A pervasive mechanism for analgesia: Activation of GIRK2 channels (original) (raw)
Abstract
G protein-coupled inwardly rectifying potassium channels (GIRKs) provide a common link between numerous neurotransmitter receptors and the regulation of synaptic transmission. We asked whether GIRKs specify a single behavioral action that is produced by drugs acting on the diverse receptors coupled with GIRKs. By using GIRK2-null mutant mice, we found marked reduction or complete elimination of the antinociceptive (hot plate test) effects of ethanol, oxotremorine, nicotine, baclofen, clonidine, and the cannabinoid receptor agonist WIN 55,212. However, ketamine analgesia remained intact. For most drugs, there was a sex difference in antinociceptive action, and the impact of deletion of the GIRK2 channel was less in female mice. The deletion of the GIRK2 channel blocks the opioid-dependent component of stress-induced analgesia (SIA), whereas nonopioid SIA was not changed. We propose that opioid, α adrenergic, muscarinic cholinergic, γ-aminobutyric acid-B, and cannabinoid receptors are coupled with postsynaptic GIRK2 channels in vivo. Furthermore, this pathway accounts for essentially all of the antinociceptive effects in males, although females appear to recruit additional signal transduction mechanisms for some analgesic drugs.
Chemically and pharmacologically diverse drugs reduce nociceptive responses in humans and in animal models of acute pain. Many of these drugs act through G protein coupled receptors and affect multiple pre- and postsynaptic signaling pathways (1, 2). A key question is whether there is a single G protein signal transduction pathway that is important for antinociceptive actions of many different drugs. One candidate for such a signaling system is the G protein-coupled inwardly rectifying potassium channel (GIRK). This assumption is based in part on the observation that weaver mutant mice have a mutation in GIRK2 and display reduced analgesia after either morphine or κ-opioid agonist (−)-U-50488 or ethanol administration (3, 4). However, the weaver mutant is not ideal for assessing the role of GIRK2 because the mutation alters the ion selectivity of GIRK2 and produces neuronal degeneration rather than a simple loss of GIRK2 function (5). Targeted mutation has produced mice lacking the GIRK2 protein; these mice lack postsynaptic responses to several neurotransmitters known to act through G protein coupled receptors, but retain normal presynaptic actions of these receptors (6–8). These mice are viable and similar to wild-type controls in many behavioral tests (9–11) and provide a unique approach to defining the role of GIRK2 in drug actions.
GIRKs are activated by M2 muscarinic, α2 adrenergic, D2 dopaminergic, histamine, 5HT1A, A1 adenosine, γ-aminobutyric acid (GABA)-B, μ, κ, and δ opioid, and somatostatin receptors (12, 13). Because many of these neurotransmitter systems are implicated in antinociception, we tested pharmacologically diverse analgesics in GIRK2 knockout mice by using the hot plate test. Because of known sex differences in sensitivity to drug-induced analgesia (14), we compared responses of male and female mice in all studies. In addition, endogenous analgesia also relies on these neuronal systems, and we studied the effects of mutation on basal antinociceptive latencies and on stress-induced analgesia.
Methods
Mice.
GIRK2-null mutant mice were generated, and their genotypes were identified by PCR analysis of tail DNA, as described by Signorini et al. (8). The genetic background of both GIRK2 mutant and wild-type control mice is 129/SvJ × C57BL/6J. Homozygous mice were obtained from the colony maintained by M.S. They were bred with wild-type mice (129/SvJ × C57BL/6J) to produce heterozygous mice. Heterozygous mating was maintained to generate GIRK2−/− and GIRK2+/+ littermates. Animals from F2-F3 generations of heterozygous mating were used in the experiments reported here. To minimize the possible effect of genetic background, only littermates were used in our experiments. Mice were group-housed, four to five to a cage based on sex and litter. Food and water were available ad libitum. The vivarium was maintained on a 12-h light/12-h dark cycle with light on at 7:00 a.m. The temperature and humidity of the room were maintained at 20°C and 50%, respectively. All experiments were performed near the midpoint of the light phase of the light/dark cycle (12:00–16:00) to minimize circadian effects of analgesic sensitivity (15). Principles of laboratory animal care of the National Institutes of Health were followed.
Analgesia.
Antinociceptive responses were measured as hot plate response latency (52.5°C) after drug treatment. The “response” was defined by the animal either licking the forepaws or hindpaws or flicking the hindpaws. In these studies, the most prominent response was forepaw licking. To avoid tissue damage, we exposed the animals to the hot plate (Columbus Instruments, Columbus, OH) for a maximum of 60 sec. In all experiments, subjects were initially tested for baseline latency, and the latency was tested again after drug injection. Data are reported as percentages of this maximum response time, which was determined from each individual mouse's basal response, the response after drug treatment, and the imposed maximum cutoff time with the following calculation (16): 100% × [(drug response time − basal response time)/(60 sec − basal response time)] = % maximum possible effect.
For stress-induced analgesia, a baseline hot plate latency was determined, and mice were subjected to a 5-min forced swim in water maintained at either 10 or 22°C. The swim was carried out in a cylindrical glass container 22.5 cm in diameter and 27.5 cm in height. The depth of the water was ≈20 cm, so that escape was impossible. Water temperature was monitored carefully by an experimenter, and maintained at ±1°C by the addition of ice or warm water. Upon completion of the swim, mice were towel-dried and placed in a towel-lined cage. They were retested on the hot plate at 5, 15, and 30 min after swimming.
Drugs.
Clonidine hydrochloride, oxotremorine sesquifumarate, nicotine, R(+) baclofen hydrochloride, ketamine, WIN 55,212-2, and ethanol were purchased from commercial sources (Research Biochemicals, Natick, MA; Sigma; Aaper Alcohol and Chemical, Shelbyville, KY). All solutions were prepared fresh daily with saline solution for injections (Baxter Scientific Products, McGaw Park, IL). WIN 55,212-2 was dissolved in dimethyl sulfoxide (Sigma) and then diluted in Chemophor EL (Sigma) and saline (final concentrations, 15% DMSO and 15% Chemophor EL). Clonidine and baclofen (10 ml/kg) were injected i.p. 30 min before testing. Ethanol (20 ml/kg) and ketamine (10 ml/kg) were injected i.p. 5 min before testing. Nicotine and oxotremorine (5 ml/kg) were injected s.c. 5 min or 30 min before testing, respectively. WIN 55,212-2 (10 ml/kg) was injected i.p. 15 min before testing. Control animals were injected with saline or a mixture of DMSO, Chemophor EL, and saline.
Statistical Analysis.
Data are reported as the mean ± SEM value. The software program prizm iii (GraphPad, San Diego) was used throughout. To evaluate differences between groups, analysis of variance (one-way or two-way ANOVA), Bonferroni post hoc, Dunnett's post hoc, and Student's t tests were carried out.
Results
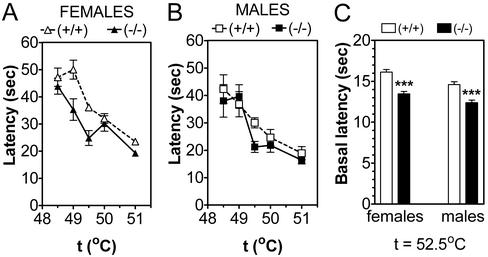
We first asked whether the GIRK2 mutation altered the responsiveness of mice to thermal stimuli, as might be expected of an endogenous mediator of pain sensitivity. To do this, we compared the hot plate response latency at different temperatures. Wild-type females were slightly less responsive to thermal stimuli than males, and this sex difference was abolished by deletion of the GIRK2 channel (Fig. 1 A and B). At low temperatures (49 and 49.5°C), GIRK2-null mutant females were more sensitive to thermal stimulus than wild-type females. When we combined data for (baseline) control responses of all mice tested at a hot plate temperature of 52.5°C (the temperature used for all subsequent experiments), females again showed slightly longer response latencies than males, and deletion of GIRK2 produced a decrease in the response latency (Fig. 1C).
Figure 1.
Deletion of GIRK2 channel produces a small thermal hyperalgesia, which is greater in females than males. (A) Response latency as a function of hot plate temperature for females, n = 10 for each genotype. There is a significant effect of the mutation. (B) Response latency as a function of hot plate temperature for males, n = 7–8 for each genotype. There is no significant effect of the mutation. (C) Response latencies for a large number of mice at a hot plate temperature of 52.5°C, n = 193–200 for each group. There is a significant effect of sex and mutation. Summary of statistics: (A and B) Wild-type mice showed an effect of gender (P = 0.0002) and temperature (P < 0.0001). GIRK2 KO showed no dependence on gender, only on temperature (P < 0.0001). Females showed effect of genotype (P < 0.0001) and temperature (P < 0.0001). Males showed dependence only on temperature (P < 0.0001) and not genotype. At 49°C (P < 0.001) and 49.5°C (P < 0.05), GIRK2 knockout females showed shorter latency than wild-type females (Bonferroni post hoc analysis). (C) There are strong effects of genotype (P < 0.0001) and gender (P < 0.0001).
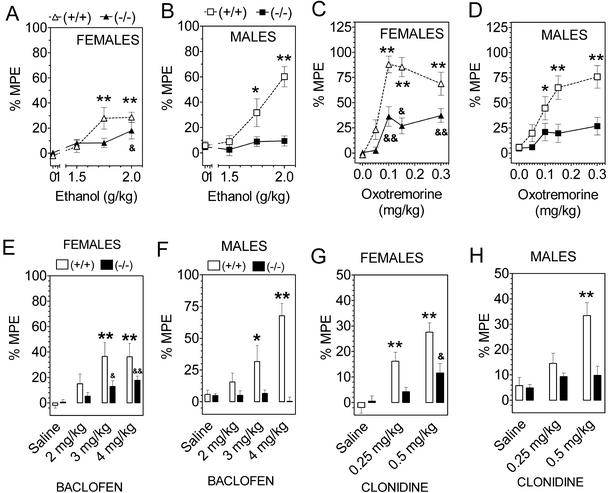
Ethanol produced hot plate antinociception in wild-type mice (Fig. 2 A and B). Mutant male mice lacking GIRK2 showed no analgesic action of ethanol, but female mice showed a small (P = 0.04) analgesic action with the highest dose of ethanol tested. The muscarinic cholinergic agonist oxotremorine produced a strong antinociceptive effect in wild-type mice, and this action was eliminated in males and reduced in females by the deletion of the GIRK2 gene (Fig. 2 C and D). GABAB receptor agonist, baclofen, produced antinociceptive effects that were completely eliminated in males but only partially reduced in the female GIRK2-null mutant mice (Fig. 2 E and F). In common with ethanol, oxotremorine, and baclofen, the α2 adrenergic agonist clonidine also produced antinociception that was eliminated in males and reduced in females by deletion of GIRK2 channels (Fig. 2 G and H).
Figure 2.
Deletion of GIRK2 channels reduces the antinociception (hot plate response given as percent of maximum possible effect) induced by ethanol, oxotremorine, baclofen, and clonidine. (A and B) Ethanol antinociception in females and males, n = 14–18 animals for each group. (C and D) Oxotremorine analgesia in females and males, n = 12–16 animals for each group. (E and F) Baclofen analgesia in females and males, n = 10–16 animals for each group. (G and H) Clonidine antinociception in females and males, n = 15–16 for each group. *, P < 0.05; **, P < 0.01; wild-type mice are different from saline control. &, P < 0.05; &&, P < 0.01; knockout mice are different from saline control (Dunnett's post hoc test). Summary of statistics. Ethanol, wild-type mice showed dependence on sex (P = 0.014) and treatment (P < 0.0001). Mutant mice showed no dependence on sex and only marginal dependence on treatment (P = 0.043). Oxotremorine, wild-type mice showed a trend for an effect of gender (P = 0.065); mutant mice had no effect of gender. Baclofen, wild-type mice show no gender dependence (P = 0.16), but mutant mice show a dependence on gender (P = 0.03). Clonidine, no dependence on gender in wild-type or mutant mice.
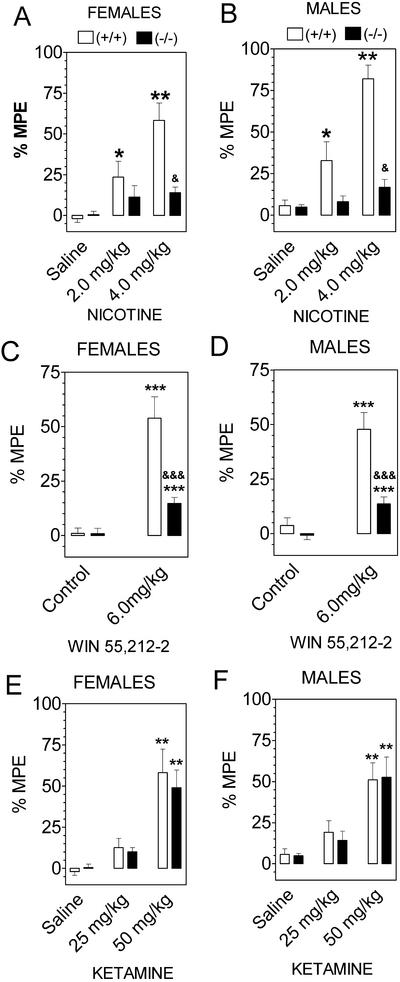
Nicotine produced antinociception in wild-type mice, and this effect was markedly reduced in null mutant mice, although some residual nicotine antinociception was seen in these mice (both male and female; Fig. 3 A and B). Likewise, the cannabinoid receptor agonist WIN 55,212 produced antinociceptive effects which were markedly reduced by deletion of GIRK2 channels (Fig. 3 C and D). In contrast to the other drugs, ketamine analgesia was not reduced in the GIRK2-null mutant mice (Fig. 3 E and F).
Figure 3.
GIRK2-null mice show marked reduction of nicotine and WIN 55,212-2-induced antinociception but no change in ketamine action (hot plate response given as a percent of maximum possible effect). (A and B) Nicotine antinociception in females and males, n = 10–16 for each group. (C and D) WIN 55,212-2 antinociception in females and males, n = 10–15 for each group. (E and F) Ketamine antinociception in females and males, n = 10–16 for each group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; wild-type mice are different from saline control. &, P < 0.05; &&&, P < 0.001; knockout mice are different from saline control (Dunnett's post hoc test). Summary of statistics: Nicotine, effect of sex in wild-type (P = 0.03) but not in GIRK2 knockout mice. WIN 55,212-2, no dependence on gender in wild-type or mutant mice.
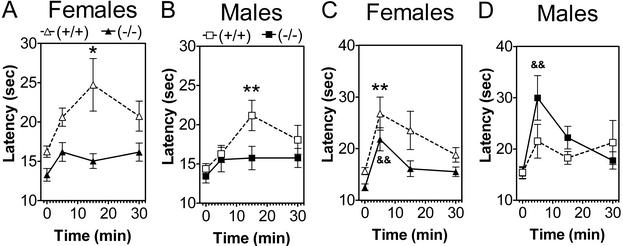
Because of the marked, but selective and gender-dependent, changes in drug-induced antinociception observed in these mutant mice, we asked whether GIRK2 is also a critical component of endogenous analgesic systems. A swim test in warm water increased response latency on the hot plate, and this analgesia was completely absent in the GIRK2-null mutant mice (Fig. 4 A and B). A swim test in cold water also produced analgesia in the wild-type mice, but the GIRK2 mutation produced only a slight reduction of analgesia in females (Fig. 4C) and increased the analgesia in males (Fig. 4D).
Figure 4.
SIA depends upon both sex and mutation. GIRK2-null mice do not display SIA after swimming in warm water but show increased SIA after swimming in cold water. (A and B) Warm water, wild-type females show greater SIA than males, and deletion of GIRK2 blocks SIA; n = 15–16 for each group. (C and D) Cold water, wild-type females and males show similar SIA, but deletion of GIRK2 reduces SIA in females and increases it in males; n = 15–16 for each group. *, P < 0.05; **, P < 0.01; wild-type mice are different from saline control. &&, P < 0.01; knockout mice are different from saline control (Dunnett's post hoc test). Summary of statistics: Warm water, wild-type mice show strong gender (P = 0.015) and time (P = 0.0006) dependence. GIRK2-null mutant mice show no gender or time dependence. Cold water, no gender dependence but dependence on time (P = 0.02) for wild-type mice. For GIRK2 mice, there are effects of gender (P = 0.002) and time (P < 0.0001).
Discussion
Our results, together with the companion publication by Mitrovic et al. (17), indicate that the GIRK2 channel is a common component for antinociception resulting from pharmacologically diverse agents, as well as some endogenous mediators of pain sensitivity. This result is somewhat surprising in view of the diverse signal transduction mechanisms available to the drugs tested in this study and previous electrophysiological evidence suggesting multiple and, perhaps, distinct actions of these drugs on brain pain pathways (1, 2). However, our studies indicate a key role for G protein activation of GIRK channels in the action of multiple analgesic drugs, although we also provide evidence that a part of the analgesic response does not require GIRK, particularly in females. As reviewed by Mitrovic et al. (17), there are sex differences in nociceptive sensitivity and antinociceptive responses in humans as well as in mice, but little is known about the mechanisms underlying such differences. Although females were slightly more sensitive to thermal stimulus than males, these differences represent a very small difference in sensitivity threshold and do not affect observed differences in drug responses. Slight gender differences in thermal response at the temperature used for the experiments were obtained only with a very big sample size. We found that deletion of GIRK2 channels abolished the antinociceptive effects of ethanol, oxotremorine, baclofen, and clonidine in males but not in females, which suggests that females can employ non-GIRK pathways of antinociception more effectively than males.
It seems likely that opioid, α adrenergic, muscarinic cholinergic, GABAB, and cannabinoid receptors are coupled with GIRK2 in nociceptive pathways, and that this coupling is responsible for most of the analgesic actions of these agents. In contrast, we suggest that nicotine indirectly activates these pathways because of release of neurotransmitters, and that ethanol may have direct activating actions on GIRK2 (4, 18), although ethanol may also activate endorphin pathways (19, 20). Deletion of GIRK2 abolishes postsynaptic responses but allows a normal magnitude of GABAB receptor-mediated presynaptic inhibition at hippocampal synapses (7). Therefore, the marked reduction of antinociception that we found in GIRK2-null mutant mice likely indicates a postsynaptic site of action for these diverse classes of drugs. The only agent that we found produces strong antinociceptive effects in the absence of GIRK2 channels was ketamine, which probably acts by inhibiting _N_-methyl-d-aspartate (NMDA) receptors and, therefore, is completely independent of GIRK2 signaling. Data from other studies that support these proposals are reviewed below and in the companion paper (17).
Administration of muscarinic agonists produces pronounced analgesic effects (21). Endogenous M2 muscarinic receptors can activate heterologous GIRK channels (GIRK1/GIRK2 subunits) in rat sympathetic neurons (22). M2 muscarinic-null mutant mice showed greatly reduced antinociceptive effects of oxotremorine, as assessed in the hot plate and tail-flick tests (23). It is likely that muscarinic analgesia is mediated predominantly via the M2 receptor subtype, at least in the mouse. Therefore, the marked reduction of oxotremorine analgesia in mice deficient in GIRK2 is likely caused by blockade of signaling from muscarinic M2 receptors.
The GABAB agonist baclofen produces analgesia by actions at both spinal and supraspinal sites (for review, see ref. 24). GABAB receptors are known to activate G proteins, thereby enhancing inwardly rectifying potassium channels (25, 26) or suppressing calcium channels (27, 28). As noted above, the reduction of baclofen-induced antinociception in GIRK2-null mutant mice probably results from blockade of postsynaptic GABAB receptor signaling. The baclofen antinociceptive response that is preserved in female mutant mice is likely mediated via presynaptic GABAB receptors signaling through Ca+2 channels or another postsynaptic GIRK channel. Lusher et al. (7) showed in GIRK2 knockout mice that there was a residual postsynaptic current activated by baclofen. This residual current might be due to a GIRK3 channel subunit either alone or together with the GIRK1, which, although it is down-regulated in the mutant mice, is still present in small amounts (8).
All α2 adrenergic receptors couple, via pertussis toxin-sensitive Gi/Go proteins, to attenuation of adenylyl cyclase, suppression of voltage-gated Ca+2 channels, and activation of inwardly rectifying K+ channels (29). Previous studies (for review, see ref. 30) indicated clonidine-induced analgesia was produced primarily through activation of postsynaptic α2 receptors, and our results indicate that these receptors act through GIRK2 channels.
Cannabinoid agonists such as delta9-THC, WIN 55,212–2, and CP 55,940 produce a characteristic combination of hypothermia, analgesia, hypoactivity, and catalepsy (for a review, see ref. 31). The CB1 receptor from brain couples with GIRK1/GIRK4 channel after coexpression in Xenopus laevis oocytes (32, 33), but the role of GIRK channels in actions of CB1 in brain has yet to be determined. Reduction of WIN 55,212-2 analgesia in GIRK2-null mutant mice argues for coupling of CB1 receptors with the GIRK2 channel in vivo.
One of our most surprising results is that nicotine analgesia requires GIRK2. Nicotine exerts antinociceptive effects by interacting with nicotinic acetylcholine receptors (nAChRs), as indicated by the observation that mice lacking either the α4- or β2-subunits of the neuronal nAChR display a reduced antinociceptive effect of nicotine on the hot plate test (34). Although nicotinic receptors do not couple with GIRK channels, nicotine-induced antinociception is inhibited by intrathecal injection of pertussis toxin, indicating a role of G proteins (35). There is clear evidence that presynaptic nAChRs increase transmitter release initiated by axonal firing (for review, see ref. 36), and regulation of release of monoamine neurotransmitters including ACh and norepinephrine may be critical for nicotine-induced analgesia (for review, see ref. 37).
Stress-induced analgesia (SIA) has been widely used to investigate brain circuitry mediating endogenous pain inhibition. It appears that forced swims of mild severity (warm water and/or short duration) selectively recruit opioid analgesic mechanisms, whereas more severe swims (cold water and/or long duration) recruit nonopioid mechanisms (38, 39). Our data show that deletion of GIRK2 channel blocks mainly the opioid-dependent SIA, which is consistent with studies of morphine antinociception in these mice (17). In contrast, the nonopioid SIA was not changed in GIRK2−/− knockout mice. Furthermore, deletion of the GIRK2 channel increased the nonopioid SIA in male mice. This increase may be the result of blockade of the opioid component of analgesia, because an activation of opioid systems inhibits nonopioid analgesia (40). The nonopioid SIA is often attributed to activation of NMDA receptors (41), and intact ketamine analgesia in GIRK2-null mutant mice supports this hypothesis.
The majority of gene-targeted mice, including those used in this study, are developed on a mixed genetic background of inbred mouse strains C57BL/6J and strain 129. These strains differ in their responses to many drugs because of the possession of polymorphic alleles (42, 43). These differences are a potential confounding variable in the analysis of any gene-targeting experiment, because a difference in response between wild-type and null allele mice may simply reflect the different underlying genetic backgrounds of the mouse strains in use rather than a difference caused by the targeted mutation. For this reason, it is useful to compare the responses of wild-type and null allele mice to the pattern of responses found in the parental mouse strains used. In the present study, genes linked to the GIRK2−/− mutation would be derived from strain 129/SvJ, whereas genes linked to the wild-type GIRK2 gene would stem from C57BL/6J. In all reports of thermal nociception, C57BL/6 mice are more sensitive than 129 mice, showing shorter latencies to make a withdrawal response (44). However, it was shown that C57BL/6 mice are relatively insensitive to a number of analgesic drugs, but the 129 strain is rather sensitive to analgesics (for review, see ref. 45). These data clearly show that the reduction or complete blockade of analgesic effects of drugs in GIRK2-null mutant mice observed in the present study do not result from hitchhiking donor genes from the 129 strain.
It is important to note that this study and that of Mitrovic et al. (17) were performed independently at different sites by using different colonies of mutant mice and somewhat different methodologies, yet the overall conclusions are remarkably consistent. This result is reassuring in view of a recent study suggesting that some behavioral and genetic effects are difficult to replicate across laboratories (46) and is another indicator of the robust role of GIRK2 channels in antinociception and sex differences in drug action.
In summary, our data, together with those of Mitrovic et al. (17), show that GIRK2 channels are critical targets for transduction of central analgesia mediated by a variety of neurotransmitter systems and suggest that development of agents that directly and selectively activate GIRK2 channels may provide a new approach for the treatment of pain.
Acknowledgments
We thank Drs. Alan Basbaum and Igor Mitrovich for sharing their unpublished data and providing many helpful discussions, Dr. John Mihic for suggestions on the manuscript, and Virginia Bleck for genotyping expertise. This work was supported by funds from the Texas Commission on Alcohol and Drug Abuse and National Institutes of Health/National Institute of Alcohol Abuse and Alcoholism Grants AA06399 and AA13520.§
Abbreviations
GIRK
G protein-coupled inwardly rectifying potassium channel
GABA
γ-aminobutyric acid
SIA
stress-induced analgesia
Footnotes
§
A preliminary report of these results was presented at the symposium “Neurobiology of Alcoholism and Addiction,” April 25–26, 2001, San Francisco, CA.
References
- 1.Vaughan C W, Ingram S L, Connor M A, Christie M J. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan C W, Connor M, Bagley E E, Christie M J. Mol Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- 3.Ikeda K, Kobayashi T, Kumanishi T, Niki H, Yano R. Neurosci Res. 2000;38:113–116. doi: 10.1016/s0168-0102(00)00144-9. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, Kumanishi T. Nat Neurosci. 1999;2:1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- 5.Patil N, Cox D R, Bhat D, Faham M, Peterson A S. Nat Genet. 1995;11:126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- 6.Slesinger P A, Stoffel M, Jan Y N, Jan L Y. Proc Natl Acad Sci USA. 1997;94:2210–2217. doi: 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luscher C, Jan L Y, Stoffel M, Malenka R C, Nicoll R A. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 8.Signorini S, Liao Y I, Duncan S A, Jan L Y, Stoffel M. Proc Natl Acad Sci USA. 1997;94:923–927. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blednov Y A, Stoffel M, Chang S R, Harris R A. Physiol Behav. 2001;74:109–117. doi: 10.1016/s0031-9384(01)00555-8. [DOI] [PubMed] [Google Scholar]
- 10.Blednov Y A, Stoffel M, Chang S R, Harris R A. J Pharmacol Exp Ther. 2001;298:521–530. [PubMed] [Google Scholar]
- 11.Blednov Y A, Stoffel M, Cooper R, Wallace D, Mane N, Harris R A. Psychopharmacology. 2002;159:370–378. doi: 10.1007/s00213-001-0937-6. [DOI] [PubMed] [Google Scholar]
- 12.North A. Br J Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hille B. Neuron. 1992;9:87–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- 14.Kest B, Sarton E, Dahan A. Anesthesiology. 2000;93:639–647. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- 15.Kavaliers M, Hirst M. Brain Res. 1983;279:387–393. doi: 10.1016/0006-8993(83)90216-0. [DOI] [PubMed] [Google Scholar]
- 16.Belknap J K, Noordewier B, Lame M. Physiol Behav. 1989;46:69–74. doi: 10.1016/0031-9384(89)90324-7. [DOI] [PubMed] [Google Scholar]
- 17.Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan L Y, Basbaum A I. Proc Natl Acad Sci USA. 2003;100:271–276. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewohl J M, Wilson W R, Mayfield R D, Brozowski S J, Morrisett R A, Harris R A. Nat Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- 19.Boada J, Feria M, Sanz E. Pharmacol Res Commun. 1981;13:673–678. doi: 10.1016/s0031-6989(81)80055-0. [DOI] [PubMed] [Google Scholar]
- 20.Pohorecky L A, Shah P. Life Sci. 1987;41:1289–1295. doi: 10.1016/0024-3205(87)90208-6. [DOI] [PubMed] [Google Scholar]
- 21.Iwamoto E T, Marion L. J Pharmacol Exp Ther. 1993;266:329–338. [PubMed] [Google Scholar]
- 22.Fernandez-Fernandez J M, Wanaverbecq N, Halley P, Caulfield M P, Brown D A. J Physiol. 1999;515:631–637. doi: 10.1111/j.1469-7793.1999.631ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond D L. In: The Pharmacology of Pain. Dickenson A, Besson J M, editors. Heidelberg: Springer; 1997. pp. 361–384. [Google Scholar]
- 25.Andrade R, Malenka R C, Nicoll R A. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- 26.Sodickson D L, Bean B P. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolphin A C, Scott R H. Br J Pharmacol. 1986;88:213–220. doi: 10.1111/j.1476-5381.1986.tb09489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mintz I M, Bean B P. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- 29.Limbird L E. FASEB J. 1988;2:2686–2695. doi: 10.1096/fasebj.2.11.2840317. [DOI] [PubMed] [Google Scholar]
- 30.Millan M J. In: The Pharmacology of Pain. Dickenson A, Besson J M, editors. Heidelberg: Springer; 1997. pp. 385–446. [Google Scholar]
- 31.Chaperon F, Thiebot M H. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- 32.McAllister S D, Griffin G, Satin L S, Abood M E. J Pharmacol Exp Ther. 1999;291:618–626. [PubMed] [Google Scholar]
- 33.Ho B Y, Uezono Y, Takada S, Takase I, Izumi F. Recept Channels. 1999;6:363–374. [PubMed] [Google Scholar]
- 34.Marubio L M, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d'Exaerde A, Huchet M, Damaj M I, Changeux J P. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- 35.Damaj M I, Welch S P, Martin B R. Pharmacol Biochem Behav. 1994;48:37–42. doi: 10.1016/0091-3057(94)90494-4. [DOI] [PubMed] [Google Scholar]
- 36.Vizi E S, Lendvai B. Brain Res Brain Res Rev. 1999;30:219–235. doi: 10.1016/s0165-0173(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 37.Furst S. Brain Res Bull. 1999;48:129–141. doi: 10.1016/s0361-9230(98)00159-2. [DOI] [PubMed] [Google Scholar]
- 38.Terman G W, Morgan M J, Liebeskind J C. Brain Res. 1986;372:167–171. doi: 10.1016/0006-8993(86)91472-1. [DOI] [PubMed] [Google Scholar]
- 39.Mogil J S, Sternberg W F, Balian H, Liebeskind J C, Sadowski B. Physiol Behav. 1996;59:123–132. doi: 10.1016/0031-9384(95)02073-x. [DOI] [PubMed] [Google Scholar]
- 40.Grisel J E, Fleshner M, Watkins L R, Maier S F. Pharmacol Biochem Behav. 1993;45:161–172. doi: 10.1016/0091-3057(93)90100-8. [DOI] [PubMed] [Google Scholar]
- 41.Vaccarino A L, Clavier M C. Pharmacol Biochem Behav. 1997;56:435–459. doi: 10.1016/s0091-3057(96)00235-3. [DOI] [PubMed] [Google Scholar]
- 42.Phillips T J, Hen R, Crabbe J C. Psychopharmacology. 1999;147:5–7. doi: 10.1007/s002130051128. [DOI] [PubMed] [Google Scholar]
- 43.Gerlai R. Trends Neurol. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 44.Mogil J S, Wilson S G, Bon K, Lee S E, Chung K, Raber P, Pieper J O, Hain H S, Belknap J K, Hubert L, et al. Pain. 1999;80:83–93. doi: 10.1016/s0304-3959(98)00196-1. [DOI] [PubMed] [Google Scholar]
- 45.Lariviere W R, Chesler E J, Mogil J S. J Pharmacol Exp Ther. 2001;297:467–473. [PubMed] [Google Scholar]
- 46.Crabbe J C, Wahlsten D, Dudek B C. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]