Sjogren Syndrome: Practice Essentials, Etiology, Epidemiology (original) (raw)
Practice Essentials
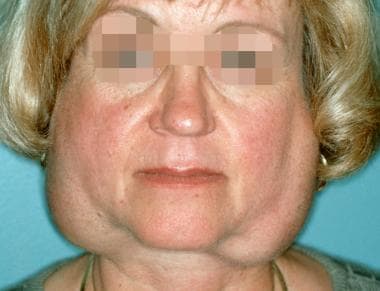
Sjögren syndrome is a systemic chronic inflammatory disorder characterized by lymphocytic infiltrates in exocrine organs. The disorder most often affects women, and the median age of onset is around 50 to 60 years. Most individuals with Sjögren syndrome present with sicca symptoms, such as xerophthalmia (dry eyes), xerostomia (dry mouth), and parotid gland enlargement, which is seen in the image below. [1] (See Presentation.)
Marked bilateral parotid gland enlargement in a patient with primary Sjögren syndrome. Sicca syndrome is a common clinical finding. (C) 1972-2004 American College of Rheumatology Clinical Slide Collection. Used with permission.
In addition, numerous extraglandular features may develop, such as the following:
- Arthralgia
- Arthritis
- Myalgia
- Pulmonary disease
- Gastrointestinal disease
- Leukopenia
- Anemia
- Lymphadenopathy
- Neuropathy
- Vasculitis
- Renal tubular acidosis
- Lymphoma
About 50% of patients with Sjögren syndrome have cutaneous findings, such as dry skin (xeroderma), palpable and nonpalpable purpura, and/or urticaria. [1] (See Etiology, Presentation, and Workup.)
Primary Sjögren syndrome occurs in the absence of another underlying rheumatic disorder, whereas secondary Sjögren syndrome is associated with another underlying rheumatic disease, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), or scleroderma. Given the overlap of Sjögren syndrome with many other rheumatic disorders, it is sometimes difficult to determine whether a clinical manifestation is solely a consequence of Sjögren syndrome or is due to one of its overlapping disorders.
Importantly, classic clinical features of Sjögren syndrome may also be seen in infections with certain viruses. These include hepatitis C virus, human immunodeficiency virus (HIV), and human T-cell lymphotrophic virus (HTLV). (See DDx.)
Most patients with primary Sjogren syndrome have two specific antibodies: against Ro (SS-A) and La (SSB) antigens. (See Workup.)
Treatment for Sjögren syndrome is largely based on symptoms (eg, lotion for dry skin, artificial tears for dry eyes). Rituximab has shown promise in the treatment of severe extraglandular manifestations (eg, vasculitis, cryoglobulinemia, peripheral neuropathy). Patients must be monitored carefully for the potential development of lymphoma, as the risk for this disease is significantly higher than in the general population. (See Treatment and Medication.)
For more information on other aspects of Sjögren syndrome, see Pediatric Sjögren Syndrome and Ophthalmologic Manifestations of Sjögren Syndrome.
Classification criteria
A number of classification criteria for Sjögren syndrome were designed primarily for clinical research studies but are also often used to help guide clinical diagnoses. The American-European Consensus Group’s criteria for the classification of Sjögren syndrome were proposed in 2002 and are the most commonly used criteria for the diagnosis of Sjögren syndrome. A new set of classification criteria was developed by the Sjögren’s International Collaborative Clinical Alliance (SICCA) investigators and accepted as a provisional criteria set by the American College of Rheumatology (ACR) in 2012.
In 2012, investigators from the SICCA team and the EULAR Sjögren’s Task Force formed the International Sjögren’s Syndrome Criteria Working Group. The Task Force's work was published in 2016 as ACR/European League Against Rheumatism (EULAR) classification criteria for primary Sjögren syndrome. [2]
American-European Consensus Group classification
The American-European Consensus Group (AECG) criteria for the classification of Sjögren syndrome are outlined below. [3, 4] These criteria allow a diagnosis of Sjögren syndrome in patients without sicca symptoms or who have not undergone a biopsy.
According to the American-European classification system (as modified by Tzioufas and Voulgarelis [5] ), diagnosis of primary Sjögren syndrome requires at least four of the criteria listed below; in addition, either criterion number 5 or criterion number 6 must be included. Sjögren syndrome can be diagnosed in patients who have no sicca symptoms if three of the four objective criteria are fulfilled. The criteria are as follows:
- Ocular symptoms - Dry eyes for more than 3 months, foreign-body sensation, use of tear substitutes more than 3 times daily
- Oral symptoms - Feeling of dry mouth, recurrently swollen salivary glands, frequent use of liquids to aid swallowing
- Ocular signs - Schirmer test performed without anesthesia (< 5 mm in 5 min), positive vital dye staining results
- Oral signs - Abnormal salivary scintigraphy findings, abnormal parotid sialography findings, abnormal sialometry findings (unstimulated salivary flow < 1.5 mL in 15 min)
- Positive minor salivary gland biopsy findings
- Positive anti–SSA or anti–SSB antibody results
Secondary Sjögren syndrome is diagnosed when, in the presence of a connective-tissue disease, symptoms of oral or ocular dryness exist in addition to criterion 3, 4, or 5, above.
Application of these criteria has yielded a sensitivity of 97.2% and a specificity of 48.6% for the diagnosis of primary Sjögren syndrome. For secondary Sjögren syndrome, the specificity is 97.2% and the sensitivity, 64.7%. [6]
Exclusion criteria include any of the following:
- Past head-and-neck irradiation
- Hepatitis C virus infection
- Acquired immunodeficiency syndrome (AIDS)
- Prior lymphoma
- Sarcoidosis
- Graft versus host disease
- Use of anticholinergic drugs
ACR/EULAR classification criteria for primary Sjogren syndrome
According to the ACR/EULAR classification criteria, individuals are classified as having primary Sjögren syndrome if they have a total score of 4 or higher, derived from the sum of the weights assigned to the following [2] :
- Focal lymphocytic sialadenitis and focus score of ≥1 foci/4 mm² in labial salivary gland biopsy samples – weight/score 3
- Anti-SSA/Ro positive – weight/score 3
- Ocular Staining Score ≥5 (or van Bijsterveld score ≥4) in at least one eye – weight/score 1
- Schirmer’s test ≤5 mm/5 min in at least one eye – weight/score 1
- Unstimulated whole saliva flow rate ≤0.1 mL/min – weight/score 1
For inclusion, patients must have at least one symptom of ocular or oral dryness, defined as a positive response to at least one of the following questions:
- Have you had daily, persistent, troublesome dry eyes for more than 3 months?
- Do you have a recurrent sensation of sand or gravel in the eyes?
- Do you use tear substitutes more than three times a day?
- Have you had a daily feeling or dry mouth for more than 3 months?
- Do you frequently drink liquids to aid in swallowing dry food?
Exclusion criteria include any of the following:
- History of head-and-neck radiation treatment
- Active hepatitis C infection (with confirmation by polymerase chain reaction [PCR]) testing
- AIDS
- Sarcoidosis
- Amyloidosis
- Graft versus host disease
- IgG4-related disease
Complications
Complications related to Sjögren syndrome include the following (see Prognosis, Treatment, and Medication):
- Emergence of disorders associated with Sjögren syndrome, such as SLE and RA
- Infection of the parotid gland, typically staphylococcal, streptococcal, or pneumococcal - clues include unilateral worsening of symptoms, along with tenderness, warmth, and erythema
- Emergence of parotid tumors - watch for unusually hard or unilateral parotid enlargement
- Pregnant patients with antiRo/SS-A antidodies are at risk for fetal loss, complete heart block in the fetus ,and neonatal lupus syndrome in the newborn
- Emergence of pseudolymphomas (pleomorphic cells that do not meet the criteria for malignancy) and non-Hodgkin B-cell lymphomas (see the image below) [5]
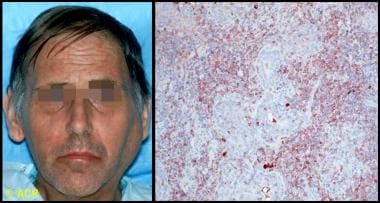
Clinical photograph and photomicrograph of a 48-year-old man with Sjögren syndrome with a large left parotid mass. On biopsy, B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) type was identified. Microscopic section of parotid biopsy, stained with immunoperoxidase for kappa light chains (brown-stained cells), showed monoclonal population of B cells, confirming the diagnosis. (C) 1972-2004 American College of Rheumatology Clinical Slide Collection. Used with permission.
Patient education
Educate patients with Sjögren syndrome on avoidance strategies and self-care issues for the treatment of dry mouth, eyes, skin, and vagina. Patient education pamphlets regarding the disease are available through the Arthritis Foundation. The Sjögren’s Syndrome Foundation, founded in 1983, is a good resource for patients.
For patient education information, see the Arthritis Center, as well as Sjögren’s Syndrome.

Etiology
The etiology of Sjögren syndrome is not well understood. The presence of activated salivary gland epithelial cells expressing major histocompatibility complex (MHC) class II molecules and the identification of inherited susceptibility markers suggest that environmental or endogenous antigens trigger a self-perpetuating inflammatory response in susceptible individuals. In addition, the continuing presence of active interferon pathways in Sjögren syndrome suggests ongoing activation of the innate immune system. [7, 8] Together, these findings suggest an ongoing interaction between the innate and acquired immune systems in Sjögren syndrome.
Association with the human leukocyte antigen
The frequency of HLA-DR52 in patients with primary Sjögren syndrome is estimated to be 87%, but it is also significantly increased in secondary Sjögren syndrome that occurs with rheumatoid arthritis or systemic lupus erythematosus.
The genetic associations in Sjögren syndrome vary among ethnic groups. In White persons, for instance, the condition is linked to human leukocyte antigen (HLA)–DR3, HLA-DQ2, and HLA-B8, [9] whereas the linkage is to HLA-DRB1*15 in Spanish persons [10] and to HLA-DR5 in Greek and Israeli persons. [11]
Some evidence indicates that the true association of Sjögren syndrome may be with HLA-DQA1, which is in linkage disequilibrium with HLA-DR3 and HLA-DR5. [12] These HLA associations appear restricted to individuals with Sjögren syndrome who have antibodies to the antigens SSA and SSB rather than to the disease manifestations themselves. [13]
Environmental triggers
Viruses are viable candidates as environmental triggers, although proof of causation has remained elusive, and certainly no single virus has been implicated. Epstein-Barr virus (EBV), HTLV-1, human herpesvirus 6 (HHV-6), HIV, hepatitis C virus (HCV), and cytomegalovirus (CMV) may have a role. Sjögrenlike syndromes are seen in patients infected with HIV, HTLV-1, and hepatitis C. [14, 15, 16] An increase of more than 50% in newly diagnosed primary Sjögren syndrome was reported in Brazil during 2020, with more cases reported during the months following the first wave of COVID-19 cases. [17]
Damage and/or cell death due to viral infection or other causes may provide triggering antigens to Toll-like receptors in or on dendritic or epithelial cells, which, by recognizing pathogen-associated patterns, are activated and begin producing cytokines, chemokines, and adhesion molecules. As T and B lymphocytes migrate into the gland, they themselves become activated by dendritic and epithelial cells, thereafter acting as antigen-presenting cells. [18]
Expressed antigens include SSA/Ro, SSB/La, alpha-fodrin and beta-fodrin, and cholinergic muscarinic receptors. [13] Dendritic cell triggering by immune complexes formed from SSA ̶ anti-SSA (or other immune complexes) may propagate the ongoing innate and acquired immune activation.
A population-based cohort study of Taiwanese residents found that exposure to carbon monoxide (CO), nitric oxide (NO), and methane (CH4) was associated with a higher risk of developing primary Sjögren syndrome. [19]

Epidemiology
In the United States, Sjögren syndrome is estimated to be the second most common rheumatologic disorder, behind SLE. Sjögren syndrome affects 0.1-4% of the population. This wide range, in part, reflects the lack of uniform diagnostic criteria. [20] Internationally, comparative studies between different ethnic groups have suggested that Sjögren syndrome is a homogeneous disease that occurs worldwide with similar prevalence and affects 1-2 million people.
The female-to-male ratio of Sjögren syndrome is 9:1. Sjögren syndrome can affect individuals of any age but is most common in elderly people. Onset typically occurs in the fourth to fifth decade of life.
The systemic phenotype of primary Sjögren syndrome is strongly influenced by personal factors (eg, age, gender, ethnicity, place of residence, according to an analysis by the Sjögren Big Data Consortium, a five-continent multicenter registry, of a cohort that included 10,007 patients (9352 female, mean age 53 years) with recorded European League Against Rheumatism's Sjögren syndrome disease activity index (ESSDAI) scores available. [21] Findings (all P < 0.001) were as follows:
- Males had a higher mean ESSDAI than females (8.1 vs 6.0, respectively).
- Patients diagnosed at < 35 years had a higher mean ESSDAI than those diagnosed at > 65 years (6.7 vs 5.6).
- By ethnicity, the highest global ESSDAI scores were reported in blacks/African Americans (6.7), followed by whites (6.5), Asians (5.4), and Hispanics (4.8).
- Black/African-American patients showed the highest frequencies in the lymphadenopathy, articular, peripheral and central nervous system, and biological domains.
- White patients showed the highest frequencies in the glandular, cutaneous, and muscular domains.
- Asian patients showed the highest frequencies in the pulmonary, renal, and hematological domains.
- Hispanic patients showed the highest frequenies in the constitutional domain.
- Systemic activity and disease activity was higher in patients from southern countries.

Prognosis
Sjögren syndrome carries a generally good prognosis. In patients who develop a disorder associated with Sjögren syndrome, the prognosis is more closely related to the associated disorder (eg, SLE, lymphoma). Interestingly, primary Sjögren syndrome is associated with lower cardiovascular risk factors and lower risk of serious cardiovascular events such as myocardial infarction and stroke, in comparison with SLE. [22]
Although salivary and lacrimal function generally stabilize, the presence of SSA and/or hypocomplementemia may predict a decline in function. [23]
Morbidity and mortality
Morbidity associated with Sjögren syndrome is mainly associated with the gradually decreased function of exocrine organs, which become infiltrated with lymphocytes. The increased mortality rate associated with the condition is primarily related to disorders commonly associated with Sjögren syndrome, such as SLE, RA, and primary biliary cirrhosis. Patients with primary Sjögren syndrome who do not develop a lymphoproliferative disorder have a normal life expectancy. [24]
Lymphoma
Among patients with Sjögren syndrome, the incidence of non-Hodgkin lymphoma is 4.3% (18.9 times higher than in the general population), with a median age at diagnosis of 58 years. The mean time to the development of non-Hodgkin lymphoma after the onset of Sjögren syndrome is 7.5 years.
The most common histologic subtype of non-Hodgkin lymphoma in Sjögren syndrome is mucosa-associated lymphoid tissue (MALT) lymphoma, which can develop in any nonlymphoid tissue infiltrated by periepithelial lymphoid tissue—most commonly the salivary glands, but also the stomach, nasopharynx, skin, liver, kidneys, and lungs. The progression of these infiltrates to lymphoma occurs slowly and in a stepwise fashion. Lymphoma is present at more than 1 site in 20% of patients at initial diagnosis.
The results of one study suggest that diagnostic labial salivary gland tissue biopsy can be used to detect germinal center ̶ like lesions, which can be a highly predictive and easily obtained marker for non-Hodgkin lymphoma in primary Sjögren syndrome patients. [25]
Risk factors for lymphoma include the following:
- Salivary gland enlargement
- Regional or generalized lymphadenopathy
- Hepatosplenomegaly
- Palpable purpura
- Leukopenia
- Renal insufficiency
- Loss of a previously positive polyclonal gammopathy
- Development of a monoclonal gammopathy or a monoclonal cryoglobulinemia
- RF positivity
- Anti-SSA/SSB positivity
- Hypocomplementemia
Demographic risk factors for the development of lymphoma include male gender and older age. [26]
Pregnancy complications
Women with Sjögren syndrome are at higher risk for experiencing complications during pregnancy. Worsening of pulmonary hypertension and increased rates of spontaneous abortion and preterm deliveries have been reported. [27]
Children born to mothers with antibodies against SSA/Ro and SSB/La are at an increased risk of neonatal lupus and congenital heart block. [26] If one such child develops congenital heart block, the risk for congenital heart block during a subsequent pregnancy is 15%.
Antiphospholipid syndrome
Patients with Sjögren syndrome who have antiphospholipid antibodies can develop the clinical features of antiphospholipid syndrome, which include increased fetal wastage and vascular thromboses.

Pathophysiology
Sjögren syndrome can occur as a primary disease of exocrine gland dysfunction or in association with several other autoimmune diseases (eg, systemic lupus erythematosus [SLE], rheumatoid arthritis, scleroderma, systemic sclerosis, cryoglobulinemia, polyarteritis nodosa). These primary and secondary types occur with similar frequency, but the sicca complex seems to cause more severe symptoms in the primary form.
Virtually all organs may be involved. The disease commonly affects the eyes, mouth, parotid gland, lungs, kidneys, skin, and nervous system.
Glandular pathology
The pathology of a typical involved salivary or lacrimal gland in Sjögren syndrome reveals aggregations of lymphocytes—periductal at first, then panlobular. These cells are primarily CD4 T cells (75%) and memory cells, with 10% B cells and immunoglobulin-secreting plasma cells. Although individual lobules can be destroyed, salivary gland biopsy samples from patients with Sjögren syndrome typically retain 40%-50% of their viable glandular structure. Therefore, inflammatory destruction of salivary and lacrimal glands may not fully account for the symptoms of Sjögren syndrome. [28]
Studies suggest that the disease process of Sjögren syndrome has a neuroendocrine component. Proinflammatory cytokines released by epithelial cells and lymphocytes may impair neural release of acetylcholine. In addition, antibodies to acetylcholine (muscarinic) receptors may interfere with the neural stimulation of local glandular secretion, [29] perhaps by interfering with aquaporin. [30] Moreover, a study reports that M3 muscarinic receptor antibodies may cause autonomic dysfunction in patients with Sjögren syndrome. [31, 32]
Current studies have also focused on the role of apoptotic mechanisms in the pathogenesis of primary Sjögren syndrome. A defect in Fas-mediated apoptosis, which is necessary for down-regulation of the immune response, can result in a chronic inflammatory destruction of the salivary gland, resembling Sjögren syndrome. [33]
Owing to these structural and functional changes in the lacrimal and salivary glands, their aqueous output is diminished. In the eye, tear hyperosmolarity results and is itself a proinflammatory stimulus, resulting in an inflammatory cascade on the ocular surface, [34] with evidence of immune activation of the conjunctival epithelium and local cytokine and metalloproteinase production. Damage to the corneal epithelium, already vulnerable due to inadequate tear film protection, ensues, with resultant epithelial erosions and surface irregularity.
Extraglandular involvement
Extraglandular involvement in Sjögren syndrome manifests in part as hypergammaglobulinemia and the production of multiple autoantibodies, especially ANA and RF. This may be due to polyclonal B-cell activation, but the cause of this expanded activation is not known.
Involvement of other organs and tissues may result from effects of these antibodies, immune complexes, or lymphocytic infiltration and occurs in one third of patients with Sjögren syndrome. Prolonged hyperstimulation of B cells may lead to disturbances in their differentiation and maturation and may account for the greatly increased incidence of lymphoma in these patients. [35]
Sex hormones
The fact that primary Sjögren syndrome occurs predominantly in women suggests that sex hormones may influence the immunologic manifestations of the disease. The prevalence of serologic markers tends to be lower in male patients than in female patients. Although the role of sex hormones (eg, estrogens, androgens) in the pathogenesis of primary Sjögren syndrome remains unknown, adrenal and gonadal steroid hormone deficiency probably affects immune function.

- Kittridge A, Routhouska SB, Korman NJ. Dermatologic manifestations of Sjögren syndrome. J Cutan Med Surg. 2011 Jan-Feb. 15(1):8-14. [QxMD MEDLINE Link].
- Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017 Jan. 76 (1):9-16. [QxMD MEDLINE Link]. [Full Text].
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002 Jun. 61(6):554-8. [QxMD MEDLINE Link].
- Langegger C, Wenger M, Duftner C, Dejaco C, Baldissera I, Moncayo R, et al. Use of the European preliminary criteria, the Breiman-classification tree and the American-European criteria for diagnosis of primary Sjögren's Syndrome in daily practice: a retrospective analysis. Rheumatol Int. 2007 Jun. 27(8):699-702. [QxMD MEDLINE Link].
- Tzioufas AG, Voulgarelis M. Update on Sjögren's syndrome autoimmune epithelitis: from classification to increased neoplasias. Best Pract Res Clin Rheumatol. 2007 Dec. 21(6):989-1010. [QxMD MEDLINE Link].
- Gálvez J, Sáiz E, López P, Pina MA, Carrillo A, Nieto A, et al. Diagnostic evaluation and classification criteria in Sjögren's Syndrome. Joint Bone Spine. 2008 Sep 29. [QxMD MEDLINE Link].
- Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions o...
- Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren's syndrome. Proc Natl Acad Sci U S A. 2006 Feb 21. 103(8):2770-5. [QxMD MEDLINE Link].
- Price EJ, Venables PJ. The etiopathogenesis of Sjögren's syndrome. Semin Arthritis Rheum. 1995 Oct. 25(2):117-33. [QxMD MEDLINE Link].
- Mattey DL, González-Gay MA, Hajeer AH, Dababneh A, Thomson W, García-Porrúa C, et al. Association between HLA-DRB1*15 and secondary Sjögren's syndrome in patients with rheumatoid arthritis. J Rheumatol. 2000 Nov. 27(11):2611-6. [QxMD MEDLINE Link].
- Papasteriades CA, Skopouli FN, Drosos AA, Andonopoulos AP, Moutsopoulos HM. HLA-alloantigen associations in Greek patients with Sjögren's syndrome. J Autoimmun. 1988 Feb. 1(1):85-90. [QxMD MEDLINE Link].
- Reveille JD, Macleod MJ, Whittington K, Arnett FC. Specific amino acid residues in the second hypervariable region of HLA-DQA1 and DQB1 chain genes promote the Ro (SS-A)/La (SS-B) autoantibody responses. J Immunol. 1991 Jun 1. 146(11):3871-6. [QxMD MEDLINE Link].
- Gottenberg JE, Busson M, Loiseau P, Cohen-Solal J, Lepage V, Charron D, et al. In primary Sjögren's syndrome, HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis Rheum. 2003 Aug. 48(8):2240-5. [QxMD MEDLINE Link].
- Haddad J, Deny P, Munz-Gotheil C, Ambrosini JC, Trinchet JC, Pateron D, et al. Lymphocytic sialadenitis of Sjögren's syndrome associated with chronic hepatitis C virus liver disease. Lancet. 1992 Feb 8. 339(8789):321-3. [QxMD MEDLINE Link].
- Itescu S, Winchester R. Diffuse infiltrative lymphocytosis syndrome: a disorder occurring in human immunodeficiency virus-1 infection that may present as a sicca syndrome. Rheum Dis Clin North Am. 1992 Aug. 18(3):683-97. [QxMD MEDLINE Link].
- Fox RI. Epidemiology, pathogenesis, animal models, and treatment of Sjögren's syndrome. Curr Opin Rheumatol. 1994 Sep. 6(5):501-8. [QxMD MEDLINE Link].
- Martelli Júnior H, Gueiros LA, de Lucena EG, Coletta RD. Increase in the number of Sjögren's syndrome cases in Brazil in the COVID-19 Era. Oral Dis. 2022 Nov. 28 Suppl 2:2588-2590. [QxMD MEDLINE Link]. [Full Text].
- Fox RI. Sjögren's syndrome. Lancet. 2005 Jul 23-29. 366(9482):321-31. [QxMD MEDLINE Link].
- Ma KS, Wang LT, Chong W, Lin CL, Li H, Chen A, et al. Exposure to environmental air pollutants as a risk factor for primary Sjögren's syndrome. Front Immunol. 2022. 13:1044462. [QxMD MEDLINE Link]. [Full Text].
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008 Jan. 58(1):15-25. [QxMD MEDLINE Link].
- Brito-Zerón P, et al; Sjögren Big Data Consortium. Epidemiological profile and north-south gradient driving baseline systemic involvement of primary Sjögren's syndrome. Rheumatology (Oxford). 2019 Dec 24. [QxMD MEDLINE Link].
- Rúa-Figueroa I, Fernández Castro M, Andreu JL, et al; Sjogrenser and Relesser Researchers and EAS-SER Group. Comorbidities in Patients With Primary Sjögren's Syndrome and Systemic Lupus Erythematosus: A Comparative Registries-Based Study. Arthritis Care Res (Hoboken). 2017 Jan. 69 (1):38-45. [QxMD MEDLINE Link]. [Full Text].
- Haldorsen K, Moen K, Jacobsen H, Jonsson R, Brun JG. Exocrine function in primary Sjögren syndrome: natural course and prognostic factors. Ann Rheum Dis. 2008 Jul. 67(7):949-54. [QxMD MEDLINE Link].
- Belenguer R, Ramos-Casals M, Brito-Zerón P, et al. Influence of clinical and immunological parameters on the health-related quality of life of patients with primary Sjögren's syndrome. Clin Exp Rheumatol. 2005 May-Jun. 23(3):351-6. [QxMD MEDLINE Link].
- Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome. Ann Rheum Dis. 2011 Aug. 70(8):1363-8. [QxMD MEDLINE Link]. [Full Text].
- Vitali C, Minniti A, Pignataro F, Maglione W, Del Papa N. Management of Sjögren's Syndrome: Present Issues and Future Perspectives. Front Med (Lausanne). 2021. 8:676885. [QxMD MEDLINE Link]. [Full Text].
- Gupta S, Gupta N. Sjögren Syndrome and Pregnancy: A Literature Review. Perm J. 2017. 21:[QxMD MEDLINE Link]. [Full Text].
- Parkin B, Chew JB, White VA, Garcia-Briones G, Chhanabhai M, Rootman J. Lymphocytic infiltration and enlargement of the lacrimal glands: a new subtype of primary Sjögren's syndrome?. Ophthalmology. 2005 Nov. 112(11):2040-7. [QxMD MEDLINE Link].
- Bacman S, Perez Leiros C, Sterin-Borda L, Hubscher O, Arana R, Borda E. Autoantibodies against lacrimal gland M3 muscarinic acetylcholine receptors in patients with primary Sjögren's syndrome. Invest Ophthalmol Vis Sci. 1998 Jan. 39(1):151-6. [QxMD MEDLINE Link].
- Steinfeld S, Cogan E, King LS, Agre P, Kiss R, Delporte C. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjögren's syndrome patients. Lab Invest. 2001 Feb. 81(2):143-8. [QxMD MEDLINE Link].
- Waterman SA, Gordon TP, Rischmueller M. Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjögren's syndrome. Arthritis Rheum. 2000 Jul. 43(7):1647-54. [QxMD MEDLINE Link].
- Dawson LJ, Stanbury J, Venn N, Hasdimir B, Rogers SN, Smith PM. Antimuscarinic antibodies in primary Sjögren's syndrome reversibly inhibit the mechanism of fluid secretion by human submandibular salivary acinar cells. Arthritis Rheum. 2006 Apr. 54(4):1165-73. [QxMD MEDLINE Link].
- Bolstad AI, Eiken HG, Rosenlund B, Alarcón-Riquelme ME, Jonsson R. Increased salivary gland tissue expression of Fas, Fas ligand, cytotoxic T lymphocyte-associated antigen 4, and programmed cell death 1 in primary Sjögren's syndrome. Arthritis Rheum. 2003 Jan. 48(1):174-85. [QxMD MEDLINE Link].
- Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004 Feb. 137(2):337-42. [QxMD MEDLINE Link].
- Ng KP, Isenberg DA. Sjögren's syndrome: diagnosis and therapeutic challenges in the elderly. Drugs Aging. 2008. 25(1):19-33. [QxMD MEDLINE Link].
- Schein OD, Hochberg MC, Munoz B, et al. Dry eye and dry mouth in the elderly: a population-based assessment. Arch Intern Med. 1999 Jun 28. 159(12):1359-63. [QxMD MEDLINE Link].
- Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001 Nov 7. 286(17):2114-9. [QxMD MEDLINE Link].
- Soy M, Piskin S. Cutaneous findings in patients with primary Sjogren's syndrome. Clin Rheumatol. 2007 Aug. 26(8):1350-2. [QxMD MEDLINE Link].
- Fox RI, Liu AY. Sjögren's syndrome in dermatology. Clin Dermatol. 2006 Sep-Oct. 24(5):393-413. [QxMD MEDLINE Link].
- Constantopoulos SH, Tsianos EV, Moutsopoulos HM. Pulmonary and gastrointestinal manifestations of Sjögren's syndrome. Rheum Dis Clin North Am. 1992 Aug. 18(3):617-35. [QxMD MEDLINE Link].
- Parambil JG, Myers JL, Lindell RM, Matteson EL, Ryu JH. Interstitial lung disease in primary Sjögren syndrome. Chest. 2006 Nov. 130(5):1489-95. [QxMD MEDLINE Link].
- Wotherspoon AC. Gastric MALT lymphoma and Helicobacter pylori. Yale J Biol Med. 1996 Jan-Feb. 69(1):61-8. [QxMD MEDLINE Link].
- Hammar O, Ohlsson B, Wollmer P, Mandl T. Impaired gastric emptying in primary Sjogren's syndrome. J Rheumatol. 2010 Nov. 37(11):2313-8. [QxMD MEDLINE Link].
- Launay D, Hachulla E, Hatron PY, Jais X, Simonneau G, Humbert M. Pulmonary arterial hypertension: a rare complication of primary Sjögren syndrome: report of 9 new cases and review of the literature. Medicine (Baltimore). 2007 Sep. 86(5):299-315. [QxMD MEDLINE Link].
- Cai FZ, Lester S, Lu T, Keen H, Boundy K, Proudman SM, et al. Mild autonomic dysfunction in primary Sjögren's syndrome: a controlled study. Arthritis Res Ther. 2008. 10(2):R31. [QxMD MEDLINE Link].
- Delalande S, de Seze J, Fauchais AL, Hachulla E, Stojkovic T, Ferriby D, et al. Neurologic manifestations in primary Sjögren syndrome: a study of 82 patients. Medicine (Baltimore). 2004 Sep. 83(5):280-91. [QxMD MEDLINE Link].
- Mori K, Iijima M, Koike H, Hattori N, Tanaka F, Watanabe H, et al. The wide spectrum of clinical manifestations in Sjögren's syndrome-associated neuropathy. Brain. 2005 Nov. 128:2518-34. [QxMD MEDLINE Link].
- Gøransson LG, Herigstad A, Tjensvoll AB, Harboe E, Mellgren SI, Omdal R. Peripheral neuropathy in primary sjogren syndrome: a population-based study. Arch Neurol. 2006 Nov. 63(11):1612-5. [QxMD MEDLINE Link].
- Ramachandiran N. Apparently persistent weakness after recurrent hypokalemic paralysis: a tale of two disorders. South Med J. 2008 Sep. 101(9):940-2. [QxMD MEDLINE Link].
- van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nat Clin Pract Urol. 2007 Sep. 4(9):484-91. [QxMD MEDLINE Link].
- Leppilahti M, Tammela TL, Huhtala H, Kiilholma P, Leppilahti K, Auvinen A. Interstitial cystitis-like urinary symptoms among patients with Sjögren's syndrome: a population-based study in Finland. Am J Med. 2003 Jul. 115(1):62-5. [QxMD MEDLINE Link].
- Choi BY, Oh HJ, Lee YJ, Song YW. Prevalence and clinical impact of fibromyalgia in patients with primary Sjögren's syndrome. Clin Exp Rheumatol. 2016 Mar-Apr. 34 (2 Suppl 96):S9-13. [QxMD MEDLINE Link].
- Prajs K, Bobrowska-Snarska D, Skala M, Brzosko M. Polyarteritis nodosa and Sjögren's syndrome: overlap syndrome. Rheumatol Int. 2010 May 18. [QxMD MEDLINE Link].
- Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008 Sep. 146(3):350-356. [QxMD MEDLINE Link].
- Versura P, Frigato M, Cellini M, Mulè R, Malavolta N, Campos EC. Diagnostic performance of tear function tests in Sjogren's syndrome patients. Eye. 2007 Feb. 21(2):229-37. [QxMD MEDLINE Link].
- Antoniazzi RP, Miranda LA, Zanatta FB, et al. Periodontal conditions of individuals with Sjögren's syndrome. J Periodontol. 2009 Mar. 80(3):429-35. [QxMD MEDLINE Link].
- Riccieri V, Sciarra I, Ceccarelli F, et al. Nailfold capillaroscopy abnormalities are associated with the presence of anti-endothelial cell antibodies in Sjogren's syndrome. Rheumatology (Oxford). 2009 Jun. 48(6):704-6. [QxMD MEDLINE Link].
- Owczarek W, Kozera-Zywczyk A, Paluchowska E, Majewski S, Patera J. [Annular erythema as the skin manifestation of primary Sjögren's syndrome--case report]. Pol Merkur Lekarski. 2010 Feb. 28(164):126-9. [QxMD MEDLINE Link].
- Bahous I. [Clinical experiences with tolectin in rheumatic diseases (author's transl)]. Schweiz Rundsch Med Prax. 1976 Aug 17. 65(33):1025-7. [QxMD MEDLINE Link].
- Crestani B, Schneider S, Adle-Biassette H, Debray MP, Bonay M, Aubier M. [Respiratory manifestations during the course of Sjogren's syndrome]. Rev Mal Respir. 2007 Apr. 24(4 Pt 1):535-51. [QxMD MEDLINE Link].
- Ren H, Wang WM, Chen XN, et al. Renal involvement and followup of 130 patients with primary Sjögren's syndrome. J Rheumatol. 2008 Feb. 35(2):278-84. [QxMD MEDLINE Link].
- Lopate G, Pestronk A, Al-Lozi M, et al. Peripheral neuropathy in an outpatient cohort of patients with Sjögren's syndrome. Muscle Nerve. 2006 May. 33(5):672-6. [QxMD MEDLINE Link].
- Invernizzi P, Battezzati PM, Crosignani A, Zermiani P, Bignotto M, Del Papa N, et al. Antibody to carbonic anhydrase II is present in primary biliary cirrhosis (PBC) irrespective of antimitochondrial antibody status. Clin Exp Immunol. 1998 Dec. 114(3):448-54. [QxMD MEDLINE Link].
- D'Arbonneau F, Ansart S, Le Berre R, Dueymes M, Youinou P, Pennec YL. Thyroid dysfunction in primary Sjögren's syndrome: a long-term followup study. Arthritis Rheum. 2003 Dec 15. 49(6):804-9. [QxMD MEDLINE Link].
- Tuchocka-Piotrowska A, Puszczewicz M, Kolczewska A, Majewski D. [Graft-versus-host disease as the cause of symptoms mimicking Sjögren's syndrome]. Ann Acad Med Stetin. 2006. 52 Suppl 2:89-93. [QxMD MEDLINE Link].
- Roberts C, Parker GJ, Rose CJ, et al. Glandular function in Sjögren syndrome: assessment with dynamic contrast-enhanced MR imaging and tracer kinetic modeling--initial experience. Radiology. 2008 Mar. 246(3):845-53. [QxMD MEDLINE Link].
- García-Carrasco M, Ramos-Casals M, Rosas J, Pallarés L, Calvo-Alen J, Cervera R, et al. Primary Sjögren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine (Baltimore). 2002 Jul. 81(4):270-80. [QxMD MEDLINE Link].
- Ramos-Casals M, Nardi N, Brito-Zerón P, et al. Atypical autoantibodies in patients with primary Sjögren syndrome: clinical characteristics and follow-up of 82 cases. Semin Arthritis Rheum. 2006 Apr. 35(5):312-21. [QxMD MEDLINE Link].
- Masaki Y, Dong L, Kurose N, Kitagawa K, Morikawa Y, Yamamoto M, et al. Proposal for a new clinical entity, IgG4-positive multi-organ lymphoproliferative syndrome: Analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2008 Aug 13. [QxMD MEDLINE Link].
- Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001 Mar 8. 344(10):732-8. [QxMD MEDLINE Link].
- Nocturne G, Virone A, Ng WF, Le Guern V, Hachulla E, Cornec D, et al. Rheumatoid Factor and Disease Activity Are Independent Predictors of Lymphoma in Primary Sjögren's Syndrome. Arthritis Rheumatol. 2016 Apr. 68 (4):977-85. [QxMD MEDLINE Link]. [Full Text].
- Tomosugi N, Kitagawa K, Takahashi N, Sugai S, Ishikawa I. Diagnostic potential of tear proteomic patterns in Sjögren's syndrome. J Proteome Res. 2005 May-Jun. 4(3):820-5. [QxMD MEDLINE Link].
- Montaño-Loza AJ, Crispín-Acuña JC, Remes-Troche JM, Uribe M. Abnormal hepatic biochemistries and clinical liver disease in patients with primary Sjögren's syndrome. Ann Hepatol. 2007 Jul-Sep. 6(3):150-5. [QxMD MEDLINE Link].
- Daniels TE. Labial salivary gland biopsy in Sjögren's syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984 Feb. 27(2):147-56. [QxMD MEDLINE Link].
- Langerman AJ, Blair EA, Sweiss NJ, Taxy JB. Utility of lip biopsy in the diagnosis and treatment of Sjogren's syndrome. Laryngoscope. 2007 Jun. 117(6):1004-8. [QxMD MEDLINE Link].
- Giuca MR, Bonfigli D, Bartoli F, Pasini M. Sjögren's syndrome: correlation between histopathologic result and clinical and serologic parameters. Minerva Stomatol. 2010 Apr. 59(4):149-54, 154-7. [QxMD MEDLINE Link].
- Ramos-Casals M, Font J. Primary Sjögren's syndrome: current and emergent aetiopathogenic concepts. Rheumatology (Oxford). 2005 Nov. 44(11):1354-67. [QxMD MEDLINE Link].
- Saraux A, Pers JO, Devauchelle-Pensec V. Treatment of primary Sjögren syndrome. Nat Rev Rheumatol. 2016 Aug. 12 (8):456-71. [QxMD MEDLINE Link].
- Willeke P, Schlüter B, Becker H, Schotte H, Domschke W, Gaubitz M. Mycophenolate sodium treatment in patients with primary Sjögren syndrome: a pilot trial. Arthritis Res Ther. 2007. 9(6):R115. [QxMD MEDLINE Link].
- Dass S, Bowman SJ, Vital EM, et al. Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis. 2008 Nov. 67(11):1541-4. [QxMD MEDLINE Link].
- Pijpe J, van Imhoff GW, Spijkervet FK, et al. Rituximab treatment in patients with primary Sjögren's syndrome: an open-label phase II study. Arthritis Rheum. 2005 Sep. 52(9):2740-50. [QxMD MEDLINE Link].
- Meijer JM, Pijpe J, Vissink A, Kallenberg CG, Bootsma H. Treatment of primary Sjogren syndrome with rituximab: extended follow-up, safety and efficacy of retreatment. Ann Rheum Dis. 2009 Feb. 68(2):284-5. [QxMD MEDLINE Link].
- Meijer JM, Meiners PM, Vissink A, Spijkervet FK, Abdulahad W, Kamminga N, et al. Effectiveness of rituximab treatment in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010 Apr. 62(4):960-8. [QxMD MEDLINE Link].
- St Clair EW, Levesque MC, Luning Prak ET, Vivino FB, Alappatt CJ, Spychala ME, et al. Rituximab therapy for primary Sjögren's syndrome: An open-label clinical trial and mechanistic analysis. Arthritis Rheum. 2013 Jan 17. [QxMD MEDLINE Link].
- Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, Berthelot JM, Perdriger A, Puéchal X, et al. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med. 2014 Feb 18. 160 (4):233-42. [QxMD MEDLINE Link].
- Thanou-Stavraki A, James JA. Primary Sjogren's syndrome: current and prospective therapies. Semin Arthritis Rheum. 2008 Apr. 37(5):273-92. [QxMD MEDLINE Link].
- Moutsopoulos NM, Katsifis GE, Angelov N, Leakan RA, Sankar V, Pillemer S, et al. Lack of efficacy of etanercept in Sjögren syndrome correlates with failed suppression of tumour necrosis factor alpha and systemic immune activation. Ann Rheum Dis. 2008 Oct. 67(10):1437-43. [QxMD MEDLINE Link].
- Mekinian A, Ravaud P, Hatron PY, Larroche C, Leone J, Gombert B, et al. Efficacy of rituximab in primary Sjogren's syndrome with peripheral nervous system involvement: results from the AIR registry. Ann Rheum Dis. 2012 Jan. 71(1):84-7. [QxMD MEDLINE Link].
- Gottenberg JE, Cinquetti G, Larroche C, Combe B, Hachulla E, Meyer O, et al. Efficacy of rituximab in systemic manifestations of primary Sjogren's syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann Rheum Dis. 2012 Dec 21. [QxMD MEDLINE Link].
- Gottenberg JE, Guillevin L, Lambotte O, Combe B, Allanore Y, Cantagrel A, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis. 2005 Jun. 64(6):913-20. [QxMD MEDLINE Link]. [Full Text].
- Brah S, Chiche L, Fanciullino R, Bornet C, Mancini J, Schleinitz N, et al. Efficacy of rituximab in immune thrombocytopenic purpura: a retrospective survey. Ann Hematol. 2012 Feb. 91(2):279-85. [QxMD MEDLINE Link].
- Greb E. Leflunomide-hydroxychloroquine combination for Sjögren’s shows potential. MD Edge Rheumatology. Available at https://www.mdedge.com/rheumatology/article/219916/lupus-connective-tissue-diseases/leflunomide-hydroxychloroquine. March 31, 2020; Accessed: March 19, 2023.
- van der Heijden EHM, Blokland SLM, Hillen MR, et al. Leflunomide–hydroxychloroquine combination therapy in patients with primary Sjögren's syndrome (RepurpSS-I): a placebo-controlled, double-blinded, randomised clinical trial. Lancet Rheumatol. March 26, 2020. [Full Text].
- Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr. 5(2):163-78. [QxMD MEDLINE Link].
- Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006 Sep. 25(8):900-7. [QxMD MEDLINE Link].
- Spiteri A, Mitra M, Menon G, et al. Tear lipid layer thickness and ocular comfort with a novel device in dry eye patients with and without Sjögren's syndrome. J Fr Ophtalmol. 2007 Apr. 30(4):357-64. [QxMD MEDLINE Link].
- Egrilmez S, Aslan F, Karabulut G, Kabasakal Y, Yagci A. Clinical efficacy of the smartplug in the treatment of primary Sjogren's syndrome with keratoconjunctivitis SICCA: one-year follow-up study. Rheumatol Int. 2010 May 21. [QxMD MEDLINE Link].
- [Guideline] Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Lin A, Rhee MK, et al. Dry Eye Syndrome Preferred Practice Pattern®. Ophthalmology. 2019 Jan. 126 (1):P286-P334. [QxMD MEDLINE Link]. [Full Text].
- Akpek EK, Lindsley KB, Adyanthaya RS, Swamy R, Baer AN, McDonnell PJ. Treatment of Sjögren's syndrome-associated dry eye an evidence-based review. Ophthalmology. 2011 Jul. 118(7):1242-52. [QxMD MEDLINE Link].
- [Guideline] Foulks GN, Forstot SL, Donshik PC, Forstot JZ, Goldstein MH, Lemp MA, et al. Clinical guidelines for management of dry eye associated with Sjögren disease. Ocul Surf. 2015 Apr. 13 (2):118-32. [QxMD MEDLINE Link].
- Jain AK, Sukhija J, Dwedi S, Sood A. Effect of topical cyclosporine on tear functions in tear-deficient dry eyes. Ann Ophthalmol (Skokie). 2007 Spring. 39(1):19-25. [QxMD MEDLINE Link].
- Daniels TE, Fox PC. Salivary and oral components of Sjögren's syndrome. Rheum Dis Clin North Am. 1992 Aug. 18(3):571-89. [QxMD MEDLINE Link].
- [Guideline] Ramos-Casals M, Brito-Zerón P, Bombardieri S, et al, EULAR-Sjögren Syndrome Task Force Group. EULAR recommendations for the management of Sjögren's syndrome with topical and systemic therapies. Ann Rheum Dis. 2020 Jan. 79 (1):3-18. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Vivino FB, Carsons SE, Foulks G, Daniels TE, Parke A, Brennan MT, et al. New Treatment Guidelines for Sjögren's Disease. Rheum Dis Clin North Am. 2016 Aug. 42 (3):531-51. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Lee AS, Scofield RH, Hammitt KM, Gupta N, Thomas DE, Moua T, et al. Consensus Guidelines for Evaluation and Management of Pulmonary Disease in Sjögren's. Chest. 2021 Feb. 159 (2):683-698. [QxMD MEDLINE Link]. [Full Text].
Author
Sriya K Ranatunga, MD, MPH Associate Professor of Clinical Rheumatology, Chief, Division of Rheumatology, Department of Internal Medicine, Southern Illinois University School of Medicine
Sriya K Ranatunga, MD, MPH is a member of the following medical societies: American College of Physicians, American College of Rheumatology, Association of American Medical Colleges
Disclosure: Nothing to disclose.
Chief Editor
Herbert S Diamond, MD Visiting Professor of Medicine, Division of Rheumatology, State University of New York Downstate Medical Center; Chairman Emeritus, Department of Internal Medicine, Western Pennsylvania Hospital
Herbert S Diamond, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Physicians, American College of Rheumatology, American Medical Association, Phi Beta Kappa
Disclosure: Nothing to disclose.
Additional Contributors
Mark L Francis, MD Professor of Medical Education, Department of Medical Education, Paul L Foster School of Medicine, Texas Tech University Health Sciences Center
Mark L Francis, MD is a member of the following medical societies: American College of Physicians, Phi Beta Kappa
Disclosure: Nothing to disclose.
Anna Tumyan, MD Assistant Professor of Internal Medicine, Division of Rheumatology, Southern Illinois University School of Medicine
Disclosure: Nothing to disclose.
Acknowledgements
Terry L Barrett, MD Clinical Professor of Dermatology and Pathology, University of Texas Southwestern School of Medicine; Director, ProPath Dermatopathology, Dallas, Texas
Terry L Barrett, MD is a member of the following medical societies: American Academy of Dermatology, American Dermatological Association, American Medical Association, American Society of Dermatopathology, College of American Pathologists, and United States and Canadian Academy of Pathology
Disclosure: Nothing to disclose.
David F Butler, MD Professor of Dermatology, Texas A&M University College of Medicine; Chair, Department of Dermatology, Director, Dermatology Residency Training Program, Scott and White Clinic, Northside Clinic
David F Butler, MD is a member of the following medical societies: Alpha Omega Alpha, American Academy of Dermatology, American Medical Association, American Society for Dermatologic Surgery, American Society for MOHS Surgery, Association of Military Dermatologists, and Phi Beta Kappa
Disclosure: Nothing to disclose.
Daniel J Dire, MD, FACEP, FAAP, FAAEM Clinical Professor, Department of Emergency Medicine, University of Texas-Houston; Clinical Professor, Department of Pediatrics, University of Texas Health Sciences Center, San Antonio, Texas
Daniel J Dire, MD, FACEP, FAAP, FAAEM is a member of the following medical societies: American Academy of Clinical Toxicology, American Academy of Emergency Medicine, American Academy of Pediatrics, American College of Emergency Physicians, and Association of Military Surgeons of the US
Disclosure: Talecris Biotherapeutics Honoraria Speaking and teaching
Dirk M Elston, MD, Director, Ackerman Academy of Dermatopathology, New York
Dirk M Elston, MD is a member of the following medical societies: American Academy of Dermatology
Disclosure: Nothing to disclose.
Gino A Farina, MD, FACEP, FAAEM Associate Professor of Clinical Emergency Medicine, Albert Einstein College of Medicine; Program Director, Department of Emergency Medicine, Long Island Jewish Medical Center
Gino A Farina, MD, FACEP, FAAEM is a member of the following medical societies: American Academy of Emergency Medicine, American College of Emergency Physicians, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Elliot Goldberg, MD Dean of the Western Pennsylvania Clinical Campus, Professor, Department of Medicine, Temple University School of Medicine
Elliot Goldberg, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Physicians, and American College of Rheumatology
Disclosure: Nothing to disclose
Rick Kulkarni, MD Attending Physician, Department of Emergency Medicine, Cambridge Health Alliance, Division of Emergency Medicine, Harvard Medical School
Rick Kulkarni, MD is a member of the following medical societies: Alpha Omega Alpha, American Academy of Emergency Medicine, American College of Emergency Physicians, American Medical Association, American Medical Informatics Association, Phi Beta Kappa, and Society for Academic Emergency Medicine
Disclosure: WebMD Salary Employment
Carlos J Lozada, MD Director of Rheumatology Fellowship Program, Professor, Department of Medicine, Division of Rheumatology and Immunology, University of Miami, Leonard M Miller School of Medicine
Carlos J Lozada, MD is a member of the following medical societies: American College of Physicians and American College of Rheumatology
Disclosure: Pfizer Honoraria Speaking and teaching; Amgen Honoraria Speaking and teaching
Jeffrey J Miller, MD Associate Professor of Dermatology, Pennsylvania State University College of Medicine; Staff Dermatologist, Pennsylvania State Milton S Hershey Medical Center
Jeffrey J Miller, MD is a member of the following medical societies: Alpha Omega Alpha, American Academy of Dermatology, Association of Professors of Dermatology, North American Hair Research Society, and Society for Investigative Dermatology
Disclosure: Nothing to disclose.
Joanna Narbutt, MD, PhD Senior Registrar, Lecturer, Department of Dermatology and Venereology, Medical University of Lodz, Poland
Disclosure: Nothing to disclose.
Darren Phelan, MD Medical Director, Department of Emergency Medicine, Sierra Nevada Memorial Hospital
Disclosure: Nothing to disclose.
Sriya K M Ranatunga, MD, MPH Associate Professor, Department of Clinical Medicine, Southern Illinois University School of Medicine
Disclosure: Nothing to disclose.
Robert A Schwartz, MD, MPH Professor and Head, Dermatology, Professor of Pathology, Pediatrics, Medicine, and Preventive Medicine and Community Health, University of Medicine and Dentistry of New Jersey-New Jersey Medical School
Robert A Schwartz, MD, MPH is a member of the following medical societies: Alpha Omega Alpha, American Academy of Dermatology, American College of Physicians, and Sigma Xi
Disclosure: Nothing to disclose.
Anna Sysa-Jedrzejowska, MD, PhD Head, Professor, Department of Dermatology and Venereology, Medical University of Lodz, Poland
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Jolanta Dorota Torzecka, MD, PhD Consulting Staff, Department of Dermatology and Venereology, Medical University of Lodz, Poland
Disclosure: Nothing to disclose.
Anna Zalewska, MD, PhD Professor of Dermatology and Venereology, Psychodermatology Department, Chair of Clinical Immunology and Microbiology, Medical University of Lodz, Poland
Disclosure: Nothing to disclose.