Pediatric Autoimmune Neutropenia: Practice Essentials, Background, Etiology (original) (raw)
Overview
Practice Essentials
Pediatric chronic autoimmune neutropenia (pediatric chronic AIN, also called chronic benign neutropenia or chronic idiopathic neutropenia) is a benign, self-limiting condition affecting infants and toddlers. [1] The name usually refers to primary, not secondary, AIN. Age of onset for primary AIN (PAIN) ranges from 4-28 months, and the duration ranges from 13-44 months. [2]
Secondary AIN (SAIN) occurs in a much older group, with average age of 10 years. A study of patients with SAIN found that 40% of them experienced severe infections, while direct anti-globulin tests (DATs) were positive in 50% of these individuals. Only 7.7% of the SAIN patients recovered from neutropenia. [3]
Neutropenia is defined as an absolute neutrophil count (ANC) of less than 1000/μL in infants and less than 1500/μL in older children. The condition is usually discovered during the workup of a febrile illness, with the treatment of AIN being focused on intercurrent infections.
Despite the fact that the condition is called autoimmune neutropenia, antibodies against neutrophils may not be demonstrated in a significant number of cases. Some authors differentiate neutropenia without demonstrable antibodies from AIN, designating the former as chronic idiopathic neutropenia. [1] Whether antibodies are found or not, their presence has no prognostic or therapeutic implications, since equal proportions of antibody-positive and antibody-negative cases recover spontaneously, and both represent primary neutropenia. Also, demonstration of antibodies varies depending on the techniques used to detect them. [4, 5, 6] In addition, the presence of antibodies has been revealed in patients who were proven to have severe congenital neutropenia due to a mutation of the ELANE gene. [7]
ANC alone does not allow differentiation of AIN from severe congenital neutropenia, since in both disorders the ANC may be extremely low. Indeed, differentiation from severe congenital neutropenia and benign chronic neutropenia may be difficult. When severe congenital neutropenia is suspected, a molecular diagnostic test is indicated.
SAIN occurs in a setting of systemic autoimmune disease, infection, neoplasia, bone marrow or organ transplantation, drug administration, or immunodeficiency. It develops in much older children than does PAIN, is more common in girls, and is clinically more prone to result in serious infections; spontaneous recovery is rare. [3]
Chronicity means that the neutropenia persists longer than 3 months, although some authors define the chronic condition as neutropenia lasting more than 6 months. [8] It is important to establish chronicity, since transient acute neutropenia associated with an infection is far more common in infants and toddlers than AIN.
PAIN in infants and children lasts, on average, 20 months. Although the clinical course is usually benign, a minority of children experience frequent infections.
Signs and symptoms of autoimmune neutropenia
Physical examination may reveal signs of a local infection, including mouth ulcer, gingivitis, upper respiratory tract infections, impetigo, otitis media, pneumonia, and, very rarely, cellulitis, abscesses, urinary tract infection (UTI), or sepsis.
Workup in autoimmune neutropenia
Points to remember in patient workup include the following:
- Determine whether the neutropenia is acute (particularly in association with a viral infection) and transient or chronic by obtaining frequent complete blood counts (CBCs)
- Determine the severity of neutropenia and the severity of concurrent infection
- Progressive neutropenia over a course of days requires rapid workup, including hematology consultation (since there is a possibility of leukemia)
- History of parent consanguinity, family history of neutropenia, or past or current life-threatening infection suggests severe congenital neutropenia or immunodeficiency disorder rather than AIN and requires genetic workup
- When the history and serial laboratory studies are consistent with AIN, a test to detect neutrophil antibodies is not needed to establish the diagnosis
- Be aware that there are multiple systemic diseases whose features include neutropenia, such as Hermansky-Pudlak syndrome, Barth syndrome, and Shwachman-Diamond syndrome; look for nonhematologic abnormalities and incorporate them into the differential diagnosis
Management of autoimmune neutropenia
PAIN has an excellent prognosis. In patients with frequent infections, prophylactic antibiotics with trimethoprim and sulfamethoxazole may help, although the benefits are anecdotal. [9] If the patient is free from significant infection, treatment is unnecessary. Spontaneous recovery after 6-24 months is typical. In severe cases of the disease, treatment usually consists of periodic administration of granulocyte colony-stimulating factor (G-CSF) (filgrastim).

Background
Immune neutropenia in children may be classified into autoimmune neutropenia and alloimmune neutropenia. The former then can be divided into primary autoimmune neutropenia (PAIN) and secondary autoimmune neutropenia (SAIN). PAIN is most common during infancy and early childhood (median age of onset 0.77 years), [3] whereas SAIN is more common in older children (median age of onset 10.07 years). SAIN occurs in a setting of systemic autoimmune disease, infection, neoplasia, bone marrow or organ transplantation, drug administration, or immunodeficiency and develops more frequently in girls. Virtually all PAIN resolves before age 5 years, while it is a rare occurrence for SAIN to resolve on its own. Serious infections in PAIN are uncommon, although a variety of infections have been reported. Some patients experience frequent oral ulcers, gingivitis, otitis media, upper respiratory infection, or tonsillopharyngitis; for these individuals, a trial of prophylactic antibiotics may be indicated. In rare cases, serious infections, such as perianal abscess with fistula formation, [10] or even multiple brain abscesses, [11] can occur. The uncommon patients who experience severe infections such as abscesses, mastoiditis, pneumonia, or sepsis would benefit from periodic administration of G-CSF.
Serious, life-threatening infections (sepsis, pneumonia, skin/soft tissue abscesses, osteomyelitis, otomastoiditis, meningitis/encephalitis) occur frequently in children with SAIN. [3] A study by Farruggia et al reported the frequency of severe infection for PAIN to be 12%, with that of SAIN being 40%. [3] Use of prophylactic antibiotics and regular administration of G-CSF (filgrastim) to prevent severe neutropenia is frequently required in SAIN. Cytopenias arising from other cell lineages, such as immune thrombocytopenic purpura (ITP) and/or autoimmune hemolytic anemia (AIHA), quite often accompany the neutropenia in SAIN. [12] A significant number of patients with SAIN may have an enlarged liver and/or spleen, while in PAIN, these organs are not enlarged. Some patients whose presentation initially indicates PAIN may have a RAG (recombination-activating gene) mutation that is associated with systemic autoimmune disease. These patients may develop autoimmune hemolytic anemia and/or autoimmune thrombocytopenia, in addition to neutropenia. [13]
Some authors divide PAIN into autoimmune neutropenia, in which patients are demonstrated to have auto-antibodies, and chronic idiopathic neutropenia (CIN), in which no such antibodies can be found. [1, 14, 8] In children, however, presentations, demographics, signs, symptoms, and prognosis are the same; [14] thus, from the point of patient management, demonstration of antibodies is not required. Antibodies found in patients with PAIN have overwhelmingly been those that act against neutrophil-specific antigens, ie, anti–human neutrophil antigen (HNA)-1 antibodies (most common), anti–HNA-3 antibodies, and anti–HNAB antibodies. Of 38 patients with persistent neutropenia who were assayed in a study, only three exhibited antibodies against major histocompatibility complex (MHC) class1 and/or MHC class 2 antigens alone, without antibodies against HNA. [15] On the other hand, in a rare instance, antibodies were demonstrated in patients who were proven to have severe congenital neutropenia due to a mutation of the ELANE gene. [7] Therefore, demonstration of anti-neutrophil antibodies does not directly indicate the etiologic role of antibodies in neutropenia.
Alloimmune neutropenia in children are almost exclusively seen in neonates secondary to a mismatch in HNA between the mother and her offspring. The neutropenia can be very severe, but spontaneous recovery is the rule.
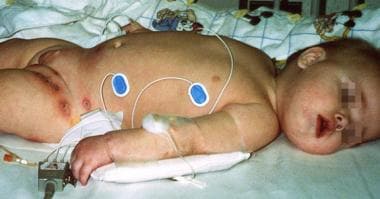
A case of secondary autoimmune neutropenia. This patient presented with recurrent otitis and areas of cellulitis in the diaper area. Pseudomonas aeruginosa and Staphylococcus aureus were isolated from the skin lesions. Autoimmune hemolytic anemia and autoimmune neutropenia were confirmed based on the presence of autoantibodies. The patient has a mutation on exon 15, A504T, which changed an asparagine residue to a valine residue.

Etiology
Lalezari and colleagues demonstrated neutrophil antibodies in 119 of 121 infants and children with chronic neutropenia, thereby establishing the autoimmune nature of the disease. [16] In the study, all patients had at one time an ANC of less than 500/µL (see the Absolute Neutrophil Count calculator).
AIN is similar to immune thrombocytopenic purpura (ITP) of young children, a more common cytopenia in pediatric patients. ITP is believed to be triggered by a viral infection and, in some individuals, by immunization. Whether AIN is commonly triggered by similar etiologies is unknown. A similar age group is affected, and recovery is expected in both disorders. Nakamura et al showed deficiencies in regulatory T cells (CD4+, CD25+) in children with AIN; [17] thus, the disease may be a phenomenon of physiologically delayed maturation of immune regulation.
Some studies, moreover, have shown an association between AIN and parvovirus B19 infection.
A literature review by Farmer et al determined that autoimmune cytopenias, including AIN, were associated with up to 84.1% of RAG protein deficiencies. [13] The median age of onset found for AIN in RAG-deficient patients, 2.6 years, was significantly older than that for other children with AIN. In the cohort with RAG protein deficiency, no patients had neutropenia alone. Instead, all of them had other cytopenias, such as autoimmune hemolytic anemia (AIHA) or ITP; granulomas; or evidence of immune deficiency or immune dysregulation (autoimmune lymphoproliferative syndrome [ALPS]–like disorder, severe combined immunodeficiency [SCID], or atypical SCID). The investigators also found that in 71.4% of cases, RAG deficiency–associated AIN was refractory to intravenous immunoglobulin, steroids, and rituximab. [13]
In AIN, antibodies most commonly include immunoglobulin G (IgG) against neutrophil glycosylated isoforms of Fc gamma RIIIb (or CD 16b) and HNA-1 and, less commonly, against HNA-4..
Although anti-HLA antibodies may be present in AIN, they are very rarely the cause of the disorder.
In PAIN, antibodies, if detected, are almost exclusively against the NA alloantigens, although at the onset of neutropenia, in rare instances, antibodies against Fc gamma RIIIb may be present (see below). On the other hand, children with SAIN usually show antibodies with pan–Fc gamma RIIIb specificity, not anti NA-alloantigens. [18]
Bone marrow examination findings in AIN are variable and not diagnostic. Bone marrow may be normal, show late-stage maturation arrest, or be hyperplastic or hypoplastic. In rare cases, granulocyte phagocytosis has been reported. [19]
In a study by Perdikogianni et al, circulating G-CSF levels (serum or plasma) were found to be normal in AIN, even during the neutropenic period, except when patients had infections. [20] On the other hand, Kobayashi et al found that the patient’s sera contained a mildly but significantly elevated concentration of G-CSF compared with the control sera. [21]
A Danish study, by Kløve-Mogensen et al, indicated that blood type O and secretion of H Leb antigens are protective against AIN. The investigators found that 36.4% of study patients with AIN were type O, compared with 46.8% of controls, while 25.2% of individuals with AIN demonstrated H Leb antigen secretion, versus 35.0% of controls. [22]

Epidemiology
The incidence of autoimmune neutropenia does not vary among different ethnic populations.
A Scottish study estimated that in the Scottish population, the incidence of autoimmune neutropenia is 1 in 100,000 children per year. [23] This may have been an underestimate, however, because laboratory tests are not usually performed in most cases of autoimmune neutropenia.
Autoimmune neutropenia has a slight preponderance in girls in one study, [16] but not in others. [24] The mean age at diagnosis of autoimmune neutropenia is 6-12 months, with a range of 3-30 months.
A high frequency of benign (ethnic) neutropenia is widely recognized in African Americans, Yemenite and Falasha Jews, Black Beduin, blacks of South African extraction, Ethiopians, West Indians, Arab Jordanians, and various tribal groups inhabiting the United Arab Emirates. [25] A study showed the median ANC of children with benign ethnic neutropenia to be 893 x 106/L. [26] The gene responsible for this form of neutropenia is identified as the DARC (Duffy antigen/receptor chemokine) gene, although the exact mechanism that causes neutropenia is still to be elucidated. [27, 28] In the age group (6 months to 3 years) when AIN is common, it may be impossible to distinguish these two entities in a given child.
Morbidity
Infections associated with primary AIN, which are usually limited and mild, include just fever, otitis media, upper respiratory tract infection, common colds, viral gastroenteritis, skin infection, stomatitis, and gingivitis. Sepsis and pneumonia are rare. Hajishengallis et al reported on the development of AIN-related periodontal disease in a child aged 2 years. [29] In a study done at St Jude Children’s Research Hospital on children with all types of chronic neutropenia, otolaryngological infections predominated, consisting of recurrent otitis media (81%), viral upper respiratory tract infection (67%), oral ulcers or gingivitis (53%), tonsillitis (39%), and sinusitis (37%). [30]
A case of secondary autoimmune neutropenia. This patient presented with recurrent otitis and areas of cellulitis in the diaper area. Pseudomonas aeruginosa and Staphylococcus aureus were isolated from the skin lesions. Autoimmune hemolytic anemia and autoimmune neutropenia were confirmed based on the presence of autoantibodies. The patient has a mutation on exon 15, A504T, which changed an asparagine residue to a valine residue.
This is in contrast to the severe, life-threatening infections (quite often systemic) experienced by infants with severe congenital neutropenia (Kostmann disease and other types), children with aplastic anemia, older children with secondary AIN, and children with neutropenia who are receiving chemotherapy. An Italian study showed that 40% of children with secondary AIN had severe infections, as compared with 12% of children with primary AIN. [3]
In another study, of 73 children with neutropenia, Fioredda et al reported a rate of 0.66 infections per patient with AIN, compared with 5.75 infections per patient with severe congenital neutropenia. [31] (The AIN figure may be an overestimate, however, since there may have been a selection bias; the neutropenia registry used in the study contained more severely affected AIN patients.) The reasons for this difference between primary autoimmune and severe congenital neutropenia may be related to the fact that individuals with AIN have an adequate bone marrow neutrophil reserve and can therefore mount some level of neutrophil response to an infection, even though these neutrophils are rapidly destroyed; this is in contrast to patients with poor or no bone marrow reserve.
Although febrile illnesses appear to be more common in children with AIN than in healthy children, AIN usually does not affect the child's growth and development, although some exceptions occur. (See History.)

Prognosis
The prognosis in primary chronic neutropenia is excellent. The condition usually lasts only 2-3 years before spontaneous resolution, and virtually all patients recover by age 5 years.
Spontaneous recovery after 6-24 months is typical. If it persists beyond age 4-5 years, consider other diagnoses (secondary neutropenia). It is important to follow these patients into recovery for this reason.

Patient Education
Thoroughly discussing the natural history with the patient’s parents and/or caregivers is important, since this can prevent undue anxiety created by low neutrophil counts.
In addition, because medical advice is usually sought after infections have occurred in a child with autoimmune neutropenia, discussing the condition’s natural history validates the experience of the parents and/or caregivers and, in turn, increases their confidence in the physician's diagnosis and treatment.
It is important for patients to maintain good dental hygiene in order to prevent gingivitis, stomatitis, and other mucous membrane infections of the mouth.

- Dale DC, Bolyard AA. An update on the diagnosis and treatment of chronic idiopathic neutropenia. Curr Opin Hematol. 2017 Jan. 24 (1):46-53. [QxMD MEDLINE Link].
- Wang LY, Wang CL, Chu CC, et al. Primary autoimmune neutropenia in children in Taiwan. Transfusion. 2009 May. 49 (5):1003-6. [QxMD MEDLINE Link].
- Farruggia P, Puccio G, Fioredda F, et al. Autoimmune neutropenia of childhood secondary to other autoimmune disorders: Data from the Italian neutropenia registry. Am J Hematol. 2017 Sep. 92 (9):E546-9. [QxMD MEDLINE Link]. [Full Text].
- Porretti L, Farruggia P, Colombo FS, et al. Diagnostic value of cell bound and circulating neutrophil antibody detection in pediatric neutropenia. Pediatr Blood Cancer. 2018 Apr. 65 (4):[QxMD MEDLINE Link].
- Ito T, Taniuchi S, Tsuji S, Iharada A, Hasui M, Kaneko K. Diagnosis of autoimmune neutropenia by neutrophil-bound IgG and IgM antibodies. J Pediatr Hematol Oncol. 2011 Oct. 33 (7):552-5. [QxMD MEDLINE Link].
- Onodera R, Kurita E, Taniguchi K, et al. Anti-human neutrophil antigen-1a, -1b, and -2 antibodies in neonates and children with immune neutropenias analyzed by extracted granulocyte antigen immunofluorescence assay. Transfusion. 2017 Nov. 57 (11):2586-94. [QxMD MEDLINE Link].
- Boxer LA, Bolyard AA, Marrero TM, et al. Is there a role for anti-neutrophil antibody testing in predicting spontaneous resolution of neutropenia in young children. Blood. 2015. 126 (23):2211.
- Angelino G, Caruso R, D'Argenio P, et al. Etiology, clinical outcome, and laboratory features in children with neutropenia: analysis of 104 cases. Pediatr Allergy Immunol. 2014 May. 25 (3):283-9. [QxMD MEDLINE Link].
- Bruin M, Dassen A, Pajkrt D, Buddelmeyer L, Kuijpers T, de Haas M. Primary autoimmune neutropenia in children: a study of neutrophil antibodies and clinical course. Vox Sang. 2005 Jan. 88(1):52-9. [QxMD MEDLINE Link].
- Adamiak T, Gheller-Rigoni A, Arca M, Grossman W, Goday PS. Perianal disease as the initial presentation of autoimmune neutropenia. J Pediatr Gastroenterol Nutr. 2010 Jan. 50 (1):99-102. [QxMD MEDLINE Link]. [Full Text].
- Tokushima-Imayoshi M, Onoue T, Matsuo M, et al. Autoimmune neutropenia of infancy with multiple brain abscesses during the course of human herpesvirus-6 infection. Int J Hematol. 2006 Aug. 84 (2):151-3. [QxMD MEDLINE Link].
- Al Ghaithi I, Wright NA, Breakey VR, et al. Combined Autoimmune Cytopenias Presenting in Childhood. Pediatr Blood Cancer. 2016 Feb. 63 (2):292-8. [QxMD MEDLINE Link].
- Farmer JR, Foldvari Z, Ujhazi B, et al. Outcomes and Treatment Strategies for Autoimmunity and Hyperinflammation in Patients with RAG Deficiency. J Allergy Clin Immunol Pract. 2019 Jul - Aug. 7 (6):1970-85.e4. [QxMD MEDLINE Link]. [Full Text].
- Lindqvist H, Carlsson G, Moell J, Winiarski J, Sundin M. Neutropenia in childhood: a 5-year experience at a tertiary center. Eur J Pediatr. 2015 Jun. 174 (6):801-7. [QxMD MEDLINE Link].
- Lee WI, Chen SH, Huang JL, et al. Identifying patients with neutrophil elastase (ELANE) mutations from patients with a presumptive diagnosis of autoimmune neutropenia. Immunobiology. 2013 May. 218 (5):828-33. [QxMD MEDLINE Link].
- Lalezari P, Khorshidi M, Petrosova M. Autoimmune neutropenia of infancy. J Pediatr. 1986 Nov. 109(5):764-9. [QxMD MEDLINE Link].
- Nakamura K, Miki M, Mizoguchi Y, Karakawa S, Sato T, Kobayashi M. Deficiency of regulatory T cells in children with autoimmune neutropenia. Br J Haematol. 2009 Jun. 145(5):642-7. [QxMD MEDLINE Link].
- Bruin MC, von dem Borne AE, Tamminga RY, Kleijer M, Buddelmeijer L, de Haas M. Neutrophil antibody specificity in different types of childhood autoimmune neutropenia. Blood. 1999 Sep 1. 94(5):1797-802. [QxMD MEDLINE Link].
- Urban C, Lackner H, Sovinz P, Beham-Schmid C. Intramedullary aggregation and phagocytosis of neutrophils in chronic benign neutropenia of childhood. Br J Haematol. 2008 Aug. 142(4):504. [QxMD MEDLINE Link].
- Perdikogianni Ch, Dimitriou H, Stiakaki E, Markaki EA, Kalmanti M. Adhesion molecules, endogenous granulocyte colony-stimulating factor levels and replating capacity of progenitors in autoimmune neutropenia of childhood. Acta Paediatr. 2003 Nov. 92(11):1277-83. [QxMD MEDLINE Link].
- Kobayashi M, Ueda K, Kojima S, Nishihira H, Ishiguro A, Shimbo T. Serum granulocyte colony-stimulating factor levels in patients with chronic neutropenia of childhood: modulation of G-CSF levels by myeloid precursor cell mass. Br J Haematol. 1999 May. 105(2):486-90. [QxMD MEDLINE Link].
- Kløve-Mogensen K, Steffensen R, Masmas TN, et al. ABO, secretor, and Lewis carbohydrate histo-blood groups are associated with autoimmune neutropenia of early childhood in Danish patients. Transfusion. 2022 Aug. 62 (8):1636-42. [QxMD MEDLINE Link]. [Full Text].
- Lyall EG, Lucas GF, Eden OB. Autoimmune neutropenia of infancy. J Clin Pathol. 1992 May. 45(5):431-4. [QxMD MEDLINE Link]. [Full Text].
- Sella R, Flomenblit L, Goldstein I, Kaplinsky C. Detection of anti-neutrophil antibodies in autoimmune neutropenia of infancy: a multicenter study. Isr Med Assoc J. 2010 Feb. 12(2):91-6. [QxMD MEDLINE Link].
- Denic S, Showqi S, Klein C, Takala M, Nagelkerke N, Agarwal MM. Prevalence, phenotype and inheritance of benign neutropenia in Arabs. BMC Blood Disord. 2009 Mar 27. 9:3. [QxMD MEDLINE Link]. [Full Text].
- Ortiz MV, Meier ER, Hsieh MM. Identification and Clinical Characterization of Children With Benign Ethnic Neutropenia. J Pediatr Hematol Oncol. 2016 Apr. 38 (3):e140-3. [QxMD MEDLINE Link]. [Full Text].
- Grann VR, Ziv E, Joseph CK, Neugut AI, Wei Y, Jacobson JS. Duffy (Fy), DARC, and neutropenia among women from the United States, Europe and the Caribbean. Br J Haematol. 2008 Oct. 143(2):288-93. [QxMD MEDLINE Link].
- Sella R, Flomenblit L, Goldstein I, Kaplinsky C. Detection of anti-neutrophil antibodies in autoimmune neutropenia of infancy: a multicenter study. Isr Med Assoc J. 2010 Feb. 12(2):91-6. [QxMD MEDLINE Link].
- Hajishengallis E, Rashewsky S, Kulkarni C, Stathopoulou P. Autoimmune Neutropenia as a Cause of Periodontal Disease in Preschool Children. J Clin Pediatr Dent. 2016 Winter. 40 (1):69-75. [QxMD MEDLINE Link].
- Shete M, Thompson JW, Naidu SI, Stocks RM, Wang WC. Olaryngologic manifestations in children with chronic neutropenia. Int J Pediatr Otorhinolaryngol. 2012 Mar. 76(3):392-5. [QxMD MEDLINE Link].
- Fioredda F, Calvillo M, Burlando O, et al. Infectious complications in children with severe congenital, autoimmune or idiopathic neutropenia: a retrospective study from the Italian Neutropenia Registry. Pediatr Infect Dis J. 2013 Apr. 32 (4):410-2. [QxMD MEDLINE Link].
- Bejjani N, Beaupain B, Bertrand Y, Bellanne-Chantelot C, Donadieu J. How to differentiate congenital from noncongenital chronic neutropenia at the first medical examination? Proposal of score: A pilot study from the French Severe Chronic Neutropenia registry. Pediatr Blood Cancer. 2017 Dec. 64 (12):[QxMD MEDLINE Link].
- Vlacha V, Feketea G. The clinical significance of non-malignant neutropenia in hospitalized children. Ann Hematol. 2007 Dec. 86(12):865-70. [QxMD MEDLINE Link].
- Alexandropoulou O, Kossiva L, Haliotis F, et al. Transient neutropenia in children with febrile illness and associated infectious agents: 2 years' follow-up. Eur J Pediatr. 2013 Jun. 172 (6):811-9. [QxMD MEDLINE Link].
- Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007 Apr 3. 146(7):486-92. [QxMD MEDLINE Link].
- Bux J, Behrens G, Jaeger G, Welte K. Diagnosis and clinical course of autoimmune neutropenia in infancy: analysis of 240 cases. Blood. 1998 Jan 1. 91(1):181-6. [QxMD MEDLINE Link].
- Audrain M, Martin J, Fromont P, Prie N, Thomas C, Muller EJ. Autoimmune neutropenia in children: analysis of 116 cases. Pediatr Allergy Immunol. 2011 Aug. 22(5):494-6. [QxMD MEDLINE Link].
- Farruggia P, Dufour C. Diagnosis and management of primary autoimmune neutropenia in children: insights for clinicians. Ther Adv Hematol. 2015 Feb. 6 (1):15-24. [QxMD MEDLINE Link].
- Lane SW, Hassell P, Kennedy GA, Fung YL, Williams BA. Characterization of the bone marrow immunofluorescence test in childhood autoimmune neutropenia. Int J Lab Hematol. 2009 Oct. 31 (5):567-71. [QxMD MEDLINE Link].
- Nixon CP, Sweeney JD. Autoimmune Cytopenias: Diagnosis & Management. R I Med J (2013). 2016 Dec 1. 99 (12):36-40. [QxMD MEDLINE Link]. [Full Text].
- A case of secondary autoimmune neutropenia. This patient presented with recurrent otitis and areas of cellulitis in the diaper area. Pseudomonas aeruginosa and Staphylococcus aureus were isolated from the skin lesions. Autoimmune hemolytic anemia and autoimmune neutropenia were confirmed based on the presence of autoantibodies. The patient has a mutation on exon 15, A504T, which changed an asparagine residue to a valine residue.


Author
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.
Gary D Crouch, MD Associate Professor, Program Director of Pediatric Hematology-Oncology Fellowship, Department of Pediatrics, Uniformed Services University of the Health Sciences
Gary D Crouch, MD is a member of the following medical societies: American Academy of Pediatrics, American Society of Hematology
Disclosure: Nothing to disclose.
Chief Editor
Acknowledgements
Gary R Jones, MD Associate Medical Director, Clinical Development, Berlex Laboratories
Gary R Jones, MD is a member of the following medical societies: American Academy of Pediatrics, American Society of Pediatric Hematology/Oncology, and Western Society for Pediatric Research
Disclosure: Nothing to disclose.