Functional Analysis of Putative Adhesion Factors in Lactobacillus acidophilus NCFM (original) (raw)
Abstract
Lactobacilli are major inhabitants of the normal microflora of the gastrointestinal tract, and some select species have been used extensively as probiotic cultures. One potentially important property of these organisms is their ability to interact with epithelial cells in the intestinal tract, which may promote retention and host-bacterial communication. However, the mechanisms by which they attach to intestinal epithelial cells are unknown. The objective of this study was to investigate cell surface proteins in Lactobacillus acidophilus that may promote attachment to intestinal tissues. Using genome sequence data, predicted open reading frames were searched against known protein and protein motif databases to identify four proteins potentially involved in adhesion to epithelial cells. Homologous recombination was used to construct isogenic mutations in genes encoding a mucin-binding protein, a fibronectin-binding protein, a surface layer protein, and two streptococcal R28 homologs. The abilities of the mutants to adhere to intestinal epithelial cells were then evaluated in vitro. Each strain was screened on Caco-2 cells, which differentiate and express markers characteristic of normal small-intestine cells. A significant decrease in adhesion was observed in the fibronectin-binding protein mutant (76%) and the mucin-binding protein mutant (65%). A surface layer protein mutant also showed reduction in adhesion ability (84%), but the effect of this mutation is likely due to the loss of multiple surface proteins that may be embedded in the S-layer. This study demonstrated that multiple cell surface proteins in L. acidophilus NCFM can individually contribute to the organism's ability to attach to intestinal cells in vitro.
Probiotics are commonly included in dairy products, especially fermented milks. One major criterion considered for selection of probiotic bacteria has been their capacity to adhere to the human intestinal epithelial cells. Adhesion is believed to be a requirement for the realization of certain probiotic effects, such as immunomodulation (37, 44) and pathogen exclusion (6, 28). However, the mechanisms of attachment are not understood. Association with the intestinal mucosa can initiate and extend transient associations, which affords these bacteria a distinct advantage when in the gastrointestinal tract. Lactobacillus acidophilus NCFM is an industrial bacterial strain used widely in dietary supplements and cultured yogurts (36). Recent studies have implicated the involvement of some surface proteins from lactobacilli in adhesion to epithelial cells (16), mucin (32), and various extracellular matrix (ECM) proteins (40). The surface layer protein, SlpA, from other lactobacilli has also been shown to bind epithelial cells and ECM components (4, 21). However, no studies have demonstrated unequivocally the function and significance of S-layers in either adherence or improved retention of probiotic cultures in the gastrointestinal tract.
Lactobacilli are normal components of the intestinal microbiota and appear to be a key factor in the processes of competitive exclusion (13) and immunomodulation (44, 45) exerted by commensal organisms. Although extensive genetic characterization of the adhesive abilities of enteropathogenic bacteria has been performed, the genetic systems responsible for intestinal adhesion of probiotic bacteria are not fully understood. The difficulties in performing human trials, the complex and kinetic nature of the intestinal environment, and the absence of mutant strains for isogenic comparisons have made it difficult to study these processes. Development of appropriate intestinal model systems has allowed us to study bacterial adhesion to epithelial and mucosal surfaces in vitro, particularly in pathogen models. In vitro model systems, together with high-throughput sequencing of Lactobacillus genomes, now provides a platform for functional analysis of genes that may contribute to adherence and aggregation processes.
In order to identify genes potentially involved in adhesion, the complete genome sequence of L. acidophilus NCFM (2) was analyzed and open reading frames (ORFs) similar to genes implicated previously in adhesion were targeted for insertional inactivation, including two streptococcal R28 homologs (LBA1633 and LBA1634), a fibronectin-binding protein (FpbA), a mucin-binding protein (Mub), and a surface layer protein (SlpA). In this study, we provide evidence that the genes encoding FbpA, Mub, and SlpA contribute to the ability of NCFM to adhere to intestinal cells in vitro.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are identified in Table 1. Lactobacillus strains were cultivated anaerobically at 37°C or 42°C in MRS broth (Difco Laboratories Inc., Detroit, MI) or, when appropriate, in MRS supplemented with 1.5% agar. Escherichia coli was propagated aerobically in Luria-Bertani (LB) medium (Difco) or on LB medium supplemented with 1.5% agar at 37°C. Brain heart infusion medium (Difco) supplemented with 1.5% agar and 150 μg/ml erythromycin (Em) was used for selection of E. coli transformants. Chloramphenicol (5.0 μg/ml) and Em (5.0 μg/ml or 150 μg/ml) were used for selection when appropriate. The number of CFU per ml was determined using a Whitley Automatic Spiral Plater (Don Whitley Scientific Ltd., West Yorkshire, England).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Origin or relevant characteristics | Source or reference |
|---|---|---|
| L. acidophilus strains | ||
| NCFM | Human intestinal isolate | 36 |
| NCK 1377 | NCFM::pTRK826 (slpA integrant) | 1 |
| NCK 1392 | NCFM containing pTRK669 | 34 |
| NCK 1398 | NCFM::pTRK685 (lacL integrant) | 34 |
| NCK 1660 | NCFM::pTRK834 (mub integrant) | This study |
| NCK 1661 | NCFM::pTRK833 (fbpA integrant) | This study |
| NCK 1662 | NCFM::pTRK835 (R28 integrant) | This study |
| NCK 1720 | NCFM::pTRK832 (R28 integrant) | This study |
| E. coli strains | ||
| EC1000 | RepA+ MC1000; Kmr; carrying a single copy of the pWV01 repA; host for pOR128-based plasmids | 26 |
| Plasmids | ||
| pOR128 | Emr ori (pWV01); replicates only with repA provided in trans | 26 |
| pTRK669 | ori (pWV01) Cmr RepA+ | 34 |
| pTRK832 | 835-bp internal region of r28 (LBA1634) cloned into BglII-XbaI sites of pORI28 | This study |
| pTRK833 | 734-bp internal region of fbpA (LBA1148) cloned into BglII-XbaI sites of pORI28 | This study |
| pTRK834 | 955-bp internal region of mub (LBA1392) cloned into NcoI-XbaI sites of pORI28 | This study |
| pTRK835 | 867-bp internal region of R28 (LBA1633) cloned into BglII-XbaI sites of pORI28 | This study |
Computational analysis.
Conserved protein domains were detected using Pfam (http://pfam.wustl.edu), and ClustalX (41) was used to align sequences. SignalP 3.0 (http://www.cbs.dtu.dk/services/SignalP-3.0/) was used to identify signal sequences, and THMM v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) predicted transmembrane domains in selected protein sequences. Putative functions were assigned to target genes manually by sequence comparison to an existing protein database using the BLAST-P algorithm (3). Phylogenetic trees of related protein sequences were constructed using ClustalX (41) and MEGA2 (25).
DNA manipulation techniques.
Total Lactobacillus genomic DNA was isolated according to the method of Walker and Klaenhammer (47). Standard protocols were used for endonuclease restriction, ligation, DNA modification, and transformation (35). Plasmid preparations for the purpose of screening E. coli transformants followed the method of Zhou et al. (48). Large-scale plasmid preparations were performed with the QIAprep Spin kit according to the manufacturer's instructions (QIAGEN Inc., Valencia, CA). PCRs were carried out according to the manufacturer's recommendations using a Taq DNA polymerase PCR system (Roche Molecular Biochemicals). PCR primers (Table 2) were synthesized by Integrated DNA Technologies (Coralville, IA) and, when appropriate, restriction sites were designed into the 5′ ends of the primers to facilitate future cloning steps. DNA fragments were extracted from 1.0% agarose gels using the Zymoclean Gel DNA Recovery kit (Zymo Research, Orange, CA). Electrocompetent Lactobacillus cells were prepared as described by Walker et al. (46). Southern hybridization of genomic DNA was carried out using Magnacharge nylon transfer membranes (MSI, Westboro, MA) according to the manufacturer's instructions.
TABLE 2.
Primer sets used
| ORF | Orientation | Internala | Externalb |
|---|---|---|---|
| LBA1148 (fpbA) | Forward | GATCTCTAGA-TGGAAGGTTCAGTCTTAC | CAACGCTCAATATCCTAGAA |
| a | |||
| Reverse | GATCAGATCT-TACGATAGCCCTCAGAAT | CTTGTGGATCAGCATTATCA | |
| b | |||
| LBA1392 (mub) | Forward | CATGCCATGG-TAAGGTCACAGGTGTAACAA | AGTGCTAGTGACCAACACTT |
| c | |||
| Reverse | GATCTCTAGA-AGCCTACTTCACTAGGAGTC | ATTGCTTAGGTGCGTTAGTA | |
| a | |||
| LBA1633 (R28) | Forward | GATCAGATCT-CAGGTTCTACAGCATCAA | TAGGCAATACTGGTAATGAA |
| b | |||
| Reverse | GATCTCTAGA-CCAACGCCTTCACTATAA | TTAACCGGAATTACAACATC | |
| a | |||
| LBA1634 (R28) | Forward | GATCAGATCT-CCAGCAGTTGTTGTCGTA | CCAGCAAGTTCAATCAAGGT |
| b | |||
| Reverse | GATCTCTAGA-ACTGTGGCCGGCTTGTTA | ACGTGGATAGTTACTGAGAC | |
| a |
Site-specific integration into L. acidophilus NCFM.
Using L. acidophilus NCFM chromosomal DNA as a template, an internal fragment of each target ORF was amplified using PCR with primers listed in Table 2. The internal fragment was cloned onto the integrative vector pORI28 (26) and subsequently transformed by electroporation into L. acidophilus NCFM containing the temperature-sensitive helper plasmid pTRK669 (34). Steps were then carried out according to the method of Russell and Klaenhammer (34) for selection of integrants. Successful integration of the plasmid was confirmed by PCR and Southern hybridization analysis of junction fragments.
Tissue culture.
The Caco-2 (ATCC HTB-37) (ATCC, Manassas, VA) cells were used only between the 40th and 60th passages. All reagents used in maintenance of Caco-2 cells were obtained from Gibco (Gibco-Invitrogen Corp., Carlsbad, CA). The cells were routinely grown in a 95% air-5% CO2 atmosphere in minimum essential medium supplemented with 20% (vol/vol) inactivated (56°C; 30 min) fetal bovine serum, 0.10 mM nonessential amino acids, and 1.0 mM sodium pyruvate. Monolayers were trypsinized for 10 min, counted using a hemocytometer, and seeded at 1.3 × 105 cells/well in 2.0 ml of cell culture medium. The medium was replaced every 2 days, and all adherence assays were performed after 14 days of incubation. Cells were grown on 15 mm Thermanox plastic coverslips (Nalge Nunc International, Rochester, NY) in treated Costar 12-well tissue culture plates (Corning Inc., Acton, MA).
Adherence assay.
The adhesion of Lactobacillus strains to Caco-2 cells was examined according to the method described previously by Chauviere et al. (8), with the following modifications. Briefly, mid-log-phase bacterial cells (optical density at 600 nm, 6.0) were prepared in MRS with 3.0 μg/ml Em to maintain selective pressure on integrants. Cells were removed by centrifugation for 10 min at 4,000 × g to eliminate any effect of low pH or extracellular proteins in culture supernatants and were washed twice with phosphate-buffered saline (PBS). Bacterial pellets were resuspended in 5 ml of fresh MRS prior to adherence. Fifteen-day Caco-2 monolayers were washed twice with PBS and treated with a bacterial suspension at a concentration of 4 × 108 CFU/ml. The bacteria were incubated on the monolayer for 1.5 h at 37°C in a mixture (1:2 [vol/vol]) of MRS and cell line culture medium. Following incubation, the monolayers were washed five times with PBS, fixed in methanol, and Gram stained. Adherent bacterial cells were then enumerated microscopically. For statistical purposes, 17 fields were examined in a predetermined fixed grid for each coverslip (Fig. 1). The pattern of the grid was selected by choosing the pattern of fields that most ideally represented the average count per field obtained by counting all fields on the coverslip. Duplicate coverslips were counted for each experiment. The final data presented collectively represented at least five experiments. Total counts for each coverslip were used, and adhesion was expressed as a percentage of that of the control strain, NCK1398 (34), which carries an insert in the lacL (β-galactosidase) gene. Using this control, all mutant cultures and the parental control could be prepared with Em to maintain selective pressure on the integrant.
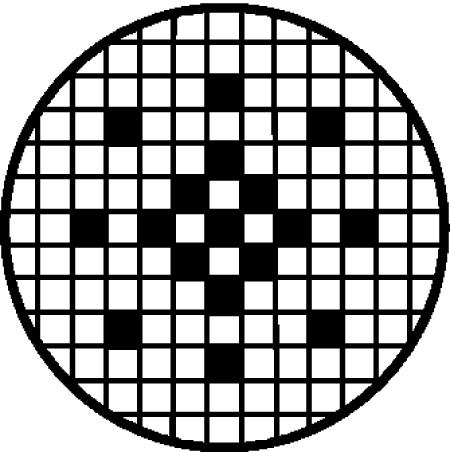
FIG. 1.
Grid pattern used for microscopic enumeration of bacterial cells adhering to Caco-2 monolayers on coverslips. Each square represents one microscopic field. The shaded squares indicate fields on the coverslips (represented by the complete circle) selected for enumeration.
RESULTS
Sequence analysis of putative adhesion ORFs.
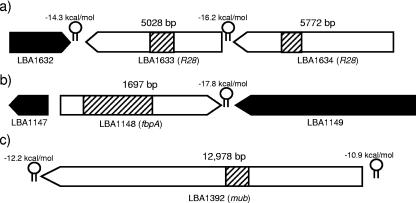
Three regions of the L. acidophilus NCFM genome were identified as harboring genes predicted to encode proteins that may participate in adhesion to the intestinal epithelium and mucosa. The first region encoded two adjacent ORFs (LBA1633 and LBA1634) showing homology to R28, a protein involved in adhesion of Streptococcus pyogenes to the vaginal epithelium (39). The second and third regions contained a putative fibronectin-binding protein (LBA1148) and a mucin-binding protein (LBA1392). Figure 2 shows the sizes of the predicted proteins and surrounding features in the genome.
FIG. 2.
Putative adhesion ORFs in the L. acidophilus NCFM genome targeted for insertional inactivation are represented by white arrows. The hatched regions in the white arrows represent the regions cloned onto pORI28 for homologous recombination and the subsequent region of integration. The hatched regions are drawn to scale within each gene, but the relative sizes of genes are not to scale. Predicted rho-independent terminator free energies are represented by hairpin structures. Potential functions of target genes are listed next to the ORF numbers.
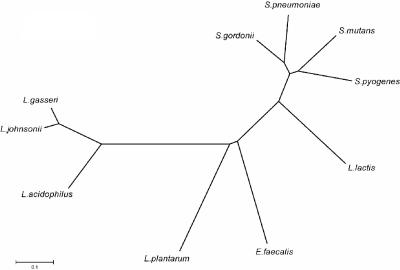
A fibronectin-binding protein (LBA1148) containing the N-terminal PFam domain (pfam05833) for fibronectin-binding protein A (FbpA) was identified; it was the only ORF in the NCFM genome containing that domain. LBA1148 is transcribed on the forward strand with its own promoter, ribosomal binding site, and terminator (Fig. 2). LBA1148 showed 41% amino acid identity to a predicted adherence protein (NP_785358) in Lactobacillus plantarum WCFS1 and a putative fibronectin-binding protein (NP_814975) in Enterococcus faecalis V583 and exhibited >65% amino acid identity to uncharacterized putative proteins in Lactobacillus gasseri (Lgas02000275) and Lactobacillus johnsonii (LJ1182). Interestingly, the fibronectin-binding proteins identified in L. acidophilus NCFM, L. gasseri, and L. johnsonii are clustered away from other fibronectin-binding proteins when compared using amino acid sequence alignments (Fig. 3). Previously studied fibronectin-binding proteins in Streptococcus mutans and Streptococcus gordonii also contain the FbpA PFam domain and show homology to LBA1148 (38% and 39% amino acid identity, respectively). Due to the similarities to other binding proteins, LBA1148 (FbpA) was inactivated by site-specific integration via a 734-bp internal region of homology. Confirmation of the integration event was performed by PCR analysis of junction fragments and by Southern hybridization (Fig. 4).
FIG. 3.
Phylogenetic tree of selected fibronectin-binding proteins in gram-positive bacteria. Protein alignment and tree construction were performed using ClustalX and MEGA2. Sequences for alignment of fibronectin-binding proteins were obtained from GenBank and were selected based on similarity to FbpA (LBA1148) in L. acidophilus NCFM.
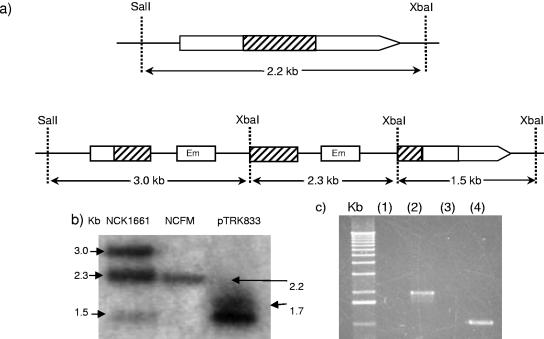
FIG. 4.
Confirmation of an integration event in the gene putatively encoding the fibronectin-binding protein, LBA1148. (a) Chromosomal insertion of pTRK833 into the target region of LBA1148. The internal target region is represented by the hatched box. Restriction sites SalI and XbaI are indicated. (b) Southern hybridization analysis of genomes of NCFM and the fibronectin-binding protein mutant, NCK1661. Chromosomal DNA was digested with SalI and XbaI, and the internal fragment (hatched region) was used as a probe. Left lane, NCK1661; middle lane, NCFM; right lane, plasmid pTRK833 (the double band represents a supercoiled and nicked plasmid). (c) PCR amplification of junction fragments in the fibronectin-binding protein mutant using primers outside of the region of homology paired with primers in the Em resistance gene of the integration plasmid. Lane 1 and lane 3, NCFM controls; lane 2, upstream junction fragment; lane 4, downstream junction fragment. The absence of a band in lanes 1 and 3 indicates that no plasmid was integrated into this region in the control.
LBA1633 and LBA1634 both show similarity to R28, a characterized adhesion protein in S. pyogenes. R28 contains 10 identical 79-residue repeats and was shown to promote the adhesion of S. pyogenes to cervical epithelial cells in vitro (39). Lactobacillus fermentum BR11 also has two tandem genes, rlp and mlp, proposed to be involved in adhesion (43); Rlp exhibits homology to R28, LBA1633, and LBA1634. Interestingly, Mlp is also similar to the mucus-binding protein (LBA1392) discussed below. Both LBA1633 and LBA1634 have exhibited LPXTG sortase target signal sequences for cell wall anchoring. A gene encoding a sortase (LBA1244) was identified, and 12 other ORFs in the L. acidophilus NCFM genome harbored the sortase target sequence. LBA1634 also contains a gram-positive cell wall anchor motif (PF00746) and a SIRK-type signal peptide sequence, suggesting that the protein is secreted and anchored to the cell wall. C-terminal transmembrane domains were found in both proteins, while only LBA1634 contains both C- and N-terminal transmembrane domains. These two ORFs contain both perfect and nonperfect internal repeats and show 39% amino acid identity to each other. LBA1633 and LBA1634 are transcribed on the complement strand and translated in different frames, and each has a terminator immediately downstream. Ribosomal binding sites, along with −10 and −35 promoter regions, were predicted upstream of each ORF. Upstream of LBA1634 is an ORF of unknown function (LBA1636) and one predicted membrane transporter (LBA1637). Downstream of LBA1633 is a predicted aldehyde dehydrogenase transcribed in the opposite direction. The similarity of LBA1633 and LBA1634 to each other and to other adhesion factors made them logical targets for characterization. Site-specific integrations (data not shown) were individually made into LBA1633 and LBA1634, and the mutants were evaluated for their ability to adhere to Caco-2 intestinal cells in vitro.
Mucin-binding proteins are common in various forms in the genome of L. acidophilus NCFM. Of the 13 ORFs that are annotated as mucus-binding proteins, 5 have BlastP e values smaller than 1e−29 (nonredundant database), and two of those contain the LPXTG sortase-targeting signal for cell wall anchoring. One of the two, LBA1392, shows homology (25% amino acid identity) to a previously characterized mucus-binding protein in L. reuteri and is of similar size (33). However, the amino acid repeat pattern in the L. reuteri protein is not conserved in LBA1392. Instead, LBA1392 contains two sets of three repeats each, ranging in size from 70 to 87 amino acids and showing 61 to 100% sequence identity between the repeats. The three repeats in each set are adjacent, and the two sets of repeats are located next to each other, covering an overall stretch of 1,586 amino acids. Transmembrane domains were identified at both the amino and carboxy termini of LBA1392, and a SIRK signal sequence and cleavage site were identified at amino acid position 50. Collectively, these predicted features strengthen the functional classification of LBA1392 as a candidate for mucin association and epithelial-cell adhesion. Mucus-binding protein homologs have been identified in several different lactobacilli (24, 31, 33) but are not generally found among the lactic acid bacteria. Although they have similar predicted functions and are found in similar probiotic lactobacilli, the lack of amino acid identity indicates substantial divergence for these proteins with presumed similar functions (Fig. 5). LBA1392 (Mub) was also insertionally inactivated, and the integration event was confirmed by both Southern hybridization and PCR (data not shown).
FIG. 5.
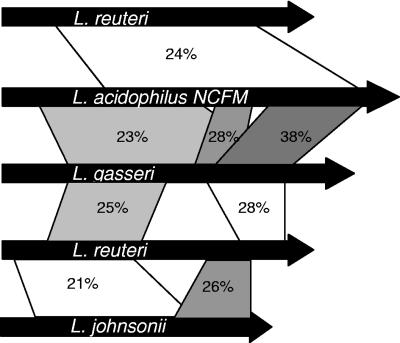
Amino acid identities compared for predicted mucin-binding proteins in four Lactobacillus species. Amino acid alignment was performed with BLAST-P, and identity is represented by the shaded regions between genes. Sequences for alignment were obtained from GenBank. Protein lengths are drawn approximately to scale; the lengths in amino acids are as follows: L. reuteri Mub, 3,269; L. acidophilus Mub, 4,326; L. gasseri Mub, 3,692; L. johnsonii Mub, 2,139.
Adhesion of mutants to epithelial cells.
The effects of insertional inactivation of LBA1633, LBA1634, FbpA (LBA1148), Mub (LBA1392), and the surface layer gene, slpA (LBA1377) (1), were determined by screening the mutants' abilities to adhere to Caco-2 cells in vitro. Caco-2 is a colonic carcinoma cell line, commonly used to study bacterial adherence, which expresses many of the markers associated with normal small-intestine villus cells (30). Log-phase bacterial cells and a 1.5-h adherence time have demonstrated the most consistent levels of adherence to Caco-2 cells in our experiments (data not shown). A derivative of L. acidophilus NCFM (NCK1398) harboring a plasmid integration into the lacL gene, encoding β-galactosidase, was used as the control so that antibiotic pressure could be maintained on all strains used in adherence comparisons. There was no significant difference in adhesion between NCK1398 and wild-type L. acidophilus NCFM (data not shown).
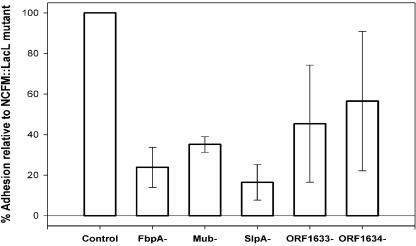
The cell morphologies of individual mutant strains were not altered under the growth conditions used in the study, with the exception of the SlpA− mutant, which grew as small curved bacilli (1). Each inactivated gene contained a predicted downstream terminator, so any effects observed were not considered likely to result from downstream polar effects. Insertional inactivation of three of the five genes targeted in this study resulted in a significant reduction (P < 0.05) in adhesion to Caco-2 intestinal cells compared to the wild-type control (Fig. 6). No reproducible effects on adhesion were observed with the two R28 homolog mutants, NCK1662 and NCK1720. Inactivation of a predicted fibronectin-binding protein resulted in a 76% decrease in adhesion. Mucin-binding proteins were predicted to be abundant in the genome of L. acidophilus NCFM. Inactivation of a single putative mucin-binding protein decreased the ability of the resulting mutant strain to adhere by 65%. The SlpA− mutant demonstrated the largest decrease in adhesion ability at 84%, although it is likely that multiple surface-associated proteins could be disrupted with the removal of the S-layer.
FIG. 6.
Adhesion properties of L. acidophilus NCFM mutants expressed as percentages of the NCFM::lacL control, enumerated microscopically. The error bars represent 1 standard deviation.
DISCUSSION
Adhesion of lactic acid bacteria to epithelial and mucosal surfaces is a complex process involving many different factors. The ability of these microorganisms to adhere to intestinal surfaces is potentially a major distinguishing feature for selection of bacteria as probiotic strains. Close interaction with host tissues may provide probiotics with a distinct advantage when establishing residence in the gastrointestinal tract or interacting with cells of the intestinal mucosa. This study exploited genome sequence data to identify four genes that may be involved in adhesion of NCFM to intestinal epithelial cells. A targeted approach for identifying adhesion factors and creating isogenic chromosomal mutations allows us to systematically evaluate cell surface proteins for their contributions to adherence or attachment capability. Previous studies have concentrated on identifying the locations of adhesive determinants (16, 20), determining the nature of adhesive determinants (8, 10, 17-19), comparing the adhesiveness of different strains (8, 42), or identifying adhesion molecules using binding assays (17, 22, 33). In contrast, this is the first study that has relied on genome sequence data to identify multiple cell surface proteins potentially involved in adhesion and specifically investigated their individual contributions via isogenic strain comparisons. In this study, a fibronectin-binding protein, a mucin-binding protein, and a surface layer protein each contributed, individually, to the ability of NCFM to adhere to Caco-2 cells in vitro.
The human Caco-2 cell line was originally isolated from a human adenocarcinoma of the colon. In culture, Caco-2 cells spontaneously differentiate postconfluence to display functional apical brush border microvilli and epithelial-cell polarization. This cell line has traditionally been employed to study the adherence of lactobacilli and to select for adhesive probiotic strains (5, 7, 8, 16, 18, 21). Additionally, the Caco-2 model is commonly used to demonstrate the competitive exclusion properties of probiotic bacteria against enteropathogenic strains, such as various Salmonella and E. coli strains (6, 11, 15). In examining the properties of probiotic bacterial adhesion to Caco-2 cells, many different protocols have emerged, each with its own benefits and shortcomings. After evaluating different protocols, we selected and adapted one based on its reproducibility and applicability to the cell surface knockout mutants constructed. Blum et al. (7) noted that the percentage of adhesion of a probiotic strain under given assay conditions does not represent an absolute value (7). Instead, adhesion should be reported as a value relative to a type strain. This study uses a β-galactosidase mutant as the control to ensure that conditions for propagation, specifically under antibiotic selection to maintain an integrated plasmid, are identical between the control strain and mutants. If the conditions of the adhesion assay are closely controlled, the effects of isogenic mutations in cell surface proteins can be accurately evaluated and compared.
We observed a significant decrease in adhesion to Caco-2 cells with inactivation of the surface layer gene, slpA. The surface layer is a self-assembled paracrystalline monolayer of proteins that coats the entire surface of some bacteria and archea. The function of surface layers can include cell shape determination, protection, and epitope display. Additionally, S-layers have been reported to be involved in epithelial-cell attachment (14, 38). However, these studies used chemical treatments, such as LiCl, to remove existing S-layers from the organism. This approach does not account for the reappearance of the S-layer after chemical removal. Use of an isogenic S-layer mutant allows a more definitive study of the effect of the S-layer on adhesion. The absence of an S-layer was confirmed in NCK1377 at the time of experimentation; however, after approximately 6 months of storage, a chromosomal inversion resulted in the reappearance of an alternate S-layer (1). It is not surprising that such a dramatic effect on adhesion was observed in the current study when the gene encoding the surface layer protein was inactivated. Removal or alteration of a surface layer can have many dramatic cellular affects, not the least of which may be on surface charge, architecture, and the presence or conformation of various surface proteins involved in attachment.
Surface proteins involved in adhesion could adhere either directly to the epithelium or to specific components of the epithelial environment. The ECM surrounding mammalian epithelial cells is composed of various secreted proteins, including laminin, collagen, and fibronectin. Fibronectin is a dimeric glycoprotein reported to be involved in adhesion of Lactobacillus cells to intestinal cells in vitro (22). Recent studies have reported fibronectin binding by lactobacilli (27, 40) and implicated involvement of fibronectin in epithelial-cell adhesion (22). Other studies have proposed involvement of bacterium-associated proteinaceous components in the adhesion of lactic acid bacteria to intestinal cells in vitro (18, 19). Multiple fibronectin-binding proteins in various Streptococcus species have been examined, one of which is the surface-associated protein FbpA in S. gordonii (9). FbpA appears to act as a bridging protein between the bacterial surface and the ECM of the host, although the precise mechanism by which this interaction occurs is not understood. By inactivating the predicted bridging protein, FbpA, in L. acidophilus NCFM, we expected to eliminate any adhesive properties due to fibronectin binding. Accordingly, we observed an approximately 76% reduction in adherence capacity, indicating that FbpA is an important, but not the only, factor contributing to the ability of lactobacilli to adhere to epithelial cells.
In addition to the ECM, epithelial cells in the mammalian gastrointestinal tract are covered with a thick layer of mucus composed mainly of glycoproteins called mucins. Mucin expression and composition are dynamic, balanced between production by goblet cells and degradation by proteases and physical erosion in the gut due to transit functions. Bacteria able to adhere to mucus but unable to associate with the epithelium may be washed away with degraded mucins, partially accounting for the transient nature of intestinal colonization observed for most probiotic bacteria. Studies have reported the abilities of many lactobacilli to bind to human intestinal mucus (23). The ability of lactobacilli to colonize this mucus layer could be vital in the realization of certain probiotic properties. A mucin-binding protein in L. reuteri, Mub, was shown not only to be associated with the cell surface via immunofluorescence, but also to bind intestinal mucins (33). When examined as part of a fusion protein, the repeat regions of L. reuteri Mub were shown to bind mucus components. Therefore, the region targeted for integration of pTRK834 (the mub integration vector) was the repeat region of mub in L. acidophilus NCFM. Although the vector was designed to integrate into the repeat region, care was taken to avoid multiple regions of homology between the vector and mub. The resulting mutant showed a consistent 65% decrease in adhesion to Caco-2 cells. Although the Caco-2 cells in this study do not produce mucins, the contribution of the mucin-binding protein to intestinal-cell binding is evident.
Both the Mub− and FbpA− mutants exhibited significant and reproducible decreases in adhesion to Caco-2 cells. Follow-up experiments will focus on the specific abilities of those mutant strains to bind mucin and fibronectin. The mutants with integrations in the contiguous R28 homologs (LBA1663 and LBA1664) did not produce convincing changes in adhesive properties. LBA1663 and LBA1664 both showed substantial homology to adhesins R28 in S. pyogenes and Rlp in L. fermentum BR11. The participation of R28 in the adhesion of S. pyogenes to vaginal epithelial cells might suggest that an in vitro model of the vaginal epithelium would be useful in characterizing these mutant strains, but that is beyond the scope of this study. Although these two ORFs in NCFM encode proteins similar to those in the Rib family, they do not contain the identical repeats present in most other Rib proteins, particularly Rib proteins in pathogenic organisms. Neighboring binding proteins with notable identity were identified in S. gordonii at two separate chromosomal loci (12, 29). Although there are terminators downstream of both genes in NCFM, the fact that these genes are adjacent and very similar could account for the substantial variation observed in attachment levels. Perhaps the cell relies upon both proteins for similar functions in adhesion, and disruption of only one leads to a variable functional attachment phenotype. A mutant strain deficient in both of these proteins would be useful in determining their effects on adhesiveness.
Significant involvement of SlpA, FbpA, and Mub in adherence of L. acidophilus NCFM to Caco-2 cells was demonstrated. Given that the inactivation of LBA1633 and LBA1634 produced neither dramatic changes nor reproducible results, the effects of the SlpA−, FbpA−, and Mub− mutations are even more striking in comparison. The genetic mechanisms and components involved in the binding of Lactobacillus to the human intestinal epithelium have yet to be fully characterized. However, with the increasing availability of genomic data and the molecular tools necessary to construct functional isogenic mutants, cell surface components can be identified and tested in vitro. No single gene inactivated in this study was able to completely eliminate adhesion of NCFM to Caco-2 cells, thereby strengthening the hypothesis that adhesion is achieved through an intimate interplay of multiple factors. Elucidation of the molecular components, and their interactions, in adhesion of lactobacilli to human epithelial cells is expected to progress rapidly through the use of functional genomics to identify and confirm those key factors.
Acknowledgments
This work was partially supported by Danisco, Inc., (Madison, WI); the Southeast Dairy Foods Research Center; Dairy Management, Inc.; and the NC Dairy Foundation. B.L.B. was partially supported by the NIH Biotechnology Training Program.
We thank M. A. Azcarate-Peril and R. Sanozky-Dawes for their helpful discussions and comments.
REFERENCES
- 1.Altermann, E., B. L. Buck, and T. R. Klaenhammer. Unpublished data.
- 2.Altermann, E., W. M. Russell, M. A. Azcarate-Peril, R. Barrangou, B. L. Buck, O. McAuliffe, N. Souther, A. Dobson, T. Duong, M. Callanan, S. Lick, A. Hamrick, R. Cano, and T. R. Klaenhammer. 2005. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. USA 102**:**3906-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., and D. J. Lipman. 1990. Protein database searches for multiple alignments. Proc. Natl. Acad. Sci. USA 87**:**5509-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avall-Jaaskelainen, S., A. Lindholm, and A. Palva. 2003. Surface display of the receptor-binding region of the Lactobacillus brevis S-layer protein in Lactococcus lactis provides nonadhesive lactococci with the ability to adhere to intestinal epithelial cells. Appl. Environ. Microbiol. 69**:**2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azuma, Y., and M. Sato. 2001. Lactobacillus casei NY1301 increases the adhesion of Lactobacillus gasseri NY0509 to human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 65**:**2326-2329. [DOI] [PubMed] [Google Scholar]
- 6.Bernet, M. F., D. Brassart, J. R. Neeser, and A. L. Servin. 1994. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35**:**483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum, S., R. Reniero, E. J. Schiffrin, R. Crittenden, T. Mattila-Sandholm, A. C. Ouwehand, S. Salminen, A. von Wright, M. Saarela, M. Saxelin, K. Collins, and L. Morelli. 1999. Adhesion studies for probiotics: need for validation and refinement. Trends Food Sci. Technol. 10**:**405-410. [Google Scholar]
- 8.Chauviere, G., M. H. Coconnier, S. Kerneis, J. Fourniat, and A. L. Servin. 1992. Adhesion of human Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J. Gen. Microbiol. 138**:**1689-1696. [DOI] [PubMed] [Google Scholar]
- 9.Christie, J., R. McNab, and H. F. Jenkinson. 2002. Expression of fibronectin-binding protein FbpA modulates adhesion in Streptococcus gordonii. Microbiology 148**:**1615-1625. [DOI] [PubMed] [Google Scholar]
- 10.Coconnier, M. H., T. R. Klaenhammer, S. Kerneis, M. F. Bernet, and A. L. Servin. 1992. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl. Environ. Microbiol. 58**:**2034-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coconnier, M. H., V. Lievin, M. Lorrot, and A. L. Servin. 2000. Antagonistic activity of Lactobacillus acidophilus LB against intracellular Salmonella enterica serovar Typhimurium infecting human enterocyte-like Caco-2/TC-7 cells. Appl. Environ. Microbiol. 66**:**1152-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demuth, D. R., Y. Duan, W. Brooks, A. R. Holmes, R. McNab, and H. F. Jenkinson. 1996. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol. Microbiol. 20**:**403-413. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez, M. F., S. Boris, and C. Barbes. 2003. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 94**:**449-455. [DOI] [PubMed] [Google Scholar]
- 14.Frece, J., B. Kos, I. K. Svetec, Z. Zgaga, V. Mrsa, and J. Suskovic. 2005. Importance of S-layer proteins in probiotic activity of Lactobacillus acidophilus M92. J. Appl. Microbiol. 98**:**285-292. [DOI] [PubMed] [Google Scholar]
- 15.Gopal, P. K., J. Prasad, J. Smart, and H. S. Gill. 2001. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food. Microbiol. 67**:**207-216. [DOI] [PubMed] [Google Scholar]
- 16.Granato, D., G. E. Bergonzelli, R. D. Pridmore, L. Marvin, M. Rouvet, and I. E. Corthesy-Theulaz. 2004. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect. Immun. 72**:**2160-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granato, D., F. Perotti, I. Masserey, M. Rouvet, M. Golliard, A. Servin, and D. Brassart. 1999. Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells. Appl. Environ. Microbiol. 65**:**1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene, J. D., and T. R. Klaenhammer. 1994. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl. Environ. Microbiol. 60**:**4487-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriksson, A., R. Szewzyk, and P. L. Conway. 1991. Characteristics of the adhesive determinants of Lactobacillus fermentum 104. Appl. Environ. Microbiol. 57**:**499-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hood, S. K., and E. A. Zottola. 1987. Electron microscopic study of the adherence properties of Lactobacillus acidophilus. J. Food Sci. 52**:**791. [Google Scholar]
- 21.Hynonen, U., B. Westerlund-Wikstrom, A. Palva, and T. K. Korhonen. 2002. Identification by flagellum display of an epithelial cell- and fibronectin-binding function in the SlpA surface protein of Lactobacillus brevis. J. Bacteriol. 184**:**3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapczynski, D. R., R. J. Meinersmann, and M. D. Lee. 2000. Adherence of Lactobacillus to intestinal 407 cells in culture correlates with fibronectin binding. Curr. Microbiol. 41**:**136-141. [DOI] [PubMed] [Google Scholar]
- 23.Kirjavainen, P. V., A. C. Ouwehand, E. Isolauri, and S. J. Salminen. 1998. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol. Lett. 167**:**185-189. [DOI] [PubMed] [Google Scholar]
- 24.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100**:**1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar, S., K. Tamura, and M. Nei. 1994. MEGA: Molecular Evolutionary Genetics Analysis software for microcomputers. Comput. Appl. Biosci. 10**:**189-191. [DOI] [PubMed] [Google Scholar]
- 26.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177**:**7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorca, G., M. I. Torino, G. Font de Valdez, and A. A. Ljungh. 2002. Lactobacilli express cell surface proteins which mediate binding of immobilized collagen and fibronectin. FEMS Microbiol. Lett. 206**:**31-37. [DOI] [PubMed] [Google Scholar]
- 28.Mack, D. R., S. Michail, S. Wei, L. McDougall, and M. A. Hollingsworth. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276**:**941-950. [DOI] [PubMed] [Google Scholar]
- 29.McNab, R., H. F. Jenkinson, D. M. Loach, and G. W. Tannock. 1994. Cell-surface-associated polypeptides CshA and CshB of high molecular mass are colonization determinants in the oral bacterium Streptococcus gordonii. Mol. Microbiol. 14**:**743-754. [DOI] [PubMed] [Google Scholar]
- 30.Pinto, M., S. Robineleon, M. D. Appay, M. Kedinger, N. Triadou, E. Dussaulx, B. Lacroix, P. Simonassmann, K. Haffen, J. Fogh, and A. Zweibaum. 1983. Enterocyte-like differentiation and polarization of the human-colon carcinoma cell-line Caco-2 in culture. Biol. Cell 47**:**323-330. [Google Scholar]
- 31.Pridmore, R. D., B. Berger, F. Desiere, D. Vilanova, C. Barretto, A. C. Pittet, M. C. Zwahlen, M. Rouvet, E. Altermann, R. Barrangou, B. Mollet, A. Mercenier, T. Klaenhammer, F. Arigoni, and M. A. Schell. 2004. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc. Natl. Acad. Sci. USA 101**:**2512-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojas, M., F. Ascencio, and P. L. Conway. 2002. Purification and characterization of a surface protein from Lactobacillus fermentum 104R that binds to porcine small intestinal mucus and gastric mucin. Appl. Environ. Microbiol. 68**:**2330-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roos, S., and H. Jonsson. 2002. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148**:**433-442. [DOI] [PubMed] [Google Scholar]
- 34.Russell, W. M., and T. R. Klaenhammer. 2001. Efficient system for directed integration into the Lactobacillus acidophilus and Lactobacillus gasseri chromosomes via homologous recombination. Appl. Environ. Microbiol. 67**:**4361-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Sanders, M. E., and T. R. Klaenhammer. 2001. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J. Dairy Sci. 84**:**319-331. [DOI] [PubMed] [Google Scholar]
- 37.Schiffrin, E. J., F. Rochat, H. Link-Amster, J. M. Aeschlimann, and A. Donnet-Hughes. 1995. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J. Dairy Sci. 78**:**491-497. [DOI] [PubMed] [Google Scholar]
- 38.Schneitz, C., L. Nuotio, and K. Lounatma. 1993. Adhesion of Lactobacillus acidophilus to avian intestinal epithelial cells mediated by the crystalline bacterial cell surface layer (S-layer). J. Appl. Bacteriol. 74**:**290-294. [DOI] [PubMed] [Google Scholar]
- 39.Stalhammar-Carlemalm, M., T. Areschoug, C. Larsson, and G. Lindahl. 1999. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol. Microbiol. 33**:**208-219. [DOI] [PubMed] [Google Scholar]
- 40.Styriak, I., R. Nemcova, Y. H. Chang, and A. Ljungh. 2003. Binding of extracellular matrix molecules by probiotic bacteria. Lett. Appl. Microbiol. 37**:**329-333. [DOI] [PubMed] [Google Scholar]
- 41.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25**:**4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuomola, E. M., and S. J. Salminen. 1998. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food Microbiol. 41**:**45-51. [DOI] [PubMed] [Google Scholar]
- 43.Turner, M. S., L. M. Hafner, T. Walsh, and P. M. Giffard. 2003. Peptide surface display and secretion using two LPXTG-containing surface proteins from Lactobacillus fermentum BR11. Appl. Environ. Microbiol. 69**:**5855-5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valeur, N., P. Engel, N. Carbajal, E. Connolly, and K. Ladefoged. 2004. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl. Environ. Microbiol. 70**:**1176-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinderola, C. G., M. Medici, and G. Perdigon. 2004. Relationship between interaction sites in the gut, hydrophobicity, mucosal immunomodulating capacities and cell wall protein profiles in indigenous and exogenous bacteria. J. Appl. Microbiol. 96**:**230-243. [DOI] [PubMed] [Google Scholar]
- 46.Walker, D. C., K. Aoyama, and T. R. Klaenhammer. 1996. Electrotransformation of Lactobacillus acidophilus group A1. FEMS Microbiol. Lett. 138**:**233-237. [DOI] [PubMed] [Google Scholar]
- 47.Walker, D. C., and T. R. Klaenhammer. 1994. Isolation of a novel IS3 group insertion element and construction of an integration vector for Lactobacillus spp. J. Bacteriol. 176**:**5330-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou, J. S., Q. Shu, K. J. Rutherfurd, J. Prasad, M. J. Birtles, P. K. Gopal, and H. S. Gill. 2000. Safety assessment of potential probiotic lactic acid bacterial strains Lactobacillus rhamnosus HN001, Lb. acidophilus HN017, and Bifidobacterium lactis HN019 in BALB/c mice. Int. J. Food Microbiol. 56**:**87-96. [DOI] [PubMed] [Google Scholar]