The transcription factors MTF-1 and USF1 cooperate to regulate mouse metallothionein-I expression in response to the essential metal zinc in visceral endoderm cells during early development (original) (raw)
Abstract
During early development of the mouse embryo, expression of the metallothionein-I (MT-I) gene is heightened specifically in the endoderm cells of the visceral yolk sac. The mechanisms of regulation of this cell-specific pattern of expression of metallothionein-I are unknown. However, it has recently been shown that MTF-1, functioning as a metalloregulatory transcription factor, activates metallothionein genes in response to the essential metal zinc. In contrast with the metallothionein genes, MTF-1 is essential for development; null mutant embryos die due to liver degeneration. We report here that MTF-1 is absolutely essential for upregulation of MT-I gene expression in visceral endoderm cells and that optimal expression also involves interactions of the basic helix–loop–helix upstream stimulatory factor-1 (USF1) with an E-box1-containing sequence at –223 bp in the MT-I promoter. Expression of MT-I in visceral endoderm cells was dependent on maternal dietary zinc. Thus, the essential metal, zinc, apparently provides the signaling ligand that activates cell- specific MT-I expression in visceral endoderm cells.
Keywords: endoderm/metallothionein-I/MTF-1/USF/zinc
Introduction
The metallothioneins (MTs) are a family of metal-binding proteins that have been conserved during evolution. In mice, four MT genes have been identified (Palmiter et al., 1993a). MT-I and -II are coordinately expressed in many cell types and are highly inducible (Searle et al., 1984). In contrast, MT-III expression has been detected only in subpopulations of cells in the brain (Palmiter et al., 1993a), the maternal deciduum (Liang et al., 1996) and several reproductive tissues (Moffatt and Seguin, 1998), and MT-IV expression has been detected in subpopulations of cells in stratified squamous epithelia (Palmiter et al., 1993a) and in the maternal deciduum (Liang et al., 1996). Neither MT-III nor MT-IV appears to be induced by agents (i.e. zinc and cadmium) that induce MT-I and -II (Palmiter et al., 1993a; Liang et al., 1996; Moffatt and Seguin, 1998).
Distinct cell-specific activation of mouse MT-I/II also occurs during embryonic development (Andrews et al., 1987a, 1993; Karasawa et al., 1991). Initially, MT-I and -II are highly expressed specifically in the endoderm of the yolk sac (Andrews et al., 1984). Visceral endoderm cells are the third cell type to differentiate from the inner cell mass (Gardner, 1982). Together with a single inner layer of mesoderm cells, they form the visceral yolk sac, which surrounds the developing embryo until late in pregnancy (Padykula et al., 1966). Visceral endoderm cells form a secretory epithelium responsible for the synthesis of the major serum and amniotic fluid proteins, such as α-fetoprotein and transferrin (Dziadek and Adamson, 1978; Janzen et al., 1982). The visceral yolk sac is also the first site of hematopoiesis (De la Chapelle et al., 1969) and plays a role in transferring maternal nutrients (e.g. amino acids and vitamins) and proteins (e.g. IgG) into the embryonic environment (Padykula et al., 1966).
There is no definitive information on the mechanisms that regulate the cell-specific activity of MT. However, many different agents and stresses can induce MT genes (Miles et al., 2000) and the mouse MT-I proximal promoter is structurally complex (Stuart et al., 1985). Metal ion regulation of MT expression is mediated by metal response elements (MREs). MRE-binding transcription factor-1 (MTF-1), a six zinc-finger protein of the Cys2His2 family, binds directly and specifically to MREs (Westin and Schaffner, 1988; Radtke et al., 1993; Koizumi et al., 1999) and regulates metal-induced and basal MT expression in cultured cells (Heuchel et al., 1994). MTF-1 is thought to function as a specific sensor of free cytoplasmic zinc (for review see Andrews, 2000). The mouse MT-I promoter contains five MREs located in the proximal 250 bp (Stuart et al., 1985) and binding sites for the transcription factors upstream stimulatory factor (USF), Sp1, Nrf2 (Dalton et al., 1994; Venugopal and Jaiswal, 1996; Li et al., 1998) and perhaps CCAAT/enhancer binding protein (C/EBP)-like proteins (Aniskovitch and Jacob, 1997).
Several transcription factors modulate MT-I gene expression (Carthew et al., 1987; Dalton et al., 1994; Li et al., 1998). Of particular importance here is USF1. USFs are basic helix–loop–helix (bHLH)–Zip proteins that form DNA-binding homodimers and heterodimers (Gregor et al., 1990; Sirito et al., 1992; Viollet et al., 1996); two USF genes (USF1 and USF2) have been described (Gregor et al., 1990; Sirito et al., 1994). The proximal promoter of the mouse MT-I gene has two binding sites for USF (Carthew et al., 1987; Li et al., 1998). One site overlaps an antioxidant response element (ARE; Dalton et al., 1994). This composite element (USF/ARE) participates in oxidant induction of MT gene expression by stress and cadmium (Dalton et al., 1994; Li et al., 1998) and can regulate basal expression of the gene in cultured cells (Carthew et al., 1987; Dalton et al., 1994). The second and higher-affinity USF-binding site is an E-box (E-box1) sequence CACATG (Li et al., 1998), but no function has been ascribed to this site.
The present study provides the first molecular explanation for a cell-specific pattern of expression of mouse MT-I during early development. Unexpectedly, it was found that MTF-1 is absolutely essential for expression of MT-I in the endoderm cells of the visceral yolk sac. Remarkably, the essential metal, zinc, appears to provide the signaling ligand for activation of this gene; this, in turn, suggests that zinc is not equally distributed and/or utilized among tissues. We also report the novel finding that MTF-1 functions in cooperation with USF1 (and perhaps other factors), which interact with the E-box1 sequence noted above, to direct this cell-specific pattern of MT-I expression.
Results
Visceral endoderm-specific MT-I expression requires the proximal promoter in transgenic embryos
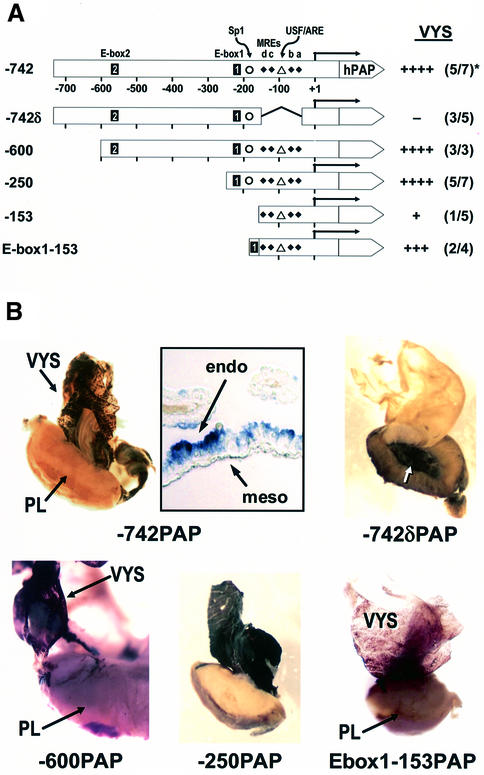
A series of mutations in the MT-I promoter was examined for the ability to direct cell-specific expression of the hPAP reporter in transgenic mouse embryos (Figure 1A). hPAP encodes an alkaline phosphatase that, unlike endogenous mouse PAP, is heat-stable. Microinjected eggs were transferred into the oviduct of recipient females; on day 14 of pregnancy, embryos were harvested for PCR genotype determination and the visceral yolk sac/placenta was fixed and analyzed by histochemical staining of hPAP. Only tissues from transgenic embryos contained detectable hPAP staining.
Fig. 1. Elements in the proximal promoter of mouse MT-I cooperate to direct high level hPAP reporter expression in the visceral yolk sac. (A) Diagrammatic representation of the mouse MT-I promoter–hPAP fusion constructs. The MT-I promoter provided the transcription start point (+1, straight arrow) and +66 bp of untranslated region, in addition to the base pairs of upstream promoter as indicated (Dalton et al., 1994). hPAP provided the translation start codon and complete coding sequence, and contains several introns (Knoll et al., 1988). A second polyadenylation signal is placed downstream of hPAP (Lin and Culp, 1991). Elements in the –742 bp promoter are as follows: E-box2 and E-box1, CAnnTG (Liang et al., 1996; Li et al., 1998); Sp1 GC-box (Briggs et al., 1986); MREs a–d, metal response elements (Stuart et al., 1984); USF/ARE, USF-binding site (not an E-box) overlapping an antioxidant response element (Dalton et al., 1994); TATA, TATA box. The –742δ promoter has a 108 bp region (bases –43 to –150 inclusive) deleted. The E-box1:–153 promoter has a single, forward-oriented copy of E-box1 inserted just upstream of the –153 bp MT-I promoter. hPAP, human placental alkaline phosphatase reporter; VYS, visceral yolk sac. The pattern of staining in the visceral yolk sac was assessed qualitatively [see (B)]: ++++, staining was always very dark and throughout the yolk sac endoderm; –, no cells stained in the visceral yolk sac in any transgenic embryos; +++, staining was dark in most cells; +, staining was weak and mosaic in the visceral yolk sac and detected in only one embryo. *Number of F0 transgenic conceptuses that expressed the hPAP gene/total number of transgenic embryos detected by PCR. (B) Histochemical detection of hPAP in F0 transgenic embryos. Promoter constructs are described above. VYS, visceral yolk sac; PL, placenta. Whole mounts of placentae were cross-sectioned before fixation and staining. The insert by the –742_PAP_ whole mount is a photomicrograph of a thin section of the visceral yolk sac that shows hPAP expression in visceral endoderm (‘endo’) cells, but not in mesodermal cells (‘meso’).
The MT-I promoter (742 bp) directed active expression of hPAP specifically in the endoderm cells of the visceral yolk sac (Figure 1B; insert). In contrast, only a few cells in the placenta or associated membranes were weakly stained (Figure 1B). Visceral endoderm expression was dependent on a 108 bp region (bases –43 to –150 inclusive) of the proximal 742 bp of the promoter (Figure 1; compare –742_PAP_ with –742δ_PAP_) (Figure 1B). This 108 bp region in –153_PAP_ did not exhibit strong activity alone, and hPAP staining was mosaic and weak in the visceral yolk sac (not shown). This suggests that promoter elements cooperate to direct expression in the visceral endoderm.
MT-I expression requires multiple promoter elements in transgenic embryos
Deletion analysis of the promoter demonstrated that the proximal 250 bp exhibited strong, endoderm-specific activity (Figure 1). A high-affinity binding site for USF is located at –223 bp in this promoter (Li et al., 1998), so we investigated whether this element was involved in cooperation with the proximal 153 bp. Addition of this element to the proximal –153 bp promoter restored high-level expression of hPAP in the visceral endoderm in two of the four transgenic embryos examined (Figure 1B). Although a limited number of embryos was examined, these results suggest a role for this element in endoderm-specific MT-I gene expression.
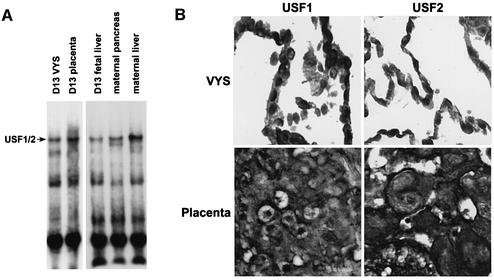
E-box1-binding activity in the visceral yolk sac was examined by electrophoretic mobility shift assay (EMSA; Figure 2A). The E-box1-binding complex detected was similar in all tissues examined and was indistinguishable from that obtained in cultured mouse cells (Li et al., 1998). Competition EMSA and supershift assays using antisera against USF1 and USF2 confirmed that this binding activity involves USF1 and USF2 and requires the E-box core sequence (data not shown; see Li et al., 1998). Immunohistochemical localization of USF1 and USF2 also confirmed the presence of these proteins in the nuclei of visceral endoderm cells (Figure 2B). Sp1 (Figure 3B) and MTF-1 DNA-binding activities were also detected in nuclear extracts from these cells (data not shown).
Fig. 2. Visceral endoderm cell nuclei contain USF1 and USF2, which can bind in vitro to E-box1. (A) Nuclear proteins were prepared from day 13 visceral yolk sac, placenta and fetal liver, as well as from the maternal liver and pancreas. Nuclear proteins were analyzed by EMSA (Dalton et al., 1996c, 1997; Li et al., 1998) for E-box1-binding and for Sp1- and MTF-1-binding activities (data not shown). The E-box1-binding complex (USF1/2) contained USF1 and USF2 and was dependent on the E-box core-binding sequence (data not shown), as described (Li et al., 1998; Sirito et al., 1998). (B) Visceral yolk sacs and placentae were fixed and paraffin sections were analyzed for USF1 and USF2 proteins using immunohistochemistry. Nuclear staining was detected in the visceral yolk sac and placenta.
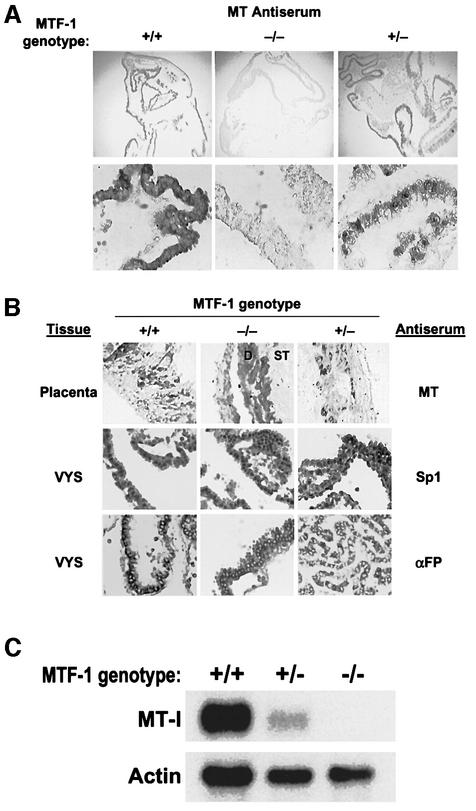
Fig. 3. Homozygous knockout of MTF-1 abolishes MT expression in the visceral endoderm. Heterozygous MTF-1 knockout mice were inbred and embryos were harvested at day 12 of gestation. The MTF-1 genotype of each embryo was determined by PCR. +/+, two normal MTF-1 alleles; +/–, heterozygous for MTF-1 knockout allele; –/–, homozygous for MTF-1 knockout alleles. The visceral yolk sacs and placentae were fixed and analyzed individually for MT, Sp1 or α-fetoprotein (αFP) using immunohistochemistry (A and B). D, deciduum basalis, ST, spongiotrophoblast layer. (A) Low- (100×) and high- (200×) magnification views of the immunohistochemical staining of MT in the visceral yolk sac. (C) Total RNA was extracted from the visceral yolk sac (four embryos) and analyzed by northern blot hybridization using MT-I and actin cRNA probes simultaneously on the same blot.
Visceral endoderm-specific MT-I expression is dependent on MTF-1 and USF1
The multiple MREs in the MT-I promoter are within the 108 bp region that is essential for expression in the visceral yolk sac. This suggested that MTF-1 may play a role in transcriptional activation of this gene in endoderm cells. MTF-1 knockout mice were used to test this possibility. Although homozygous _MTF-1_–/– knockout embryos die on day 14, they develop normally until then. Heterozygous MTF-1 knockout (MTF-1+/–) mice were inbred and the embryos were examined at day 12 of pregnancy. The genotype of each embryo was determined by PCR and MT protein was immunolocalized in the visceral yolk sac/placenta (Figure 3A). MT immunostaining was strong in the visceral endoderm from normal and heterozygous embryos, but was absent from the visceral endoderm of all of the homozygous embryos.
The loss of MT protein from the visceral endoderm of the homozygous MTF-1 knockout embryos was specific (Figure 3B). Immunohistochemical detection of Sp1, another zinc-finger transcription factor, was unaffected, as was immunostaining for α-fetoprotein, the major visceral endoderm secretory protein (Figure 3B). In addition, MT-immunoreactive cells in the outer placenta, probably decidua basalis cells, were detected in all the samples.
Relative levels of MT-I mRNA were determined in total yolk sac RNA from normal heterozygous and homozygous MTF-1 knockout embryos. Northern blotting revealed high levels of MT-I mRNA in the yolk sac RNA from normal embryos and diminished levels in that from heterozygous embryos. In contrast, little or none was detected in yolk sac RNA from homozygous embryos (Figure 3C). Actin mRNA levels were similar in these RNA samples.
The finding that E-box1 may play a role in regulating endoderm-specific expression of MT-I suggested that USF family members could be involved in this process (Li et al., 1998). USF1 and USF2 have some overlapping biological activities (Sirito et al., 1998) and both are expressed in the visceral endoderm (Figure 2B). The in vivo roles of these proteins were examined using USF1 or USF2 knockout mice (Figure 4). Homozygous USF1 knockout mice are viable and fertile as adults (Sirito et al., 1998). In contrast, ∼50% of USF2 null mutant mice die at birth (Sirito et al., 1998). Nearly all USF2 knockout males die as they become sexually mature and the few survivors are often infertile (M.Sawadogo and M.Sirito, unpublished data). Furthermore, embryos with targeted mutations of both the USF1 and USF2 genes die in utero (Sirito et al., 1998). Homozygous null mutant USF1 mice, or control mice with the same genetic background, were inbred and tissues were harvested at day 14 of pregnancy. Heterozygous USF2+/– mice were inbred and the littermates were individually genotyped at day 14 of pregnancy. Samples of identical genotype were pooled. Relative levels of MT-I mRNA were determined in total yolk sac RNA from control and homozygous USF1 knockout embryos (Figure 4A) and from USF2 (Figure 4B) knockout and normal embryo littermates. MT-I mRNA levels (relative to β-actin) were reduced 4.0-fold in yolk sacs from homozygous USF1 knockout embryos relative to those from control embryos (Figure 4A), but were unaffected in the placenta (1.1-fold increase). In contrast, MT-I mRNA levels were essentially unchanged (1.8-fold increase) in the yolk sacs from homozygous USF2 knockout embryos (Figure 4B). Actin mRNA levels were similar in these yolk sacs. Although there was some degradation of this mRNA in the USF2 knockout sample, this did not affect interpretation of the results (Figure 4B). The relative abundance of MT-I mRNA in the visceral yolk sac total RNA was identical among each of the control samples examined (MTF+/+ littermates; USF1+/+ genetic control and USF2+/+ littermates). This suggests that MT-I expression in the visceral yolk sac is unaffected by the genetic background of the mouse strains in these experiments.
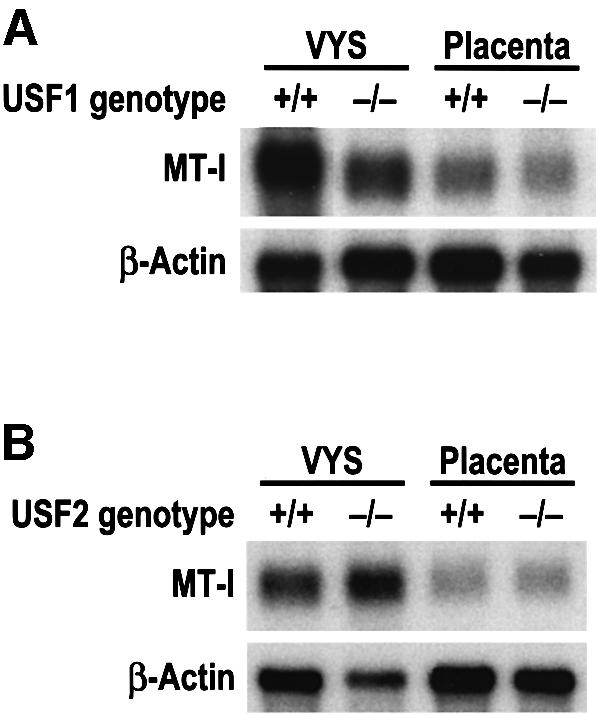
Fig. 4. Homozygous knockout of USF1, but not USF2, attenuates MT expression in the visceral endoderm. Heterozygous USF1 (A) or USF2 (B) knockout and control embryos and tissues were harvested at day 14 of gestation. +/+, embryo with two normal USF alleles; –/–, embryo homozygous for USF knockout alleles. Total RNA was extracted from the visceral yolk sac (VYS) and placenta (six per genotype) and analyzed by northern blot hybridization using MT-I and actin cRNA probes simultaneously on the same blot. Hybrids were quantified by radioanalytical analysis of the membranes. These RNA samples were northern blotted at least three separate times with similar results.
High-level MT-I expression in visceral endoderm is regulated by maternal dietary zinc
The effects of maternal dietary zinc deficiency during pregnancy on MT-I expression in the visceral endoderm were examined (Figure 5). Pregnant females were fed a normal diet or a zinc-deficient diet beginning on day 8 of pregnancy. RNA was extracted from visceral yolk sacs (∼20 per sample) and placentae were collected at days 12 and 14 of pregnancy and analyzed by northern blotting (Figure 5A) with quantitative radio-analytic analysis of the membranes (Figure 5B). The results demonstrated that MT-I mRNA levels were reduced by ∼40% on day 12 and by >20-fold on day 14 in mice fed the zinc-deficient diet (Figure 5). MT protein was reduced >10-fold, based on a [109Cd]hemoglobin exchange assay (Andrews and Geiser, 1999) (data not shown). Zinc deficiency had little effect on placental MT-I mRNA levels (Figure 5).
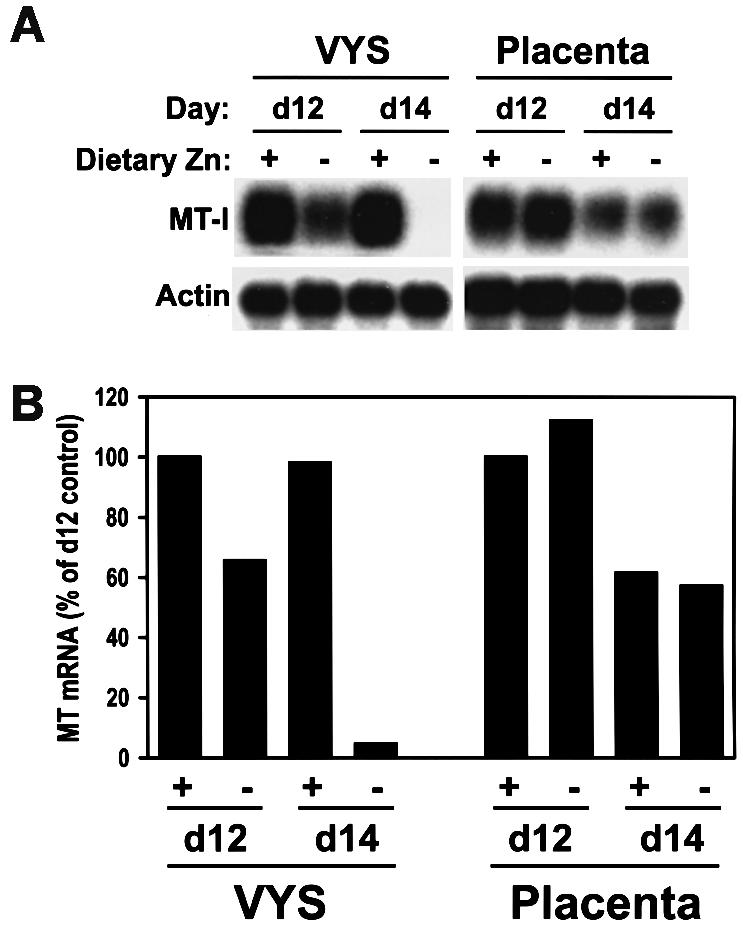
Fig. 5. Dietary zinc modulates MT-I expression in the visceral yolk sac. CD-1 mice were exposed to dietary zinc deficiency beginning on day 8 of pregnancy and the effects on MT-I mRNA levels in the visceral yolk sac and placenta were determined on days 12 and 14. Under these conditions, ∼20% of the embryos developed abnormally by day 14, but there was no increase in embryo mortality (Andrews and Geiser, 1999). Total RNA was extracted from visceral yolk sacs (VYS) and placentae (∼20 per sample) collected from mice fed a zinc-adequate (Zn-A) diet (+, containing 50 p.p.m. Zn2+) or a zinc-deficient (Zn-D) diet (−, containing 1 p.p.m. Zn2+). RNA was analyzed by northern blot hybridization using mouse MT-I and actin cRNA probes simultaneously. Hybrids were detected by autoradiography (A) and quantified by radioanalytic analysis of the membrane (B).
Discussion
It has been known for >15 years that the mouse MT-I and -II genes are expressed at high levels only in a few, specific cell types in the developing conceptus (Andrews et al., 1987a, 1993; Karasawa et al., 1991; Liang et al., 1996). Although these genes are expressed at basal levels and are inducible by metal ions as early as the blastocyst stage of development (Andrews et al., 1991), the mechanisms regulating cell-specific patterns of heightened expression during development remain undiscovered. The endoderm cells of the visceral yolk sac are the first cell type in the developing mouse embryo to activate the MT-I gene naturally and dramatically. This pattern of developmental activation of mouse MT-I gene expression is mimicked during the in vitro differentiation of F9 embryonal carcinoma cells along the visceral endoderm pathway (Andrews et al., 1984). It was felt, therefore, that visceral endoderm expression of MT is a preprogrammed developmental event independent of maternal signals. In the present study we demonstrated that the transcription factor MTF-1, in cooperation with USF1, is essential for this cell-specific pattern of MT-I expression. MTF-1 is also essential for zinc and cadmium induction, as well as for basal expression of mouse MT-I in embryonic stem cells (Heuchel et al., 1994) and in mouse embryo fibroblasts (Koizumi et al., 1999), but these cell types express MT-I at high levels under normal culture conditions. Thus, it was unexpected that MTF-1 would play such a dominant role in regulating MT-I expression in the visceral endoderm. However, this finding, and the demonstration that dietary zinc status modulates endoderm expression of MT-I, strongly suggests that zinc is, in fact, the maternally derived factor responsible for this upregulation of MT-I. Zinc was also present in significant concentrations in the medium used to culture F9 cells.
The finding that maternal dietary zinc deficiency dramatically reduces the expression of MT-I in visceral endoderm cells is consistent with a prominent role of MTF-1 as a metalloregulatory sensor of zinc. The zinc-finger domain of MTF-1 has been highly conserved during evolution (Auf der Maur et al., 1999), and the DNA-binding activity of native and recombinant MTF-1 is reversibly modulated by zinc interactions with the finger domain (Heuchel et al., 1994; Dalton et al., 1997). The zinc-fingers are heterogeneous in function and at least two exhibit low-affinity binding of zinc (Chen et al., 1998, 1999). Dissection of the zinc-finger domain revealed that finger 1 of MTF-1 constitutes a unique metal-sensing domain that, in cooperation with the transactivation domains, produces a zinc-sensing metalloregulatory transcription factor (Bittel et al., 2000).
The question remains as to why MTF-1 activity is zinc-activated naturally in visceral endoderm cells. It seems likely that the endoderm is particularly rich in zinc, perhaps due to an enhanced zinc-uptake system. The visceral yolk sac may play a role in the movement of zinc between the maternal and embryonic compartments. It has been shown to participate in the uptake of vitamins, amino acids and peptides (Padykula et al., 1966). Although little is known about mechanisms of zinc uptake in mouse cells (Eng et al., 1998), we recently demonstrated that the mouse ZnT1 gene, which encodes a transmembrane protein that functions in the efflux of zinc (Palmiter and Findley, 1995), is highly expressed in the visceral yolk sac (Langmade et al., 2000). MTF-1 also modulates the level of expression of this gene in response to zinc (Langmade et al., 2000). Thus, MTF-1 may coordinate the expression of a cohort of genes involved in zinc homeostasis during development.
Another possible explanation for MTF-1 activity in the visceral endoderm is that, in these cells, there may be a unique set of co-activator proteins that facilitate activation of the MT-I gene. A novel finding in this study was that optimal expression of the MT-I promoter in the visceral endoderm required cooperative interactions between MTF-1 and USF1. Neither the proximal promoter (MREs) nor the distal promoter (E-box1) functioned alone, and mutations of the USF1 gene significantly attenuated MT-I gene expression in visceral endoderm cells. Previous studies have implicated USF in the regulation of basal expression (Stuart et al., 1984; Carthew et al., 1987) and cadmium induction of the mouse MT-I gene (Li et al., 1998). However, the E-box1 region of the MT-I promoter is constitutively occupied in vivo in several cell-types; in vivo footprinting studies and in vitro assays suggest that USF binds to this element (Li et al., 1998). However, E-box1 has little effect on the basal expression or metal induction of the MT-I promoter in the few transfected cell types that have been studied (Li et al., 1998). Our studies suggest that E-box1 may function in a cell-specific manner.
In spite of its ubiquitous expression in most cells, USF has been implicated in cell-specific regulation of the human insulin gene (Read et al., 1993), the prostaglandin endoperoxide synthase-2 promoter in ovarian granulosa cells (Morris and Richards, 1996), pancreatic expression of a homeobox gene (STF-1) (Sharma et al., 1996) and α-myosin heavy chain expression (Ojamaa et al., 1995). In each of these promoters, USF interacts with nearby elements to exert its effect, suggesting protein–protein interactions. Three isoforms of USF (USF1, USF2a and USF2b) have been described (Sirito et al., 1992; Viollet et al., 1996). The 43 kDa USF1 and the 44 kDa USF2 polypeptides are encoded by separate genes (Gregor et al., 1990; Sirito et al., 1994) while alternative splicing gives rise to the USF2a and USF2b isoforms (Viollet et al., 1996). USFs (Sirito et al., 1992; Viollet et al., 1996) can interact with each other and with several different bZip transcription factors (Kurschner and Morgan, 1997; Pognonec et al., 1997). Remarkably, USF1, but not USF2, was found to be important in regulating MT expression. This suggests that USF1 homodimers, or heterodimers not including USF2, are involved. Whether USF interacts directly with MTF-1 remains to be examined. The cell-specific nature of the cooperative interaction of E-box1 and MTF-1 suggests that protein(s) in addition to USF must also be important for optimal activation of MT-I expression in the visceral endoderm.
It is possible that chromatin structure and/or DNA methylation of the MT-I gene contribute significantly to its patterns of cell-specific expression. Inhibition of histone deacetylase activity renders cultured cells hypersensitive to induction of MT gene expression by metals (Andrews and Adamson, 1987), which suggests that the threshold for sensitivity to metals may have different set points in different cells based on nucleosome structure. In that regard, USF is also a component of the human β-globin locus control region (Bresnick and Felsenfeld, 1993) and may participate in the establishment of an active chromatin configuration (Salminen et al., 1996). The mouse MT-I and -II genes are flanked by regions with clusters of DNase I hypersensitive sites that function as locus control regions, which permit copy number-dependent and integration site-independent expression of transgenes in transgenic mice (Palmiter et al., 1993b). Our results demonstrate that these regions from the mouse MT locus are not essential for proper cell-specific expression of the MT-I promoter during early development. Interestingly, structural genes in the visceral endoderm are generally hypomethylated (Rossant et al., 1986), and DNA methylation has been correlated with inactivation of the mouse MT-I gene (Lieberman et al., 1983; Lu et al., 1999). Thus, several mechanisms may contribute to regulating MT gene expression in the visceral endoderm.
A final interesting point of discussion is the finding that MTF-1 is not essential for development and differentiation of the visceral yolk sac endoderm. Visceral endoderm cells lacking MTF-1 had apparently normal morphology and normal expression of α-fetoprotein and actin. Targeted mutation of mouse MTF-1 has revealed that it is an essential gene and homozygous null mutant embryos die on day 14 of development. The liver fails to develop properly in these mice, while development of the nervous system is not impaired (Günes et al., 1998; Lichtlen et al., 1999). Major functions of the fetal liver (e.g. serum protein synthesis and hematopoiesis) are performed earlier in development by the visceral yolk sac.
In summary, these studies demonstrated that MTF-1 is essential for the heightened expression of MT-I in the visceral endoderm of the developing mouse embryo and suggest that zinc modulates the ability of MTF-1 to transactivate gene expression in these cells. It was also found that MTF-1 cooperates with USF1 to regulate endoderm-specific expression of the mouse MT-I gene.
Materials and methods
Animals
All experiments involving animals were conducted in accordance with NIH guidelines for the care and use of experimental animals and were approved by our Institutional Animal Care and Use Committee(s). The targeted disruption of MTF-1 in the mouse has been described in detail previously (Günes et al., 1998; Lichtlen et al., 1999). Mice with targeted disruption of the mouse USF1 or USF2 genes have been described in detail previously (Sirito et al., 1998). MTF-1 or USF2 heterozygous knockout mice were inbred. The day of a vaginal plug was considered day 1 of pregnancy. For each implantation site on day 14, the embryo, visceral yolk sac and placenta were harvested. For genotyping the conceptus, the DNA from each embryo was extracted and analyzed by PCR. Tissues of identical genotype were pooled for analysis. Mice homozygous for USF1 knockout and a genetic control strain were generated by inbreeding littermates derived from inbred heterozygous USF1 knockout mice. Offspring with two normal USF1 alleles and those with two knockout USF1 alleles were inbred; the visceral yolk sacs and placenta were harvested at day 14 of pregnancy.
For immunohistochemistry, the visceral yolk sac attached to the placenta was dissected out and fixed as described below. For RNA extraction, each visceral yolk sac was separated from the placenta, frozen in liquid nitrogen and stored at –80°C until genotype analysis was complete. Tissues of the same genotype (at least six yolk sacs each, from two females) were pooled and RNA was extracted as described below.
In experiments involving dietary zinc deficiency, mouse diets were purchased from Harlan Teklad (Madison, WI) and have been described in detail previously (Dalton et al., 1996a; Andrews and Geiser, 1999). There was 0.5–1.5 µg/g (1 p.p.m.) zinc in the zinc-deficient (Zn-D) diet and 50 µg/g (50 p.p.m.) in the zinc-adequate (Zn-A) diet. These diets each contained ∼18 µg/g Cu2+ and were otherwise identical. CD-1 females (48–60 days old; Charles River Breeding Laboratories, Raleigh, NC) were mated with CD-1 males. On day 1 of pregnancy, mice were placed in pairs in cages and provided free access to Zn-A feed and deionized distilled water until day 8 of pregnancy. On day 8, pairs of mice were placed in cages with stainless steel false bottoms to reduce recycling of zinc (Cook Mills and Fraker, 1993) and the diet was changed to the Zn-D diet or mice were maintained on the Zn-A diet. On day 14, MT expression in placenta and visceral yolk sac was measured as described below. Embryonic tissues were pooled from ∼20 implantation sites for each group. Samples were frozen in liquid nitrogen, stored at –80°C and later assayed in sextuplicate for 109Cd-binding activity and MT mRNA levels.
In experiments involving hPAP transgenic mice, DNA microinjection and manipulation were carried out by the Transgenic and Gene Targeting Facility at the University of Kansas Medical Center. These transgenic experiments involved analysis of F0 transgenic mice harvested on day 14 after embryo transfer. From each implantation site, the embryo was recovered for PCR genotyping of genomic DNA. The visceral yolk sac attached to the placenta was dissected out, the placenta was cross-sectioned and the tissues were frozen in liquid nitrogen and stored at –80°C until histochemical detection of hPAP, as described below. Routinely, 10–25% of these embryos were identified as transgenic.
Construction of MT-I promoter–hPAP fusions
The MT-I promoter constructs have been described in detail previously (Dalton et al., 1994; Li et al., 1998). Promoter constructs each provided the transcription start site and 66 bp of untranslated region. These were moved from CAT or luciferase reporter constructs (Dalton et al., 1994; Li et al., 1998) into the hPAP reporter vector described below.
To construct an hPAP reporter vector, a custom-made polylinker (_Not_I, _Nhe_I, _Xho_I, _Hin_dIII, _Xba_I, Not_I) was first inserted between the Sac_I and Xba_I sites of pGEM-7Zf(+) (Promega Corporation, Madison, WI), with elimination of the vector Xba_I site. The 5.5 kb Hin_dIII–_Xba_I fragment from pRSV-hPAP (American Type Culture Collection, Rockville Pike, MD) (Henthorn et al., 1988; Knoll et al., 1988; Lin and Culp, 1991), containing the complete hPAP gene (Henthorn et al., 1988; Knoll et al., 1988) and the bovine growth hormone 3′ polyadenylation site, was then inserted between the corresponding sites in the polylinker. This provided the recipient for the following MT-I promoter constructs described previously: –742_PAP, –742δ_PAP (bases –43 to –150 inclusive are deleted); –600_PAP, –250_PAP and –153_PAP. These promoter fragments (through base +66) were excised with _Nhe_I (or _Xho_I depending on the parental plasmid) and _Hin_dIII, and inserted into these sites in the pGEM-7Zf-hPAP construct.
The E-box1:–153_PAP_ fusion gene was constructed by inserting an E-box1 oligonucleotide duplex (exclusive of restriction-site bases: TGT TCCACACGTCACATGGGTCGTCCTATCCGAGCC) between the _Nhe_I site and a Bgl_II site adjacent to base –153 in –153_PAP of the promoter insert. The E-box1 oligonucleotide encompassed bases –200 to –235 in the MT-I promoter (Li et al., 1998).
To prepare DNA for microinjection, the ∼6 kb MT-I promoter– _hPAP_–polyadenylation-site cassette was excised with _Not_I and separated from the 3 kb pGEM-7Zf vector by agarose gel electrophoresis. DNA was extracted from the gel using a Qiagen gel extraction kit and further purified by phenol–chloroform–isoamyl alcohol extractions. DNA was ethanol-precipitated and redissolved in microinjection buffer (10 mM Tris–HCl, 0.25 mM EDTA pH 7.4). DNA concentration was measured by fluorimetry.
Genotyping of embryos by PCR
PCR was used to determine the genotype of DNA from individual embryos using reaction conditions described previously (Lee et al., 1996). The MTF-1, USF1 and USF2 knockout alleles were determined as described in detail previously (Heuchel et al., 1994; Sirito et al., 1998). Genotyping of F0 _MT_-hPAP transgenic mice was performed using a sense-strand primer beginning at –5 bp in the mouse MT-I promoter (Searle et al., 1984) and an antisense-strand primer beginning at +329 bp in hPAP (Knoll et al., 1988).
Histochemical detection of hPAP
hPAP in whole mounts and tissue sections was detected as described (Deprimo et al., 1996). Tissue samples were fixed in 2% (v/v) formaldehyde, 0.2% (v/v) glutaraldehyde in phosphate-buffered saline (PBS) at 4°C for 60 min. Samples were washed three times with cold PBS and then incubated in 1 ml of PBS for 45 min at 65°C to destroy endogenous peroxidase activity. Samples were then incubated for ≤18 h at 4°C in 1 ml of a freshly prepared solution of 1 mg/ml nitroblue tetrazolium, 1 mg/ml 5-bromo-1-chloro-3-indolyl phosphate (Promega) in 0.1 M Tris–HCl pH 10.0. For detection of hPAP in tissue sections, stained whole mounts were washed in PBS, dehydrated in ethanol, infiltrated and embedded in methacrylate using a JB-4 embedding kit (Polysciences; Worthington, PA). Sections (1–2 µm) were mounted on to poly-lysine-coated slides and not counterstained before photography.
Immunohistochemistry
A rabbit polyclonal antiserum against a synthetic peptide corresponding to the N-terminal 15 amino acids of mouse MT-I (N-Ac-MDPNASASTGGSATC-amide) was characterized previously (Dalton et al., 1996b). Rabbit polyclonal antisera against Sp1 (PEP 2), USF1 and USF2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and antiserum against α-fetoprotein was purchased from Zymed Labs (San Francisco, CA).
Visceral yolk sacs and placentae were fixed overnight in Bouin’s fluid at 4°C, embedded in paraffin, sectioned and used for light-level immunohistochemistry as described (Dalton et al., 1996b). Slides were blocked for 1 h at room temperature with 10% goat serum in PBS–Triton X-100 and incubated overnight at 4°C with antisera against MT (1:50), Sp1 (1:100), α-fetoprotein (1:100), USF1 or USF2 (1:250). Slides were then incubated with anti-rabbit IgG conjugated to peroxidase and stained with a DAB kit (Zymed Labs, San Francisco, CA). Controls included non-immune serum and omission of primary antisera.
Extraction and northern blot analysis of RNA
Placentae and visceral yolk sacs (20 per group) from zinc-deficient or zinc-adequate mothers were rapidly thawed and RNA was extracted in buffers containing guanidinium isothiocyanate using a MIDI RNA isolation kit (Qiagen) according to the manufacturer’s instructions. RNA from the visceral yolk sacs from knockout mice was isolated using a MINI RNA isolation kit according to the manufacturer’s instructions (Qiagen). To remove residual DNA, all samples of total RNA were precipitated once with 3 M ammonium acetate at –2°C, followed by a final ethanol precipitation, as described (Andrews et al., 1987b).
Total RNA (1 µg in 5 µl) was denatured and size separated by electrophoresis in a 0.75% agarose–formaldehyde gel. RNA was transferred and cross-linked to nylon membranes, and northern blots were prehybridized, hybridized and washed as described (Dalton et al., 1994). Hybrids were detected by autoradiography at –70°C with intensifying screens and quantified by radioimage analysis (Molecular Dynamics, Sunnyvale, CA). In each experiment, a duplicate gel was stained with acridine orange to verify integrity and equal loading of RNA, and blots were co-hybridized with MT-I and actin probes as an internal control.
The mouse MT-I cDNA used as a template for the synthesis of 32P-labeled cRNA probe was described previously (Dalton et al., 1994). The mouse actin cDNA template was purchased from Ambion (Austin, TX) and also used for the synthesis of a 32P-labeled cRNA probe.
EMSA
EMSA was performed using nuclear extracts as described previously (Dalton et al., 1996c, 1997; Bittel et al., 1998; Li et al., 1998). Proteins from nuclear extracts (5–10 µg in 2–3 µl) were incubated in buffer containing 12 mM HEPES pH 7.9, 60 mM KCl, 0.5 mM dithiothreitol, 12% glycerol, 5 mM MgCl2, 0.2 µg poly(dI)/(dC)/µg protein and 8 fmol end-labeled double-stranded oligonucleotide (5000 c.p.m./fmol), in a total volume of 20 µl for 20 min on ice (Dalton et al., 1996c, 1997). In antibody supershift experiments, 1 µg of USF1- or USF2-specific antibody was incubated with nuclear extracts for 2 h on ice before addition of the labeled binding site (data not shown). EMSA was performed using the E-box1 and mutant E-box1 oligonucleotides described in detail previously (Li et al., 1998). An Sp1-binding site oligonucleotide and the MTF-1-binding site MREs have been described previously (Radtke et al., 1993; Dalton et al., 1996c). Protein–DNA complexes were separated electrophoretically and detected by autoradiography as described previously (Dalton et al., 1997; Li et al., 1998).
Measurement of MT protein levels
Steady-state levels of total MT were quantified in tissue homogenates using the [109Cd]hemoglobin-exchange assay (Eaton and Cherian, 1991).
Acknowledgments
Acknowledgements
We are indebted to Mr Jim Geiser and Mrs Hui-Xin Yang for technical support with animal husbandry and genotyping, to Mr Steve Eklund for excellent molecular biology technical support and to Dr Wenhao Xu of the KUMC Transgenic and Gene Targeting Facility. This work was supported by NCI grants CA 61262 (to G.K.A.) and CA 79578 (to M.S.).
References
- Andrews G.K. (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol., 59, 95–104. [DOI] [PubMed] [Google Scholar]
- Andrews G.K. and Adamson,E.D. (1987) Butyrate selectively activates the metallothionein gene in teratocarcinoma cells and induces hypersensitivity to metal induction. Nucleic Acids Res., 15, 5461–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G.K. and Geiser,J. (1999) Expression of metallothionein-I and -II genes provides a reproductive advantage during maternal dietary zinc deficiency. J. Nutr., 129, 1643–1648. [DOI] [PubMed] [Google Scholar]
- Andrews G.K., Adamson,E.D. and Gedamu,L. (1984) The ontogeny of expression of murine metallothionein: comparison with the α-fetoprotein gene. Dev. Biol., 103, 294–303. [DOI] [PubMed] [Google Scholar]
- Andrews G.K., Gallant,K.R. and Cherian,M.G. (1987a) Regulation of the ontogeny of rat liver metallothionein mRNA by zinc. Eur. J. Biochem., 166, 527–531. [DOI] [PubMed] [Google Scholar]
- Andrews G.K., Huet,Y.M., Lehman,L.D. and Dey,S.K. (1987b) Metallothionein gene regulation in the preimplantation rabbit blastocyst. Development, 100, 463–469. [DOI] [PubMed] [Google Scholar]
- Andrews G.K., Huet-Hudson,Y.M., Paria,B.C., McMaster,M.T., De,S.K. and Dey,S.K. (1991) Metallothionein gene expression and metal regulation during preimplantation mouse embryo development. Dev. Biol., 145, 13–27. [DOI] [PubMed] [Google Scholar]
- Andrews G.K., McMaster,M.T., De,S.K., Paria,B.C. and Dey,S.K. (1993) Cell-specific expression and regulation of the mouse metallothionein-I and -II genes in the reproductive tract and preimplantation embryo. In Suzuki,K.T., Imura,N. and Kimura,M. (eds), Metallothionein III: Biological Roles and Medical Implications. Birkhauser Verlag, Basel, Switzerland, pp. 351–362.
- Aniskovitch L.P. and Jacob,S.T. (1997) Purification and characterization of a rat liver protein that recognizes CCAAT-homologous sequences of the metallothionein promoter and _trans_-activates this promoter. Arch. Biochem. Biophys., 341, 337–346. [DOI] [PubMed] [Google Scholar]
- Auf der Maur A., Belser,T., Elgar,G., Georgiev,O. and Schaffner,W. (1999) Characterization of the transcription factor MTF-1 from the Japanese pufferfish (Fugu rubripes) reveals evolutionary conservation of heavy metal stress response. Biol. Chem., 380, 175–185. [DOI] [PubMed] [Google Scholar]
- Bittel D., Dalton,T., Samson,S., Gedamu,L. and Andrews,G.K. (1998) The DNA-binding activity of metal response element-binding transcription factor-1 is activated in vivo and in vitro by zinc, but not by other transition metals. J. Biol. Chem., 273, 7127–7133. [DOI] [PubMed] [Google Scholar]
- Bittel D., Smirnova,I. and Andrews,G.K. (2000) Functional heterogeneity in the zinc fingers of the metalloregulatory transcription factor, MTF-1. J. Biol. Chem., 275, 37194–37201. [DOI] [PubMed] [Google Scholar]
- Bresnick E.H. and Felsenfeld,G. (1993) Evidence that the transcription factor USF is a component of the human β-globin locus control region heteromeric protein complex. J. Biol. Chem., 268, 18824–18834. [PubMed] [Google Scholar]
- Briggs M.R., Kadonaga,J.T., Bell,S.P. and Tjian,R. (1986) Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science, 234, 47–52. [DOI] [PubMed] [Google Scholar]
- Carthew R.W., Chodosh,L.A. and Sharp,P.A. (1987) The major late transcription factor binds to and activates the mouse metallothionein I promoter. Genes Dev., 1, 973–980. [DOI] [PubMed] [Google Scholar]
- Chen X.H., Agarwal,A. and Giedroc,D.P. (1998) Structural and functional heterogeneity among the zinc fingers of human MRE-binding transcription factor-1. Biochemistry, 37, 11152–11161. [DOI] [PubMed] [Google Scholar]
- Chen X.H., Chu,M.H. and Giedroc,D.P. (1999) MRE-binding transcription factor-1: weak zinc-binding finger domains 5 and 6 modulate the structure, affinity and specificity of the metal-response element complex. Biochemistry, 38, 12915–12925. [DOI] [PubMed] [Google Scholar]
- Cook Mills J.M. and Fraker,P.J. (1993) Functional capacity of the residual lymphocytes from zinc-deficient adult mice. Br. J. Nutr., 69, 835–848. [DOI] [PubMed] [Google Scholar]
- Dalton T.P., Palmiter,R.D. and Andrews,G.K. (1994) Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res., 22, 5016–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton T.P., Fu,K., Palmiter,R.D. and Andrews,G.K. (1996a) Transgenic mice that over-express metallothionein-I resist dietary zinc deficiency. J. Nutr., 126, 825–833. [DOI] [PubMed] [Google Scholar]
- Dalton T.P., Fu,K., Enders,G.C., Palmiter,R.D. and Andrews,G.K. (1996b) Analysis of the effects of over-expression of metallothionein-I in transgenic mice on the reproductive toxicology of cadmium. Environ. Health Perspect., 104, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton T.P., Li,Q.W., Bittel,D., Liang,L.C. and Andrews,G.K. (1996c) Oxidative stress activates metal-responsive transcription factor-1 binding activity—Occupancy in vivo of metal response elements in the metallothionein-I gene promoter. J. Biol. Chem., 271, 26233–26241. [DOI] [PubMed] [Google Scholar]
- Dalton T.P., Bittel,D. and Andrews,G.K. (1997) Reversible activation of the mouse metal response element-binding transcription factor-1 DNA binding involves zinc interactions with the zinc-finger domain. Mol. Cell. Biol., 17, 2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Chapelle A., Fantoni,A. and Marks,P.A. (1969) Differentiation of mammalian somatic cells: DNA and hemoglobin synthesis in fetal mouse yolk sac erythroid cells. Proc. Natl Acad. Sci. USA, 63, 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprimo S.E., Stambrook,P.J. and Stringer,J.R. (1996) Human placental alkaline phosphatase as a histochemical marker of gene expression in transgenic mice. Transgene Res., 5, 459–466. [DOI] [PubMed] [Google Scholar]
- Dziadek M. and Adamson,E. (1978) Localization and synthesis of α foetoprotein in post-implantation mouse embryos. J. Embryol. Exp. Morphol., 43, 289–313. [PubMed] [Google Scholar]
- Eaton D.L. and Cherian,M.G. (1991) Determination of metallothionein in tissues by cadmium–hemoglobin affinity assay. Methods Enzymol., 205, 83–88. [DOI] [PubMed] [Google Scholar]
- Eng B.H., Guerinot,M.L., Eide,D. and Saier,M.H.,Jr (1998) Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J. Membr. Biol., 166, 1–7. [DOI] [PubMed] [Google Scholar]
- Gardner R.L. (1982) Investigation of cell lineage and differentiation in the extraembryonic endoderm of the mouse embryo. J. Embryol. Exp. Morphol., 68, 175–198. [PubMed] [Google Scholar]
- Gregor P.D., Sawadogo,M. and Roeder,R.G. (1990) The adenovirus major late transcription factor USF is a member of the helix–loop–helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev., 4, 1730–1740. [DOI] [PubMed] [Google Scholar]
- Günes Ç., Heuchel,R., Georgiev,O., Müller,K.H., Lichtlen,P., Blüthmann,H., Marino,S., Aguzzi,A. and Schaffner,W. (1998) Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J., 17, 2846–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P., Zervos,P., Raducha,M., Harris,H. and Kadesch,T. (1988) Expression of a human placental alkaline phosphatase gene in transfected cells: use as a reporter for studies of gene expression. Proc. Natl Acad. Sci. USA, 85, 6342–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuchel R., Radtke,F., Georgiev,O., Stark,G., Aguet,M. and Schaffner,W. (1994) The transcription factor MTF-I is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J., 13, 2870–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen R.G., Andrews,G.K. and Tamaoki,T. (1982) Synthesis of secretory proteins in developing mouse yolk sac. Dev. Biol., 90, 18–23. [DOI] [PubMed] [Google Scholar]
- Karasawa M., Nishimura,N., Nishimura,H., Tohyama,C., Hashiba,H. and Kuroki,T. (1991) Localization of metallothionein in hair follicles of normal skin and the basal cell layer of hyperplastic epidermis: possible association with cell proliferation. J. Invest. Dermatol., 97, 97–100. [DOI] [PubMed] [Google Scholar]
- Knoll B.J., Rothblum,K.N. and Longley,M. (1988) Nucleotide sequence of the human placental alkaline phosphatase gene. Evolution of the 5′ flanking region by deletion/substitution [published erratum appears in J. Biol. Chem, 1989, 264, 2391]. J. Biol. Chem., 263, 12020–12027. [PubMed] [Google Scholar]
- Koizumi S., Suzuki,K., Ogra,Y., Yamada,H. and Otsuka,F. (1999) Transcriptional activity and regulatory protein binding of metal-responsive elements of the human metallothionein-IIA gene. Eur. J. Biochem., 259, 635–642. [DOI] [PubMed] [Google Scholar]
- Kurschner C. and Morgan,J.I. (1997) USF2/FIP associates with the b-Zip transcription factor, c-Maf, via its bHLH domain and inhibits c-Maf DNA binding activity. Biochem. Biophys. Res. Commun., 231, 333–339. [DOI] [PubMed] [Google Scholar]
- Langmade J., Ravindra,R., Daniels,P.J. and Andrews,G.K. (2000) The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J. Biol. Chem., 275, 34803–34809. [DOI] [PubMed] [Google Scholar]
- Lee D.K., Fu,K., Liang,L., Dalton,T.P., Palmiter,R.D. and Andrews,G.K. (1996) Transgenic mouse blastocysts that over-express metallo thionein-I resist cadmium toxicity in vitro. Mol. Reprod. Dev., 43, 158–166. [DOI] [PubMed] [Google Scholar]
- Li Q.W., Hu,N.M., Daggett,M.A.F., Chu,W.A., Bittel,D., Johnson,J.A. and Andrews,G.K. (1998) Participation of upstream stimulatory factor (USF) in cadmium-induction of the mouse metallothionein-I gene. Nucleic Acids Res., 26, 5182–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Fu,K., Lee,D.K., Sobieski,R.J., Dalton,T.P. and Andrews,G.K. (1996) Activation of the complete metallothionein gene locus in the maternal deciduum. Mol. Reprod. Dev., 43, 25–37. [DOI] [PubMed] [Google Scholar]
- Lichtlen P., Georgiev,O., Schaffner,W., Aguzzi,A. and Brandner,S. (1999) The heavy metal-responsive transcription factor-1 (MTF-1) is not required for neural differentiation. Biol. Chem., 380, 711–715. [DOI] [PubMed] [Google Scholar]
- Lieberman M.W., Beach,L.R. and Palmiter,R.D. (1983) Ultraviolet radiation-induced metallothionein-I gene activation is associated with extensive DNA demethylation. Cell, 35, 207–214. [DOI] [PubMed] [Google Scholar]
- Lin W.C. and Culp,L.A. (1991) Selectable plasmid vectors with alternative and ultrasensitive histochemical marker genes. Biotechniques, 11, 344–348, 350. [PubMed] [Google Scholar]
- Lu Z.H., Cobine,P., Dameron,C.T. and Solioz,M. (1999) How cells handle copper: a view from microbes. J. Trace Elem. Exp. Med., 12, 347–360. [Google Scholar]
- Miles A.T., Hawksworth,G.M., Beattie,J.H. and Rodilla,V. (2000) Induction, regulation, degradation and biological significance of mammalian metallothioneins. Crit. Rev. Biochem. Mol. Biol., 35, 35–70. [DOI] [PubMed] [Google Scholar]
- Moffatt P. and Seguin,C. (1998) Expression of the gene encoding metallothionein-3 in organs of the reproductive system. DNA Cell Biol., 17, 501–510. [DOI] [PubMed] [Google Scholar]
- Morris J.K. and Richards,J.S. (1996) An E-box region within the prostaglandin endoperoxide synthase-2 (PGS-2) promoter is required for transcription in rat ovarian granulosa cells. J. Biol. Chem., 271, 16633–16643. [DOI] [PubMed] [Google Scholar]
- Ojamaa K., Samarel,A.M. and Klein,I. (1995) Identification of a contractile-responsive element in the cardiac α-myosin heavy chain gene. J. Biol. Chem., 270, 31276–31281. [DOI] [PubMed] [Google Scholar]
- Padykula H.A., Deren,J.J. and Wilson,T.H. (1966) Development of structure and function in the mammalian yolk sac. I. Developmental morphology and vitamin B12 uptake of the rat yolk sac. Dev. Biol., 13, 311–348. [DOI] [PubMed] [Google Scholar]
- Palmiter R.D. and Findley,S.D. (1995) Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J., 14, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R.D., Sandgren,E.P., Koeller,D.M., Findley,S.D. and Brinster,R.L. (1993a) Metallothionein genes and their regulation in transgenic mice. In Suzuki,K.T., Imura,N. and Kimura,M. (eds), Metallothionein III: Biological Roles and Medical Implications. Birkhauser Verlag, Basel, Switzerland, pp. 399–406.
- Palmiter R.D., Sandgren,E.P., Koeller,D.M. and Brinster,R.L. (1993b) Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol. Cell. Biol., 13, 5266–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pognonec P., Boulukos,K.E., Aperlo,C., Fujimoto,M., Ariga,H., Nomoto,A. and Kato,H. (1997) Cross-family interaction between the bHLHZip USF and bZip Fra1 proteins results in down-regulation of AP1 activity. Oncogene, 14, 2091–2098. [DOI] [PubMed] [Google Scholar]
- Radtke F., Heuchel,R., Georgiev,O., Hergersberg,M., Gariglio,M., Dembic,Z. and Schaffner,W. (1993) Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J., 12, 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read M.L., Clark,A.R. and Docherty,K. (1993) The helix–loop–helix transcription factor USF (upstream stimulating factor) binds to a regulatory sequence of the human insulin gene enhancer. Biochem. J., 295, 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J., Sanford,J.P., Chapman,V.M. and Andrews,G.K. (1986) Undermethylation of structural gene sequences in extraembryonic lineages of the mouse. Dev. Biol., 117, 567–573. [DOI] [PubMed] [Google Scholar]
- Salminen M., Lopez,S., Maire,P., Kahn,A. and Daegelen,D. (1996) Fast-muscle-specific DNA–protein interactions occurring in vivo at the human aldolase A M promoter are necessary for correct promoter activity in transgenic mice. Mol. Cell. Biol., 16, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle P.F., Davison,B.L., Stuart,G.W., Wilkie,T.M., Norstedt,G. and Palmiter,R.D. (1984) Regulation, linkage and sequence of mouse metallothionein I and II genes. Mol. Cell. Biol., 4, 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Leonard,J., Lee,S., Chapman,H.D., Leiter,E.H. and Montminy,M.R. (1996) Pancreatic islet expression of the homeobox factor STF-1 relies on an E-box motif that binds USF. J. Biol. Chem., 271, 2294–2299. [DOI] [PubMed] [Google Scholar]
- Sirito M., Walker,S., Lin,Q., Kozlowski,M.T., Klein,W.H. and Sawadogo,M. (1992) Members of the USF family of helix–loop– helix proteins bind DNA as homo- as well as heterodimers. Gene Expr., 2, 231–240. [PMC free article] [PubMed] [Google Scholar]
- Sirito M., Lin,Q., Maity,T. and Sawadogo,M. (1994) Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res., 22, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirito M., Lin,Q., Deng,J.M., Behringer,R.R. and Sawadogo,M. (1998) Overlapping roles and asymmetrical cross-regulation of the USF proteins in mice. Proc. Natl Acad. Sci. USA, 95, 3758–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G.W., Searle,P.F., Chen,H.Y., Brinster,R.L. and Palmiter,R.D. (1984) A 12-base-pair DNA motif that is repeated several times in metallothionein gene promoters confers metal regulation to a heterologous gene. Proc. Natl Acad. Sci. USA, 81, 7318–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G.W., Searle,P.F. and Palmiter,R.D. (1985) Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. Nature, 317, 828–831. [DOI] [PubMed] [Google Scholar]
- Venugopal R. and Jaiswal,A.K. (1996) Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl Acad. Sci. USA, 93, 14960–14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B., Lefrancois Martinez,A.M., Henrion,A., Kahn,A., Raymondjean,M. and Martinez,A. (1996) Immunochemical characterization and transacting properties of upstream stimulatory factor isoforms. J. Biol. Chem., 271, 1405–1415. [DOI] [PubMed] [Google Scholar]
- Westin G. and Schaffner,W. (1988) A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J., 7, 3763–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]