Pharmacological blockade of group II metabotropic glutamate receptors reduces the growth of glioma cells in vivo (original) (raw)
Abstract
U87MG human glioma cells in cultures expressed metabotropic glutamate (mGlu) receptors mGlu2 and mGlu3. Addition of the mGlu2/3 receptor antagonist LY341495 to the cultures reduced cell growth, expression of cyclin D1/2, and activation of the MAP kinase and phosphatidylinositol-3-kinase pathways. This is in line with the evidence that activation of mGlu2/3 receptors sustains glioma cell proliferation. U87MG cells were either implanted under the skin (1 × 106 cells/0.5 ml) or infused into the caudate nucleus (0.5 × 106 cells/5 μl) of nude mice. Animals were treated for 28 days with mGlu receptor antagonists by means of subcutaneous osmotic minipumps. Treatments with LY341495 or (2_S_)-α-ethylglutamate (both infused at a rate of 1 mg/kg per day) reduced the size of tumors growing under the skin. Infusion of LY341495 (10 mg/kg per day) also reduced the growth of brain tumors, as assessed by magnetic resonance imaging analysis carried out every seven days. The effect of drug treatment was particularly evident during the exponential phase of tumor growth, that is, between the third and the fourth week following cell implantation. Immunohistochemical analysis showed that U87MG cells retained the expression of mGlu2/3 receptors when implanted into the brain of nude mice. These data suggest that mGlu2/3 receptor antagonists are of potential use in the experimental treatment of malignant gliomas.
Keywords: glioma, glutamate, antagonist, MRI, tumor, growth
The role for extracellular glutamate in the growth and invasive migration of malignant glioma has been outlined in a recent review (Sontheimer, 2003). As opposed to typical solid tumors that grow in soft tissues, brain gliomas expand by creating new space as a result of tissue destruction. They accomplish this task by releasing glutamate, which kills surrounding neurons via an excitotoxic mechanism. Accordingly, glioma cell lines that lack glutamate transporters at the cell surface expand more rapidly as a result of glutamate accumulation in the extracellular space (Takano et al., 2001). Interestingly, glioma cells express ionotropic glutamate receptors, which respond to extracellular glutamate promoting tumor growth and/or migration (Ishiuchi et al., 2002; Rzeski et al., 2001). We and others have found that glioma cells also express metabotropic glutamate (mGlu)5 receptors (Aronica et al., 2003; Condorelli et al., 1997; D’Onofrio et al., 2003). These receptors form a family of eight subtypes, of which mGlu1 and mGlu5 are coupled to Gq proteins, whereas all other subtypes (mGlu2, mGlu3, mGlu4, mGlu6, mGlu7, and mGlu8) are coupled to Gi proteins in recombinant cells (D’Onofrio et al., 2001). The mGlu3 receptors are consistently found in primary cultures obtained from human glioblastomas, as well as in a number of glioma cell lines. The mGlu2 and mGlu5 receptors are found only occasionally in glioma cells (Condorelli et al., 1997; D’Onofrio et al., 2003). Pharmacological blockade of mGlu2/3 receptors produces antiproliferative effects in cultured glioma cells and reduces the activation of the MAP kinase (MAPK) pathway induced by epidermal growth factor (EGF) or other mitogens (D’Onofrio et al., 2001). This is particularly interesting from a therapeutic standpoint because ligands of mGlu2 and mGlu3 receptors represent an expanding class of drugs endowed with a high receptor affinity, brain penetration, and a good profile of safety and tolerability (Bruno et al., 2001; Schoepp et al., 1999). However, the efficacy of mGlu2/3 receptor antagonists cannot be easily predicted from in vitro data because these drugs in vivo might produce effects that are detrimental to the final outcome of malignant gliomas, such as a facilitation of excitotoxic neuronal death (Battaglia et al., 1998; Bruno et al., 2001; Kingston et al., 1999; Poli et al., 2003) or a reduced expression of the excitatory amino acid carrier 1 glutamate transporter (Aronica et al., 2003). In addition, it is not known whether glioma cells proliferating in their native environment (i.e., inside the brain) still express mGlu2/3 receptors and retain the sensitivity to mGlu2/3 receptor antagonists. Here we show that a chronic treatment with the potent and brain-permeant mGlu2/3 receptor antagonist (2_S_)-2-amino-2-[(1_S_,2_S_)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495) (Kingston et al., 1998) reduces the growth of glioma cells implanted either under the skin or inside the brain of mice.
Methods
Materials
LY341495, (2_S_)-α -ethylglutamate (EGlu), and (R_S_)-α-methyl-4-tetrazolylphenylglycine (MTPG) were obtained from Tocris Cookson Ltd. (Bristol, UK). The (−)-2-oxa-4-aminobicyclo[3,1,0]hexane-4,6-dicarboxylic acid (LY379268) was a gift. All other chemicals were purchased from Sigma (Milan, Italy).
Cell Cultures
U87MG human glioblastoma continuous cell lines were obtained from the American Type Culture Collection (Manassas, Va.). Cells were grown in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal calf serum (Life Technologies Ltd., Milan, Italy) and were plated in Falcon Primaria culture dishes (Becton Dickinson, Lincoln Park, N.J.). The medium was changed every three days. After 14 to 15 days, cells were trypsinized and replated into 24-well plates at a density of 25 × 103 cells per well and shifted into Dulbecco’s Modified Eagle’s Medium Glutamax without serum (Life Technologies Ltd.). Glutamax was used instead of glutamine, which is a potential chemical source of glutamate.
Cell Cycle Analysis
Fluorescence-activated cell sorting (FACS) analysis of cell cycle and DNA ploidy was carried out with a Coulter Elite flow cytometer (Beckman Coulter, Milan, Italy) after staining with propidium iodide (50 μg/ml) and treatment for 1 h with RNAse (100 μg/ml, as described previously [Copani et al., 1999]).
RNA Isolation and RT-PCR Analysis
Two micrograms of total RNA extracted from glioma cultures (Ultraspec, Biotech, Houston, Tex.) and 100 ng of random hexamers dissolved in 12 μl of RNAse-free water were heated at 65°C for 15 min and then cooled on ice for 1 min. Five microliters of Moloney murine leukemia virus buffer 5×, deoxynucleotide-5'-triphosphate (500 nM) and dithiotreitol (10 nM), then 1 μl of Moloney murine leukemia virus (200 U), were added (Life Technologies Ltd.), and the mix was incubated for 60 min at 42°C. Polymerase chain reaction analysis was carried out in 50 μl of a Tris-HCl (10 mM, pH 8.8) buffer containing MgCl2 (1.5 mM); Triton X-100 (0.1%); deoxythymidine-5'-triphosphate, deoxyguano-sine-5'-triphosphate, deoxycytidine-5'-triphosphate, and deoxyadenosine-5'-triphosphate (200 μM each); forward and reverse primers (25 pmol each); and 10 μl of single-stranded cDNA. Taq polymerase (1 U) (Promega, Milan, Italy) was added in hot-start conditions (95°C for 3–5 min), and 35 cycles were carried out as follows: 95°C for 1 min, 58°C for 1 min, and 72°C for 1 min. Amplification products were separated by agarose gel (2%) and stained with ethidium bromide. Primers used were as follows.
mGlu1 receptor:
forward: GCTGTACCTACTATGCCTTC
reverse: AGACCATGACACAGACTTGC
mGlu2 receptor:
forward: 5'-GAGAAGGTGGGCCGTGCCATGAG-3'
reverse: 5'-CGCTGCCTGCCCGCAGATAGGT-3'
mGlu3 receptor:
forward: 5'-GCTCCAACATCCGCAAGTCCTA-3'
reverse: 5'-TGTCAATGGCCAGGTGCTTGTC-3'
mGlu4 receptor:
forward: 5'-TGAGCTACGTGCTGCTGGCG-3'
reverse: TGTCGGCTGACTGTGAGGTG
mGlu5 receptor:
forward: 5'-CCCAGAATGAGAAGAGTACC-3'
reverse: 5'-TCTGCGAAGGTCGTCATGGT-3'
mGlu7 receptor:
forward: 5'-CACGAAGATATTGCGGAAGGAG-3'
reverse: CTGAAGTTCATCTCTGTCCACTGC-3’
Western Blot Analysis
Cells were washed twice with phosphate-buffered saline and lysed in Tris-buffered saline buffer-1% Triton-X 100 (NaCl, 150 mM; Tris-HCl, 50 mM, pH 8; EDTA, 5 mM; NaF, 1 mM; Na4P2O7, 1 mM; Na3VO4, 0.4 mM) for the detection of mGlu receptors. Proteins (30–50 μg) were separated on sodium dodecyl sulfate polyacrylamide gel (7.5%) and transferred on Immuno PVDF membrane (Biorad, Milan, Italy) for 1 h. Filters were blocked overnight in Tween-20 Tris-buffered saline (TTBS) (100 mM Tris-HCl, 0.9% NaCl, 1% Tween 20, pH 7.4) containing 5% nonfat dry milk. Blots were then incubated for 1 h at room temperature with primary mGlu2/3 or mGlu5 receptor polyclonal antibodies (1:1000) (Chemicon, DBA, Milan, Italy). Blots were washed three times with TTBS buffer and then incubated for 1 h with secondary antibody (peroxidase-coupled antirabbit [Amer-sham, Milan, Italy], 1:10,000) with TTBS. Immunostaining was revealed by enhanced ECL Western Blotting analysis system (Amersham).
Detection of Cyclin D1/2, Phosphorylated ERK1/ERK2, and Phosphorylated Akt in Human Continuous Cell Cultures of U87MG
Cultures were starved in serum-free medium for 72 h and then stimulated with EGF (100 ng/ml) plus LY341495 (1 μM) and/or LY379268 (1 μM) for 10 min for the detection of phosphorylated extracellular signal-regulated kinase 1 (ERK1)//ERK2 or phosphorylated Akt, and for 8 h for the detection of cyclin D1/2. We used phospho-ERK1/ERK2 (1:2000) and nonphosphorylated ERK1/ ERK2 (1:1000) monoclonal antibodies (New England Biolabs, Beverly, Mass.), phospho-Akt (1:1000) and nonphosphorylated Akt (1:1000) polyclonal antibodies (Cell Signaling Technology, Beverly, Mass.), and poly-clonal antibodies raised against synthetic peptides corresponding to the 11 C-terminal amino acids of human cyclin D1, which cross-reacts with cyclin D2 (1.5 μg/ml, Santa Cruz, Calif.). Western blot analysis was carried out as described above.
Determination of Extracellular L-Glutamate Levels
Analysis of glutamate in the medium of cultured U87MG cells was performed by precolumn derivatization with _o_-phthalaldehyde and mercaptoethanol, followed by high-performance liquid chromatography with fluorescence detection, as described previously (D’Onofrio et al., 2003).
U87MG Cell Implantation
Male CD1 nude mice (20–22-g body weight; Charles River, Calco, CO, Italy) were kept under controlled conditions (temperature, 22°C; humidity, 40%) on a 12-h light/dark cycle with food and water ad libitum. Experiments were performed following the guidelines for animal care and use promulgated by the National Institutes of Health (Bethesda, Md.). Mice were subcutaneously implanted with 1 × 106 U87MG cells/0.5 ml. Mice were implanted at the same time with osmotic minipumps releasing 250 nl/h for 28 days (Alzet, Cupertino, Calif.) filled with saline, LY341495, or EGlu. Implantation was performed on mice under ketamine (100 mg/kg, i.p.)/xylazine (10 mg/kg, i.p.) anesthesia. The weight of subcutaneous tumors was assessed after one month. In another set of experiments, U87MG cells were stereo-taxically implanted into the left caudate nucleus (by using the following coordinates: 0.6 mm anterior to the bregma; 1.7 mm lateral to the midline; and 4.5 mm ventral from the surface of skull [Franklin and Paxinos, 1997]) of mice under ketamine (100 mg/kg, i.p.)/xyla-zine (10 mg/kg, i.p.) anesthesia. Cells (0.5 × 106 cells/5 μl) were implanted at an infusion rate of 1 μl/min. The needle was left in place 5 min after cell infusion before it was withdrawn. Mice were chronically treated with saline or LY341495 by means of small osmotic mini-pumps (Alzet) releasing 500 nl/h for 7 days. Minipumps were removed just prior to each session of MRI analysis and then substituted after the analysis while the animals were under isoflurane anesthesia, as described below.
MRI Analysis
MRI analysis was carried out at 4.7 tesla (T) by using a Varian Inova SIS 200/183 system (Varian, Palo Alto, Calif.) equipped with a 4.0-cm-diameter, circularly polarized coil. T1-weighted multislice spin-echo scout images (xz, xy) were obtained with TR/TE = 500/16 ms, NS = 4, slice thickness of 1.0 mm, and matrix 128 × 512. T2-weighted (x,y) images were acquired only on the first day of analysis: TR/TE = 2500/70 ms, NS = 4, slice thickness of 1.0 mm, and matrix 128 × 512. The total volume of each tumor was measured by using a series of axial, post-gadolinium-enhanced diethylenetriamine pentaacetic acid (3.0 ml/kg, s.c.), T1-weighted, spin-echo sequence images. Tumor volume was evaluated by using a dedicated image browser program (Varian); the area of the lesion was indicated manually, and the mean value from the evaluations was calculated by two independent investigators. The area of interest was manually defined on each single slice and the total volume automatically measured; two separate measures were performed by two independent blind investigators. Animals were anesthetized with 1% isoflurane in O2 (1 liter/min), and body temperature was maintained constant by means of a water bed at 37°C. The presence of a brain tumor was demonstrated in 49 of 55 mice injected in the striatum with U87MG cells. Mice were subjected to MRI analysis every seven days after cell implantation.
Immunohistochemistry and Histological Analysis
Brains were fixed in 4% formaldehyde in 0.1 M phosphate buffer (pH 7.2). Serial sections were stained with hematoxylin and eosin. Immunohistochemistry for the proliferation marker, Ki-67 (Ventana, Tucson, Ariz.), and for the mGlu2/3 receptor (1:100) (Chemicon) was performed automatically with a Nexes instrument (Ventana). Antibody detection was performed by using a multilink streptavidin-biotin complex method, and antibodies were visualized by a diaminobenzidine chromagen method. Negative control samples were incubated with primary antibodies only. The number of Ki-67-positive cells was determined in four random fields (1 cm2 each) by using the ImageJ 1.31v software (National Institutes of Health).
Results
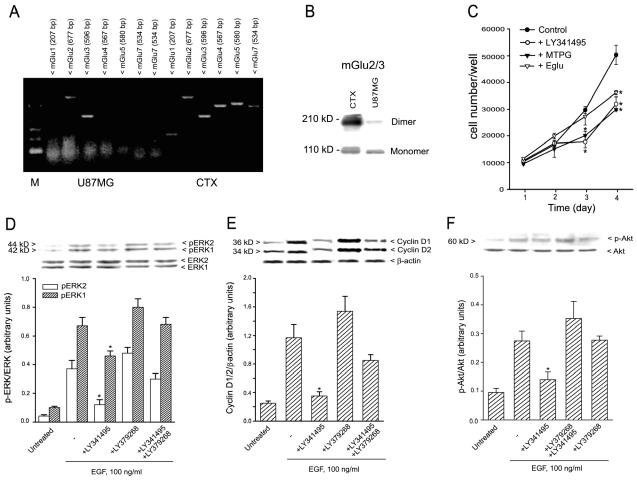
We previously observed that glioma cells in culture respond to the mGlu2/3 receptor antagonist LY341495 with a reduced proliferation rate (D’Onofrio et al., 2003). However, our initial attempts to implant nude mice with cells isolated from surgically removed human gliomas failed because of the limited growth rate of these cells. We therefore used U87MG glioma cells for in vivo studies. These cells expressed both mGlu2 and mGlu3 receptor mRNA (Fig. 1A), as well as the mGlu2/3 receptor protein (Fig. 1B). We did not detect any expression of mGlu1, mGlu4, mGlu5, or mGlu7 mRNA (Fig. 1A) or mGlu5 receptor protein (not shown). A daily addition of LY341495 (1 μM), MTPG (100 μM), or EGlu (100 μM) to cultured U87MG cells reduced cell proliferation in a time-dependent manner. In cultures treated with LY341495 or MTPG, a reduction in cell proliferation was seen after three and four days (particularly between the second and third day of exposure (Fig. 1C). EGlu was less effective than the other two antagonists and reduced cell proliferation only after four days of exposure (Fig. 1C). FACS analysis of cell cycle in cells treated with LY341495 for two days showed a significant reduction in the percentage of cells in the S and G2M phases of the cell cycle that was associated with an increased percentage of cells in the G0/G1 phase. The distribution of the cell cycle phases in cells treated with LY341495 for four days did not differ from the distribution in control cells (Table 1). LY341495 treatment did not induce apoptosis of U87MG cells, as assessed by FACS analysis of prediploid DNA (not shown).
Fig. 1.
Response of U87MG cells to treatment with group II mGlu receptor ligands. A. Reverse transcriptase polymerase chain reaction analysis of mGlu1, 2, 3, 4, 5, and 7 receptor RNA in U87MG cells. The adult male rat cerebral cortex (CTX) is shown as a positive control. The absence of genomic DNA contamination was proved in parallel samples incubated without the reverse transcriptase (not shown). B. Western blotting of mGlu2/3 receptors in U87MG cells. Thirty micrograms of proteins were loaded for each lane. CTX is shown as a positive control. The blot was repeated three times with similar results. C. Exposure to the mGlu2/3 receptor antagonists LY341495 (1 μM), MTPG (100 μM), or Eglu (100 μM) reduced U87MG cell growth in culture. Drugs were applied to the cultures every 24 h for four days. Values are means ± the standard error of the mean (SEM) of three individual determinations. *P < 0.05 (one-way ANOVA + Student-Newman-Keuls test to isolate the differences) versus the respective controls. D–F. Expression of phosphoERK1/2 (panel D), cyclin D1/D2 (panel E), and phospho-Akt (panel F) in cultured U87MG stimulated with EGF and treated with LY341495 (1 μM) and/or LY379268 (1 μM). Densitometric values are means + SEM of three to four determinations. *P < 0.05 (one-way ANOVA + Student-Newman-Keuls test) as compared with all other groups treated with EGF.
Table 1.
FACS analysis of U87MG cell cultures
| Percent of Cell Population | ||||||
|---|---|---|---|---|---|---|
| Treatment | G 0 /G 1 | 48 h S | G 2 -M | G 0 /G 1 | 96 h S | G 2 -M |
| Control | 42.4 ± 1.7 | 42.8 ± 0.9 | 14.6 ± 1 | 62.4 ± 0.5 | 28.7 ± 0.7 | 9.2 ± 0.2 |
| LY341495 | 53.1 ± 2.3* | 36.5 ± 1.7* | 10.3 ± 0.7* | 62.6 ± 0.8 | 29 ± 0.4 | 8.4 ± 0.7 |
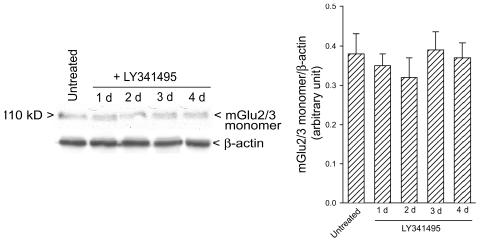
LY341495 (1 μM) reduced the activation of the MAPK pathway (as assessed by Western blot analysis of phos-phorylated ERK1/ERK2), the activation of the PI-3-K pathway (as assessed by Western blot analysis of phosphorylated Akt), and the expression of cyclin D1/2 induced by the mitogen EGF (100 ng/ml) in cultured U87MG cells deprived of serum for 72 h. All of these effects were reversed by the coapplication of the potent mGlu2/3 receptor agonist, LY379268, which was inactive per se (Figs. 1D–F). In cells deprived of serum for 72 h, extracellular glutamate concentrations were 92 ± 21 μM (n = 5). Cultures treated with LY341495 were also used to assess whether antagonist treatment could induce adaptive changes in the expression of mGlu2/3 receptors. Western blot analysis showed that exposure to LY341495 did not change mGlu2/3 levels (at least up to four days of treatment) (Fig. 2).
Fig. 2.
Western blot analysis of mGlu2/3 receptors in cultured U87MG cells treated for one to four days with LY341495. Densito-metric values are means + SEM (n = 3).
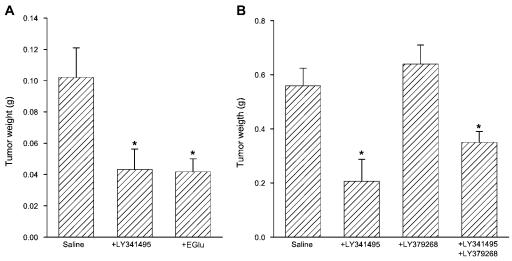
To examine whether pharmacological blockade of mGlu2/3 receptors could reduce glioma cell growth in vivo, we first implanted U87MG under the skin of nude mice (1 × 106/0.5 ml). At the same time, mice were subcutaneously implanted with osmotic minipumps releasing saline, LY341495 (1 mg/kg per day), EGlu (1 mg/kg per day), LY379268 (1 mg/kg per day), or LY341495 + LY379268 for 28 days. Analysis of tumor weight after one month showed that chronic infusion with mGlu2/3 receptor antagonists LY341495 or EGlu reduced glioma cell growth (Figs. 3A, B). Infusion with the receptor agonist LY379268 did not affect tumor growth and failed to reverse the lowering effect of LY341495, although the weight of tumors in mice treated with LY341495 + LY379268 tended to be higher than that in mice treated with LY341495 alone (Fig. 3B).
Fig. 3.
Effects of continuous systemic administration of mGlu2/3 receptor ligands on the growth of U87MG cells implanted under the skin of nude mice. Animals were treated for 28 days with saline, LY341495 (1 mg/kg per day), Eglu (1 mg/kg per day), LY379268 (1 mg/kg per day), or LY341495 + LY379268, all by means of subcutaneous osmotic minipumps. Two independent experiments are shown. A. n = 10. B. n = 8 to 10. Values are means + SEM; *P < 0.05 (one-way ANOVA + Student-Newman-Keuls test) versus mice treated with saline.
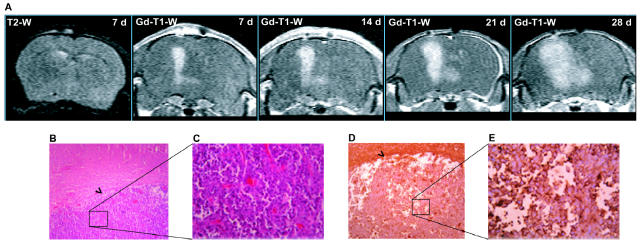
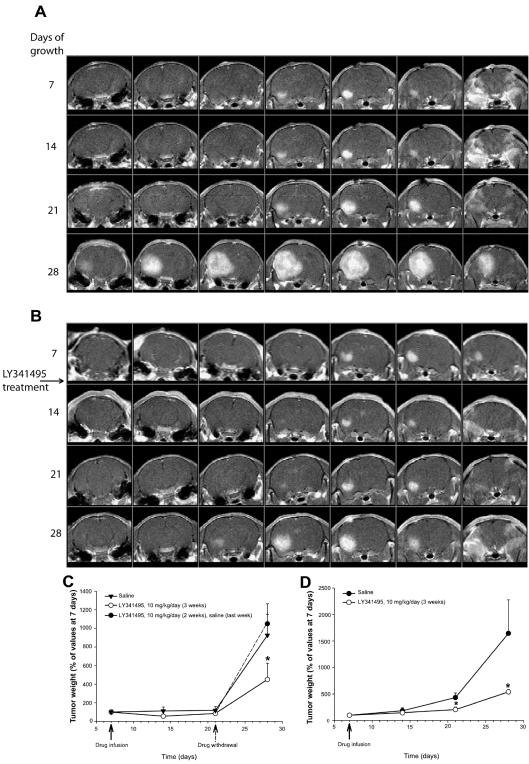
In another series of experiments, we implanted U87MG cells into the left caudate nucleus of nude mice (0.5 × 106 cells/5 μl for 5 min). Mice were subjected to MRI analysis every seven days after cell implantation. T2-weighted images were acquired only after the first seven days and did not show the presence of edema at the injection site of U87MG cells. Contrast-enhanced T1-weighted images showed the presence of a tumor, which grew progressively from day 7 to day 28 following cell implantation (Fig. 4A). In most of the animals, the exponential phase of tumor growth was observed between 21 and 28 days after cell implantation. Histological examination of xenografts carried out after the last MRI analysis at 28 days showed a homogenous tumor mass with sharp borders, which was clearly delimited from the adjacent brain tissue. Cytologically, tumors were composed of large pleomorphic cells with abundant eosinophilic cytoplasms (Figs. 4B, C). Most glioma cells were immunopositive for mGlu2/3 receptors (Figs. 4D, E), as well as for the proliferation marker, Ki-67. After the first MRI analysis at seven days, selected mice with similar tumor sizes were subdivided into two groups and subcutaneously implanted with osmotic minipumps releasing either saline or LY341495 (10 mg/ kg per day) subcutaneously for seven days. Minipumps were removed just prior to the next MRI analysis (for technical reasons) and replaced afterward.
Fig. 4.
Growth to day 28 of a brain tumor initiated with U87MG cells. A. MRI analysis of a brain tumor originating from unilateral intra-striatal infusion of U87MG cells in a control nude mouse. The T2-weighted (T2-W) image shows the absence of brain edema at seven days following cell implantation. Sequential contrast-enhanced T1-W images (from 7 to 28 days following cell implantation) show the progressive expansion of the brain tumor. B–E. Hematoxylineosin staining after 28 days shows (B) the sharp border between the tumor and the surrounding brain tissue (objective = 10×), (C) tumor cells at higher (40×) magnification, (D) the mGlu2/3 receptor immunoreactivity at 10×, and (E) the immunoreactivity at 40× magnification. Arrowheads in panels B and D show the borders between tumors and normal brain.
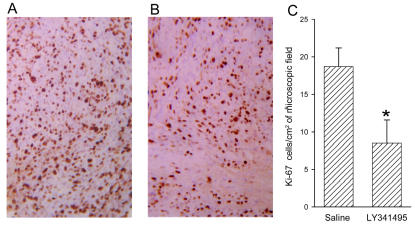
Results of two independent experiments are shown in Figs. 5C and 5D. Treatment with LY341495 reduced the growth rate of brain tumors in both experiments. The action of the drug was particularly evident between day 21 and day 28 following cell implantation, that is, during the exponential phase of growth. In the second experiment, four mice were withdrawn from LY341495 on day 21. In these mice, tumor volume increased to the same extent as in control mice from day 21 to day 28, which indicated that the effect of LY341495 was reversible (Fig. 5D). Mice were not allowed to survive beyond 28 days after cell implantation to avoid the occurrence of severe neurologic symptoms. Only five mice died during the 28 days of the experiments. All of these mice were from the control groups treated with saline. Histological analysis did not show any difference between the morphology of tumor cells of mice treated with saline and that of mice treated with LY341495. No detectable edema was found around or inside the tumor specimens from control and LY341495-treated mice. Treatment with LY341495 significantly reduced the number of Ki-67-positive cells in tumor specimens (Fig. 6).
Fig. 5.
Tumor growth over 28 days in LY341495-treated and control mice. A–B. Sequential frontal contrast-enhanced T1-W images of brain tumor originated from unilateral intrastriatal infusion of U87MG cells in mice. A. Representative control mouse. B. Mouse treated with LY341495 (10 mg/kg/day). The treatment was started 7 days following cell implantation. C–D. Data (means + SEM) from two independent experiments. C. Ten mice were treated with saline or LY341495 (10 mg/kg per day). Four mice treated with LY341495 were withdrawn from the drug at day 21 (black circle and dashed line in the graph). D. Twelve mice were treated with saline, and six were treated with LY341495. *P < 0.05 (one-way ANOVA + Student-Newman-Keuls test in panel C; and Student t test in panel D) versus the corresponding values obtained in mice treated with saline (C and D) or in mice withdrawn from LY341495 (C).
Fig. 6.
Ki-67 immunostaining of U87MG cells implanted in the mouse brain. A–B. A representative immunostaining of tumor developed in mice treated with saline (A) or LY341495 (B) released subcutaneously by osmotic minipumps (objective = 20×). C. Quantification of immunostaining, where data are normalized per square centimeter of microscopic field and represent the means ± SEM of cell counts obtained from five mice per group (four random microscopic fields for each mouse). *P < 0.05 (Student t test), if compared with mice treated with saline.
Discussion
There have been few significant advances in the treatment of malignant gliomas over the last two decades. Compared with other brain tumors (e.g., primary CNS lymphomas, anaplastic oligodendrogliomas, and pediatric embryonal tumors), tumors of the astrocytic lineage show a poor responsiveness to cytotoxic chemotherapy because of the development of chemoresistance (Bredel and Zentner, 2002). This encourages the search for alternative drug treatments aimed at improving the prognosis of malignant gliomas. Recent evidence suggests that gliomas may be “perverting glutamate and ion homeostasis” to support their unusual growth and invasive migration (Sontheimer, 2003). Glioma cell growth inside the brain is favored by an active release of glutamate combined with a low expression of sodium-dependent membrane glutamate transporters (Takano et al., 2001; Ye et al., 1999). This leads to excitotoxic death of surrounding neurons, a process that creates the necessary space for tumor expansion (Ye and Sontheimer, 1999). Drugs that protect neurons against excitotoxic death (e.g., _N_-methyl-d-aspartate receptor antagonists) or inhibit the glutamate/cystine membrane exchanger (i.e., the main system accounting for the release of glutamate) are potential candidates for the treatment of malignant gliomas. Inhibitors of the glutamate/cystine cotransporter will also limit the intracellular synthesis of glutathione, a molecule involved in mechanisms of chemoresistance (Reichelt et al., 1997). Interestingly, glioma cells express amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors lacking the GluR2 subunit, which acts as a negative dominant in controlling the Ca2+ permeability of AMPA-gated ion channels (Burnashev et al., 1992; Sommer et al., 1991). Activation of AMPA receptors in glioma cells generates Ca2+ transients that support cell migration and survival, thus facilitating tumor expansion (Ishiuchi et al., 2002).
Using glioma cultures from bioptic human samples or glioma cell lines, we have shown that pharmacological blockade of mGlu3 metabotropic glutamate receptors reduces cell proliferation and MAPK activation. This effect was seen in all selected glioma cell cultures expressing mGlu3 receptors (D’Onofrio et al., 2003). Here, we have shown that a continuous systemic infusion of the potent mGlu2/3 receptor antagonist LY341495 (Johnson et al., 1999; Kingston et al., 1998) reduced the growth of glioma cells implanted either under the skin or inside the brain of recipient mice. These two models are complementary because the growth of cells implanted in a soft tissue (i.e., subcutaneously) relies exclusively on the rate of proliferation and on an adequate energy supply, whereas the growth of a brain glioma is the result of multiple processes such as the excitotoxic death of surrounding neurons, the expression of enzymes that degrade the extracellular matrix, and the expression of ion channels that drive the movement of water out of the cell (Bowman et al., 1999). However, one must consider that glioma cells implanted in the mouse brain tend to grow uniformly, with the resulting brain tumor appearing as a homogenous mass at MRI and histological analysis. This contrasts with primitive advanced-stage gliomas, which disseminate asymmetrically, with cells migrating along blood vessels and fiber tracts (Giese, 2003). Thus, in our model, the contribution of cell shrinkage to the overall tumor growth is minimized.
We used a cell line (U87MG) for in vivo studies because cells from surgically removed human gliomas (that we had used in vitro) showed a limited growth when implanted either subcutaneously or inside the brain of nude mice. U87MG cells expressed both mGlu2 and mGlu3 receptors, two highly homologous receptors that share the same pharmacological profile (Sallese et al., 2001), but not other mGlu receptor subtypes. This is noteworthy because the antagonist LY341495 binds with nanomolar affinity to mGlu2 and -3 receptors, but it recruits additional mGlu receptor subtypes at higher concentrations (Schoepp et al., 1999). Because of its very high affinity, LY341495 can antagonize the activation of mGlu2/3 receptors by micromolar concentrations of ambient glutamate (the affinity of glutamate for mGlu2 and mGlu3 receptors is usually in the range of 2 to 10 μM [Schoepp et al., 1999]).
U87MG cells in vitro responded to a single pulse of LY341495 with a reduced activation of the MAPK and PI-3-K pathways (which are both implicated in glioma cell proliferation) and a reduced expression of cyclin D1 (an early marker of the G1/S phase transition) in response to the mitogen EGF. All of these effects were reversed by the combined application of LY379268, a potent subtype-selective agonist with a nanomolar affinity for mGlu2 and mGlu3 receptors (reviewed by Schoepp et al. [1999]), suggesting that endogenous activation of mGlu2/3 receptors supports proliferation of U87MG cells. This was confirmed by experiments in which U87MG cells were continuously exposed to mGlu2/3 receptor blockers up to four days. Inhibition of cell growth was seen after exposure to LY341495, MTPG, and EGlu, three drugs that share the ability to antagonize both mGlu2 and mGlu3 receptors (see Shoepp et al. [1999]). In cultured U87MG cells treated with LY341495 in particular, cell growth was substantially reduced between the second and the third day of drug exposure and recovered afterward. This is different from what has been observed in primary cultures of human brain gliomas, in which the inhibitory action of LY341495 on cell growth was fully maintained after four days of exposure (D’Onofrio et al., 2003). The transient nature of the effect of LY341495 on U87MG cell growth was confirmed by FACS analysis of the cell cycle, which showed the ability of the drug to reduce the percentage of cells in the S and G2M phases of the cell cycle after two days but not after four days of exposure. Whether this reflects the development of mGlu2 or mGlu3 receptor supersensitivity in response to the long-term receptor blockade in vitro is unknown because information on the adaptive changes in the activity of these receptors upon continuous drug exposure is lacking. However, it is noteworthy that no changes in the expression of mGlu2/3 receptors (detected by Western blot analysis) were found in cultured U87MG cells exposed to LY341495 up to four days. Thus, the mechanisms underlying the transient effect of mGlu2/3 receptor blockade on the proliferation rate of U87MG cells in culture remain obscure.
When cells were implanted subcutaneously, LY341495 reduced tumor growth even in the presence of equimolar doses of LY379268. The incomplete knowledge available on the pharmacokinetics of mGlu receptor ligands would prohibit accurate pharmacological experiments that might disclose an interaction between LY341495 and LY379268 in vivo. However, a critical role for mGlu2/3 receptors is suggested by the reduced tumor growth observed in mice treated with EGlu, which selectively antagonizes mGlu2/3 receptors (Jane et al., 1996). The finding that systemic infusion of LY341495, a drug that can cross the blood-brain barrier (Monn et al., 1999), limits tumor growth inside the mouse brain is particularly encouraging from a therapeutic standpoint and suggests that inhibition of cell proliferation overcomes any possible detrimental effect of the drug on tumor growth, such as the facilitation of excito-toxic neuronal death (Battaglia et al., 1998; Poli et al., 2003). We have no information on the final outcome of brain tumors in mice treated with LY341495 because animals were not allowed to survive beyond the fourth week of tumor growth. However, it is noteworthy that none of the five mice that died during the four weeks of observation belonged to the groups treated with LY341495. We expect that treatment with systemically active mGlu2/3 receptor antagonists will be even more effective in reducing the growth of primary brain gliomas, because the antiproliferative action of LY341495 was persistent in primary cultures of human gliomas (whereas it was transient in cultured U87MG cells). We did not test this directly because the growth of glioma cells isolated from human brain tumors was very slow in the brain of recipient nude mice. Other questions that need to be addressed are whether LY341495 synergizes with classical cytotoxic agents used in clinical practice and whether mGlu2/3 receptors present in glioma cells control the expression of proteins or enzymes involved in mechanisms of chemoresistance.
Acknowledgment
The LY379268 was kindly provided by Eli Lilly Research Laboratories, Indianapolis, Indiana. We thank Teodoro Squatriti for technical assistance in animal preparation for MRI analysis.
Footnotes
1
This work was partially supported by a grant of the Istituto Superiore di Sanità, ISS-0D/C.
5
Abbreviations used are as follows: AMPA, amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; EGF, epidermal growth factor; Eglu, (2_S_)-α-ethylglutamate; ERK, extracellular signal-regulated kinase; LY341495, (2_S_)-2-amino-2-[(1_S_,2_S_)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid; LY379268, (−)-2-oxa-4-aminobicyclo[3,1,0]hexane-4,6-dicarboxylic acid; FACS, fluorescence-activated cell sorting; MAPK, mitogen-activated protein kinase; mGlu, metabotropic glutamate; MTPG, (R_S_)-α-methyl-4-tetrazolylphenylglycine; SEM, standard error of the mean; TTBS, Tween-20 Tris-buffered saline.
References
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: Opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003;17:2106–2118. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Bruno V, Ngomba RT, Di Grezia R, Copani A, Nicoletti F. Selective activation of group-II metabotropic glutamate receptors is protective against excitotoxic neuronal death. Eur J Pharmacol. 1998;356:271–274. doi: 10.1016/s0014-2999(98)00551-2. [DOI] [PubMed] [Google Scholar]
- Bowman CL, Yohe L, Lohr JW. Enzymatic modulation of cell volume in C6 glioma cells. Glia. 1999;27:22–31. doi: 10.1002/(sici)1098-1136(199907)27:1<22::aid-glia3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Bredel M, Zentner J. Braintumour drug resistance: The bare essentials. Lancet Oncol. 2002;3:397–406. doi: 10.1016/s1470-2045(02)00786-6. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, D’Onofrio M, Di Iorio P, De Blasi A, Melchiorri D, Flor PJ, Nicoletti F. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab. 2001;21:1013–1033. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Dell’Albani P, Corsaro M, Giuffrida R, Caruso A, Trovato Salinaro A, Spinella F, Nicoletti F, Albanese V, Giuffrida Stella A. Metabotropic glutamate receptor expression in cultured rat astrocytes and human gliomas. Neurochem Res. 1997;22:1127–1133. doi: 10.1023/a:1027317319166. [DOI] [PubMed] [Google Scholar]
- Copani A, Condorelli F, Caruso A, Cancheri C, Sala A, Giuffrida Stella AM, Canonico PL, Nicoletti F, Sortino MA. Mitotic signaling by beta-amyloid causes neuronal death. FASEB J. 1999;13:2225–2234. [PubMed] [Google Scholar]
- D’Onofrio M, Cuomo L, Battaglia G, Ngomba RT, Storto M, Kingston AE, Orzi F, De Blasi A, Di Iorio P, Nicoletti F, Bruno V. Neuroprotection mediated by group-II metabotropic glutamate receptors requires the activation of the MAP kinase and phosphatidylinositol-3-kinase pathways. J Neurochem. 2001;78:435–445. doi: 10.1046/j.1471-4159.2001.00435.x. [DOI] [PubMed] [Google Scholar]
- D’Onofrio M, Arcella A, Bruno V, Ngomba RT, Battaglia G, Lombari V, Ragona G, Calogero A, Nicoletti F. Pharmacological blockade of mGlu2/3 metabotropic glutamate receptors reduces cell proliferation in cultured human glioma cells. J Neurochem. 2003;84:1288–1295. doi: 10.1046/j.1471-4159.2003.01633.x. [DOI] [PubMed] [Google Scholar]
- Franklin, K.B.J., and Paxinos, G. (1997) The Mouse Brain in Stereotaxic Coordinates San Diego, Calif.: Academic Press.
- Giese A. Glioma invasion—pattern of dissemination by mechanisms of invasion and surgical intervention, pattern of gene expression and its regulatory control by tumor suppressor p53 and proto-oncogene ETS-1. Acta Neurochir Suppl. 2003;88:153–162. doi: 10.1007/978-3-7091-6090-9_21. [DOI] [PubMed] [Google Scholar]
- Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M, Sasaki T, Osawa S. Blockade of Ca2+ permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8:971–978. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- Jane DE, Thomas NK, Tse HW, Watkins JC. Potent antagonists at the L-AP4- and (1S,3S)-ACPD-sensitive presynaptic metabotropic glutamate receptors in the neonatal rat spinal cord. Neuropharmacology. 1996;35:1029–1035. doi: 10.1016/s0028-3908(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Johnson BG, Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H]- LY341495 as a novel antagonist radio- ligand for group II metabotropic glutamate (mGlu) receptors: Characterization of binding to membranes of mGlu receptor subtype expressing cells. Neuropharmacology. 1999;38:1519–1529. doi: 10.1016/s0028-3908(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Kingston AE, O’Neill MJ, Lam A, Bales KR, Monn JA, Schoepp DD. Neuroprotection by metabotropic glutamate receptor agonists LY354740, LY379268 and LY389795. Eur J Pharmacol. 1999;377:155–165. doi: 10.1016/s0014-2999(99)00397-0. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Jr, Wright RA, Johnson BG, Andis SL, Kingston A, Tomlinson R, Lewis R, Griffey KR, Tizzano JP, Schoepp DD. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid ( LY354740): Identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- Poli A, Beraudi A, Villani L, Storto M, Battaglia G, Di Giorgi Gerevini V, Cappuccio I, Caricasole A, D’Onofrio M, Nicoletti F. Group II metabotropic glutamate receptors regulate the vulnerability to hypoxic brain damage. J Neurosci. 2003;23:6023–6029. doi: 10.1523/JNEUROSCI.23-14-06023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt W, Stabel-Burow J, Pannicke T, Weichert H, Heinemann U. The glutathione level of retinal Muller glial cells is dependent on the high-affinity sodium-dependent uptake of glutamate. Neuroscience. 1997;77:1213–1224. doi: 10.1016/s0306-4522(96)00509-x. [DOI] [PubMed] [Google Scholar]
- Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proc Natl Acad Sci USA. 2001;11:6372–6377. doi: 10.1073/pnas.091113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallese M, Iacovelli L, Storto M, De Blasi A. Receptor specificity of G-protein-coupled receptor kinases in target cells. Trends Pharmacol Sci. 2001;22:168–169. doi: 10.1016/s0165-6147(00)01641-2. (letter) [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. Malignant gliomas: Perverting glutamate and ion homeostasis for selective advantage. Trends Neurosci. 2003;26:543–549. doi: 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: Reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cysteine-glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]