Stroma-Derived Three-Dimensional Matrices Are Necessary and Sufficient to Promote Desmoplastic Differentiation of Normal Fibroblasts (original) (raw)
Abstract
Stromagenesis is a host reaction of connective tissue that, when induced in cancer, produces a progressive and permissive mesenchymal microenvironment, thereby supporting tumor progression. The stromal microenvironment is complex and comprises several cell types, including fibroblasts, the primary producers of the noncellular scaffolds known as extracellular matrices. The events that support tumor progression during stromagenesis are for the most part unknown due to the lack of suitable, physiologically relevant, experimental model systems. In this report, we introduce a novel **in vivo**-like three-dimensional system derived from tumor-associated fibroblasts at diverse stages of tumor development that mimic the stromagenic features of fibroblasts and their matrices observed in vivo. Harvested primary stromal fibroblasts, obtained from different stages of tumor development, did not retain in vivo stromagenic characteristics when cultured on traditional two-dimensional substrates. However, they were capable of effectively maintaining the tumor-associated stromal characteristics within three-dimensional cultures. In this study, we demonstrate that **in vivo**-like three-dimensional matrices appear to have the necessary topographical and molecular information sufficient to induce desmoplastic stroma differentiation of normal fibroblasts.
During tumor progression, the transformation of proliferating epithelial cells into increasingly more aggressive phenotypes is accompanied by basement membrane discontinuity, severe immune responses, and the formation of new blood vessels. Among these host responses are additional alterations to the mesenchyme in the vicinity of the tumor.1 Mesenchymal alterations, known as stromagenesis, occur parallel to tumorigenesis and resemble an inflammatory response during wound healing or fibrosis.1–7 Hence, stromagenesis is a host process of the connective tissue that is induced by neoplasia, accompanies tumor development, and can be sorted into three distinctive phases: normal, primed, and activated, or tumor-associated.8,9
The predominant cell type within a normal stroma, quiescent fibroblasts, and their extracellular matrix (ECM), are believed to constrain tumor progression by acting as a natural barrier.2,10–13 However, early hyperplastic tumors can trigger mesenchymal alterations resulting in a primed stroma that can provoke, stimulate, and support (instead of constraining) tumor progression.5,7,14–18 These mesenchymal host responses can initiate a vicious cycle of paracrine and autocrine feedback signals between tumor cells and their microenvironment.19 Eventually, the parallel tumor-associated stromal progression upsets tissue homeostasis, further aggravating both tumors and associated stroma. During this parallel progression, the stroma becomes fully activated and fibroblastic stromal cells begin to express an array of specific genes such as type I collagen.20 Throughout this later activated stroma phase the tumor becomes invasive and metastatic.21,22 The activated stromal characteristics can be manifested as desmoplastic or oncofetal tumor microenvironments.2
Desmoplastic stroma, perhaps the best characterized stromal phenotype, is associated with invasive cancers of the breast, ovaries, prostate, pancreas, gastrointestinal tract, lung, squamous cell carcinomas (SCCs), and others.2,3,22 The desmoplastic stromal phenotype is known to be scar-like, highly fibrotic, and can comprise more than 50% of the tumor mass. Fibroblasts are the most abundant stromal cells within desmoplastic reactions.2,3,6,17,23 A defining feature of desmoplasia is the phenotypic switching of quiescent fibroblasts into differentiated myofibroblast cells that express smooth muscle α-actin (α-SMA).1 Although it has been shown that a small population of skin fibroblasts can express α-SMA,24 in most cases, quiescent normal fibroblasts become highly proliferative and well-organized tumor-associated fibroblasts (TAFs).1 Indeed, α-SMA is a commonly used marker to identify neomyofibroblasts within desmoplastic stroma,2,22 whereas the presence of additional markers such as desmin assist in further characterizing the type of myofibroblastic cells found in the tumor-associated stroma.25 An additional aberrant stromal phenotype was initially identified in breast tumor-derived fibroblasts that exhibited an enhanced fetal-like or oncofetal migratory behavior within three-dimensional (3-D) collagen gels. Oncofetal TAFs express fetal mesenchymal autocrine and paracrine factors including migration-stimulating factor and several oncofetal splice variants of fibronectin.26
During desmoplasia, myofibroblastic cells within the tumor-associated stroma produce an organized fibrotic ECM rich in fibronectin and elevated levels of type I collagen.6,21 Moreover, highly proliferative fibroblasts and a tumor-associated stroma rich in fibronectin and type I collagen are often associated with a bad prognosis,27 and with increased cancer progression.6,23,28 Furthermore, it has been suggested that a primed permissive stroma may be inherited or acquired and that the tumor-associated stroma promotes epithelial transformation through mutagenesis.5
A lack of in vitro models available to study stromal effects in cancer progression has been well documented in the literature.29 In previous studies, we introduced a system that accurately mimics mesenchymal matrices in vivo30,31 by exploiting a 3-D cell-derived culture system to analyze adhesion structures and signaling pathways reminiscent of in vivo adhesions.32 Our previous studies of adhesion structures within these connective tissue-like 3-D microenvironments indicate that signaling cascades of fibroblasts plated within 3-D cultures markedly differ from signaling of fibroblasts plated onto traditional 2-D surfaces. In this study, we have developed an _in vivo_-like 3-D progressive system derived from TAFs at diverse stages of cancer development. We show that this desmoplastic and physiologically relevant stromagenic 3-D system can be used for sorting fibroblasts as normal, primed, or activated (tumor-associated). In this study, we investigated the effect of cell-derived stromagenic 3-D matrices on the differentiation of stromagenic fibroblasts as a model for progressive desmoplasia.
Materials and Methods
Antibodies and Reagents
All chemicals and reagents of analytical grade were used and obtained from Fisher Chemicals (Fairlawn, NJ), unless otherwise specified. Polyclonal rabbit anti-fibronectin (745) (1:500, immunohistochemistry; 1:500, immunofluorescence; and 1:10,000, Western blot) was kindly provided by Dr. Kenneth Yamada (National Institute of Dental and Craniofacial Research/National Institutes of Health, Bethesda, MD). Antibodies against α-SMA (1:200, immunohistochemistry; and 1:4000, Western blot) and desmin (1:100, immunohistochemistry; and 1:500, Western blot) were obtained from AbCam Inc. (Cambridge, MA). Anti-actin (1:3000, Western blot), anti-vimentin (1:2500, Western blot), and horseradish peroxidase conjugated anti-mouse (1:5000) and rabbit (1:5000) were from Sigma-Aldrich (St. Louis, MO). Anti-keratin-10 (1:2000, Western blot) was obtained from Covance Research Products (Berkeley, CA). Antibodies against type I collagen (1:100, immunohistochemistry; 1:100, immunofluorescence; and 1:2500, Western blot) and GAPDH (1:10,000, Western blot) were from Chemicon Int. (Temecula, CA). Rhodamine red-, Cy2-, and Cy5-conjugated affinity purified F(ab′)2 fragments donkey anti-mouse, -rabbit, and -rat (1:200) were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Two-Stage Carcinogenesis
Mouse skin SCC tumors were induced by a well-characterized two-stage carcinogenesis protocol as previously described.33 Briefly, initiation of tumors was accomplished by topically applying a single dose of 100 nmol of 7,12-dimethylbenz[a]anthracene in 0.2 ml of acetone to shaved dorsal skin. One week after initiation, the skin was topically treated with 6 nmol of 12-_O_-tetradecanoylphorbol-13-acetate in 0.2 ml of acetone twice weekly for a period of 30 weeks. After treatment, mice were sacrificed by cervical dislocation while anesthetized. Normal tissue samples were taken from untreated shaved skin, treated tissues were derived from 3-week treated skin samples, and tumor samples were from SCC tumors at the end of the 30-week treatment period.
Immunohistochemistry of Tissue Sections
Immunohistochemistry of paraffin-embedded sections obtained from murine normal skin, treated skin, and SCCs (see Two-Stage Carcinogenesis) was performed using polyclonal rabbit anti-fibronectin, anti-type I collagen, anti-α-SMA, and anti-desmin as primary antibodies (see Antibodies and Reagents for details). An avidin-biotin-peroxidase kit (Vectastain Elite; Vector Laboratories, Burlingame, CA), followed by the chromogen 3′,3′-diaminobenzidine, was used following the manufacturer’s instructions. When appropriate, negative controls, not incubated with the primary antibodies, were incubated with normal rabbit or mouse serum. All sections were counterstained with hematoxylin and mounted.
Harvesting Primary Stromagenic Fibroblasts
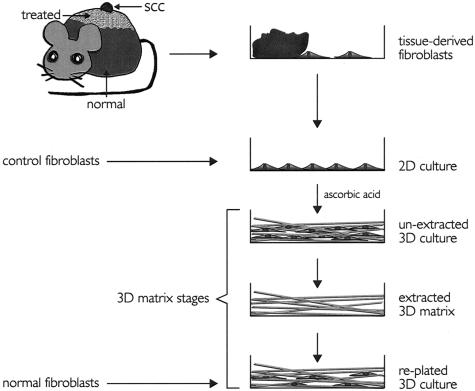
As shown in Figure 2, normal, treated, and tumor skin samples were rinsed in phosphate-buffered saline (PBS); 0.137 mol/L NaCl, 2.7 mmol/L KCl, 1.4 mmol/L KH2PO4, and 1.44 mmol/L Na2HPO4 at pH 7.4 and finely chopped into 1-mm2 pieces. The chopped tissue pieces were deposited onto a cell culture dish and allowed to dry for a period of 1 to 2 minutes to facilitate their adhesion onto the culture dish. The adherent tissue samples were then carefully covered with maintenance medium; high glucose Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Maintenance medium was replaced by fresh medium every other day until primary fibroblasts proceeded out of the samples onto the culture plates reaching 70% confluence. Tissue samples were then removed from the cultures and harvested fibroblasts were aliquoted and frozen for storage or used between passages 2 and 6 for experimental procedures. All batches were tested for vimentin expression and homogeneity before experimental use (see Results). The stromagenic 3-D matrices obtained from normal, treated, or tumor skin samples (see Stromal Fibroblast-Derived 3-D Matrix Production) were sorted as normal, primed, or tumor-associated ECMs, respectively.
Figure 2.
Model illustrating production and staging of fibroblast-derived 3-D matrices. Mouse skin SCCs were induced by a two-step process (see Materials and Methods). Indicated (normal, treated, and SCC) tissue samples were placed onto tissue culture plates where fibroblasts were allowed to crawl out. Tissue samples were removed and cells were stored or allowed to reach confluence for experimental purposes (2-D culture). Stromagenic and control 2-D cultures were kept for 8 days under conditions permissive for matrix production (unextracted 3-D cultures). Unextracted cultures were used for experimental purposes or submitted to alkaline detergent extraction (extracted 3-D matrices). Extracted 3-D matrices were stored at 4°C until needed for experimental use. Finally HFFs (normal fibroblasts) were replated into extracted 3-D matrices (replated 3-D culture). Note that normal, treated, or TAFs, as well as control fibroblasts, were used to form all three unextracted, extracted, and replated 3-D matrix stages.
Stromal Fibroblast-Derived 3-D Matrix Production
Primary cell-derived 3-D matrices were produced by the three above-mentioned normal, treated, or tumor-associated stromagenic fibroblasts, and by two additional control cell lines; primary human foreskin fibroblasts (HFFs) that were kindly provided by S.S. Yamada (National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD), and NIH-3T3s (American Type Culture Collection, Manassas, VA). NIH-3T3s were cultured in maintenance medium (see Harvesting Primary Stromagenic-Fibroblasts) for a minimum of 20 passages before matrix production to overcome their normal contact growth inhibition.30 All 3-D matrices were produced using our published protocols.30,31 Briefly, confluent fibroblastic cultures were treated with fresh maintenance media supplemented with 50 μg/ml of cell culture-tested ascorbic acid (Sigma-Aldrich) every other day for 8 days. We had previously documented that a substantial accumulation of cell-derived 3-D matrix occurs when preconditioned NIH-3T3s are induced with ascorbic acid for 5 to 8 days.30–32 After 8 days of ascorbic acid treatment, all unextracted cultures were qualitatively examined under the microscope. Alkaline detergent treatment [0.5% (v/v) Triton X-100 and 20 mmol/L NH4OH] of these unextracted cultures and washing three times with PBS, gave rise to cell-free _in vivo_-like 3-D matrices that remained attached to the culture plates. The resulting extracted 3-D matrices (Figure 2) were stored at 4°C in PBS (see Harvesting Primary Stromagenic Fibroblasts) containing 100 U/ml penicillin and 100 μg/ml streptomycin until needed. Primary normal fibroblasts were seeded within these extracted 3-D matrices to accomplish the replated 3-D matrix stage (Figure 2).
Indirect Immunofluorescence
Unextracted cultures prepared onto (22 mm; Carolina Biological Supply, Burlington, NC) coverslips were fixed and permeabilized in a solution of PBS supplemented with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), 5% sucrose (w/v), and 0.5% Triton X-100 for 3 minutes at room temperature. Cells were subsequently fixed with PBS containing 4% paraformaldehyde and 5% sucrose (w/v) for an additional 20 minutes. Fixed and permeabilized cells were blocked with M.O.M. blocking solution (Vector Laboratories) containing 20% preimmune donkey serum (Jackson ImmunoResearch Laboratories) for 1 hour at 37°C in a humidified chamber. Primary antibodies were diluted (see Antibodies and Reagents) in PBS containing 0.05% Tween-20 and 5% preimmune donkey serum and incubated onto samples for 45 minutes at room temperature. Cells were then rinsed three times for 7 minutes, with PBS containing 0.05% Tween-20. Secondary antibody, fluorophore-conjugated, affinity-purified F(ab′)2 fragments of donkey anti-mouse, anti-rabbit, or anti-rat, were diluted (see Antibodies and Reagents) in PBS containing 0.05% Tween-20 and 10% preimmune donkey serum and incubated with samples for 20 minutes. Secondary antibodies were precleared by centrifugation at 13,500 rpm for 10 minutes to remove any residual debris. Coverslips were rinsed as before and samples were mounted using Prolong Gold anti-fade reagent (Invitrogen, Carlsbad, CA) following manufacturer’s instructions.
Western Blotting
Cells were lysed using a modified RIPA buffer consisting of 50 mmol/L Tris pH 8.0, 10% glycerol, 1% deoxycholate salt (w/v), 150 mmol/L NaCl, 5 mmol/L benzamidine, 48 mmol/L NaF, 1 mmol/L sodium pyrophosphate, 1 mmol/L nitrophenol phosphate, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L sodium orthovanadate, and mammalian protease inhibitor cocktail (Sigma-Aldrich). Cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using precast Tris-glycine 4 to 12% or 8 to 16% gels (Invitrogen). The proteins were then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA) using XCell Surelock Novex Mini-Cell, (Invitrogen) following the manufacturer’s instructions. Membranes were incubated for 1 hour in blocking buffer [Tris-buffered saline (TBS), pH 8.0, 100 mmol/L Tris-Cl, pH 8.0, and 0.9% NaCl (w/v) supplemented with 5% nonfat dry milk]. After blocking, membranes were incubated for 1 hour at room temperature with assorted primary antibodies in TBS containing 0.05% Tween-20 (TBST) and 3% nonfat dry-milk, followed by three consecutive rinses for 15 minutes each in TBST containing 1% nonfat dry-milk. Horseradish peroxidase-conjugated secondary antibodies were diluted (see Antibodies and Reagents) in TBST containing 3% nonfat dry-milk and incubated with polyvinylidene difluoride membranes at room temperature for an additional 1 hour. After rinsing three times with TBST as described above, specific proteins were revealed by chemiluminescence to horseradish peroxidase with ECL-Plus (Amersham Biosciences, Piscataway, NJ) using Kodak BioMax XAR photographic film (Eastman Kodak, Rochester, NY). Individual protein bands were scanned and their optical densities quantified using Scion Image Software (Scion Corp., NIH, Bethesda, MD).
Cell Density Assay
Four independent, double-repetitive, experiments of assorted unextracted cultures were prepared by fluorescently labeling the unextracted culture’s cell-nuclei with Hoechst 33342 (Molecular Probes, Eugene, OR) following the manufacturer’s instructions. Five random regions capturing 0.3 μm-thick z-slices (from the dorsal to ventral surfaces) of each unextracted culture were acquired with a Nikon TE-2000U microscope (Optical Apparatus Co., Ardmore, PA) equipped with epifluorescence optics, a Roper Scientific Cool Snap HQ camera, shutters for transmitted and fluorescent light sources (Sutter Instruments, Novato, CA), a Z-motorized focus control (Prior Scientific, Rockland, MA), excitation and emission filter wheels, and a polydichroic cube. Images were analyzed by flipping through the slices and manually scoring individual nuclei using the count objects command of MetaMorph offline 6.2r1 imaging analysis software (Molecular Devices, Sunnyvale, CA). Statistical analysis was performed by Welch correction analyses of variance tests using Instat Statistical Software (GraphPad Software, San Diego, CA).
3-D Matrix Thickness Measurements
Four independent, double-repetitive experiments of assorted unextracted cultures were prepared by fluorescently labeling fibronectin (see Indirect Immunofluorescence for details). Thickness measurements were made at five individual region locations on each sample by subtracting the distance between the top and bottom sections of assembled fibronectin matrix using the Nikon TE-2000U fluorescent microscope (Optical Apparatus Co). Statistical analyses of thickness measurements were performed as described above using Instat Statistical Software (GraphPad Software).
Stromal 3-D Matrix Fiber Organizational Distribution and Statistics
Two independent experiments that included duplicate samples of all unextracted cultures were prepared by fluorescently labeling fibronectin (see Indirect Immunofluorescence for details). Four individually captured regions (0.5 μm-thick z-slices) from each unextracted culture sample were acquired using a Perkin-Elmer spinning disk confocal (PerkinElmer Life Sciences, Boston, MA) mounted onto a Nikon TE-2000S microscope (Optical Apparatus Co) rendering a total of 16 sample regions per unextracted culture type. Acquired z-slices were overlaid as maximum projections that rendered reconstituted views of the corresponding 3-D fibronectin fibers for each region.
Reconstituted 3-D projections were subjected to identical modifications and digital filters using MetaMorph offline 6.2r1 imaging analysis software (Molecular Devices). Nonspecific background was reduced by selectively darkening objects with a pixel area greater than 15 using the flatten background function. A binary image that rendered selected fibers as objects was created by selecting 35% threshold at the maximum internal intensity option using the internal threshold function. Counts of all of the fibers recognized by the software, as well as their orientation (angle relative to _x_-axis), were measured using the integrated morphometry analysis function. The relative angles were approximated to the nearest 10th degree using the rounding function on Microsoft’s Excel software. Microsoft’s Excel was also used to find the mode angle for each sample image. To allow for experimental comparison, the mode angle, defined as the angle to which the maximum number of fibers was oriented, was arbitrarily set to 0°. To determine the relative distribution of fibers organized in a parallel pattern, the percentage of fibers that were arranged in parallel within ±100 of the mode angle was calculated for each analyzed region. Percents and total counts were then statistically analyzed by a Mann-Whitney variance test using Instat Statistical Software (GraphPad Software).
Isolation of Assembled Matrix Proteins
Deoxycholate insoluble matrix proteins were isolated and quantified as modified by Quade and McDonald.34 Briefly, unextracted (or when necessary, extracted) cultures were lysed in PBS (see Harvesting Primary Stromagenic Fibroblasts for details) containing 3% Triton X-100, 10 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L phenylmethyl sulfonyl fluoride, and mammalian protease inhibitor cocktail (Sigma-Aldrich). The insoluble matrix remaining on the culturing plates was then treated with 50 mmol/L Tris (pH 7.4) containing 10 mmol/L MnCl2, 1 mol/L NaCl, 100 μg/ml DNase, and 1 mmol/L phenylmethyl sulfonyl fluoride followed by a brief wash with 50 mmol/L Tris (pH 8.8), 2% deoxycholate (w/v), 10 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L phenylmethyl sulfonyl fluoride, and mammalian protease inhibitor cocktail (Sigma-Aldrich). Lastly, the deoxycholate-resistant matrix was washed three times in PBS and collected by scraping the matrix from the dish using 50 mmol/L Tris (pH 8.8), 1% sodium dodecyl sulfate, 150 mmol/L NaCl, 5 mmol/L ethylenediamine tetraacetic acid, 5 mmol/L dithiothreitol, and 13 mmol/L iodoacetamide. The fibronectin and type I collagen content of the deoxycholate-resistant matrix was quantified by Western blot analysis as described above (see Western Blotting).
Results
SCC Stroma in Vivo Exhibits a Desmoplastic Phenotype
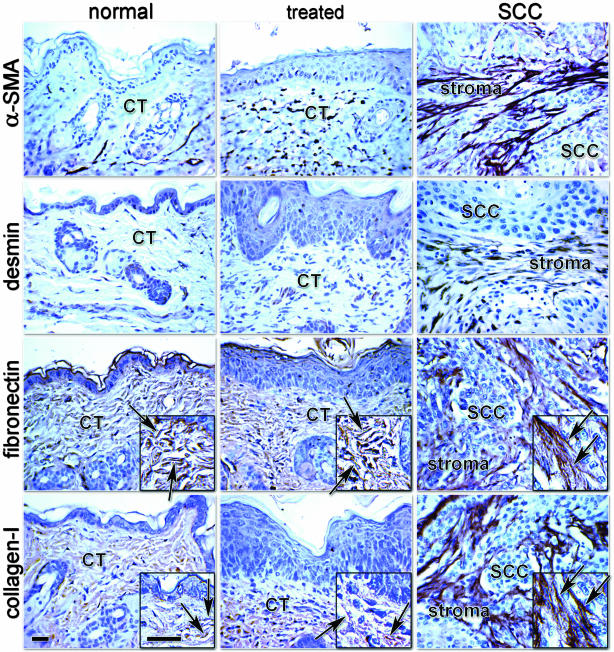
With the purpose of harvesting fibroblasts for the production of the _in vivo_-like 3-D stromagenic system, we used a murine two-stage carcinogenesis protocol that results in SSC formation (see Materials and Methods).33 Initially, we engaged in characterizing the in vivo tumor-associated stroma by immunohistochemistry. Expression of α-SMA in the tumor-associated stroma indicates a desmoplastic phenotype, which contains differentiated myofibroblasts.21,25 The identification of desmin-positive tumor-associated stroma assist to further characterize the type of differentiated myofibroblasts observed within the desmoplastic reaction.21,25 As shown in Figure 1, after induction by the two-stage treatment, the SCC-associated stroma demonstrated strong positive reactivity for both α-SMA and desmin in vivo.
Figure 1.
SCC stroma exhibits a desmoplastic phenotype in vivo. Immunohistochemistry counterstained with hematoxylin; Gill’s no. 3 nuclei stain, of normal skin (normal), treated skin (treated), and SCC showing α-SMA, desmin, fibronectin, and type I collagen. Connective tissue (CT), tumor-associated stroma (stroma), and SCC are denoted. Arrows identify ECM fibers. Note the levels of expression of α-SMA and desmin within the tumor-associated stroma. Also note the disorganization of the ECM fibers of both fibronectin and collagen within normal and treated CT, depicted by the insets versus the parallel patterned ECM organization within the chemically induced mouse SCC-associated stroma. Scale bars, 30 μm.
In addition to differentiated myofibroblastic cells, the desmoplastic stroma exhibits a dense cellular population with a vast fibrous ECM organized in a parallel pattern.35 As depicted in Figure 1, fibronectin expression in the ECM of the tumor-associated stroma is in fact densely packed, fibrous, and highly organized (see insets in Figure 1 for details). Type I collagen expression in the SCC stroma appears to be abundant, as well as highly organized. In contrast, both fibronectin and type I collagen were detected in normal and treated murine dermis within a mesh-like ECM that lacks clear organization.
Unextracted Stromagenic Cultures Acquire Spindle-Shape Morphology and High Levels of Organization in Association with SCCs
Parallel to the initial immunohistochemistry characterization, fresh unfixed samples of murine normal, and treated skin, as well as SCCs, were placed onto tissue culture plates where fibroblasts associated with the tissue samples were allowed to crawl out onto the plates (see Materials and Methods for details). Tissue samples were removed and fibroblasts were used for experimental purposes between passages 2 and 6 (Figure 2, 2-D culture). Cell cultures were kept confluent for 8 days in media containing ascorbic acid thus inducing ECM production (Figure 2, unextracted 3-D cultures). After the matrix production, the unextracted 3-D cultures were either used for experimental purposes or submitted to alkaline detergent extraction (Figure 2, extracted 3-D matrices). Extracted 3-D matrices were stored at 4°C until needed for experimental use. To test the ECM effects on undifferentiated fibroblasts, normal primary fibroblasts were replated into extracted normal, primed (derived from treated), or tumor-associated 3-D matrices (Figure 2, replated 3-D culture).
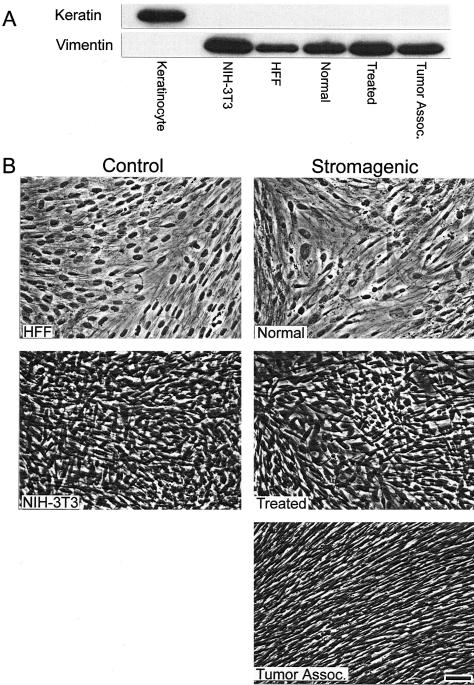
To establish the physiological relevance of the 3-D system, we originally engaged in characterizing the stromagenic properties of unextracted cultures (Figure 2, 3-D matrix staging). First, we verified that the three murine stromagenic cell types (normal, treated, and tumor-associated) were fibroblastic as opposed to epithelial. Primary HFFs and NIH-3T3s were used as positive fibroblastic cell-line controls. We determined whether the stromagenic cultures were homogenous and, if so, which of the various cells expressed fibroblastic but not epithelial markers. As shown in Figure 3A, a representative Western blot confirms that all stromagenic cells express the fibroblastic marker vimentin but lacked the expression of keratin, which was used as an epithelial marker. The morphology of both the stromagenic and control unextracted cultures is shown in Figure 3B and suggests that all cultures are homogenous.
Figure 3.
Unextracted control and stromagenic 3-D cultures are fibroblastic and homogenous. A: Western blot showing the expression of epithelial marker keratin-10 and fibroblastic marker vimentin. Control cells were epithelial primary mouse keratinocytes and fibroblastic HFFs and NIH-3T3. Stromagenic normal, treated, and tumor-associated cells were positive for vimentin and negative for keratin expression. B: Phase contrast images of unextracted fibroblastic cultures showing morphological differences between stromagenic cell types and control fibroblasts. Note organizational level of tumor-associated unextracted 3-D cultures and similarities between HFF and normal and between NIH-3T3 and treated unextracted 3-D cultures in appearance, as well as the overcoming of growth inhibition by contact of treated and tumor-associated cultures. Scale bar, 25 μm.
Clear morphological differences are observed between the stromagenic normal, treated, and tumor-associated unextracted cultures. Tumor-associated unextracted cultures are comprised of fibroblastic cells that are spindle shaped, dense, and organized in a parallel pattern. Moreover, primary normal control unextracted HFF cultures are morphologically indistinguishable from their normal murine counterparts (Figure 3B). Similarly, the morphology and organization of primed unextracted stromagenic cultures appear analogous to conditioned NIH-3T3 cultures.
Unextracted Stromagenic Cultures Are Increasingly Dense and Produce Thicker Matrices
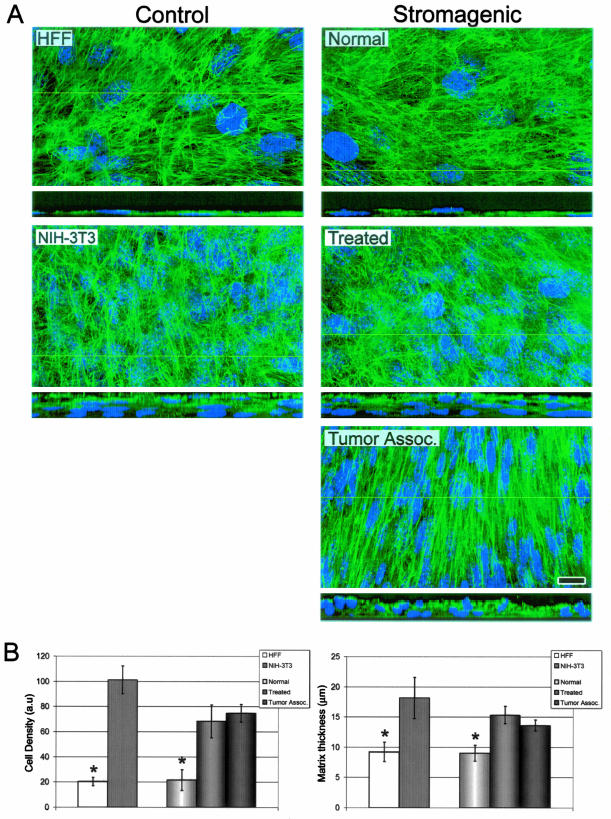
In contrast to quiescent fibroblasts in normal connective tissue, stromal TAFs in vivo are highly proliferative.21 Moreover, desmoplastic stroma consist of dense differentiated myofibroblastic populations that produce ECMs with a relatively thick fibronectin matrix and a parallel-patterned mesenchymal ECM.35 The morphological observations seen with our stromagenic system (Figure 3), suggested that the unextracted cultures differed in cell density. This progressive increase in cell density from normal to TAFs confers a progressive thickness to cell-derived 3-D matrix deposition. To test this prediction, we scored both cell density and 3-D matrix thickness in the control (HFF and NIH-3T3) and normal, treated, or tumor-associated unextracted cultures (Figure 4).
Figure 4.
Normal unextracted 3-D cultures reach cell densities and matrix thicknesses that significantly differ from stromagenic-treated and tumor-associated unextracted cultures. A: Maximum projection reconstructed confocal images of indirect immunofluorescently labeled fibronectin (in green) and cell nuclei (in blue) of assorted control and stromagenic unextracted 3-D cultures. The yellow lines in the main panels indicate the position where insets, at the bottom of the main panels, show 90° views of the unextracted cultures. Note, within the insets, that normal-derived monolayers and stromagenic-derived polylayers vary in matrix thicknesses. B: Bar graphs representing the average measured cell density and matrix thickness for all assorted cell types (see Table 1 for details). Asterisks indicate statistical significant differences from all other data in the graphs. Scale bar, 15 μm.
Unextracted nuclei and matrices were fluorescently labeled and digitally acquired for qualitative and quantitative analyses. Laser-scanned spinning disk confocal images qualitatively show both progressive fibroblastic density and 3-D matrix thicknesses (Figure 4A). The qualitative observations were confirmed by quantitative nuclei count analyses. Both treated and tumor-associated unextracted cultures were threefold denser than normal cultures (Figure 4B). Statistical analyses established that both treated and tumor-associated unextracted cultures, reached cell densities that significantly differ from unextracted normal cultures. Moreover, the progressive cell densities observed coincided with the statistically significant progression in 3-D matrix thickness during stromagenesis (Figure 4B). As expected, normal unextracted cultures were not capable of overcoming growth inhibition by contact (Figure 4A); as monolayers, their cell-derived matrices were relatively thin with an average thickness of only 9.0 μm. In contrast to the relatively thin matrix deposition by primary normal fibroblasts (∼9 μm), primed stromal fibroblasts (treated) and TAFs deposited matrices that averaged 15.3 μm and 13.6 μm, respectively (Figure 4B).
Stromagenic 3-D Fibronectin Fibers Are Increasingly Deposited and Progressively Organized in Parallel Patterns
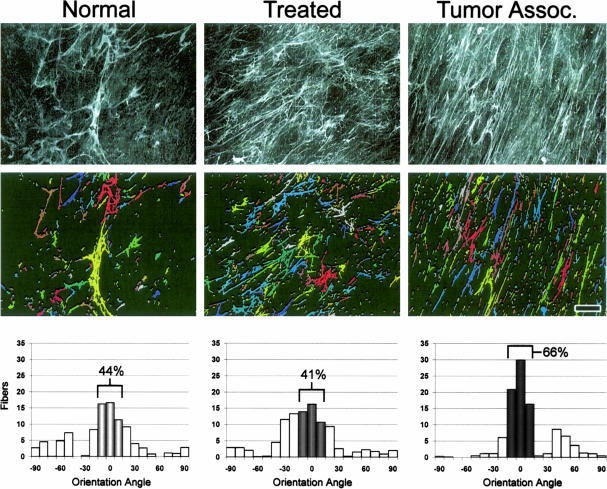
As mentioned before, and shown in Figure 1, the desmoplastic reaction associated with the SCC from which fibroblasts for the 3-D system were harvested (see Materials and Methods), exhibited a rich, fibrous, and parallel patterned ECM. The progressive morphological cell organization observed in our stromagenic system (Figure 3), together with the apparent progressive parallel arrangement of ECM shown in Figure 4, suggests that the unextracted cultures preserve the parallel patterning and increased accumulation of ECM proteins typically observed during desmoplasia in vivo. To evaluate this observation and to sort primary fibroblastic stroma cell types into a normal, primed, or tumor-associated stromagenic phases, we quantified the deposited fibers and measured their scattering organization (Figure 5).
Figure 5.
Stromagenic fibronectin 3-D fibers are progressively organized in parallel patterns. Deconvoluted maximum projection reconstructed images of indirect immunofluorescently labeled fibronectin fibers of normal, treated, and tumor-associated unextracted 3-D cultures (top). Images were analyzed to create a binary image output that identifies individual fibers by presenting them as variable pseudo-colored objects (middle; see Materials and Methods for details). Angles at which fibers were orientated relative to the _x_-axis were rounded to the nearest 10th degree and are shown in the graphs (bottom). The sample mode was arbitrarily set to 0°, thus normalizing the data for comparison purposes (see Table 1 for details and statistics). Numbers indicate percentages of angles positioned within 10° of the angle for which most of the fibers were orientated. Note that less than 50% of fibers were found to be organized in normal and primed (treated) matrices, while more than 60% of fibers were found to be organized parallel within tumor-associated 3-D matrices. Scale bar, 15 μm.
Fibronectin fibers were detected by indirect immunofluorescence (Figure 5, top), and 16 representative images were obtained from each unextracted culture type. Digital analyses counting fibronectin fibers and measuring their relative orientation angle were performed on each acquired and digitally filtered image (Figure 5, middle). To allow comparative analyses among the various images, the mode angle, representing the angle at which the largest score of fibers was observed, was arbitrarily set to 0°. The average percentage of fibers oriented within 10° of the arbitrary angle mode (−10° to 10°) was measured next. Unextracted cultures with low levels of 3-D matrix organization reached low percentages out of the total count of fibers near the mode, while more organized 3-D matrices achieve greater organizational levels due to their fibronectin parallel patterning organization and larger fiber counts (Figure 5).
The total count and orientation measurements show statistically significant parallel patterning organization for 3-D matrices in a progressive stromagenic manner, as well as a tendency for producing a greater total amount of fibers per se (more than two times more tumor-associated stromal fibers than normal). The total fiber count for normal, treated, and TAF cultures was of 253 (SD ±163), 497 (SD ±184), and 573 (SD ± 102), respectively. The percentage number of fibers within 10° of the mode was 49% (SD ± 14.5), 38% (SD ± 8.5), and 64% (SD ± 8.7), respectively for unextracted normal, treated, and TAFs cultures. The graphs in Figure 5 (bottom) are representative of the example images shown in the top panels of the figure, identified by the software, and shown in the middle panels. Both the total count and the orientation tendencies of the stromagenic 3-D matrices were conserved in the extracted cell-free 3-D matrices (data not shown; see Figure 2, for 3-D matrix staging) indicating that, after organizing their microenvironments, fibroblasts are no longer needed to conserve the organizational patterning of the 3-D matrices leaving stable 3-D substrates for storage and further experimental use. Taken together, the fiber count and mean percentage of parallel patterning measured in the stromagenic 3-D system confirmed that these statistically relevant differences are measurable parameters.
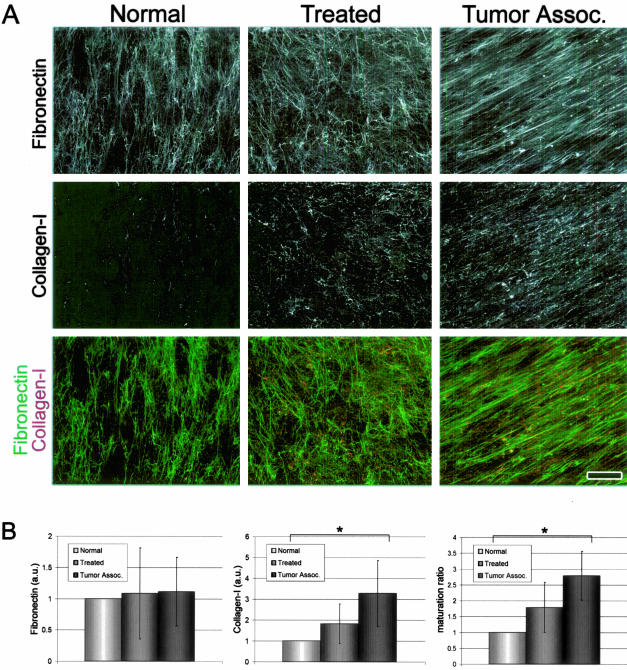
The Maturation State of 3-D Matrices Intensifies in a Stromagenic-Dependent Manner
Contrary to conditions in vitro in which purified type I collagen spontaneously polymerizes into mesh-like 3-D gels, in vivo type I collagen polymerization is cell-driven and dependent on fibronectin fibrillogenesis.36–38 An increasing type I collagen to fibronectin ratio is indicative of a mature 3-D matrix state in vitro. Moreover, desmoplastic stromal ECM in vivo is often characterized by an elevated level of type I collagen (Figure 1). To investigate whether the various unextracted stromagenic cultures mimic the progressive expression of type I collagen during desmoplasia in vivo, we calculated the ratios of type I collagen to fibronectin in unextracted ECMs. As predicted, Figure 6A shows that the ECM of unextracted cultures contains type I collagen in a stromagenic phase-progressive manner while the fibronectin in vitro expression of polymerized ECMs remains stable. The graphs shown in Figure 6B quantitatively demonstrate that TAFs produce ECMs at threefold greater maturation rates than normal fibroblasts. Moreover, this difference in the ratio of type I collagen to fibronectin was found to be statistically significant (P < 0.01). The type I collagen to fibronectin ratio observed at the unextracted culture stages remained unchanged after cellular lysis to yield the extracted 3-D matrices (data not shown).
Figure 6.
The maturation state of 3-D matrices intensifies in a stromagenic-dependent manner. A: Maximum projection reconstructed confocal images of indirect immunofluorescence showing ECM protein assembly in stromagenic unextracted 3-D cultures. Samples were labeled for fibronectin (top) or type I collagen (middle). Overlaid images showing fibronectin (in green) and type I collagen (collagen-I, in red) are shown at the bottom. Images were submitted to identical filters (see Materials and Methods for details). B: Graph bar representing quantification of fibronectin fibers, type I collagen fibers, and matrix maturation ratio for each stromagenic cell type (see Table 1 for details and statistics). Asterisks mark samples statistically significantly different from all others. Note the increase in type I collagen levels and 3-D matrix maturation during stromagenesis. Scale bar, 25 μm.
Extracted 3-D Matrices Derived from Assorted Stromagenic Phases Differentially Modify Primary Normal Fibroblasts
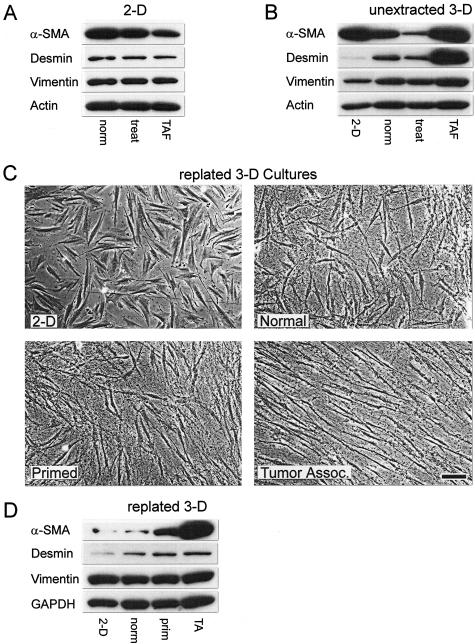
To evaluate whether harvested TAFs retained α-SMA and desmin expression, all stromagenic fibroblasts were tested by Western blot analysis for the expression of these myofibroblastic markers and were compared to the mesenchymal marker vimentin while the total protein load was normalized to actin. In our initial analysis of α-SMA and desmin expression, we obtained lysates of the various stromagenic fibroblasts cultured under classic 2-D conditions; on fibronectin-coated plates overnight. To our surprise, on 2-D culture, we observed that TAFs’ α-SMA expression was down-regulated when compared to normal skin fibroblast α-SMA levels of expression, whereas vimentin and desmin levels showed no change (Table 1 and Figure 7A). To investigate whether the apparent decrease in expression of the desmoplastic stromal marker α-SMA was due to 2-D conditions, we measured all marker protein levels in our progressive stromagenesis 3-D system at the unextracted culture stage (see Figure 2, for 3-D matrix staging). As shown and quantified in Figure 7B and Table 1, respectively, and contrary to 2-D conditions, unextracted tumor-associated 3-D cultures retained both α-SMA and desmin expression, akin to the expression of these markers in vivo (Figure 1), whereas the levels of vimentin remained unchanged, as expected.
Table 1.
Microenvironment-Dependent α-SMA, Desmin, and Vimentin Expression Levels
| Normal | Treated | Tumor-associated | ||||
|---|---|---|---|---|---|---|
| Avg. | SE (n) | Avg. | SE (n) | Avg. | SE (n) | |
| 2-D cultures | ||||||
| α-SMA | 1 | 0 (6) | 0.696** | 0.08 (6) | 0.528** | 0.09 (6) |
| Desmin | 1 | 0 (6) | 0.842 | 0.13 (6) | 0.951 | 0.18 (6) |
| Vimentin | 1 | 0 (6) | 1.236 | 0.08 (6) | 1.029 | 0.12 (6) |
| 3-D cultures | ||||||
| α-SMA | 0.636* | 0.02 (4) | 0.266* | 0.05 (4) | 0.682* | 0.10 (4) |
| Desmin | 2.741** | 0.14 (6) | 5.637** | 1.62 (6) | 24.722** | 5.84 (6) |
| Vimentin | 1.170** | 0.02 (6) | 1.051 | 0.17(6) | 1.235 | 0.14 (6) |
| Replated in 3-D matrices | ||||||
| α-SMA | 0.837** | 0.03 (12) | 1.130 | 0.34 (6) | 3.761** | 1.00 (6) |
| Desmin | 1.217 | 0.24 (6) | 1.324 | 0.46 (6) | 1.669 | 0.44 (6) |
| Vimentin | 0.997 | 0.07 (6) | 0.924 | 0.09 (6) | 0.874 | 0.12 (6) |
Figure 7.
Extracted 3-D matrices derived from assorted stromagenic phases differentially modify primary normal fibroblasts. Western blots showing protein levels of α-SMA, desmin, and vimentin expression on 2-D (A) or within pre-extracted 3-D (B) cultures for normal (norm), treated (treat), or TAFs. C: Phase contrast images of normal primary human fibroblasts plated on 2-D coated fibronectin or within normal, primed, or tumor-associated extracted 3-D matrices. D: Western blot showing α-SMA, desmin, and vimentin expression levels of normal primary human fibroblasts plated on 2-D coated fibronectin or within normal (norm), primed (prim), or tumor-associated (TA) extracted 3-D matrices (see Table 1 for statistics). Note how different stromagenic phases differentially modify the normal primary fibroblast morphology, organization, and desmoplastic marker expression. Scale bar, 25 μm.
Because extracting the 3-D matrices did not affect the molecular or physical matrix characteristics (see above), we proceeded to replate control normal primary fibroblasts within the assorted stromagenic 3-D matrices. Figure 7C shows that similar to unextracted cultures normal fibroblasts present increased spindle-shaped morphology and parallel cell-to-cell organization dependent on the stromagenic phase of the particular 3-D matrix (compare Figure 7C and Figure 3B). We then evaluated whether the extracted 3-D matrices can induce α-SMA and desmin protein expression to levels similar to the unextracted 3-D cultures while keeping the levels of vimentin unchanged. As Figure 7D illustrates and Table 1 summarizes, extracted 3-D matrices alone are sufficient to regulate normal fibroblast α-SMA and desmin expression in a stromagenic-progressive manner.
Discussion
Host stromagenesis is a cancer-induced connective tissue process that supports tumor progression by providing the tumor with a progressive and permissive mesenchymal microenvironment.2–9,13,15,17,18,29,35,39–42 Fibroblastic cells are responsible for both the production and regulatory dynamics of the stromal ECM.2–4,6,17 Moreover, fibroblasts are capable of mechanosensing their microenvironment through integrin receptors. By transmitting information bidirectionally, fibroblasts can cause modifications to cells and to their surrounding matrices.43–45 Due to lack of a suitable, physiologically relevant, experimental system, the events that trigger stromal priming and the mechanisms that incite and support tumor progression are mostly unknown. Here, we introduce a new _in vivo_-like 3-D stromagenic system derived from TAFs at diverse stages of tumor development. Harvested primary stromal fibroblasts, obtained from stroma at different stages of tumor progression, do not retain in vivo stromagenic characteristics when cultured on traditional 2-D conditions, eg, expression of desmoplastic marker α-SMA. However, the 3-D system herein presented, successfully mimics in vivo fibroblastic stromagenic progression of both cellular and ECM components. Our working hypothesis postulated that even though these fibroblasts do not retain stromagenic features on 2-D cultures, they are still capable of deriving 3-D cultures, which effectively retain the original stromagenic characteristics. The data presented herein does not address whether stromagenesis in vivo is due to a progressive stromal phenotypic switch or to alternative mechanisms such as recruiting myofibroblastic cells from remote or neighboring reservoirs. More importantly for this study, the use of cell-free stroma fibroblast-derived 3-D matrices, have demonstrated that _in vivo_-like 3-D matrices alone contain sufficient topographical and molecular information required for triggering desmoplastic differentiation of normal fibroblasts in vitro.
As previously described, pathological stromagenesis associated with tumor progression can be either oncofetal or desmoplastic.2 Our first observation, based on the immunohistochemical analyses of desmoplastic marker α-SMA, confirmed that in vivo, stroma associated with chemically induced skin SCC, is desmoplastic. Moreover, the presence of desmin within the desmoplastic assists to further characterize the type of myofibroblastic cells observed.25 Fibroblasts used to produce the stromagenic 3-D system were harvested from discrete tissue samples derived from regions of normal skin, carcinogen-treated skin, or SCCs and corresponded to the three stromagenic phases of normal, primed, and tumor-associated or activated stroma, respectively.8,9
Because the in vivo tumor-associated stroma was identified as desmoplastic, as opposed to oncofetal, consistent with validation of the system as physiologically relevant, we wanted to ensure that our cell-derived 3-D system retained its pathological characteristics typical for desmoplasia in vivo. The three progressive stromagenic fibroblasts were cultured under conditions permissive for matrix production (see Materials and Methods30,31). The resultant unextracted 3-D cultures were tested for their progressive stromagenic mimetic capabilities. The system proved to be faithful to its cellular in vivo counterparts, as assessed by its effects on altering fibroblastic spindle-shape morphology and organization, increasing cell density, and regulating desmoplastic marker expression. Moreover, the 3-D ECM component of the system resembled their in vivo progressive counterparts by expressing desmoplastic characteristics, such as highly organized parallel-patterned matrix fibers and substantial ECM maturation reflected by increased ratios of type I collagen to fibronectin. These experiments not only confirmed that unextracted 3-D cultures imitate the in vivo stromagenic environment but also facilitated sorting or classifying fibroblasts as normal, primed, or tumor-associated by measuring their characteristics in our 3-D system.
We, and others, have previously postulated that fibroblastic cells are directly affected by the composition, three-dimensionality, and rigidity of their substratum.32,43,44,46–50 Additionally, we proposed that fibroblast-derived 3-D matrices resemble in vivo mesenchymal microenvironments,32 which are often found to be more pliable than traditional culturing substrates and, thus, differentially transmit signals to cells.32,46–48,51 In this study, we successfully established a progressive 3-D system derived from fibroblastic cells associated with diverse stages of carcinogenesis. The system presents significant differences in 2-D versus 3-D culturing conditions in which 3-D conditions successfully mimic desmoplastic stroma settings, eg, higher α-SMA and desmin expression while vimentin levels remained unchanged. The differences between 2-D versus 3-D cultures suggested that harvested stromagenic fibroblasts may retain their tumor-associated differentiation characteristics although a 3-D matrix is necessary to trigger these characteristics. These observations also emphasize the need to use physiologically relevant 3-D systems for stromagenic studies and underline the in vivo microenvironmental relevance of our system. Similar to our previous results,32 we were able to show that even though primary fibroblasts apparently lose their differentiated characteristics under traditional 2-D culturing conditions, when presented with cell-derived 3-D matrices, these cells are capable of retaining their physiological characteristics observed in vivo.
After depositing and organizing a substantial ECM, unextracted tumor-associated 3-D cultures effectively induced re-expression of α-SMA and desmin while the same fibroblasts, under traditional 2-D conditions, express only moderate levels of these marker proteins, while vimentin remained unchanged. These observations suggest that 3-D matrices produced and organized by TAFs are necessary to induce desmoplastic marker expression. Furthermore, by replating normal primary fibroblasts within cell-extracted TAF-derived 3-D matrices, we learned that stromagenic 3-D matrices are sufficient to induce increased spindle-shaped morphology, cell-to-cell parallel organizational patterning, as well as α-SMA and desmin expression that resemble both in vivo desmoplastic stroma and stromagenic unextracted cultures. Similar α-SMA and desmin expression in unextracted tumor-associated stromagenic and normal replated (within TAF-derived 3-D matrices) cultures suggests that stroma-derived 3-D matrices are sufficient to induce desmoplastic differentiation in advanced phases of stromagenesis. Taken together, our results suggest that tumor-associated stroma-derived 3-D matrices are both necessary and sufficient for the induction of desmoplastic differentiation.
It has been reported that during wound healing, inflammation, and fibroblastic stroma progression, fibroblasts differentiate through various stages.41,52–55 α-SMA expression levels can be used to identify these differentiation stages. For example, protomyofibroblasts often down-regulate α-SMA expression before progressing into de novo differentiated myofibroblasts and up-regulating α-SMA protein levels back.23 We believe that in our unextracted 3-D stromagenic system, the primed-phase 3-D cultures are analogous to in vivo protomyofibroblasts, which slightly down-regulate α-SMA expression while tumor-associated 3-D cultures successfully mimic de novo myofibroblast redifferentiation accompanied by substantial increase in α-SMA protein expression. It is known that functionally differentiated myofibroblasts, generate greater contractile force than protomyofibroblasts, which is reflected by a higher organization of extracellular fibronectin into fibrils.25 Moreover, α-SMA inhibition has been found to have an important role in myofibroblastic-dependent wound contraction resulting in decreased expression of type I collagen.56 The changing expression of both α-SMA and desmin together with the organization and molecular composition of the ECM observed herein, suggests that fibroblasts might undergo dynamic differentiation during stromagenesis and proposes that our 3-D system mimics the myofibroblastic characteristics of in vivo desmoplastic stromagenesis associated with carcinogenesis, as well as other wound closure and fibrocontractive cirrhotic changes.23,25,56
Another established pathological characteristic of stroma progression is the extensive cross linking of ECM fibers. Transglutaminase is an enzyme known to catalyze the cross-linking of several abundant ECM proteins including fibronectin and fibrin. Elevated expression of transglutaminase-2 has been shown to be associated with wound healing and tumor-associated stroma.57,58 The different characteristics of the stromagenic matrices in vitro, and the potential increase in tension that should be induced by the de novo expression of α-SMA observed in this study, may represent different pliability characteristics of the three stromagenic phases in vivo. The presence of greater amounts of type I collagen may contribute to a physical architecture that transmits different mechanical cues to their resident cells. It has been proposed that substrate tension up-regulates type I collagen polymerization59 and that different tensile forces exerted on the ECM can induce variation of signaling cascades within their resident cells.32,51,57,58
Studies reviewing variations in ECM rigidity during stromagenesis that selectively induce differential signaling pathways and analyses of stroma permissiveness during carcinogenesis are beyond the scope of this study. Nevertheless, these studies are potentially achievable by the use of this newly introduced stromagenic _in vitro_-like 3-D system. In vitro systems such as those described in this study should facilitate the possibility of conducting more meticulous examinations of stromagenic events that could assist in developing stroma targeted therapeutic drugs in the future.
Acknowledgments
We thank Drs. J. Chernoff, J. Cheng, and D.A. Beacham for critical comments; P. Ovseiovich-Berdichevsky for graphic design consultation; M. Lech for technical assistance; and K.B. Buchheit for proofreading.
Footnotes
Address reprint requests to Edna Cukierman, Basic Science/Tumor Cell Biology, Fox Chase Cancer Center, 333 Cottman Ave., Room W428, Philadelphia, PA 19111-2497. E-mail edna.cukierman@fccc.edu.
Supported by the American Association of Cancer Research (AACR) [Pennsylvania Department of Health career development award to E.C. (the Department specifically disclaims responsibility for any analyses, interpretations, or conclusions)], the National Institutes of Health/National Cancer Institute (grant CA006927 and grant 1 R21 CA109442-01 to E.C.), and the Commonwealth of Pennsylvania.
References
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part I): active stromal participants in tumor development and progression? Histol Histopathol. 2002;17:599–621. doi: 10.14670/HH-17.599. [DOI] [PubMed] [Google Scholar]
- Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part II): functional impact on tumor tissue. Histol Histopathol. 2002;17:623–637. doi: 10.14670/HH-17.623. [DOI] [PubMed] [Google Scholar]
- Silzle T, Randolph GJ, Kreutz M, Kunz-Schughart LA. The fibroblast: sentinel cell and local immune modulator in tumor tissue. Int J Cancer. 2004;108:173–180. doi: 10.1002/ijc.11542. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Li G, Satyamoorthy K, Meier F, Berking C, Bogenrieder T, Herlyn M. Function and regulation of melanoma-stromal fibroblast interactions: when seeds meet soil. Oncogene. 2003;22:3162–3167. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation. 2002;70:486–497. doi: 10.1046/j.1432-0436.2002.700903.x. [DOI] [PubMed] [Google Scholar]
- Beacham DA, Cukierman E: Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol (in press) [DOI] [PubMed] [Google Scholar]
- Cukierman E. A visual-quantitative analysis of fibroblastic stromagenesis in breast cancer progression. J Mammary Gland Biol Neoplasia. 2004;9:311–324. doi: 10.1007/s10911-004-1403-y. [DOI] [PubMed] [Google Scholar]
- Bauer G. Elimination of transformed cells by normal cells: a novel concept for the control of carcinogenesis. Histol Histopathol. 1996;11:237–255. [PubMed] [Google Scholar]
- Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C. The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci. 2004;117:1495–1502. doi: 10.1242/jcs.01000. [DOI] [PubMed] [Google Scholar]
- Abelev GI. Differentiation antigens: dependence on carcinogenesis mechanisms and tumor progression (a hypothesis). Mol Biol. 2003;37:2–8. [PubMed] [Google Scholar]
- Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol Med Today. 2000;6:324–329. doi: 10.1016/s1357-4310(00)01756-1. [DOI] [PubMed] [Google Scholar]
- Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- Mareel M, Leroy A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol Rev. 2003;83:337–376. doi: 10.1152/physrev.00024.2002. [DOI] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta V, Giannelli G. Cancer invasion: watch your neighbourhood! Tumori. 2003;89:343–348. doi: 10.1177/030089160308900401. [DOI] [PubMed] [Google Scholar]
- Sung SY, Chung LW. Prostate tumor-stroma interaction: molecular mechanisms and opportunities for therapeutic targeting. Differentiation. 2002;70:506–521. doi: 10.1046/j.1432-0436.2002.700905.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Liyanarachchi S, Davuluri RV, Auer H, Martin EW, Jr, de la Chapelle A, Frankel WL. Role of cancer-associated stromal fibroblasts in metastatic colon cancer to the liver and their expression profiles. Oncogene. 2004;23:7366–7377. doi: 10.1038/sj.onc.1208013. [DOI] [PubMed] [Google Scholar]
- Noel A, Kebers F, Maquoi E, Foidart JM. Cell-cell and cell-matrix interactions during breast cancer progression. Curr Top Pathol. 1999;93:183–193. [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–2483. [PubMed] [Google Scholar]
- Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- Dugina V, Alexandrova A, Chaponnier C, Vasiliev J, Gabbiani G. Rat fibroblasts cultured from various organs exhibit differences in alpha-smooth muscle actin expression, cytoskeletal pattern, and adhesive structure organization. Exp Cell Res. 1998;238:481–490. doi: 10.1006/excr.1997.3868. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Schor SL, Ellis IR, Jones SJ, Baillie R, Seneviratne K, Clausen J, Motegi K, Vojtesek B, Kankova K, Furrie E, Sales MJ, Schor AM, Kay RA. Migration-stimulating factor: a genetically truncated onco-fetal fibronectin isoform expressed by carcinoma and tumor-associated stromal cells. Cancer Res. 2003;63:8827–8836. [PubMed] [Google Scholar]
- Bondeson L, Lindholm K. Prediction of invasiveness by aspiration cytology applied to nonpalpable breast carcinoma and tested in 300 cases. Diagn Cytopathol. 1997;17:315–320. doi: 10.1002/(sici)1097-0339(199711)17:5<315::aid-dc2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Han S, Sidell N, Roser-Page S, Roman J. Fibronectin stimulates human lung carcinoma cell growth by inducing cyclooxygenase-2 (COX-2) expression. Int J Cancer. 2004;111:322–331. doi: 10.1002/ijc.20281. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- Cukierman E. Cell migration analyses within fibroblast-derived 3-D matrices. Guan J, editor. Totowa: Humana Press,; Cell MigrationDevelopmental Methods and Protocols. 2004:pp 79–93. doi: 10.1385/1-59259-860-9:079. [DOI] [PubMed] [Google Scholar]
- Cukierman E. Preparation of extracellular matrices produced by cultured fibroblasts. Bonifacino JS, Dasso M, Lippincott-Schwartz J, Harford JB, Yamada KM, editors. Philadelphia: John K. Wiley & Sons; Current Protocols in Cell Biology. 2002:pp 10.19.11–10.19.14. [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- Quade BJ, McDonald JA. Fibronectin’s amino-terminal matrix assembly site is located within the 29-kDa amino-terminal domain containing five type I repeats. J Biol Chem. 1988;263:19602–19609. [PubMed] [Google Scholar]
- Labat-Robert J. Fibronectin in malignancy: effect of aging. Semin Cancer Biol. 2002;12:187–195. doi: 10.1016/S1044-579X(02)00022-6. [DOI] [PubMed] [Google Scholar]
- Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins a11b1 and a2b1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA, Kelley DG, Broekelmann TJ. Role of fibronectin in collagen deposition: Fab′ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol. 1982;92:485–492. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupa SM, Menard S, Forti S, Tagliabue E. New insights into the role of extracellular matrix during tumor onset and progression. J Cell Physiol. 2002;192:259–267. doi: 10.1002/jcp.10142. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Meier F, Herlyn M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation. 2002;70:522–536. doi: 10.1046/j.1432-0436.2002.700906.x. [DOI] [PubMed] [Google Scholar]
- Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- Buttery RC, Rintoul RC, Sethi T. Small cell lung cancer: the importance of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1154–1160. doi: 10.1016/S1357-2725(03)00261-9. [DOI] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Pankov R, Cukierman E. Dimensions and dynamics in integrin function. Braz J Med Biol Res. 2003;36:959–966. doi: 10.1590/s0100-879x2003000800001. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–640. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- Geiger B. Cell biology: encounters in space. Science. 2001;294:1661–1663. doi: 10.1126/science.1066919. [DOI] [PubMed] [Google Scholar]
- Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004;279:33024–33034. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- Walpita D, Hay E. Studying actin-dependent processes in tissue culture. Nat Rev Mol Cell Biol. 2002;3:137–141. doi: 10.1038/nrm727. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Cheng JD, Weiner LM. Tumors and their microenvironments: tilling the soil. Clin Cancer Res. 9:1590–1647. [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-[kappa]B functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Eikmans M, Baelde JJ, de Heer E, Bruijn JA. ECM homeostasis in renal diseases: a genomic approach. J Pathol. 2003;200:526–536. doi: 10.1002/path.1417. [DOI] [PubMed] [Google Scholar]
- van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82:539–548. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G, Chaponnier C. The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol. 2002;157:657–663. doi: 10.1083/jcb.200201049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P, Grenard P, Aeschlimann P, Langley M, Blain E, Errington R, Kipling D, Thomas D, Aeschlimann D. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. J Cell Sci. 2004;117:3389–3403. doi: 10.1242/jcs.01188. [DOI] [PubMed] [Google Scholar]
- Verderio EA, Johnson T, Griffin M. Tissue transglutaminase in normal and abnormal wound healing. Amino Acids. 2004;26:387–404. doi: 10.1007/s00726-004-0094-4. [DOI] [PubMed] [Google Scholar]
- He YL, Macarak EJ, Korostoff JM, Howard PS. Compression and tension: differential effects on matrix accumulation by periodontal ligament fibroblasts in vitro. Connect Tissue Res. 2004;45:28–39. doi: 10.1080/03008200490278124. [DOI] [PubMed] [Google Scholar]