Disparity of cycad leaves dispels the living fossil metaphor (original) (raw)
Abstract
The living fossil metaphor is tightly linked with the cycads. This group of gymnosperms is supposed to be characterised by long-term morphological stasis, particularly after their peak of diversity and disparity in the Jurassic. However, no formal test of this hypothesis exists. Here, we use a recent phylogenetic framework and an improved character matrix to reconstruct the Disparity Through Time for cycad leaves using a Principal Coordinate Analysis and employing Pre-Ordination Ancestral State Reconstruction to test the impact of sampling on the results. Our analysis shows that the cycad leaf morsphospace expanded up to the present, with numerous shifts in its general positioning, independently of sampling biases. Moreover, they also show that Zamiaceae expanded rapidly in the Early Cretaceous and continued to expand up to the present, while now-extinct clades experienced a slow contraction from their peak in the Triassic. We also show that rates of evolution were constantly high up to the Early Cretaceous, and then experienced a slight decrease in the Paleogene, followed by a Neogene acceleration. These results show a much more dynamic history for cycads, and suggest that the ‘living fossil’ metaphor is actually a hindrance to our understanding of their macroevolution.
Similar content being viewed by others
Introduction
The idea of living fossils is as old as evolutionary thought itself, being introduced by Darwin1 to refer to species or groups that have experienced minimal change across time, and thus closely resembled their fossil ancestors. Since its inception though, various authors have employed different criteria to classify organisms as living fossils. While most scholars emphasize morphological stasis, others stress different criteria such as lack of species diversity, persistence of a lineage through geological time, phylogenetic uniqueness, geographically restricted distribution, and others, leading to a confusing and vague definition2,3,4. Surprisingly though, this metaphor still holds substantial power in swaying research programmes and generating hypotheses5.
Cycads are a charismatic group both for scientists and the general public. They are a group of gymnosperm plants characterised by a palm-like habit and large compound leaves, as well as having plants of separate sexes (dioecy). The order Cycadales includes around 375 species in two families[6](/article/10.1038/s42003-024-06024-9#ref-CR6 "Calonje M., Stevenson D. W. & Osborne R. The World List of Cycads, online edition. https://www.cycadlist.org
(2022)."), the Cycadaceae (including the genus _Cycas_ L. and \~ 119 species) and the Zamiaceae (including the other 9 genera and the majority of the species diversity). Nowadays, they are distributed in tropical and subtropical climates, with major centres of diversity in Mexico and Central America, South Africa, and Australia[7](/article/10.1038/s42003-024-06024-9#ref-CR7 "Norstog, K. J. & Nichols, T. J. The Biology of the Cycads. (Cornell University Press, New York, 1997)."). Among the extant plants, the diversity and disparity of cycads is dwarfed by other groups such as the angiosperms and the polypodiaceous ferns, but it is similar to that of other groups of comparable age, such as Araucariales (around 200 species)[8](/article/10.1038/s42003-024-06024-9#ref-CR8 "Leslie, A. B. et al. An overview of extant conifer evolution from the perspective of the fossil record. Am. J. Bot. 105, 1531–1544 (2018).") or Gleicheniales (also around 200 species)[9](/article/10.1038/s42003-024-06024-9#ref-CR9 "Choo, T. Y. & Escapa, I. H. Assessing the evolutionary history of the fern family Dipteridaceae (Gleicheniales) by incorporating both extant and extinct members in a combined phylogenetic study. Am. J. Bot. 105, 1315–1328 (2018)."). Even so, cycads are often considered together with Ginkgoales (including the only extant species _Ginkgo biloba_ L.) as examples of plant living fossils.Indeed, cycads’ reputation as “dinosaur plants”, apparently unchanged since their origin in the Palaeozoic and dominance during the Mesozoic, still dominates the discourse surrounding this group10,11,12. Although the results of molecular and total-evidence phylogenies indicate that in terms of species diversity, the cycads are a rather young group, diversifying in the late Cenozoic12,13, they are still considered to be morphologically similar, if not identical, to their fossil relatives14. Their peak of diversity and morphological disparity is supposed to be in the Jurassic (201.4–145 Ma), followed by a decline leading to a depauperate modern flora15.
Recent discoveries and analysis have challenged this view, indicating a much complex pattern of morphological evolution than expected from the living fossil metaphor. Cycad fossils with unexpected morphology, such as the diminutive male cone from the Early Cretaceous of California, suggest the presence of much-hidden diversity of Zamiaceae during this period, possibly indicating a radiation16. This pattern is further supported by phylogenetic analyses including fossil cycad leaves17. This analysis found a geographical expansion of the Zamiaceae across the Jurassic and the Cretaceous, corresponding with the appearance of fossils that can be confidently assigned to this family. Taken together, these results suggest that the dynamics of cycad diversification and disparification might be complex, and that the living fossil metaphor might be a detrimental constraint on cycad research.
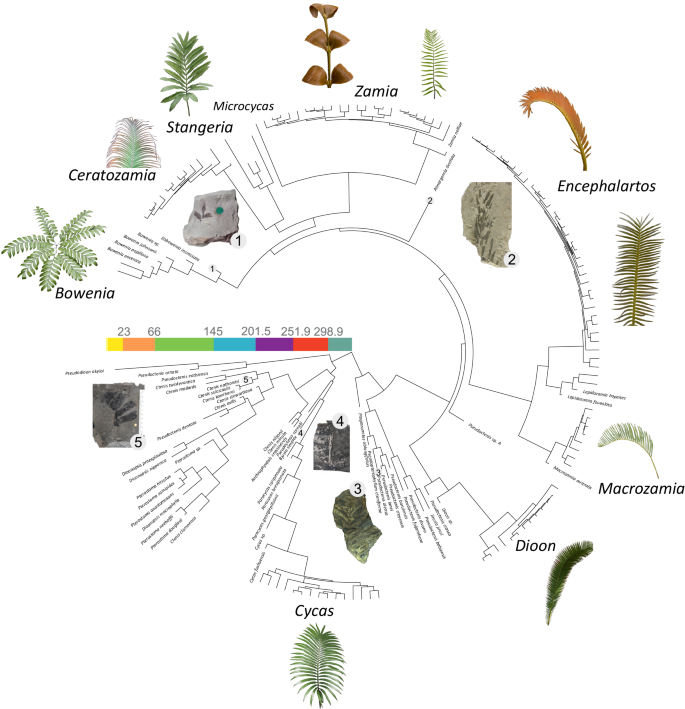
Here, we use a recent phylogenetic hypothesis for cycad leaf fossils (Fig. 1) to conduct the first formal analysis of the macroevolutionary dynamics of leaf disparity in the Cycadales by reconstructing leaf disparity through time. We show that cycad disparity has increased through time, mostly due to the origin and expansion of the Zamiaceae after the Jurassic Period, and that the rates of evolution have not declined up to the present but instead show a recent increase. This presents a much more dynamic history for this group of plants, and suggests that the living fossil metaphor should be abandoned in favour of more productive ones.
Fig. 1: Trimmed time-calibrated consensus tree from Coiro et al.17 used in this study.
Leaves of Bowenia spectabilis Hook. ex Hook.f., Ceratozamia chimalapensis Pérez-Farr. & Vovides_, Stangeria eriopus_ (Kunze) Baill., Zamia imperialis A.S.Taylor, J.L.Haynes & Holzman_, Zamia_ sp_., Encephalartos lehmannii_ Lehm., Encephalartos inopinus R.A.Dyer_, Macrozamia secunda_ C.Moore_, Dioon edule_ Lindl., and Cycas thouarsii R.Br. are shown as examples of extant cycad leaf diversity. Fossils of Eobowenia incrassata (S.Archang.) M.Coiro & C.Pott (1), Almargemia dentata Florin (2), Pseudoctenis oleosa Harris (3), Bjuvia simplex Florin (4), and Ctenis nathorstii Möller (5) are shown as examples of cycad fossil leaf diversity. Images are not to scale. Extant cycad images courtesy of Michael Calonje, except Dioon edule who has been taken by the authors. Image of Almargemia dentata from Coiro & Pott21, used under a CC BY 4.0 license.
Results
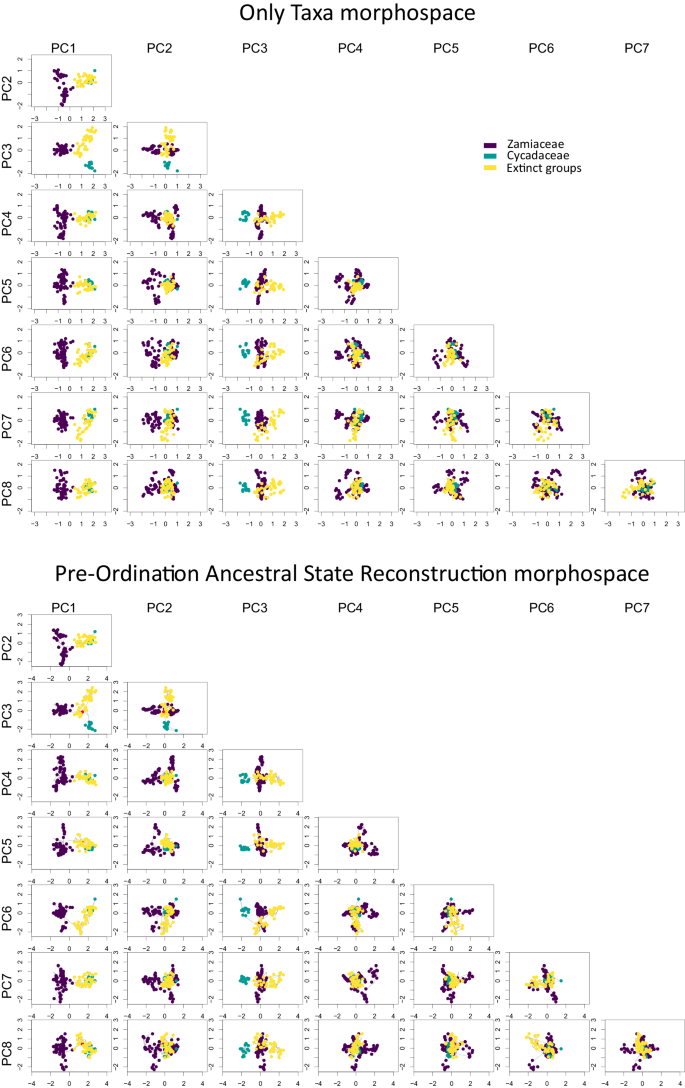
Principal Coordinate Analysis of the morphological matrix resulted in a matrix of 337 rows, corresponding to 169 tips, 168 internal nodes and 335 columns (PCoA axes). A Scree plot (Supplementary Fig. 1) shows that the first 10 axes account for around 10% of the variation, compatible with a similar analysis of other matrices18.
The only taxa (OT) morphospace and the Pre-Ordination Ancestral State Reconstruction (POASR) morphospace show almost identical distributions (Fig. 2). PC1 separates Zamiaceae from the rest of the cycad leaves, and PC3 separates the leaves of Cycadaceae from the rest, while PC2 has a less clear phylogenetic signal. Both sums of variance and the mean distance from the centre do not show strong sensitivity to rarefaction neither in the OT analysis (Supplementary Fig. 2) nor the POASR analysis (Supplementary Fig. 3), with the exception of some of the bins with the smaller sample size.
Fig. 2: Scatter plots for the first eight Principal Coordinate Analysis axes (PC1-PC8) for the Only Taxa morphospace (top) and the Pre-Ordination Ancestral State Reconstruction morphospace (bottom).
Tips and nodes for Zamiaceae are coloured in purple, Cycadaceae in green, and extinct cycads in yellow. The figure shows that the first PcoA axis (PC1) separates Zamiaceae from the rest of the cycads, and that in general the three groups of cycads occupy different parts of the morphospace. On the bottom graphs, the root node is coloured in red, and the phylogenetic relationships are indicated with grey lines.
Disparity through time
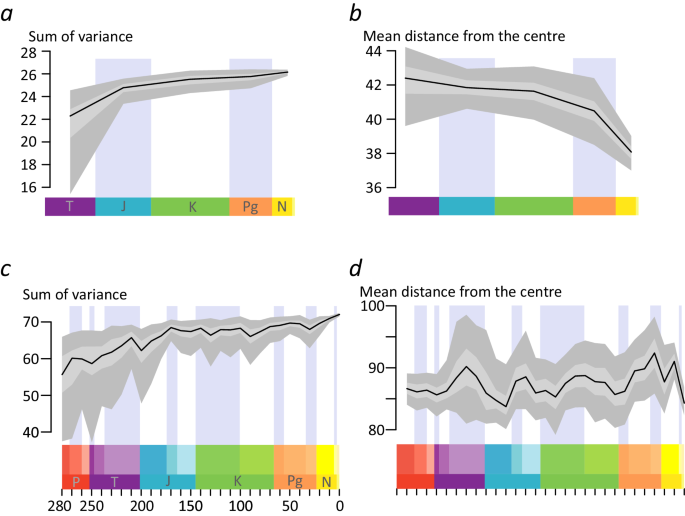
In the OT analysis, the size of cycad leaf morphospace, expressed as the sum of variance, shows an initial increase between the Triassic and the Jurassic (Fig. 3a). Although the increase slows down, it continues up to the present, with a small plateau between the Cretaceous and the Paleogene. The mean distance from the centre, indicating the position of leaf morphospace, shows smaller shifts between the Triassic and the Cretaceous, followed by larger shifts towards the Paleogene and the Neogene-Quaternary (Fig. 3b).
Fig. 3: Disparity-Through-Time (DTT) plots of cycad leaves, showing a more dynamic pattern than expected from stasis and/or post-Jurassic decline.
a DTT plot of the sum of the variance of the ‘OT’ analysis, i.e. including only the tips of the cycad tree, showing an increase of the morphospace of cycad leaves up to the present. b DTT plot of the mean distance from the centre of the ‘OT’ analysis, showing the placement of the morphospace moving through time up to the present. c DTT plot of the sum of variance of the Pre-Ordination Ancestral Reconstruction analysis. d DTT plot of the mean distance from the centre of the pre-ordination ancestral reconstruction analysis. Both c and d show a similar but more complex pattern than a and b. Black line indicates the bootstrapped median, light grey indicates the 25%-75% percentiles, dark grey indicates the 2.5–97.5% percentiles.
In the analysis using data reconstructed for the nodes, the sum of variance shows an initial expansion during the Permian and the Triassic, with a peak around 210 Ma followed by a drop (Fig. 3c). A subsequent increase up to a peak in the Mid Jurassic (170 Ma) is followed by oscillations leading to overall stasis. After a dip during the Late Cretaceous, a slower increase leads to a further peak in the Eocene, followed by a drop in the Oligocene and a recovery up to the highest levels of disparity in the present.
The position of the morphospace is also quite dynamic, with major shifts in distance from the centre in the transition between the Early and Late Triassic, Late Triassic and Early Jurassic, Early Jurassic and Mid Jurassic, Late Jurassic and Early Cretaceous, Late Cretaceous and the Oligocene, as well as Oligocene to early Miocene, early to late Miocene, and late Miocene to the present (Fig. 3d). The difference between the mean distance to the centre in the present and at the origin shows a shift in the position of the extant morphospace compared to the original morphospace, similar to the tip only analysis. The results from the POASR analyses are also robust to topological uncertainty (Supplementary Fig. 4).
Clade-specific disparity through time
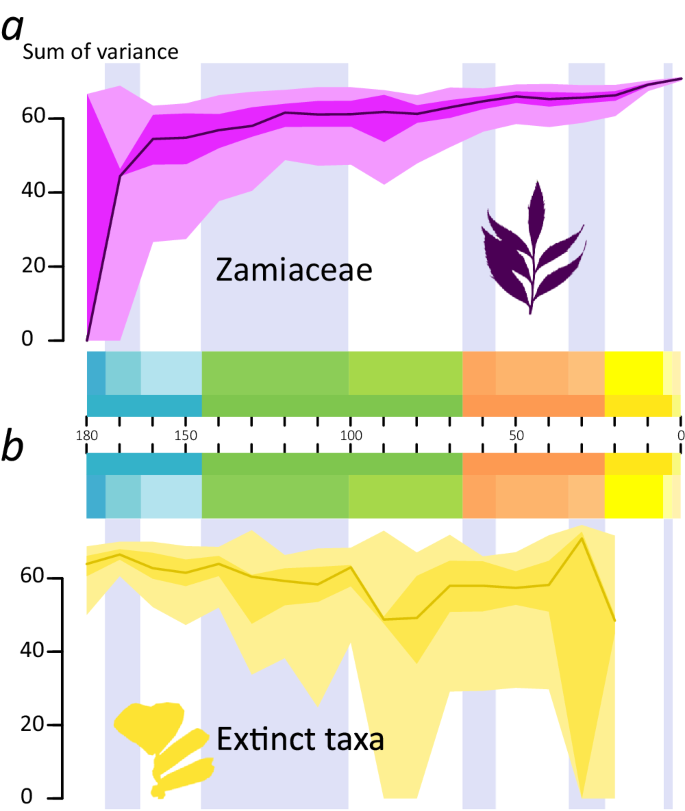
Portioning the disparity according to taxonomic grouping shows that while the Mid Jurassic peak is driven by extinct taxa, the subsequent growth of leaf morphological space is mostly driven by the expansion of Zamiaceae (Fig. 4a, Supplementary Fig. 5). This group shows a first period of expansion up to the Early Cretaceous, with a peak at 120 Ma, followed by two other major periods of expansion between the Late Cretaceous and the Eocene and between the Miocene and the present. The space occupied by extinct taxa shows a progressive shrinkage from a Mid Jurassic peak (Fig. 4b, Supplementary Fig. 6). Smaller peaks are found in between the Early and Late Cretaceous and in the late Eocene. The space reaches its smallest extent before the complete extinction of the Ctenis clade.
Fig. 4: Disparity-Through-Time (DTT) plots of different cycad groups from the Middle Jurassic to the Present.
a DTT plot of the sum of the variance of leaves of the Zamiaceae using the morphospace from the Pre-Ordination Ancestral Reconstruction analysis. The graph shows that in the Zamiaceae there is a fast increase during the early phase of the evolution of the group, followed by a slower increase up to a peak in the Early Cretaceous. b DTT plot of the sum of the variance of leaves of extinct cycads, i.e. excluding the crown groups of Zamiaceae and Cycadaceae. In the extinct taxa, it shows a gradual decrease with a low valley in the Late Cretaceous, with the lowest disparity reached before their extinction in the Neogene. The black line indicates the bootstrapped median, light grey indicates the 25–75% percentiles, dark grey indicates the 2.5–97.5% percentiles.
Rate analysis
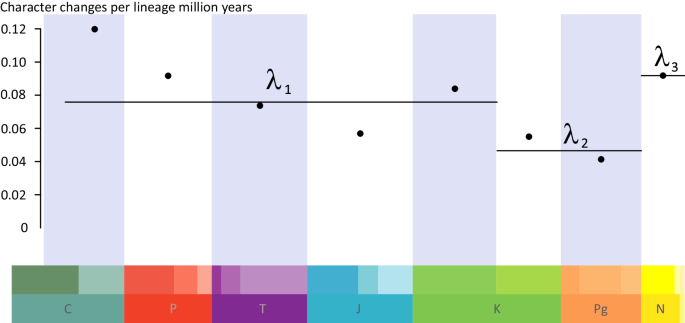
Point rate estimates for our selected time bins show the highest rates in the Carboniferous, with a slowdown leading to a minimum in the Jurassic and then a rise in the Early Cretaceous (Fig. 5). Rates decrease in the Late Cretaceous and Paleogene, only to jump up during the Neogene-Present. However, AICc selection favours a much simpler model, with a single rate of 0.07 character changes per Ma from the Carboniferous to the Early Cretaceous, a lower rate of 0.05 in the Late Cretaceous and Paleogene, and the highest rate of 0.09 during the Neogene and Present.
Fig. 5: Rates through time plot for all the characters across the cycad phylogeny, showing high rates between the Carboniferous and the Early Cretaceous (λ1), lower rates during the Late Cretaceous and the Paleogene (λ2), and the highest rates during the Neogene (λ3).
Points represent estimates for each time bin, while lines represent estimates from the best model including three rates.
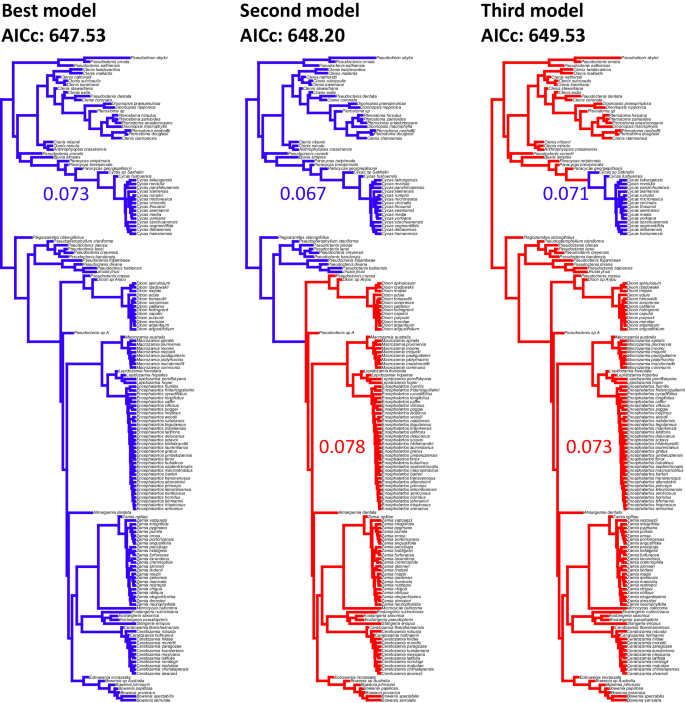
The analysis of rates in different clades corresponding to the acquisition of nitrogen fixation favored a model with a single rate across the tree (Fig. 6). However, a model with the Zamiaceae crown group having a higher rate than the background was not significantly worse (ΔAICc = 0.67). On the other hand, a model with the genus Cycas having a lower rate than the background was significantly worse than the best model (ΔAICc = 2).
Fig. 6: Model for rates of morphological evolution across the tree, testing whether lineages inferred to have acquired nitrogen fixation have different rates than the other cycads.
Though the best model has a single rate across the tree, the second model shows an increased rate in crown group Zamiaceae. A model with Cycadaceae having lower rates is significantly worse than the best model (ΔAICc =2).
Time series analysis
Our time series analysis did not find a correlation between temperature or CO2 and our disparity metrics through time. All correlations coefficients were lower than 0.5 (Supplementary Fig. 7).
Discussion
Our analysis does not support the hypothesis of stasis and reduction in cycad leaf disparity through time15. The size of the leaf morphospace does instead increase up to the present, with major periods of expansion corresponding to the transition between the Mid and Late Triassic, the Early and Mid-Jurassic, and the Oligocene to the Miocene and present. While the expansion in the Triassic corresponds with an early burst of disparity within the crown-group Cycadales, a common pattern in many clades19, the one in the Jurassic and the later increases are driven by the origin and expansion of the Zamiaceae. Indeed, during the Early Cretaceous we see the appearance of fossil leaves in the stem groups of Dioon and Bowenia20,21, as well as forms with less clear affinities22. This strengthens the hypothesis that Zamiaceae underwent an evolutionary radiation during the Jurassic-Cretaceous16, a pattern suggested by the point estimates of the evolutionary rates in the Early Cretaceous. This radiation would be quasi-contemporaneous to those of other plant groups such as Gnetales23,24, Podocarpaceae25, and angiosperms26,27, suggesting the possibility of a global turnover event across seed plants. Even though our analyses do not seem to indicate that temperature or CO2 were directly driving cycad disparity, it cannot exclude the impact of more complex factors such as aridity. Moreover, some important traits such as the size of the leaves, vein density, or stomatal size and density were not included in our dataset, opening the possibility of a more thorough analysis of the physiological variation of cycads through time. Further investigation on cycads and on the other groups seemingly radiating during the same period should help to test the generalities of this phenomenon and help to disentangle its causes.
Contrary to expectations of a demise of cycads caused by the competition of more efficient and fast-growing flowering plants15,28, leaf disparity does not seem to decrease in response to the rise and expansion of the angiosperms during the Cretaceous29. Recent work has suggested that some Cycadales, namely Zamiaceae and some crown group Cycadaceae, avoided competition with angiosperms by evolving nitrogen fixation, while the non-fixing cycads declined30. Our data could seem to partially support this since Zamiaceae show both an increase starting from the Early Cretaceous onwards while the morphospace of other cycads slowly declined (Fig. 4). Moreover, there is an indication (albeit weak) that Zamiaceae have higher rates of morphological evolution compared to the rest of the Cycadales. On the other hand, competition can generate complex dynamics31, and thus the expectation of a simple decrease in disparity indicating dismissal by competition might be naive. Further investigation on the ecology and ecophysiology of the cycads from the Ctenis clade and other extinct groups might help to truly understand the causes of their decline and extinction32.
The Neogene burst in cycad disparity and evolutionary rates corresponds broadly with the origin of extant species diversity within the extant 10 genera12,13,17, suggesting that the increase in species diversity correlated with an increased variation in leaf morphology. This is in agreement with the observation of the variability of morphology and anatomy in some of the extant genera including Cycas33, Zamia34, Dioon35,36, and Ceratozamia11. The generation of this level of variation in a relatively short time span seems to be at odds with the widespread assumptions on the biology of cycads: these plants have exceedingly long lifespans, small population sizes, and rather slow rates of molecular evolution28,37. The availability of genomic data for the cycads38 and the comparison between genomes of closely related species might help to find the causes of this apparent conundrum, making cycads a potentially fundamental system for understanding the genetic basis for morphological differentiation and adaptive evolution.
Interestingly, no reduction of disparity can be observed in response to major extinction events such as the Permian-Triassic and the Cretaceous-Palaeogene extinctions. This agrees with other lines of evidence showing a less severe effect of mass extinction on plant groups39,40,41. However, the patterns associated with random or trait-selective extinction events are rather varied and context-dependent42, and a reduction in disparity might not be observed even after a mass extinction event. Moreover, our sampling strategy may be too coarse to detect the actual response of cycads to these mass extinctions. Further analyses are needed to more confidently test this.
It has to be kept in mind that trends in leaf disparity might not fully capture changes in disparity at the whole-plant level. It has been argued that whole-plant morphology is necessary to investigate the trajectories of disparity through time43. However, the fossil record of plants is by its nature fragmentary, and the process of reconstructing whole plants is extremely complex44. In the cycads, the issue is complicated by their dioecious nature, with the only whole-plant reconstruction known in some detail only bearing male strobili45,46,47. On the other hand, leaf fossils are relatively well preserved, and thus allow us to sample morphology over time in a more continuous way. Moreover, leaf morphology has important consequences for plant ecology and adaptation48,49, and thus represents a worthy line of investigation by itself. Based on these considerations, as well as more recent studies approaching single organ disparity50,51, we think that focusing on single organs can still bring fundamental insights in the macroevolutionary dynamics of plants50,51,52.
In conclusion, phylogeny, biogeography, and disparity all agree in showing that the “living fossil” metaphor is inappropriate for the cycads. While metaphors can be powerful tools that drive entire research programmes forward (i.e. the ‘adaptive radiation’ metaphor53,54,55), they can become deleterious by highlighting some aspects of a group’s biology while hiding others that might be as, if not more, important5. This seems to be the case with the ‘living fossil’ metaphor and the cycads: this metaphor has highlighted the case of apparent ‘stasis’ while hiding the dynamic history of diversification and disparification of this group through time12,13,17. We suggest that stasis in cycads should be considered at the level of the single traits3,56, instead of assuming it based on vague whole-plant morphological similarity, while the dynamic history of this group of “slow-growing”, dioecious plants should be the focus of more research at the organismic and molecular level. This new pluralistic view will bring better insights into this charismatic and endangered lineage.
Material and Methods
Analysis environment
All analyses were conducted in R ver. 4.2.1.
Morphological matrix and tree
To analyse cycad leaf disparity through time, we used the matrix and trees from ref. 17. This tree was generated using a combination of molecular data (15 nuclear loci, one mitochondrial locus, and two plastidial loci) from 321 extant species and morphological data (31 characters) from the extant species and 60 leaf fossil taxa spanning from the Permian to the Miocene. These data were analysed in a Bayesian dated framework using the Fossilized Birth-Death prior57,58.
The matrix of 31 morphological traits, scored from both direct observation and a review of the large literature on the morphology and anatomy of cycad leaves20,21,22,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91 was expanded to include 14 new characters, for a total of 45 (Supplementary Methods S1). To avoid issues with high levels of missing data, we reduced the taxon sampling to include only extant taxa that were scored for cuticular characters in the original matrix, leading to 109 extant taxa (from the original 321[6](/article/10.1038/s42003-024-06024-9#ref-CR6 "Calonje M., Stevenson D. W. & Osborne R. The World List of Cycads, online edition. https://www.cycadlist.org
(2022).")) and 60 fossil taxa. The extant taxa included all extant genera, with 2 out of 2 species of _Bowenia_ Hook. ex Hook.f., 11 out of 40 species of _Ceratozamia_ Brongn., 15 out of 119 species of _Cycas_, 13 out of 18 species of _Dioon_ Lindl., 33 out of 65 species of _Encephalartos_ Lehm., 2 out of 2 species of _Lepidozamia_ Regel, 8 out of 41 species of _Macrozamia_ Miq., and 23 out of 86 species of _Zamia_ L.The tree was trimmed to only include the 169 species from the morphological matrix using the function drop.tip from the R package ape92. A random sample of 100 trees from the posterior distribution from ref. 17 were also used to test the sensitivity of our results to topological uncertainty. All zero-length branches were transformed to 0.001-length branches.
Morphospace
The function ordinate_cladistic_matrix from the package Claddis93,94 was used to generate two morphospaces: one was built using only data from the scored taxa, using the prior estimates from17 as first and last occurrence of the fossil tips, with extant species set to 0-0 (Only Taxa, OT); the other used the tree to conduct ancestral state reconstruction, to increase sample size for the past time bins, thus combating sampling bias (Pre-Ordination Ancestral State Reconstruction, POASR). For both datasets, the distance calculations used the Maximum Observable Rescaled Distance (MORD)94. This metric is based on the Gower Coefficient (GC)[95](/article/10.1038/s42003-024-06024-9#ref-CR95 "Guillerme T. & Cooper N. Time for a rethink: time sub-sampling methods in disparity-through-time analyses. Palaeontology, 1–13. https://doi.org/10.1111/pala.12364
(2018)."), a measure of distance rescaled by the number of characters that can be scored in both taxa compared. The MORD further rescales the GC to the maximum possible distance, leading to a value between 0 and 1.The MORD has been shown to perform better than the GC and the General Euclidean Distance (GED) for analyses of disparity. The calculation of the distances was followed by a Principal Coordinate Analysis (PCoA), both operations included in the ordinate_cladistic_matrix function. To test robustness to topological uncertainty, we analysed morphospaces across 100 trees taken from the posterior of ref. 17. For this analysis, a combination of the function multi.tree and char.diff from the package dispRity96 and the function pcoa from the package ape92 was used to replicate the same process followed by the function ordinate_cladistic matrix from the package Claddis93,94 while optimizing the speed of the analysis.
Disparity through time
For the Disparity Through Time (DTT) analysis (i.e., the amount of morphological variation across time bins), the OT morphospace was split into time bins equivalent to Periods, given the paucity of data, starting from the Triassic and ending in a combined Miocene-Pliocene-Quaternary bin (from 23 to 0 Ma) (Table 1). This was done using the function chrono.subset from the package dispRity96. For the analysis including reconstructed data, the morphospace was split into 10 Ma time bins from 300 Ma to 0 (Table 2). The binning was conducted using the continuous method96, and assuming both a gradual split model and a punctuated model. Matrices were then bootstrapped for 500 replicates using the function boot_matrix. We also tested the impact of sampling by rarefying the data to 6 and 3 samples.
Table 1 Number of data points (tips) per time bin in the Only Taxa analysis
Table 2 Number of data points (tips plus nodes) per time bin in the Pre-Ordination Ancestral State Reconstruction analysis according to the type of model used to integrate node data (gradual vs punctuated) in the different sampling strategies
Following the recommendations presented in ref. 96, we tested the appropriateness of the metrics representing the expansion of cycad leaf morphospace using the web-based shiny app “moms”. After this analysis, we selected the sum of variance as an estimate of the size of the morphospace and the mean distance from the centre of the morphospace (the 0,0 point) as a measure of position. These metrics were then calculated using the function dispRity.
Clade-specific disparity through time
To test the contribution of the different clades to the disparity dynamics of the cycads, we generated DTT curves for the Zamiaceae and for the taxa that are not strongly supported as close relatives of the extant clades. These were defined as taxa or clades that did not result in a clade with Zamiaceae or Cycadaceae with pp=1 in the consensus tree from ref. 17. DTT for the Cycadaceae could not be reconstructed due to the lack of data caused by the sparse fossil record of the Cycadaceae, including only 5 species over the whole timespan of the crown group leading to only 1 or 2 points per time bin.
The morphospace from the pre-ordination ancestral reconstruction analysis and the tree were trimmed to only include members of either Zamiaceae or completely extinct clades. For the Zamiaceae analysis, we used time bins of 10 Ma from 180 to 0, while for the extinct clade analysis the time bins extended to the age of the tree root.
Rate analysis
To test the variation in evolutionary rates through geological time, we used the time bin method test_rates in the library Claddis92. Time bins were set to be equal to Periods (Carboniferous 330.36-299, Permian 299-252, Triassic 252-201, Jurassic 201-145, Paleogene 66-23) except for the Cretaceous, where we considered the Series level (Early Cretaceous 145-100, Late Cretaceous 100-66), and the combined Neogene-Pliocene-Holocene (23-0). All combinations of rate models between the 8 time bins were tested, for a total of 128 comparisons. The best model was selected using AICc to avoid overparameterization.
We further tested whether the acquisition of nitrogen fixation had an effect on the rates of character evolution in cycads. We used the clade method in the test_rates function to test whether the two clades that are inferred by30 to have acquired nitrogen fixation (namely the crown group Zamiaceae and the clade including the Late Cretaceous Cycas from Sakhalin (Cycas sp. Sakhalin) and extant Cycas) had different rates of morphological evolution compared with a model with a single rate across the tree. AICc was used to select the best model.
Time series analysis
To test the correlation between the disparity of cycad leaves and macroclimatic factors, we downloaded CO2 and temperature data through geological time from ref. 97. We selected this dataset since it spans the same time span of our analyses, and includes temperature and CO2 estimates in 10 Ma intervals, allowing a direct comparison. To deal with spurious correlation due to strong autocorrelation in time series data, the CO2 and temperature data were analyzed using an Autoregressive Integrated Moving Average (ARIMA) model, implemented in the function auto.arima from the package forecast98. The same ARIMA model was then used to analyze the bootstrapped median values for the sum of variance and the centroid distance disparity metrics obtained from the gradual and punctuated models of the POASR analysis using the function Arima from the package forecast. The residuals of the ARIMA model for temperature or CO2 were then correlated with the residuals of the same ARIMA model applied to the different dependent variables using cross-correlation as implemented in the function ccf from the package stats.
Reporting summary
Further information on experimental design is available in the Nature Portfolio Reporting Summary linked to this Article.
Data availability
Code availability
References
- Darwin, C. R. On the origin of species by means of natural selection. (John Murray, London, 1859).
Google Scholar - Turner, D. D. In defense of living fossils. Biol. Philos. 34, 23 (2019).
Article Google Scholar - Lidgard, S. & Love, A. C. Rethinking living fossils. BioScience 68, 760–770 (2018).
Article PubMed PubMed Central Google Scholar - Lidgard, S. & Love, A. C. The living fossil concept: reply to Turner. Biol. Philos. 36, 13 (2021).
Article Google Scholar - Olson, M. E., Arroyo-Santos, A. & Vergara-Silva, F. A user’s guide to metaphors in ecology and evolution. Trends Ecol. Evol. 34, 605–615 (2019).
Article PubMed Google Scholar - Calonje M., Stevenson D. W. & Osborne R. The World List of Cycads, online edition. https://www.cycadlist.org (2022).
- Norstog, K. J. & Nichols, T. J. The Biology of the Cycads. (Cornell University Press, New York, 1997).
Google Scholar - Leslie, A. B. et al. An overview of extant conifer evolution from the perspective of the fossil record. Am. J. Bot. 105, 1531–1544 (2018).
Article PubMed Google Scholar - Choo, T. Y. & Escapa, I. H. Assessing the evolutionary history of the fern family Dipteridaceae (Gleicheniales) by incorporating both extant and extinct members in a combined phylogenetic study. Am. J. Bot. 105, 1315–1328 (2018).
Article CAS PubMed Google Scholar - Clugston, J. A. & Kenicer, G. J. Sexing cycads—a potential saviour. Nat. Plants 8, 326–327 (2022).
Article PubMed Google Scholar - Medina-Villarreal A., González-Astorga J. & de los Monteros A. E. Evolution of Ceratozamia cycads: a proximate-ultimate approach. Mol. Phylogenet. Evol. 139, 106530 (2019).
- Nagalingum, N. S. et al. Recent synchronous radiation of a living fossil. Science 334, 796–799 (2011).
Article ADS CAS PubMed Google Scholar - Condamine, F. L., Nagalingum, N. S., Marshall, C. R. & Morlon, H. Origin and diversification of living cycads: a cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evolut. Biol. 15, 65 (2015).
Article Google Scholar - Brenner, E. D., Stevenson, D. W. & Twigg, R. W. Cycads: Evolutionary innovations and the role of plant-derived neurotoxins. Trends Plant Sci. 8, 446–452 (2003).
Article CAS PubMed Google Scholar - Harris, T. The fossil cycads. Palaeontology 4, 313–323 (1961).
Google Scholar - Elgorriaga, A. & Atkinson, B. A. Cretaceous pollen cone with three-dimensional preservation sheds light on the morphological evolution of cycads in deep time. N. Phytol. 238, 1695–1710 (2023).
Article CAS Google Scholar - Coiro, M., Allio, R., Mazet, N., Seyfullah, L. J. & Condamine, F. L. Reconciling fossils with phylogenies reveals the origin and macroevolutionary processes explaining the global cycad biodiversity. N. Phytol. 240, 1616–1635 (2023).
Article Google Scholar - Lloyd, G. T. Journeys through discrete-character morphospace: synthesizing phylogeny, tempo, and disparity. Palaeontology 61, 637–645 (2018).
Article Google Scholar - Hughes, M., Gerber, S. & Wills, M. A. Clades reach highest morphological disparity early in their evolution. Proc. Natl. Acad. Sci. USA 2013, 36–38 (2013).
Google Scholar - Archangelsky, S. & Baldoni, A. Notas sobre la flora de la zona de Tico, provincia de Santa Cruz. X. Dos nuevas especies de Pseudoctenis (Cycadales). Ameghiniana 9, 241–257 (1972).
Google Scholar - Coiro, M. & Pott, C. Eobowenia gen. nov. from the Early Cretaceous of Patagonia: Indication for an early divergence of Bowenia? BMC Evolut. Biol. 17, 97 (2017).
Article Google Scholar - Florin R. Studien über die Cycadales des Mesozoikums, nebst Erörterungen über die Spaltöffnungsapparate der Bennettitales_._ Svenska Vetenskapsakademiens Handligar 12, 1–134 (1933).
- Coiro, M., Roberts, E. A., Hofmann, C.-C. & Seyfullah, L. J. Cutting the long branches: Consilience as a path to unearth the evolutionary history of Gnetales. Front. Ecol. Evol. 10, 1082639 (2022).
Article Google Scholar - Crane, P. R. The fossil history of the Gnetales. Int. J. Plant Sci. 157, 50–57 (1996).
Article Google Scholar - Andruchow-Colombo A., Escapa I. H., Aagesen L. & Matsunaga K. K. In search of lost time: tracing the fossil diversity of Podocarpaceae through the ages. Bot. J. Linnean Soc. 203, 315–336 (2023).
- Herendeen, P. S., Friis, E. M., Pedersen, K. R. & Crane, P. R. Palaeobotanical redux: Revisiting the age of the angiosperms. Nat. Plants 3, 1–8 (2017).
Article Google Scholar - Coiro, M., Doyle, J. A. & Hilton, J. How deep is the conflict between molecular and fossil evidence on the age of angiosperms? N. Phytol. 223, 83–99 (2019).
Article Google Scholar - Gorelick, R. Did insect pollination cause increased seed plant diversity? Biol. J. Linn. Soc. 74, 407–427 (2001).
Article Google Scholar - Benton, M. J., Wilf, P. & Sauquet, H. The Angiosperm Terrestrial Revolution and the origins of modern biodiversity. N. Phytol. 233, 2017–2035 (2022).
Article Google Scholar - Kipp M. A. et al. Nitrogen isotopes reveal independent origins of N2-fixing symbiosis in extant cycad lineages. Nat. Ecol. Evol. 8, 57–69 (2024).
- Strotz, L. C. & Lieberman, B. S. The end of the line: competitive exclusion and the extinction of historical entities. R. Soc. Open Sci. 10, 221210 (2023).
Article ADS PubMed PubMed Central Google Scholar - Wing, S. L., Hickey, L. J. & Swisher, C. L. Implications of an exceptional fossil flora for Late Cretaceous vegetation. Nature 363, 342–344 (1993).
Article ADS Google Scholar - Griffith, M. P., Magellan, T. M. & Tomlinson, P. B. Variation in leaflet structure in Cycas (Cycadales: Cycadaceae): Does anatomy follow phylogeny and geography? Int. J. Plant Sci. 175, 241–255 (2014).
Article Google Scholar - Glos R. A., et al. Leaflet anatomical diversity in Zamia (Cycadales: Zamiaceae) shows little correlation with phylogeny and climate. Bot. Rev. 88, 437–452 (2022).
- Vovides, A. P. et al. Epidermal morphology and leaflet anatomy of Dioon (Zamiaceae) with comments on climate and environment. Flora.: Morphol. Distrib. Funct. Ecol. Plants 239, 20–44 (2018).
Article Google Scholar - Barone Lumaga, M. R., Coiro, M., Truernit, E., Erdei, B. & De Luca, P. Epidermal micromorphology in Dioon: Did volcanism constrain Dioon evolution? Bot. J. Linn. Soc. 179, 236–254 (2015).
Article Google Scholar - Gorelick, R. & Olson, K. Is lack of cycad (Cycadales) diversity a result of a lack of polyploidy? Bot. J. Linn. Soc. 165, 156–167 (2011).
Article Google Scholar - Liu, Y. et al. The Cycas genome and the early evolution of seed plants. Nat. Plants 8, 389–401 (2022).
Article CAS PubMed PubMed Central Google Scholar - Nowak, H., Schneebeli-Hermann, E. & Kustatscher, E. No mass extinction for land plants at the Permian–Triassic transition. Nat. Commun. 10, 384 (2019).
Article ADS CAS PubMed PubMed Central Google Scholar - McElwain, J. C. & Punyasena, S. W. Mass extinction events and the plant fossil record. Trends Ecol. Evol. 22, 548–557 (2007).
Article PubMed Google Scholar - Thompson, J. B. & Ramírez-Barahona, S. No phylogenetic evidence for angiosperm mass extinction at the Cretaceous–Palaeogene (K-Pg) boundary. Biol. Lett. 19, 20230314 (2023).
Article PubMed PubMed Central Google Scholar - Puttick, M. N., Guillerme, T. & Wills, M. A. The complex effects of mass extinctions on morphological disparity. Evolution 74, 2207–2220 (2020).
Article PubMed Google Scholar - Oyston J. W., Hughes M., Gerber S. & Wills M. A. Why should we investigate the morphological disparity of plant clades? Ann. Bot. 117, 859–879 (2015).
- Bateman, R. M. & Hilton, J. Palaeobotanical systematics for the phylogenetic age: applying organ-species, form-species and phylogenetic species concepts in a framework of reconstructed fossil and extant whole-plants. Taxon 58, 1254–1280 (2009).
Article Google Scholar - Hermsen, E. & Taylor, E. Morphology and ecology of the Antarcticycas plant. Rev. Palaeobot. Palynol. 153, 105–120 (2009).
Article Google Scholar - Hermsen, E. J., Taylor, T. N., Taylor, E. L. & Stevenson, D. W. Cataphylls of the Middle Triassic cycad Antarcticycas schopfii and new insights into cycad evolution. Am. J. Bot. 93, 724–738 (2006).
Article PubMed Google Scholar - Smoot, E., Taylor, T. & Delevoryas, T. Structurally preserved fossil plants from Antarctica. I. Antarcticycas, gen. nov., a Triassic cycad stem from the Beardmore Glacier area. Am. J. Bot. 72, 1410–1423 (1985).
Article Google Scholar - Blonder, B., Violle, C., Bentley, L. P. & Enquist, B. J. Venation networks and the origin of the leaf economics spectrum. Ecol. Lett. 14, 91–100 (2011).
Article PubMed Google Scholar - Osnas, J. L. D., Lichstein, J. W., Reich, P. B. & Pacala, S. W. Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science 340, 741–744 (2013).
Article ADS CAS PubMed Google Scholar - Chartier, M. & Jabbour, F. The floral morphospace–a modern comparative approach to study angiosperm evolution. N. Phytol. 204, 841–853 (2014).
Article Google Scholar - Chartier, M. et al. How (much) do flowers vary? Unbalanced disparity among flower functional modules and a mosaic pattern of morphospace occupation in the order Ericales. Proc. R. Soc. B: Biol. Sci. 284, 20170066 (2017).
Article Google Scholar - Lopez Martinez A. M., et al. Angiosperm flowers reached their highest morphological diversity early in their evolutionary history. New Phytol. 241, 1348–1360 (2024).
- Gavrilets, S. & Losos, J. B. Adaptive radiation: contrasting theory with data. Science 323, 732–737 (2009).
Article ADS CAS PubMed Google Scholar - Givnish, T. J. Adaptive radiation versus ‘radiation’ and ‘explosive diversification’: Why conceptual distinctions are fundamental to understanding evolution. N. Phytol. 207, 297–303 (2015).
Article Google Scholar - Bouchenak-Khelladi Y., Onstein R. E., Xing Y., Schwery O. & Linder H. P. On the complexity of triggering evolutionary radiations. New Phytol. 207, 313–326 (2015).
- Tomescu, A. M. F., Escapa, I. H., Rothwell, G. W., Elgorriaga, A. & Cúneo, N. R. Developmental programmes in the evolution of Equisetum reproductive morphology: a hierarchical modularity hypothesis. Ann. Bot. 119, 489–505 (2017).
Article PubMed PubMed Central Google Scholar - Heath, T. A., Huelsenbeck, J. P. & Stadler, T. The fossilized birth–death process for coherent calibration of divergence-time estimates. Proc. Natl. Acad. Sci. 111, E2957–E2966 (2014).
Article ADS CAS PubMed PubMed Central Google Scholar - Zhang, C., Stadler, T., Klopfstein, S., Heath, T. A. & Ronquist, F. Total-evidence dating under the fossilized birth-death process. Syst. Biol. 65, 228–249 (2016).
Article PubMed Google Scholar - Greguss P. 1968 Xylotomy of the living Cycads, with a description of their leaves and epidermis. Budapest: Akademiai Kiado.
- Harris T. M. 1964 The Yorkshire Jurassic Flora. II. Caytoniales, Cycadales & Pteridosperms. London: British Museum (Natural History).
- Harris T. M. The Fossil Flora of Scoresby Sound, East Greenland: Part 2: Description of seed plants incertae sedis together with a discussion of certain Cycadophyta. Meddelelser om Gronland 35, 1–104 (1932).
- Watson, J. & Cusack, H. A. Cycadales of the English Wealden. Palaeontogr. Soc. Monogr. 622, 1–189 (2005).
Google Scholar - Archangelsky, A., Andreis, R. R., Archangelsky, S. & Artabe, A. Cuticular characters adapted to volcanic stress in a new Cretaceous cycad leaf from Patagonia, Argentina. Considerations on the stratigraphy and depositional history of the Baqueró Formation. Rev. Palaeobot. Palynol. 89, 213–233 (1995).
Article Google Scholar - Barale G. 1981 La paléoflore jurassique du Jura français: étude systématique, aspects stratigraphiques et paléoécologiques. Villeurbanne: Département des sciences de la terre, Université Claude-Bernard Lyon.
- Barthel, M. Eozane Floren des Geiseltales: Farne und Cycadeen. Abhandlungen des. Zent. Geologischen Inst. Palaontol. Abhandl 26, 439–498 (1976).
Google Scholar - Berthelin, M. & Pons, D. Signification des caracteres partages entre Bennettitales et Cycadales. Implications de la decouverte d’une Cycadale nouvelle du Cenomanien de l’Anjou (France). Annales de. Paleontologie 85, 227–239 (1999).
Article Google Scholar - Carpenter, R. Macrozamia from the early Tertiary of Tasmania and a study of the cuticles of extant species. Aust. Syst. Bot. 4, 433–444 (1991).
Article Google Scholar - Carpenter, R. J., Macphail, M. K., Jordan, G. J. & Hill, R. S. Fossil evidence for open, Proteaceae-dominated heathlands and fire in the Late Cretaceous of Australia. Am. J. Bot. 102, 1–16 (2015).
Article Google Scholar - Cookson, I. C. On Macrozamia hopeites -an early Tertiary cycad from Australia. Phytomorphology 3, 306–312 (1953).
Google Scholar - Doludenko, M. P. & Svanidze, C. I. Pozdnejurskaja flora Gruzii. Trans. Geol. Inst., Acad. Sci. USSR 178, 1–116 (1969).
Google Scholar - Douglas J. G. 1969 The Mesozoic floras of Victoria. Melbourne: Department of Mines.
- Erdei, B., Akgün, F. & Barone Lumaga, M. R. Pseudodioon akyoli gen. et sp. nov., an extinct member of Cycadales from the Turkish Miocene. Plant Syst. Evol. 285, 33–49 (2009).
Article Google Scholar - Erdei, B., Calonje, M., Hendy, A. & Espinosa, N. A review of the Cenozoic fossil record of the genus Zamia L. (Zamiaceae, Cycadales) with recognition of a new species from the late Eocene of Panama – Evolution and biogeographic inferences. Bull. Geosci. 93, 185–204 (2018).
Article Google Scholar - Erdei, B. & Manchester, S. R. Ctenis clarnoensis sp n., an unusual cycadalean foliage from the Eocene Clarno formation, Oregon. Int. J. Plant Sci. 176, 31–43 (2015).
Article Google Scholar - Erdei, B., Manchester, S. R. & Kvaček, Z. Dioonopsis Horiuchi et Kimura Leaves from the Eocene of Western North America: A cycad shared with the Paleogene of Japan. Int. J. Plant Sci. 173, 81–95 (2012).
Article Google Scholar - Feng, Z., Lv, Y., Guo, Y., Wei, H.-B. & Kerp, H. Leaf anatomy of a late Palaeozoic cycad. Biol. Lett. 13, 20170456 (2017).
Article PubMed PubMed Central Google Scholar - Hill, R. Three new Eocene cycads from eastern Australia. Aust. J. Bot. 28, 105 (1980).
Article MathSciNet Google Scholar - Conran J. G., Bannister J. M., Kaulfuss U. & Lee D. E. Pterostoma neehoffii (cf. Zamiaceae): a new species of extinct cycad from the middle Miocene of New Zealand and an overview of fossil New Zealand cycads. N. Z. J. Bot. 58, 30–47 (2020).
- Hill, R. S. & Pole, M. S. Two new species of Pterostoma R. S. Hill from Cenozoic sediments in Australasia. Rev. Palaeobot. Palynol. 80, 123–130 (1994).
Article Google Scholar - Horiuchi, J. & Kimura, T. Dioonopsis nipponica gen. et. sp. nov., a new cycad from the Palaeogene of Japan. Rev. Palaeobot. Palynol. 51, 213–225 (1987).
Article Google Scholar - Krassilov, V. A. Late Cretaceous gymnosperms from Sakhalin and the terminal Cretaceous event. Palaeontology 21, 893–905 (1978).
Google Scholar - Kvaček, Z. A new Tertiary Ceratozamia (Zamiaceae, Cycadopsida) from the european Oligocene. Flora 197, 303–316 (2002).
Article Google Scholar - Kvaček, Z. New fossil records of Ceratozamia (Zamiaceae, Cycadales) from the European Oligocene and lower Miocene. Acta Palaeobot. 54, 231–247 (2014).
Article Google Scholar - Kvac̆ek, J. New Cycad Foliage of Pseudoctenis babinensis From the Bohemian Cenomanian. Acta Musei Nationalis Pragae, Ser. B Historia Naturalis 64, 125–131 (2008).
Google Scholar - Kvaček, Z. & Manchester, S. Eostangeria Barthel (extinct Cycadales) from the Paleogene of western North America and Europe. Int. J. Plant Sci. 160, 621–629 (1999).
Article Google Scholar - Uzunova, K., Palamarev, E. & Kvaček, Z. Eostangeria ruzinciniana (Zamiaceae) from the Middle Miocene of Bulgaria and its relationship to similar taxa of fossil Eostangeria, and extant Chigua and Stangeria (Cycadales). Acta Palaeobot. 41, 177–193 (2001).
Google Scholar - Passalia, M. G. On the presence of the cycad Pseudoctenis dentata Archangelsky and Baldoni in the Punta del Barco Formation (late Aptian), Santa Cruz Province, Argentina. Ameghiniana 50, 257–264 (2013).
Article Google Scholar - Pole, M. & Douglas, B. Plant macrofossils of the upper Cretaceous Kaitangata Coalfield, New Zealand. Aust. J. Bot. 12, 331–364 (1999).
Article Google Scholar - Pott, C., Kerp, J. H. F. & Krings, M. Pseudoctenis cornelii nov. spec.(cycadalean foliage) from the Carnian (Upper Triassic) of Lunz, Lower Austria. Ann. des. Naturhistorischen Mus. Wien. 109 A, 1–17 (2007).
Google Scholar - Su, K., Quan, C. & Liu, Y.-S. Cycas fushunensis sp. nov. (Cycadaceae) from the Eocene of northeast China. Rev. Palaeobot. Palynol. 204, 43–49 (2014).
- Van Konijnenburg-Van Cittert, J. H. A. & Van Der Burgh, J. The flora from the Kimmeridgian (Upper Jurassic) of Culgower, Sutherland, Scotland. Rev. Palaeobot. Palynol. 61, 1–51 (1989).
Article Google Scholar - Paradis E. & Schliep K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019).
- Lloyd, G. T. Estimating morphological diversity and tempo with discrete character-taxon matrices: Implementation, challenges, progress, and future directions. Biol. J. Linn. Soc. 118, 131–151 (2016).
Article Google Scholar - Gower J. C. A general coefficient of similarity and some of its properties. Biometrics 27, 857–871 (1971).
- Guillerme T. & Cooper N. Time for a rethink: time sub-sampling methods in disparity-through-time analyses. Palaeontology, 1–13. https://doi.org/10.1111/pala.12364 (2018).
- Guillerme, T., Puttick, M. N., Marcy, A. E. & Weisbecker, V. Shifting spaces: Which disparity or dissimilarity measurement best summarize occupancy in multidimensional spaces? Ecol. Evol. 10, 7261–7275 (2020).
Article PubMed PubMed Central Google Scholar - Mayhew, P. J., Bell, M. A., Benton, T. G. & McGowan, A. J. Biodiversity tracks temperature over time. Proc. Natl. Acad. Sci. 109, 15141–15145 (2012).
Article ADS CAS PubMed PubMed Central Google Scholar - Hyndman, R. J. & Khandakar, Y. Automatic time series forecasting: the forecast package for R. J. Stat. Softw. 26, 1–22 (2008).
Google Scholar
Acknowledgements
This research was funded in whole or in part by the Austrian Science Fund (FWF), grant doi: 10.55776/M3168. The computational results presented have been achieved in part using the Vienna Scientific Cluster (VSC). We are deeply grateful to Thomas Guillerme for implementing functions in dispRity allowing us to analyse data across multiple trees, and further suggestions on the analyses. We also thank Jamie B. Thompson and an anonymous reviewers for comments that greatly improved the manuscript. We also thank Michael Calonje for allowing us to use many of the cycad leaf images included in Fig. 1.
Author information
Authors and Affiliations
- Department of Palaeontology, University of Vienna, Vienna, Austria
Mario Coiro & Leyla Jean Seyfullah - Ronin Institute for Independent Scholarship, Montclair, NJ, USA
Mario Coiro
Authors
- Mario Coiro
You can also search for this author inPubMed Google Scholar - Leyla Jean Seyfullah
You can also search for this author inPubMed Google Scholar
Contributions
M.C. and L.J.S. conceived the idea. MC collected the data, analysed the data, generated the graphs and drafted the figures. M.C. and L.S. wrote the manuscript.
Corresponding author
Correspondence toMario Coiro.
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Katie Davis and Luke R. Grinham. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coiro, M., Seyfullah, L.J. Disparity of cycad leaves dispels the living fossil metaphor.Commun Biol 7, 328 (2024). https://doi.org/10.1038/s42003-024-06024-9
- Received: 03 August 2023
- Accepted: 07 March 2024
- Published: 14 March 2024
- DOI: https://doi.org/10.1038/s42003-024-06024-9
- Springer Nature Limited