Isolated populations and complex disease gene identification (original) (raw)
Over the past few years, understanding how genetic variation in individuals and in populations contributes to the biological pathways involved in determining human traits and mechanisms of disease has become a reachable goal for genetic research. Following on from the achievements in molecular studies of monogenic disorders, recent studies have used strategies of hypothesis-free fine mapping of genes and loci to identify underlying factors in common complex diseases with major impacts on public health. These diseases, which include cancers, coronary heart disease, schizophrenia, autism and multiple sclerosis, arise from complex interactions between environmental factors and variation in several different genes. Until recently, detection of the genes underlying these diseases met with only limited success, but the past two years have witnessed the identification of more than 100 well established loci. These successes mainly involved the collection of very large study cohorts for any individual trait and international collaborations on an unprecedented scale [1].
The detection of genes underlying common complex diseases might not always need large global population samples. Samples of individuals from genetically isolated populations, or 'population isolates', have already proved immensely useful in the identification of rare recessive disease genes. Such genes are only detectable in isolated populations with a limited number of founders, where rare disease alleles are enriched, thus resulting in homozygote individuals affected by the disease. Impressive accomplishments in disease-locus mapping and gene identification using genome-wide scans of only a handful of affected individuals in such populations have been reported, typically based on linkage analyses and homozygosity scanning [2, 3]. It is becoming increasingly apparent that studies locating genes underlying complex phenotypes also benefit from the study of samples from homogeneous populations with a limited number of founders - 'founder populations' (Table 1).
Table 1 Recent genetic studies of complex diseases and traits in special populations
Success stories from population isolates
One of the most impressive examples of the resourceful use of known genealogy, large extended families and vast amounts of medical data in genetic studies is provided by the company deCODE genetics in Iceland, where more than 50% of the adult population have volunteered their medical and genetic information to be used in genetic research [[4](/article/10.1186/gb-2008-9-8-109#ref-CR4 "Statistics Iceland. [ http://www.statice.is/
]"), [5](/article/10.1186/gb-2008-9-8-109#ref-CR5 "deCODE genetics. [
http://www.decode.com
]")\]. Although the Icelandic population does not represent a population isolate as conventionally defined, genetic drift over generations has reduced the amount of variation within it relative to the rest of Europe \[[6](/article/10.1186/gb-2008-9-8-109#ref-CR6 "Helgason A, Nicholson G, Stefansson K, Donnelly P: A reassessment of genetic diversity in Icelanders: strong evidence from multiple loci for relative homogeneity caused by genetic drift. Ann Hum Genet. 2003, 67: 281-297. 10.1046/j.1469-1809.2003.00046.x.")\]. This, among other benefits of a geographically isolated population, has enabled the identification by means of linkage, and more recently by genome-wide association (GWA) studies, of an impressive number of variants contributing to the development of common/complex disease \[[5](/article/10.1186/gb-2008-9-8-109#ref-CR5 "deCODE genetics. [
http://www.decode.com
]")\]. Among these are gene loci for myocardial infarction and stroke (_ALOX5AP_ and chromosomal region 9p21) \[[7](/article/10.1186/gb-2008-9-8-109#ref-CR7 "Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K: The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004, 36: 233-239. 10.1038/ng1311."), [8](/article/10.1186/gb-2008-9-8-109#ref-CR8 "Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K: A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007, 316: 1491-1493. 10.1126/science.1142842.")\], type 2 diabetes (_TCF7L2_ and _CDKAL1_) \[[9](/article/10.1186/gb-2008-9-8-109#ref-CR9 "Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K: Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006, 38: 320-323. 10.1038/ng1732."), [10](/article/10.1186/gb-2008-9-8-109#ref-CR10 "Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, Doumatey A, So WY, Ma RC, Andersen G, Borch-Johnsen K, et al: A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007, 39: 770-775. 10.1038/ng2043.")\], atrial fibrillation (4q25) \[[11](/article/10.1186/gb-2008-9-8-109#ref-CR11 "Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, et al: Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007, 448: 353-357. 10.1038/nature06007.")\] and prostate cancer (2p15 and Xp11.22) \[[12](/article/10.1186/gb-2008-9-8-109#ref-CR12 "Gudmundsson J, Sulem P, Rafnar T, Bergthorsson JT, Manolescu A, Gudbjartsson D, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Blondal T, Jakobsdottir M, Stacey SN, Kostic J, Kristinsson KT, Birgisdottir B, Ghosh S, Magnusdottir DN, Thorlacius S, Thorleifsson G, Zheng SL, Sun J, Chang BL, Elmore JB, Breyer JP, McReynolds KM, Bradley KM, Yaspan BL, Wiklund F, Stattin P, Lindström S, et al: Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat Genet. 2008, 40: 281-283. 10.1038/ng.89.")\]. In addition to disease genes, the Icelandic population has revealed genes contributing to a number of complex traits, such as adult stature (several loci, including _ZBTB38_) \[[13](/article/10.1186/gb-2008-9-8-109#ref-CR13 "Gudbjartsson DF, Walters GB, Thorleifsson G, Stefansson H, Halldorsson BV, Zusmanovich P, Sulem P, Thorlacius S, Gylfason A, Steinberg S, Helgadottir A, Ingason A, Steinthorsdottir V, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Pedersen O, Aben KK, Witjes JA, Swinkels DW, den Heijer M, Franke B, Verbeek AL, Becker DM, Yanek LR, Becker LC, et al: Many sequence variants affecting diversity of adult human height. Nat Genet. 2008, 40: 609-615. 10.1038/ng.122.")\] as well as skin and hair pigmentation (_SLC24A4_, _KITLG_, _TYR_, _OCA2_, _MC1R_ and 6p25.3) \[[14](/article/10.1186/gb-2008-9-8-109#ref-CR14 "Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, Manolescu A, Karason A, Palsson A, Thorleifsson G, Jakobsdottir M, Steinberg S, Pálsson S, Jonasson F, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Aben KK, Kiemeney LA, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K: Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007, 39: 1443-1452. 10.1038/ng.2007.13."), [15](/article/10.1186/gb-2008-9-8-109#ref-CR15 "Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Jakobsdottir M, Steinberg S, Gudjonsson SA, Palsson A, Thorleifsson G, Pálsson S, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Aben KK, Vermeulen SH, Goldstein AM, Tucker MA, Kiemeney LA, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K: Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008, 40: 835-837. 10.1038/ng.160.")\]. The continuing work by deCODE genetics on 50 common diseases is sure to result in a slew of additional gene findings and help to characterize the allelic spectrum of disease-predisposing variants. The wisely designed strategy of fully harvesting the unique population and the combined power of linkage and association has been the basis of the success of genetic research in Iceland.Another population isolate with proven value in gene mapping is the population of Finland, where genes for 35 monogenic diseases that are more frequent than in other populations have been identified [16]. Features of the Finnish population have also been an advantage in studies of schizophrenia spectrum disorders: a balanced translocation (1;11)(q42.1;q14.3) segregating with schizophrenia was first described in a large Scottish family [17] and evidence for association of the gene DISC1 with the disorder was subsequently obtained in Finnish families with diagnosed schizophrenia [18, 19]. Large pedigrees from the Finnish population were also used successfully in a study of familial combined hyperlipidemia that identified the gene for upstream stimulatory factor 1 (USF1) as a risk factor for this complex disease [20]. This association was subsequently replicated in other populations, and evidence of the functional significance of the gene variants and their association with cardiovascular disease and dyslipidemia at the population level has also been obtained [21–23]. Another excellent example from Finland is a gene conferring susceptibility to asthma (NPSR1), discovered in Kainuu and North Karelia subpopulations of Eastern Finland representing regions of the late-settlement [24].
The communal lifestyle and genetic isolation of the Hutterites, who live in the northern United States and western Canada, have especially aided studies of asthma and related traits [25]. Recently, the chitinase 3-like 1 gene was identified as an asthma-susceptibility gene in Hutterites, and the finding subsequently replicated in two population cohorts of European descent [25]. Studies of type 2 diabetes and obesity have used Pima Indians [26], as well as other genetic isolates, such as Finland and Sardinia [27, 28]. Genes contributing to neuropsychiatric disorders are sought, and previous gene discoveries are confirmed, in studies of special populations, such as people from the Antioquia in Colombia and the Central Valley of Costa Rica [29], Basques from Spain [30], the Micronesian population of the islands of Kosrae [31] and Palau [32], Bulgarian Gypsies [33], and sub-isolates from Sweden [34] and Israel [35]. Other special populations utilized in recent genetic studies of complex diseases include French Canadians [36], Ashkenazi Jews [37], Mennonites [38], Newfoundlanders [39], sub-isolates from the Netherlands [40] and the Amish [41].
The important observation from all these studies is that the genetic variants identified within isolates and/or exceptional families seemingly segregating a common disease in a near-Mendelian fashion are not restricted therein, but are being replicated in large-scale population samples and uncovering new pathways behind these disease processes.
Reduced haplotype complexity
The increasing information in public databases on single nucleotide polymorphisms (SNPs) and their haplotype-tagging properties [42–44] as well as advances in genome-wide data collection using advanced technology platforms [45] have facilitated the recent deluge of studies utilizing the genome-wide SNP-association strategy to identify loci influencing disease phenotypes. This GWA approach is essentially 'hypothesis free'. It circumvents the necessity of understanding disease pathogenesis, which has previously guided studies of candidate genes selected for their biological relevance. In a GWA study, a dense set of SNPs totaling up to 1 million across the genome is genotyped using a standard platform and tested for association with a disease or quantitative trait. Successful gene identification by GWA studies, which operates very much under the common-disease, common-variant hypothesis, requires that the susceptibility variant itself, or a variant highly correlated with it, is among the markers typed.
As a result of the International HapMap Project [44], the linkage disequilibrium (LD) patterns of most genomic regions are known and SNP genotyping platforms have been designed to detect a restricted number of haplotype-tagging variants with the hypothesis that they should capture most of the common variation within genomic regions [46, 47]. Ultimately, the LD structure of each study population determines the number of genotyped SNPs needed for complete coverage in a GWA study.
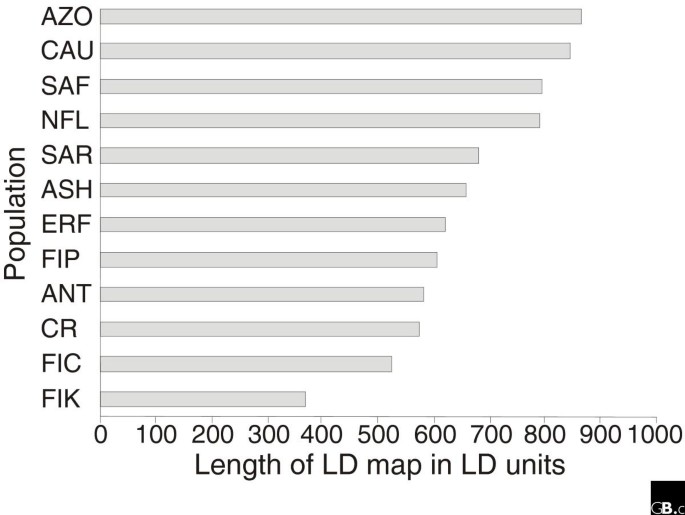
Several studies have been undertaken to characterize differences in the magnitude and distribution of LD in global populations [48–51]. Even though the density of SNPs required for 100% coverage of the genome in whole-genome genotyping efforts in various global populations remains unknown, on the basis of the size of LD blocks in 'young isolates', populations that are relatively recently (less than 2,000 years ago) inhabited or isolated, it has been concluded that GWA studies in populations such as that of Finland, the Dutch isolate referred to above, Costa Rica, Antioquia, Sardinia or the Ashkenazim require some 30% fewer markers than in more outbred populations, and that the current GWA panels provide excellent genome-wide coverage with a very small number of gaps (Figure 1) [48]. In an isolated population there are a potentially fewer number of haplotypes being segregated through the population and the haplotype-tagging SNPs should also be able to detect those haplotypes that carry more rare alleles. In a more outbred population with considerably higher numbers of haplotypes for a given locus, the causative allele is more likely to be located on several haplotypic backgrounds, thereby diluting its signal to an extent that precludes its identification by genetic means. The value of population isolates and their genomic LD patterns may thus be even greater when lower-frequency (less than 5%) variants are considered [52].
Figure 1
Considerable differences in LD map length across populations. The length of the LD map in LD units (as defined in [88]) in 12 different population samples is depicted in order of decreasing map length. AZO, Azores; CAU, outbred European-derived sample; SAF, Afrikaner; NFL, Newfoundland; SAR, province of Nuoro in Sardinia; ASH, Ashkenazi; ERF, a village in southwestern Netherlands; FIP, Finland nationwide; ANT, Antioquia; CR, Central Valley of Costa Rica; FIC, early-settlement Finland; FIK, Finnish sub-isolate of Kuusamo. Adapted from [48].
The problem of GWA studies carried out in genetic isolates is that the strong LD that initially helped identify the disease locus may in the end hamper efforts to distinguish the biologically relevant variants from insignificant polymorphisms in complete LD with them. Comparing the GWA data across isolates from different populations should help pin down the potential causative variants for functional studies.
Restricted allelic and locus heterogeneity
Extensive allelic and locus heterogeneity, a key feature of common complex diseases, can obscure the association signal within disease-associated genomic regions. This problem is reduced in population isolates. When combined with geographic isolation that prevents the influx of new alleles, genetic drift acts to randomly raise some alleles to fixation and send others to extinction, thus reducing heterogeneity. A representative example of such drift, and of the founder effect, is the enrichment of various recessive diseases in founder populations, such as Ashkenazi Jews [53] and Finns [54], and an exceptionally low prevalence of other diseases in Finns, such as cystic fibrosis or phenylketonuria, which are common in other European populations. In founder populations, these recessive diseases are often characterized by a presence of one founder mutation, whereas numerous mutations in the same genes are identified in the global population [16]. Although allelic heterogeneity is expected to exist behind common diseases even in isolated populations, it is a reasonable expectation that the number of predisposing alleles will be more restricted than in more heterogeneous populations.
Furthermore, isolated populations may facilitate studies of the possible joint actions of associated gene loci as well as studies of the population effect of these associated markers, even before the actual causative variant has been identified. This may be possible as in isolated populations with a high degree of LD, the tagging of specific allele is more reliable than in heterogeneous populations in which broader allelic diversity of associated alleles can obscure these examinations.
In contrast to the 'gene-breaking' mutations underlying most monogenic diseases, variants that affect susceptibility to complex diseases are suggested to be ones that leave gene structure untouched and instead affect the dynamics of gene expression. Such variation can be situated in enhancer elements in the vicinity of the phenotype-causing genes or in the promoters of these genes where various transcription factors bind (_cis_-acting variants). SNPs elsewhere in the genome (_trans_-acting variants) may affect the phenotype via the function of the protein or RNA that the _trans_-acting gene encodes. These _cis_- and _trans_-acting variants account for much of the variation in gene expression between individuals. A good example of the identification of a trait-associated variant in a strong _cis_-regulatory element, using LD and samples from a population isolate, was the finding of the DNA variant behind lactose tolerance/intolerance: the variant was initially found among Finns and later confirmed to represent the common Caucasian mutation. This led to the identification of a regulatory DNA region with enrichment of mutations underlying the trait in numerous global populations [55].
Identification of rare variants
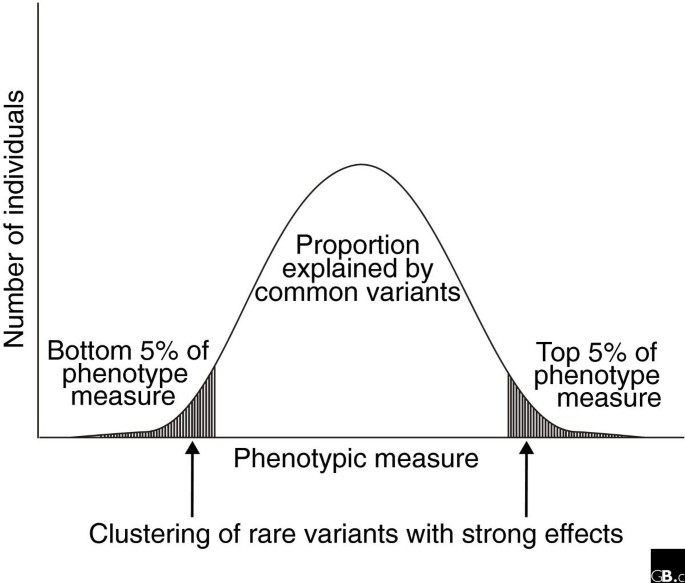
Susceptibility to common complex diseases probably involves the contribution of both common variants and rare mutations [56] and the relative significance of each in particular traits and disease phenotypes will have to be determined by large-scale resequencing studies of associated loci in large study samples. Whereas several common variants are likely to explain a substantial fraction of the heritable variation in complex traits, rare variants probably contribute significantly by having greater effects on the phenotype, as proposed for extreme lipid levels [57, 58] (Figure 2). Furthermore, although rare variants are by definition rare by themselves, in a particular population there could exist a myriad of these variants and in combination they might explain a considerable proportion of the variance in a trait of interest [58]. Consequently, in addition to the interrogation of common polymorphisms, the rare variants implicated in many Mendelian diseases along with structural variation in the genome are now studied with increasing interest [59]. Identification of rare high-impact alleles may be of critical importance for our detailed understanding of the biology behind common diseases or traits.
Figure 2
Contribution of rare and common variants to the distribution of a quantitative phenotype. Although common genetic variants explain the majority of the phenotypic variance in the population, the contribution of rare variants with strong effects may be observed at the extreme ends of the phenotypic distribution.
A whole-genome strategy based on common haplotype-tagging SNPs is unlikely to be very successful in detecting rare variants that increase disease susceptibility [60]. The statistical power to detect susceptibility alleles is positively correlated with the frequency and the penetrance of the allele. Even though detection of rare alleles with high penetrance is essentially as feasible as the detection of common alleles with more modest penetrance, it is unclear how well these rare variants are captured with the GWA arrays designed to tag common SNPs. Thus, while genome-wide association studies are likely to continue to identify the 'low-hanging fruit', study of linkage and association in exceptional families as well as in population isolates may be necessary to identify and define those risk alleles (the majority) that, although significant, are lost in the sea of peaks that fail to reach genome-wide significance in GWA studies as a result of their rarity or population-specific effect [60]. The founder effect, genetic bottlenecks and genetic drift have worked to increase the frequency of certain rare alleles in the population isolate, thus improving the power to detect those in genome-wide studies.
Notably, owing to founder effect and genetic drift, each genetic isolate typically has a unique profile of rare disease alleles [61]. Some rare variants that are readily detected in one population isolate may go unnoticed in others, necessitating the use of multiple isolates to get a picture of the full spectrum of variants with effects on phenotype [62]. Importantly, if the impact of the rare variants on the disease phenotype is really high, measuring them in a clinical setting might turn out to be of critical importance for 'family-specific' or personalized medicine, revealing individuals with the highest genetic risk. The existence of such population- or family-specific alleles is entirely possible - even expected -and personalized medicine just might become more personal than we ever dreamed of.
Population isolates help to minimize the environmental component of disease
In contrast to monogenic diseases, where the genetic composition of an individual often solely determines the disease phenotype, environmental factors are critical risk factors for complex diseases. The incidence and prevalence of many common diseases may vary between founder populations [63], and establishing whether this variation in disease incidence is the result of genetic background or of environmental factors characteristic for the population can be challenging because of complex interactions between genetic risk factors and environmental exposures [63–65]. Natural selection induced by the environment can, for instance, modify allele frequencies and may lead to distinctive disease susceptibilities in different populations [66, 67]. Furthermore, inbreeding in founder populations can increase the incidence of some common diseases, for instance via increased homozygosity of rare variants with large recessive effects [68]. In addition to increasing the incidence of the disease in a given population, environmental factors may have an effect on the severity of the disease phenotype.
Data from model animals suggest that the impact of gene-environment interaction on the phenotype may be considerable [69]. Therefore, accurate determination of phenotype, minimally perturbed by differences in environment, is of great importance for GWA studies - arguably even more so than in linkage studies using family data. Although there is variation in environmental exposures between individuals even in the most homogeneous populations, in population isolates the cultural, environmental and phenotypic homogeneity can facilitate disease-gene identification by reducing variance caused by environmental background. More uniform patterns of, for example, nutrition or exposure to pathogens or homogeneous diagnostic standards, more easily obtained for small populations, provide the best human approximation to controlled experiments in uniform conditions in inbred strains of experimental animals.
The importance of knowing the study population
Population isolates with diverse ethnic backgrounds and different degrees of inbreeding have been described from around the world. Each has its unique characteristics, and may have its own advantages and disadvantages in research into complex diseases (Table 2). Such facts should be considered in study design. Several factors, such as the demographic history of the population, age distribution, number of founders, growth pattern, and degree of genetic and cultural isolation since foundation, determine the features of the genetic landscape of a population isolate [70].
Table 2 Use of isolated versus outbred populations
Relatively young and small founding populations that have experienced population bottleneck events in their history followed by recent expansion in population size should be ideal for initial locus identification using GWA scans. This is because the population history has created a setting in which the genomes are characterized by a high degree of LD and low genetic diversity [48]. Distinguishing the biologically relevant variants at the associated loci would require older isolates with shorter LD intervals. In small, very ancient isolates with limited population growth, such as the Saami of northern Scandinavia, LD is the result of genetic drift, not a founder effect. These old isolates may be very useful for identifying common disease alleles by drift mapping [71]. Population isolates may also contain sub-isolates, which display different LD intervals of disease alleles as well as different mutation frequencies [72]: these sub-isolates may thus be ideal for complex disease gene mapping even when the founder population itself lacks any obvious advantage.
Population isolates have thus earned their place as an indispensable resource for medical genetics through their use in identifying numerous Mendelian disease genes. Their utility is increasingly valued also in complex disease gene mapping. Genetic, environmental and phenotypic homogeneity, good genealogical records, high participation rates in genetic studies, extended LD in the genome, as well as reduced allelic and locus heterogeneity are highly beneficial features for such studies.
Not all genetic isolates are alike: each population has its own advantages and disadvantages for studies of complex diseases, and thus knowing the genetic makeup of the study population is crucial. The choice and design of statistical methods also deserve particular care in studies utilizing population isolates [73] and the study strategy should also differ depending on the allelic architecture of the disease. The global wealth of population isolates with well established history and carefully phenotyped study samples is paving the way to a more comprehensive understanding of complex disease genetics. The scientific community might observe the resource of population isolates to be harnessed not only in medical genetics but also in public-health genomics.
References
- Wellcome Trust Case Control Consortium: Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007, 447: 661-678. 10.1038/nature05911.
Article Google Scholar - Nikali K, Suomalainen A, Terwilliger J, Koskinen T, Weissenbach J, Peltonen L: Random search for shared chromosomal regions in four affected individuals: the assignment of a new hereditary ataxia locus. Am J Hum Genet. 1995, 56: 1088-1095.
PubMed CAS PubMed Central Google Scholar - Neufeld EJ, Mandel H, Raz T, Szargel R, Yandava CN, Stagg A, Faure S, Barrett T, Buist N, Cohen N: Localization of the gene for thiamine-responsive megaloblastic anemia syndrome, on the long arm of chromosome 1, by homozygosity mapping. Am J Hum Genet. 1997, 61: 1335-1341. 10.1086/301642.
Article PubMed CAS PubMed Central Google Scholar - Statistics Iceland. [http://www.statice.is/]
- deCODE genetics. [http://www.decode.com]
- Helgason A, Nicholson G, Stefansson K, Donnelly P: A reassessment of genetic diversity in Icelanders: strong evidence from multiple loci for relative homogeneity caused by genetic drift. Ann Hum Genet. 2003, 67: 281-297. 10.1046/j.1469-1809.2003.00046.x.
Article PubMed CAS Google Scholar - Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K: The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004, 36: 233-239. 10.1038/ng1311.
Article PubMed CAS Google Scholar - Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K: A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007, 316: 1491-1493. 10.1126/science.1142842.
Article PubMed CAS Google Scholar - Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K: Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006, 38: 320-323. 10.1038/ng1732.
Article PubMed CAS Google Scholar - Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, Doumatey A, So WY, Ma RC, Andersen G, Borch-Johnsen K, et al: A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007, 39: 770-775. 10.1038/ng2043.
Article PubMed CAS Google Scholar - Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, et al: Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007, 448: 353-357. 10.1038/nature06007.
Article PubMed CAS Google Scholar - Gudmundsson J, Sulem P, Rafnar T, Bergthorsson JT, Manolescu A, Gudbjartsson D, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Blondal T, Jakobsdottir M, Stacey SN, Kostic J, Kristinsson KT, Birgisdottir B, Ghosh S, Magnusdottir DN, Thorlacius S, Thorleifsson G, Zheng SL, Sun J, Chang BL, Elmore JB, Breyer JP, McReynolds KM, Bradley KM, Yaspan BL, Wiklund F, Stattin P, Lindström S, et al: Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat Genet. 2008, 40: 281-283. 10.1038/ng.89.
Article PubMed CAS PubMed Central Google Scholar - Gudbjartsson DF, Walters GB, Thorleifsson G, Stefansson H, Halldorsson BV, Zusmanovich P, Sulem P, Thorlacius S, Gylfason A, Steinberg S, Helgadottir A, Ingason A, Steinthorsdottir V, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Pedersen O, Aben KK, Witjes JA, Swinkels DW, den Heijer M, Franke B, Verbeek AL, Becker DM, Yanek LR, Becker LC, et al: Many sequence variants affecting diversity of adult human height. Nat Genet. 2008, 40: 609-615. 10.1038/ng.122.
Article PubMed CAS Google Scholar - Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, Manolescu A, Karason A, Palsson A, Thorleifsson G, Jakobsdottir M, Steinberg S, Pálsson S, Jonasson F, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Aben KK, Kiemeney LA, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K: Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007, 39: 1443-1452. 10.1038/ng.2007.13.
Article PubMed CAS Google Scholar - Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Jakobsdottir M, Steinberg S, Gudjonsson SA, Palsson A, Thorleifsson G, Pálsson S, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Aben KK, Vermeulen SH, Goldstein AM, Tucker MA, Kiemeney LA, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K: Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008, 40: 835-837. 10.1038/ng.160.
Article PubMed CAS Google Scholar - Peltonen L, Jalanko A, Varilo T: Molecular genetics of the Finnish disease heritage. Hum Mol Genet. 1999, 8: 1913-1923. 10.1093/hmg/8.10.1913.
Article PubMed CAS Google Scholar - St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C, Evans HJ: Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990, 336: 13-16. 10.1016/0140-6736(90)91520-K.
Article PubMed CAS Google Scholar - Ekelund J, Hovatta I, Parker A, Paunio T, Varilo T, Martin R, Suhonen J, Ellonen P, Chan G, Sinsheimer JS, Sobel E, Juvonen H, Arajärvi R, Partonen T, Suvisaari J, Lönnqvist J, Meyer J, Peltonen L: Chromosome 1 loci in Finnish schizophrenia families. Hum Mol Genet. 2001, 10: 1611-1617. 10.1093/hmg/10.15.1611.
Article PubMed CAS Google Scholar - Hennah W, Varilo T, Kestilä M, Paunio T, Arajärvi R, Haukka J, Parker A, Martin R, Levitzky S, Partonen T, Meyer J, Lönnqvist J, Peltonen L, Ekelund J: Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet. 2003, 12: 3151-3159. 10.1093/hmg/ddg341.
Article PubMed CAS Google Scholar - Pajukanta P, Lilja HE, Sinsheimer JS, Cantor RM, Lusis AJ, Gentile M, Duan XJ, Soro-Paavonen A, Naukkarinen J, Saarela J, Laakso M, Ehnholm C, Taskinen MR, Peltonen L: Familial combined hyperlipidemia is associated with upstream transcription factor 1 (USF1). Nat Genet. 2004, 36: 371-376. 10.1038/ng1320.
Article PubMed CAS Google Scholar - Komulainen K, Alanne M, Auro K, Kilpikari R, Pajukanta P, Saarela J, Ellonen P, Salminen K, Kulathinal S, Kuulasmaa K, Silander K, Salomaa V, Perola M, Peltonen L: Risk alleles of USF1 gene predict cardiovascular disease of women in two prospective studies. PloS Genet. 2006, 2: e69-10.1371/journal.pgen.0020069.
Article PubMed PubMed Central Google Scholar - Naukkarinen J, Gentile M, Soro-Paavonen A, Saarela J, Koistinen HA, Pajukanta P, Taskinen MR, Peltonen L: USF1 and dyslipidemias: converging evidence for a functional intronic variant. Hum Mol Genet. 2005, 14: 2595-2605. 10.1093/hmg/ddi294.
Article PubMed CAS Google Scholar - Reiner AP, Carlson CS, Jenny NS, Durda JP, Siscovick DS, Nickerson DA, Tracy RP: USF1 gene variants, cardiovascular risk, and mortality in European Americans: analysis of two US cohort studies. Arterioscler Thromb Vasc Biol. 2007, 27: 2736-2742. 10.1161/ATVBAHA.107.154559.
Article PubMed CAS Google Scholar - Laitinen T, Polvi A, Rydman P, Vendelin J, Pulkkinen V, Salmikangas P, Mäkelä S, Rehn M, Pirskanen A, Rautanen A, Zucchelli M, Gullstén H, Leino M, Alenius H, Petäys T, Haahtela T, Laitinen A, Laprise C, Hudson TJ, Laitinen LA, Kere J: Characterization of a common susceptibility locus for asthma-related traits. Science. 2004, 304: 300-304. 10.1126/science.1090010.
Article PubMed CAS Google Scholar - Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, Radford S, Parry RR, Heinzmann A, Deichmann KA, Lester LA, Gern JE, Lemanske RF, Nicolae DL, Elias JA, Chupp GL: Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008, 358: 1682-1691. 10.1056/NEJMoa0708801.
Article PubMed CAS PubMed Central Google Scholar - Baier LJ, Hanson RL: Genetic studies of the etiology of type 2 diabetes in Pima Indians: hunting for pieces to a complicated puzzle. Diabetes. 2004, 53: 1181-1186. 10.2337/diabetes.53.5.1181.
Article PubMed CAS Google Scholar - Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, et al: A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007, 316: 1341-1345. 10.1126/science.1142382.
Article PubMed CAS PubMed Central Google Scholar - Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orrú M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR: Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007, 3: e115-10.1371/journal.pgen.0030115.
Article PubMed PubMed Central Google Scholar - Herzberg I, Jasinska A, García J, Jawaheer D, Service S, Kremeyer B, Duque C, Parra MV, Vega J, Ortiz D, Carvajal L, Polanco G, Restrepo GJ, López C, Palacio C, Levinson M, Aldana I, Mathews C, Davanzo P, Molina J, Fournier E, Bejarano J, Ramírez M, Ortiz CA, Araya X, Sabatti C, Reus V, Macaya G, Bedoya G, Ospina J, et al: Convergent linkage evidence from two Latin-American population isolates supports the presence of a susceptibility locus for bipolar disorder in 5q31-34. Hum Mol Genet. 2006, 15: 3146-3153. 10.1093/hmg/ddl254.
Article PubMed CAS Google Scholar - Parsons MJ, Mata I, Beperet M, Iribarren-Iriso F, Arroyo B, Sainz R, Arranz MJ, Kerwin R: A dopamine D2 receptor gene-related polymorphism is associated with schizophrenia in a Spanish population isolate. Psychiatr Genet. 2007, 17: 159-163. 10.1097/YPG.0b013e328017f8a4.
Article PubMed Google Scholar - Wijsman EM, Rosenthal EA, Hall D, Blundell ML, Sobin C, Heath SC, Williams R, Brownstein MJ, Gogos JA, Karayiorgou M: Genome-wide scan in a large complex pedigree with predominantly male schizophrenics from the island of Kosrae: evidence for linkage to chromosome 2q. Mol Psychiatry. 2003, 8: 695-705. 10.1038/sj.mp.4001356. 643
Article PubMed CAS Google Scholar - Devlin B, Bacanu SA, Roeder K, Reimherr F, Wender P, Galke B, Novasad D, Chu A, Cuenco KT, Tiobek S, Otto C, Byerley W: Genome-wide multipoint linkage analyses of multiplex schizophrenia pedigrees from the oceanic nation of Palau. Mol Psychiatry. 2002, 7: 689-694. 10.1038/sj.mp.4001056.
Article PubMed CAS Google Scholar - Kaneva R, Milanova V, Angelicheva D, Macgregor S, Kostov C, Vladimirova R, Aleksiev S, Angelova M, Stoyanova V, Loh A, Hallmayer J, Kalaydjieva L, Jablensky A: Bipolar disorder in the Bulgarian Gypsies: Genetic heterogeneity in a young founder population. Am J Med Genet B Neuropsychiatr Genet. 2008, doi: 10.1002/ajmg.b.30775.
Google Scholar - Venken T, Claes S, Sluijs S, Paterson AD, van Duijn C, Adolfsson R, Del-Favero J, Van Broeckhoven C: Genomewide scan for affective disorder susceptibility loci in families of a northern Swedish isolated population. Am J Hum Genet. 2005, 76: 237-248. 10.1086/427836.
Article PubMed CAS PubMed Central Google Scholar - Kohn Y, Danilovich E, Filon D, Oppenheim A, Karni O, Kanyas K, Turetsky N, Korner M, Lerer B: Linkage disequlibrium in the DTNBP1 (dysbindin) gene region and on chromosome 1p36 among psychotic patients from a genetic isolate in Israel: findings from identity by descent haplotype sharing analysis. Am J Med Genet B Neuropsychiatr Genet. 2004, 128B: 65-70. 10.1002/ajmg.b.30044.
Article PubMed Google Scholar - Seda O, Tremblay J, Gaudet D, Brunelle PL, Gurau A, Merlo E, Pilote L, Orlov SN, Boulva F, Petrovich M, Kotchen TA, Cowley AW, Hamet P: Systematic, genome-wide, sex-specific linkage of cardiovascular traits in French Canadians. Hypertension. 2008, 51: 1156-1162. 10.1161/HYPERTENSIONAHA.107.105247.
Article PubMed CAS Google Scholar - Gan-Or Z, Giladi N, Rozovski U, Shifrin C, Rosner S, Gurevich T, Bar-Shira A, Orr-Urtreger A: Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008, 70: 2277-2283. 10.1212/01.wnl.0000304039.11891.29.
Article PubMed CAS Google Scholar - Orton NC, Innes AM, Chudley AE, Bech-Hansen NT: Unique disease heritage of the Dutch-German Mennonite population. Am J Med Genet A. 2008, 146A: 1072-1087. 10.1002/ajmg.a.32061.
Article PubMed Google Scholar - Martin GR, Loredo JC, Sun G: Lack of association of ghrelin precursor gene variants and percentage body fat or serum lipid profiles. Obesity (Silver Spring). 2008, 16: 908-912. 10.1038/oby.2007.125.
Article CAS Google Scholar - Henneman P, Aulchenko YS, Frants RR, Willems van Dijk K, Oostra BA, van Duijn CM: Prevalence and heritability of the metabolic syndrome and its individual components in a Dutch isolate: The Erasmus Rucphen Family (ERF) study. J Med Genet. 2008, doi:10.1136/jmg.2008.058388
Google Scholar - Hsueh WC, Silver KD, Pollin TI, Bell CJ, O'Connell JR, Mitchell BD, Shuldiner AR: A genome-wide linkage scan of insulin level derived traits: the Amish Family Diabetes Study. Diabetes. 2007, 56: 2643-2648. 10.2337/db06-1023.
Article PubMed CAS Google Scholar - Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, et al: Initial sequencing and analysis of the human genome. Nature. 2001, 409: 860-921. 10.1038/35057062.
Article PubMed CAS Google Scholar - Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K: dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001, 29: 308-311. 10.1093/nar/29.1.308.
Article PubMed CAS PubMed Central Google Scholar - The International HapMap Project. Nature. 2003, 426: 789-796. 10.1038/nature02168.
- Pe'er I, de Bakker PI, Maller J, Yelensky R, Altshuler D, Daly MJ: Evaluating and improving power in whole-genome association studies using fixed marker sets. Nat Genet. 2006, 38: 663-667. 10.1038/ng1816.
Article PubMed Google Scholar - Johnson GC, Esposito L, Barratt BJ, Smith AN, Heward J, Di Genova G, Ueda H, Cordell HJ, Eaves IA, Dudbridge F, Twells RC, Payne F, Hughes W, Nutland S, Stevens H, Carr P, Tuomilehto-Wolf E, Tuomilehto J, Gough SC, Clayton DG, Todd JA: Haplotype tagging for the identification of common disease genes. Nat Genet. 2001, 29: 233-237. 10.1038/ng1001-233.
Article PubMed CAS Google Scholar - Cardon LR, Abecasis GR: Using haplotype blocks to map human complex trait loci. Trends Genet. 2003, 19: 135-140. 10.1016/S0168-9525(03)00022-2.
Article PubMed CAS Google Scholar - Service S, DeYoung J, Karayiorgou M, Roos JL, Pretorious H, Bedoya G, Ospina J, Ruiz-Linares A, Macedo A, Palha JA, Heutink P, Aulchenko Y, Oostra B, van Duijn C, Jarvelin MR, Varilo T, Peddle L, Rahman P, Piras G, Monne M, Murray S, Galver L, Peltonen L, Sabatti C, Collins A, Freimer N: Magnitude and distribution of linkage disequilibrium in population isolates and implications for genome-wide association studies. Nat Genet. 2006, 38: 556-560. 10.1038/ng1770.
Article PubMed CAS Google Scholar - Gu S, Pakstis AJ, Li H, Speed WC, Kidd JR, Kidd KK: Significant variation in haplotype block structure but conservation in tagSNP patterns among global populations. Eur J Hum Genet. 2007, 15: 302-312. 10.1038/sj.ejhg.5201751.
Article PubMed CAS Google Scholar - Shifman S, Kuypers J, Kokoris M, Yakir B, Darvasi A: Linkage disequilibrium patterns of the human genome across populations. Hum Mol Genet. 2003, 12: 771-776. 10.1093/hmg/ddg088.
Article PubMed CAS Google Scholar - Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D: The structure of haplotype blocks in the human genome. Science. 2002, 296: 2225-2229. 10.1126/science.1069424.
Article PubMed CAS Google Scholar - Kruglyak L: Prospects for whole-genome linkage disequilibrium mapping of common disease genes. Nat Genet. 1999, 22: 139-144. 10.1038/9642.
Article PubMed CAS Google Scholar - Charrow J: Ashkenazi Jewish genetic disorders. Fam Cancer. 2004, 3: 201-206. 10.1007/s10689-004-9545-z.
Article PubMed CAS Google Scholar - Norio R: Finnish disease heritage I: characteristics, causes, background. Hum Genet. 2003, 112: 441-456.
PubMed Google Scholar - Enattah NS, Trudeau A, Pimenoff V, Maiuri L, Auricchio S, Greco L, Rossi M, Lentze M, Seo JK, Rahgozar S, Khalil I, Alifrangis M, Natah S, Groop L, Shaat N, Kozlov A, Verschubskaya G, Comas D, Bulayeva K, Mehdi SQ, Terwilliger JD, Sahi T, Savilahti E, Perola M, Sajantila A, Järvelä I, Peltonen L: Evidence of still-ongoing convergence evolution of the lactase persistence T-13910 alleles in humans. Am J Hum Genet. 2007, 81: 615-625. 10.1086/520705.
Article PubMed CAS PubMed Central Google Scholar - Sandilands A, Terron-Kwiatkowski A, Hull PR, O'Regan GM, Clayton TH, Watson RM, Carrick T, Evans AT, Liao H, Zhao Y, Campbell LE, Schmuth M, Gruber R, Janecke AR, Elias PM, van Steensel MA, Nagtzaam I, van Geel M, Steijlen PM, Munro CS, Bradley DG, Palmer CN, Smith FJ, McLean WH, Irvine AD: Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007, 39: 650-654. 10.1038/ng2020.
Article PubMed CAS Google Scholar - Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN: Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003, 33: 177-182. 10.1038/ng1071.
Article PubMed CAS Google Scholar - Cohen JC, Kiss RS, Pertsemlidis A, Marcel YL, McPherson R, Hobbs HH: Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science. 2004, 305: 869-872. 10.1126/science.1099870.
Article PubMed CAS Google Scholar - Stranger BE, Forrest MS, Dunning M, Ingle CE, Beazley C, Thorne N, Redon R, Bird CP, de Grassi A, Lee C, Tyler-Smith C, Carter N, Scherer SW, Tavaré S, Deloukas P, Hurles ME, Dermitzakis ET: Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science. 2007, 315: 848-853. 10.1126/science.1136678.
Article PubMed CAS PubMed Central Google Scholar - Zeggini E, Rayner W, Morris AP, Hattersley AT, Walker M, Hitman GA, Deloukas P, Cardon LR, McCarthy MI: An evaluation of HapMap sample size and tagging SNP performance in large-scale empirical and simulated data sets. Nat Genet. 2005, 37: 1320-1322. 10.1038/ng1670.
Article PubMed CAS Google Scholar - Arcos-Burgos M, Muenke M: Genetics of population isolates. Clin Genet. 2002, 61: 233-247. 10.1034/j.1399-0004.2002.610401.x.
Article PubMed CAS Google Scholar - Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, Cohen JC: Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet. 2007, 39: 513-516. 10.1038/ng1984.
Article PubMed CAS PubMed Central Google Scholar - Schulz LO, Bennett PH, Ravussin E, Kidd JR, Kidd KK, Esparza J, Valencia ME: Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care. 2006, 29: 1866-1871. 10.2337/dc06-0138.
Article PubMed Google Scholar - Yepiskoposyan L, Harutyunyan A: Population genetics of familial Mediterranean fever: a review. Eur J Hum Genet. 2007, 15: 911-916. 10.1038/sj.ejhg.5201869.
Article PubMed CAS Google Scholar - Richards EJ: Inherited epigenetic variation - revisiting soft inheritance. Nat Rev Genet. 2006, 7: 395-401. 10.1038/nrg1834.
Article PubMed CAS Google Scholar - Barreiro LB, Laval G, Quach H, Patin E, Quintana-Murci L: Natural selection has driven population differentiation in modern humans. Nat Genet. 2008, 40: 340-345. 10.1038/ng.78.
Article PubMed CAS Google Scholar - Le Souef PN, Candelaria P, Goldblatt J: Evolution and respiratory genetics. Eur Respir J. 2006, 28: 1258-1263. 10.1183/09031936.06.00088006.
Article PubMed CAS Google Scholar - Rudan I, Rudan D, Campbell H, Carothers A, Wright A, Smolej-Narancic N, Janicijevic B, Jin L, Chakraborty R, Deka R, Rudan P: Inbreeding and risk of late onset complex disease. J Med Genet. 2003, 40: 925-932. 10.1136/jmg.40.12.925.
Article PubMed CAS PubMed Central Google Scholar - Valdar W, Solberg LC, Gauguier D, Cookson WO, Rawlins JN, Mott R, Flint J: Genetic and environmental effects on complex traits in mice. Genetics. 2006, 174: 959-984. 10.1534/genetics.106.060004.
Article PubMed CAS PubMed Central Google Scholar - Wright AF, Carothers AD, Pirastu M: Population choice in mapping genes for complex diseases. Nat Genet. 1999, 23: 397-404. 10.1038/70501.
Article PubMed CAS Google Scholar - Terwilliger JD, Zollner S, Laan M, Pääbo S: Mapping genes through the use of linkage disequilibrium generated by genetic drift: 'drift mapping' in small populations with no demographic expansion. Hum Hered. 1998, 48: 138-154. 10.1159/000022794.
Article PubMed CAS Google Scholar - Fraumene C, Petretto E, Angius A, Pirastu M: Striking differentiation of sub-populations within a genetically homogeneous isolate (Ogliastra) in Sardinia as revealed by mtDNA analysis. Hum Genet. 2003, 114: 1-10. 10.1007/s00439-003-1008-3.
Article PubMed CAS Google Scholar - Bourgain C, Genin E: Complex trait mapping in isolated populations: Are specific statistical methods required?. Eur J Hum Genet. 2005, 13: 698-706. 10.1038/sj.ejhg.5201400.
Article PubMed Google Scholar - Balaci L, Spada MC, Olla N, Sole G, Loddo L, Anedda F, Naitza S, Zuncheddu MA, Maschio A, Altea D, Uda M, Pilia S, Sanna S, Masala M, Crisponi L, Fattori M, Devoto M, Doratiotto S, Rassu S, Mereu S, Giua E, Cadeddu NG, Atzeni R, Pelosi U, Corrias A, Perra R, Torrazza PL, Pirina P, Ginesu F, Marcias S, et al: IRAK-M is involved in the pathogenesis of early-onset persistent asthma. Am J Hum Genet. 2007, 80: 1103-1114. 10.1086/518259.
Article PubMed CAS PubMed Central Google Scholar - Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Center JR, Nguyen TV, Bagger Y, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K: Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008, 358: 2355-2365. 10.1056/NEJMoa0801197.
Article PubMed CAS Google Scholar - Stacey SN, Manolescu A, Sulem P, Thorlacius S, Gudjonsson SA, Jonsson GF, Jakobsdottir M, Bergthorsson JT, Gudmundsson J, Aben KK, Strobbe LJ, Swinkels DW, van Engelenburg KC, Henderson BE, Kolonel LN, Le Marchand L, Millastre E, Andres R, Saez B, Lambea J, Godino J, Polo E, Tres A, Picelli S, Rantala J, Margolin S, Jonsson T, Sigurdsson H, Jonsdottir T, Hrafnkelsson J, et al: Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2008, 40: 703-706. 10.1038/ng.131.
Article PubMed CAS Google Scholar - Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, Masson G, Jakobsdottir M, Thorlacius S, Helgason A, Aben KK, Strobbe LJ, Albers-Akkers MT, Swinkels DW, Henderson BE, Kolonel LN, Le Marchand L, Millastre E, Andres R, Godino J, Garcia-Prats MD, Polo E, Tres A, Mouy M, Saemundsdottir J, Backman VM, Gudmundsson L, Kristjansson K, Bergthorsson JT, Kostic J, et al: Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2007, 39: 865-869. 10.1038/ng2064.
Article PubMed CAS Google Scholar - Helgadottir A, Thorleifsson G, Magnusson KP, Grétarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jääskeläinen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsäter A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, et al: The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008, 40: 217-224. 10.1038/ng.72.
Article PubMed CAS Google Scholar - Engert JC, Lemire M, Faith J, Brisson D, Fujiwara TM, Roslin NM, Brewer CG, Montpetit A, Darmond-Zwaig C, Renaud Y, Doré C, Bailey SD, Verner A, Tremblay G, St-Pierre J, Bétard C, Platko J, Rioux JD, Morgan K, Hudson TJ, Gaudet D: Identification of a chromosome 8p locus for early-onset coronary heart disease in a French Canadian population. Eur J Hum Genet. 2008, 16: 105-114. 10.1038/sj.ejhg.5201920.
Article PubMed CAS Google Scholar - Raelson JV, Little RD, Ruether A, Fournier H, Paquin B, Van Eerdewegh P, Bradley WE, Croteau P, Nguyen-Huu Q, Segal J, Debrus S, Allard R, Rosenstiel P, Franke A, Jacobs G, Nikolaus S, Vidal JM, Szego P, Laplante N, Clark HF, Paulussen RJ, Hooper JW, Keith TP, Belouchi A, Schreiber S: Genome-wide association study for Crohn's disease in the Quebec Founder Population identifies multiple validated disease loci. Proc Natl Acad Sci USA. 2007, 104: 14747-14752. 10.1073/pnas.0706645104.
Article PubMed CAS PubMed Central Google Scholar - Chen WM, Erdos MR, Jackson AU, Saxena R, Sanna S, Silver KD, Timpson NJ, Hansen T, Orrù M, Grazia Piras M, Bonnycastle LL, Willer CJ, Lyssenko V, Shen H, Kuusisto J, Ebrahim S, Sestu N, Duren WL, Spada MC, Stringham HM, Scott LJ, Olla N, Swift AJ, Najjar S, Mitchell BD, Lawlor DA, Smith GD, Ben-Shlomo Y, Andersen G, Borch-Johnsen K, et al: Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest. 2008, 118: 2620-2628.
PubMed CAS PubMed Central Google Scholar - Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K: Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007, 317: 1397-1400. 10.1126/science.1146554.
Article PubMed CAS Google Scholar - Sanna S, Jackson AU, Nagaraja R, Willer CJ, Chen WM, Bonnycastle LL, Shen H, Timpson N, Lettre G, Usala G, Chines PS, Stringham HM, Scott LJ, Dei M, Lai S, Albai G, Crisponi L, Naitza S, Doheny KF, Pugh EW, Ben-Shlomo Y, Ebrahim S, Lawlor DA, Bergman RN, Watanabe RM, Uda M, Tuomilehto J, Coresh J, Hirschhorn JN, Shuldiner AR, et al: Common variants in the GDF5-UQCC region are associated with variation in human height. Nat Genet. 2008, 40: 198-203. 10.1038/ng.74.
Article PubMed CAS PubMed Central Google Scholar - Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsäter A, Flex A, Aben KK, de Vegt F, et al: A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008, 452: 638-642. 10.1038/nature06846.
Article PubMed CAS PubMed Central Google Scholar - Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, et al: Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007, 39: 977-983. 10.1038/ng2062.
Article PubMed CAS Google Scholar - Palo OM, Antila M, Silander K, Hennah W, Kilpinen H, Soronen P, Tuulio-Henriksson A, Kieseppä T, Partonen T, Lönnqvist J, Peltonen L, Paunio T: Association of distinct allelic haplotypes of DISC1 with psychotic and bipolar spectrum disorders and with underlying cognitive impairments. Hum Mol Genet. 2007, 16: 2517-2528. 10.1093/hmg/ddm207.
Article PubMed CAS Google Scholar - Chavarría-Siles I, Walss-Bass C, Quezada P, Dassori A, Contreras S, Medina R, Ramírez M, Armas R, Salazar R, Leach RJ, Raventos H, Escamilla MA: TGFB-induced factor (TGIF): a candidate gene for psychosis on chromosome 18p. Mol Psychiatry. 2007, 12: 1033-1041. 10.1038/sj.mp.4001997.
Article PubMed Google Scholar - Maniatis N, Collins A, Xu CF, McCarthy LC, Hewett DR, Tapper W, Ennis S, Ke X, Morton NE: The first linkage disequilibrium (LD) maps: delineation of hot and cold blocks by diplotype analysis. Proc Natl Acad Sci USA. 2002, 99: 2228-2233. 10.1073/pnas.042680999.
Article PubMed CAS PubMed Central Google Scholar