Insulin resistance and cancer: Epidemiological evidence (original) (raw)
Abstract
Over the last 60 years, Japanese people have experienced a rapid and drastic change in lifestyle, including diet. Suspicions have been raised that so‐called ‘Westernization’, characterized by a high‐calorie diet and physical inactivity, is associated with increasing trends in the incidence of cancer of the colon, liver, pancreas, prostate, and breast, as well as type 2 diabetes. Epidemiological evidence from our prospective study, the Japan Public Health Center‐based Prospective (JPHC) study, and systematic literature reviews generally support the idea that factors related to diabetes or insulin resistance are associated with an increased risk of colon (mostly in men), liver, and pancreatic cancers. These cancers are inversely associated with physical activity and coffee consumption, which are known to decrease the risk of type 2 diabetes. The suggested mechanism of these effects is that insulin resistance and the resulting chronic hyperinsulinemia and increase in bioavailable insulin‐like growth factor 1 (IGF1) stimulate tumor growth. In contrast, associations with diabetes are less clear for cancer of the colon in women, and breast and prostate, which are known to be related to sex hormones. The effect of insulin resistance or body fat on sex‐hormone production and bioavailability may modify their carcinogenic effect differently from cancers of the colon in men, and liver and pancreas. In conclusion, there is substantial evidence to show that cancers of the colon, liver, and pancreas are associated with insulin resistance, and that these cancers can be prevented by increasing physical activity, and possibly coffee consumption.
(Cancer Sci 2010; 101: 1073–1079)
Japanese people have experienced a rapid and drastic change in lifestyle, including diet. The industrialization and economic growth occurring following World War II has been accompanied by the so‐called ‘Westernization’ of lifestyle, characterized by a high‐calorie diet and physical inactivity. Paralleling this change, the mortality of infectious diseases has decreased while that of lifestyle‐related diseases such as cancer and heart disease has increased. Cancer has been the leading cause of death since 1981, and now accounts for 30% of all deaths. Among various cancer sites, cancers of the colon, pancreas, breast, and prostate, which are known to be more common in Western countries, have increased in Japan. Most but not all of these cancers have been shown to be associated with greater body (or abdominal) fatness and physical inactivity.
Here, we review the associations observed in our prospective study, the Japan Public Health Center‐based Prospective (JPHC) study, between body fatness, diabetes, physical activity, coffee consumption (which may affect diabetes), and the risk of cancer, as well as the epidemiological literature on this topic. Evidence is discussed with a special focus on the role of insulin resistance.
In brief, the JPHC study conducted a baseline survey of registered residents aged 40–69 years in 11 public health center areas nationwide in 1990–1994. Approximately 110 000 subjects returned the questionnaire, giving a response rate of 81.0%, and 50 000 provided blood and health check‐up data.( 1 ) The subjects have been followed for vital status and the occurrence of cancer and other diseases, and 5‐ and 10‐year follow‐up surveys have been conducted to update information on lifestyle and health condition.
Time trend analysis
According to the annual National Nutrition Survey by the Ministry of Health, Labour and Welfare (MHLW) (Fig. 1), and allowing for the lack of age‐adjustment, total energy intake (per capita per day) increased from 1903 kcal in 1946 to peak at 2287 kcal in 1971. It then followed a downward trend, decreasing to 1891 kcal in 2006. The rapid increase in fat intake (14.7 g in 1946 to 48.7 g in 1971) owed mainly to the increase in total energy during the period of post‐war reconstruction and high economic growth. The constant decrease in carbohydrate intake and leveling off of fat intake resulted in a decreasing trend in total energy intake after the mid‐1970s, probably when physical activity decreased (due to the spread of electrification and automobile use in daily life and at the workplace) and the westernization of Japanese dietary habits plateaued.
Figure 1.
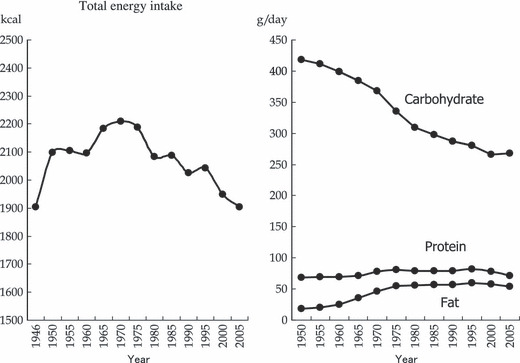
Time trends in nutrient intake (per capita per day) in Japan. Data source: The National Nutrition Survey by the Ministry of Health, Labour and Welfare of Japan.
In Japan, age‐adjusted mortality rates of colon, liver, and pancreatic cancers steadily increased up to the mid‐1990s, and then flattened out or decreased. The exception to this trend was liver cancer, which switched from a decrease to an increase around the 1970s, probably due to endemic hepatitis virus infection (Fig. 2). Cancer incidence rates for Japanese people are only available for the years after 1975 and the quality of incidence data is not considered high enough to allow comparison of long‐term trends.( 2 ) However, allowing for this insufficient quality, incidence rates of these cancers have fluctuated in parallel with mortality rates. For cancer of the liver and pancreas in particular, mortality rate can be deemed as a substitute for incidence rate due to their poor prognoses.
Figure 2.
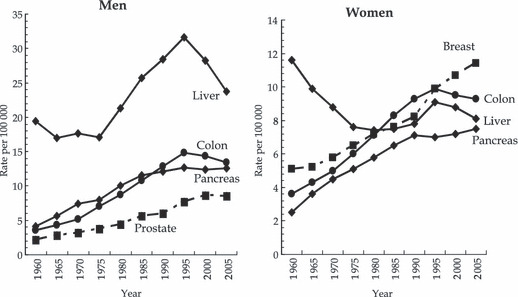
Trends in mortality rates of selected cancers in Japan (rate adjusted by Japanese model population in 1985). Data source: Vital Statistics Reports by the Ministry of Health, Labour and Welfare of Japan.
These trends in dietary and cancer mortality pattern appear to be parallel with a lag time of approximately 20 years. Cancers of the colon, liver, and pancreas might therefore be associated with energy imbalance, with incubation periods of 20 years. Cancers of the breast and prostate have shown different trends, however, increasing even after the mid‐1990s. The etiology of these cancers may differ somewhat from those of the colon, liver, and pancreas.
This parallel trend in suspected energy imbalance and cancers of the colon, liver, and pancreas may indirectly support a close link with insulin resistance, as characterized by energy imbalance.
Body fatness, abdominal fatness, and cancer
In the JPHC study, overweight and obesity as measured by body mass index (BMI) were associated with specific sites of cancer such as male colorectum,( 3 ) postmenopausal breast,( 4 ) and liver among subjects with hepatitis C virus (HCV) infection.( 5 ) By contrast, they had little impact on cancer of the pancreas( 6 ) or cancer at all sites.( 7 )
The World Cancer Research Fund and American Institute for Cancer Research have evaluated causal relationships between several factors and each cancer based on systematic reviews of epidemiological evidence as well as mechanistic interpretations and data from animal experimental models.( 8 ) Greater body fatness convincingly increased the risk of cancers of the esophagus (adenocarcinoma only), colorectum, pancreas, postmenopausal breast, endometrium, kidney, probably the gallbladder, and possibly the liver. In addition, greater abdominal fatness as measured by waist circumference or waist to hip ratio convincingly increased the risk of colorectal cancer and probably cancers of the pancreas, postmenopausal breast, and endometrium.
Based on a meta‐analysis of 221 datasets (141 articles) reporting 282 000 incidence cases,( 9 ) the magnitude of risk was greater for esophageal adenocarcinoma (relative risk [RR] = 1.52, P < 0.0001, with a 5 kg/m2 increase in BMI), thyroid (RR = 1.33, P < 0.0001), colon (RR = 1.24, P < 0.0001), renal (RR = 1.24, P < 0.0001), and liver (RR = 1.24, P = 0.12) cancers in men, and endometrial (RR = 1.59, P < 0.0001), gallbladder (RR = 1.59, P = 0.04), esophageal adenocarcinoma (RR = 1.51, P < 0.0001), and renal (1.34, P < 0.0001) cancers in women. The relative risk was 1.09 (P < 0.0001) for female colon cancer, with this gender difference being highly statistically significant (P < 0.0001).
The proportions of all cancer deaths in the US population attributable to overweight and obesity (BMI >25 kg/m2) have been estimated at 4.2–14.2% in men and 14.3–19.8% in women.( 10 ) Incident cancer burden attributable to excess body mass index across 30 European countries has been estimated at 2.5% (95% confidence interval [CI], 1.5–3.6%) in men and 4.1% (95% CI, 2.3–5.9%) in women.( 11 ) In contrast, respective figures in the JPHC study have been negligible, since no statistically significant increase in risk for cancer at any site has been observed in overweight and obesity categories in either men or women.( 7 )
Possible mechanisms of the effect of body fatness on cancer risk are explained by increased bioavailable growth‐factor production (e.g. insulin, insulin‐like growth factor [IGF] 1) due to insulin resistance, sex‐hormone production (e.g. estrogen, testosterone), chronic local inflammation characterized by the creation of reactive oxygen species and the induction of cell cycling for tissue growth and repair, and chronic gastroesophageal reflux (for adenocarcinoma of the esophagus).
Diabetes and cancer: Epidemiological evidence
In the JPHC study, a history of diabetes was associated with an increased risk of several cancer sites after adjustment for potential confounding factors such as age, study area, history of cerebrovascular disease, history of ischemic heart disease, smoking, ethanol intake, body mass index, leisure‐time physical activity, green vegetable intake, and coffee intake (Fig. 3).( 12 ) In men, statistically significant increases were observed for cancers of the colon, liver, pancreas, and kidney. Hazard ratios (HRs) were almost double for cancers of the liver, pancreas, and kidney, and remained so after the exclusion of cases occurring within 5 years of follow‐up. In women, HRs were statistically significant for cancers of the stomach and liver.
Figure 3.
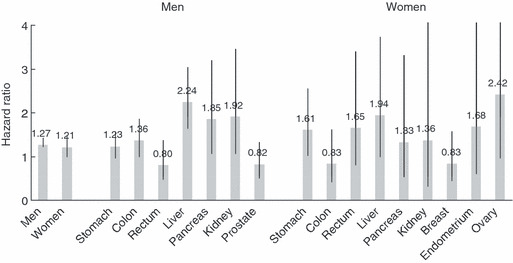
History of diabetes and risk of cancer: the Japan Public Health Center‐based Prospective (JPHC) study. Adjusted for age, study area, history of cerebrovascular disease, history of ischemic heart disease, smoking, ethanol intake, body mass index, leisure‐time physical activity, green vegetable intake, and coffee intake. Data source: Inoue et al. ( 12 )
Although not statistically significant due to the small number of cases, elevated HRs were also shown for cancers of the pancreas, endometrium, ovary, and kidney. In contrast, no association was seen with colon and breast in women. These increases in risk in several cancers resulted in a 27% increase in total cancer in men and a 21% increase in women.
In Japan, only a few cohort studies have evaluated the association between diabetes and total cancer risk. A report from the Takayama study on the history of diabetes and mortality risk also showed an increased risk of cancer mortality with a history of diabetes (HR = 1.88; 95% CI, 1.16–3.05) among women.( 13 ) However, the Japan Collaborative Cohort (JACC) study on the association between a history of diabetes and cancer mortality showed no significant impact of a history on total cancer risk in either men (HR = 0.98) or women (HR = 0.83), although an increased risk was observed for liver cancer in both sexes (HR = 2.30; 95% CI, 1.47–3.59 in men and HR = 2.70 95% CI, 1.20–6.05 in women) and non‐Hodgkin lymphoma in men (HR = 2.77; 95% CI, 1.04–7.38), while a decreased risk was seen for stomach cancer in both sexes (HR = 0.67; 95% CI, 0.46–0.99 in men and HR = 0.49; 95% CI, 0.23–1.04 in women).( 14 )
Findings similar to those of the JPHC study were seen in a 10‐year prospective cohort study of 1.3 million Koreans aged 30 to 95 years.( 15 ) Elevated fasting serum glucose levels (≥140 mg/dL) were associated with higher death rates for all cancers combined (HR = 1.29; 95% CI, 1.22–1.37 in men and HR = 1.23; 95% CI, 1.09–1.39 in women) compared with subjects with the lowest level (<90 mg/dL). By cancer site, the association was strongest for pancreatic cancer (HR = 1.91; 95% CI, 1.52–2.41 in men and HR = 2.05; 95% CI, 1.43–2.93 in women). Significant associations were also found for cancers of the esophagus (1.44), liver (1.57), and colorectum (1.31) in men and liver (1.33) in women. In contrast, the HR of colorectal cancer mortality was 0.85 in women.
Meta‐analyses, derived mainly from Western populations, support the idea that diabetes (e.g. self‐report, or elevated blood glucose level in fasting or after glucose load) was associated with an increased risk of cancers of the liver (summary HR = 2.5; 95% CI, 1.8–2.9 among 13 case‐control and 12 cohort studies),( 16 ) pancreas (HR = 1.82; 95% CI, 1.66–1.99 among 17 case‐control and 19 cohort studies),( 17 ) colorectum (HR = 1.30; 95% CI, 1.20–1.40 among six case‐control and nine cohort studies),( 18 ) breast (HR = 1.20; 95% CI, 1.12–1.29 among five case‐control and 15 cohort studies),( 19 ) and endometrium (HR = 2.10; 95% CI, 1.75–2.53 among 13 case‐control and three cohort studies).( 20 ) In contrast, diabetes was inversely associated with the risk of prostate cancer (HR = 0.84; 95% CI, 0.76–0.93 among seven case‐control and 12 cohort studies).( 21 )
Possible mechanisms relating diabetes to cancer risk
The findings of a nested case‐control study within the JPHC study showed that plasma levels of C‐peptide, a marker of insulin resistance, were strongly associated with the risk of colorectal cancer in men, specifically colon cancer, but not in women (Fig. 4).( 22 ) Similar findings were observed in two nested case‐control studies within cohort studies in the USA: C‐peptide was strongly associated with the risk of colorectal cancer in the Physicians’ Health Study (men),( 23 ) but not in the Nurses’ Health Study (women).( 24 )
Figure 4.
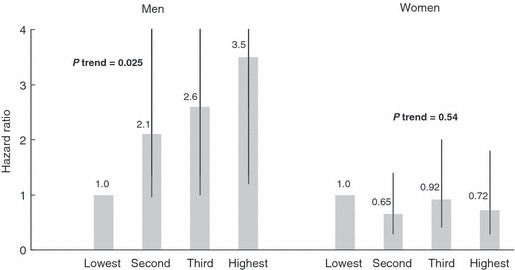
Plasma C‐peptide and risk of colon cancer: a nested case control study within the Japan Public Health Center‐based Prospective (JPHC) Study. Matched by sex, age, date of blood drawn, time since last meal, and study area. Adjusted for smoking, ethanol intake, body mass index, leisure‐time physical activity, and family history of colorectal cancer. Data source: Otani et al. ( 22 )
These data support the ideas that insulin resistance and the chronic hyperinsulinemia which results may increase the risk of colon cancer, and that this carcinogenic effect may be modified by hormone production and bioavailability in women. The mechanisms of insulin resistance and cancer risk were first proposed with regard to the pathogenesis of colon cancer, and subsequently expanded to include those of breast, pancreas, and endometrium. They have been reviewed in detail.( 25 )
In brief, an increase in insulin levels leads to a decrease in the liver synthesis and blood levels of insulin‐like growth factor binding protein 1 (IGFBP1), and is associated with a decrease in IGFBP2 in the blood. These effects in turn result in an increase in bioavailable IGF1. Insulin and IGF1 signal through insulin receptors and IGF1 receptors, respectively, to promote cellular proliferation and inhibit apoptosis in many tissue types. These effects might contribute to tumorigenesis. Insulin resistance also causes a decrease in circulating levels of sex‐hormone‐binding globulin (SHBG) and an increase in the bioavailability of estradiol and testosterone. In parallel, adipose tissue produces the enzymes aromatase and 17β‐hydroxysteroid dehydrogenase and increases estradiol production. Estradiol production is accordingly higher in obese individuals, who are characterized by insulin resistance. The combined effect of increased bioavailability and formation of estradiol and testosterone can impact target cells where these hormones bind to estrogen and androgen receptors. The effect of sex steroid binding with receptors can vary, depending on tissue type, but in some tissues (e.g. breast epithelium and endometrium), binding promotes cellular proliferation and inhibits apoptosis.
However, the magnitude of associations between diabetes and typical sex hormone‐related cancers such as breast and prostate are less clear (summary HR by meta‐analysis = 1.20 for breast( 19 ) and 0.84 for prostate( 21 )) than those for other diabetes‐related cancers (summary HR around 2.0 for liver,( 16 ) pancreas,( 17 ) and endometrium( 20 )). The pathway of insulin resistance to carcinogenesis in sex hormone‐related cancers therefore appears complicated. Although endometrial cancer is known to be hormone‐related, the effects of combined estrogen‐progestagen oral contraceptives and tamoxifen, a selective estrogen‐receptor modulator, act in opposing directions in the endometrium and breast( 26 ) and are more closely associated with body fatness. For colon cancer, the association with diabetes and body fatness is less clear in women, because female sex hormones may modify the effect of insulin resistance in colon epithelium. A randomized controlled trial showed that the use of estrogen plus progestin was associated with a decreased risk of colorectal cancer in women.( 27 )
Physical activity and cancer
Physical activity is well known to reduce the risk of type 2 diabetes,( 28 ) as well as colon and female hormone‐related cancers, such as postmenopausal breast and endometrium, independently of other factors such as body fatness. Due to the small number of studies, however, evidence suggesting that physical activity protects against cancers of the liver and pancreas is limited.
In the JPHC study, physical activity (calculated as metabolic equivalents (METs) score) showed a statistically significant inverse trend for colon and pancreatic cancers in men and for stomach cancer in women (Fig. 5).( 29 ) A similar tendency was seen for liver cancer in both men and women. Among other sites, a statistically significant decrease was seen for stomach cancer in women, whereas no decreasing trend was seen for breast cancer. The decreases in these cancers led to a statistically significant inverse trend for cancer as a whole in both men and women.
Figure 5.
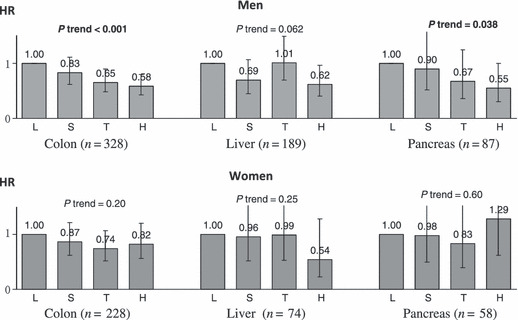
Physical activity and cancers of the colon, liver, and pancreas: the Japan Public Health Center‐based Prospective (JPHC) study. HR, hazard ratio. Data source: Inoue et al. ( 29 )
Physical activity may reduce the risk of colon, liver, and pancreatic cancers, which are diabetes‐related, by inhibiting insulin resistance and hyperinsulinemia.
Coffee consumption and cancer
Many recent reports have provided evidence of an inverse association between coffee consumption and diabetes( 30 ) and several sites of cancer.
In the JPHC study, coffee consumption was inversely associated with diabetes( 31 ) and several cancer sites such as liver,( 32 ) colon (women),( 33 ) pancreas (men) (Fig. 6),( 34 ) and endometrium.( 35 ) For liver cancer, a similar risk tendency was observed in those with either or both HCV and hepatitis B virus (HBV) infection.( 36 )
Figure 6.
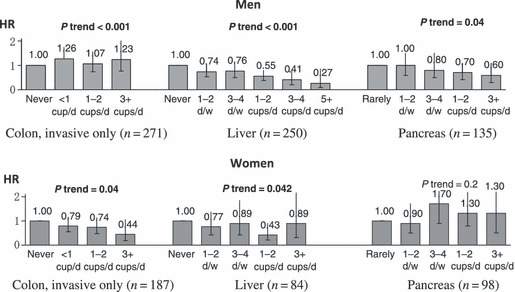
Coffee consumption and cancers of the colon, liver, and pancreas: the Japan Public Health Center‐based Prospective (JPHC) study. HR, hazard ratio. Data sources: colon;( 33 ) liver;( 32 ) pancreas.( 34 )
A meta‐analysis has supported the association of coffee consumption with a decreased risk of cancers of the liver( 37 ) (summary HR = 0.45; 95% CI, 0.38–0.53 for the highest consumption category and HR = 0.70; 95% CI, 0.57–0.85 for moderate consumption against lowest consumption among seven case‐control and five cohort studies) and endometrium( 38 ) (HR = 0.64; 95% CI, 0.48–0.86 for the highest consumption category and HR = 0.87; 95% CI, 0.78–0.97 for moderate consumption against lowest consumption among six case‐control and two cohort studies). The association was less clear for colon cancer,( 39 ) particularly in men (HR = 1.00; 95% CI, 0.81–1.24 in men and HR = 0.79; 95% CI, 0.60–1.04 in women among six studies).
Biologically, the beneficial effects of coffee on carcinogenesis are thought to derive from three major components of coffee: chlorogenic acids, caffeine, and the coffee diterpenes cafestol and kahweol. Because diterpene content is negligible in instant, drip‐filtered, and percolated brews, the preparation methods most commonly used in Japan, the main putative candidates in our population are chlorogenic acids and caffeine.
Chlorogenic acid has relatively strong antioxidant effects and improves insulin resistance by increasing insulin sensitivity or inhibiting glucose absorption in the intestine. The latter effect may explain the observation that higher coffee consumption was associated with lower postload, rather than fasting, glucose concentrations.( 40 , 41 )
Several short‐term studies have shown that acute caffeine ingestion can decrease glucose disposal.( 42 , 43 , 44 ) In US studies, however, decaffeinated coffee consumption was inversely associated with the risk of type 2 diabetes, and our cross‐sectional study showed that the inverse association with hyperglycemia was stronger for coffee than for caffeine.( 45 ) These observations suggest that coffee components other than caffeine may have beneficial effects on the risk of type 2 diabetes. Coffee also contains substantial amounts of magnesium, which has been linked to better insulin sensitivity and insulin secretion.
Based on substantial evidence and biological plausibility, coffee consumption is considered to have a favorable effect on insulin resistance, and may thereby reduce the risk of diabetes‐related cancers such as colon, liver, pancreas, and endometrium.
Metabolic factors, their aggregate, and cancer
The MHLW has repeatedly warned about a rapid increase in type 2 diabetes, which is also caused by energy imbalance. The 2007 National Health and Nutrition survey estimated that a total of 22 million people possibly had diabetes, nearly twofold the 14 million estimated in 1997. In 2008, the MHLW implemented a policy against metabolic syndrome, which is defined by the presence of increased waist circumference (necessary factor) and at least two of the following metabolic factors: lipid abnormality (elevated triglyceride levels or low high density lipoprotein (HDL) cholesterol), elevated blood pressure, and elevated fasting glucose level. It is expected that the control of abdominal fatness as indicated by waist circumference would result in a decrease in risk factors, and thereby prevent future ischemic cardiovascular diseases (CVD) such as ischemic heart disease as well as ischemic stroke.
This now‐implemented policy against metabolic syndrome may also influences cancer control. Estimating its impact on cancer requires the elucidation of how the respective metabolic factors, both individually and in the aggregate, namely metabolic syndrome are related to not only CVD but also cancer, the leading cause of death in Japan.
Using data from approximately 30 000 subjects who provided health check‐up data at baseline or in a 5‐year follow‐up survey, we tested the association between these metabolic factors, both individually and in the aggregate, and the risk of cancer.( 46 ) Each metabolic factor increased the risk of specific sites of cancer with statistical significance, albeit that statistical power was not strong enough due to the relatively small number of subjects and cancer cases. For liver cancer, high glucose (HR = 1.76; 95% CI, 1.07–2.89), low HDL (HR = 2.25; 95% CI, 1.34–3.79) and overweight (HR = 2.18; 95% CI, 1.33–3.58) were associated with an increased risk in men, and overweight was also associated in women (HR = 1.95; 95% CI, 1.03–3.69). In addition, high triglyceride (HR = 1.71; 95% CI, 1.11–2.62) was associated with the risk of colon cancer in men and overweight (HR = 1.75; 95% CI, 1.21–2.55) with the risk of breast cancer. However, the presence of metabolic factors in the aggregate (two or more factors in addition to overweight) was shown to be a statistically significant risk only for male liver (HR = 1.99; 95% CI, 1.11–3.58) and female pancreatic cancer (HR = 1.99; 95% CI, 1.00–3.96). The observed HR was not as high as those observed for each factor individually, nor did the individual factors or their aggregate predict the subsequent occurrence of cancer as a whole.
These studies on the association between metabolic syndrome and cancer have focused mainly on specific sites of cancer, including colon, prostate, breast, and endometrium, which are common in Western populations and are considered to have an etiologic link with the respective metabolic factors or their aggregate. Among them, two large prospective studies, in Italy( 47 ) and the USA, showed that the clustering of metabolic factors increased the risk of colorectal cancer mortality compared with the individual factors alone among men.( 48 )
The only study focusing on the impact of metabolic factors in the aggregate on total cancer risk to date, in Italy, reported a null association for total cancer incidence,( 49 ) which accords with our result. Although the extent to which the grand sum of the effect on these sites affects total cancer incidence has yet to be clarified, it appears that metabolic factors in the aggregate have little impact on the risk of cancer as a whole in Japan. The association between specific components of metabolic syndrome and specific cancers suggests the presence of an etiologic link.
Conclusion
Descriptive (time‐trend) and analytic (cohort and case‐control studies) evidence obtained to date suggest that cancers of the colon, liver, and pancreas may be associated with insulin resistance or energy imbalance. These cancers may be preventable by increasing physical activity, and possibly also coffee consumption, as well as by maintaining energy balance. Insulin resistance may be a stronger predictor of these cancers than body fatness itself. Metabolic factors in the aggregate appear to have little impact on the risk of cancer as a whole in Japan.
Acknowledgments
We sincerely thank the members and coworkers of the Japan Public Health Center‐Based Prospective Study Group. This work was supported by Grants‐in‐Aid for Cancer Research and for the Third‐Term Comprehensive Ten‐Year Strategy for Cancer Control from the Ministry of Health, Labour, and Welfare of Japan.
References
- 1.Tsugane S, Sobue T. Baseline survey of JPHC study – design and participation rate. Japan Public Health Center‐based prospective study on cancer and cardiovascular diseases. J Epidemiol 2001; 6 (Suppl): S24–9. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T; Japan Cancer Surveillance Research Group . Cancer incidence and incidence rates in Japan in 2003: based on data from 13 population‐based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) Project. Jpn J Clin Oncol 2009; 39: 850–8. [DOI] [PubMed] [Google Scholar]
- 3.Otani T, Iwasaki M, Inoue M, Tsugane S. Body mass index, body height, and subsequent risk of colorectal cancer in middle‐aged and elderly Japanese men and women: Japan public health center‐based prospective study. Cancer Causes Control 2005; 16: 839–50. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki M, Otani T, Inoue M, Sasazuki S, Tsugane S. Body size and risk for breast cancer in relation to estrogen and progesterone receptor status in Japan. Ann Epidemiol 2007; 17: 304–12. [DOI] [PubMed] [Google Scholar]
- 5.Inoue M, Kurahashi N, Iwasaki M_et al._Japan Public Health Center‐based Prospective Study Group . Metabolic factors and subsequent risk of hepatocellular carcinoma by hepatitis virus infection status: a large‐scale population‐based cohort study of Japanese men and women (JPHC Study Cohort II). Cancer Causes Control 2009; 20: 741–50. [DOI] [PubMed] [Google Scholar]
- 6.Luo J, Iwasaki M, Inoue M_et al._Body mass index, physical activity and the risk of pancreatic cancer in relation to smoking status and history of diabetes: a large‐scale population‐based cohort study in Japan – the JPHC study. Cancer Causes Control 2007; 18: 603–12. [DOI] [PubMed] [Google Scholar]
- 7.Inoue M, Sobue T, Tsugane S. Impact of body mass index on the risk of total cancer incidence and mortality among middle‐aged Japanese: data from a large‐scale population‐based cohort study – the JPHC study. Cancer Causes Control 2004; 15: 671–80. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR, 2007. [Google Scholar]
- 9.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet 2008; 371: 569–78. [DOI] [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348: 1625–38. [DOI] [PubMed] [Google Scholar]
- 11.Renehan AG, Soerjomataram I, Tyson M_et al._Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer 2009; 126: 692–702. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large‐scale population‐based cohort study in Japan. Arch Intern Med 2006; 166: 1871–7. [DOI] [PubMed] [Google Scholar]
- 13.Oba S, Nagata C, Nakamura K, Takatsuka N, Shimizu H. Self‐reported diabetes mellitus and risk of mortality from all causes, cardiovascular disease, and cancer in Takayama: a population‐based prospective cohort study in Japan. J Epidemiol 2008; 18: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M, Mori M, Fujino Y_et al._Japan Collaborative Cohort Study Group . Site‐specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pac J Cancer Prev 2006; 7: 253–9. [PubMed] [Google Scholar]
- 15.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005; 293: 194–202. [DOI] [PubMed] [Google Scholar]
- 16.El‐Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4: 369–80. [DOI] [PubMed] [Google Scholar]
- 17.Huxley R, Ansary‐Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type‐II diabetes and pancreatic cancer: a meta‐analysis of 36 studies. Br J Cancer 2005; 92: 2076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta‐analysis. J Natl Cancer Inst 2005; 97: 1679–87. [DOI] [PubMed] [Google Scholar]
- 19.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta‐analysis. Int J Cancer 2007; 121: 856–62. [DOI] [PubMed] [Google Scholar]
- 20.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta‐analysis. Diabetologia 2007; 50: 1365–74. [DOI] [PubMed] [Google Scholar]
- 21.Kasper JS, Giovannucci E. A meta‐analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006; 15: 2056–62. [DOI] [PubMed] [Google Scholar]
- 22.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma C‐peptide, insulin‐like growth factor‐I, insulin‐like growth factor binding proteins and risk of colorectal cancer in a nested case‐control study: the Japan public health center‐based prospective study. Int J Cancer 2007; 120: 2007–12. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Giovannucci E, Pollak M_et al._A prospective study of plasma C‐peptide and colorectal cancer risk in men. J Natl Cancer Inst 2004; 96: 546–53. [DOI] [PubMed] [Google Scholar]
- 24.Wei EK, Ma J, Pollak MN_et al._A prospective study of C‐peptide, insulin‐like growth factor‐I, insulin‐like growth factor binding protein‐1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2005; 14: 850–5. [DOI] [PubMed] [Google Scholar]
- 25.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004; 4: 579–91. [DOI] [PubMed] [Google Scholar]
- 26.Grosse Y, Baan R, Straif K_et al._WHO International Agency for Research on Cancer Monograph Working Group . A review of human carcinogens‐Part A: pharmaceuticals. Lancet Oncol 2009; 10: 13–4. [DOI] [PubMed] [Google Scholar]
- 27.Chlebowski RT, Wactawski‐Wende J, Ritenbaugh C_et al._Women’s Health Initiative Investigators . Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004; 350: 991–1004. [DOI] [PubMed] [Google Scholar]
- 28.Jeon CY, Lokken RP, Hu FB, Van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007; 30: 744–52. [DOI] [PubMed] [Google Scholar]
- 29.Inoue M, Yamamoto S, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Daily total physical activity level and total cancer risk in men and women: results from a large‐scale population‐based cohort study in Japan. Am J Epidemiol 2008; 168: 391–403. [DOI] [PubMed] [Google Scholar]
- 30.Van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA 2005; 294: 97–104. [DOI] [PubMed] [Google Scholar]
- 31.Kato M, Noda M, Inoue M, Kadowaki T, Tsugane S; JPHC Study Group . Psychological factors, coffee and risk of diabetes mellitus among middle‐aged Japanese: a population‐based prospective study in the JPHC study cohort. Endocr J 2009; 56: 459–68. [DOI] [PubMed] [Google Scholar]
- 32.Inoue M, Yoshimi I, Sobue T, Tsugane S. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst 2005; 97: 293–300. [DOI] [PubMed] [Google Scholar]
- 33.Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. Coffee consumption and risk of colorectal cancer in a population‐based prospective cohort of Japanese men and women. Int J Cancer 2007; 121: 1312–8. [DOI] [PubMed] [Google Scholar]
- 34.Luo J, Inoue M, Iwasaki M_et al._Green tea and coffee intake and risk of pancreatic cancer in a large‐scale, population‐based cohort study in Japan (JPHC study). Eur J Cancer Prev 2007; 16: 542–8. [DOI] [PubMed] [Google Scholar]
- 35.Shimazu T, Inoue M, Sasazuki S_et al._Coffee consumption and risk of endometrial cancer: a prospective study in Japan. Int J Cancer 2008; 123: 2406–10. [DOI] [PubMed] [Google Scholar]
- 36.Inoue M, Kurahashi N, Iwasaki M_et al._Japan Public Health Center‐Based Prospective Study Group . Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer Epidemiol Biomarkers Prev 2009; 18: 1746–53. [DOI] [PubMed] [Google Scholar]
- 37.Bravi F, Bosetti C, Tavani A_et al._Coffee drinking and hepatocellular carcinoma risk: a meta‐analysis. Hepatology 2007; 46: 430–5. [DOI] [PubMed] [Google Scholar]
- 38.Bravi F, Scotti L, Bosetti C_et al._Coffee drinking and endometrial cancer risk: a metaanalysis of observational studies. Am J Obstet Gynecol 2009; 200: 130–5. [DOI] [PubMed] [Google Scholar]
- 39.Je Y, Liu W, Giovannucci E. Coffee consumption and risk of colorectal cancer: a systematic review and meta‐analysis of prospective cohort studies. Int J Cancer 2009; 124: 1662–8. [DOI] [PubMed] [Google Scholar]
- 40.Van Dam RM, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ. Coffee consumption and incidence of impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes: the Hoorn Study. Diabetologia 2004; 47: 2152–9. [DOI] [PubMed] [Google Scholar]
- 41.Yamaji T, Mizoue T, Tabata S_et al._Coffee consumption and glucose tolerance status in middle‐aged Japanese men. Diabetologia 2004; 47: 2145–51. [DOI] [PubMed] [Google Scholar]
- 42.Keijzers GB, De Galan BE, Tack CJ, Smits P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care 2002; 25: 364–9. [DOI] [PubMed] [Google Scholar]
- 43.Greer F, Hudson R, Ross R, Graham T. Caffeine ingestion decreases glucose disposal during a hyperinsulinemic‐euglycemic clamp in sedentary humans. Diabetes 2001; 50: 2349–54. [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Hudson R, Kilpatrick K, Graham TE, Ross R. Caffeine ingestion is associated with reductions in glucose uptake independent of obesity and type 2 diabetes before and after exercise training. Diabetes Care 2005; 28: 566–72. [DOI] [PubMed] [Google Scholar]
- 45.Isogawa A, Noda M, Takahashi Y, Kadowaki T, Tsugane S. Coffee consumption and risk of type 2 diabetes mellitus. Lancet 2003; 361: 703–4. [DOI] [PubMed] [Google Scholar]
- 46.Inoue M, Noda M, Kurahashi N_et al._Impact of metabolic factors on subsequent cancer risk: results from a large‐scale population‐based cohort study in Japan (JPHC Study). Eur J Cancer Prev 2009; 18: 240–7. [DOI] [PubMed] [Google Scholar]
- 47.Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F; Risk Factors and Life Expectancy Research Group . Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev 2001; 10: 937–41. [PubMed] [Google Scholar]
- 48.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev 2002; 11: 385–91. [PubMed] [Google Scholar]
- 49.Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer 2008; 44: 293–7. [DOI] [PubMed] [Google Scholar]