The structure of the inter-SH2 domain of class IA phosphoinositide 3-kinase determined by site-directed spin labeling EPR and homology modeling (original) (raw)
Abstract
Phosphoinositide (PI) 3-kinases catalyze the phosphorylation of the D3 position of the inositol ring of PI, and its phosphorylated derivatives and play important roles in many intracellular signal transducing pathways. Class IA PI3-kinases contain distinct regulatory (p85) and catalytic (p110) subunits. p110 is stabilized and inhibited by constitutive association with p85, and is disinhibited when the SH2 domains of p85 bind to tyrosyl-phosphorylated proteins. Because the two subunits do not dissociate, disinhibition of p110 presumably occurs by an allosteric mechanism. To explore the means by which p85 regulates the activity of p110, structures of the inter-SH2 domain of p85 were determined with and without phosphopeptide by using a combination of site directed spin labeling EPR and homology modeling and molecular dynamics. The inter-SH2 domain is assigned as a rigid anti-parallel coiled-coil whose primary function is to bind p110, facilitating inhibition of p110 by the N-terminal SH2 domain of p85.
Keywords: nitroxide‖distance measurement‖SDSL-EPR‖p85‖p110
Phosphoinositide (PI) 3-kinases are important regulators of proliferation, apoptosis, motility, and vesicular trafficking (1). Class IA PI3-kinases contain distinct regulatory (p85) and catalytic (p110) subunits. It has been shown previously that p85 plays two roles with regard to regulation of p110: stabilization of p110 against thermal denaturation and degradation, and inhibition of p110 catalytic activity (2). The N-terminal half of p85 contains an SH3 domain and a Rac/CDC42-binding domain, which is flanked by two proline-rich domains (3, 4). These domains are important for cytoskeletal regulation but not mitogenic signaling (5), and are not present in three of the regulatory subunit variants (p50α, p55α, and p55γ). The C-terminal half of p85 contains two src homology 2 (SH2) domains (the N-terminal or nSH2 domain and the C-terminal or cSH2 domain) separated by the inter-SH2 (iSH2) domain, a putative coiled-coil that contains the binding site for the p110 catalytic subunit (6). Although CD studies of the iSH2 domain show it to be highly helical (7), its structure has not been further characterized.
The binding of tyrosine-phosphorylated proteins or peptides to the SH2 domains of p85 releases its inhibition of p110, leading to an activation of p85/p110 heterodimers (8, 9) through unknown conformational changes. The minimal fragment of p85 capable of regulating p110 is the nSH2 domain linked to the iSH2 domain (10) (Fig. 1a). Although the iSH2 domain is sufficient to bind p110 (6, 11, 12) it has minimal effects on p110 activity (10). In contrast, the nSH2-iSH2 fragment (heretofore referred to as p85ni) inhibits p110, and p85ni/p110 complexes are activated on tyrosine phosphopeptide binding. Given that the nSH2 domain is the sole phosphopeptide binding domain in p85ni, several explanations for inhibition/disinhibition regulation are possible. These include phosphopeptide-induced conformational changes in the nSH2 domain, the iSH2 domain, the adjoining segment, or a combination of these regions.
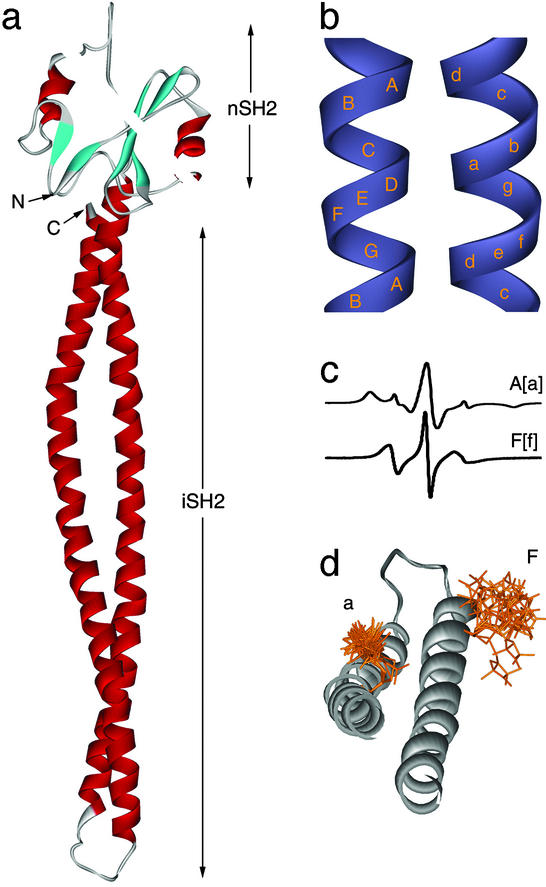
Figure 1.
(a) Homology model of the p85 iSH2 domain (bottom) concatenated to the NMR structure of the amino terminal-SH2 domain (13, 14) (top). The NMR structure is included solely to indicate the relative sizes of the two domains. (b) The relative positions of Cαs in the heptad repeats for the major N-terminal (uppercase letters) and minor C-terminal (lowercase letters) helices in coiled-coils. Approximately 14 Å separates the centers of each helix. (c) The room temperature EPR spectra are typical of A and F positions. (d) Superimposed USA/MD structures of doubly nitroxide-labeled p85 depict the distribution of distances between spin label pairs. The extreme distances (minimum and maximum nitroxide separation) are informative as predicted bounds for experiment. Δ503(F) on the upper right shows the large conformational distribution expected for exposed helical sites. For Δ535(a), the first three bonds are restricted by tertiary contacts, consistent with an interhelical site.
The structure of the isolated nSH2 domain has been determined by solution NMR and x-ray crystallography (13, 14). The present work therefore focuses on the structure of the iSH2 domain of p85ni, which has not been previously determined. To this end, we have used a hybrid experimental and computational approach, combining site-directed spin labeling (SDSL) EPR spectroscopy (15–17) with homology modeling and molecular dynamics (MD) (18, 19). Recently, analogous approaches using spin-labeling and computer simulation have proven very effective at predicting tertiary structure (20–22). The techniques presented here involve the generation of a series of computer-generated model structures of the spin-labeled protein. A preliminary backbone structure is provided from homology modeling, whereas the amino acid and spin label side chains are added and minimized by using MD calculations. The accuracy of these models is investigated experimentally by using SDSL-EPR to measure the mobility of single nitroxide labels and the distance between pairs of labeled residues.
Our work predicts the iSH2 domain to be a long antiparallel coiled-coil that undergoes no significant conformational changes on phosphopeptide binding. In conjunction with previous studies (6, 7, 10), these results lead to a model in which the iSH2 forms a rigid tether for p110, allowing additional inhibitory contacts between the nSH2 and p110.
Experimental Procedures
Construction and Labeling of p85ni Mutants.
Residues 320–600 of human p85α (p85ni) contain a single cysteine (Cys-498). Mutants were constructed by PCR using a Cys498Ser template, and produced as GST-fusions in bacteria. After removal of GST, mutants were labeled with (1-oxyl-2,2,5,5-tetramethylpyrolinyl-3-methyl)-methanethiosulfonate (MTSSL) (Toronto Research, Toronto) as described by Berliner et al. (23), dialyzed extensively against PBS (pH 7.4), and analyzed by mass spectrometry. Proteins were ≈90–95% pure as determined by densitometry of Coomassie-stained gels. Only samples with complete labeling (>90% as determined by matrix-assisted laser desorption ionization MS) were used in the study. MTSSL-labeled p85ni mutants (denoted with Δ) were tested for inhibition of p110 and disinhibition by tyrosyl phosphopeptides as described (10). CD spectra of each mutant were compared with wild type and in each case were within 10% as measured at 208 and 222 nm. One mutant, Δ489/Δ549, showed a slight increase in intensity at 208 nM with the same ellipticity at 222 nM. Further details of mutant protein preparation and purification are described in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
EPR.
Continuous wave EPR measurements were carried out on a Varian E112 X-Band Spectrometer. Room temperature fluid solution studies used ≈20 μl of sample in 50-μl glass capillary tubes with protein concentrations ranging from 15–100 μM with the following EPR parameters: 2-mW microwave power; 1-kHz modulation frequency; 1.6-G modulation amplitude; 1-s time constant; 4-min scan time; 4–32 scans averaged per spectrum. For double mutants at room temperature, 40% sucrose was added to slow protein motion and allow for distance determination (24). For studies at 77 K, the power was reduced to 0.05 mW and the sample volume was increased to 100–300 μl in 4-mm quartz EPR tubes. When indicated, samples were preincubated with phosphopeptide from the human PDGF receptor p85 binding site (GG[P]YMDMSKDE). To control for adventitious labeling, the Cys498Ser mutant was investigated at room temperature and no spin label was detected.
Homology Modeling and Side-Chain Dynamics.
Homology modeling and MD of the p85ni domains were carried out by using the HOMOLOGY module of insight ii (Accelrys, San Diego). The p85 iSH2 domain was manually aligned to the alanine coiled-coil Colicin E3 (PDB ID 1JCH) (25). The force fields for the model were then defined in insight ii, and 200 steps of conjugate gradient minimization were carried out to relax the side chains. For modeling of the nitroxide side chain distributions, the Cysteine-Spin label (CSL) was built and minimized by using the program amber (University of California, San Francisco) and imported into the residue library of insight ii where the appropriate native side chains were replaced with CSL. These constructs were then used as the basis for structure calculations. Unrestrained simulated annealing and MD (USA/MD) calculations were applied to each doubly spin labeled p85 molecule in a manner similar to that described by Constantine (26). For the simulations, 100 structures were generated of each mutant by using unrestrained simulated annealing with torsion angle dynamics (27). Nitroxide–nitroxide distances were extracted from the trial Protein Data Bank files. Based on guassian calculations kindly provided by Keith Constantine, it was assumed that the nitrogen–nitrogen distance was a good measure of the dipole vector for the paramagnetic electrons with 80% of the spin density residing at the ring nitrogen. The distances of the ensemble of structures were then plotted in 1- or 2-Å bins by using the program matlab.
Experimentally Determined Distance Distributions.
Distances were determined by using a Fourier-deconvolution method similar to that described by Rabenstein and Shin (28). A summed set of Pake patterns (24) was fit to the experimental broadening function by using a simulated annealing protocol (29). The Pake pattern summation was calculated assuming multiple (2 or 3) Gaussian distributions of nitroxide–nitroxide distances. These Gaussian distributions in distance had variable widths, amplitudes, and centers, which were sampled in the simulated annealing protocol to obtain the lowest energy, best fits to the dipolar broadening function. Distances were extracted from the electron-electron dipolar coupling constants D by using standard equations (28, 30). The uncertainties inherent in this approximate determination of nitroxide–nitroxide distance from D were well within the distance ranges defined by the conformational flexibility of the nitroxide side chain. Although the distances we report for p85ni are rigid limit calculations, this method is equally applicable to smaller volumes of 40% sucrose solutions at room temperature (24). The signal-to-noise allowed broadening resulting from distances of up to ≈25 Å to be detected, consistent with previous studies (24, 28). All spectra with reported dipolar broadening were checked for reproducibility by using multiple sample preparations and multiple acquisitions.
Results
Homology Modeling and SDSL-EPR.
Based on previous predictions that the p85 sequence contains a coiled-coil (6), we used homology modeling to generate a preliminary structure of the iSH2 region (Fig. 1a). The veracity of this model was tested by comparing the results of single and double nitroxide-labeled EPR spectra to predicted values for side chain motion and inter-nitroxide distances generated by USA/MD (26, 31). Single paramagnetic reporters offer detailed information on local nitroxide and backbone dynamics (16). Furthermore, the presence or absence of tertiary contacts can be deduced and used to broadly determine secondary structure (32). When a second nitroxide label is introduced to a sample and the distance between electrons is less than ≈25 Å, the appearance of a dipolar interaction becomes measurable in continuous wave EPR spectra and can be accurately quantitated (28, 33). Side chain flexibility is expected to give rise to a distribution in inter-nitroxide distances, which is observed in the USA/MD structures and accounted for in the fitting of the experimental data (see Experimental Methods).
The Structure of the iSH2 Domain.
The inherent periodicity of coiled-coils defines the chemical environment of specific amino acids in a predictable manner (Fig. 1b). Additionally, interhelical pairs should retain the same distance relationship irrespective of residue number so long as the heptad register is maintained. Fig. 1d shows 10 superimposed double-label structures obtained from USA/MD for a representative mutant containing a nitroxide-labeled cysteine at F and a positions, denoted Δ503/Δ535 (for definitions, see Figs. 1 and 2). In the model, it is clear that the A[or a] position is more restricted because of interhelical contacts along the hydrophobic interface. In the solution EPR spectra of an a position, this restricted conformational space corresponds to broadening of the individual spectral peaks as well as an increase in the total width of the spectrum, with the appearance of features similar to those observed in frozen solution (powder) spectra (Fig. 1c). In contrast, the solvent-exposed F position is less restricted in the absence of intramolecular interactions. This mobility is indicated by a relatively narrow solution EPR spectrum displaying no powder-like components. Interestingly, our models indicate subpopulations of F side-chains can exist in the g+g+ conformation reported for solvent exposed, helical, R1-side chains in crystal structures of T4 lysozyme (15).
Figure 2.
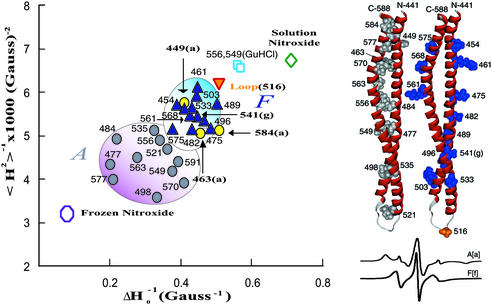
(Left) Mobility map of p85ni in PBS (inverse derivative line width, ΔH0−1 vs. second moment, <H2>−1) for residues predicted to be at tertiary contact sites (A positions, gray circles), and solvent exposed helical sites (F positions, blue triangles) in p85ni. Positions Δ449(a), Δ463(a), and Δ584(a) (yellow circles) have parameters more typical of solvent exposure. Δ516 (orange inverted triangle) is in the putative loop. Δ556 and Δ549 in Gdn⋅HCl (blue square) serve as references for unfolded protein. The nitroxide spin label at room temperature (green diamond) and in frozen solution (open circle) represent the upper and lower bounds for nitroxide mobility, respectively. The mobility parameter ΔH0−1 is measured from the maximum to the minimum of the central peak of the derivative spectra and is dominated by the most mobile (narrowest) component. Conversely, the inverse second moment <H2>−1 is more sensitive to restricted motions. (Right) Homology model of the iSH2 domain is shown (441–588) with _A_[a] positions (gray), _F_[f] and G[g] positions (blue) and the loop region (orange). Room temperature solution EPR spectra corresponding to representative _A_[a] and _F_[f] positions are shown below.
Heptad periodicity in the protein primary sequence of the general form (HPPHPPP)n, in which H and P are hydrophobic and polar amino acids, respectively, is a hallmark of coiled-coils (34, 35). The hydrophobic residues form the interface between the helices and the polar residues are exposed to solvent. We therefore made a series of 25 cysteine mutants at proposed A and F positions from residue 449 through residue 591 as well as at a putative loop residue (Δ516). Introduction of the spin label at these positions had no effect on inhibition of p110 by p85ni, activation of p85ni/p110 dimers by phosphopeptide, or overall conformation as determined by CD (data not shown). In a manner similar to that used by McHaourab et al. (16), we calculated motional parameters for each mutant and plotted the data to search for grouping based on position.
The correlation amongst single mutants at presumed F positions was very pronounced, and was consistent with a large degree of conformational freedom and solvent exposure (Fig. 2, see Fig. 6, which is published as supporting information on the PNAS web site, for the spectra used to generate this plot). In contrast, single mutants at presumed A positions formed a well demarcated, relatively immobile group. In each case the motional parameters were indicative of more restricted motion than those computed at F positions. Three A positions with unexpected mobility, Δ449, Δ463, and Δ584, were located at the putative ends of the coiled-coil, near the nSH2 domain. Both Δ449 and Δ463 had immobilized subpopulations, indicating that they may be at the terminus of the coil and/or that substitution of the R1 side chain is less tolerated at these positions than at locations more distal to the nSH2 domain. This explanation is less applicable for residue Δ584, because residue Δ591 retained _A_-like character. Δ584 may be in a locally unfolded region, or Δ591 may be immobilized through contacts other than with the major helix, potentially with the nSH2 domain. Finally, the Δ516 mutant, which is predicted to be positioned near the center of loop adjoining the major and minor helices, was less restricted than any of the F or A positions, presumably because of the contribution of additional backbone flexibility.
Additionally we prepared Δ556 and Δ549 in 6 M guanidine. Under these conditions the motion of the spin label is expected to be approximately isotropic with added contributions from the unfolded backbone as well as protein tumbling. As such, it serves as a control for labeled sites in unfolded or unstructured regions and as an upper bound for labeled-protein mobility. All of the labeled mutants were more constrained than Δ556(Gdn⋅HCl) or Δ549(Gdn⋅HCl).
The differences in mobility between A and other positions were readily observable without the addition of sucrose, which is often used to slow the overall protein rotational correlation time. For this reason, our mutants showed a small constant positive offset in both measures of motion compared with studies using sucrose. However, the relationship of tertiary contact sites to helical exposed positions, loop regions, and denatured sites was consistent with data published elsewhere (16, 32).
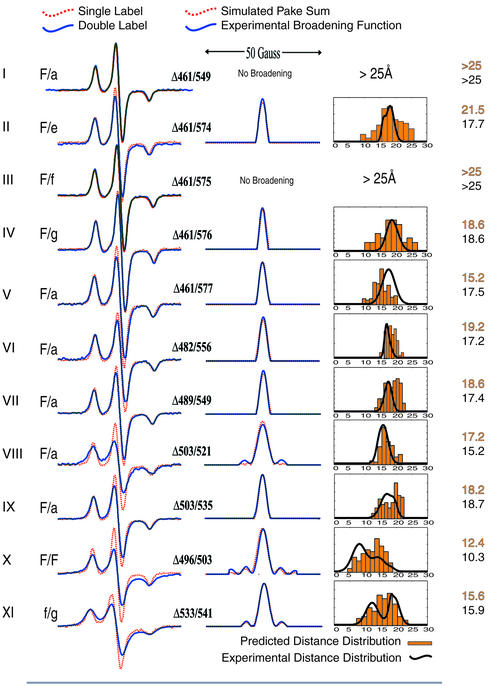
To further elucidate the structure of the iSH2 domain, we studied 11 doubly labeled p85 mutants (Fig. 3 and Table 1). In each case we compared predicted and experimental distance distributions rather than a single mean distance to more accurately reflect the conformational space sampled by the side chain in solution. We prepared nine double mutant constructs predicted to show dipolar interactions and two constructs for which no detectable interaction was predicted. Simulations of the normalized sum of the respective singles spectra convolved with broadening functions corresponding to 25 Å or longer were not differentiable from the summed singles spectra themselves within the experimental signal-to-noise range. Thus, mutants I (an F/a pair separated by an additional turn of helix with respect to other F/a mutants studied) and III (an F/f pair) yielded no discernable broadening of the spectra, consistent with their calculated separation of >25 Å; these pairs served as negative controls for the interacting mutants. Pairs II–V represent a “helical walk,” whereby Δ461(F) was held constant while the labeled position on the minor helix progressed from Δ574(e) through Δ577(a). The experimentally observed distance distributions matched very closely with those predicted from the model and are indicative of both the helical progression of the mutants as well as their organization into a coiled-coil. Mutants V through IX are all F/a pairs distributed throughout the iSH2 domain. In each case not only are the interspin distances maintained, but they also match those predicted from the model. Lastly, X and XI are intrahelical F/F and f/g pairs that were studied to confirm the helicity of this region. In both cases the predicted and observed distance distributions agree. The distribution widths of these mutants are greater than for F/a mutants, as expected given the additional mobility of the second solvent exposed label.
Figure 3.
Comparison of predicted and measured distances. (Left) The 77 K EPR spectra of the doubly label mutants (blue line) with the normalized sum of the corresponding single labels (red dotted line). In this depiction, spin-interactions are manifest as a diminution of the central peak of the double mutant spectra. (Center) Broadening functions derived by Fourier-deconvolution of experimental spectra (blue line) and the fit resulting from simulated annealing with multiple, floating distance distributions (red dotted line). The experimental single label spectra were convolved with the calculated broadening functions (a sum of pake patterns) and compared with the experimentally obtained double label spectra, with good agreement in all cases. (Right) Histogram plot (orange) of distances from 100 modeled structures from USA/MD. Overlaid are the distance distributions (black) calculated by fitting the Fourier-deconvoluted broadening function derived from experimental data (see Center). In all cases the two data sets were within one standard deviation of each other and the limits of each were similar. The distances in the last column are the means of each set (predicted above, measured below).
Table 1.
Summary of distances of USA/MD and experimental dipole data
| Double | Model distance | Experimental distance | ||||
|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | |
| 461/549 | >25 | – | – | >25 | – | – |
| 461/574 | 21.5 | 10.1 | 26.2 | 17.8 | 12.0 | 25.6 |
| 461/575 | >25 | – | – | >25 | – | – |
| 461/576 | 18.6 | 10.0 | 27.0 | 18.6 | 13.3 | 24.6 |
| 461/577 | 15.2 | 9.1 | 20.4 | 17.5 | 12.4 | 22.8 |
| 482/556 | 19.2 | 10.9 | 21.4 | 17.2 | 14.6 | 20.6 |
| 489/549 | 18.6 | 13.1 | 22.9 | 17.4 | 13.2 | 20.8 |
| 503/521 | 17.2 | 11.5 | 24.6 | 15.2 | 11.0 | 19.8 |
| 503/535 | 18.2 | 12.0 | 22.4 | 18.7 | 11.4 | 22.4 |
| 496/503 | 12.4 | 4.3 | 18.8 | 10.3 | 6.2 | 23.0 |
| 533/541 | 15.6 | 7.2 | 23.7 | 15.9 | 1.6 | 21.8 |
The p85 iSH2 Domain Is a Rigid Tether for p110 Binding.
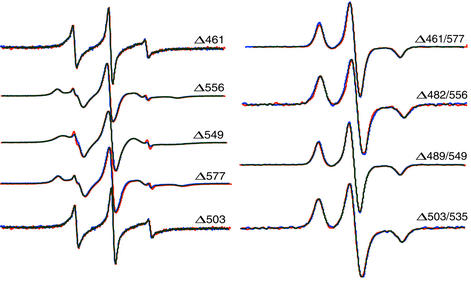
To test for structural perturbations in the iSH2 domain on phosphopeptide binding to the nSH2 domain, we incubated mutants spanning the length of the iSH2 domain with phosphopeptide and acquired their EPR spectra. Fig. 4 shows the solution spectra of five singly labeled samples, and the frozen spectra of four doubly labeled samples; spectra obtained in the absence or presence of peptide are superimposed. In no cases were there discernable differences in the individual spectra, indicating that no significant (> ≈1 Å) conformational changes occur in the iSH2 domain on phosphopeptide binding. This finding is in agreement with an 19F-NMR study showing that two residues at the C-terminal end of the iSH2 domain (Trp-583 and Trp-597) did not undergo conformational changes on peptide binding to the nSH2 domain; the CD spectra of an nSH2–iSH2–cSH2 fragment was also unchanged (7). Similar results were obtained in preliminary studies with two samples (Δ461/577 and Δ489/549) in which peptide binding was studied in the presence of an iSH2 binding fragment from p110 (data not shown). These data support a model in which the iSH2 domain serves as a rigid tether for p110, rather than a conformational switch.
Figure 4.
Solution spectra of single mutants (Left) and frozen solution spectra of double mutants (Right), with (red) and without (blue) phosphopeptide.
Discussion
Taken together, four separate lines of evidence support the model that the p85 iSH2 domain is an anti-parallel coiled-coil. Firstly, empirical sequence algorithms and predictive methods anticipate coiled-coil structure in this region (6). Secondly, the α-helical content, derived from CD measurements, is consistent with the presence of a coiled-coil (7). Thirdly, single-label EPR measurements confirm that heptad periodicity occurs within the domain. Finally, distances derived from double mutant experiments are in good agreement with those predicted for a coiled-coil.
It might have been expected that regulation of p110 by p85 could be described by an allosteric mechanism in which phosphopeptide binding to the nSH2 domain of p85 transmits a conformational change to the iSH2 domain, releasing p110 from p85 inhibition. However, given the absence of significant structural changes in the iSH2 domain in the presence of peptide (Fig. 4), it is more likely that the iSH2 domain serves as a rigid tether that is the primary binding site for p110 attachment. This interaction would stabilize p110, but play little or no other role in activity regulation. In contrast, the nSH2 domain is predicted to form a second inhibitory contact with p110 (Fig. 5). The binding between the nSH2 domain and p110 is presumably weak relative to that between the iSH2 domain and p110, as free nSH2 does not bind p110 (6, 11, 12); interaction between the nSH2 domain and p110 is thus facilitated by binding of the iSH2 domain to p110. It is also possible that binding of the iSH2 domain to p110 induces a conformational change in the p110 domain that increases the affinity of p110 for the nSH2 domain.
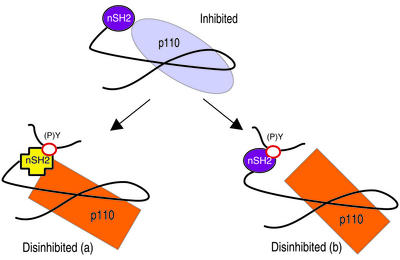
Figure 5.
Models of p110 regulation by p85. The iSH2 domain forms a rigid tether for p110. Inhibition is mediated by a secondary contact between the nSH2 and p110. Phosphopeptide binding alters (a) or disrupts (b) the nSH2-p110 contact.
During activation of PI3-kinase, phosphopeptide binding to the nSH2 domain could cause a local conformational change that is not transmitted to the iSH2 domain, but is sufficient to abrogate inhibition of p110. This could occur through either a conformational change in nSH2 (Fig. 5, model a), or a disruption of interactions between the nSH2 domain and p110 (Fig. 5, model b). NMR and crystallographic studies on the isolated p85 nSH2 domain show relatively small conformational changes on phosphopeptide binding (13, 14, 36). However, in the phosphatase SHP-2, phosphopeptide binding to the SH2 domains causes similarly small conformational changes that are nonetheless sufficient to disinhibit catalytic activity (37). Alternatively, conformational flexibility in the linker between nSH2 and iSH2 (approximately residues 431–442) could allow for reorientation of the nSH2 and iSH2 domains through changes in nSH2-iSH2 contacts. Finally, peptide binding to the nSH2 domain could simply compete with p110 for nSH2 binding.
In contrast to the results of this work, it has recently been suggested that the iSH2 domain of p85 is itself a modular structure, with discrete p110-binding, GTPase-responsive and inhibitory domains, the latter consisting of the C-terminal end of the iSH2 domain and the cSH2 domain (residues 572–724) (38). The coiled-coil model described here does not support the likelihood of discrete structural modules within the iSH2 domain. In addition, we have previously shown that removal of the cSH2 domain has no effect on inhibition of p110 by p85 (10). These discrepancies could be caused by the fact that Chan et al. (38) examined the effect of p85 overexpression on inhibition of V12Ras-induced Akt activation in cells, whereas our work has focused on regulation of p110 catalytic activity by purified recombinant fragments of p85. Based on data in the present study, we note that truncation of p85 at residue 572 would remove up to 30 aa of the minor helix, which could destabilize the conformation of the opposing region of the major helix. This region of the major helix is adjacent to the nSH2 domain, and could be important in facilitating inhibitory contacts between the nSH2 domain and p110. In this model, the activated phenotype of the truncated p85 (39) would be caused by loss of these inhibitory contacts. Our data does not bear on the possibility of additional regulatory roles for the cSH2 domain in intact cells, as has recently been suggested (40).
In summary, we have used SDSL-EPR to test the validity of a model structure generated by using homology modeling and MD. Analyzing the mobility and internitroxide distances for a relatively small number of strategically selected mutants has provided confirmation for this model. We have accounted for side chain flexibility and the resulting distribution in internitroxide distances in both the analysis of the experimental data and in the model structures. Our data supports the hypothesis that the iSH2 domain is a coiled-coil that forms a rigid tether for p110. This finding suggests that inhibition of p110 by p85 is caused by additional contacts between the nSH2 domain and p110 that are influenced by phosphopeptide binding. This study has highlighted the potential of homology modeling in combination with SDSL-EPR for macromolecular structural determinations, either as the primary basis of assignment or as a complement to other techniques.
Supplementary Material
Supporting Information
Acknowledgments
We would like to thank Keith Constantine (Bristol-Myers Squibb Pharmaceutical Research Institute, Lawrenceville, NJ) for advice concerning MD calculations, Y. K. Shin (Iowa State University, Ames) for discussions concerning Fourier deconvolution, Wayne Hubbell (University of California, Los Angeles) for providing PDB coordinates of spin-labeled T4 lysozyme, Mark Girvin, Denis Rousseau, Steve Almo (Albert Einstein College of Medicine) for helpful discussions, and A. J. Ebrahim for excellent technical assistance. Mass spectrometry was performed by Fang Wang and Edward Nieves in the Laboratory for Macromolecular Analysis and Proteomics at the Albert Einstein College of Medicine, which is supported in part by the Albert Einstein Comprehensive Cancer Center (CA13330) and the Diabetes Research and Training Center (DK20541). This work was supported by National Institutes of Health Grants GM60609 (to G.J.G.) and GM55692 (to J.M.B.). E.A.-S. is supported by the Medical Scientist Training Program at Albert Einstein College of Medicine.
Abbreviations
SDSL
site-directed spin labeling
PI
phosphoinositide
SH2
src homology domain 2
iSH2
inter-SH2
nSH2
N-terminal SH2
cSH2
C-terminal SH2
MTSSL
methanethiosulfonate spin label
MD
molecular dynamics
USA
unrestrained simulated annealing
Gdn⋅HCl
guanidine hydrochloride
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cantley L C. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr G A, Backer J M. Mol Cell Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B, Leevers S J, Ahmadi K, Timms J, Katso R, Driscoll P C, Woscholski R, Parker P J, Waterfield M D. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 4.Fruman D A, Meyers R E, Cantley L C. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 5.Hill K M, Huang Y, Yip S C, Yu J, Segall J E, Backer J M. J Biol Chem. 2001;276:16374–16378. doi: 10.1074/jbc.M006985200. [DOI] [PubMed] [Google Scholar]
- 6.Dhand R, Hara K, Hiles I, Bax B, Gout I, Panayotou G, Fry M J, Yonezawa K, Kasuga M, Waterfield M D. EMBO J. 1994;13:511–521. doi: 10.1002/j.1460-2075.1994.tb06289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien R, Rugman P, Renzoni D, Layton M, Handa R, Hilyard K, Waterfield M D, Driscoll P C, Ladbury J E. Protein Sci. 2000;9:570–579. doi: 10.1110/ps.9.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backer J M, Myers M G, Jr, Shoelson S E, Chin D J, Sun X J, Miralpeix M, Hu P, Margolis B, Skolnik E Y, Schlessinger J, et al. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter C L, Auger K R, Duckworth B C, Hou W M, Schaffhausen B, Cantley L C. Mol Cell Biol. 1993;13:1657–1665. doi: 10.1128/mcb.13.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Wjasow C, Backer J M. J Biol Chem. 1998;273:30199–203. doi: 10.1074/jbc.273.46.30199. [DOI] [PubMed] [Google Scholar]
- 11.Holt K H, Olson L, Moye-Rowley W S, Pessin J E. Mol Cell Biol. 1994;14:42–49. doi: 10.1128/mcb.14.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klippel A, Escobedo J A, Hu Q, Williams L T. Mol Cell Biol. 1993;13:5560–5566. doi: 10.1128/mcb.13.9.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolte R T, Eck M J, Schlessinger J, Shoelson S E, Harrison S C. Nat Struct Biol. 1996;3:364–374. doi: 10.1038/nsb0496-364. [DOI] [PubMed] [Google Scholar]
- 14.Hensmann M, Booker G W, Panayotou G, Boyd J, Linacre J, Waterfield M, Campbell I D. Protein Sci. 1994;3:1020–1030. doi: 10.1002/pro.5560030704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langen R, Oh K J, Cascio D, Hubbell W L. Biochemistry. 2000;39:8396–8405. doi: 10.1021/bi000604f. [DOI] [PubMed] [Google Scholar]
- 16.McHaourab H S, Lietzow M A, Hideg K, Hubbell W L. Biochemistry. 1996;35:7692–7704. doi: 10.1021/bi960482k. [DOI] [PubMed] [Google Scholar]
- 17.Hubbell W L, Cafiso D S, Altenbach C. Nat Struct Biol. 2000;7:735–739. doi: 10.1038/78956. [DOI] [PubMed] [Google Scholar]
- 18.Sali A. Curr Opin Biotechnol. 1995;6:437–451. doi: 10.1016/0958-1669(95)80074-3. [DOI] [PubMed] [Google Scholar]
- 19.Sali A. Mol Med Today. 1995;1:270–277. doi: 10.1016/s1357-4310(95)91170-7. [DOI] [PubMed] [Google Scholar]
- 20.Brown L J, Sale K L, Hills R, Rouviere C, Song L, Zhang X, Fajer P G. Proc Natl Acad Sci USA. 2002;99:12765–12770. doi: 10.1073/pnas.202477399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao W, Poirier M A, Bennett M K, Shin Y K. Nat Struct Biol. 2001;8:308–311. doi: 10.1038/86174. [DOI] [PubMed] [Google Scholar]
- 22.Poirier M A, Xiao W, Macosko J C, Chan C, Shin Y K, Bennett M K. Nat Struct Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- 23.Berliner L J, Grunwald J, Hankovszky H O, Hideg K. Anal Biochem. 1982;119:450–455. doi: 10.1016/0003-2697(82)90612-1. [DOI] [PubMed] [Google Scholar]
- 24.Altenbach C, Oh K J, Trabanino R J, Hideg K, Hubbell W L. Biochemistry. 2001;40:15471–15482. doi: 10.1021/bi011544w. [DOI] [PubMed] [Google Scholar]
- 25.Soelaiman S, Jakes K, Wu N, Li C, Shoham M. Mol Cell. 2001;8:1053–1062. doi: 10.1016/s1097-2765(01)00396-3. [DOI] [PubMed] [Google Scholar]
- 26.Constantine K L. Biophys J. 2001;81:1275–1284. doi: 10.1016/S0006-3495(01)75785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilges M, Gronenborn A M, Brunger A T, Clore G M. Protein Eng. 1988;2:27–38. doi: 10.1093/protein/2.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Rabenstein M D, Shin Y K. Proc Natl Acad Sci USA. 1995;92:8239–8243. doi: 10.1073/pnas.92.18.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Press W H. Numerical Recipes in C the Art of Scientific Computing. 2nd Ed. New York: Cambridge Univ. Press; 2000. [Google Scholar]
- 30.Berliner L J, Eaton G R, Eaton S S. Distance Measurements in Biological Systems by EPR, Biological Magnetic Resonance. Vol. 19. New York: Kluwer Academic/Plenum; 2000. [Google Scholar]
- 31.Brunger A T, Adams P D, Clore G M, DeLano W L, Gros P, Grosse-Kunstleve R W, Jiang J S, Kuszewski J, Nilges M, Pannu N S, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 32.Isas J M, Langen R, Haigler H T, Hubbell W L. Biochemistry. 2002;41:1464–1473. doi: 10.1021/bi011856z. [DOI] [PubMed] [Google Scholar]
- 33.Steinhoff H J, Radzwill N, Thevis W, Lenz V, Brandenburg D, Antson A, Dodson G, Wollmer A. Biophys J. 1997;73:3287–3298. doi: 10.1016/S0006-3495(97)78353-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crick F H C. Acta Crystallogr. 1953;6:689–697. [Google Scholar]
- 36.Gunther U L, Liu Y, Sanford D, Bachovchin W W, Schaffhausen B. Biochemistry. 1996;35:15570–15581. doi: 10.1021/bi961783x. [DOI] [PubMed] [Google Scholar]
- 37.Hof P, Pluskey S, Dhe-Paganon S, Eck M J, Shoelson S E. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 38.Chan T O, Rodeck U, Chan A M, Kimmelman A C, Rittenhouse S E, Panayotou G, Tsichlis P N. Cancer Cell. 2002;1:181–191. doi: 10.1016/s1535-6108(02)00033-8. [DOI] [PubMed] [Google Scholar]
- 39.Jimenez C, Jones D R, Rodriguez-Viciana P, Gonzalez-Garcia A, Leonardo E, Wennstrom S, von Kobbe C, Toran J L, R-, Borlado L, Calvo V, et al. EMBO J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jimenez C, Hernandez C, Pimentel B, Carrera A C. J Biol Chem. 2002;277:41556–41562. doi: 10.1074/jbc.M205893200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information