Polymyositis: Practice Essentials, Pathophysiology, Etiology (original) (raw)
Overview
Practice Essentials
Polymyositis is an idiopathic inflammatory myopathy characterized by the following [1] :
- Progressive symmetric, predominantly proximal muscle weakness
- Elevated skeletal muscle enzyme levels
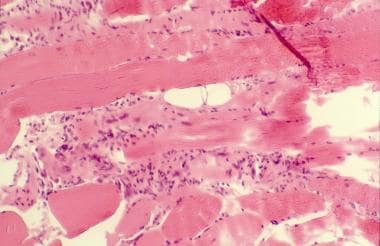
- Characteristic electromyography (EMG) and muscle biopsy findings (see the image below)
Polymyositis. Histopathology slide shows endomysial mononuclear inflammatory infiltrate and muscle fiber necrosis.
Polymyositis is one of several idiopathic inflammatory myopathies. [2] Clinically similar to polymyositis, dermatomyositis is an idiopathic inflammatory myopathy that also features symmetrical muscle weakness that develops over weeks to months, but has characteristic skin manifestations and muscle biopsy findings that confirm it as a distinct clinical entity. [3, 4] Inclusion body myositis is a slowly progressive idiopathic inflammatory myopathy that is the most common inflammatory myopathy in patients older than age 50 years. It more commonly presents as asymmetrical distal weakness and also has distinct biopsy findings.
Bohan and Peter classified the idiopathic inflammatory myopathies as follows [5] :
- I - Primary idiopathic polymyositis
- II - Primary idiopathic dermatomyositis
- III - Polymyositis or dermatomyositis associated with malignancy [6]
- IV - Childhood polymyositis or dermatomyositis
- V - Polymyositis or dermatomyositis associated with another connective-tissue disease
- VII - Miscellaneous (eg, eosinophilic myositis, myositis ossificans, focal myositis, giant cell myositis)
Necrotizing autoimmune myopathy (NAM) is a more recently recognized form of idiopathic inflammatory myopathy that is identified by finding macrophage-predominant myocyte destruction, with few to no lymphocytes, on muscle biopsy. NAM has been associated with malignancy and statin use. [7]
See Etiology, Presentation, and Workup.
Treatment of polymyositis is empirical because of the rarity of the disease and the paucity of randomized controlled trials. Prednisone is the first-line treatment of choice. Immunosuppressive agents are indicated in patients who do not improve within a reasonable period (ie, 4 wk) or in whom adverse effects from corticosteroids develop. Limited data support the use of other agents.
See Treatment and Medication.

Pathophysiology
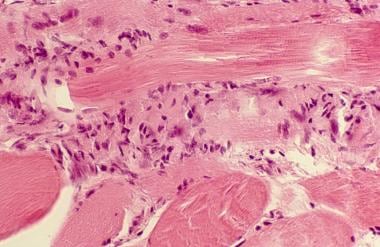
The pathogenesis of polymyositis appears to be a T-cell–mediated cytotoxic process directed against unidentified muscle antigens. Supporting this conclusion is the involvement of CD8 T cells, which, along with macrophages, initially surround healthy nonnecrotic muscle fibers and eventually invade and destroy them. [8] (See the image below.)
Polymyositis. Close view of muscle biopsy, showing chronic inflammatory infiltrate consisting of T lymphocytes, especially CD8+ T lymphocytes.
The factors triggering a T-cell–mediated process in polymyositis are unclear. Viruses have been implicated; so far, however, the only viruses that have been etiologically connected with the disease are the human retroviruses human immunodeficiency virus (HIV) and human T-cell lymphotrophic virus type I (HTLV-I), the simian retroviruses, and coxsackievirus B. Those viruses may directly invade the muscle tissue, damaging the vascular endothelium and releasing cytokines, which then induce abnormal expression of the major histocompatibility complex (MHC) and render the muscle susceptible to destruction.
An autoimmune response to nuclear and cytoplasmic autoantigens is detected in about 60-80% of patients with polymyositis and dermatomyositis. Some serum autoantibodies are shared with other autoimmune diseases (ie, myositis-associated antibodies [MAAs]), and some are unique to myositis (ie, myositis-specific antibodies [MSAs]). MSAs are found in approximately 40% of patients with polymyositis or dermatomyositis, whereas MAAs are found in 20-50% of these patients.
Myositis-specific antibodies
The identified MSA targets include the following 3 distinct groups of proteins:
- Aminoacyl–transfer ribonucleic acid (tRNA) synthetases (anti–Jo-1)
- Nuclear Mi-2 protein
- Components of the signal-recognition particle (SRP)
Most of the anti-tRNA synthetase antibodies are directed toward functional and highly conserved domains of the enzyme. As many as 6 of 20 aminoacyl-tRNA synthetases have been described, but anti-histidyl-tRNA synthetase (Jo-1) is most common (20-30%). Autoantibodies directed toward the other synthetases specific for alanine (anti-PL12), glycine (anti-EJ), isoleucine (anti-OJ), threonine (anti-PL7), and asparagine (anti-KS) have been reported in only about 1% of patients.
Anti–Jo-1 autoantibodies were originally described as precipitating autoantibodies in sera of patients with polymyositis. Subsequently, the anti-Jo-1 antibodies were recognized to be specific for patients with polymyositis. The target for the anti-Jo-1 antibodies was the aminoacyl-tRNA synthetases, a family of distinct cellular enzymes.
The Jo-1 antigen is histidyl-tRNA synthetase. This enzyme is partially responsible for attaching tRNA to its cognate ribosomal RNA (rRNA). The Jo-1 antigen migrates as a 53-kd protein on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
The presence of autoantibodies against the Jo-1 antigen has been reported in 15-30% of polymyositis patients by immunodiffusion. Anti–Jo-1 antibodies are almost completely specific for myositis and are more common in polymyositis than in dermatomyositis; they are rare in children. The presence of anti–Jo-1 antibodies defines a distinct group of polymyositis patients with interstitial lung disease, arthritis, and fevers. The anti–Jo-1 response appears to be self-antigen driven, having a broad spectrotype over time and undergoing isotype switching. Anti–Jo-1 antibodies also inhibit the function of histidyl-tRNA synthetase in humans more than they do in other species.
Anti–Mi-2 antibodies recognize a major protein of a nuclear complex formed by at least 7 proteins that is involved in the transcription process. Autoantibodies recognizing Mi-2 are considered specific serologic markers of dermatomyositis. They are detected in about 10% of patients with myositis and are associated with relatively acute onset, a good prognosis, and a good response to therapy.
Anti-SRP antibodies are directed toward an RNA-protein complex that consists of 6 proteins and a 300-nucleotide RNA molecule (7SL RNA) and present in 5% of myositis patients [9] . Patients with anti-SRP antibodies have acute polymyositis with cardiac involvement, a poor prognosis, and a poor response to therapy.
Myositis-associated antibodies
The MAA are found in the sera of 20-50% of patients and are commonly encountered in other connective tissue diseases. The most important antigenic targets of the MAA are the following:
- PM/Scl nucleolar antigen
- Nuclear Ku antigen
- Small nuclear ribonucleoproteins (snRNP)
- Cytoplasmic ribonucleoproteins (RoRNP)
Anti-PM/Scl autoantibodies are generally found in patients affected by polymyositis overlapping with scleroderma. Anti-Ku antibodies are found in patients with myositis overlapping with other connective tissue diseases.
Antibodies directed against snRNP are frequently found in patients with myositis and in patients with connective tissue–disease overlap syndrome, whereas antibodies toward Ro/SSA 60 kD, Ro/SSA 52 kD, and La/SSB protein components of the RoRNP complex are almost exclusively found in patients with Sjögren syndrome and systemic lupus erythematosus (SLE).

Etiology
Polymyositis is an immune-mediated syndrome secondary to defective cellular immunity that is most commonly associated with other systemic autoimmune diseases. It may be due to diverse causes that occur alone or in association with viral infections, malignancies, or connective-tissue disorders.
Risk factors
An increased association of myositis has been found with human leukocyte antigen (HLA) haplotypes A1, B8, and DR3, which also increase the risk for autoimmune diseases. Environmental triggers, especially infectious agents, have been suggested as etiologic agents. These include the following:
- Coxsackievirus B1
- HIV
- HTLV-1
- Hepatitis B virus
- Influenza
- Echovirus
- Adenovirus
- Severe acute respiratory syndrome coronavirus 2 [10]
Many drugs are known to cause myopathy. Most of those drugs, such as hydroxychloroquine and colchicine, cause a toxic or metabolic myopathy. However, several drugs may rarely induce an immune-mediated myopathy or myositis; in these cases, muscle biopsy shows chronic inflammatory changes consistent with polymyositis. Drugs such as D-penicillamine, hydralazine, procainamide, phenytoin, and angiotensin-converting enzyme (ACE) inhibitors have been associated with this type of inflammatory myopathy. Statins can cause severe muscle inflammation and rhabdomyolysis.

Epidemiology
Frequency
Idiopathic inflammatory myopathies are relatively rare diseases, with an incidence in the United States that ranges from 0.5-8.4 cases per million population. Polymyositis is more common in the United States within the Black population, with the estimated Black-to-White incidences for polymyositis and dermatomyositis being 5:1 and 3:1, respectively. Internationally, polymyositis is less common among the Japanese.
Sex- and age-related demographics
Polymyositis and dermatomyositis are more common in women than in men (2:1 ratio), while inclusion body myositis is twice as common in men.
Polymyositis usually affects adults older than 20 years, especially those aged 45-60 years. Polymyositis rarely affects children. The age of onset of polymyositis with another collagen vascular disease is related to the associated condition.
Although dermatomyositis is primarily a disease of adults, it can be seen in children, usually those aged 5-14 years. Eighty percent of patients with inclusion body myositis are older than 50 years at onset.

Prognosis
In most patients, polymyositis responds well to treatment, although residual weakness occurs in approximately 30% of patients. Osteoporosis, a common complication of long-term corticosteroid therapy, may cause significant morbidity. A study from Taiwan determined that the risk of osteoporosis was 2.99 times higher in patients with polymyositis, and that the risk was independent of corticosteroid and immunosuppressant treatment. [11]
Poor prognostic factors include the following:
- Advanced age
- Female sex
- African-American race
- Interstitial lung disease
- Presence of anti-Jo-1 (lung disease) and anti-SRP antibodies (severe muscle disease, cardiac involvement)
- Associated malignancy
- Delayed or inadequate treatment
- Dysphagia, dysphonia
- Cardiac and pulmonary involvement
Watanabe et al reported that negative assays for myositis-specific autoantibodies and the absence of severe muscle weakness requiring assistance at diagnosis are independent predictive factors for sustained remission in adult patients with polymyositis/dermatomyositis. [12]
Complications
Complications of polymyositis may include the following:
- Interstitial lung disease (ILD)
- Aspiration pneumonia
- Heart block
- Arrhythmias
- Congestive heart failure
- Pericarditis
- Dysphagia
- Malabsorption
- Pneumonia
- Infection [13]
- Myocardial infarction [14]
- Carcinoma - Especially in the breast and lung [15]
- Steroid myopathy or other complications of steroid therapy
Carruthers et al reported that patients with polymyositis are at increased risk for venous thromboembolism (VTE), with hazard ratios of 7.0 for VTE, 6.16 for deep venous thrombosis, and 7.23 for pulmonary embolism. Overall, the highest calculated incidence rate ratios were observed in the first year after diagnosis of polymyositis. [16]
A meta-analysis estimated that worldwide, the prevalence of ILD in patients with polymyositis and dermatomyositis is 41%, with the highest prevalence among Asians (50%). ILD was more frequently associated with anti-Jo-1 and anti-melanoma differentiation–associated gene 5 antibodies than other myositis-specific autoantibodies. [17]
The incidence of lung, bladder, and non-Hodgkin lymphoma may be increased in patients with polymyositis, especially in the first year after diagnosis. Nicoletis et al reported that a high pretreatment neutrophil-to-lymphocyte ratio (≥5.5) is associated with an increased risk of cancer in patients with polymyositis/dermatomyositis. These authors concluded that in patients age 60 years and older, a high neutrophil-to-lymphocyte ratio should prompt investigation for cancer, both at diagnosis of polymyositis and during follow-up. [18]
Polymyositis shares pathologic characteristics and immunologic features with amyotrophic lateral sclerosis (ALS), and a nationwide cohort study from Taiwan found that a diagnosis of polymyositis increased the likelihood of a subsequent diagnosis of ALS (P < 0.001). The association was independent of sex, age, and concomitant autoimmune diseases. [19]
Five-year survival rates in polymyositis have been estimated at more than 80%. Mortality is most often related to associated malignancy or pulmonary complications; elderly patients with cardiac involvement or dysphagia also have a higher mortality rate. [20] However, Qiao et al reported that in the United States, the number of death certificates listing polymyositis as the underlying cause of death fell by 5.2% annually from 2005–2006 to 2019–2020, and the number that listed multiple causes of death including polymyositis fell by 4.1% annually from 1991–1992 to 2019–2020. [21]

Patient Education
Patients with polymyositis should be educated early about the disease, its complications, and treatment options and should be provided with realistic expectations about outcomes. Most patients show significant improvement with treatment. Stress the need for close follow-up care, continued physical therapy, and long-term therapy with monitoring of several parameters including medication toxicity and screening for malignancy.
For patient education information, see Polymyositis: Causes, Symptoms, and Treatment. In addition, patients may visit The Myositis Association Web site.

- Yang SH, Chang C, Lian ZX. Polymyositis and dermatomyositis - challenges in diagnosis and management. J Transl Autoimmun. 2019 Dec. 2:100018. [QxMD MEDLINE Link]. [Full Text].
- Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher-Stine L, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers. 2021 Dec 2. 7 (1):86. [QxMD MEDLINE Link].
- Waldman R, DeWane ME, Lu J. Dermatomyositis: Diagnosis and treatment. J Am Acad Dermatol. 2020 Feb. 82 (2):283-296. [QxMD MEDLINE Link].
- Cheeti A, Brent LH, Panginikkod S. Autoimmune Myopathies. 2024 Jan. [QxMD MEDLINE Link]. [Full Text].
- Bohan A. History and classification of polymyositis and dermatomyositis. Clin Dermatol. 1988 Apr-Jun. 6(2):3-8. [QxMD MEDLINE Link].
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010 Apr. 9(6):449-53. [QxMD MEDLINE Link].
- Carroll MB, Newkirk MR, Sumner NS. Necrotizing Autoimmune Myopathy: A Unique Subset of Idiopathic Inflammatory Myopathy. J Clin Rheumatol. 2016 Oct. 22 (7):376-80. [QxMD MEDLINE Link].
- Kamiya M, Mizoguchi F, Takamura A, Kimura N, Kawahata K, Kohsaka H. A new in vitro model of polymyositis reveals CD8+ T cell invasion into muscle cells and its cytotoxic role. Rheumatology (Oxford). 2020 Jan 1. 59 (1):224-232. [QxMD MEDLINE Link]. [Full Text].
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin Rev Allergy Immunol. 2017 Feb. 52 (1):1-19. [QxMD MEDLINE Link].
- Reddy SA, Rivera Vargas N, Varshney A, Karasik O. A Case of COVID-19-Triggered Polymyositis Leading to Rhabdomyolysis. Cureus. 2024 Jul. 16 (7):e64267. [QxMD MEDLINE Link].
- Lee CW, Muo CH, Liang JA, Sung FC, Hsu CY, Kao CH. Increased osteoporosis risk in dermatomyositis or polymyositis independent of the treatments: a population-based cohort study with propensity score. Endocrine. 2015 Oct 1. [QxMD MEDLINE Link].
- Watanabe E, Gono T, Kuwana M, Terai C. Predictive factors for sustained remission with stratification by myositis-specific autoantibodies in adult polymyositis/dermatomyositis. Rheumatology (Oxford). 2020 Mar 1. 59 (3):586-593. [QxMD MEDLINE Link].
- Marie I, Hachulla E, Chérin P, et al. Opportunistic infections in polymyositis and dermatomyositis. Arthritis Rheum. 2005 Apr 15. 53(2):155-65. [QxMD MEDLINE Link].
- Rai SK, Choi HK, Sayre EC, Aviña-Zubieta JA. Risk of myocardial infarction and ischaemic stroke in adults with polymyositis and dermatomyositis: a general population-based study. Rheumatology (Oxford). 2015 Sep 30. [QxMD MEDLINE Link].
- Yang Z, Lin F, Qin B, Liang Y, Zhong R. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015 Feb. 42 (2):282-91. [QxMD MEDLINE Link].
- Carruthers EC, Choi HK, Sayre EC, Aviña-Zubieta JA. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: a general population-based study. Ann Rheum Dis. 2014 Sep 5. [QxMD MEDLINE Link].
- Sun KY, Fan Y, Wang YX, Zhong YJ, Wang GF. Prevalence of interstitial lung disease in polymyositis and dermatomyositis: A meta-analysis from 2000 to 2020. Semin Arthritis Rheum. 2021 Feb. 51 (1):175-191. [QxMD MEDLINE Link].
- Nicoletis I, Pasco J, Maillot F, Goupille P, Corcia P, Grammatico-Guillon L, et al. High pre-treatment neutrophil-to-lymphocyte ratio in patients with dermatomyositis/polymyositis predicts an increased risk of cancer. Eur J Dermatol. 2020 Apr 10. [QxMD MEDLINE Link].
- Tseng CC, Chang SJ, Tsai WC, Ou TT, Wu CC, Sung WY, et al. Increased Incidence of Amyotrophic Lateral Sclerosis in Polymyositis: A Nationwide Cohort Study. Arthritis Care Res (Hoboken). 2017 Aug. 69 (8):1231-1237. [QxMD MEDLINE Link].
- Bronner IM, van der Meulen MF, de Visser M, Kalmijn S, van Venrooij WJ, Voskuyl AE, et al. Long-term outcome in polymyositis and dermatomyositis. Ann Rheum Dis. 2006 Nov. 65(11):1456-61. [QxMD MEDLINE Link]. [Full Text].
- Qiao P, Guo Q, Gao J, Ma D, Liu S, Gao X, et al. Long-term secular trends in dermatomyositis and polymyositis mortality in the USA from 1981 to 2020 according to underlying and multiple cause of death mortality data. Arthritis Res Ther. 2023 Jan 31. 25 (1):16. [QxMD MEDLINE Link]. [Full Text].
- Schnabel A, Hellmich B, Gross WL. Interstitial lung disease in polymyositis and dermatomyositis. Curr Rheumatol Rep. 2005 Apr. 7(2):99-105. [QxMD MEDLINE Link].
- Ruiz-Lozano RE, Velazquez-Valenzuela F, Roman-Zamudio M, Andrade-Leal SK, Rodriguez-Garcia A. Polymyositis and dermatomyositis: ocular manifestations and potential sight-threatening complications. Rheumatol Int. 2022 Jul. 42 (7):1119-1131. [QxMD MEDLINE Link].
- Kuo SH, Vullaganti M, Jimenez-Shahed J, Kwan JY. Camptocormia as a presentation of generalized inflammatory myopathy. Muscle Nerve. 2009 Dec. 40(6):1059-63. [QxMD MEDLINE Link].
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007 Aug. 28 (4):451-8. [QxMD MEDLINE Link].
- Jakubaszek M, Kwiatkowska B, Maślińska M. Polymyositis and dermatomyositis as a risk of developing cancer. Reumatologia. 2015. 53 (2):101-5. [QxMD MEDLINE Link].
- Wang J, Guo G, Chen G, Wu B, Lu L, Bao L. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013 Oct. 169 (4):838-47. [QxMD MEDLINE Link].
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin Rev Allergy Immunol. 2017 Feb. 52 (1):1-19. [QxMD MEDLINE Link].
- Mammen AL. Statin-Associated Autoimmune Myopathy. N Engl J Med. 2016 Feb 18. 374 (7):664-9. [QxMD MEDLINE Link].
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin Rev Allergy Immunol. 2017 Feb. 52 (1):1-19. [QxMD MEDLINE Link].
- Huang ZG, Gao BX, Chen H, Yang MX, Chen XL, Yan R, et al. An efficacy analysis of whole-body magnetic resonance imaging in the diagnosis and follow-up of polymyositis and dermatomyositis. PLoS One. 2017. 12 (7):e0181069. [QxMD MEDLINE Link]. [Full Text].
- Elessawy SS, Abdelsalam EM, Abdel Razek E, Tharwat S. Whole-body MRI for full assessment and characterization of diffuse inflammatory myopathy. Acta Radiol Open. 2016 Sep 21. 5 (9):2058460116668216. [QxMD MEDLINE Link]. [Full Text].
- Chahin N, Engel AG. Correlation of muscle biopsy, clinical course, and outcome in PM and sporadic IBM. Neurology. 2008 Feb 5. 70(6):418-24. [QxMD MEDLINE Link].
- Xiong A, Qiang Y, Cao Y, Shuai Y, Chen H, Xiang Q, et al. The therapeutic efficacy and safety of intravenous immunoglobulin in dermatomyositis and polymyositis: A systematic review and meta-analysis. Mod Rheumatol. 2023 Apr 13. 33 (3):533-542. [QxMD MEDLINE Link].
- Hengstman GJ, van den Hoogen FH, Barrera P, Netea MG, Pieterse A, van de Putte LB, et al. Successful treatment of dermatomyositis and polymyositis with anti-tumor-necrosis-factor-alpha: preliminary observations. Eur Neurol. 2003. 50(1):10-5. [QxMD MEDLINE Link].
- Anandacoomarasamy A, Howe G, Manolios N. Advanced refractory polymyositis responding to infliximab. Rheumatology (Oxford). 2005 Apr. 44(4):562-3. [QxMD MEDLINE Link].
- Aggarwal R, Oddis CV. Therapeutic advances in myositis. Curr Opin Rheumatol. 2012 Nov. 24(6):635-41. [QxMD MEDLINE Link].
- Dastmalchi M, Grundtman C, Alexanderson H, Mavragani CP, Einarsdottir H, Helmers SB, et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis. 2008 Dec. 67(12):1670-7. [QxMD MEDLINE Link].
- Uthman I, El-Sayad J. Refractory polymyositis responding to infliximab. Rheumatology (Oxford). 2004 Sep. 43(9):1198-9. [QxMD MEDLINE Link].
- Schiffenbauer A, Garg M, Castro C, Pokrovnichka A, Joe G, Shrader J, et al. A randomized, double-blind, placebo-controlled trial of infliximab in refractory polymyositis and dermatomyositis. Semin Arthritis Rheum. 2017 Oct 16. [QxMD MEDLINE Link].
- Tjärnlund A, Tang Q, Wick C, Dastmalchi M, Mann H, Tomasová Studýnková J, et al. Abatacept in the treatment of adult dermatomyositis and polymyositis: a randomised, phase IIb treatment delayed-start trial. Ann Rheum Dis. 2017 Oct 9. [QxMD MEDLINE Link].
- Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013 Feb. 65(2):314-24. [QxMD MEDLINE Link]. [Full Text].
- Ueno KI, Shimojima Y, Kishida D, Sekijima Y, Ikeda SI. Advantage of administering tacrolimus for improving prognosis of patients with polymyositis and dermatomyositis. Int J Rheum Dis. 2016 Jul 26. [QxMD MEDLINE Link].
- Ge Y, Zhou H, Shi J, Ye B, Peng Q, Lu X, et al. The efficacy of tacrolimus in patients with refractory dermatomyositis/polymyositis: a systematic review. Clin Rheumatol. 2015 Sep 2. [QxMD MEDLINE Link].
- Caramaschi P, Volpe A, Carletto A, Bambara LM, Biasi D. Long-standing refractory polymyositis responding to mycophenolate mofetil: a case report and review of the literature. Clin Rheumatol. 2007 Oct. 26 (10):1795-6. [QxMD MEDLINE Link].
- Levine T. Treating refractory dermatomyositis or polymyositis with adrenocorticotropic hormone gel: a retrospective case series. Drug Des Devel Ther. 2012. 6:133-9. [QxMD MEDLINE Link].
- Alexanderson H. Exercise: an important component of treatment in the idiopathic inflammatory myopathies. Curr Rheumatol Rep. 2005 Apr. 7(2):115-24. [QxMD MEDLINE Link].
- Kannappan R, Kumar R, Cichelli K, Brent LH. A Review of Myositis-Associated Interstitial Lung Disease. J Clin Med. 2024 Jul 11. 13 (14):[QxMD MEDLINE Link]. [Full Text].
- Shimojima Y, Ishii W, Matsuda M, Kishida D, Ikeda SI. Effective Use of Calcineurin Inhibitor in Combination Therapy for Interstitial Lung Disease in Patients With Dermatomyositis and Polymyositis. J Clin Rheumatol. 2017 Mar. 23 (2):87-93. [QxMD MEDLINE Link].
- Chen D, Wang XB, Zhou Y, Zhu XC. Efficacy of infliximab in the treatment for dermatomyositis with acute interstitial pneumonia: a study of fourteen cases and literature review. Rheumatol Int. 2013 Oct. 33(10):2455-8. [QxMD MEDLINE Link].
- Takada K, Katada Y, Ito S, Hayashi T, Kishi J, Itoh K, et al. Impact of adding tacrolimus to initial treatment of interstitial pneumonitis in polymyositis/dermatomyositis: a single-arm clinical trial. Rheumatology (Oxford). 2020 May 1. 59 (5):1084-1093. [QxMD MEDLINE Link].
- Marie I, Menard JF, Hatron PY, Hachulla E, Mouthon L, Tiev K, et al. Intravenous immunoglobulins for steroid-refractory esophageal involvement related to polymyositis and dermatomyositis: a series of 73 patients. Arthritis Care Res (Hoboken). 2010 Dec. 62 (12):1748-55. [QxMD MEDLINE Link]. [Full Text].
- Polymyositis. MRI of thighs showing increased signal in the quadriceps muscles bilaterally consistent with inflammatory myositis.
- Polymyositis. Histopathology slide shows endomysial mononuclear inflammatory infiltrate and muscle fiber necrosis.
- Polymyositis. Close view of muscle biopsy, showing chronic inflammatory infiltrate consisting of T lymphocytes, especially CD8+ T lymphocytes.
- Polymyositis. Hematoxylin and eosin frozen section shows polymyositis. Endomysial chronic inflammation is present among intact myofibers, which are remarkable only for increased variability of fiber size. Image courtesy of Roberta J. Seidman, MD.
- Polymyositis. Hematoxylin and eosin paraffin section shows polymyositis. Patient had dense endomysial inflammation that contains an abundance of plasma cells, which can be observed in patients with chronic polymyositis. Two necrotic myofibers, characterized by dense eosinophilic staining, are observed. Focal fatty infiltration of the muscle is present in the lower left quadrant of the photomicrograph. Image courtesy of Roberta J. Seidman, MD.
- Polymyositis. Hematoxylin and eosin paraffin section shows polymyositis. Photomicrograph illustrates attack on a nonnecrotic myofiber by autoaggressive T lymphocytes. On the left, the central myofiber is intact. On the right, it is obliterated by a segmental inflammatory attack. If immunohistochemistry were performed, expected findings would include an admixture of CD8 T lymphocytes and macrophages in the inflammatory process. Image courtesy of Roberta J. Seidman, MD.
- Polymyositis. Hematoxylin and eosin paraffin shows dermatomyositis. In dermatomyositis, inflammation is characteristically perivascular and perimysial. Vessel oriented approximately vertically in the center has a mild perivascular chronic inflammatory infiltrate. The endothelium is plump. The wall is not necrotic. A few lymphocytes in the wall of the vessel are probably in transit from the lumen to the external aspect of the vessel. Some observers may interpret this finding as vasculitis, but it is certainly neither necrotizing vasculitis nor arteritis. Image courtesy of Roberta J. Seidman, MD.
- Polymyositis. Hematoxylin and eosin paraffin section shows polymyositis. Longitudinal section shows a dense, chronic, endomysial inflammatory infiltrate. Image courtesy of Roberta J. Seidman, MD.


Author
Mythili Seetharaman, MD Consultant Rheumatologist, Orthopedic Associates of Allentown, Affiliated with St Luke's University Hospital
Mythili Seetharaman, MD is a member of the following medical societies: American College of Rheumatology, American Medical Association, Pennsylvania Rheumatology Society
Disclosure: Nothing to disclose.
Chief Editor
Herbert S Diamond, MD Visiting Professor of Medicine, Division of Rheumatology, State University of New York Downstate Medical Center; Chairman Emeritus, Department of Internal Medicine, Western Pennsylvania Hospital
Herbert S Diamond, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Physicians, American College of Rheumatology, American Medical Association, Phi Beta Kappa
Disclosure: Nothing to disclose.
Acknowledgements
Michael S Beeson, MD, MBA, FACEP, Professor of Emergency Medicine, Northeastern Ohio Universities College of Medicine and Pharmacy; Attending Faculty, Akron General Medical Center
Michael S Beeson, MD, MBA, FACEP is a member of the following medical societies: American College of Emergency Physicians, Council of Emergency Medicine Residency Directors, National Association of EMS Physicians, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Thomas H Brannagan III, MD, Associate Professor of Clinical Neurology and Director, Peripheral Neuropathy Center, Columbia University, College of Physicians and Surgeons; Co-Director, EMG Laboratory, New York-Presbyterian Hospital, Columbia Campus, New York
Thomas H Brannagan III, MD is a member of the following medical societies: American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and Peripheral Nerve Society
Disclosure: Nothing to disclose.
Lawrence H Brent, MD, Associate Professor of Medicine, Jefferson Medical College of Thomas Jefferson University; Chair, Program Director, Department of Medicine, Division of Rheumatology, Albert Einstein Medical Center
Lawrence H Brent, MD is a member of the following medical societies: American Association for the Advancement of Science, American Association of Immunologists, American College of Physicians, and American College of Rheumatology
Disclosure: Genentech Honoraria Speaking and teaching; Genentech Grant/research funds Other; Amgen Honoraria Speaking and teaching; Pfizer Honoraria Speaking and teaching; Abbott Immunology Honoraria Speaking and teaching; Takeda Honoraria Speaking and teaching; UCB Speaking and teaching; Omnicare Consulting fee Consulting; Centocor Consulting fee Consulting
Zaineb Daud, MD, Consulting Staff, Department of Neurology, Medical College of Pennsylvania Hahnemann University
Disclosure: None
Gino A Farina, MD, FACEP, FAAEM, Associate Professor of Clinical Emergency Medicine, Albert Einstein College of Medicine; Program Director, Department of Emergency Medicine, Long Island Jewish Medical Center
Gino A Farina, MD, FACEP, FAAEM is a member of the following medical societies: American Academy of Emergency Medicine, American College of Emergency Physicians, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Francisco de Assis Aquino Gondim, MD, MSc, PhD, Associate Professor of Neurology, Department of Neurology and Psychiatry, St Louis University School of Medicine
Francisco de Assis Aquino Gondim, MD, MSc, PhD is a member of the following medical societies: American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and Movement Disorders Society
Disclosure: Nothing to disclose.
Aamir Hashmat, MD, Consulting Staff, Neurology and Neurodiagnostics Lab, Department of Neurology, Jeff Anderson Regional Medical Center
Aamir Hashmat, MD is a member of the following medical societies: American Academy of Neurology, American Epilepsy Society; American Medical Association, AO Foundation
Disclosure: None
Milind J Kothari, DO, Professor and Vice-Chair, Department of Neurology, Pennsylvania State University College of Medicine; Consulting Staff, Department of Neurology, Penn State Milton S Hershey Medical Center
Milind J Kothari, DO is a member of the following medical societies: American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Neurological Association
Disclosure: Nothing to disclose.
Kristine M Lohr, MD, MS, Professor, Department of Internal Medicine, Center for the Advancement of Women's Health and Division of Rheumatology, Director, Rheumatology Training Program, University of Kentucky College of Medicine
Kristine M Lohr, MD, MS is a member of the following medical societies: American College of Physicians, American College of Rheumatology, and American Medical Women's Association
Disclosure: Nothing to disclose.
Glenn Lopate, MD, Associate Professor, Department of Neurology, Division of Neuromuscular Diseases, Washington University School of Medicine; Director of Neurology Clinic, St Louis ConnectCare; Consulting Staff, Department of Neurology, Barnes-Jewish Hospital
Glenn Lopate, MD is a member of the following medical societies: American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and Phi Beta Kappa
Disclosure: Nothing to disclose.
Nicholas Lorenzo, MD, Consulting Staff, Neurology Specialists and Consultants
Nicholas Lorenzo, MD is a member of the following medical societies: Alpha Omega Alpha and American Academy of Neurology
Disclosure: Nothing to disclose.
Henry Rosenkranz, MD, FAAEM, FACEP, Department of Emergency Medicine, Norwood Hospital
Henry Rosenkranz, MD, FAAEM, FACEP is a member of the following medical societies: American Academy of Emergency Medicine and American College of Emergency Physicians
Disclosure: Nothing to disclose.
Erik D Schraga, MD, Staff Physician, Department of Emergency Medicine, Mills-Peninsula Emergency Medical Associates
Francisco Talavera, PharmD, PhD, Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Reference Salary Employment