Meniere Disease (Idiopathic Endolymphatic Hydrops): Background, Anatomy, Pathophysiology (original) (raw)
Overview
Background
Ménière disease is a disorder of the inner ear that is also known as idiopathic endolymphatic hydrops. Endolymphatic hydrops refers to a condition of increased hydraulic pressure within the inner ear endolymphatic system. Excess pressure accumulation in the endolymph can cause a tetrad of symptoms: (1) fluctuating hearing loss, (2) occasional episodic vertigo (usually a spinning sensation, sometimes violent), (3) tinnitus or ringing in the ears (usually low-tone roaring), and (4) aural fullness (eg, pressure, discomfort, fullness sensation in the ears).
The term endolymphatic hydrops is often used synonymously with Ménière disease and Ménière syndrome, both of which are both believed to result from increased pressure within the endolymphatic system. However, Ménière disease is idiopathic by definition, whereas Ménière syndrome can occur secondary to various processes interfering with normal production or resorption of endolymph (eg, endocrine abnormalities, trauma, electrolyte imbalance, autoimmune dysfunction, medications, parasitic infections, hyperlipidemia). With the growing understanding of the pathophysiology and disease processes involved with Ménière disease a re-evaluation and possible redefinition of this condition are well underway. [1]
The distinction in nomenclature is analogous to that applied to Bell palsy. When the source of facial paralysis is known, Bell palsy is not the diagnosis. Similarly, when the cause of vertigo is known, Ménière disease is not the diagnosis. In other words, Ménière syndrome is endolymphatic hydrops caused by a specific condition, and Ménière disease is endolymphatic hydrops of unknown etiology (ie, idiopathic endolymphatic hydrops).
Evaluation and management of dizziness and vertigo can be one of the most difficult medical tasks. Sources of imbalance can range from simple conditions (eg, dehydration) to serious conditions (eg, brain tumors). Central nervous system (CNS) problems must be distinguished from circulation anomalies, chemical and hormonal imbalances, and peripheral inner ear disorders. Often, this distinction is not clear. (For related information, see Dizziness, Vertigo, and Imbalance.)
Medical therapy can be directed toward treatment of the actual symptoms of the acute attack or directed toward prophylactic prevention of the attacks. If endolymphatic hydrops is attributable to a given disease process—that is, if it is Ménière syndrome rather than Ménière disease—the first-line management is diagnosis and treatment of the primary disease (eg, thyroid disease). Surgical therapy for Ménière disease is reserved for medical treatment failures and is otherwise controversial.
Go to Surgical Treatment of Meniere Disease for complete information on this topic.

Anatomy
The relevant anatomy centers on the petrous bone and the inner ear. The ear is divided into 3 sections: external, middle, and inner. The external ear consists of the auricle, external ear canal, and tympanic membrane. The tympanic membrane separates the external ear from the structures of the middle ear. The middle ear is an air-containing space that houses the 3 hearing bones: the malleus, the incus, and the stapes. The inner ear is completely encased in bone and consists of the cochlear-vestibular apparatus and its associated nerves.
The cochlear-vestibular apparatus is a complex structure arranged in a complex yet elegant spatial orientation. Because it is completely encased in bone, this structure is housed in a series of winding tunnels and interconnecting spaces. The mazelike orientation of these tunnels is appropriately named the labyrinth. The bone that encases it is the bony labyrinth.
The cochlea is a snail-shaped chamber that houses the organ of Corti. It is responsible for translating mechanical vibrations into electrical impulses and sending them to the brain through the cochlear nerve.
The vestibular system consists of a large chamber (ie, the vestibule) from which 3 semicircular canals protrude. Within the vestibule, 2 sensors (the utricle and the saccule), detect linear acceleration, and the semicircular canals detect rotational movements in the 3 planes of rotation. The vestibular apparatus gives off 2 nerves: the superior and the inferior vestibular nerves. Together with the cochlear and facial nerves, the vestibular nerves travel through the internal auditory canal to the cerebellopontine angle.
The cochlea and the vestibular system are joined in the middle and share a dual-chambered hydraulic system. These hydraulic chambers are bathed by 2 fluids: endolymph and perilymph. Endolymph is produced primarily by the stria vascularis in the cochlea and also by the planum semilunatum and the dark cells in the vestibular labyrinth. [2] Perilymph is protein-poor extracellular fluid. A membrane (ie, the membranous labyrinth) separates the fluids and completely surrounds and contains the endolymph.
The system may be visualized as a water balloon floating in a pool. In this analogy, the water inside the balloon is the endolymph, and the balloon itself is the membranous labyrinth that contains the endolymph. The surrounding pool water is the perilymph, which supports the delicate nerve tissues of the membranous labyrinth. The walls of the pool represent the limits of the bony labyrinth space, and the ground encasing the pool is the bone that encases the labyrinthine space.
The endolymphatic sac is a reservoir pouch that resides on the posterior surface of the petrous bone against the posterior fossa dura. It is connected via the vestibular duct to drain into the endolymphatic space of the cochlea.
Endolymphatic flow has been described as following a “lake-river-pond” model. The endolymph flows from the endolymphatic fluid space (the lake) through the vestibular aqueduct (the river) to the endolymphatic sac (the pond). [3] If there is obstruction, then endolymphatic hydrops will occur.

Pathophysiology
The exact pathophysiology of Ménière disease is controversial. The underlying mechanism is believed to be distortion of the membranous labyrinth resulting from overaccumulation of endolymph. Some authors have questioned whether endolymphatic hydrops is actually a marker of disease rather than a cause. A study looking at temporal bones found that all patients with Ménière’s disease had hydrops in at least 1 ear but that hydrops was also found in patients who exhibited no signs of the disease. [4]
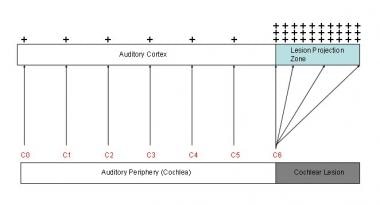
The endolymph and perilymph (ie, fluids that fill the chambers of the inner ear) are separated by thin membranes that house the neural apparatus of hearing and balance. Fluctuations in pressure stress these nerve-rich membranes, causing hearing disturbance, tinnitus (see the image below), vertigo, imbalance, and a pressure sensation in the ear.
Tinnitus model. Two phenomena in auditory cortex are associated with peripheral deafferentation: (1) hyperactivity in lesion projection zone and (2) increased cortical representation of lesion-edge frequencies (here, C6) in lesion projection zone. These 2 phenomena are presumed to be neurophysiologic correlates of tinnitus. Red letters correspond to octave intervals of fundamental frequency.
Attacks of hydrops probably are caused by an increase in endolymphatic pressure, which, in turn, causes a break in the membrane that separates the perilymph (potassium-poor extracellular fluid) from the endolymph (potassium-rich intracellular fluid). The resultant chemical mixture bathes the vestibular nerve receptors, leading to a depolarization blockade and transient loss of function. The sudden change in the rate of vestibular nerve firing creates an acute vestibular imbalance (ie, vertigo).
The physical distention caused by increased endolymphatic pressure also leads to a mechanical disturbance of the auditory and otolithic organs. Because the utricle and saccule are responsible for linear and translational motion detection (as opposed to angular and rotational acceleration), irritation of these organs may produce nonrotational vestibular symptoms.
This physical distention causes mechanical disturbance of the organ of Corti as well. Distortion of the basilar membrane and the inner and outer hair cells may cause hearing loss and/or tinnitus. Since the apex of the cochlea is wound much tighter than the base, the apex is more sensitive to pressure changes than the base. This explains why hydrops preferentially affects low frequencies (at the apex) as opposed to high frequencies (at the relatively wider base). Symptoms improve after the membrane is repaired as sodium and potassium concentrations revert to normal.
Various extrinsic mechanisms are thought to contribute to the development of endolymphatic hydrops, including infection, trauma, and allergens

Etiology
By definition, Ménière disease is idiopathic. In other words, if the cause is known, the disease process can no longer be called Ménière disease. However, because the root of the problem is elevated endolymphatic pressure, it is worthwhile to consider other causes of endolymphatic hydrops. Ménière disease must be distinguished from these causes.
Disorders that may give rise to elevated endolymphatic pressure include metabolic disturbances, hormonal imbalance, trauma, and various infections (eg, otosyphilis and Cogan’s syndrome [interstitial keratitis]). [5, 6]
Autoimmune diseases, such as lupus and rheumatoid arthritis, may cause an inflammatory response within the labyrinth. An autoimmune etiology was postulated after there was found to be an association with the presence of thyroid autoantibodies in patients with Ménière disease. [7, 8]
In addition, allergy has been implicated in many patients with difficult-to-treat Ménière disease. Food triggers are also important factors in the generation of hydrops.

Epidemiology
In the United States, a prevalence of 1,000 cases of endolymphatic hydrops per 100,000 population is a reasonable approximation, though it is probably an underestimate. Familial predisposition may be a factor, since half of patients have a significant family history. [9]
The reported prevalence of Ménière disease (ie, idiopathic endolymphatic hydrops) varies widely, from 15 per 100,000 in the United States to 157 per 100,000 in the United Kingdom. [10] This difference in prevalence based on geographic area is likely due to reporting biases and not geographic patterns of disease. Bilateral disease is found in 10% of patients with Ménière disease at initial diagnosis; with disease progression, it may be found in more than 40%. [11]
Ménière disease can be seen at almost all ages: it has been described in children as young as 4 years and in elderly persons older than 90 years. [3] The typical onset begins at early to middle adulthood. The peak incidence of Ménière’s disease is in the 40- to 60-year-old age group. [10] The mean age among treatment groups in some studies ranged from 49-67 years.
Ménière disease appears to be more common in females than in males, with reported ratios ranging from 1.3:1 [10] to 1.8:1. These figures may reflect reporting bias—that is, they may in part be the result of more females seeking treatment.The disease primarily affects whites, [12] although this finding too may reflect reporting bias. [13] The female predilection of Ménière disease is shared with migraine headache and, in fact, there is a growing body of evidence that Ménière disease and migraine headache may be related and/or different spectrums of the same disease. [14]

Prognosis
Patient presentation and progression of Ménière disease vary widely. The disease can be classified into several stages of progression. Early stages involve cochlear hydrops, which proceeds to affect the vestibular system. Ménière disease is most bothersome during these early stages.
As patients progress to later stages, the hydrops fills the vestibule so completely that no further room is available for pressure fluctuation and the vertigo spells disappear. The acute attacks are replaced by constant imbalance and progressive hearing loss.
The prognosis of patients with Ménière disease varies. Periods of remission punctuated by exacerbations of symptoms are typical. [15] Some patients have minimal symptoms, whereas others have severe attacks. Episodes may occur as infrequently as once or twice a year or they may occur on a regular basis.
The pattern of exacerbation and remission makes evaluation of treatment and prognosis difficult. In general, the patient’s condition tends to spontaneously stabilize over time. Ménière disease is said to “burn out” over time. The spontaneous remission rate is high: over 50% within 2 years and over 70% after 8 years. [10] This spontaneous stabilization comes at a price, however: many patients are left with poor balance and poor hearing.
Most of the remaining patients (ie, those whose disease does not spontaneously stabilize) are well managed with medications. Surgical treatment is required for 5-10% of patients.
Ménière disease is not directly associated with mortality; however, it is associated with drop attacks, which could lead to accidental trauma resulting in morbidity or mortality. Failure to warn patients of the possibility of drop attacks, which could result in injury, is a pitfall.
The main morbidity associated with Ménière disease is the debilitating nature of vertigo and the progressive and possibly permanent loss of hearing. In a Finnish study using a questionnaire, 22% of respondents listed problems with mobility and 19% listed mental effects of their illness. [16]

Patient Education
Proper education in terms of dietary control and avoidance techniques is helpful. Vestibular rehabilitation can be useful in teaching patients to cope with the vertigo and imbalance. Patients should be warned of the possibility of falls.
Patients should be instructed that if their symptoms significantly worsen or if they develop any new symptoms suggestive of another disease process they should return immediately to the emergency department for reevaluation.
For patient education resources, see the Brain and Nervous System Center and the Ear, Nose, and Throat Center, as well as Dizziness, Ménière Disease, and Tinnitus.

- Gürkov R, Pyykö I, Zou J, Kentala E. What is Menière's disease? A contemporary re-evaluation of endolymphatic hydrops. J Neurol. 2016 Apr. 263 Suppl 1:S71-81. [QxMD MEDLINE Link].
- Sajjadi H, Paparella MM. Meniere's disease. Lancet. 2008 Aug 2. 372(9636):406-14. [QxMD MEDLINE Link].
- Paparella MM. Pathogenesis and pathophysiology of Meniére's disease. Acta Otolaryngol Suppl. 1991. 485:26-35. [QxMD MEDLINE Link].
- Merchant SN, Adams JC, Nadol JB Jr. Pathophysiology of Meniere's syndrome: are symptoms caused by endolymphatic hydrops?. Otol Neurotol. 2005 Jan. 26(1):74-81. [QxMD MEDLINE Link].
- Paparella MM, Djalilian HR. Etiology, pathophysiology of symptoms, and pathogenesis of Meniere's disease. Otolaryngol Clin North Am. 2002 Jun. 35(3):529-45, vi. [QxMD MEDLINE Link].
- Grasland A, Pouchot J, Hachulla E, Blétry O, Papo T, Vinceneux P. Typical and atypical Cogan's syndrome: 32 cases and review of the literature. Rheumatology (Oxford). 2004 Aug. 43(8):1007-15. [QxMD MEDLINE Link].
- Fattori B, Nacci A, Dardano A, Dallan I, Grosso M, Traino C, et al. Possible association between thyroid autoimmunity and Menière's disease. Clin Exp Immunol. 2008 Apr. 152(1):28-32. [QxMD MEDLINE Link]. [Full Text].
- Nacci A, Dallan I, Monzani F, Dardano A, Migliorini P, Riente L, et al. Elevated antithyroid peroxidase and antinuclear autoantibody titers in Ménière's disease patients: more than a chance association?. Audiol Neurootol. 2010. 15(1):1-6. [QxMD MEDLINE Link].
- Klockars T, Kentala E. Inheritance of Meniere's disease in the Finnish population. Arch Otolaryngol Head Neck Surg. 2007 Jan. 133(1):73-7. [QxMD MEDLINE Link].
- Minor LB, Schessel DA, Carey JP. Ménière's disease. Curr Opin Neurol. 2004 Feb. 17(1):9-16. [QxMD MEDLINE Link].
- Kitahara M. Bilateral aspects of Meniére's disease. Meniére's disease with bilateral fluctuant hearing loss. Acta Otolaryngol Suppl. 1991. 485:74-7. [QxMD MEDLINE Link].
- Morrison AW, Johnson KJ. Genetics (molecular biology) and Meniere's disease. Otolaryngol Clin North Am. 2002 Jun. 35(3):497-516. [QxMD MEDLINE Link].
- Mancini F, Catalani M, Carru M, Monti B. History of Meniere's disease and its clinical presentation. Otolaryngol Clin North Am. 2002 Jun. 35(3):565-80. [QxMD MEDLINE Link].
- Ghavami Y, Mahboubi H, Yau AY, Maducdoc M, Djalilian HR. Migraine features in patients with Meniere's disease. Laryngoscope. 2016 Jan. 126 (1):163-8. [QxMD MEDLINE Link].
- Havia M, Kentala E. Progression of symptoms of dizziness in Ménière's disease. Arch Otolaryngol Head Neck Surg. 2004 Apr. 130(4):431-5. [QxMD MEDLINE Link].
- Stephens D, Pyykko I, Varpa K, Levo H, Poe D, Kentala E. Self-reported effects of Ménière's disease on the individual's life: a qualitative analysis. Otol Neurotol. 2010 Feb. 31(2):335-8. [QxMD MEDLINE Link].
- Monsell EM. New and revised reporting guidelines from the Committee on Hearing and Equilibrium. American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc. Otolaryngol Head Neck Surg. 1995 Sep. 113(3):176-8. [QxMD MEDLINE Link].
- Paparella MM. Benign paroxysmal positional vertigo and other vestibular symptoms in Ménière disease. Ear Nose Throat J. 2008 Oct. 87(10):562. [QxMD MEDLINE Link].
- Bhattacharyya N, Baugh RF, Orvidas L, Barrs D, Bronston LJ, Cass S, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008 Nov. 139(5 Suppl 4):S47-81. [QxMD MEDLINE Link].
- Baloh RW, Jacobson K, Winder T. Drop attacks with Menière's syndrome. Ann Neurol. 1990 Sep. 28(3):384-7. [QxMD MEDLINE Link].
- Kentala E, Havia M, Pyykkö I. Short-lasting drop attacks in Meniere's disease. Otolaryngol Head Neck Surg. 2001 May. 124(5):526-30. [QxMD MEDLINE Link].
- White J. Benign paroxysmal positional vertigo: how to diagnose and quickly treat it. Cleve Clin J Med. 2004 Sep. 71(9):722-8. [QxMD MEDLINE Link].
- Sajjadi H. Medical management of Meniere's disease. Otolaryngol Clin North Am. 2002 Jun. 35(3):581-9, vii. [QxMD MEDLINE Link].
- Bronstein A. Visual symptoms and vertigo. Neurol Clin. 2005 Aug. 23(3):705-13, v-vi. [QxMD MEDLINE Link].
- Lorenzi MC, Bento RF, Daniel MM, Leite CC. Magnetic resonance imaging of the temporal bone in patients with Ménière's disease. Acta Otolaryngol. 2000 Aug. 120(5):615-9. [QxMD MEDLINE Link].
- Sepahdari AR, Ishiyama G, Vorasubin N, Peng KA, Linetsky M, Ishiyama A. Delayed intravenous contrast-enhanced 3D FLAIR MRI in Meniere's disease: correlation of quantitative measures of endolymphatic hydrops with hearing. Clin Imaging. 2014 Oct 16. [QxMD MEDLINE Link].
- Hagiwara M, Roland JT Jr, Wu X, Nusbaum A, Babb JS, Roehm PC, et al. Identification of Endolymphatic Hydrops in Ménière's Disease Utilizing Delayed Postcontrast 3D FLAIR and Fused 3D FLAIR and CISS Color Maps. Otol Neurotol. 2014 Dec. 35(10):e337-42. [QxMD MEDLINE Link].
- de Sousa LC, Piza MR, da Costa SS. Diagnosis of Meniere's disease: routine and extended tests. Otolaryngol Clin North Am. 2002 Jun. 35(3):547-64. [QxMD MEDLINE Link].
- Coelho DH, Lalwani AK. Medical management of Ménière's disease. Laryngoscope. 2008 Jun. 118(6):1099-108. [QxMD MEDLINE Link].
- Cope D, Bova R. Steroids in otolaryngology. Laryngoscope. 2008 Sep. 118(9):1556-60. [QxMD MEDLINE Link].
- Herraiz C, Plaza G, Aparicio JM, Gallego I, Marcos S, Ruiz C. Transtympanic steroids for Ménière's disease. Otol Neurotol. 2010 Jan. 31(1):162-7. [QxMD MEDLINE Link].
- Phillips JS, Prinsley PR. Prescribing practices for Betahistine. Br J Clin Pharmacol. 2008 Apr. 65(4):470-1. [QxMD MEDLINE Link]. [Full Text].
- Mira E, Guidetti G, Ghilardi L, Fattori B, Malannino N, Maiolino L, et al. Betahistine dihydrochloride in the treatment of peripheral vestibular vertigo. Eur Arch Otorhinolaryngol. 2003 Feb. 260(2):73-7. [QxMD MEDLINE Link].
- Huang W, Liu F, Gao B, Zhou J. Clinical long-term effects of Meniett pulse generator for Meniere's disease. Acta Otolaryngol. 2008 Oct 15. 1-7. [QxMD MEDLINE Link].
- Dornhoffer JL, King D. The effect of the Meniett device in patients with Meniere's disease: long-term results. Otol Neurotol. 2008 Sep. 29(6):868-74. [QxMD MEDLINE Link].
- Mattox DE, Reichert M. Meniett device for Meniere's disease: use and compliance at 3 to 5 years. Otol Neurotol. 2008 Jan. 29(1):29-32. [QxMD MEDLINE Link].
- Odkvist LM, Arlinger S, Billermark E, Densert B, Lindholm S, Wallqvist J. Effects of middle ear pressure changes on clinical symptoms in patients with Ménière's disease--a clinical multicentre placebo-controlled study. Acta Otolaryngol Suppl. 2000. 543:99-101. [QxMD MEDLINE Link].
- Silverstein H, Smouha E, Jones R. Natural history vs. surgery for Meniere's disease. Otolaryngol Head Neck Surg. 1989 Jan. 100(1):6-16. [QxMD MEDLINE Link].
- Wetmore SJ. Endolymphatic sac surgery for Ménière's disease: long-term results after primary and revision surgery. Arch Otolaryngol Head Neck Surg. 2008 Nov. 134(11):1144-8. [QxMD MEDLINE Link].
- Smith WK, Sandooram D, Prinsley PR. Intratympanic gentamicin treatment in Meniere's disease: patients' experiences and outcomes. J Laryngol Otol. 2006 Sep. 120(9):730-5. [QxMD MEDLINE Link].
- Banerjee AS, Johnson IJ. Intratympanic gentamicin for Ménière's disease: effect on quality of life as assessed by Glasgow benefit inventory. J Laryngol Otol. 2006 Oct. 120(10):827-31. [QxMD MEDLINE Link].
- Hsieh LC, Lin HC, Tsai HT, Ko YC, Shu MT, Lin LH. High-dose intratympanic gentamicin instillations for treatment of Meniere's disease: long-term results. Acta Otolaryngol. 2009 Dec. 129(12):1420-4. [QxMD MEDLINE Link].
- Barrs DM. Intratympanic corticosteroids for Meniere's disease and vertigo. Otolaryngol Clin North Am. 2004 Oct. 37(5):955-72, v. [QxMD MEDLINE Link].
- Monsell EM, Wiet RJ. Endolymphatic sac surgery: methods of study and results. Am J Otol. 1988 Sep. 9(5):396-402. [QxMD MEDLINE Link].
- Glasscock ME 3rd, Jackson CG, Poe DS, Johnson GD. What I think of sac surgery in 1989. Am J Otol. 1989 May. 10(3):230-3. [QxMD MEDLINE Link].
- Bretlau P, Thomsen J, Tos M, Johnsen NJ. Placebo effect in surgery for Meniere's disease: nine-year follow-up. Am J Otol. 1989 Jul. 10(4):259-61. [QxMD MEDLINE Link].
- Pullens B, Giard JL, Verschuur HP, van Benthem PP. Surgery for Ménière's disease. Cochrane Database Syst Rev. 2010 Jan 20. CD005395. [QxMD MEDLINE Link].
- Sood AJ, Lambert PR, Nguyen SA, Meyer TA. Endolymphatic sac surgery for Ménière's disease: a systematic review and meta-analysis. Otol Neurotol. 2014 Jul. 35(6):1033-45. [QxMD MEDLINE Link].
- Fife TA, Lewis MP, May JS, Oliver ER. Cochlear Implantation in Ménière's Disease. JAMA Otolaryngol Head Neck Surg. 2014 May 1. [QxMD MEDLINE Link].
- Hansen MR, Gantz BJ, Dunn C. Outcomes after cochlear implantation for patients with single-sided deafness, including those with recalcitrant Ménière's disease. Otol Neurotol. 2013 Dec. 34(9):1681-7. [QxMD MEDLINE Link]. [Full Text].
- Shea JJ Jr, Ge X. Streptomycin perfusion of the labyrinth through the round window plus intravenous streptomycin. Otolaryngol Clin North Am. 1994 Apr. 27(2):317-24. [QxMD MEDLINE Link].
- Pyykko I, Ishizaki H, Kaasinen S, Aalto H. Intratympanic gentamicin in bilateral Meniere's disease. Otolaryngol Head Neck Surg. 1994 Feb. 110(2):162-7. [QxMD MEDLINE Link].
- Basura GJ, Adams ME, Monfared A, et al. Clinical Practice Guideline: Ménière's Disease Executive Summary. Otolaryngol Head Neck Surg. 2020 Apr. 162 (4):415-434. [QxMD MEDLINE Link].
- Transtympanic instillation device is used to administer drugs to inner ear.
- Intraoperative view of the left ear treated with labyrinthectomy; endolymphatic sac can be seen in this view.
- Tinnitus model. Two phenomena in auditory cortex are associated with peripheral deafferentation: (1) hyperactivity in lesion projection zone and (2) increased cortical representation of lesion-edge frequencies (here, C6) in lesion projection zone. These 2 phenomena are presumed to be neurophysiologic correlates of tinnitus. Red letters correspond to octave intervals of fundamental frequency.


Author
Chief Editor
Nicholas Lorenzo, MD, CPE, MHCM, FAAPL Co-Founder and Former Chief Publishing Officer, eMedicine and eMedicine Health, Founding Editor-in-Chief, eMedicine Neurology; Founder and Former Chairman and CEO, Pearlsreview; Founder and CEO/CMO, PHLT Consultants; Former Chief Medical Officer, MeMD Inc
Nicholas Lorenzo, MD, CPE, MHCM, FAAPL is a member of the following medical societies: Alpha Omega Alpha, American Academy of Neurology, American Association for Physician Leadership
Disclosure: Nothing to disclose.
Additional Contributors
Acknowledgements
Christopher I Doty, MD, FACEP, FAAEM Assistant Professor of Emergency Medicine, Residency Program Director, Department of Emergency Medicine, Kings County Hospital Center, State University of New York Downstate Medical Center
Christopher I Doty, MD, FACEP, FAAEM is a member of the following medical societies: American Academy of Emergency Medicine, American College of Emergency Physicians, American Medical Association, Council of Emergency Medicine Residency Directors, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Gerard J Gianoli, MD Clinical Associate Professor, Department of Otolaryngology-Head and Neck Surgery, Tulane University School of Medicine; Vice President, The Ear and Balance Institute; Chief Executive Officer, Ponchartrain Surgery Center
Gerard J Gianoli, MD is a member of the following medical societies: American Academy of Otolaryngology-Head and Neck Surgery, American College of Surgeons, American Neurotology Society, American Otological Society, Society of University Otolaryngologists-Head and Neck Surgeons, and Triological Society
Disclosure: Vesticon, Inc. None Board membership
Michael E Hoffer, MD Director, Spatial Orientation Center, Department of Otolaryngology, Naval Medical Center of San Diego
Michael E Hoffer, MD is a member of the following medical societies: American Academy of Otolaryngology-Head and Neck Surgery
Disclosure: American biloogical group Royalty Other
Glenn Lopate, MD Associate Professor, Department of Neurology, Division of Neuromuscular Diseases, Washington University School of Medicine; Director of Neurology Clinic, St Louis ConnectCare; Consulting Staff, Department of Neurology, Barnes-Jewish Hospital
Glenn Lopate, MD is a member of the following medical societies: American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and Phi Beta Kappa
Disclosure: Baxter Grant/research funds Other; Amgen Grant/research funds None
Spiros Manolidis, MD Associate Professor of Otolaryngology and Neurological Surgery, Columbia University
Spiros Manolidis, MD is a member of the following medical societies: American Academy of Otolaryngology-Head and Neck Surgery, American Auditory Society, American Head and Neck Society, American Medical Association, Canadian Society of Otolaryngology-Head & Neck Surgery, Society of University Otolaryngologists-Head and Neck Surgeons, and Texas Medical Association
Disclosure: Nothing to disclose.
Arlen D Meyers, MD, MBA Professor, Department of Otolaryngology-Head and Neck Surgery, University of Colorado School of Medicine
Arlen D Meyers, MD, MBA is a member of the following medical societies: American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology-Head and Neck Surgery, and American Head and Neck Society
Disclosure: Covidien Corp Consulting fee Consulting; US Tobacco Corporation Unrestricted gift Unknown; Axis Three Corporation Ownership interest Consulting; Omni Biosciences Ownership interest Consulting; Sentegra Ownership interest Board membership; Syndicom Ownership interest Consulting; Oxlo Consulting; Medvoy Ownership interest Management position; Cerescan Imaging Honoraria Consulting; GYRUS ACMI Honoraria Consulting
Mark S Slabinski, MD, FACEP, FAAEM Vice President, EMP Medical Group
Mark S Slabinski, MD, FACEP, FAAEM is a member of the following medical societies: Alpha Omega Alpha, American Academy of Emergency Medicine, American College of Emergency Physicians, American Medical Association, and Ohio State Medical Association
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
R Gentry Wilkerson, MD Assistant Professor, Director of Research, Emergency Medicine Residency Program, University of South Florida College of Medicine, Tampa General Hospital
R Gentry Wilkerson, MD is a member of the following medical societies: American College of Emergency Physicians
Disclosure: Nothing to disclose.