Peptidyl-prolyl cis/trans isomerases and transcription: is there a twist in the tail? (original) (raw)
Abstract
Eukaryotic transcription is regulated predominantly by the post-translational modification of the participating components. One such modification is the cis–trans isomerization of peptidyl-prolyl bonds, which results in a conformational change in the protein involved. Enzymes that carry out this reaction include the yeast peptidyl-prolyl cis/trans isomerase Ess1 and its human counterpart Pin1, both of which recognize phosphorylated target motifs exclusively. Consequently, they operate together with proline-directed serine–threonine kinases and phosphatases. High-profile client proteins involved in transcription include steroid hormone receptors, cell-cycle regulators and immune mediators. Other key targets are elements of the transcription machinery, including the multiply phosphorylated carboxy-terminal domain of RNA polymerase II. Changes in isomerase activity have been shown to alter the transactivation potential, protein stability or intracellular localization of these client proteins. The resulting disruption to developmental processes and cell proliferation has been linked, in some cases, to human cancers.
Keywords: co-activators, Ess1, hormone receptors, Pin1, RNA polymerase II
Introduction
Although the template-dependent synthesis of RNA molecules by polymerase enzymes is a linear and highly conserved process, it is subject to a plethora of regulatory inputs. The complexities of transcriptional control are not restricted to metazoans; they operate even in unicellular organisms, such as yeast. A common theme is the post-translational modification of participating components, including subunits of RNA polymerases (Sims et al, 2004). Prominent among these post-translational modifications is phosphorylation, which can regulate transcriptional events in several ways. A more recently identified mechanism involves conformational change, which can be regulated in a phosphorylation-dependent manner by some parvulin-like peptidyl-prolyl isomerases (PPIs). PPIs are enzymes that catalyse the cis–trans isomerization of peptide bonds that are amino-terminal to proline residues in polypeptide chains (Schiene & Fischer, 2000).
The yeast parvulin-like PPI Ess1/Ptf1 (hereafter called Ess1) and its human orthologue Pin1 both consist of two domains—an N-terminal WW domain and a carboxy-terminal catalytic domain—joined by a short linker. The WW domain recognizes serine–proline (S–P) or threonine–proline (T–P) motifs, but only when the S or T is phosphorylated—that is, _p_S-P or _p_T-P (Landrieu et al, 2001; Lu et al, 2002; Verdecia et al, 2000). These motifs are generated by proline-directed protein kinases, such as cyclin-dependent kinases (CDKs), and mitogen-activated protein kinases (MAPKs; Chen et al, 2001; Loyer et al, 2005; Sanchez & Dynlacht, 2005). The cis and trans forms of the peptide bond between _p_S/_p_T and P exist in equilibrium; however, in proteins the trans form predominates and only 5–10% of peptidyl-proline bonds exist in the cis form (Reimer et al, 1998). Rotation around the bond is restricted due to its partial double-bond character and the potential effects of adjacent charged side chains, and can be rate limiting during protein folding. The catalytic domains of Ess1 and Pin1 accelerate isomerization of this bond whereby isomerization is contingent on prior phosphorylation. Although Pin1 increases the rate of isomerization considerably in vitro, it is not clear whether its function in vivo is always catalytic, rather than stoichiometric; the occupation of target sites by PPIs could result in some of the biological effects ascribed to them by out-competing the binding of other factors.
Ess1 was first identified as an essential gene in yeast, emphasizing the importance of this control mechanism (Hanes et al, 1989). Deletion of Ess1 in yeast, or its unique counterpart Pin1 in HeLa cells, causes cell-cycle arrest in late mitosis (Lu et al, 1996). However, homozygous Pin1−/− mice are viable and fertile, even though embryonal fibroblasts derived from these mice grow slowly and show cell-cycle re-entry defects (Fujimori et al, 1999). Furthermore, Pin1 has been shown to interact with several mitotic regulators, including Cdc25, a Cdc2-directed phosphatase, and Plk1, in a phosphorylation-dependent manner (Crenshaw et al, 1998; Shen et al, 1998). As well as these proteins, Pin1 substrates include several transcription factors for which the consequences of Pin1 action seem to be modulation of their transactivation potential, primarily through changes in protein stability and, in some cases, intracellular localization (Table 1). Furthermore, as well as constituting a select group of high-profile transcription factors involved in cell proliferation, survival and oncogenesis, known clients of Pin1 include RNA polymerase II (RNAP II) itself, implying that Pin1 might also have a more direct, facilitating role in the mechanism of eukaryotic gene transcription.
Table 1.
Targets, facilitating kinases and consequences of Pin1-mediated transcriptional control
| Client | Motif | Kinase | Consequence | References |
|---|---|---|---|---|
| cJun | S63-P, S73-P | JNK/SAPKs | Transactivation | Wulf et al, 2001 |
| cFos | T232-P, T325-P,T331-P, S374-P | ERKs | Transactivation | Monje et al, 2005 |
| RARα | S77-P | Cdk7 | Transactivation, degradation | Brodani et al, 2005 |
| SRC-3/AIB1 | ND | ND | Transactivation, degradation | Yi et al, 2005 |
| c-Myc | S58-P, S62-P | ERKs | Dephosphorylation by PP2A, degradation | Yeh et al, 2004 |
| β-catenin | S246-P | ND | Stabilization, nuclear localization | Ryo et al, 2001 |
| p53 | S33-P, T81-P, S315-P, S46-P | p38mapk, Cdc2, JNK/SAPKs | Stabilization, transactivation | Zacchi et al, 2002; Zheng et al, 2002; Wulf et al, 2002 |
| p73 | S412-P, T442-P, T482-P | p38mapk | Stabilization, transactivation | Mantovani et al, 2004 |
| p65/Rel | T254-P | ND | Stabilization, transactivation | Ryo et al, 2003 |
| IRF3 | S339-P | ND | Degradation | Saitoh et al, 2006 |
| Rpb1 | S2-P, S5-P, Cdc2/cyclin B | Cdk7, Cdk9 | Dephosphorylation by Fcp1 | Xu et al, 2003; Wilcox et al, 2004 |
Controlling transactivation by activator protein 1 subunits
The N-terminal transactivation domain of cJun, which is a component of the transcription factor AP1 (activator protein 1), has been shown to require phosphorylation by Jun N-terminal kinases/stress-activated protein kinases (JNK/SAPKs) and the subsequent action of Pin1 to attain full activity (Wulf et al, 2001). Target genes of cJun include cyclin D1, and positive correlations between Pin1 and cyclin D1 expression levels in breast and oral squamous carcinomas have been observed (Miyashita et al, 2003; Wulf et al, 2001). The cJun paradigm also applies to at least one other AP1 component, as cFos interacts with Pin1 through _p_S/T–P motifs generated by extracellular signal-regulated kinase (ERK) phosphorylation of the C-terminal transactivation domain. Pin1 expression also augmented transcriptional responses to platelet-derived growth factor by cFos and its isolated transactivation domain (as a GAL4 fusion), although it remains unclear whether this required the isomerase activity of Pin1 (Monje et al, 2005). The suggestion that Pin1 is needed for cJun (and cFos) to acquire their fully active conformation has yet to be substantiated; given that both are conditional substrates for ubiquitin-dependent and ubiquitin-independent proteasomal degradation (Acquaviva et al, 2002), the underlying mechanism could involve changes in their stability. Other AP1 components might also require Pin1 for activation, potentially implicating it in tumour progression and metastasis.
Stability and activity of steroid hormone receptors
The modulation of transcription factor activity through changes in stability was first exemplified by β-catenin and its role in Wingless/Int-related (Wnt) signalling (Logan & Nusse, 2004). Pin1 was reported to act as a positive regulator of β-catenin by antagonizing its interaction with the adenomatous polyposis coli protein (APC) complex, resulting in the stabilization and nuclear accumulation of β-catenin and the stimulation of its target genes, such as c-myc, fibronectin and cyclin D1, together with T-cell factor 4 (Tcf4; Ryo et al, 2001; Shaw, 2002). As with cJun, links between Pin1, Wnt signalling and cyclin D1 expression in several types of cancer have been reported (Kuramochi et al, 2006; Pang et al, 2006; Zhou & Gao, 2006).
Recently, a second mechanism has been described through which Pin1 action upregulates Tcf4/β-catenin in phosphatase and tensin homologue (PTEN)-negative prostate cancer cells (Chen et al, 2006). Despite elevated Akt–protein kinase B signalling and consequent inactivation of glycogen synthase kinase 3β (GSK3β)—the kinase presumed to be responsible for β-catenin phosphorylation (Ryo et al, 2001)—overexpression of Pin1 in these cells led to an increase in Tcf4/β-catenin transcriptional activity without affecting β-catenin levels. As the androgen receptor (AR) also uses β-catenin as a co-activator and might therefore compete with Tcf4 for limiting β-catenin, Chen and colleagues explored the effect of Pin1 on AR/β-catenin interactions. Pin1 was found to antagonize the interaction of β-catenin with the ligand-binding domain of AR, thereby preventing transactivation by the AR/β-catenin complex and resulting in concomitant Tcf4/β-catenin activation. However, it is unclear whether the mechanism involves isomerization of β-catenin, because the Pin1-insensitive β-catenin S246A mutant was not tested and the protein kinase acting in place of GSK3β was not identified. Indeed, significant inhibition was achieved with a catalytically inactive Pin1 mutant, which questions whether isomerization is the primary mechanism for AR/β-catenin downregulation.
Pin1 also encroaches on the regulation of other hormone receptor systems. Both the stabilization of and ligand-independent transcriptional activation by the retinoic-acid receptor RARα require phosphorylation on S77 within its AF1 domain by Cdk7 (Kopf et al, 2000; Rochette-Egly et al, 1997), which is an atypical cyclin-dependent kinase and a component of the basal transcription factor TFIIH (Loyer et al, 2005). However, Pin1 overexpression destabilized RARα with a consequent loss of reporter transactivation (Brondani et al, 2005). An S77A mutation in RARα prevented its interaction with Pin1 in vitro and stabilized the receptor in COS cells, although at the expense of its transcriptional activity. This scenario is reminiscent of the relationship between transcriptional activation and proteasome-mediated protein degradation, which has been elegantly shown for the oestrogen receptor (Reid et al, 2003). In the case of RARα, the role of Pin1 would be to switch the phosphorylated receptor from the transcriptionally competent to the ubiquitination-sensitive conformation. Too much Pin1 activity would trigger premature ubiquitination of RARα, thereby suppressing transcription.
The influence of Pin1 on a steroid-receptor coactivator (SRC) has also been reported. When co-expressed in HeLa cells, SRC-3, which is also known as AIB1/ACTR/pCIP/RAC3/TRAM1 (Xu & Li, 2003), and Pin1 synergistically activated progesterone receptor-mediated reporter expression (Yi et al, 2005). Again, Pin1 expression had a dual effect: its interaction with SRC-3 increased the amount of p300/CBP that was associated with SRC-3 and recruited to an endogenous oestrogen-responsive promoter. Pin1 also reduced the level of SRC-3 in cells in a manner that was reversible by the proteasome inhibitor MG132, again indicating that Pin1 acts as a linchpin between transcriptional activation and activator degradation.
Conformation and stability of cell-cycle regulators
Cis–trans isomerization as a mechanism to control protein stability extends to several transcription factors that are prominent in cell-cycle control. In response to DNA damage, phosphorylation of p53 by stress-dependent kinases generates target motifs for Pin1, which alters the conformation of p53 such that it is less-readily bound by the E3 ubiquitin ligase Mdm2 (Zacchi et al, 2002; Zheng et al, 2002). The net result is stabilization and nuclear accumulation of p53, the activation of checkpoint controls and ultimately the upregulation of target genes, including mdm2, BCL2-associated X protein (Bax) and killer/DR5, to increase the amount of apoptosis. These responses to genotoxic insults are impaired in Pin1−/− mouse embryonic fibroblasts (MEFs; Berger et al, 2005; Wulf et al, 2002; Zacchi et al, 2002; Zheng et al, 2002).
Genotoxic stress also triggers apoptosis in cells lacking p53 through the action of its relatives p63 and p73 (Murray-Zmijewski et al, 2006). Intriguingly, Pin1 seems to be equally important in the regulation of p73. Mantovani and colleagues were able to show, in a p53-null background, that loss of Pin1 destabilized p73 under both normal and stress conditions, and compromised expression from p73 target gene promoters such as Bax, p53-induced gene 3 (PIG3) and p53-regulated apoptosis-inducing protein 1 (p53AIP1; Mantovani et al, 2004). Treatment of cells with cisplatin or doxorubicin induced phosphorylation of p73 on Y99 by the c-Abl tyrosine kinase (Tsai & Yuan, 2003; Yuan et al, 1999), and subsequently on three S/T residues within the proline-rich region present in the α and β isoforms of p73 by p38mapk (Sanchez-Prieto et al, 2002). The _p_S/T–P motifs bind Pin1, and this was enhanced by overexpression of either c-Abl or a combination of p38mapk and active MAPK kinase 6 (MKK6). Pin1 binding could be correlated with a conformational change in p73, the association of p73 with p300 and its acetylation on lysine residues. Acetylation might be important for targeting p73 to pro-apoptotic promoters (Costanzo et al, 2002). Overexpression of p300 also stabilized p73 in a Pin1-dependent manner, indicating that lysine acetylation might antagonize ubiquitination of p73 and its degradation by the proteasome (Mantovani et al, 2004).
In direct contrast to its role in promoting p53 stability, Pin1 has been shown to contribute to the ubiquitin-mediated degradation of other transcription factors, as reported for chorion factor 2 (CF2), which is a protein involved in Drosophila egg development (Hsu et al, 2001; Shaw, 2002). Another transcription factor influenced by this mechanism is the cell-cycle regulator c-Myc. In response to Ras signalling, S62 in the N terminus of c-Myc is phosphorylated by ERKs, which enhances c-Myc stability. Subsequently, as Ras signalling subsides, GSK3β becomes active and phosphorylates c-Myc a second time on T58 (Sears et al, 2000). Phosphorylation of T58 is associated with the destabilization of c-Myc: T58 mutations have been observed in Burkitt's lymphomas, and linked to the increased stability and transforming potential of c-Myc (Dang et al, 2005). However, destabilization of c-Myc also requires dephosphorylation of S62 by protein phosphatase 2A (PP2A), which is a conformation-sensitive phosphatase (Zhou et al, 2000). It seems that Pin1 binds to c-Myc at the _p_S62-P and _p_T58-P motifs to catalyse cis–trans isomerization of the seryl-prolyl bond and create an ideal substrate for PP2A (Yeh et al, 2004). Once c-Myc is dephosphorylated at S62, it is reportedly targeted for ubiquitin-mediated degradation by the F-box protein Fbw7 (Welcker et al, 2004a,b; Yada et al, 2004); however, the recent demonstration that a second E3 ubiquitin ligase, HectH9, controls transcriptional activation by c-Myc indicates that the mechanism involves further levels of complexity (Adhikary et al, 2005).
Immune mediators, conformation and stability
Nuclear factor-κB (NFκB) subunits p65/Rel and p50 accumulate in the nucleus after the cytokine-induced degradation of their cytoplasmic inhibitors, referred to as IκBs (Li & Verma, 2002). Subsequent downregulation of NFκB is achieved, in part, by newly synthesized IκB, which can retrieve NFκB from the nucleus. However, a second mechanism seems to regulate p65/Rel. The correlation between high Pin1 expression and elevated nuclear p65 levels in breast cancer tissues prompted a study into the effect of Pin1 expression on NFκB activity; it was found to enhance NFκB DNA binding and reporter activity (Ryo et al, 2003). A single motif in p65/Rel (_p_T254-P) was recognized by Pin1. A T254A mutation in p65/Rel abolished Pin1 binding and destabilized the protein. Conversely, polyubiquitination of p65, which is mediated by the E3 ubiquitin ligase Socs1, could be inhibited by overexpression of Pin1. However, it is not clear whether isomerization by Pin1 is required for this effect or whether binding alone could be sufficient to stabilize p65/Rel. The T254A mutant of p65/Rel was also not protected by de novo IκB synthesis; however, this could have been due to its failure to activate NFκB-responsive genes and IκB expression, rather than it being refractory to IκB downregulation.
The interferon-regulatory factor 3 (IRF3), which is involved in gene regulation during innate immune responses, seems to have the opposite relationship with Pin1 (Saitoh et al, 2006). Downregulation of Pin1 expression or mutation of its conserved recognition motif (S339-P) in IRF3 prolonged double-stranded RNA (dsRNA)-induced expression from the IRF3-responsive interferon-β (IFN-β) gene promoter. Conversely, Pin1 overexpression curtailed expression by accelerating the ubiquitin-dependent degradation of active IRF3 by the 26S proteasome. In line with these observations, Pin1-deficient mice overproduced IFN-β following injection of dsRNA, and bone marrow-derived Pin1−/− macrophages secreted three times more IFN-β than macrophages from Pin1+/+ mice, which is consistent with a role for Pin1 as a negative regulator of IRF3 signalling.
The reciprocal effects of Pin1 on NFκB and IRF3 might have important consequences for viral immunity. Activation of type I interferon expression by inflammatory mediators and dsRNA involves the participation of NFκB and IRF3, respectively, in the assembly of a multiprotein complex at interferon gene promoters. Given the above findings, Pin1 might act to integrate signal input to such promoters by counterbalancing the activity of the two transcription factors within the complex. In this context, Pin1 might even affect the balance between cell death and survival, as discussed elsewhere (Goutagny et al, 2006), although this provocative idea will require further work to be substantiated.
The relationship between Pin1 and RNA polymerase II
Despite the array of high-profile transcription factors that have been identified as Pin1 client proteins, perhaps the most interesting target in the context of this review is RNAP II (Fig 1). The C-terminal domain (CTD) of the largest mammalian polymerase subunit (Rpb1) is a 50-fold repeat of the conserved heptamer Y-S2-P-T-S5-P-S. This domain is essential for cell viability and gene transcription (Allison et al, 1988). The heptamer repeat is a substrate for several kinases and phosphatases. Notable among the former are the cyclin-dependent kinases Cdk7, Cdk8 and Cdk9, as well as the mitotic kinase Cdc2/cyclin B (Loyer et al, 2005). Cdk7, with cyclin H, is part of the basal transcription factor TFIIH (Feaver et al, 1994; Roy et al, 1994) and phosphorylates S5 during transcriptional initiation. Cdk9, with cyclin T, is part of the elongation factor p-TEFb (Marshall et al, 1996) and phosphorylates S2 during elongation. Cdk8, with cyclin C, is part of the Srb/mediator complex (Akoulitchev et al, 2000) and, like Cdk7, phosphorylates S5 during transcriptional initiation (Liu et al, 2004). By contrast, the Cdc2/cyclin B complex phosphorylates both S2 and S5, thereby inhibiting transcription (Gebara et al, 1997).
Figure 1.
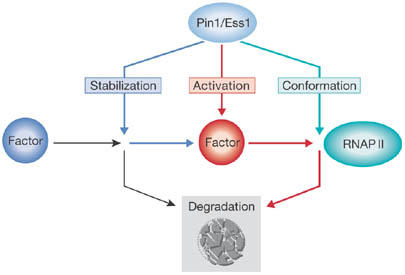
The peptidyl-prolyl isomerase Pin1/Ess1 acts in three ways in transcriptional activation. In the first scenario (blue), PPI activity protects client proteins such as β-catenin and p53 from proteasomal degradation, allowing them to participate in promoter binding and gene activation. In the second scenario (red), conformational change triggers ubiquitin-dependent degradation by the proteasome (grey box), which might (for example, in the case of retinoic-acid receptor α or steroid-receptor coactivator-3) or might not (for example, in the case of c-Myc or interferon-regulatory factor 3) be linked to transcriptional potency. The third scenario (turquoise) involves direct interaction of Pin1 with the carboxy-terminal domain of RNA polymerase II (RNAP II), which has been implicated in downregulation of the polymerase during mitosis, polymerase recycling and elongation. PPI, peptidyl-prolyl isomerase.
At least four CTD phosphatases have been identified so far (Loyer et al, 2005). TFIIF-associating CTD phosphatase1 (Fcp1) is the best characterized and is implicated in polymerase recycling (Archambault et al, 1998; Cho et al, 1999; Kobor et al, 1999). Notably, Fcp1 is one of several multicopy suppressors identified for yeast mutants lacking Ess1 (Pin1; Wu et al, 2000). This genetic linkage of Pin1 to RNAP II has been substantiated by the demonstration of Pin1 binding to phospho-CTD fragments (Kops et al, 2002; Myers et al, 2001) and inhibiting CTD dephosphorylation by Fcp1 in vitro (Palancade et al, 2004). Importantly, Pin1 has been implicated in the accumulation of the hyper-phosphorylated inactive form of RNAP II that is present in mitotic cells (Xu et al, 2003). Pin1 achieves this by inhibiting CTD dephosphorylation by Fcp1, as well as by augmenting CTD phosphorylation by cdc2/cyclin B. This might be the crucial function of Pin1 in Saccharomyces cerevisiae, as Ess1-deficient yeast cells arrest in mitosis (Hanes et al, 1989; Lu et al, 1996). However, Pin1−/− MEFs complete mitosis successfully but show defects in cell cycle re-entry (Fujimori et al, 1999), suggesting a second crucial role for Pin1. In Pin1−/− mice, this is reflected in growth and fertility defects owing to a prolonged cell cycle (rather than cell-cycle arrest or apoptosis), culminating in the complete loss of primordial germ cells from the testes and tubule degeneration by 14 months of age (Liou et al, 2002; Atchison et al, 2003; Atchison & Means, 2003).
Subsequent analyses have suggested a positive role for Pin1 in transcription initiation by revealing a genetic linkage between Ess1 and several yeast CTD kinases, including Kin28 and Srb10 (orthologues of mammalian kinases Cdk7 and Cdk8). In contrast to Fcp1, which was identified as a multicopy suppressor, the defects caused by the loss of Ess1 were improved by reducing the levels of these CTD kinases (Wilcox et al, 2004). For example, phosphorylation of the CTD by Srb10, which is a subunit of the mediator complex, is thought to inhibit polymerase recycling by preventing pre-initiation complex formation (Hengartner et al, 1998). Pin1 might reverse the effect of Srb10 by stimulating CTD dephosphorylation of S5 by Fcp1 (Kops et al, 2002), which is consistent with the antagonistic roles of Srb10 and Fcp1. This idea is further supported by the ability of alanine substitutions at S5 in the CTD to confer viability on ess1 mutants (Wilcox et al, 2004), indicating that Pin1 activity is required for S5 dephosphorylation. An inability to turnover RNAP II rapidly could be the underlying defect that prevents the rapid expression of immediate early genes at the onset of G1, which corresponds to the phenotype of Pin1−/− MEFs. It would also exacerbate reductions in cyclin D1 expression, the importance of which is highlighted by the similar phenotypes of _Pin1_-deficient and _cyclin D1_-deficient mice (Liou et al, 2002).
The two scenarios described above raise an important question: is it possible that during mitosis the crucial role for Pin1 is to inhibit CTD dephosphorylation by Fcp1, whereas in G1 it has the equally important but opposite role of stimulating CTD dephosphorylation by Fcp1 to promote RNAP II recycling? In fact, Pin1 would do the same thing in both cases—that is, isomerize the _p_S-P bonds—but the consequence would only be the same if the prevailing CTD phosphorylation and conformation were the same in both situations. During mitosis, the combined activities of Cdc2/cyclin B and Pin1 might allow an Fcp1-resistant and, hence, hyper-phosphorylated CTD conformation to predominate, whereas early in G1, Pin1 activity might generate the Fcp1-sensitive CTD conformation required for RNAP II recycling. It should be noted that the relative importance of these two events might differ in yeast and mammalian cells, and that genetic interactions between Ess1 and other CTD kinases have highlighted additional roles for Pin1 in transcription elongation (Hani et al, 1999; Wilcox et al, 2004), further complicating the interplay between Pin1 and the CTD of RNAP II.
Perspectives
Studies of Pin1 have revealed three scenarios for the involvement of peptidyl-prolyl isomerization in transcriptional regulation (Fig 1). The first relates to transcription factors that are constitutively or rapidly turned over, such as p53; Pin1 stabilizes these, allowing subsequent transactivation to occur. The second involves transcription factors for which Pin1 stimulates their degradation, as exemplified by RARα; in some cases, degradation has been tentatively linked to transactivation, hinting at a co-activator function for Pin1. The third and most complex scenario, involving the CTD of RNAP II, is one in which Pin1, in cooperation with CTD kinases and phosphatases, determines the phosphorylation and conformation of the CTD, thereby controlling the association of factors during transcription and the cell cycle.
Although the importance of parvulin-like PPIs for eukaryotic gene expression is beyond doubt, the relative importance of the numerous roles identified so far remains to be established. Presently, it is unclear why Ess1/Pin1 orthologues are essential in some but not all organisms. One explanation might be compensatory expression of other PPIs, such as cyclophilins and FK506-binding proteins (Shaw, 2002). In this regard, the existence of _Pin1_-like genes has also been proposed (Liou et al, 2002), However, the tiny amount of Ess1 activity necessary for growth in S. cerevisiae compared with the much higher levels prevailing in wild-type cells (Gemmill et al, 2005) challenges this explanation, instead suggesting conditional requirements for PPI activity directed towards specific targets in individual cell types, rather than a panoply of targets in every cell. Yet more complexity has been highlighted by the report of Pin1 phosphorylation on Ser16 in its WW domain (Lu et al, 2002), which provides scope for the regulation of Pin1 target selection during the cell cycle. In the fascinating tale of Pin1 and transcriptional regulation, there are surely more twists and turns to come.

Peter E. Shaw
Acknowledgments
Research in the author's laboratory is funded by the Biotechnology and Biological Sciences Research Council and the British Heart Foundation.
References
- Acquaviva C, Bossis G, Ferrara P, Brockly F, Jariel-Encontre I, Piechaczyk M (2002) Multiple degradation pathways for Fos family proteins. Ann NY Acad Sci 973: 426–434 [DOI] [PubMed] [Google Scholar]
- Adhikary S et al. (2005) The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell 123: 409–421 [DOI] [PubMed] [Google Scholar]
- Akoulitchev S, Chuikov S, Reinberg D (2000) TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 407: 102–106 [DOI] [PubMed] [Google Scholar]
- Allison LA, Wong JK, Fitzpatrick VD, Moyle M, Ingles CJ (1988) The C-terminal domain of the largest subunit of RNA polymerase II of Saccharomyces cerevisiae, Drosophila melanogaster, and mammals: a conserved structure with an essential function. Mol Cell Biol 8: 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault J, Pan G, Dahmus GK, Cartier M, Marshall N, Zhang S, Dahmus ME, Greenblatt J (1998) FCP1, the RAP74-interacting subunit of a human protein phosphatase that dephosphorylates the carboxyl-terminal domain of RNA polymerase IIO. J Biol Chem 273: 27593–27601 [DOI] [PubMed] [Google Scholar]
- Atchison FW, Means AR (2003) Spermatogonial depletion in adult Pin1-deficient mice. Biol Reprod 69: 1989–1997 [DOI] [PubMed] [Google Scholar]
- Atchison FW, Capel B, Means AR (2003) Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development 130: 3579–3586 [DOI] [PubMed] [Google Scholar]
- Berger M, Stahl N, Del Sal G, Haupt Y (2005) Mutations in proline 82 of p53 impair its activation by Pin1 and Chk2 in response to DNA damage. Mol Cell Biol 25: 5380–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondani V, Schefer Q, Hamy F, Klimkait T (2005) The peptidyl-prolyl isomerase Pin1 regulates phospho-Ser77 retinoic acid receptor α stability. Biochem Biophys Res Commun 328: 6–13 [DOI] [PubMed] [Google Scholar]
- Chen SY, Wulf G, Zhou XZ, Rubin MA, Lu KP (2006) Activation of β-catenin signaling in prostate cancer by peptidyl-prolyl isomerase Pin1-mediated abrogation of the androgen receptor-β-catenin interaction. Mol Cell Biol 26: 929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH (2001) MAP kinases. Chem Rev 101: 2449–2476 [DOI] [PubMed] [Google Scholar]
- Cho H, Kim TK, Mancebo H, Lane WS, Flores O, Reinberg D (1999) A protein phosphatase functions to recycle RNA polymerase II. Genes Dev 13: 1540–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo A et al. (2002) DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol Cell 9: 175–186 [DOI] [PubMed] [Google Scholar]
- Crenshaw DG, Yang J, Means AR, Kornbluth S (1998) The mitotic peptidyl-prolyl isomerase, Pin1, interacts with Cdc25 and Plx1. EMBO J 17: 1315–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, O'Donnell KA, Juopperi T (2005) The great MYC escape in tumorigenesis. Cancer Cell 8: 177–178 [DOI] [PubMed] [Google Scholar]
- Feaver WJ, Svejstrup JQ, Henry NL, Kornberg RD (1994) Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell Cycle 79: 1103–1109 [DOI] [PubMed] [Google Scholar]
- Fujimori F, Takahashi K, Uchida C, Uchida T (1999) Mice lacking Pin1 develop normally, but are defective in entering cell cycle from go arrest. Biochem Biophys Res Comm 265: 658–663 [DOI] [PubMed] [Google Scholar]
- Gebara MM, Sayre MH, Corden JL (1997) Phosphorylation of the carboxy-terminal repeat domain in RNA polymerase II by cyclin-dependent kinases is sufficient to inhibit transcription. J Cell Biochem 64: 390–402 [PubMed] [Google Scholar]
- Gemmill TR, Wu X, Hanes SD (2005) Vanishingly low levels of Ess1 prolyl-isomerase activity are sufficient for growth in Saccharomyces cerevisiae. J Biol Chem 280: 15510–15517 [DOI] [PubMed] [Google Scholar]
- Goutagny N, Severa M, Fitzgerald KA (2006) Pin-ning down immune responses to RNA viruses. Nat Immunol 7: 555–557 [DOI] [PubMed] [Google Scholar]
- Hanes SD, Shank PR, Bostian KA (1989) Sequence and mutational analysis of ESSI, a gene essential for growth in Saccharomyces cerevisiae. Yeast 5: 55–72 [DOI] [PubMed] [Google Scholar]
- Hani J, Schelber B, Bernhardt A, Domdey H, Fischer G, Wiebauer K, Rahfeld J (1999) Mutations in a peptidyl-cis/_trans_-isomerase gene lead to a defect in 3′-end formation of a pre-mRNA in Saccharomyces cerevisiae. J Biol Chem 274: 108–116 [DOI] [PubMed] [Google Scholar]
- Hengartner CJ, Myer VE, Liao SM, Wilson CJ, Koh SS, Young RA (1998) Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell 2: 43–53 [DOI] [PubMed] [Google Scholar]
- Hsu T, McRackan D, Vincent TS, Gert De Couet H (2001) Drosophila Pin1 prolyl isomerase Dodo is a MAP kinase signal responder during oogenesis. Nat Cell Biol 3: 538–543 [DOI] [PubMed] [Google Scholar]
- Kobor MS et al. (1999) An unusual eukaryotic protein phosphatase required for transcription by RNA polymerase II and CTD dephosphorylation in S. cerevisiae. Mol Cell 4: 55–62 [DOI] [PubMed] [Google Scholar]
- Kopf E, Plassat JL, Vivat V, de The H, Chambon P, Rochette-Egly C (2000) Dimerization with retinoid X receptors and phosphorylation modulate the retinoic acid-induced degradation of retinoic acid receptors α and γ through the ubiquitin-proteasome pathway. J Biol Chem 275: 33280–33288 [DOI] [PubMed] [Google Scholar]
- Kops O, Zhou XZ, Lu KP (2002) Pin1 modulates the dephosphorylation of the RNA polymerase II C-terminal domain by yeast Fcp1. FEBS Lett 513: 305–311 [DOI] [PubMed] [Google Scholar]
- Kuramochi J, Arai T, Ikeda S, Kumagai J, Uetake H, Sugihara K (2006) High Pin1 expression is associated with tumor progression in colorectal cancer. J Surg Oncol 94: 155–160 [DOI] [PubMed] [Google Scholar]
- Landrieu I, Odaert B, Wieruszeski J, Drobecq H, Rousselot-Pailley P, Inze D, Lippens G (2001) p13SUC1 and the WW domain of PIN1 bind to the same phosphothreonine-proline epitope. J Biol Chem 276: 1434–1438 [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM (2002) NF-kB regulation in the immune system. Nat Rev Immunol 2: 725–734 [DOI] [PubMed] [Google Scholar]
- Liou Y, Ryo A, Huang H, Lu P, Bronson R, Fujimori F, Uchida T, Hunter T, Lu KP (2002) Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc Natl Acad Sci USA 99: 1335–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kung C, Fishburn J, Ansari AZ, Shokat KM, Hahn S (2004) Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol Cell Biol 24: 1721–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Loyer P, Trembley JH, Katona R, Kidd VJ, Lahti JM (2005) Role of CDK/cyclin complexes in transcription and RNA splicing. Cell Signal 17: 1033–1051 [DOI] [PubMed] [Google Scholar]
- Lu KP, Hanes SD, Hunter T (1996) A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380: 544–547 [DOI] [PubMed] [Google Scholar]
- Lu P-J, Zhou XZ, Liou Y-C, Noel JP, Lu KP (2002) Critical role of WW domain phosphorylation in regulating phosphoserine binding activity and Pin1 function. J Biol Chem 277: 2381–2384 [DOI] [PubMed] [Google Scholar]
- Mantovani F, Piazza S, Gostissa M, Strano S, Zacchi P, Mantovani R, Blandino G, Del Sal G (2004) Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol Cell 14: 625–636 [DOI] [PubMed] [Google Scholar]
- Marshall NF, Peng J, Xie Z, Price DH (1996) Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J Biol Chem 271: 27176–27183 [DOI] [PubMed] [Google Scholar]
- Miyashita H, Mori S, Motegi K, Fukumoto M, Uchida T (2003) Pin1 is overexpressed in oral squamous cell carcinoma and its levels correlate with cyclin D1 overexpression. Oncol Rep 10: 455–461 [PubMed] [Google Scholar]
- Monje P, Hernandez-Losa J, Lyons RJ, Castellone MD, Gutkind JS (2005) Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J Biol Chem 280: 35081–35084 [DOI] [PubMed] [Google Scholar]
- Murray-Zmijewski F, Lane DP, Bourdon JC (2006) p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ 13: 962–972 [DOI] [PubMed] [Google Scholar]
- Myers JK, Morris DP, Greenleaf AL, Oas TG (2001) Phosphorylation of RNA polymerase II CTD fragments results in tight binding to the WW domain from the yeast prolyl isomerase Ess1. Biochemistry 40: 8479–8486 [DOI] [PubMed] [Google Scholar]
- Palancade B, Marshall NF, Tremeau-Bravard A, Bensaude O, Dahmus ME, Dubois MF (2004) Dephosphorylation of RNA polymerase II by CTD-phosphatase FCP1 is inhibited by phospho-CTD associating proteins. J Mol Biol 335: 415–424 [DOI] [PubMed] [Google Scholar]
- Pang RW, Lee TK, Man K, Poon RT, Fan ST, Kwong YL, Tse E (2006) PIN1 expression contributes to hepatic carcinogenesis. J Pathol 210: 19–25 [DOI] [PubMed] [Google Scholar]
- Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F (2003) Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Mol Cell 11: 695–707 [DOI] [PubMed] [Google Scholar]
- Reimer U, Scherer G, Drewello M, Kruber S, Schutkowski M, Fischer G (1998) Side-chain effects on peptidyl-prolyl cis/trans isomerases. J Mol Biol 279: 449–460 [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C, Adam S, Rossignol M, Egly JM, Chambon P (1997) Stimulation of RAR a activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell Cycle 90: 97–107 [DOI] [PubMed] [Google Scholar]
- Roy R, Adamczewski JP, Seroz T, Vermeulen W, Tassan JP, Schaeffer L, Nigg EA, Hoeijmakers JH, Egly JM (1994) The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell Cycle 79: 1093–1101 [DOI] [PubMed] [Google Scholar]
- Ryo A, Nakamura M, Wulf G, Liou Y, Lu KP (2001) Pin1 regulates turnover and subcellular localization of β-catenin by inhibiting its interaction with APC. Nat Cell Biol 3: 793–801 [DOI] [PubMed] [Google Scholar]
- Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, Rottapel R, Yamaoka S, Lu KP (2003) Regulation of NF-κB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell 12: 1413–1426 [DOI] [PubMed] [Google Scholar]
- Saitoh T, Tun-Kyi A, Ryo A, Yamamoto M, Finn G, Fujita T, Akira S, Yamamoto N, Lu KP, Yamaoka S (2006) Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat Immunol 7: 598–605 [DOI] [PubMed] [Google Scholar]
- Sanchez I, Dynlacht BD (2005) New insights into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol 16: 311–321 [DOI] [PubMed] [Google Scholar]
- Sanchez-Prieto R, Sanchez-Arevalo VJ, Servitja JM, Gutkind JS (2002) Regulation of p73 by c-Abl through the p38 MAP kinase pathway. Oncogene 21: 974–979 [DOI] [PubMed] [Google Scholar]
- Schiene C, Fischer G (2000) Enzymes that catalyse the restructuring of proteins. Curr Opin Struct Biol 10: 40–45 [DOI] [PubMed] [Google Scholar]
- Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR (2000) Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14: 2501–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PE (2002) Peptidyl-prolyl isomerases: a new twist to transcription. EMBO Rep 3: 521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Stukenberg PT, Kirschner MW, Lu KP (1998) The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev 12: 706–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims R, Mandal SS, Reinberg D (2004) Recent highlights of RNA-polymerase-II-mediated transcription. Curr Opin Cell Biol 16: 263–271 [DOI] [PubMed] [Google Scholar]
- Tsai KK, Yuan ZM (2003) c-Abl stabilizes p73 by a phosphorylation-augmented interaction. Cancer Res 63: 3418–3424 [PubMed] [Google Scholar]
- Verdecia MA, Bowman ME, Lu KP, Hunter T, Noel JP (2000) Structural basis for phosphoserine-proline recognition by group IV WW domains. Nat Struct Biol 7: 639–643 [DOI] [PubMed] [Google Scholar]
- Welcker M, Orian A, Grim JE, Eisenman RN, Clurman BE (2004a) A nucleolar isoform of the Fbw7 ubiquitin ligase regulates c-Myc and cell size. Curr Biol 14: 1852–1857 [DOI] [PubMed] [Google Scholar]
- Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, Clurman BE (2004b) The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci USA 101: 9085–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CB, Rossettini A, Hanes SD (2004) Genetic interactions with C-terminal domain (CTD) kinases and the CTD of RNA Pol II suggest a role for ESS1 in transcription initiation and elongation in Saccharomyces cerevisiae. Genetics 167: 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wilcox CB, Devasahayam G, Hackett RL, Arevalo-Rodriguez M, Cardenas ME, Heitman J, Hanes SD (2000) The Ess1 prolyl isomerase is linked to chromatin remodeling complexes and the general transcription machinery. EMBO J 19: 3727–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Petkova V, Lu KP (2001) Pin1 is overexpressed in breast cancer and cooperates with Ras signalling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J 20: 3459–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf GM, Liou YC, Ryo A, Lee SW, Lu KP (2002) Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J Biol Chem 277: 47976–47979 [DOI] [PubMed] [Google Scholar]
- Xu J, Li Q (2003) Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol 17: 1681–1692 [DOI] [PubMed] [Google Scholar]
- Xu YX, Hirose Y, Zhou XZ, Lu KP, Manley JL (2003) Pin1 modulates the structure and function of human RNA polymerase II. Genes Dev 17: 2765–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K, Nakayama KI (2004) Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J 23: 2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E et al. (2004) A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol 6: 308–318 [DOI] [PubMed] [Google Scholar]
- Yi P, Wu RC, Sandquist J, Wong J, Tsai SY, Tsai MJ, Means AR, O'Malley BW (2005) Peptidyl-prolyl isomerase 1 (Pin1) serves as a coactivator of steroid receptor by regulating the activity of phosphorylated steroid receptor coactivator 3 (SRC-3/AIB1). Mol Cell Biol 25: 9687–9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZM, Shioya H, Ishiko T, Sun X, Gu J, Huang YY, Lu H, Kharbanda S, Weichselbaum R, Kufe D (1999) p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature 399: 814–817 [DOI] [PubMed] [Google Scholar]
- Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio F, Volinia S, Ronai Z, Blandino G, Schneider C, Del Sal G (2002) The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419: 853–857 [DOI] [PubMed] [Google Scholar]
- Zheng H, You H, Zhou XZ, Murray SA, Uchida T, Wulf G, Gu L, Tang X, Lu KP, Xiao ZX (2002) The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature 419: 849–853 [DOI] [PubMed] [Google Scholar]
- Zhou CX, Gao Y (2006) Aberrant expression of β-catenin, Pin1 and cylin D1 in salivary adenoid cystic carcinoma: relation to tumor proliferation and metastasis. Oncol Rep 16: 505–511 [PubMed] [Google Scholar]
- Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, Kullertz G, Lu KP (2000) Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell 6: 873–883 [DOI] [PubMed] [Google Scholar]