Will Multiple Coreceptors Need To Be Targeted by Inhibitors of Human Immunodeficiency Virus Type 1 Entry? (original) (raw)
Abstract
Despite being able to use the Bonzo coreceptor as efficiently as CCR5 in transfected cells, pediatric human immunodeficiency virus type 1 isolate P6 was unable to replicate in peripheral blood mononuclear cells (PBMC) lacking the CCR5 receptor. Furthermore, its replication in wild-type PBMC was completely inhibited by inhibitors of CCR5-mediated entry. Similarly, maternal isolate M6 could use CCR5, CXCR4, Bonzo, and other coreceptors in transfected cells but was completely sensitive to inhibitors of CCR5- and CXCR4-mediated entry when grown in PBMC. The ability of these viruses to use coreceptors in addition to CCR5 and CXCR4 in vitro was, therefore, irrelevant to their drug sensitivity in primary cells. We argue that CCR5 and CXCR4 should remain the primary targets for antiviral drug development, pending strong evidence to the contrary.
The entry of human immunodeficiency virus type 1 (HIV-1) into target cells is now known to involve sequential interactions of the viral envelope glycoproteins with CD4 and a coreceptor (5, 7, 16, 35). The coreceptors are members of the seven-transmembrane-spanning, G-protein-coupled receptor superfamily. The first of these proteins identified as being an HIV-1 coreceptor was the CXC chemokine receptor CXCR4, which mediates entry of syncytium-inducing (SI) or T-cell-line-tropic HIV-1 isolates (23). Subsequently, the CC chemokine receptor CCR5 was shown to be the major coreceptor for non-syncytium-inducing (NSI) or macrophage-tropic viruses (2, 11, 14, 18, 19). A nomenclature for HIV-1 phenotype based on coreceptor usage has been proposed, in which viruses able to use CXCR4 are designated X4, those able to use CCR5 are designated R5, and dual-tropic viruses that can use both receptors are called R5X4 (6).
There is strong genetic evidence that CCR5 is the most important coreceptor for the macrophage-tropic viruses that are commonly transmitted between individuals (13, 27, 31, 44). There is also good circumstantial evidence that CXCR4 is the most relevant coreceptor for the T-cell-line-tropic isolates that emerge in a substantial fraction of individuals after several years of HIV-1 infection (8, 12, 28, 49). These coreceptors are, therefore, of clear and obvious interest as targets for antiviral drug development. However, at least 10 other members of the G-protein-coupled receptor superfamily have been shown to have HIV-1 coreceptor activity to greater or lesser extents, when transfected into barren target cells and tested in viral entry and/or fusion assays in vitro. These include CCR2b (18), CCR3 (1, 4, 11, 25, 39), BOB/GPR15 (15, 21, 22), Bonzo/STRL33/TYMSTR (3, 15, 21, 30, 32), GPR1 (21, 22), CCR8 (26, 41), US28 (38), V28/CX3CR1 (41), APJ (10, 20), and ChemR23 (43). Of these, CCR3 functions most efficiently, with the broadest range of isolates. The question then arises as to whether any among this eclectic gallimaufry of coreceptors is of importance when considering drug development strategies. Will HIV-1 when faced with, e.g., a CCR5-specific inhibitor simply evade the drug by using a different coreceptor in vivo? To a substantial extent, this question can be answered only by clinical trials of coreceptor-targeted inhibitors in humans or animals. However, to gain some insights into the nature of the problem, we have studied an unusual series of pediatric HIV-1 isolates that are able to use the coreceptors CCR5, Bonzo, and in the case of SI variants, CXCR4, CCR8, V28/CX3CR1, and APJ with approximately equivalent efficiencies in vitro (53). Specifically, we have addressed the issue of whether the ability of these viruses to use Bonzo and other coreceptors affects their sensitivity to inhibitors directed against CCR5 and CXCR4 in primary, CD4+ T cells.
Growth of Bonzo coreceptor-using HIV-1 in wild-type and Δ32-CCR5 peripheral blood mononuclear cells (PBMC).
Viruses designated M6 were isolated from an HIV-1-infected mother who has since died of AIDS (9, 53). They are of the SI phenotype and can use CCR5, CXCR4, Bonzo, CCR8, V28/CX3CR1, and APJ when these coreceptors are expressed in transfected GHOST or U87-CD4 cells in vitro (53). The P6 isolates are from the mother’s younger, vertically infected child, are of the NSI phenotype, and use both CCR5 and Bonzo in vitro (53). We have previously shown that Bonzo usage by the M6 and P6 isolates is efficient, to an extent comparable with CCR5 use, which is unusual (21, 53).
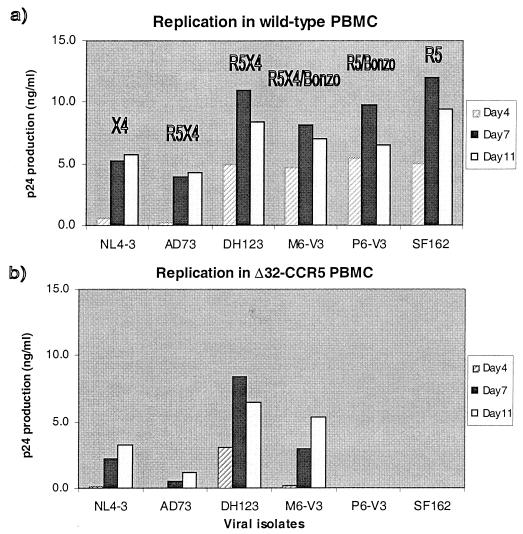
We first addressed whether these isolates were able to replicate in PBMC from a human homozygous for defective CCR5 alleles (Δ32-CCR5), using procedures described previously (51, 53). The maternal (M6) isolate replicated both in the Δ32-CCR5 cells and in PBMC from a wild-type donor (Fig. 1). The same was also true of other X4 and R5X4 viruses, NL4-3, AD73, and DH123, which is consistent with the ability of all these viruses to use CXCR4, a protein expressed normally on Δ32-CCR5 cells (31, 51). In contrast, the infant (P6) isolate, like the control SF162 R5 isolate, was completely unable to replicate in the Δ32-CCR5 cells, although these viruses grew efficiently in wild-type cells (Fig. 1). Similar results were obtained in more-stringent cocultivation assays, in that no replication of P6 and SF162 could be detected in Δ32-CCR5 cells even when they were subsequently cocultivated for 7 days with phytohemagglutinin-activated PBMC from a donor wild type for CCR5 (data not shown). Thus, the ability of the P6 isolate to use Bonzo in transfected cells is irrelevant to its replication in primary PBMC; the absence of CCR5 from the Δ32-CCR5 cells is clearly not overcome by Bonzo usage.
FIG. 1.
Replication of HIV-1 isolates in PBMC from wild-type and Δ32-CCR5 donors. The HIV-1 isolates indicated were tested for their ability to replicate in mitogen-stimulated PBMC from wild-type (a) and Δ32-CCR5 (b) donors. Virus replication was assessed by p24 antigen production on days 4, 7, and 11, as described previously (51, 53). The coreceptors that can be used by each isolate is given in panel a. For M6-V3 and DH123, this is incomplete, and a more-detailed description is provided elsewhere (53). Similar results were obtained with cells from two donors and when other isolates of the P6 and M6 series were tested.
Sensitivity of Bonzo coreceptor-using HIV-1 to CCR5- and CXCR4-directed inhibitors.
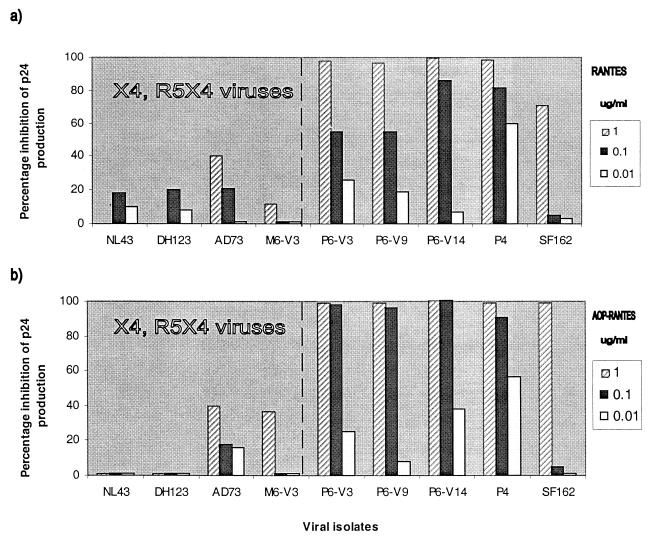
We next assessed the sensitivity of the Bonzo-using P6 and M6 isolates to RANTES and aminoxypentane-RANTES (AOP-RANTES), two inhibitors of HIV-1 entry via CCR5 (14, 19, 33, 48, 51) (Fig. 2). Like other isolates able to use CXCR4, replication of the M6 isolate in CCR5 wild-type donor PBMC was only weakly inhibited by RANTES and AOP-RANTES, since entry via CXCR4 is unhindered (Fig. 2). In contrast, the replication of P6 isolates (R5/Bonzo) was as sensitive as the replication of the P4 and SF162 isolates (R5) to RANTES and AOP-RANTES (Fig. 2). The inhibition was dose dependent and eventually complete for all these isolates, showing that Bonzo usage did not permit HIV-1 to evade CCR5-directed inhibitors in PBMC. Of note is the fact that, although the natural ligands for Bonzo are unknown, there is no evidence that Bonzo is a RANTES receptor; RANTES does not block P6 replication in GHOST-Bonzo cells (data not shown).
FIG. 2.
Sensitivity of HIV-1 isolates to RANTES and AOP-RANTES in wild-type PBMC. The HIV-1 isolates indicated were tested for their ability to replicate in mitogen-stimulated PBMC from a wild-type donor in the presence of the indicated concentrations of RANTES (a) or AOP-RANTES (b). Virus replication was assessed by p24 antigen production on day 7 and related to the amount produced in the absence of inhibitor (defined as 100%). The isolates to the left of the broken line in each panel can use CXCR4; those to the right cannot. Similar results were obtained with cells from two donors and with other isolates of the P6 and M6 series.
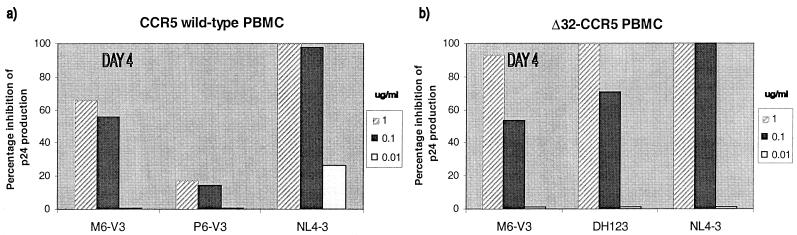
To test the sensitivity of HIV-1 replication to a CXCR4-specific inhibitor, we used the bicyclam AMD3100 (17, 45, 46) (Fig. 3). In PBMC from CCR5 wild-type donors, AMD3100 significantly but only partially inhibited the replication of the dual-tropic M6 isolate, which can still enter the cells via CCR5. The X4 virus NL4-3 was completely inhibited by AMD3100, but the R5/Bonzo virus P6 was insensitive, as expected (Fig. 3a). When PBMC from a Δ32-CCR5 donor were used, M6 and the control DH123 and NL4-3 isolates were all completely (or almost completely) inhibited by AMD3100 (Fig. 3b). In other experiments, the M6 isolate, which can use Bonzo as well as CXCR4, was indistinguishable from DH123, which cannot use Bonzo, in its sensitivity to AMD3100 (data not shown). Thus, the ability to use Bonzo does not allow HIV-1 to evade a CXCR4-specific inhibitor. Furthermore, although both DH123 and M6 can also use several other coreceptors in transfected cells, including CCR8, V28/CX3CR1, and APJ (53), they are still sensitive to a CXCR4-specific inhibitor in Δ32-CCR5 PBMC (Fig. 3b).
FIG. 3.
Sensitivity of HIV-1 isolates to AMD3100 in PBMC from wild-type and Δ32-CCR5 donors. The HIV-1 isolates indicated were tested for their ability to replicate in mitogen-stimulated PBMC from wild-type (a) and Δ32-CCR5 (b) donors in the presence of the indicated concentrations of AMD3100. Virus replication was assessed by p24 antigen production on day 4 and related to the amount produced in the absence of inhibitor (defined as 100%). Inhibition of M6 replication by AMD3100 exceeded 99% when the cultures were retested on day 7.
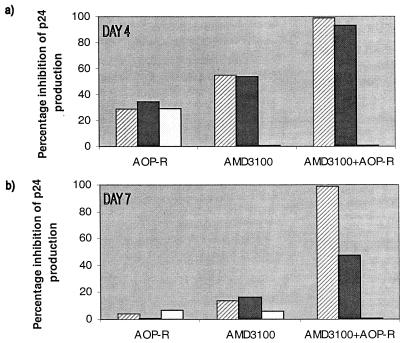
The combination of AOP-RANTES with AMD3100 was used to assess the effects of inhibitors directed at both CCR5 and CXCR4 simultaneously on the infection of healthy donor PBMC by the maternal isolate M6 (Fig. 4). Alone, AOP-RANTES and AMD3100 could only partially inhibit M6 infectivity, but when they were used together, inhibition was complete (Fig. 4). Similar results were obtained with the DH123 isolate (data not shown). Thus, despite the ability of M6 to enter transfected cells via other coreceptors, such as Bonzo, CCR8, V28/CX3CR1, and APJ (53), a combination of a CCR5 inhibitor and a CXCR4 inhibitor was sufficient to block its replication in PBMC from a wild-type CCR5 donor.
FIG. 4.
Complete inhibition of HIV-1 by a combination of AOP-RANTES and AMD3100. Replication of the HIV-1 isolate M6-V3 in PBMC from a wild-type donor was tested in the presence of AOP-RANTES (AOP-R) and AMD3100. These compounds were each tested at 1, 0.1, and 0.01 μg/ml, as indicated by the hatched solid, and white bars, respectively. The same final concentrations of each compound were present in the AOP-RANTES plus AMD3100 combination. Virus replication was assessed by p24 antigen production on days 4 and 7 and related to the amount produced in the absence of inhibitor (defined as 100%).
Discussion.
Although SI, T-cell-line-tropic viruses are often able to use multiple coreceptors for entry into transfected cell lines, the efficient use of coreceptors other than CCR5 by NSI, macrophage-tropic HIV-1 strains is rare (21, 53). CCR3 usage by NSI viruses can be demonstrated in transfected cells (1, 4, 11, 25, 39). However, among the many viruses we have tested, only a single set of NSI isolates (P6 series) from an HIV-1-infected child was able to use a receptor other than CCR5 as efficiently as CCR5 itself could be used; these isolates entered Bonzo- or CCR5-expressing cells with approximately equivalent efficiency (53). SI isolates (M6 series) from the mother of this child used CCR5, CXCR4, Bonzo, and other coreceptors (CCR8, V28/CX3CR1, and APJ). The availability of these Bonzo-using isolates allowed us to address whether coreceptors other than CCR5 and CXCR4 are likely to be of significant importance for antiviral drug development strategies aimed at the HIV-1 coreceptors. For example, had we found that an otherwise effective, CCR5-specific inhibitor such as AOP-RANTES was unable to completely inhibit the replication of the P6 isolates in vitro, it would have implied that Bonzo provided an alternative, unhindered route for HIV-1 entry into PBMC. An inference would then be that the acquisition of Bonzo use might be an evolutionary pathway for viral escape from a CCR5-specific inhibitor. Similar arguments could be made for an SI virus such as M6, under the selection pressure of a combination of CCR5- and CXCR4-specific inhibitors.
We observed, however, that the ability of the P6 and M6 viruses to use Bonzo did not overtly affect their sensitivity to AOP-RANTES and AMD3100, alone or in combination, when these were used as prototypic inhibitors of entry via CCR5 and CXCR4, respectively. We cannot exclude the possibility of subtle influences of the usage of Bonzo (or other coreceptors) on drug sensitivity, but the P6 and M6 viruses were neither unusually sensitive or insensitive to AOP-RANTES or AMD3100 in our experience. These experiments therefore suggest that the ability of the P6 and M6 isolates to use Bonzo in transfected cells is irrelevant to their replication in PBMC. This conclusion is further supported by the observation that P6 viruses were unable to replicate in PBMC from a Δ32-CCR5 homozygous individual. Bonzo (STRL33) is expressed at the mRNA level in PBMC (15, 21, 30) but is not used for entry of the P6 isolate. Furthermore, the replication of the M6 isolate in the Δ32-CCR5 PBMC was completely blocked by the CXCR4-specific inhibitor AMD3100, despite M6 also being able to use Bonzo, CCR8, V28/CX3CR1, and APJ in transfected cells (53). The same reservations about the relevance of Bonzo usage for HIV-1 replication in PBMC may therefore apply also to CCR8, V28/CX3CR1, and APJ.
Viruses isolated from an HIV-1-infected individual who was homozygous for Δ32-CCR5 alleles were found to use only CXCR4 when tested against a variety of coreceptor-expressing cell lines in vitro (34). Of note is that the relative levels of mRNAs for several coreceptors in PBMC have been reported to be as follows: CXCR4, 150; CCR5, 100; CCR2b, 15; and CCR3, 10 (36). In addition, fluorescence-activated cell sorting analysis shows that CCR3 is expressed in PBMC at only about 1% of the level of CCR5 expression (42). All of these observations are consistent with a paramount role of CCR5 and CXCR4 for HIV-1 replication in peripheral blood cells. In principle, any of the coreceptors that have been described as functional for HIV-1 entry in transfected cells could provide an escape route for HIV-1 under drug selection pressure in vivo. However, we could find no reason to believe this is likely to occur. We note that an SDF-1 escape mutant of NL4/3, derived in vitro, still retained CXCR4 usage, in a manner that was less sensitive to the inhibitor (29, 45). Coreceptors other than CCR5 and CXCR4 might be important for HIV-1 replication in nonlymphoid cells, including those found at mucosal surfaces (20, 24, 25, 36, 40, 47, 50), in minor lymphocyte subsets (42, 54), or in specialist lymphoid tissues, such as the thymus (especially in infants) (52). However, it has been shown that 99% of virus production in HIV-1-infected people is produced by CD4+ T lymphocytes (37). Inhibition of HIV-1 replication in these cells is of major clinical benefit, so usage of coreceptors other than CCR5 and CXCR4 should not be the primary concern in the development of antiviral compounds directed at blocking HIV-1 entry into CD4+ T cells.
The priority in the development of coreceptor-targeted antiviral compounds should, we believe, remain squarely on CCR5 and CXCR4 pending strong evidence to the contrary. A major issue will be whether the use of a CCR5-specific inhibitor will drive the evolution of an NSI virus towards CXCR4 usage and the consequent acquisition of the SI phenotype. This will require careful evaluation in vitro and in vivo should suitable drugs be developed. However, it is likely that any coreceptor-targeted inhibitors will be used in combination with existing antiviral drugs such as protease and reverse transcriptase inhibitors. Their suppression of HIV-1 replication will reduce the probability of phenotypic evolution. Nonetheless, the powerful potential of HIV-1 to evade any inhibitors of its replication must always be recognized, so the routes it might take in such evasion need to be carefully defined.
Acknowledgments
We appreciate the contributions of the donors of HIV-1 isolates and clones used in this study, in particular the participants in the Pediatric AIDS Foundation’s ARIEL Project for isolates M6 and P6. We particularly thank Amanda Proudfoot (Serono Research Institute, Geneva, Switzerland) for the gifts of RANTES and AOP-RANTES, Bahige Baroudy (Schering Plough Research Institute, Nutley, N.J.) for AMD3100, and Dan Littman and Vineet KewalRamani for cell lines. We are grateful to Cecilia Cheng-Mayer and Alexandra Trkola for interesting discussions and to Tom Ketas and Ivor Biggun for PBMC preparations.
This study was supported in part by NIH grant AI41420 and by the Pediatric AIDS Foundation, for which J.P.M. is an Elizabeth Glaser Scientist.
REFERENCES
- 1.Alkhatib G, Berger E A, Murphy P M, Pease J E. Determinants of HIV-1 co-receptor function on CC chemokine receptor 3. Importance of both extracellular and transmembrane/cytoplasmic regions. J Biol Chem. 1997;272:20420–20426. doi: 10.1074/jbc.272.33.20420. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Liao F, Berger E A, Farber J M, Peden K C W. A new SIV coreceptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 4.Bazan H A, Alkhatib G, Broder C C, Berger E A. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl. A):S3–S16. [PubMed] [Google Scholar]
- 6.Berger E A, Doms R W, Fenyö E-M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 7.Bieniasz P D, Cullen B R. Chemokine receptors and human immunodeficiency virus infection. Front Biosci. 1998;3:44–58. doi: 10.2741/a265. [DOI] [PubMed] [Google Scholar]
- 8.Björndal Å, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Krogstad P, Korber B T, Koup R A, Muldoon M, Macken C, Song J L, Jin Z, Zhao J-Q, Clapp S, Chen I S Y, Ho D D, Ammann A J the Ariel Project Investigators. Maternal HIV-1 viral load and vertical transmission of infection: the Ariel Project for the prevention of HIV transmission from mother to infant. Nat Med. 1997;3:549–552. doi: 10.1038/nm0597-549. [DOI] [PubMed] [Google Scholar]
- 10.Choe H, Farzan M, Konkel M, Martin K, Sun Y, Marcon L, Cayabyab M, Berman M, Dorf M E, Gerard N, Gerard G, Sodroski J. The orphan seven-transmembrane receptor Apj supports the entry of primary T-cell-line-tropic and dualtropic human immunodeficiency virus type 1. J Virol. 1998;72:6113–6118. doi: 10.1128/jvi.72.7.6113-6118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard G, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 12.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien S J Hemophilia Growth and Development Study; Multicenter AIDS Cohort Study; Multicenter Hemophilia Cohort Study; San Francisco City Cohort; ALIVE Study. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion of the CKR5 structural allele. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 14.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major coreceptor 5 for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 15.Deng H K, Unutmaz D, Kewalramani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 16.Doms R W, Peiper S C. Unwelcome guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 17.Donzella G A, Schols D, Lin S W, Esté J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, Moore J P. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 18.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S, Parmentier M, Collman R G, Doms R W. A dual-tropic, primary HIV-1 isolate that uses fusion and the β-chemokine receptors CKR-5, CKR-2b as fusion cofactors. Cell. 1996;85:1149–1159. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 19.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 20.Edinger A L, Hoffman T L, Sharron M, Lee B, Yi Y, Choe W, Kolson D C, Mitrovic B, Zhou Y, Faulds D, Collman R G, Hesselgesser J, Horuk R, Doms R W. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998;72:7934–7940. doi: 10.1128/jvi.72.10.7934-7940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edinger A L, Hoffman T L, Sharron M, Lee B, O’Dowd B, Doms R W. Use of GPR1, GPR15, and STRL33 as co-receptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology. 1998;249:367–378. doi: 10.1006/viro.1998.9306. [DOI] [PubMed] [Google Scholar]
- 22.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane G protein coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 24.Ghorpade A, Xia M Q, Hyman B T, Persidsky Y, Nukuna A, Bock P, Che M, Limoges J, Gendelman H E, Mackay C R. Role of the β-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J Virol. 1998;72:3351–3361. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 26.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, Doranz B J, Doms R W. The CC chemokine I-309 inhibits CCR8 dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbaksh K, Kuntsman K, Erickson D, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 28.Jansson M, Popovic M, Karlsson A, Cocchi F, Rossi P, Albert J, Wigzell H. Sensitivity to inhibition by β-chemokines correlates with biological phenotypes of primary HIV-1 isolates. Proc Natl Acad Sci USA. 1996;93:15382–15387. doi: 10.1073/pnas.93.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labrosse B, Brelot A, Heveker N, Sol N, Schols D, DeClerq E, Alizon M. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J Virol. 1998;72:6381–6388. doi: 10.1128/jvi.72.8.6381-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–378. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 32.Loetscher M, Amara A, Oberlin E, Brass N, Legler D F, Loetscher P, D’Apuzzo M, Meese E, Rousset D, Virelizier J-L, Baggiolini M, Arenzana-Seisdedos F, Moser B. TYMSTR, a putative chemokine receptor selectively expressed in activated T cells, exhibits HIV-1 coreceptor function. Curr Biol. 1997;7:652–660. doi: 10.1016/s0960-9822(06)00292-2. [DOI] [PubMed] [Google Scholar]
- 33.Mack M, Luckow B, Nelson P J, Lihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells T N C, Schlöndorff D, Proudfoot A E I. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michael N L, Nelson J A E, KewalRamani V N, Chang G, O’Brien S J, Mascola J R, Volsky B, Louder M, White II G C, Littman D R, Swanstrom R, O’Brien T R. Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 Δ32. J Virol. 1998;72:6040–6047. doi: 10.1128/jvi.72.7.6040-6047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 36.Patterson B K, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski M A, Barcia P. Repertoire of chemokine receptor expression in the female genital tract. Am J Pathol. 1998;153:481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perelson A, Neumann A U, Markowitz M, Leonard J, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 38.Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 39.Ross T M, Cullen B R. The ability of HIV type 1 to use CCR-3 as a coreceptor is controlled by envelope V1/V2 sequences acting in conjunction with a CCR-5 tropic V3 loop. Proc Natl Acad Sci USA. 1998;95:7682–7686. doi: 10.1073/pnas.95.13.7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubbert A, Combadiere C, Ostrowski M, Arthos J, Dybul M, Machado E, Cohn M A, Hoxie J A, Murphy P M, Fauci A S, Weissman D. Dendritic cells express multiple chemokine receptors used as coreceptors for HIV entry. J Immunol. 1998;160:3933–3941. [PubMed] [Google Scholar]
- 41.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sallusto F, Mackay C R, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 43.Samson M, Edinger A L, Stordeur P, Rucker J, Verhasselt V, Sharron M, Govaerts C, Mollereau C, Vassart G, Doms R W, Parmentier M. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur J Immunol. 1998;28:1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 44.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C-M, Saragosti S, Lapoumèroulie C, Cogniaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection of Caucasian individuals bearing mutant alleles of the CCR5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 45.Schols D, Este J A, Cabrera C, DeClerq E. T-cell-line-tropic human immunodeficiency virus type 1 that is made resistant to stromal cell-derived factor 1α contains mutations in the envelope gp120 but does not show a switch in coreceptor use. J Virol. 1998;72:4032–4037. doi: 10.1128/jvi.72.5.4032-4037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schols D, Struyf S, Van Damme J, Esté J A, Henson G, De Clerq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shieh J T C, Albright A V, Sharron M, Gartner S, Strizki J, Doms R W, Gonzalez-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simmons G, Clapham P R, Picard C, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E I. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 49.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spira A I, Marx P A, Patterson B K, Mahone J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination following intravaginal inoculation of SIV into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trkola A, Paxton W A, Monard S P, Hoxie J A, Siani M A, Thompson D A, Wu L, Mackay C R, Horuk R, Moore J P. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaitseva M B, Lee S, Rabin R L, Tiffany H L, Farber J M, Peden K W C, Murphy P M, Golding H. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]
- 53.Zhang, Y.-J., T. Dragic, Y. Cao, L. Kostrikis, D. S. Kwon, D. R. Littman, V. N. KewalRamani, and J. P. Moore. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J. Virol. **72:**9337–9344. [DOI] [PMC free article] [PubMed]
- 54.Zingoni A, Soto H, Hedrick J A, Stoppacciaro F, Storlazzi C T, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M, Santoni A, Zlotnick A, Napolitano M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]