema
See Workup for more detail.
Management
Surgery is the only curative modality for localized colon cancer (stage I-III). Surgical resection potentially provides the only curative option for patients with limited metastatic disease in liver and/or lung (stage IV disease). Surgical options include the following:
- Right hemicolectomy: For lesions in the cecum and right colon
- Extended right hemicolectomy: For lesions in the proximal or middle transverse colon
- Left hemicolectomy: For lesions in the splenic flexure and left colon
- Sigmoid colectomy: For sigmoid colon lesions
- Total abdominal colectomy with ileorectal anastomosis: For selected patients with hereditary nonpolyposis colon cancer, attenuated familial adenomatous polyposis, metachronous cancers in separate colon segments, or acute malignant colon obstructions with unknown status of the proximal bowel
Other therapeutic options for patients who are not surgical candidates include the following:
- Cryotherapy
- Radiofrequency ablation
- Hepatic arterial infusion of chemotherapeutic agents
Adjuvant (postoperative) therapy is used in selected patients with stage II colon cancer who are at high risk of recurrence, and is standard for stage III colon cancer. Regimens used for systemic chemotherapy may include the following:
- 5-Fluorouracil (5-FU)
- Capecitabine
- Oxaliplatin
- Combinations of multiple agents (eg, capecitabine or 5-FU with oxaliplatin, FOLFOX, FOLFIRI, cetuximab or panitumumab with encorafenib)
Regimens used for adjuvant chemotherapy commonly include 5-FU with leucovorin or capecitabine, either alone or in combination with oxaliplatin. [2, 3, 4]
For metastatic colon cancer, systemic chemotherapy is standard, with neoadjuvant chemotherapy used to convert unresectable isolated liver metastases to resectable liver metastases. Biologic agents have assumed a major role, typically as targeted therapy based on genetic analysis of the tumor. Biologic agents employed to treat colon cancer include the following:
- Bevacizumab (Avastin)
- Cetuximab (Erbitux)
- Ipilimumab (Yervoy)
- Nivolumab (Opdivo)
- Panitumumab (Vectibix)
- Pembrolizumab (Keytruda)
- Ramucirumab (Cyramza)
- Tucatinib (Tukysa)
- Fruquintinib (Fruzaqla)
See Treatment and Medication for more detail.
For more information, see Colorectal Cancer Guidelines.
Go to Oncology Decision Point Colorectal Cancer for expert commentary on treatment decisions and related guidelines.
For patient education information, see Colon Cancer.

Background
Invasive colorectal cancer is a preventable disease. Early detection through widely applied screening programs is the most important factor in the recent decline of colorectal cancer in developed countries (see Overview/Epidemiology).
Fundamental advances in understanding the biology and genetics of colorectal cancer are taking place. This knowledge is slowly making its way into the clinic and being employed to better stratify individual risks of developing colorectal cancer, discover better screening methodologies, allow for better prognostication, and improve the ability to predict benefit from new anticancer therapies.
In the past 10 years, an unprecedented advance in systemic therapy for colorectal cancer has dramatically improved outcome for patients with metastatic disease. Until the mid-1990s, the only approved agent for colorectal cancer was 5-fluorouracil. Since then, new agents in a variety of classes have become available, including the following:
- Cytotoxic agents (eg, irinotecan, oxaliplatin) [5]
- Oral fluoropyrimidines (ie, capecitabine)
- Biologic agents (eg, bevacizumab, cetuximab, panitumumab, pembrolizumab, nivolumab) [1]
- Most recently, anti-angiogenic agents (ie, ziv-aflibercept, regorafenib)
Although surgery remains the definitive treatment modality, these new agents will likely translate into improved cure rates for patients with early-stage disease (stage II and III) and prolonged survival for those with stage IV disease. Further advances are likely to come from the development of new targeted agents and from better integration of systemic therapy with other modalities such as surgery, radiation therapy, and liver-directed therapies.

Pathophysiology
Genetically, colorectal cancer represents a complex disease, and genetic alterations are often associated with progression from premalignant lesion (adenoma) to invasive adenocarcinoma. Sequence of molecular and genetic events leading to transformation from adenomatous polyps to overt malignancy has been characterized by Vogelstein and Fearon. [6]
The early event is a mutation of APC (adenomatous polyposis gene), which was first discovered in individuals with familial adenomatous polyposis (FAP). The protein encoded by APC is important in the activation of oncogene c-myc and cyclin D1, which drives the progression to malignant phenotype. Although FAP is a rare hereditary syndrome accounting for only about 1% of cases of colon cancer, APC mutations are very frequent in sporadic colorectal cancers.
Other important genes in colon carcinogenesis include the KRAS oncogene, chromosome 18 loss of heterozygosity (LOH) leading to inactivation of SMAD4 (DPC4), and DCC (deleted in colon cancer) tumor suppression genes. Chromosome arm 17p deletion and mutations affecting the p53 tumor suppressor gene confer resistance to programmed cell deat—h (apoptosis) and are thought to be late events in colon carcinogenesis.
A subset of colorectal cancers is characterized with deficient DNA mismatch repair. This phenotype has been linked to mutations of genes such as MSH2, MLH1, and PMS2. These mutations result in so-called high frequency microsatellite instability (H-MSI), which can be detected with an immunocytochemistry assay. H-MSI is a hallmark of hereditary nonpolyposis colon cancer syndrome (HNPCC, Lynch syndrome), which accounts for about 6% of all colon cancers. H-MSI is also found in about 20% of sporadic colon cancers.
In addition to mutations, epigenetic events such as abnormal DNA methylation can also cause silencing of tumor suppressor genes or activation of oncogenes. These events compromise the genetic balance and ultimately lead to malignant transformation.
Cancer cells produce extracellular vesicles (EVs)—principally, microvesicles and exosomes—that can promote the growth, survival, invasiveness, and metastatic activity of tumors. [7] Zhao et al reported that in an animal model of aggressive late-stage colorectal cancer, tumor-secreted EVs promoted resistance to immune checkpoint blockade. The colorectal cancer cells in this model secrete exosomes that carry immunosuppressive microRNAs these block CD28 on T cells and CD80 on dendritic cells that infiltrate the tumors, disabling T-cell–mediated anti-tumor immune response. [8]
Further, these authors found that intravenous injections of tumor-secreted exosomes without immunosuppressive microRNAs, in combination with immune checkpoint inhibitors, resulted in an enhanced anti-tumor immune response. This offers a potential therapeutic strategy for late-stage colorectal cancer. [8]

Etiology
Colorectal cancer is a multifactorial disease process. Genetic factors, environmental exposures (including diet), and inflammatory conditions of digestive tract are all involved in the development of colorectal cancer.
Although much about colorectal cancer genetics remains unknown, current research indicates that genetic factors have the greatest correlation to colorectal cancer. Hereditary mutation of the APC gene is the cause of familial adenomatous polyposis (FAP), in which affected individuals carry an almost 100% risk of developing colon cancer by age 40 years.
Hereditary nonpolyposis colon cancer syndrome (HNPCC, Lynch syndrome) poses about a 40% lifetime risk for developing colorectal cancer; individuals with this syndrome are also at increased risk for urothelial cancer, endometrial cancer, and other less common cancers. Lynch syndrome is characterized by deficient mismatch repair (dMMR) due to inherited mutation in one of the mismatch repair genes, such as hMLH1, hMSH2, hMSH6, hPMS1, hPMS2, and possibly other undiscovered genes.
HNPCC is a cause of about 6% of all colon cancers. Although the use of aspirin may reduce the risk of colorectal neoplasia in some populations, a study by Burn et al found no effect on the incidence of colorectal cancer in carriers of Lynch syndrome with use of aspirin, resistant starch, or both. [9]
Dietary factors are the subject of intense and ongoing investigations. [10] Epidemiologic studies have linked increased risk of colorectal cancer with a diet high in red meat and animal fat, low-fiber diets, and low overall intake of fruits and vegetables. A study by Aune et al found that a high intake of fiber was associated with a reduced risk of colorectal cancer. In particular, cereal fiber and whole grains were found to be effective. [11] A study by Pala et al found that high yogurt intake was also associated with a decreased risk for colorectal cancer. [12]
A cohort study by Tabung et al that followed 121,050 adults for 26 years found that in both men and women, intake of proinflammatory diets (replete in red, processed, and organ meat, for example) was associated with a significantly higher risk of developing colorectal cancer. Risk was especially high in overweight and obese men and, paradoxically, in lean women. Risk was also increased in men and women who do not drink alcohol. [13]
Factors associated with lower risk include folate intake, calcium intake, and estrogen replacement therapy. However, most of these studies were retrospective epidemiologic studies and have yet to be validated in prospective, placebo-controlled, interventional trials.
Obesity and lifestyle choices such as cigarette smoking, alcohol consumption, and sedentary habits have also been associated with increased risk for colorectal cancer. A meta-analysis of prospective cohort studies found a modest but significant elevation of colorectal cancer risk in current smokers; risk was higher for men and for rectal cancers than colon cancers, and persisting in former smokers. [14]
In a large prospective study, Cho and colleagues reported that high alcohol consumption was associated with elevated risk for colorectal cancer, in individuals with a family history of the disease. The association was significant only for the highest alcohol intake category of 30 g or more daily; no significant linear trend was evident. In comparison with nondrinkers with no family history, individuals who consumed 30 g/d or more and who had a family history of colorectal cancer had a relative risk for colon cancer of 2.80. [15]
Current screening guidelines recommend that clinicians be aware of increased colorectal cancer risk in patients who smoke or are obese, but do not highlight the increased risk in patients with diabetes. A meta-analysis of case-control and cohort studies identified diabetes as an independent risk factor for colon and rectal cancer. Subgroup analyses confirmed the consistency of the findings across study type and population. This information may have an impact on screening guidelines and on building risk models of colorectal cancer. [16]
Association between body mass index (BMI) and risk of colorectal adenomas and cancer has been reported, but few studies have had adequate sample size for conducting stratified analyses. Jacobs et al pooled data from 8,213 participants in seven prospective studies and found that BMI was significantly related to most histologic characteristics of metachronous adenomas in men but not in women. The researchers concluded that body size may affect colorectal carcinogenesis at comparatively early stages, particularly in men. [17]
A nationwide cohort study from France of incident colorectal cancer in obese patients, which compared outcomes in 74,131 patients who underwent bariatric surgery with 971, 217 patients who did not have surgery, found that in the bariatric surgery cohort, risk of colorectal cancer was the same as that in the general population. In the obese patients who did not undergo bariatric surgery, the risk was 34% above that of the general population. [18]
Activation of the WNT signaling pathway, which most often results from APC loss, plays a critical role in the development of colorectal cancer, and CTNNB1 (β-catenin) is a major mediator of the WNT pathway. WNT-CTNNB1 signaling also appears to be involved in obesity, glucose metabolism, and metabolic diseases such as obesity and type II diabetes. Consequently, Morikawa et al hypothesized that the association of obesity and physical activity with colorectal cancer risk might differ by tumor subtypes according to CTNNB1 status. [19]
Using a molecular pathological epidemiology database, these researchers determined that risk of CTNNB1-negative cancer was significantly higher with greater BMI and lower with increased physical activity level. These researchers found no association between either BMI or physical activity level and CTNNB1-positive cancer risk. [19]
Greater adult-attained height is associated with an increased risk of colorectal cancer and adenoma, according to a systematic review and meta-analysis by Zhou et al that included 47 observational studies involving 280,644 colorectal cancer and 14,139 colorectal adenoma cases.The study found that overall, the risk of colorectal cancer is 24% higher in the tallest individuals within the highest percentile of height, compared with the shortest individuals within the lowest percentile. Every 3.9-inch (10-centimeter) increase in height was associated with a 14% higher risk for colon cancer and 6% higher odds of adenoma. Zhou et al recommend considering height as a risk factor for colorectal cancer screening. [20]
Excessive consumption of beverages sweetened with high-fructose corn syrup (HFCS) is associated with increased risk of colorectal cancer. In a study of adenomatous polyposis coli (APC) mutant mice, which are predisposed to develop intestinal tumors, daily administration of 20 g of weight-adjusted HFCS (the equivalent of 1 soda a day) resulted in a substantial increase in in polyps that rapidly developed into advanced, high-grade dysplastic lesions. Carbon labeling showed uptake in fructose within the intestinal tumors themselves. Within the tumors, fructose was converted to fructose-1-phosphate, leading to activation of glycolysis and increased synthesis of fatty acids that support tumor growth. [21]
Inflammatory bowel diseases such as ulcerative colitis and Crohn disease also increase the risk of developing colorectal adenocarcinoma. The risk for developing colorectal malignancy increases with the duration of inflammatory bowel disease and the greater extent of colon involvement.
A matched case-control study of incident colorectal cancer cases in the United Kingdom from 1989 to 2012 found that use of oral antibiotics was associated with increased risk of colon cancer, particularly in the proximal colon. The association involved antibiotic exposure occurring more than 10 years before colon cancer diagnosis. Risk was dose dependent but was observed after even a single course of antibiotics. In addition, risk was greatest with anti-anaerobic antibiotics. The authors note that such antibiotics markedly disrupt the gut microbiome, which consists predominantly of anaerobes, and this disruption may facilitate the acquisition or development of a carcinogenic colon microbiota. [22]

Epidemiology
The incidence and mortality from colon cancer have been on a slow decline over the past several decades in the United States, with the incidence falling on average 2.4% each year and death rates falling on average 2.2% each year over 2007-2019. [23] However, colorectal cancers remain the third most common cancer and third most common cause of cancer-related mortality in US men and women. [24] In addition, rates of colon cancer in younger persons have been increasing. [25]
The American Cancer Society estimates that 106,970 new cases of colon cancer will be diagnosed in the United States in 2023. Estimates for mortality from colon and rectal cancer (the two are combined because of classification difficulties) are for 52,550 deaths in 2023. [24]
A case-control study using national Veterans Affairs–Medicare data concluded that colonoscopy was associated with significant reductions in colorectal cancer mortality in veterans. Mortality benefit was greater for left-sided cancer than right-sided cancer. [26] Case patients (n= 4964) were veterans aged 52 years or older who were diagnosed with colorectal cancer in 2002 to 2008 and died of the disease by the end of 2010. Case patients were matched to four control patients (n= 9,856) without prior colorectal cancer. Risk of mortality from left-sided cancer was reduced in those who had undergone colonoscopy (odds ratio [OR], 0.28 [CI, 0.24 to 0.32]), as was risk for mortality from right-sided cancer (OR, 0.54 [CI, 0.47 to 0.63]). [26]
Worldwide, an estimated 1,931,590 new cases of colorectal cancer occurred in 2020 (10% of all cancers). Geographically, the incidence varies as much as six-fold. The highest estimated rates are in southern Europe (per 100,000 population, 40.6 in men and 24.5 in women), and the lowest in south-central Asia (per 100,000 population, 6.6 in men and 4.4 in women). [27]
Worldwide, colorectal cancer caused approximately 935,173 deaths in 2020, accounting for 9.4% of cancer mortality overall. As with incidence rates, mortality rates worldwide vary six-fold, with the highest estimated mortality rates in central and eastern Europe (14.5 per 100,000 population), and the lowest in south-central Asia (3.2 per 100,000 population). [27]
An epidemiologic study from the European Union (EU) concluded that in 2018, colorectal cancer would account for the second highest number of cancer deaths, at 98,000 deaths in men and 79,400 in women. However, while the total number of colorectal deaths in the EU has risen since 2012 because of the aging population, since 2012 the age-standardized death rate has fallen by 6.7% (to 15.8 per 100,000 in men and 7.5% (to 9.2 per 100,000) in women. [28]
Racial, sexual, and age-related disparities in incidence
Since 1989, colorectal cancer incidence rates have been higher for Blacks than for Whites in both men and women. Currently, incidence rates of colorectal cancer are 21% higher in Black men and 18% higher in Black women compared with White men and women, respectively. [29]
Colorectal mortality rates are 44% higher in Black men and 31% higher in Black women compared with White men and women. However, from 2010 to 2019, colorectal cancer death rates declined faster in Blacks than in Whites (2.8% vs 1.8% per year), narrowing the racial disparity in both men and women. [29]
Asians/Pacific Islanders have the lowest incidence and mortality from colorectal cancer. Hispanics have the second lowest. [23]
The incidence of colorectal cancer is relatively equal in men and women. The American Cancer Society estimates that colon cancer will be diagnosed in 54,420 men and 52,550 women in the United States in 2023. [24]
Age is a well-known risk factor for colorectal cancer, as it is for many other solid tumors. The timeline for progression from early premalignant lesion to malignant cancer ranges from 10-20 years. Median age at diagnosis is 66 years. [23]
However, in contrast to the decline in colon cancer incidence rates in persons age 55 and older, which began in the mid-1980s, rates of colon cancer in younger persons have been increasing. In adults age 20 to 39 years, colon cancer incidence rates have increased by 1.0% to 2.4% annually since the mid-1980s; in those age 40 to 54 years, the incidence has increased by 0.5% to 1.3% annually since the mid-1990s. Currently, adults born circa 1990 have double the risk of colon cancer compared with those born circa 1950. Increased obesity is one likely factor. [25]
From 2011 through 2016, the incidence of colorectal cancer continued to decline in those aged 65 years and older, by 3.3% annually. Rates increased by 1% annually in those aged 50 to 64 years, and rose approximately 2% annually in those younger than 50 years. The American Cancer Society estimated that 17,930 of the 147,950 individuals expected to be diagnosed with colon and rectal cancer in 2020, and 3640 of the 53,200 expected to die from the disease, would be younger than 50 years of age. [30]
Tumor site tends to vary by patient age. From 2012 to 2016, the proximal colon was the site of colon cancer in 23% of those under 50 years of age, 31% of those 50-64 years, and 49% of those 65 and older. Incidence trends varied by race/ethnicity: in those 50-64 years old, rates increased in whites by 1.3% per year but decreased in blacks by 1.6% per year, and were stable in Hispanics. In those younger than 50, rates rose by 2% annually in whites and by 0.5% annually in Blacks. [30]
A review of Surveillance, Epidemiology and End Results (SEER) data found that US cases of colorectal cancer in persons aged 40-49 years have increased significantly since 1995, with the greatest average annual percentage increase for distant cancers, at 2.9%, while localized and regional disease each increased < 1.5% per year. In addition, the proportion of distant colorectal cancers in this age group increased significantly from 1990-1994 to 2011–2015, from 22% to 27%, while the proportion of localized cases did not change, and the proportion of regional cases decreased. These authors point out that these results indicate a true increase in risk, because if the increase had reflected earlier detection due to wider use of colonoscopy, earlier stage at diagnosis would be expected. [31]

Prognosis
The approximate 5-year survival rate for colorectal cancer patients in the United States (all stages included) is 64.6%. [23] Survival is inversely related to stage: approximate 5-year relative survival rates are as follows:
- Localized disease: 90.2%
- Regional disease: 71.8%
- Distant disease: 14.3%
A study by Chua et al found that approximately one in every three patients who undergo resection for colorectal liver metastases become actual 5-year survivors. [32] Of those, approximately half survive 10 years and are cured of colorectal liver metastases. A multivariate analysis of 1001 patients who underwent potentially curative resection of liver metastases identified five factors as independent predictors of worse outcome [33] :
- Size greater than 5 cm
- Disease-free interval of less than a year
- More than one tumor
- Primary lymph-node positivity
- Carcinoembryonic antigen (CEA) level greater than 200 ng/mL
Aggarwal et al found that circulating tumor cells measured at baseline after the initiation of new therapy in patients with metastatic colorectal cancer independently predicted survival; in patients with a baseline carcinoembryonic antigen (CEA) value of 25 ng/mL or higher, those with low baseline levels of circulating tumor cells (< 3) had longer survival. Both the number of circulating tumor cells and the CEA level measured at 6-12 weeks independently predicted survival. [34]
Research suggests a role for intra-tumoral immune response as a predictor of clinical outcome in patients with colorectal cancer, in addition to more traditional pathological and molecular markers. Katz et al reported that in patients with colorectal liver metastases, high numbers of T regulatory cells relative to CD4 or CD8 T cells predicted poor outcome [35]
A study by Yothers et al found that black patients with resected stage II and stage III colon cancer had worse overall and recurrence-free survival compared with white patients who underwent the same therapy. Five-year overall survival rate was 68.2% for blacks and 72.8% for whites; the three-year recurrence-free survival was 68.4% in blacks and 72.1% in whites. [36]
A study by Campbell et al found that prediagnosis body mass index (BMI) is an important predictor of survival among patients with nonmetastatic colorectal cancer, whereas postdiagnosis BMI is not. [37] A separate study from Campbell et al found that spending 6 or more hours per day sitting was associated with higher all-cause mortality compared with sitting less than 3 hours per day. The study concluded that increased recreational physical activity in patients with colorectal carcinoma reduces mortality. [38]
Morikawa et al reported that in patients with colorectal cancer that tested negative for cadherin-associated protein β 1 (CTNNB1 or β-catenin), high physical activity (≥18 metabolic equivalent task [MET] hours/week) after diagnosis was associated with significantly better cancer-specific survival. No association between physical activity and survival was seen in CTNNB1–positive cases. [39]
A review of eight trials by Rothwell et al found that allocation to aspirin reduced death caused by cancer. Benefit was apparent after 5 years of follow-up. The 20-year risk of cancer death was also lower in the aspirin group for all solid cancers. A latent period of 5 years was observed before risk of death was decreased for esophageal, pancreatic, brain, and lung cancers. A more delayed latent period was observed for stomach, colorectal, and prostate cancer. The overall effect on 20-year risk of cancer death was greatest for adenocarcinomas. [40]
A study by Burn et al found that 600 mg of aspirin per day for a mean of 25 months reduced cancer incidence after 55.7 months among known carriers of hereditary colorectal cancer. However, further studies are needed to determine the optimum dose and duration of treatment. [41]
Patients with preexisting mental disorders have an overall higher mortality rate than their counterparts. This higher mortality rate can be attributed to a lack of surgery, chemotherapy, and radiation therapy, especially in patients with psychotic disorders and dementia. Improved public health initiatives are needed to improve colon cancer detection and treatment in older adults with mental disorders. [42]
A study by Phipps et al found that smoking is also associated with increased mortality after colorectal cancer diagnosis, especially in patients whose cancer has high microsatellite instability. [43] A study by Dehal et al found that patients with colorectal cancer and type 2 diabetes mellitus have a higher risk of mortality than those without, most notably a higher risk due to cardiovascular disease. [44]

- PDQ Adult Treatment Editorial Board. Colon Cancer Treatment–Health Professional Version. National Cancer Institute. Available at https://www.cancer.gov/types/colorectal/hp/colon-treatment-pdq. January 20, 2023; Accessed: January 25, 2023.
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004 Jan 1. 22(1):23-30. [QxMD MEDLINE Link].
- Haller DG, Catalano PJ, Macdonald JS, O'Rourke MA, Frontiera MS, Jackson DV. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005 Dec 1. 23(34):8671-8. [QxMD MEDLINE Link].
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3. 350(23):2335-42. [QxMD MEDLINE Link].
- Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008 Dec 10. 26(35):5721-7. [QxMD MEDLINE Link].
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1. 319(9):525-32. [QxMD MEDLINE Link].
- Chang WH, Cerione RA, Antonyak MA. Extracellular Vesicles and Their Roles in Cancer Progression. Methods Mol Biol. 2021. 2174:143-170. [QxMD MEDLINE Link]. [Full Text].
- Zhao X, Yuan C, Wangmo D, Subramanian S. Tumor secreted extracellular vesicles regulate T-cell costimulation and can be manipulated to induce tumor specific T-cell responses. Gastroenterology. 2021 Apr 22. [QxMD MEDLINE Link].
- Burn J, Bishop DT, Mecklin JP, Macrae F, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008 Dec 11. 359(24):2567-78. [QxMD MEDLINE Link].
- Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007 Aug 15. 298(7):754-64. [QxMD MEDLINE Link].
- Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011 Nov 10. 343:d6617. [QxMD MEDLINE Link]. [Full Text].
- Pala V, Sieri S, Berrino F, et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int J Cancer. 2011 Dec 1. 129(11):2712-9. [QxMD MEDLINE Link].
- Tabung FK, Liu L, Wang W, Fung TT, Wu K, Smith-Warner SA, et al. Association of Dietary Inflammatory Potential With Colorectal Cancer Risk in Men and Women. JAMA Oncol. 2018 Jan 18. [QxMD MEDLINE Link].
- Tsoi KK, Pau CY, Wu WK, Chan FK, Griffiths S, Sung JJ. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2009 Jun. 7(6):682-688.e1-5. [QxMD MEDLINE Link].
- Cho E, Lee JE, Rimm EB, Fuchs CS, Giovannucci EL. Alcohol consumption and the risk of colon cancer by family history of colorectal cancer. Am J Clin Nutr. 2012 Feb. 95(2):413-9. [QxMD MEDLINE Link]. [Full Text].
- Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer?. Am J Gastroenterol. 2011 Nov. 106(11):1911-21; quiz 1922. [QxMD MEDLINE Link].
- Jacobs ET, Ahnen DJ, Ashbeck EL, Baron JA, Greenberg ER, Lance P, et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: a pooling study. Am J Epidemiol. 2009 Mar 15. 169(6):657-66. [QxMD MEDLINE Link].
- Bailly L, Fabre R, Pradier C, Iannelli A. Colorectal Cancer Risk Following Bariatric Surgery in a Nationwide Study of French Individuals With Obesity. JAMA Surg. 2020 Mar 11. [QxMD MEDLINE Link].
- Morikawa T, Kuchiba A, Lochhead P, et al. Prospective Analysis of Body Mass Index, Physical Activity, and Colorectal Cancer Risk Associated with ß-Catenin (CTNNB1) Status. Cancer Res. 2013 Mar 1. 73(5):1600-10. [QxMD MEDLINE Link]. [Full Text].
- Zhou E, Wang L, Santiago CN, Nanavati J, Rifkin S, Spence E, et al. Adult-Attained Height and Colorectal Cancer Risk: A Cohort Study, Systematic Review and Meta-Analysis. Cancer Epidemiol Biomarkers Prev. 2022 Mar 1. [QxMD MEDLINE Link]. [Full Text].
- Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science. 2019 Mar 22. 363 (6433):1345-1349. [QxMD MEDLINE Link].
- Zhang J, Haines C, Watson AJM, Hart AR, Platt MJ, Pardoll DM, et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989-2012: a matched case-control study. Gut. 2019 Aug 19. [QxMD MEDLINE Link].
- Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Colorectal Cancer. National Cancer Institute. Available at https://seer.cancer.gov/statfacts/html/colorect.html. Accessed: January 25, 2023.
- Cancer Facts & Figures 2023. American Cancer Society. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf. Accessed: January 25, 2023.
- Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst. 28 February 2017. 109:[Full Text].
- Kahi CJ, Pohl H, Myers LJ, Mobarek D, Robertson DJ, Imperiale TF. Colonoscopy and Colorectal Cancer Mortality in the Veterans Affairs Health Care System: A Case-Control Study. Ann Intern Med. 2018 Mar 13. [QxMD MEDLINE Link].
- World Health Organization, International Agency for Research on Cancer. Colorectal Cancer. Available at https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf. Accessed: January 25, 2023.
- Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C, et al. European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann Oncol. 2018 Mar 19. [QxMD MEDLINE Link]. [Full Text].
- Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/Black People 2022. CA Cancer J Clin. 2022 May. 72 (3):202-229. [QxMD MEDLINE Link]. [Full Text].
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020 Mar 5. [QxMD MEDLINE Link]. [Full Text].
- Meester RGS, Mannalithara A, Lansdorp-Vogelaar I, Ladabaum U. Trends in Incidence and Stage at Diagnosis of Colorectal Cancer in Adults Aged 40 Through 49 Years, 1975-2015. JAMA. 2019 May 21. 321 (19):1933-1934. [QxMD MEDLINE Link].
- Chua TC, Saxena A, Chu F, Zhao J, Morris DL. Predictors of cure after hepatic resection of colorectal liver metastases: an analysis of actual 5- and 10-year survivors. J Surg Oncol. 2011 Jun. 103(8):796-800. [QxMD MEDLINE Link].
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999 Sep. 230(3):309-18; discussion 318-21. [QxMD MEDLINE Link].
- Aggarwal C, Meropol NJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol. 2012 Oct 1. [QxMD MEDLINE Link].
- Katz SC, Bamboat ZM, Maker AV, Shia J, Pillarisetty VG, Yopp AC, et al. Regulatory T Cell Infiltration Predicts Outcome Following Resection of Colorectal Cancer Liver Metastases. Ann Surg Oncol. 2012 Sep 26. [QxMD MEDLINE Link].
- Yothers G, Sargent DJ, Wolmark N, et al. Outcomes Among Black Patients With Stage II and III Colon Cancer Receiving Chemotherapy: An Analysis of ACCENT Adjuvant Trials. J Natl Cancer Inst. 2011 Oct 19. 103(20):1498-1506. [QxMD MEDLINE Link]. [Full Text].
- Campbell PT, Newton CC, Dehal AN, et al. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012 Jan 1. 30(1):42-52. [QxMD MEDLINE Link].
- Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013 Mar 1. 31(7):876-85. [QxMD MEDLINE Link].
- Morikawa T, Kuchiba A, Yamauchi M, et al. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011 Apr 27. 305(16):1685-94. [QxMD MEDLINE Link]. [Full Text].
- Rothwell PM, Fowkes GR, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomized trials. Lancet. Dec 7/2010; Early online publication. [Full Text].
- Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011 Dec 17. 378(9809):2081-7. [QxMD MEDLINE Link]. [Full Text].
- Baillargeon J, Kuo YF, Lin YL, et al. Effect of mental disorders on diagnosis, treatment, and survival of older adults with colon cancer. J Am Geriatr Soc. 2011 Jul. 59(7):1268-73. [QxMD MEDLINE Link].
- Phipps AI, Baron J, Newcomb PA. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: The Seattle Colon Cancer Family Registry. Cancer. 2011 Nov 1. 117(21):4948-57. [QxMD MEDLINE Link]. [Full Text].
- Dehal AN, Newton CC, Jacobs EJ, et al. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012 Jan 1. 30(1):53-9. [QxMD MEDLINE Link].
- Arhi CS, Ziprin P, Bottle A, Burns EM, Aylin P, Darzi A. Colorectal cancer patients under the age of 50 experience delays in primary care leading to emergency diagnoses: a population-based study. Colorectal Dis. 2019 Aug 6. [QxMD MEDLINE Link]. [Full Text].
- Fritz CDL, Otegbeye EE, Zong X, Demb J, Nickel KB, Olsen MA, et al. Red-flag Signs and Symptoms for Earlier Diagnosis of Early-Onset Colorectal Cancer. J Natl Cancer Inst. 2023 May 4. [QxMD MEDLINE Link].
- [Guideline] Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021 Mar 1. 116 (3):458-479. [QxMD MEDLINE Link]. [Full Text].
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004 Feb 18. 96(4):261-8. [QxMD MEDLINE Link]. [Full Text].
- Thirunavukarasu P, Sukumar S, Sathaiah M, Mahan M, Pragatheeshwar KD, Pingpank JF, et al. C-stage in Colon Cancer: Implications of Carcinoembryonic Antigen Biomarker in Staging, Prognosis, and Management. J Natl Cancer Inst. 2011 Apr 20. 103(8):689-97. [QxMD MEDLINE Link].
- When Should You Start Getting Screened for Colorectal Cancer?. American Cancer Society. Available at https://www.cancer.org/latest-news/american-cancer-society-updates-colorectal-cancer-screening-guideline.html?utm_campaign&. February 4, 2021; Accessed: January 25, 2023.
- Littlejohn C, Hilton S, Macfarlane GJ, Phull P. Systematic review and meta-analysis of the evidence for flexible sigmoidoscopy as a screening method for the prevention of colorectal cancer. Br J Surg. 2012 Sep 21. [QxMD MEDLINE Link].
- Holme O, et al for the NORCCAP Study Group. Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A Randomized Trial. Ann Intern Med. 24 April 2018. [Full Text].
- Wilschut JA, Habbema JD, van Leerdam ME, et al. Fecal occult blood testing when colonoscopy capacity is limited. J Natl Cancer Inst. 2011 Dec 7. 103(23):1741-51. [QxMD MEDLINE Link].
- Jensen CD, Corley DA, Quinn VP, Doubeni CA, Zauber AG, Lee JK, et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med. 2016 Jan 26. [QxMD MEDLINE Link]. [Full Text].
- Corley DA, Jensen CD, Quinn VP, Doubeni CA, Zauber AG, Lee JK, et al. Association Between Time to Colonoscopy After a Positive Fecal Test Result and Risk of Colorectal Cancer and Cancer Stage at Diagnosis. JAMA. 2017 Apr 25. 317 (16):1631-1641. [QxMD MEDLINE Link].
- Gandhi S, Narula N, Mosleh W, Marshall JK, Farkouh M. Meta-analysis: colonoscopic post-polypectomy bleeding in patients on continued clopidogrel therapy. Aliment Pharmacol Ther. 2013 May. 37(10):947-52. [QxMD MEDLINE Link].
- Nelson R. FDA Approves Cologuard for Colorectal Cancer Screening. Medscape Medical News. Available at https://www.medscape.com/viewarticle/829757. August 11, 2014; Accessed: January 30, 2018.
- Brooks M. FDA Clears First Blood-Based Colorectal Cancer Screening Test. Medscape Medical News. Available at https://www.medscape.com/viewarticle/861942. April 14, 2016; Accessed: January 30, 2018.
- [Guideline] Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008 May-Jun. 58(3):130-60. [QxMD MEDLINE Link].
- Karsenti D, Tharsis G, Burtin P, Venezia F, Tordjman G, Gillet A, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019 Jan 28. 25 (4):447-456. [QxMD MEDLINE Link]. [Full Text].
- Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011 May 23. 171(10):906-12. [QxMD MEDLINE Link].
- [Guideline] Johnson DA, Barkun AN, Cohen LB, Dominitz JA, Kaltenbach T, Martel M, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2014 Oct. 109 (10):1528-45. [QxMD MEDLINE Link]. [Full Text].
- Given Imaging Receives FDA Clearance for PillCam® COLON in Patients Following Incomplete Colonoscopy. Med Device Online. Available at https://www.meddeviceonline.com/doc/given-imaging-fda-pillcam-colon-incomplete-colonoscopy-0001. February 3, 2014; Accessed: January 25, 2023.
- Spada C, De Vincentis F, Cesaro P, Hassan C, Riccioni ME, Minelli Grazioli L, et al. Accuracy and safety of second-generation PillCam COLON capsule for colorectal polyp detection. Therap Adv Gastroenterol. 2012 May. 5(3):173-8. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, et al. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020 Feb 5. 143(3):844-57. [QxMD MEDLINE Link].
- [Guideline] Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017 May 1. 35 (13):1453-1486. [QxMD MEDLINE Link]. [Full Text].
- American Joint Committee on Cancer. Colon and Rectum. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, et al, eds. AJCC Cancer staging manual. 8th ed. New York: Springer; 2017.
- Ogino S, Kawasaki T, Kirkner GJ, Ohnishi M, Fuchs CS. 18q loss of heterozygosity in microsatellite stable colorectal cancer is correlated with CpG island methylator phenotype-negative (CIMP-0) and inversely with CIMP-low and CIMP-high. BMC Cancer. 2007 May 2. 7:72. [QxMD MEDLINE Link].
- Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS Mutations and Sensitivity to Epidermal Growth Factor Receptor Inhibitors in Colorectal Cancer: Practical Application of Patient Selection. J Clin Oncol. 2009 Jan 5. [QxMD MEDLINE Link].
- Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010 Jul 10. 28 (20):3219-26. [QxMD MEDLINE Link]. [Full Text].
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003 Jul 17. 349(3):247-57. [QxMD MEDLINE Link].
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011 Feb 20. 29(6):610-8. [QxMD MEDLINE Link].
- Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003 Aug 1. 21(15):2912-9. [QxMD MEDLINE Link].
- Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010 Jan 10. 28(2):264-71. [QxMD MEDLINE Link]. [Full Text].
- Hugen N, van de Velde CJ, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014 Mar. 25(3):651-7. [QxMD MEDLINE Link].
- Boggs W. Histology influences colorectal cancer metastatic pattern. Reuters Health Information. March 11, 2014. [Full Text].
- [Guideline] Baxter NN, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, et al. Adjuvant Therapy for Stage II Colon Cancer: ASCO Guideline Update. J Clin Oncol. 2021 Dec 22. 22(16):JCO2102538. [QxMD MEDLINE Link]. [Full Text].
- Boller AM, Nelson H. Colon and rectal cancer: laparoscopic or open?. Clin Cancer Res. 2007 Nov 15. 13(22 Pt 2):6894s-6s. [QxMD MEDLINE Link].
- Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007 Oct. 246(4):655-62; discussion 662-4. [QxMD MEDLINE Link].
- Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007 Jul 20. 25(21):3061-8. [QxMD MEDLINE Link].
- Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008 Apr 16. CD003432. [QxMD MEDLINE Link].
- Lacy AM, Delgado S, Castells A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008 Jul. 248(1):1-7. [QxMD MEDLINE Link].
- Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005 Jul. 6(7):477-84. [QxMD MEDLINE Link].
- Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG. Outcome of Primary Tumor in Patients With Synchronous Stage IV Colorectal Cancer Receiving Combination Chemotherapy Without Surgery As Initial Treatment. J Clin Oncol. 2009 Jun 1. [QxMD MEDLINE Link].
- Nitzkorski JR, Farma JM, Watson JC, Siripurapu V, Zhu F, Matteotti RS, et al. Outcome and natural history of patients with stage IV colorectal cancer receiving chemotherapy without primary tumor resection. Ann Surg Oncol. 2012 Feb. 19(2):379-83. [QxMD MEDLINE Link].
- Venderbosch S, de Wilt JH, Teerenstra S, et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol. 2011 Nov. 18(12):3252-60. [QxMD MEDLINE Link]. [Full Text].
- Di Benedetto F, Berretta M, D'Amico G, et al. Liver resection for colorectal metastases in older adults: a paired matched analysis. J Am Geriatr Soc. 2011 Dec. 59(12):2282-90. [QxMD MEDLINE Link].
- Brouquet A, Overman MJ, Kopetz S, et al. Is resection of colorectal liver metastases after a second-line chemotherapy regimen justified?. Cancer. 2011 Oct 1. 117(19):4484-92. [QxMD MEDLINE Link]. [Full Text].
- House MG, Kemeny NE, Gonen M, et al. Comparison of adjuvant systemic chemotherapy with or without hepatic arterial infusional chemotherapy after hepatic resection for metastatic colorectal cancer. Ann Surg. 2011 Dec. 254(6):851-6. [QxMD MEDLINE Link].
- van Hooft JE, Bemelman WA, Oldenburg B, et al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011 Apr. 12(4):344-52. [QxMD MEDLINE Link].
- Sargent D, Sobrero A, Grothey A, O'Connell MJ, Buyse M, Andre T, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009 Feb 20. 27(6):872-7. [QxMD MEDLINE Link].
- Twelves C, Scheithauer W, McKendrick J, Seitz JF, Van Hazel G, Wong A, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol. 2012 May. 23 (5):1190-1197. [QxMD MEDLINE Link]. [Full Text].
- Schmoll HJ, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine Plus Oxaliplatin Compared With Fluorouracil/Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer: Final Results of the NO16968 Randomized Controlled Phase III Trial. J Clin Oncol. 2015 Nov 10. 33 (32):3733-40. [QxMD MEDLINE Link]. [Full Text].
- van Erning FN, Creemers GJ, De Hingh IH, Loosveld OJ, Goey SH, Lemmens VE. Reduced risk of distant recurrence after adjuvant chemotherapy in patients with stage III colon cancer aged 75 years or older. Ann Oncol. 2013 Aug 8. [QxMD MEDLINE Link].
- André T, Iveson T, Labianca R, Meyerhardt JA, Souglakos I, Yoshino T, et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) Collaboration: Prospective Combined Analysis of Phase III Trials Investigating Duration of Adjuvant Therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) Regimen for Patients with Stage III Colon Cancer: Trial Design and Current Status. Curr Colorectal Cancer Rep. 2013. 9:261-269. [QxMD MEDLINE Link]. [Full Text].
- Lieu C, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, et al. Duration of Oxaliplatin-Containing Adjuvant Therapy for Stage III Colon Cancer: ASCO Clinical Practice Guideline. J Clin Oncol. 2019 Jun 1. 37 (16):1436-1447. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Available at https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Version 2.2022 — October 27, 2022; Accessed: January 25, 2023.
- Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007 Dec 15. 370(9604):2020-9. [QxMD MEDLINE Link].
- O'Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011 Sep 1. 29(25):3381-8. [QxMD MEDLINE Link]. [Full Text].
- Petrelli F, Labianca R, Zaniboni A, et al. Assessment of Duration and Effects of 3 vs 6 Months of Adjuvant Chemotherapy in High-Risk Stage II Colorectal Cancer: A Subgroup Analysis of the TOSCA Randomized Clinical Trial. JAMA Oncol. 2020 Feb 13. [QxMD MEDLINE Link].
- Kim GP, Sargent DJ, Mahoney MR, Rowland KM Jr, Philip PA, Mitchell E, et al. Phase III noninferiority trial comparing irinotecan with oxaliplatin, fluorouracil, and leucovorin in patients with advanced colorectal carcinoma previously treated with fluorouracil: N9841. J Clin Oncol. 2009 Jun 10. 27(17):2848-54. [QxMD MEDLINE Link]. [Full Text].
- Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011 May 21. 377(9779):1749-59. [QxMD MEDLINE Link].
- Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015 May 14. 372 (20):1909-19. [QxMD MEDLINE Link].
- Prager GW, Taieb J, Fakih M, Ciardiello F, et al. Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N Engl J Med. 2023 May 4. 388 (18):1657-1667. [QxMD MEDLINE Link]. [Full Text].
- Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis Markers. 2004. 20 (4-5):199-206. [QxMD MEDLINE Link]. [Full Text].
- Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013 Jan. 14(1):29-37. [QxMD MEDLINE Link].
- Kabbinavar FF, Hurwitz HI, Yi J, Sarkar S, Rosen O. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol. 2009 Jan 10. 27(2):199-205. [QxMD MEDLINE Link].
- Goey KKH, Elias SG, van Tinteren H, Laclé MM, Willems SM, Offerhaus GJA, et al. Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Ann Oncol. 2017 Sep 1. 28 (9):2128-2134. [QxMD MEDLINE Link]. [Full Text].
- Tebbutt NC, Murphy F, Zannino D, et al. Risk of arterial thromboembolic events in patients with advanced colorectal cancer receiving bevacizumab. Ann Oncol. 2011 Aug. 22(8):1834-8. [QxMD MEDLINE Link].
- Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011 Jan 1. 29(1):11-6. [QxMD MEDLINE Link].
- Stark, Angela. FDA approves first biosimilar for the treatment of cancer. FDA News Release. 09/14/2017. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm576112.htm.
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004 Jul 22. 351(4):337-45. [QxMD MEDLINE Link].
- Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011 May 20. 29(15):2011-9. [QxMD MEDLINE Link].
- Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009 Apr 2. 360(14):1408-17. [QxMD MEDLINE Link]. [Full Text].
- Lin AY, Buckley NS, Lu AT, et al. Effect of KRAS mutational status in advanced colorectal cancer on the outcomes of anti-epidermal growth factor receptor monoclonal antibody therapy: a systematic review and meta-analysis. Clin Colorectal Cancer. 2011 Mar 1. 10(1):63-9. [QxMD MEDLINE Link].
- Chustecka Z. FDA Approves Panitumumab for Use With FOLFOX in mCRC. Medscape Medical News. Available at https://www.medscape.com/viewarticle/825699. Accessed: June 2, 2014.
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010 Nov 1. 28(31):4697-705. [QxMD MEDLINE Link].
- Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010 Nov 1. 28(31):4706-13. [QxMD MEDLINE Link].
- Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014 May. 15(6):569-79. [QxMD MEDLINE Link].
- Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009 Feb 10. 27(5):672-80. [QxMD MEDLINE Link].
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009 Feb 10. 27(5):663-71. [QxMD MEDLINE Link].
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013 Sep 12. 369(11):1023-34. [QxMD MEDLINE Link].
- Kim TW, Elme A, Kusic Z, Park JO, Udrea AA, Kim SY, et al. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild-type KRAS or RAS metastatic colorectal cancer. Br J Cancer. 2016 Nov 8. 115 (10):1206-1214. [QxMD MEDLINE Link]. [Full Text].
- Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015 Apr 10. [QxMD MEDLINE Link].
- Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017 Jul 19. [QxMD MEDLINE Link]. [Full Text].
- Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018 Mar 10. 36 (8):773-779. [QxMD MEDLINE Link].
- Opdivo (nivolumab) [package insert]. Bristol-Myers Squibb Company: Princeton, NJ 08543 USA. 7/2017. Available at [Full Text].
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015 Jun 25. 372 (26):2509-20. [QxMD MEDLINE Link]. [Full Text].
- Stivarga (regorafenib) [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc. September 2012. Available at [Full Text].
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013 Jan 26. 381 (9863):303-12. [QxMD MEDLINE Link].
- Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018 Jun 26. 319 (24):2486-2496. [QxMD MEDLINE Link]. [Full Text].
- Dasari A, Lonardi S, Garcia-Carbonero R, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. 2023 Jul 1. 402 (10395):41-53. [QxMD MEDLINE Link]. [Full Text].
- Patel A, Sun W. Ziv-aflibercept in metastatic colorectal cancer. Biologics. 2014. 8:13-25. [QxMD MEDLINE Link]. [Full Text].
- Van Cutsem E, et al. Aflibercept Plus FOLFIRI vs. Placebo Plus FOLFIRI in Second-Line Metastatic Colorectal Cancer: a Post Hoc Analysis of Survival from the Phase III VELOUR Study Subsequent to Exclusion of Patients who had Recurrence During or Within 6 Months of Completing Adjuvant Oxaliplatin-Based Therapy. Target Oncol. 2016 Jun. 11 (3):383-400. [QxMD MEDLINE Link]. [Full Text].
- Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012 Oct 1. 30(28):3499-506. [QxMD MEDLINE Link].
- FDA approves an oncology drug that targets a key genetic driver of cancer, rather than a specific type of tumor. U.S. Food & Drug Administration. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-oncology-drug-targets-key-genetic-driver-cancer-rather-specific-type-tumor. November 26, 2018; Accessed: June 5, 2019.
- Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med. 2019 Oct 24. 381 (17):1632-1643. [QxMD MEDLINE Link].
- Davenport L. Updated BEACON: Doublet as Good as Triplet in Metastatic CRC. Medscape Medical News. Available at https://www.medscape.com/viewarticle/931555. June 2, 2020; Accessed: February 11, 2021.
- Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016 Apr 20. [QxMD MEDLINE Link]. [Full Text].
- Otto MA. Tucatinib Plus Trastuzumab Approved for HER2+ Colorectal Cancer. Medscape Medical News. Available at https://www.medscape.com/viewarticle/987242. January 20, 2023; Accessed: January 240, 2023.
- Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010 Aug 10. 28(23):3687-94. [QxMD MEDLINE Link].
- Song M, Wu K, Meyerhardt JA, Ogino S, Wang M, Fuchs CS, et al. Fiber Intake and Survival After Colorectal Cancer Diagnosis. JAMA Oncol. 2017 Nov 2. [QxMD MEDLINE Link]. [Full Text].
- Van Blarigan EL, Ou FS, Bainter TM, Fuchs CS, Niedzwiecki D, Zhang S, et al. Associations Between Unprocessed Red Meat and Processed Meat With Risk of Recurrence and Mortality in Patients With Stage III Colon Cancer. JAMA Netw Open. 2022 Feb 1. 5 (2):e220145. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, et al. Follow-Up Care, Surveillance Protocol, and Secondary Prevention Measures for Survivors of Colorectal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol. 2013 Nov 12. [QxMD MEDLINE Link].
- [Guideline] Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013 Oct. 24 Suppl 6:vi64-72. [QxMD MEDLINE Link].
- Kirkegaard H, Johnsen NF, Christensen J, Frederiksen K, Overvad K, Tjonneland A. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. BMJ. 2010 Oct 26. 341:c5504. [QxMD MEDLINE Link]. [Full Text].
- Kim J, Boushey CJ, Wilkens LR, Haiman CA, Le Marchand L, Park SY. Plant-based dietary patterns defined by a priori indices and colorectal cancer risk by sex and race/ethnicity: the Multiethnic Cohort Study. BMC Med. 2022 Nov 29. 20 (1):430. [QxMD MEDLINE Link]. [Full Text].
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006 Aug 1. 24(22):3535-41. [QxMD MEDLINE Link].
- Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N Engl J Med. 2015 Oct 15. 373 (16):1519-30. [QxMD MEDLINE Link]. [Full Text].
- Barry EL, Peacock JL, Rees JR, Bostick RM, Robertson DJ, Bresalier RS, et al. Vitamin D Receptor Genotype, Vitamin D3 Supplementation, and Risk of Colorectal Adenomas: A Randomized Clinical Trial. JAMA Oncol. 2016 Dec 15. [QxMD MEDLINE Link].
- McCullough ML, Zoltick ES, Weinstein SJ, Fedirko V, Wang M, et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J Natl Cancer Inst. 2018 Jun 14. [QxMD MEDLINE Link].
- Arber N, Spicak J, Rácz I, Zavoral M, Breazna A, Gerletti P, et al. Five-year analysis of the prevention of colorectal sporadic adenomatous polyps trial. Am J Gastroenterol. 2011 Jun. 106(6):1135-46. [QxMD MEDLINE Link].
- Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007 May 12. 369(9573):1603-13. [QxMD MEDLINE Link].
- Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009 Feb 18. 101(4):256-66. [QxMD MEDLINE Link].
- Zhang X, Smith-Warner SA, Chan AT, et al. Aspirin Use, Body Mass Index, Physical Activity, Plasma C-Peptide, and Colon Cancer Risk in US Health Professionals. Am J Epidemiol. 2011 Aug 15. 174(4):459-67. [QxMD MEDLINE Link].
- Nishihara R, Lochhead P, Kuchiba A, Jung S, Yamauchi M, Liao X, et al. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA. 2013 Jun 26. 309(24):2563-71. [QxMD MEDLINE Link].
- Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-Day, Low-Dose Aspirin and Cancer Risk: Long-Term Observational Follow-up of a Randomized Trial. Ann Intern Med. 2013 Jul 16. 159(2):77-85. [QxMD MEDLINE Link].
- Meyskens FL Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa). 2008 Jun. 1(1):32-8. [QxMD MEDLINE Link].
- [Guideline] American Cancer Society Guideline for Colorectal Cancer Screening. American Cancer Society. Available at https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html. May 30, 2018; Accessed: May 25, 2019.
- [Guideline] US Preventive Services Task Force., Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021 May 18. 325 (19):1965-1977. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Qaseem A, Crandall CJ, Mustafa RA, Hicks LA, Wilt TJ, Clinical Guidelines Committee of the American College of Physicians., et al. Screening for Colorectal Cancer in Asymptomatic Average-Risk Adults: A Guidance Statement From the American College of Physicians. Ann Intern Med. 2019 Nov 5. 171 (9):643-654. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colorectal Cancer Screening. NCCN. Available at https://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf. Version 3.2022 — September 30, 2022; Accessed: January 25, 2023.
- [Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High Risk Assessment: Colorectal. NCCN. Available at https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Version 2.2022 — December 7, 2022; Accessed: January 25, 2023.
- [Guideline] Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020 Oct. 31 (10):1291-1305. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Stjepanovic N, Moreira L, Carneiro F, Balaguer F, Cervantes A, Balmaña J, et al. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019 Oct 1. 30 (10):1558-1571. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Rubenstein JH, Enns R, Heidelbaugh J, Barkun A, Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Lynch Syndrome. Gastroenterology. 2015 Sep. 149 (3):777-82; quiz e16-7. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Balmaña J, Balaguer F, Cervantes A, Arnold D, ESMO Guidelines Working Group. Familial risk-colorectal cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2013 Oct. 24 Suppl 6:vi73-80. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MW, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015 Jan 10. 33 (2):209-17. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb. 110 (2):223-62; quiz 263. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Rutter MD, East J, Rees CJ, Cripps N, Docherty J, Dolwani S, et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 2020 Feb. 69 (2):201-223. [QxMD MEDLINE Link]. [Full Text].
- Church J, Simmang C, Standards Task Force, American Society of Colon and Rectal Surgeons, Collaborative Group of the Americas on Inherited Colorectal Cancer and the Standards Committee of The American Society of Colon and Rectal Surgeons. Practice parameters for the treatment of patients with dominantly inherited colorectal cancer (familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer). Dis Colon Rectum. 2003 Aug. 46 (8):1001-12. [QxMD MEDLINE Link].
- Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis Colon Rectum. 2022 Feb 1. 65 (2):148-177. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, et al. Practice Guideline for the Surveillance of Patients After Curative Treatment of Colon and Rectal Cancer. Dis Colon Rectum. 2015 Aug. 58 (8):713-25. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Kahi CJ, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colonoscopy Surveillance After Colorectal Cancer Resection: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2016 Mar. 150 (3):758-768.e11. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Allegra CJ, Rumble RB, Hamilton SR, Mangu PB, Roach N, Hantel A, et al. Extended RAS Gene Mutation Testing in Metastatic Colorectal Carcinoma to Predict Response to Anti-Epidermal Growth Factor Receptor Monoclonal Antibody Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J Clin Oncol. 2016 Jan 10. 34 (2):179-85. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016 Aug. 27 (8):1386-422. [QxMD MEDLINE Link]. [Full Text].
- Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004 Jan 15. 22(2):229-37. [QxMD MEDLINE Link].
- Arkenau HT, Arnold D, Cassidy J, Diaz-Rubio E, Douillard JY, Hochster H, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. J Clin Oncol. 2008 Dec 20. 26(36):5910-7. [QxMD MEDLINE Link].
- Sehgal R, Lembersky BC, Rajasenan KK, et al. A Phase I/II Study of Capecitabine Given on a Week on/Week off Schedule Combined With Bevacizumab and Oxaliplatin for Patients With Untreated Advanced Colorectal Cancer. Clin Colorectal Cancer. 2011 Jun. 10(2):117-20. [QxMD MEDLINE Link].
- Rozyltrek (entrectinib) [package insert]. South San Francisco, CA: Genentech USA, Inc. August, 2019. Available at [Full Text].
Author
Coauthor(s)
Vassiliki Liana Tsikitis, MD, MBA, MCR, FACS, FASCRS Professor of Surgery, Head, Division of Gastrointestinal and General Surgery, Vice Chair, Diversity Equity and Inclusion, Department of Surgery, Oregon Health and Science University School of Medicine
Vassiliki Liana Tsikitis, MD, MBA, MCR, FACS, FASCRS is a member of the following medical societies: Alpha Omega Alpha, American College of Surgeons, American Society of Colon and Rectal Surgeons, Association of Women Surgeons, European Society of Coloproctology, International Society of Surgery, Mayo Clinic Alumni Association, Pacific Coast Surgical Association, Society for Surgery of the Alimentary Tract, Society of University Surgeons, The Priestley Society, Western Surgical Association
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Chief Editor
N Joseph Espat, MD, MS, FACS Harold J Wanebo Professor of Surgery, Assistant Dean of Clinical Affairs, Boston University School of Medicine; Chairman, Department of Surgery, Director, Adele R Decof Cancer Center, Roger Williams Medical Center
N Joseph Espat, MD, MS, FACS is a member of the following medical societies: Alpha Omega Alpha, American Association for Cancer Research, American College of Surgeons, American Medical Association, American Society for Parenteral and Enteral Nutrition, American Society of Clinical Oncology, Americas Hepato-Pancreato-Biliary Association, Association for Academic Surgery, Central Surgical Association, Chicago Medical Society, International Hepato-Pancreato-Biliary Association, Pancreas Club, Sigma Xi, The Scientific Research Honor Society, Society for Leukocyte Biology, Society for Surgery of the Alimentary Tract, Society of American Gastrointestinal and Endoscopic Surgeons, Society of Surgical Oncology, Society of University Surgeons, Southeastern Surgical Congress, Southern Medical Association, Surgical Infection Society
Disclosure: Nothing to disclose.
Additional Contributors