Bladder Cancer: Practice Essentials, Background, Anatomy (original) (raw)
Practice Essentials
Bladder cancer is a common urologic cancer that has the highest recurrence rate of any malignancy. The most common type is urothelial carcinoma (UC). Other types include squamous cell carcinoma (see the image below) and adenocarcinomas.
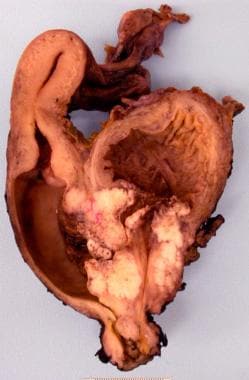
Bladder cancer. Cross-section through the bladder, uterus, and vagina with squamous cell carcinoma of the bladder infiltrating through the bladder wall into the vaginal wall.
Signs and symptoms
Clinical manifestations of bladder cancer are as follows:
- Painless gross hematuria - Approximately 80-90% of patients; classic presentation
- Irritative bladder symptoms (eg, dysuria, urgency, frequency of urination) - 20-30% of patients
- Pelvic or bony pain, lower-extremity edema, or flank pain - In patients with advanced disease
- Palpable mass on physical examination - Rare in superficial bladder cancer
See Presentation for more detail.
Diagnosis
Urine studies include the following:
- Urinalysis with microscopy
- Urine culture to rule out infection, if suspected
- Voided urinary cytology
- Urinary tumor marker testing
Urinary cytology:
- Standard noninvasive diagnostic method
- Low sensitivity for low-grade and early-stage cancers
- Fluorescence in situ hybridization (FISH) may improve the accuracy of cytology
Cystoscopy:
- The primary modality for the diagnosis of bladder carcinoma
- Permits biopsy and resection of papillary tumors
Upper urinary tract imaging:
- Usually necessary for the hematuria workup, but may be omitted on the basis of risk stratification
- American Urological Association Best Practice Policy recommends CT scanning of the abdomen and pelvis with contrast, with preinfusion and postinfusion phases
- Imaging is ideally performed with CT urography, using multidetector CT
- Ultrasonography is commonly used, but it may miss urothelial tumors of the upper tract and small stones
The diagnostic strategy for patients with negative cystoscopy is as follows:
- Negative urine cytology and FISH - Routine follow-up
- Negative urine cytology, positive FISH - Increased frequency of surveillance
- Positive urine cytology, positive or negative FISH - Cancer until proven otherwise
No blood tests are specific for bladder cancer, but a general evaluation is necessary prior to initiating therapy with intravesical bacillus Calmette-Guérin (BCG). Laboratory tests include the following:
- Complete blood count (CBC)
- Liver function tests
- Bony fraction of alkaline phosphatase assay (if bone metastasis suspected)
- Kidney function studies
See Workup for more detail.
Management
The treatment of non–muscle-invasive bladder cancer (NMIBC; Ta, T1, carcinoma in situ [CIS]) begins with transurethral resection of bladder tumor (TURBT). Subsequent treatment is as follows:
- Small-volume, low-grade Ta bladder cancer - An immediate single, postoperative dose of intravesical chemotherapy
- Intermediate-risk bladder cancer (recurrent low grade, high volume low grade, high-grade Ta) – Intravesical chemotherapy or BCG
- High-risk Ta, T1, and CIS urothelial carcinoma - Intravesical BCG
- Persistent or recurrent high-risk disease - Repeat resection prior to additional intravesical therapy; consider cystectomy for high-risk disease
Nogapendekin alfa inbakicept is an interleukin-15 agonist indicated for intravesical treatment in combination with BCG for adults with BCG-unresponsive NMIBC with CIS, with or without papillary tumors.
The treatment of muscle-invasive bladder cancer is as follows:
- Radical cystoprostatectomy in men
- Radical cystectomy with anterior pelvic exenteration in women
- Bilateral pelvic lymphadenectomy (PLND), standard or extended
- Creation of a urinary diversion (eg, ileal conduit, Indiana pouch, orthotopic bladder substitution)
- Neoadjuvant chemotherapy - May improve cancer-specific survival
Alternatively, a bladder-sparing approach of TURBT followed by concurrent radiation therapy and systemic chemotherapy (trimodality therapy) may be used.
Chemotherapeutic regimens for locally advanced or metastatic bladder cancer include the following [1] :
- Gemcitabine and cisplatin (GC) with avelumab maintenance
- Dose-dense methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin (DDMVAC) with avelumab maintenance
Inhibitors of programmed cell death 1 (PD-1) protein and its ligands PD-L1 and PD-L2, are first-line agents in patients with metastatic urothelial carcinoma who are not candidates for platinum-containing chemotherapy, and second-line agents for those with disease progression despite cisplatin-based chemotherapy. Agents in this category include the following [1] :
- Pembrolizumab
- Atezolizumab
- Nivolumab
- Avelumab
Erdafitinib, a fibroblast growth factor receptor inhibitor, is approved for locally advanced or metastatic urothelial carcinoma that has FGFR2 or FGFR3 genetic alterations and has progressed during or following at least 1 line of platinum-containing chemotherapy.
See Treatment and Medication for more detail.
See Bladder Cancer Treatment Protocols for more information on this topic. Go to Oncology Decision Point for expert commentary on bladder cancer treatment decisions and related guidelines. To view a multidisciplinary tumor board case discussion, see Memorial Sloan Kettering e-Tumor Boards: Muscle Invasive Bladder Cancer.
For patient education information, see Bladder Cancer.

Background
Bladder cancer is a common urologic cancer. Almost all bladder cancers originate in the urothelium, which is a 3- to 7-cell mucosal layer within the muscular bladder.
Urothelial carcinoma
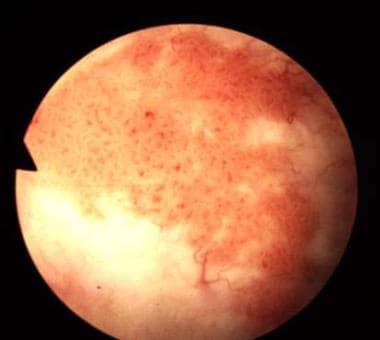
In North America, South America, Europe, and Asia, the most common type of urothelial tumor is urothelial carcinoma (UC); it constitutes more than 90% of bladder cancers in those regions. UC can arise anywhere in the urinary tract, including the renal pelvis, ureter, bladder, and urethra, but it is usually found in the urinary bladder. Carcinoma in situ (CIS) is frequently found in association with high-grade or extensive UC. (See the image below.)
Bladder cancer. The classic appearance of carcinoma in situ is as a flat, velvety patch. However, using special staining techniques such as 5-aminolevulinic acid, it has been shown that significant areas of carcinoma in situ are easily overlooked by conventional cystoscopy. Courtesy of Abbott and Vysis Inc.
Squamous cell carcinoma
Squamous cell carcinoma (SCC) is the second most common cell type associated with bladder cancer in industrialized countries. In the United States, around 5% of bladder cancers are SCCs. [2] Worldwide, however, SCC is the most common form of bladder cancer, accounting for 75% of cases in developing nations (see Epidemiology).
In the United States, the development of SCC is associated with persistent inflammation from long-term indwelling Foley catheters and bladder stones, as well as, possibly, infections. In developing nations, SCC is often associated with bladder infection by Schistosoma haematobium (see Etiology).
Other types of bladder cancer
Approximately 2% of bladder cancers are adenocarcinomas. Nonurothelial primary bladder tumors are extremely rare and may include small cell carcinoma, carcinosarcoma, primary lymphoma, and sarcoma (see Pathophysiology). Small cell carcinoma of the urinary bladder accounts for only 0.3-0.7% of all bladder tumors. High-grade urothelial carcinomas can also show divergent histologic differentiation, such as squamous, glandular, neuroendocrine, and sarcomatous features.
Phenotypes
Clinical and pathologic data indicate that at least 3 different phenotypes, as follows, exist in urothelial carcinoma [2, 3] :
- Low-grade proliferative lesions that develop into non–muscle-invasive tumors; these account for approximately 80% of bladder cancers
- Highly proliferative invasive tumors with a propensity to metastasize
- CIS, which can penetrate the lamina propria and eventually progress
Clinical course
The clinical course of bladder cancer is marked by a broad spectrum of aggressiveness and risk. Low-grade, superficial bladder cancers have minimal risk of progression to death; however, high-grade non–muscle-invasive cancers frequently progress, and muscle-invasive cancers are often lethal (see Prognosis).
The classic presentation of bladder cancer is painless gross hematuria, which is seen in approximately 80-90% of patients. Physical examination results are often unremarkable (see Presentation). Cystoscopy, cytology, and biopsy when necessary are the principal diagnostic tests (see Workup).
At presentation, 55-60% of patients have low-grade, noninvasive disease, which is usually treated conservatively with transurethral resection of bladder tumor (TURBT) and followed up with periodic cystoscopy. Intravesical agents may also be given selectively to decrease the frequency of recurrences. The remaining patients have high-grade disease, of which 50% is muscle invasive and is typically treated with radical cystectomy or with trimodality therapy (ie, TURBT followed by concurrent radiation therapy and systemic chemotherapy; see Treatment).
CIS is managed by TURBT and instillation of chemotherapeutic or immunotherapeutic agents—most commonly, immunotherapy with bacillus Calmette-Guérin (BCG)—into the bladder via catheter. These intravesical treatments are not effective in patients whose cancer has invaded the bladder wall muscle; those cases require cystectomy or a combination of radiation therapy and chemotherapy (see Treatment).
Bladder cancer has the highest recurrence rate of any malignancy. Although most patients with bladder cancer can be treated with organ-sparing therapy, most experience either recurrence or progression, creating a great need for accurate and diligent surveillance (see Treatment).
For more information on bladder cancer, see the following:

Anatomy
The bladder is an extraperitoneal muscular urine reservoir that lies behind the pubis symphysis in the pelvis. At the dome of the bladder lies the median umbilical ligament, a fibrous cord that is anchored to the umbilicus and that represents the obliterated urachus (allantois). The ureters, which transport urine from kidney to bladder, approach the bladder obliquely and posterosuperiorly, entering at the trigone (the area between the interureteric ridge and the bladder neck). The intravesical ureteral orifices are roughly 2-3 cm apart and form the superolateral borders of the trigone.
In males, the seminal vesicles, vas deferens, ureters, and rectum border the inferoposterior aspect of the bladder. Anterior to the bladder is the space of Retzius, which is composed of fibroadipose tissue and the prevesical fascia. The dome and posterior surface of the bladder are covered by parietal peritoneum, which reflects superiorly to the seminal vesicles and is continuous with the anterior rectal peritoneum. In females, the posterior peritoneal reflection is continuous with the uterus and vagina.
The vascular supply to the bladder arrives primarily via the internal iliac (hypogastric) arteries, branching into the superior, middle, and inferior vesical arteries, which are often recognizable as lateral and posterior pedicles. The arterial supply also arrives via the obturator and inferior gluteal artery and, in females, via the uterine and vaginal arteries. Bladder venous drainage is a rich network that often parallels the named arterial vessels, most of which ultimately drain into the internal iliac vein.
Initial lymphatic drainage from the bladder is primarily into the external iliac, obturator, internal iliac (hypogastric), and common iliac nodes. Following the drainage to these sentinel pelvic regions, spread may continue to the presacral, paracaval, interaortocaval, and para-aortic lymph node chains.
Almost all bladder cancers originate in the urothelium, which is a 3- to 7-cell mucosal layer within the muscular bladder. Squamous cell carcinoma of the bladder can involve multiple sites; however, the lateral wall and trigone are more commonly involved by this tumor. All small cell carcinomas of the urinary system identified so far have been located in the urinary bladder, most commonly in the dome and vesical lateral wall. [4]
See Bladder Anatomy.

Pathophysiology
Bladder cancer is often described as a polyclonal field change defect with frequent recurrences due to a heightened potential for malignant transformation. However, bladder cancer has also been described as resulting from implantation of malignant cells that have migrated from a previously affected site. The latter occurs less often and may account for only a small percentage of cases.
Use of the common term superficial bladder cancer should be discouraged. The term implies a harmless nature, which is misleading in many instances. Because it was used to describe the disparate disorders of low-grade papillary bladder cancer and the markedly more aggressive form, carcinoma in situ (CIS), the World Health Organization (WHO) has recommended it be abandoned.
In its place, the term non–muscle-invasive bladder cancer should be used and qualified with the appropriate American Joint Committee on Cancer stage (ie, Ta, T1, Tis). Stage T1 cancer invades lamina propria but not the muscle of the bladder. High-grade T1 tumor associated with CIS carries a relatively high risk for disease recurrence and progression (approximately 60%).
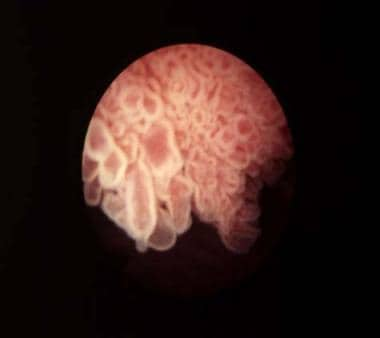
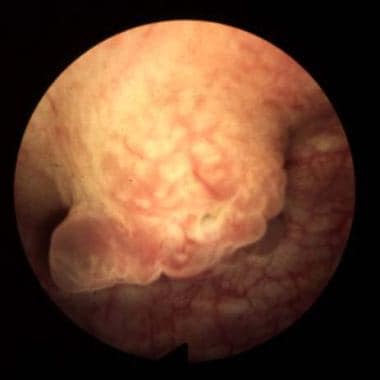
The current WHO/International Society of Urological Pathology (ISUP) system classifies bladder cancers as low grade or high grade. [5] Tumors are also classified by growth patterns: papillary (70%), sessile or mixed (20%), and nodular (10%). (See the images below.)
Bladder cancer. Papillary bladder tumors such as this one are typically of low stage and grade (Ta-G1). Courtesy of Abbott and Vysis Inc.
Bladder cancer. Sessile lesions as shown usually invade muscle, although occasionally a tumor is detected at the T1-G3 stage prior to muscle invasion. Courtesy of Abbott and Vysis Inc.
Urothelial carcinoma
Urothelial carcinoma (UC) arises from stem cells that are adjacent to the basement membrane of the epithelial surface. Depending on the genetic alterations that occur, these cells may follow different pathways in the expression of their phenotype.
The most common molecular biologic pathway for UCs involves the development of a papillary tumor that projects into the bladder lumen and, if untreated, eventually penetrates the basement membrane, invades the lamina propria, and then continues into the bladder muscle, where it can metastasize.
This progression occurs with high-grade cancers only. Low-grade cancers rarely, if ever, progress and are thought to have a distinct molecular pathway, different from the high-grade cancers and CIS.
CIS, which constitutes 10% of UCs, follows a different molecular pathway. This is a flat, noninvasive, high-grade UC that spreads along the surface of the bladder, staying superficial to the basement membrane. Over time, this may progress to an invasive form of cancer that behaves the same as invasive UC.
Many urothelial tumors are primarily UC but contain areas of squamous differentiation, squamous cell carcinoma (SCC), or adenocarcinoma.
Squamous cell carcinoma
SCC of the urinary bladder is a malignant neoplasm that is derived from bladder urothelium and has a pure squamous phenotype. [6, 7, 8] SCC of the bladder is essentially similar to squamous cell tumors arising in other organs. Because many urothelial carcinomas contain a minor squamous cell component, a diagnosis of SCC of the bladder should be rendered only when the tumor is solely composed of squamous cell components, with no conventional urothelial carcinoma component.
Reportedly, SCC has less of a tendency for nodal and vascular distant metastases than does urothelial carcinoma. [9, 10]
Rare forms of bladder cancer
Adenocarcinomas account for less than 2% of primary bladder tumors. These lesions are observed most commonly in exstrophic bladders and are often associated with malignant degeneration of a persistent urachal remnant.
Other rare forms of bladder cancer include leiomyosarcoma, rhabdosarcoma, carcinosarcoma, lymphoma, and small cell carcinoma. Leiomyosarcoma is the most common sarcoma of the bladder. Rhabdomyosarcomas most commonly occur in children. Carcinosarcomas are highly malignant tumors that contain a combination of mesenchymal and epithelial elements. Primary bladder lymphomas arise in the submucosa of the bladder. Except for lymphomas, all these rare bladder cancers carry a poor prognosis.
Small cell carcinoma of the urinary bladder is a poorly differentiated, malignant neoplasm that originates from urothelial stem cells and has variable expression of neuroendocrine markers. Morphologically, it shares features of small cell carcinoma of other organs, including the lung.
Genetic factors in pathogenesis
Divergent, yet interconnected and overlapping, molecular pathways are likely responsible for the development of noninvasive and invasive bladder tumors. Somatic mutations in fibroblast growth receptor3 (FGFR-3) and tumor protein p53 (TP53) in tumor cells appear to be important early molecular events in the noninvasive and invasive pathways, respectively.
FGFR-3, Ras, and PIK3CA mutations occur with high frequency in noninvasive tumors, leading to upregulation of Akt and mitogen-activated protein kinase (MAPK). [11, 12] Loss of heterozygosity (LOH) on chromosome 9 is among the most frequent genetic alterations in bladder tumors and is considered an early event. [13]
Large numbers of genomic changes have been detected using karyotyping and comparative genomic hybridization (CGH) analysis in urothelial carcinoma. Numerically common are losses of 2q, 5q, 8p, 9p, 10q, 18q, and Y. Gains of 1q, 5p, 8q, and 17q are frequently present, and high-level amplifications can be found; however, the target genes in the regions of amplifications have not been conclusively identified. [14]
Alterations in the TP53 gene are noted in approximately 60% of invasive bladder cancers. Progression-free survival is significantly shorter in patients with TP53 mutations and is an independent predictor of death among patients with muscle-invasive bladder cancer. [15]
Additionally, alterations in retinoblastoma (Rb), PTEN, and p16 are common in high-grade invasive cancers. [16] Overexpression of JUN, MAP2K6, STAT3, and ICAM1 and molecules associated with survival (Bcl-2, caspase-3, p53, survivin), as well as insensitivity to antigrowth signals (p53, p21, p16, pRB), has been demonstrated. [17]
In advanced disease, multiple mechanisms may lead to tumor progression. These include those that promote proliferation, survival, invasion, and metastasis, as well as those that involve deficiencies in DNA damage repair and the finding of stemlike cells.
In addition to tumor cell alterations, the microenvironment may promote tumor growth by paracrine influences, including vascular endothelial growth factor (VEGF) production and aberrant E-cadherin expression. Finally, a growing body of research indicates that epigenetic alterations may silence tumor suppressor genes and that they represent important events in tumor progression. [18, 19, 20]

Etiology
Up to 80% of bladder cancer cases are associated with environmental exposure. Tobacco use is by far the most common cause of bladder cancer in the United States and is increasing in importance in some developing countries. Smoking duration and intensity are directly related to increased risk. [21, 22, 2]
The risk of developing bladder carcinoma is 2-6 times greater in smokers than in nonsmokers. This risk appears to be similar between men and women. [23] Nitrosamine, 2-naphthylamine, and 4-aminobiphenyl are possible carcinogenic agents found in cigarette smoke.
A number of occupations involve exposure to substances that may increase risk for bladder cancer. Of occupationally related bladder cancer cases, the incidence rate is highest in workers exposed to aromatic amines, while mortality is greatest in those exposed to polycyclic aromatic hydrocarbons and heavy metals. [24]
Numerous occupations associated with diesel exhaust, petroleum products, and solvents (eg, auto work, truck driving, plumbing, leather and apparel work, rubber and metal work) have also been associated with an increased risk of bladder cancer. In addition, increased bladder carcinoma risk has been reported in persons, including the following, who work with organic chemicals and dyes:
- Beauticians
- Dry cleaners
- Painters
- Paper production workers
- Rope-and-twine industry workers
- Dental workers
- Physicians
- Barbers
People living in urban areas are also more likely to develop bladder cancer. The etiology in these cases is thought to be multifactorial, potentially involving exposure to numerous carcinogens.
Arsenic exposure may be a factor in the development of bladder cancer. Results of a population-based case-control study in Maine, New Hampshire, and Vermont support an association between low-to-moderate levels of arsenic in drinking water and bladder cancer risk in those states, where incidence rates of bladder cancer have long been about 20% higher than in the United States overall. [25] A likely source of the arsenic is residue of arsenic-based pesticides, which were used extensively on crops such as blueberries, apples, and potatoes in that region from the 1920s through to the 1950s. [26]
Several medical risk factors are associated with an increased risk of bladder cancer, including the following:
- Radiation treatment of the pelvis
- Chemotherapy with cyclophosphamide - Increases the risk of bladder cancer via exposure to acrolein, a urinary metabolite of cyclophosphamide [27]
- Spinal cord injuries requiring long-term indwelling catheters - A 16- to 20-fold increase in the risk of developing SCC of the bladder
Although certain common genetic polymorphisms appear to increase susceptibility in persons with occupational exposure associated with increased bladder cancer risk, [28] no convincing evidence exists for a hereditary factor in the development of bladder cancer. Nevertheless, familial clusters of bladder cancer have been reported.
Schistosomiasis
In many developing countries, particularly in the Middle East, Schistosoma haematobium infection causes most cases of bladder SCC. In a study from Egypt, 82% of patients with bladder carcinoma harbored S haematobium eggs in the bladder wall. In egg-positive patients, the tumor tended to develop at a younger age (with SCC predominant) than it did in egg-negative persons. A higher degree of adenocarcinoma has also been reported in schistosomal-associated bladder carcinomas. [29]
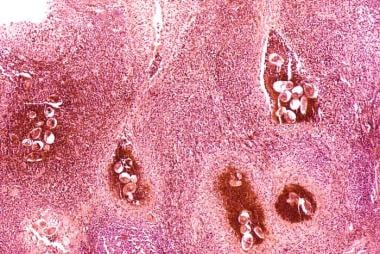
Along with S haematobium, the species S mansoni and S japonicum are responsible for schistosomiasis in humans. The eggs reside in the pelvic and mesenteric venous plexus. In the bladder, a severe inflammatory response and fibrosis secondary to the deposition of Schistosoma eggs is common. (See the image below.)
Bladder cancer. Histopathology of bladder shows eggs of Schistosoma haematobium surrounded by intense infiltrates of eosinophils and other inflammatory cells.
The eggs are found embedded in the lamina propria and muscularis propria of the bladder wall. Many of the eggs are destroyed by host reaction and become calcified, resulting in a lesion commonly known as a sandy patch, which appears as a granular, yellow-tan surface lesion.
In normal epithelial cells, S haematobium total antigen reportedly induces increased proliferation, migration, and invasion and decreases apoptosis. [30] Keratinous squamous metaplasia has been associated with the increased risk of developing SCC, with approximately one half of the cases arising subsequent to the metaplasia. [31, 32]
The majority of schistosomiasis-related cases of SCC will arise in the setting of chronic cystitis. [33] Chronic irritation secondary to lithiasis, [6, 7] urinary retention, and indwelling catheters has also been linked to the development of SCC. [7]
Other squamous cell carcinoma risk factors
Having bladder diverticula may increase an individual’s chance of developing SCC. [34] Rarely, bacillus Calmette-Guerin (BCG) treatment for CIS has been reported to lead to development of SCC. [35] Development of bladder cancer at a younger age has been associated with bladder exstrophy. [36, 37, 38, 39] SCC has also been described in urachal remnants. [40, 41, 42, 43, 44]
Coffee consumption does not increase the risk of developing bladder cancer. Early studies of rodents and a minority of human studies suggested a weak connection between artificial sweeteners (eg, saccharin, cyclamate) and bladder cancer; however, most recent studies show no significant correlation.

Epidemiology
Occurrence in the United States
The American Cancer Society estimates that 83,190 new cases of bladder cancer will be diagnosed in the United States in 2024 and that 16,840 people will die of the disease. [45] The incidence of bladder cancer increases with age, with the median age at diagnosis being 73 years; bladder cancer is rarely diagnosed before age 40 years. [46]
Bladder cancer is about 4 times more common in men than in women. [45] The male predominance in bladder cancer in the United States reflects the prevalence of transitional cell carcinoma (TCC). With small cell carcinoma—in contrast to TCC—the male-to-female incidence ratio is 1:2.
Bladder cancer is the fourth most common cancer in men in the United States, after prostate, lung, and colorectal cancer, but it is not among the top 10 cancers in women. Accordingly, more men than women are expected to die of bladder cancer in 2024, with 12,290 deaths in men versus 4550 in women. [45] Nevertheless, women generally have a worse prognosis than men.
The incidence of bladder cancer is twice as high in White men as in Black men in the United States. However, Blacks have a worse prognosis than Whites. [46, 47]
From 2000 to 2019, incidence and death rates for bladder cancer decreased in most racial and ethnic groups in both men and women in the US. On average, incidence rates decreased by 1.88% annually in men and 1.34% in women; death rates decreased by 2.16% in men and 2.44% in women. However, incidence rates showed a steady increase in American Indian and Alaska Native men and women, and death rates stabilized in Asian American and Pacific Islander men and Hispanic women. [48]
Limited data indicate that small cell carcinoma of the urinary bladder probably has the same epidemiologic characteristics as urothelial carcinoma. Patients are more likely to be male and older than 50 years. [49, 50]
International occurrence
Worldwide, bladder cancer is diagnosed in approximately 275,000 people each year, and about 108,000 die of this disease. In industrialized countries, 90% of bladder cancers are TCC. In developing countries—particularly in the Middle East and Africa—the majority of bladder cancers are SCCs, and most of these cancers are secondary to Schistosoma haematobium infection. Urothelial carcinoma is reported to be the most common urologic cancer in China.
In Africa, the highest incidence of SCC has been seen in schistosomal-endemic areas, notably Sudan and Egypt, where SCC ranges from two thirds to three quarters of all malignant tumors of the bladder. Since the turn of the century, a few studies from Egypt have shown a reversal of this trend due to the better control of schistosomiasis in the region, whereas in other parts of Africa the association is unchanged. [10, 51, 52] Increased smoking incidence is believed to have contributed to the shift in Egypt toward TCC, which has a stronger smoking association.

Prognosis
Untreated bladder cancer produces significant morbidity, including the following:
- Hematuria
- Dysuria
- Irritative urinary symptoms
- Urinary retention
- Urinary incontinence
- Ureteral obstruction
- Pelvic pain
The recurrence rate for superficial TCC of the bladder is high. As many as 80% of patients have at least one recurrence.
The most significant prognostic factors for bladder cancer are grade, depth of invasion, and the presence of CIS. In patients undergoing radical cystectomy for muscle-invasive bladder cancer, the presence of nodal involvement is the most important prognostic factor. To date, there is no convincing evidence of genetic factors affecting outcome. [53]
Early-stage non–muscle-invasive bladder cancer has a good prognosis. The 5-year survival rate decreases with increasing stage, as follows:
- Ta, T1, CIS – 82-100%
- T2 – 63-83%
- T3a – 67-71%
- T3b – 17-57%
- T4 – 0-22%
Prognosis for patients with metastatic urothelial cancer is poor, with only 5-10% of patients living 2 years after diagnosis.
The risk of progression, defined as an increased tumor grade or stage, depends primarily on the tumor grade, as follows:
- Grade I – 2-4%
- Grade II – 5-7%
- Grade III – 33-64%
Prognosis in carcinoma in situ
CIS in association with T1 papillary tumor carries a poorer prognosis. It has a recurrence rate of 63-92% and a rate of progression to muscle invasion of 50-75% despite intravesical BCG. Diffuse CIS is an especially ominous finding; in one study, 78% of cases progressed to muscle-invasive disease. [54]
Prognosis in squamous cell carcinoma
Tumor stage, lymph node involvement, and tumor grade have been shown to be of independent prognostic value in SCC. [55, 56] However, pathologic stage is the most important prognostic factor. In one relatively large series of 154 cases, the overall 5-year survival rate was 56% for pT1 and 68% for pT2 tumors. However, the 5-year survival rate for pT3 and pT4 tumors was only 19%. [53]
Several studies have demonstrated grading to be a significant morphologic parameter in SCC. [53] In one series, 5-year survival rates for grade 1, 2, and 3 SCC was 62%, 52%, and 35%, respectively. [53] In the same study of patients undergoing cystectomy, the investigators suggested that a higher number of newly formed blood vessels predicts unfavorable disease outcome.
In SCC, the survival rate appears to be better with radical surgery than with radiation therapy and/or chemotherapy. In locally advanced tumors, however, neoadjuvant radiation improves the outcome. [57] Sex and age have not been prognostically significant in SCC. [53]
Prognosis in small cell carcinoma
Patients with small cell carcinoma of the bladder usually have disease in an advanced stage at diagnosis, and they have a poor prognosis. [58, 59, 60] Overall median survival is only 1.7 years. The 5-year survival rates for stage II, III, and IV disease are 64%, 15%, and 11%, respectively. [61]
Recurrent bladder cancer
Bladder cancer has the highest recurrence rate of any malignancy (ie, 70% within 5 y). Although most patients with bladder cancer can be treated initially with organ-sparing therapy, most experience either recurrence or progression. The underlying genetic changes that result in a bladder tumor occur in the entire urothelium, making the whole lining of the urinary system susceptible to tumor recurrence.
Risk factors for recurrence and progression include the following [62, 63] :
- Female sex
- Larger tumor size
- Multifocality
- Larger number of tumors
- High tumor grade
- Advanced stage
- Presence of CIS
The time interval to recurrence is also significant. Patients with tumor recurrences within 2 years, and especially with recurrences within 3-6 months, have an aggressive tumor and an increased risk of disease progression.

- [Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Bladder Cancer. NCCN.org. Available at https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Version 4.2024 — May 9, 2024; Accessed: May 10, 2024.
- Escudero DO, Shirodkar SP, Lokeshwar VB. Bladder Carcinogenesis and Molecular Pathways. Lokeshwar VB. Bladder Tumors: Molecular Aspects and Clinical Management. New York: Springer Science; 2010. 23-41.
- Spruck CH 3rd, Ohneseit PF, Gonzalez-Zulueta M, Esrig D, Miyao N, Tsai YC, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res. 1994 Feb 1. 54(3):784-8. [QxMD MEDLINE Link].
- Trias I, Algaba F, Condom E, Español I, Seguí J, Orsola I, et al. Small cell carcinoma of the urinary bladder. Presentation of 23 cases and review of 134 published cases. Eur Urol. 2001 Jan. 39(1):85-90. [QxMD MEDLINE Link].
- Compérat EM, Burger M, Gontero P, Mostafid AH, Palou J, Rouprêt M, et al. Grading of Urothelial Carcinoma and The New "World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016". Eur Urol Focus. 2019 May. 5 (3):457-466. [QxMD MEDLINE Link]. [Full Text].
- Bessette PL, Abell MR, Herwig KR. A clinicopathologic study of squamous cell carcinoma of the bladder. J Urol. 1974 Jul. 112(1):66-7. [QxMD MEDLINE Link].
- Faysal MH. Squamous cell carcinoma of the bladder. J Urol. 1981 Nov. 126(5):598-9. [QxMD MEDLINE Link].
- Lagwinski N, Thomas A, Stephenson AJ, Campbell S, Hoschar AP, El-Gabry E, et al. Squamous cell carcinoma of the bladder: a clinicopathologic analysis of 45 cases. Am J Surg Pathol. 2007 Dec. 31(12):1777-87. [QxMD MEDLINE Link].
- El-Sebaie M, Zaghloul MS, Howard G, Mokhtar A. Squamous cell carcinoma of the bilharzial and non-bilharzial urinary bladder: a review of etiological features, natural history, and management. Int J Clin Oncol. 2005 Feb. 10(1):20-5. [QxMD MEDLINE Link].
- Heyns CF, van der Merwe A. Bladder cancer in Africa. Can J Urol. 2008 Feb. 15(1):3899-908. [QxMD MEDLINE Link].
- Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol. 2007 Sep. 213(1):91-8. [QxMD MEDLINE Link]. [Full Text].
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005 Apr. 16(2):139-49. [QxMD MEDLINE Link].
- Fadl-Elmula I. Chromosomal changes in uroepithelial carcinomas. Cell Chromosome. 2005 Aug 7. 4:1. [QxMD MEDLINE Link]. [Full Text].
- Knowles MA. Molecular subtypes of bladder cancer: Jekyll and Hyde or chalk and cheese?. Carcinogenesis. 2006 Mar. 27(3):361-73. [QxMD MEDLINE Link].
- Salinas-Sánchez AS, Lorenzo-Romero JG, Giménez-Bachs JM, Sánchez-Sánchez F, Donate-Moreno MJ, Rubio-Del-Campo A, et al. Implications of p53 gene mutations on patient survival in transitional cell carcinoma of the bladder: a long-term study. Urol Oncol. 2008 Nov-Dec. 26(6):620-6. [QxMD MEDLINE Link].
- Miyamoto H, Shuin T, Ikeda I, Hosaka M, Kubota Y. Loss of heterozygosity at the p53, RB, DCC and APC tumor suppressor gene loci in human bladder cancer. J Urol. 1996 Apr. 155(4):1444-7. [QxMD MEDLINE Link].
- Karam JA, Lotan Y, Karakiewicz PI, Ashfaq R, Sagalowsky AI, Roehrborn CG, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007 Feb. 8(2):128-36. [QxMD MEDLINE Link].
- Campbell SC, Volpert OV, Ivanovich M, Bouck NP. Molecular mediators of angiogenesis in bladder cancer. Cancer Res. 1998 Mar 15. 58(6):1298-304. [QxMD MEDLINE Link].
- Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009 Aug 18. 106(33):14016-21. [QxMD MEDLINE Link]. [Full Text].
- Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006 Feb 10. 24(5):778-89. [QxMD MEDLINE Link].
- Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000 Apr 15. 86(2):289-94. [QxMD MEDLINE Link].
- Fortuny J, Kogevinas M, Chang-Claude J, González CA, Hours M, Jöckel KH, et al. Tobacco, occupation and non-transitional-cell carcinoma of the bladder: an international case-control study. Int J Cancer. 1999 Jan 5. 80(1):44-6. [QxMD MEDLINE Link].
- Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011 Aug 17. 306(7):737-45. [QxMD MEDLINE Link]. [Full Text].
- Cumberbatch MG, Cox A, Teare D, Catto JW. Contemporary Occupational Carcinogen Exposure and Bladder Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2015 Dec. 1 (9):1282-90. [QxMD MEDLINE Link].
- Baris D, Waddell R, Beane Freeman LE, Schwenn M, Colt JS, et al. Elevated Bladder Cancer in Northern New England: The Role of Drinking Water and Arsenic. J Natl Cancer Inst. 2016 Sep. 108 (9):[QxMD MEDLINE Link].
- Nelson R. Arsenic-Contaminated Well Water Boosts Bladder Cancer Risk. Medscape Medical News. Available at https://www.medscape.com/viewarticle/862914. May 5, 2016; Accessed: May 7, 2016.
- Stein JP, Skinner EC, Boyd SD, Skinner DG. Squamous cell carcinoma of the bladder associated with cyclophosphamide therapy for Wegener's granulomatosis: a report of 2 cases. J Urol. 1993 Mar. 149(3):588-9. [QxMD MEDLINE Link].
- Figueroa JD, Koutros S, Colt JS, Kogevinas M, Garcia-Closas M, et al. Modification of Occupational Exposures on Bladder Cancer Risk by Common Genetic Polymorphisms. J Natl Cancer Inst. 2015 Nov. 107 (11):[QxMD MEDLINE Link].
- El-Bolkainy MN, Mokhtar NM, Ghoneim MA, Hussein MH. The impact of schistosomiasis on the pathology of bladder carcinoma. Cancer. 1981 Dec 15. 48(12):2643-8. [QxMD MEDLINE Link].
- Botelho M, Ferreira AC, Oliveira MJ, Domingues A, Machado JC, da Costa JM. Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. Int J Parasitol. 2009 Aug. 39(10):1083-91. [QxMD MEDLINE Link].
- Ahmad I, Barnetson RJ, Krishna NS. Keratinizing squamous metaplasia of the bladder: a review. Urol Int. 2008. 81(3):247-51. [QxMD MEDLINE Link].
- Khan MS, Thornhill JA, Gaffney E, Loftus B, Butler MR. Keratinising squamous metaplasia of the bladder: natural history and rationalization of management based on review of 54 years experience. Eur Urol. 2002 Nov. 42(5):469-74. [QxMD MEDLINE Link].
- Newman DM, Brown JR, Jay AC, Pontius EE. Squamous cell carcinoma of the bladder. J Urol. 1968 Oct. 100(4):470-3. [QxMD MEDLINE Link].
- Faysal MH, Freiha FS. Primary neoplasm in vesical diverticula. A report of 12 cases. Br J Urol. 1981 Apr. 53(2):141-3. [QxMD MEDLINE Link].
- Yurdakul T, Avunduk MC, Piskin MM. Pure squamous cell carcinoma after intravesical BCG treatment. A case report. Urol Int. 2005. 74(3):283-5. [QxMD MEDLINE Link].
- STUART WT. Carcinoma of the bladder associated with exstrophy. Report of a case and review of the literature. Va Med Mon (1918). 1962 Jan. 89:39-42. [QxMD MEDLINE Link].
- Ribeiro JC, Silva C, Sousa L, García P, Santos A. [Squamous cell carcinoma in bladder extrophy]. Actas Urol Esp. 2005 Jan. 29(1):110-2. [QxMD MEDLINE Link].
- Gupta S, Gupta IM. Ectopia vesicae complicated by squamous cell carcinoma. Br J Urol. 1976 Aug. 48(4):244. [QxMD MEDLINE Link].
- Rieder JM, Parsons JK, Gearhart JP, Schoenberg M. Primary squamous cell carcinoma in unreconstructed exstrophic bladder. Urology. 2006 Jan. 67(1):199. [QxMD MEDLINE Link].
- Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant urachal lesions. J Urol. 1984 Jan. 131(1):1-8. [QxMD MEDLINE Link].
- Lin RY, Rappoport AE, Deppisch LM, Natividad NS, Katz W. Squamous cell carcinoma of the urachus. J Urol. 1977 Dec. 118(6):1066-7. [QxMD MEDLINE Link].
- SHAW RE. Squamous-cell carcinoma in a cyst of the urachus. Br J Urol. 1958 Mar. 30(1):87-9. [QxMD MEDLINE Link].
- Chow YC, Lin WC, Tzen CY, Chow YK, Lo KY. Squamous cell carcinoma of the urachus. J Urol. 2000 Mar. 163(3):903-4. [QxMD MEDLINE Link].
- Fujiyama C, Nakashima N, Tokuda Y, Uozumi J. Squamous cell carcinoma of the urachus. Int J Urol. 2007 Oct. 14(10):966-8. [QxMD MEDLINE Link].
- Cancer Facts & Figures 2024. American Cancer Society. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf. Accessed: May 10, 2024.
- Cancer Stat Facts: Bladder Cancer. National Cancer Institute. Available at https://seer.cancer.gov/statfacts/html/urinb.html. Accessed: January 23, 2023.
- Dawson C, Whitfield H. ABC of Urology. Urological malignancy--II: Urothelial tumours. BMJ. 1996 Apr 27. 312(7038):1090-4. [QxMD MEDLINE Link]. [Full Text].
- Schafer EJ, Jemal A, Wiese D, Sung H, Kratzer TB, Islami F, et al. Disparities and Trends in Genitourinary Cancer Incidence and Mortality in the USA. Eur Urol. 2022 Dec 21. [QxMD MEDLINE Link]. [Full Text].
- Abrahams NA, Moran C, Reyes AO, Siefker-Radtke A, Ayala AG. Small cell carcinoma of the bladder: a contemporary clinicopathological study of 51 cases. Histopathology. 2005 Jan. 46(1):57-63. [QxMD MEDLINE Link].
- Lohrisch C, Murray N, Pickles T, Sullivan L. Small cell carcinoma of the bladder: long term outcome with integrated chemoradiation. Cancer. 1999 Dec 1. 86(11):2346-52. [QxMD MEDLINE Link].
- Gouda I, Mokhtar N, Bilal D, El-Bolkainy T, El-Bolkainy NM. Bilharziasis and bladder cancer: a time trend analysis of 9843 patients. J Egypt Natl Canc Inst. 2007 Jun. 19(2):158-62. [QxMD MEDLINE Link].
- Felix AS, Soliman AS, Khaled H, Zaghloul MS, Banerjee M, El-Baradie M, et al. The changing patterns of bladder cancer in Egypt over the past 26 years. Cancer Causes Control. 2008 May. 19(4):421-9. [QxMD MEDLINE Link].
- Elsobky E, El-Baz M, Gomha M, Abol-Enein H, Shaaban AA. Prognostic value of angiogenesis in schistosoma-associated squamous cell carcinoma of the urinary bladder. Urology. 2002 Jul. 60(1):69-73. [QxMD MEDLINE Link].
- Griffiths TR, Charlton M, Neal DE, Powell PH. Treatment of carcinoma in situ with intravesical bacillus Calmette-Guerin without maintenance. J Urol. 2002 Jun. 167(6):2408-12. [QxMD MEDLINE Link].
- Pycha A, Mian C, Posch B, Haitel A, Mokhtar AA, El-Baz M, et al. Numerical chromosomal aberrations in muscle invasive squamous cell and transitional cell cancer of the urinary bladder: an alternative to classic prognostic indicators?. Urology. 1999 May. 53(5):1005-10. [QxMD MEDLINE Link].
- Shaaban AA, Javadpour N, Tribukait B, Ghoneim MA. Prognostic significance of flow-DNA analysis and cell surface isoantigens in carcinoma of bilharzial bladder. Urology. 1992 Mar. 39(3):207-10. [QxMD MEDLINE Link].
- Ghoneim MA, Ashamallah AK, Awaad HK, Whitmore WF Jr. Randomized trial of cystectomy with or without preoperative radiotherapy for carcinoma of the bilharzial bladder. J Urol. 1985 Aug. 134(2):266-8. [QxMD MEDLINE Link].
- Cheng L, Pan CX, Yang XJ, Lopez-Beltran A, MacLennan GT, Lin H, et al. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer. 2004 Sep 1. 101(5):957-62. [QxMD MEDLINE Link].
- Shahab N. Extrapulmonary small cell carcinoma of the bladder. Semin Oncol. 2007 Feb. 34(1):15-21. [QxMD MEDLINE Link].
- Mackey JR, Au HJ, Hugh J, Venner P. Genitourinary small cell carcinoma: determination of clinical and therapeutic factors associated with survival. J Urol. 1998 May. 159(5):1624-9. [QxMD MEDLINE Link].
- Choong NW, Quevedo JF, Kaur JS. Small cell carcinoma of the urinary bladder. The Mayo Clinic experience. Cancer. 2005 Mar 15. 103(6):1172-8. [QxMD MEDLINE Link].
- van Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009 Sep. 56(3):430-42. [QxMD MEDLINE Link].
- Fernandez-Gomez J, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, Hernandez R, et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin: multivariate analysis of data from four randomized CUETO trials. Eur Urol. 2008 May. 53(5):992-1001. [QxMD MEDLINE Link].
- [Guideline] Holzbeierlein JM, Bixler BR, Buckley DI, Chang SS, Holmes R, James AC, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. J Urol. 2024 Apr. 211 (4):533-538. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016 Oct. 196 (4):1021-9. [QxMD MEDLINE Link].
- [Guideline] Barocas DA, Boorjian SA, Alvarez RD, Downs TM, Gross CP, Hamilton BD, et al. Microhematuria: AUA/SUFU Guideline. J Urol. 2020 Oct. 204 (4):778-786. [QxMD MEDLINE Link]. [Full Text].
- Cha EK, Tirsar LA, Schwentner C, Christos PJ, Mian C, Hennenlotter J, et al. Immunocytology is a strong predictor of bladder cancer presence in patients with painless hematuria: a multicentre study. Eur Urol. 2012 Jan. 61(1):185-92. [QxMD MEDLINE Link].
- Strittmatter F, Buchner A, Karl A, Sommer ML, Straub J, Tilki D, et al. Individual learning curve reduces the clinical value of urinary cytology. Clin Genitourin Cancer. 2011 Sep. 9(1):22-6. [QxMD MEDLINE Link].
- Lotan Y, Roehrborn CG. Cost-effectiveness of a modified care protocol substituting bladder tumor markers for cystoscopy for the followup of patients with transitional cell carcinoma of the bladder: a decision analytical approach. J Urol. 2002 Jan. 167(1):75-9. [QxMD MEDLINE Link].
- [Guideline] Holzbeierlein J, Bixler BR, Buckley DI, Chang SS, Holmes RS, James AC, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/SUO GUIDELINE (2017; Amended 2020, 2024). J Urol. 2024 Apr 25. 101097JU0000000000003981. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022 Jan. 81 (1):75-94. [QxMD MEDLINE Link]. [Full Text].
- Apolo AB, Vogelzang NJ, Theodorescu D. New and promising strategies in the management of bladder cancer. Am Soc Clin Oncol Educ Book. 2015. 35:105-12. [QxMD MEDLINE Link]. [Full Text].
- Rose KM, Huelster HL, Meeks JJ, Faltas BM, Sonpavde GP, Lerner SP, et al. Circulating and urinary tumour DNA in urothelial carcinoma - upper tract, lower tract and metastatic disease. Nat Rev Urol. 2023 Jul. 20 (7):406-419. [QxMD MEDLINE Link].
- Murphy WM, Crabtree WN, Jukkola AF, Soloway MS. The diagnostic value of urine versus bladder washing in patients with bladder cancer. J Urol. 1981 Sep. 126(3):320-2. [QxMD MEDLINE Link].
- Lokeshwar VB, Soloway MS. Current bladder tumor tests: does their projected utility fulfill clinical necessity?. J Urol. 2001 Apr. 165(4):1067-77. [QxMD MEDLINE Link].
- Grossman HB, Soloway M, Messing E, Katz G, Stein B, Kassabian V, et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006 Jan 18. 295(3):299-305. [QxMD MEDLINE Link].
- Al-Sukhun S, Hussain M. Molecular biology of transitional cell carcinoma. Crit Rev Oncol Hematol. 2003 Aug. 47(2):181-93. [QxMD MEDLINE Link].
- Halling KC, Kipp BR. Bladder cancer detection using FISH (UroVysion assay). Adv Anat Pathol. 2008 Sep. 15(5):279-86. [QxMD MEDLINE Link].
- Sanguedolce F, Russo D, Calò B, Cindolo L, Carrieri G, Cormio L. Diagnostic and prognostic roles of CK20 in the pathology of urothelial lesions. A systematic review. Pathol Res Pract. 2019 Jun. 215 (6):152413. [QxMD MEDLINE Link].
- Sugeeta SS, Sharma A, Ng K, Nayak A, Vasdev N. Biomarkers in Bladder Cancer Surveillance. Front Surg. 2021. 8:735868. [QxMD MEDLINE Link]. [Full Text].
- Tomiyama E, Fujita K, Hashimoto M, Uemura H, Nonomura N. Urinary markers for bladder cancer diagnosis: A review of current status and future challenges. Int J Urol. 2024 Mar. 31 (3):208-219. [QxMD MEDLINE Link].
- Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007 Dec. 178(6):2314-30. [QxMD MEDLINE Link].
- American Joint Committee on Cancer. Urinary bladder. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017.
- Svatek RS, Daneshmand S, Singh P. Bladder Neoplasms: Muscle Invasive Bladder Cancer. AUA. Available at https://university.auanet.org/modules/webapps/core/index.cfm#/corecontent/177. March 1, 2021; Accessed: January 23, 2023.
- PDQ Adult Treatment Editorial Board. Bladder Cancer Treatment (PDQ®): Health Professional Version. January 18, 2023. [QxMD MEDLINE Link]. [Full Text].
- Chedgy EC, Black PC. Radical Cystectomy and the Multidisciplinary Management of Muscle-Invasive Bladder Cancer. JAMA Oncol. 2016 May 5. [QxMD MEDLINE Link]. [Full Text].
- Mitin T. Rethinking Radical Cystectomy as the Best Choice for Most Patients With Muscle-Invasive Bladder Cancer. JAMA Oncol. 2016 May 5. [QxMD MEDLINE Link]. [Full Text].
- Teo MT, Dyrskjøt L, Nsengimana J, Buchwald C, Snowden H, Morgan J, et al. Next-generation sequencing identifies germline MRE11A variants as markers of radiotherapy outcomes in muscle-invasive bladder cancer. Ann Oncol. 2014 Apr. 25 (4):877-83. [QxMD MEDLINE Link]. [Full Text].
- Serretta V, Galuffo A, Pavone C, Allegro R, Pavone-MacAluso M. Gemcitabine in intravesical treatment of Ta-T1 transitional cell carcinoma of bladder: Phase I-II study on marker lesions. Urology. 2005 Jan. 65(1):65-9. [QxMD MEDLINE Link].
- Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol. 2005 Jul. 174(1):86-91; discussion 91-2. [QxMD MEDLINE Link].
- Witjes JA, Hendricksen K. Intravesical pharmacotherapy for non-muscle-invasive bladder cancer: a critical analysis of currently available drugs, treatment schedules, and long-term results. Eur Urol. 2008 Jan. 53(1):45-52. [QxMD MEDLINE Link].
- Zaharoff DA, Hoffman BS, Hooper HB, Benjamin CJ Jr, Khurana KK, Hance KW, et al. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Res. 2009 Aug 1. 69(15):6192-9. [QxMD MEDLINE Link]. [Full Text].
- Islam MA, Bhuiyan ZH, Shameem IA. Intravesical adjuvant therapy using mitomycin C. Mymensingh Med J. 2006 Jan. 15(1):40-4. [QxMD MEDLINE Link].
- Herr HW, Dalbagni G, Donat SM. Bacillus Calmette-Guérin without maintenance therapy for high-risk non-muscle-invasive bladder cancer. Eur Urol. 2011 Jul. 60(1):32-6. [QxMD MEDLINE Link].
- Schmidbauer J, Witjes F, Schmeller N, Donat R, Susani M, Marberger M. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004 Jan. 171(1):135-8. [QxMD MEDLINE Link].
- Jichlinski P, Guillou L, Karlsen SJ, Malmström PU, Jocham D, Brennhovd B, et al. Hexyl aminolevulinate fluorescence cystoscopy: new diagnostic tool for photodiagnosis of superficial bladder cancer--a multicenter study. J Urol. 2003 Jul. 170(1):226-9. [QxMD MEDLINE Link].
- Hungerhuber E, Stepp H, Kriegmair M, Stief C, Hofstetter A, Hartmann A, et al. Seven years' experience with 5-aminolevulinic acid in detection of transitional cell carcinoma of the bladder. Urology. 2007 Feb. 69(2):260-4. [QxMD MEDLINE Link].
- Kausch I, Sommerauer M, Montorsi F, Stenzl A, Jacqmin D, Jichlinski P, et al. Photodynamic diagnosis in non-muscle-invasive bladder cancer: a systematic review and cumulative analysis of prospective studies. Eur Urol. 2010 Apr. 57(4):595-606. [QxMD MEDLINE Link].
- Waknine Y. FDA Approves Cysview for Cystoscopic Detection of Papillary Bladder Cancer. Medscape Medical News, June 4, 2010. Available at https://www.medscape.com/viewarticle/722923. Accessed: January 10, 2013.
- Booth CM, Tannock IF. Benefits of Adjuvant Chemotherapy for Bladder Cancer. JAMA Oncol. 2015 Sep. 1 (6):727-8. [QxMD MEDLINE Link].
- Standard or Extended Pelvic Lymphadenectomy in Treating Patients Undergoing Surgery for Invasive Bladder Cancer. ClinicalTrials.gov. ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT01224665. Accessed: May 15, 2024.
- Mukesh M, Cook N, Hollingdale AE, Ainsworth NL, Russell SG. Small cell carcinoma of the urinary bladder: a 15-year retrospective review of treatment and survival in the Anglian Cancer Network. BJU Int. 2009 Mar. 103(6):747-52. [QxMD MEDLINE Link].
- Brinkman MT, Karagas MR, Zens MS, Schned A, Reulen RC, Zeegers MP. Minerals and vitamins and the risk of bladder cancer: results from the New Hampshire Study. Cancer Causes Control. 2010 Apr. 21(4):609-19. [QxMD MEDLINE Link]. [Full Text].
- Boorjian SA, Alemozaffar M, Konety BR, Shore ND, Gomella LG, Kamat AM, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021 Jan. 22 (1):107-117. [QxMD MEDLINE Link]. [Full Text].
- FDA approves nogapendekin alfa inbakicept-pmln for BCG-unresponsive non-muscle invasive bladder cancer. U.S. Food & Drug Administration. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nogapendekin-alfa-inbakicept-pmln-bcg-unresponsive-non-muscle-invasive-bladder-cancer. April 22, 2024; Accessed: May 16, 2024.
- Chamie K, Chang SS, Kramolowsky EV, Gonzalgo ML, Huang M, Bhar P, et al. Quality of Life in the Phase 2/3 Trial of N-803 Plus Bacillus Calmette-Guérin in Bacillus Calmette-Guérin‒Unresponsive Nonmuscle-Invasive Bladder Cancer. Urol Pract. 2024 Mar. 11 (2):367-375. [QxMD MEDLINE Link]. [Full Text].
- O'Donnell MA, Lilli K, Leopold C. Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer. J Urol. 2004 Sep. 172(3):888-93. [QxMD MEDLINE Link].
- Nepple KG, Lightfoot AJ, Rosevear HM, O'Donnell MA, Lamm DL. Bacillus Calmette-Guérin with or without interferon a-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol. 2010 Nov. 184(5):1915-9. [QxMD MEDLINE Link].
- Kamat AM, Dickstein RJ, Messetti F, Anderson R, Pretzsch SM, Gonzalez GN, et al. Use of fluorescence in situ hybridization to predict response to bacillus Calmette-Guérin therapy for bladder cancer: results of a prospective trial. J Urol. 2012 Mar. 187(3):862-7. [QxMD MEDLINE Link]. [Full Text].
- Mulcahy N. FDA Approves Pembrolizumab for High-Risk Bladder Cancer. Medscape Medical News. Available at https://www.medscape.com/viewarticle/923568. January 8, 2020; Accessed: January 9, 2020.
- Balar AV, Kulkarni GS, Uchio EM, Boormans J, Mourey L, Krieger LEM, et al. Keynote 057: Phase II trial of Pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to bacillus calmette-guérin (BCG). Journal of Clinical Oncology 37, no. 7_suppl (March 01, 2019) 350-350. [Full Text].
- Barlow L, McKiernan JM, Benson MC. Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guérin therapy. J Urol. 2013 Mar. 189(3):834-9. [QxMD MEDLINE Link].
- Schmidbauer J, Witjes F, Schmeller N, Donat R, Susani M, Marberger M. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004 Jan. 171(1):135-8. [QxMD MEDLINE Link].
- Jichlinski P, Guillou L, Karlsen SJ, Malmström PU, Jocham D, Brennhovd B, et al. Hexyl aminolevulinate fluorescence cystoscopy: new diagnostic tool for photodiagnosis of superficial bladder cancer--a multicenter study. J Urol. 2003 Jul. 170(1):226-9. [QxMD MEDLINE Link].
- Hungerhuber E, Stepp H, Kriegmair M, Stief C, Hofstetter A, Hartmann A, et al. Seven years' experience with 5-aminolevulinic acid in detection of transitional cell carcinoma of the bladder. Urology. 2007 Feb. 69(2):260-4. [QxMD MEDLINE Link].
- Fradet Y, Grossman HB, Gomella L, Lerner S, Cookson M, Albala D, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol. 2007 Jul. 178(1):68-73; discussion 73. [QxMD MEDLINE Link].
- Jocham D, Witjes F, Wagner S, Zeylemaker B, van Moorselaar J, Grimm MO, et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol. 2005 Sep. 174(3):862-6; discussion 866. [QxMD MEDLINE Link].
- Stenzl A, Burger M, Fradet Y, Mynderse LA, Soloway MS, Witjes JA, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol. 2010 Nov. 184(5):1907-13. [QxMD MEDLINE Link].
- Hermann GG, Mogensen K, Carlsson S, Marcussen N, Duun S. Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in Ta/T1 patients: a randomized two-centre study. BJU Int. 2011 Oct. 108(8 Pt 2):E297-303. [QxMD MEDLINE Link].
- Tilki D, Reich O, Svatek RS, Karakiewicz PI, Kassouf W, Novara G, et al. Characteristics and outcomes of patients with clinical carcinoma in situ only treated with radical cystectomy: an international study of 243 patients. J Urol. 2010 May. 183(5):1757-63. [QxMD MEDLINE Link].
- Djaladat H, Bruins HM, Miranda G, Cai J, Skinner EC, Daneshmand S. Reproductive organ involvement in female patients undergoing radical cystectomy for urothelial bladder cancer. J Urol. Dec 2012. 188:2134-2138.
- Liedberg, F., Jancke, G., Sörenby, A., et al. Should we refrain from performing oophorectomy in conjunction with radical cystectomy for bladder cancer?. Eur Urol. 2017. 71:851-853.
- Nik, N.N., Vang, R., Shih, I.M., et al. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu Rev Pathol. 2014. 9:27-45.
- Davis JW, Castle EP, Pruthi RS, Ornstein DK, Guru KA. Robot-assisted radical cystectomy: an expert panel review of the current status and future direction. Urol Oncol. 2010 Sep-Oct. 28(5):480-6. [QxMD MEDLINE Link].
- Chang SS, Cookson MS. Radical cystectomy for bladder cancer: the case for early intervention. Urol Clin North Am. 2005 May. 32(2):147-55. [QxMD MEDLINE Link].
- Sánchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003 Jan. 169(1):110-5; discussion 115. [QxMD MEDLINE Link].
- Jürgen E Gschwend 1, Matthias M Heck 2, Jan Lehmann 3, Herbert Rübben 4, Peter Albers 5, Johannes M Wolff 6, et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. European Urology. Apr 2019. 75:604-611. [QxMD MEDLINE Link].
- Raghavan D, Burgess E, Gaston KE, Haake MR, Riggs SB. Neoadjuvant and adjuvant chemotherapy approaches for invasive bladder cancer. Semin Oncol. 2012 Oct. 39(5):588-97. [QxMD MEDLINE Link].
- Winquist E, Kirchner TS, Segal R, Chin J, Lukka H. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol. 2004 Feb. 171(2 Pt 1):561-9. [QxMD MEDLINE Link].
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003 Aug 28. 349(9):859-66. [QxMD MEDLINE Link].
- Herr HW, Faulkner JR, Grossman HB, Natale RB, deVere White R, Sarosdy MF, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004 Jul 15. 22(14):2781-9. [QxMD MEDLINE Link].
- von der Maase H1, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000 Sep;18. 17:3068-77.
- Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011 Jun 1. 29(16):2171-7. [QxMD MEDLINE Link]. [Full Text].
- Mooso BA, Vinall RL, Mudryj M, Yap SA, deVere White RW, Ghosh PM. The role of EGFR family inhibitors in muscle invasive bladder cancer: a review of clinical data and molecular evidence. J Urol. 2015 Jan. 193 (1):19-29. [QxMD MEDLINE Link].
- Sternberg CN, Skoneczna I, Kerst JM, Albers P, Fossa SD, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015 Jan. 16 (1):76-86. [QxMD MEDLINE Link].
- Saxman SB, Propert KJ, Einhorn LH, Crawford ED, Tannock I, Raghavan D, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997 Jul. 15(7):2564-9. [QxMD MEDLINE Link].
- Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006 Jan. 42(1):50-4. [QxMD MEDLINE Link].
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005 Jul 20. 23(21):4602-8. [QxMD MEDLINE Link].
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005 Jul 20. 23(21):4602-8. [QxMD MEDLINE Link].
- Iwasaki K, Obara W, Kato Y, Takata R, Tanji S, Fujioka T. Neoadjuvant gemcitabine plus carboplatin for locally advanced bladder cancer. Jpn J Clin Oncol. 2013 Feb. 43(2):193-9. [QxMD MEDLINE Link].
- Bellmunt J, Théodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009 Sep 20. 27(27):4454-61. [QxMD MEDLINE Link].
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016 Mar 4. [QxMD MEDLINE Link].
- Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017 Jan 7. 389 (10064):67-76. [QxMD MEDLINE Link].
- Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018 Feb 24. 391 (10122):748-757. [QxMD MEDLINE Link].
- Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017 Jan 25. [QxMD MEDLINE Link].
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021 Jun 3. 384 (22):2102-2114. [QxMD MEDLINE Link]. [Full Text].
- Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol. 2017 Apr 4. JCO2016716795. [QxMD MEDLINE Link].
- Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017 Nov. 18 (11):1483-1492. [QxMD MEDLINE Link].
- Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017 Mar 16. 376 (11):1015-1026. [QxMD MEDLINE Link].
- Hoimes CJ, Flaig TW, Milowsky MI, Friedlander TW, Bilen MA, Gupta S, et al. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J Clin Oncol. 2023 Jan 1. 41 (1):22-31. [QxMD MEDLINE Link]. [Full Text].
- Loriot Y, et al; BLC2001 Study Group. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019 Jul 25. 381 (4):338-348. [QxMD MEDLINE Link].
- Rosenberg JE, O'Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2019 Oct 10. 37 (29):2592-2600. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. 2020 Apr 29. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol. 2021 Jan. 79 (1):62-79. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022 Mar. 33 (3):244-258. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] U.S. Preventive Services Task Force. Final Recommendation Statement: Bladder Cancer in Adults: Screening. Available at https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/bladder-cancer-in-adults-screening. August 15, 2011; Accessed: May 10, 2024.
- Clinical Preventive Service Recommendation: Bladder Cancer. American Academy of Family Physicians. Available at https://www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/bladder-cancer.html. Accessed: February 2, 2023.
- Bladder Cancer. American Cancer Society. Available at https://www.cancer.org/cancer/bladder-cancer.html. Accessed: May 10, 2024.
- Flaig TW. NCCN Guidelines Updates: Management of Muscle-Invasive Bladder Cancer. J Natl Compr Canc Netw. 2019 May 1. 17 (5.5):591-593. [QxMD MEDLINE Link]. [Full Text].
Author
Kara N Babaian, MD, FACS Assistant Professor, Director of Urologic Oncology, Division of Urology, Southern Illinois University School of Medicine
Kara N Babaian, MD, FACS is a member of the following medical societies: American College of Surgeons, American Society of Clinical Oncology, American Urological Association, European Association of Urology, North Central Section of the American Urological Association (AUA), Society of Urologic Oncology, Society of Women in Urology
Disclosure: Participation in a clinical trial for: Janssen.
Coauthor(s)
Parker G Adams, BS DO Candidate, Kansas City University of Medicine and Biosciences College of Osteopathic Medicine
Parker G Adams, BS is a member of the following medical societies: American Urological Association
Disclosure: Nothing to disclose.
Courtney McClure, DO Resident Physician, Department of Urology, Ascension Healthcare
Disclosure: Nothing to disclose.
Brandon Tompkins, BS DO Candidate, Kansas City University of Medicine and Biosciences College of Osteopathic Medicine
Disclosure: Nothing to disclose.
Chief Editor
Bradley Fields Schwartz, DO, FACS Professor of Urology, Frank and Linda Vala Endowed Chair of Urology, Director, Center for Laparoscopy and Endourology, Department of Surgery, Division of Urology, Southern Illinois University School of Medicine
Bradley Fields Schwartz, DO, FACS is a member of the following medical societies: American College of Surgeons, American Urological Association, Association of Military Osteopathic Physicians and Surgeons, Endourological Society, Society of Laparoscopic and Robotic Surgeons, Society of University Urologists
Disclosure: Serve(d) as a director, officer, partner, employee, advisor, consultant or trustee for: Endourological Society Board of Directors
Serve(d) as a speaker or a member of a speakers bureau for: Cook Medical
Received research grant from: Cook Medical.
Additional Contributors
Gary D Steinberg, MD, FACS Professor and Director, Perlmutter Cancer Center, Goldstein Bladder Cancer Program, NYU Langone Health, NYU Urology Associates
Gary D Steinberg, MD, FACS is a member of the following medical societies: American Association for Cancer Research, American College of Surgeons, American Society of Clinical Oncology, American Urological Association, International Society of Urology, Society of Laparoscopic and Robotic Surgeons, Society of Urologic Oncology
Disclosure: Serve(d) as a director, officer, partner, employee, advisor, consultant or trustee for: Heat Biologics; CG Oncology; PhotoCure; Merck; Roche/Genentech; Ciclomed; Taris Biomedical (now Janssen); MDxHealth; Fidia Farmaceuticals; Urogen, Ferring; Aduro; Boston Scientific; Bristol Myers Squibb; Astra Zeneca; Pfizer, Janssen; others
Member of Clinical Trial Protocol Committees for: for: Merck; BMS; Janssen; CG Oncology; Pfizer; PhotoCure; Fidia; Seagen; Protara.
Kush Sachdeva, MD Southern Oncology and Hematology Associates, Inspira Health Network
Disclosure: Nothing to disclose.
Bagi RP Jana, MD, MBA, MHA, FACP Professor, Medical Director, Department of General Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center-Houston; Professor, Department of General Oncology, The University of Texas Medical Branch; Medical Oncology Section Chief-MDACC/UTMB Health Galveston Medical Oncology and Hematology Program, Department of General Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center-Galveston
Bagi RP Jana, MD, MBA, MHA, FACP is a member of the following medical societies: American Cancer Society, American Medical Association, American Society of Clinical Oncology, SWOG
Disclosure: Nothing to disclose.
Acknowledgements
Sujeet S Acharya, MD Resident Physician, Department of Surgery, Section of Urology, University of Chicago Division of the Biological Sciences, The Pritzker School of Medicine
Disclosure: Nothing to disclose.
Brendan Curti, MD Director, Genitourinary Oncology Research, Robert W Franz Cancer Research Center, Earle A Chiles Research Institute, Providence Cancer Center
Brendan Curti, MD is a member of the following medical societies: American College of Physicians, American Society of Clinical Oncology, Oregon Medical Association, and Society for Biological Therapy
Disclosure: Nothing to disclose.
Edward M Gong, MD Fellow, Department of Surgery, Division of Urology, Children's Hospital Boston
Disclosure: Nothing to disclose.
Mark H Katz, MD Fellow in Urologic Oncology and Minimally Invasive Surgery, University of Chicago Medical Center
Mark H Katz, MD is a member of the following medical societies: Alpha Omega Alpha, American Urological Association, Endourological Society, and Society of Urologic Oncology
Disclosure: Nothing to disclose.
Hyung L Kim, MD Associate Professor, Cedars-Sinai Medical Center
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Dan Theodorescu, MD, PhD Paul A Bunn Professor of Cancer Research, Professor of Surgery and Pharmacology, Director, University of Colorado Comprehensive Cancer Center
Dan Theodorescu, MD, PhD is a member of the following medical societies: American Cancer Society, American College of Surgeons, American Urological Association, Medical Society of Virginia, Society for Basic Urologic Research, and Society of Urologic Oncology
Disclosure: Key Genomics Ownership interest Co-Founder-50% Stock Ownership; KromaTiD, Inc Stock Options Board membership