An Essential Role for Katanin in Severing Microtubules in the Neuron (original) (raw)
Abstract
Several lines of evidence suggest that microtubules are nucleated at the neuronal centrosome, and then released for transport into axons and dendrites. Here we sought to determine whether the microtubule-severing protein known as katanin mediates microtubule release from the neuronal centrosome. Immunomicroscopic analyses on cultured sympathetic neurons show that katanin is present at the centrosome, but is also widely distributed throughout the neuron. Microinjection of an antibody that inactivates katanin results in a dramatic accumulation of microtubules at the centrosome, indicating that katanin is indeed required for microtubule release from the centrosome. However, the antibody also causes an inhibition of axon outgrowth that is more immediate than expected on this basis alone. It may be that katanin severs microtubules throughout the cell body to keep them sufficiently short to be efficiently transported into developing processes. Consistent with this idea, there were significantly fewer free ends of microtubules in the cell bodies of neurons that had been injected with the katanin antibody compared with controls. These results indicate that microtubule-severing by katanin is essential for releasing microtubules from the neuronal centrosome, and also for regulating the length of the microtubules after their release.
Keywords: katanin, microtubule, neuron, centrosome, axon
Axons and dendrites contain dense arrays of highly organized microtubules. The microtubules within these processes have a consistent 13-protofilament lattice and are tightly regulated with regard to their polarity orientation. We have proposed a model whereby these features of the axonal and dendritic microtubule arrays are established within the cell body of the neuron. Microtubules are nucleated at the centrosome by gamma tubulin (Ahmad et al., 1994), rapidly released (Ahmad and Baas, 1995), and then transported into neuronal processes by the motor proteins known as cytoplasmic dynein (Ahmad et al., 1998) and CHO1/MKLP1 (Sharp et al., 1997). Gamma tubulin is organized into ring structures within the pericentriolar region of the centrosome, and nucleation from these structures presumably constrains the lattice structure of each microtubule to 13 protofilaments (see Baas, 1997). Within the cell body, the microtubules remain relatively short, presumably so that their ends can be efficiently directed into the hillock region of the developing process (Yu and Baas, 1994). The specific orientation of each microtubule is determined by the motor protein that conveys it into the developing process, such that axons contain uniformly plus-end-distal microtubules while dendrites contain microtubules of both orientations (for review see Baas and Yu, 1996).
One important feature of this model that has not been explored is the mechanism by which microtubules are released from the centrosome after their nucleation. We have argued that most of the cellular mechanisms that regulate the microtubule arrays of the postmitotic neuron are variations of mechanisms that organize microtubules in mitotic cells (for review see Baas, 1999). On this basis, it seems reasonable that microtubules may be released from the neuronal centrosome by katanin, a protein thought to release microtubules from the centrosome during mitosis. Katanin was originally purified from sea urchin eggs, and was shown to sever microtubules by disrupting contacts within the polymer lattice using energy derived from ATP hydrolysis (McNally and Vale, 1993). Subsequently, the protein was shown to be present in a region just outside of the pericentriolar material within these eggs, an ideal location for it to sever microtubules from the centrosome after their nucleation (McNally et al., 1996). The 60- and 80-kD katanin subunits have now been identified in vertebrate cells such as human fibroblasts, and it has been determined that the smaller subunit has the microtubule-severing activity (Hartman et al., 1998; McNally and Thomas, 1998). The larger subunit does not sever microtubules on its own, but enhances the microtubule severing capacity of the smaller subunit. In addition, the larger subunit has a region that binds sites at the centrosome, ensuring that a portion of the total katanin within the cell will be targeted to the centrosome. It has been proposed that katanin severs microtubules from the centrosome during mitosis so that tubulin subunits can undergo a flux through the polymer. It seems reasonable that katanin might also sever centrosomal microtubules in certain types of interphase cells such as epithelial cells that must deploy microtubules through the cytoplasm (Keating et al., 1997).
In the present study, we have sought to test the hypothesis that katanin is essential for the release of microtubules from the neuronal centrosome. For these analyses, we microinjected into cultured neurons a function-blocking antibody to the smaller katanin subunit. This antibody has been shown to interfere with the capacity of katanin to sever microtubules (McNally and Thomas, 1998). The results of these studies demonstrate that microtubule-severing by katanin is essential for microtubule release from the neuronal centrosome, and for regulating microtubule lengths throughout the neuronal cell body. In addition, our results demonstrate that these katanin-mediated events are essential for the normal outgrowth of neuronal processes.
Materials and Methods
Cell Culture
Cultures of sympathetic neurons from the superior cervical ganglia of newborn rat pups were prepared as follows. After dissection, the ganglia were treated with 0.25% collagenase and 0.25% trypsin for 15 min, and then triturated with a pasteur pipet into a single cell dispersion. The cells were then plated onto “special dishes” that were prepared by adhering a glass coverslip to the bottom of a 35-mm plastic petri dish into which had been drilled a 1-cm-diam hole. For the preparation of these dishes, we used glass coverslips that had been photoetched with a pattern of demarcated boxes that assist in the relocation of individual cells (Bellco Glass, Inc.). Before plating the cells, the glass-bottomed well of the special dish was treated for 3 h with 1 mg/ml polylysine, rinsed extensively, and then treated with 10 μg/ml laminin (Sigma Chemical Co.) for 4 h. Cells were plated in medium consisting of Leibovitz' L-15 (Sigma Chemical Co.) supplemented with 0.6% glucose, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% fetal bovine serum (Hyclone), and 100 ng/ml nerve growth factor (Upstate Biotechnology Inc.). Most of the experiments and immunolocalization studies were performed on freshly plated neurons. Some of the immunofluorescence analyses were performed on older cultures, and, for these studies, we used a medium better suited to long-term culture (see Baas and Ahmad, 1992).
Western Blot Analyses
Western blots were used to document the presence of the katanin subunits in sympathetic neurons, and to test the specificities of the katanin polyclonal antibodies. Analyses were performed on superior cervical ganglia obtained from rat fetuses or pups at various ages. Electrophoresis and immunoblotting were performed as previously described (McNally and Thomas, 1998).
Immunofluorescence Analyses on Katanin Distribution
Immunofluorescence microscopy was used to determine the distribution of both katanin subunits in cultured neurons at various stages of development. For these analyses, neurons were either fixed directly or preextracted in a microtubule-stabilizing buffer before fixation. Fixation was either in −20°C methanol or 4% formaldehyde. Cultures were then treated for 30 min in a blocking solution containing 2% normal goat serum and 1% BSA in PBS, and exposed overnight at 4°C to one of the two primary antibodies (used at 1:100) diluted in blocking solution. The primary antibodies were the affinity-purified rabbit polyclonal antibodies specific for either the 60- or 80-kD subunit of katanin (see McNally and Thomas, 1998). The following morning, the cultures were rinsed three times in PBS, treated again for 30 min in blocking solution, exposed for 1 h at 37°C to an appropriate Cy-3–conjugated second antibody (purchased from Jackson ImmunoResearch Laboratories, Inc.) diluted in blocking solution, rinsed four times for 5 min each in PBS, and mounted in a medium that reduces photobleaching. To optimize the possibility of visualizing any potential association of katanin with specific cytoplasmic structures, optical sections were acquired using the 410 Laser Scanning Confocal Microscope (Carl Zeiss, Inc.). The optical sections were 0.5 μm in width, and all sections comprising an individual cell were examined. Images were depicted in “glow-scale pseudocolor,” which displays the highest fluorescence intensity in white, the lowest in red, and intermediate intensities in shades of yellow and orange (see Sharp et al., 1995).
Immunoelectron Microscopic Analyses on Katanin Distribution
Immunoelectron microscopy was used to obtain a higher resolution perspective on the distribution of katanin in cultured neurons, and also to determine whether a portion of the katanin is localized to the region of the centrosome. For these analyses, we used the same procedure that we previously used to localize gamma tubulin in cultured neurons (Baas and Joshi, 1992). The antibody against the 60-kD subunit was used for these analyses at a concentration of 1:50. The negatives obtained from the electron microscope were scanned with a flat-bed scanner to obtain a digitized image, after which each gold particle was manually highlighted with the pencil tool in Adobe Photoshop to enhance the contrast of the particles. For most centrosomes observed, we obtained a rough estimate of the number of microtubules attached to it and the number of gold particles associated with it. Estimates were obtained by counting and adding together the microtubules and gold particles observed on available sections.
Immunofluorescence Analyses on Microtubule Distribution
For immunofluorescence visualization of microtubules, we used our previously described procedure (Ahmad and Baas, 1995). Cultures were rinsed briefly in the microtubule-stabilizing buffer termed PHEM (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2, pH 6.9), and then extracted for 3 min with 0.5% Triton X-100 in PHEM containing 10 μM taxol (provided as a gift from the National Cancer Institute). This treatment removes unassembled tubulin while preserving microtubules (Black et al., 1986). The cultures were then fixed by the addition of an equal volume of PHEM containing 8% paraformaldehyde and 0.3% glutaraldehyde. After 10 min fixation, the cultures were rinsed twice in PBS, treated three times for 5-min each in PBS containing 10 mg/ml sodium borohydride, and rinsed again in PBS. Cultures were then treated for 30 min in a blocking solution containing 2% normal goat serum and 1% BSA in PBS, and exposed overnight at 4°C to the primary antibody diluted in blocking solution. The following morning, the cultures were rinsed three times in PBS, treated again for 30 min in blocking solution, exposed for 1 h at 37°C to an appropriate Cy-3–conjugated second antibody (see above) diluted in blocking solution, rinsed four times for 5 min each in PBS, and mounted in a medium that reduces photobleaching. The primary antibody was the mouse monoclonal antibody against β tubulin purchased from Amersham Corp. and used at 1:500.
Electron Microscopic Analyses on Microtubule Distribution
To investigate whether microtubules were attached to the centrosome in control neurons and neurons that had been injected with the function-blocking katanin antibody (see below), we used standard transmission electron microscopy. Cultures were first extracted by the same procedure used in the immunofluorescence analyses because we have found that extraction significantly enhances the clarity of microtubules in the electron micrographs. For these analyses, neurons were extracted, fixed, prepared, and visualized as described in an earlier report from our laboratory (Yu et al., 1993).
Experimental Regimes
In one set of experiments, a volume of roughly 4 pl of the function-blocking antibody against the 60-kD subunit of katanin was microinjected into cultured sympathetic neurons at a concentration of 6 mg/ml. Cells were microinjected roughly 45 min after plating, before the outgrowth of any neuronal processes. (Some cells already had short processes by this time, but these cells were not selected for microinjection.) At this point, the cultures were returned to the incubator for 6 h, and then prepared for immunofluorescence visualization of microtubules. In another set of experiments, we took advantage of a pharmacological regime that reveals the outward transport of microtubules from the centrosome (Ahmad and Baas, 1995; Ahmad et al., 1998). In this regime, nocodazole (Aldrich Chemical Co.) is introduced into the cultures 30 min after plating at a final concentration of 10 μg/ml, and the cultures are returned to the incubator. After 6 h, the cultures are rinsed twice with warm drug-free medium, placed in a third rinse of warm drug-free medium, and then returned to the incubator. After 3 min of recovery, vinblastine sulfate (Sigma Chemical Co.) is added to a final concentration of 50 nM, and the cultures are once again returned to the incubator for 30 or 60 min. Cultures are then prepared for immunofluorescence visualization of microtubule distribution. In the present study, we wished to determine the effects on microtubule distribution of inhibiting katanin function. To accomplish this, we microinjected the function-blocking katanin antibody either immediately after adding the nocodazole or roughly 1 h before removing the nocodazole.
In some experiments, we microinjected a function-blocking antibody to a protein called centrin, which is also present at the centrosomes of many cell types and has been proposed as a candidate for releasing microtubules from the centrosome (Yu et al., 1993). This antibody was prepared and characterized as previously described (Sanders and Salisbury, 1994), and was microinjected in the same manner as the katanin antibody. The antibody was provided as a kind gift from Dr. Jeffrey Salisbury of the Mayo Clinic (Rochester, MN).
Quantification of Microtubule Levels and Free Ends
To obtain information on the relative lengths of the microtubules within the cell bodies of control and antibody-injected neurons, we quantified the total amount of microtubule polymer and the number of free ends of microtubules. To quantify the total amount of microtubule polymer, we extracted and fixed cultures 4–5 h after a portion of the neurons in the culture had been microinjected with the katanin antibody. Total microtubule levels in the cell body were quantified using MetaMorph software (Universal Imaging Corp.), and expressed in arbitrary fluorescence units (AFUs).1 For illustrative purposes, fluorescence images were converted to a standard pseudocolor scale in which red represents the highest and purple the lowest intensity. To quantify the number of free ends of microtubules, we microinjected roughly 4 pl of rhodamine-labeled tubulin (prepared by the method of Keating et al., 1997) at a concentration of 4 mg/ml into the cell bodies of uninjected neurons and neurons that had been injected with the katanin antibody 4–5 h before the injection of the tubulin. We then waited 1 min to permit the incorporation of fluorescent tubulin onto the ends of the microtubules, after which the cells were extracted in our standard microtubule-stabilizing buffer (see above), and rapidly imaged with a cooled CCD (see below). For these analyses, images were acquired using a cooled CCD to visualize the entire cell body and to optimize resolution. The number of microtubule ends was obtained by counting individual fluorescent points from images displayed on a high-resolution computer monitor.
Results
Katanin Expression and Distribution in Neurons
Western blot analyses were performed to determine whether katanin is expressed in vertebrate neurons and to confirm the specificity of the antibodies. Because katanin is a relatively low-abundance protein, analyses were performed directly on samples of superior cervical ganglia, rather than on cultures generated from the ganglia. The blots reveal the presence of both 60- and 80-kD subunits of katanin within the developing ganglia (Fig. 1 A). A single band for each subunit was detected in samples from superior cervical ganglia obtained from embryonic (E18), newborn, and 4-d rat pups. The bands were entirely similar to those obtained from cultured HeLa cells, which were used as a positive control. No other bands appeared within these samples, nor were any bands detected in control blots incubated without the primary antibody. Given that neurons are the principle cells that compose these ganglia (especially at E18), these results strongly suggest that katanin is expressed in neuronal cells, and demonstrate that the antibodies do not cross-react with other proteins expressed by neurons or nonneuronal cells within the ganglia. Immunofluorescence and immunelectron microscopy on cultures derived from the ganglia confirm that the neurons express both the 60- and 80-kD katanin subunits.
Figure 1.
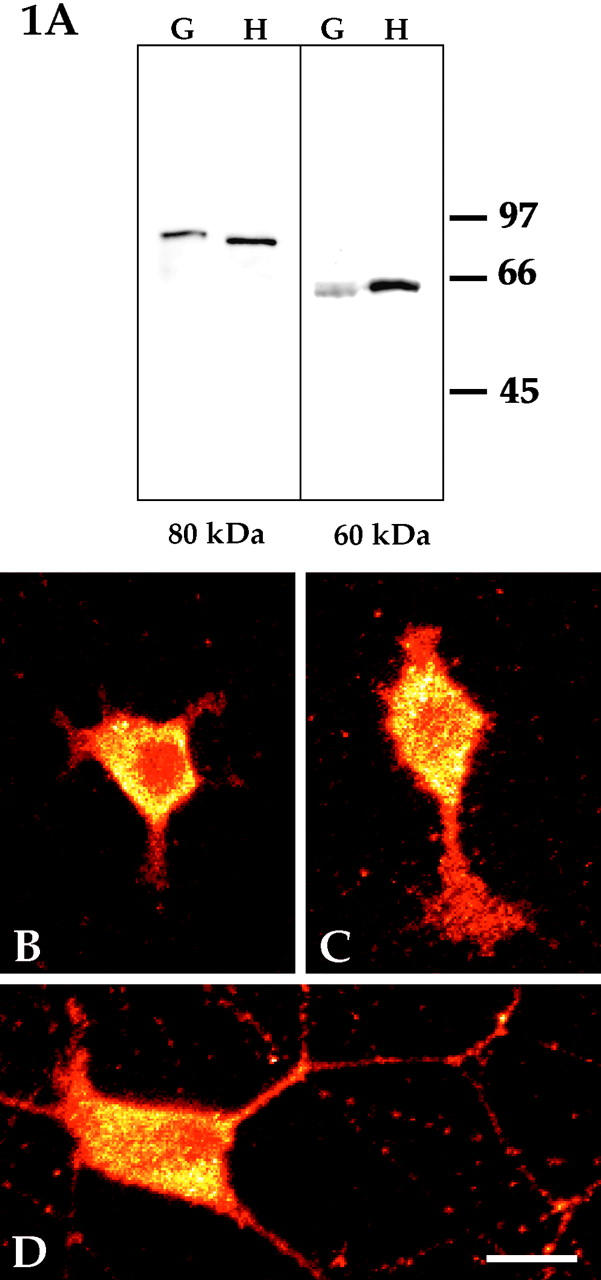
Western blotting and immunofluorescence analyses of katanin in rat sympathetic neurons. Western blot analyses were performed using affinity-purified polyclonal antibodies that recognize either the 80- (A, left) or 60-kD (A, right) katanin subunit. The blots reveal the presence of both subunits within developing rat sympathetic ganglia. Shown here are samples of superior cervical ganglia from 4-d animals (G) alongside samples from HeLa cells used as a positive control (H). Similar results were obtained with ganglia obtained from E18 and newborn rat pups. Molecular weight standards are indicated in the margin. Immunofluorescence analyses on cultured rat sympathetic neurons revealed the presence of both katanin subunits throughout the neuron at all stages of development. B and C show freshly plated neurons, while D shows a 3-d culture. B is a cell that had flattened against the substrate, but had not yet begun to extend processes. C is a cell that had begun to extend short processes. D is a cell that had extended a complex axonal arbor (and had begun to show signs of dendritic differentiation). Optical sections were obtained with a confocal microscope, and images were depicted in glow-scale pseudocolor to assist in determining whether there was a focal enrichment of katanin in the cell body that might correspond to the centrosome. No such enrichment was observed, indicating that katanin is not specifically concentrated at the centrosome. Shown here are analyses with the antibody to the 60-kD katanin subunit. Similar results were obtained with the antibody to the 80-kD subunit (not shown). Bar, 15 μm.
Immunofluorescence analyses were performed on freshly plated neurons (Fig. 1, B and C), and on neurons that had been permitted to develop in culture for 1 (not shown), 3 (Fig. 1 D), or 7 (not shown) d. Similar results were obtained whether we used the antibody to the smaller or larger katanin subunit. All images presented in the figures were obtained using the antibody to the smaller subunit. Optical sections were obtained with the confocal microscope to assist in determining whether any particular regions within the cell body stained more intensely for katanin, and also to permit a better comparison of fluorescence intensities within the cell body and the processes. Images were depicted in glow-scale pseudocolor to further assist in determining whether there was a focal enrichment of katanin in the cell body that might correspond to the centrosome. In all of these cases, the immunofluorescence images showed a widespread distribution of both katanin subunits throughout the neuron. Katanin immunoreactivity was detected in the cell body and in all regions of developing axons and dendrites at all stages of development. The appearance of the staining was generally diffuse, suggesting that neurons might contain a soluble pool of katanin as well as a pool bound to structures such as the centrosome. However, the immunofluorescence images were entirely similar in neurons that had been extracted before fixation in our standard microtubule-stabilizing buffer supplemented with various concentrations of detergents such as Triton X-100 and saponin (data not shown). These results suggest either that virtually all of the katanin is bound to detergent-insoluble structures, or that soluble katanin precipitates under these extraction conditions. No enrichment at any discrete site within the cell body was observed, indicating that katanin is not specifically concentrated at the centrosome. The images suggest that katanin is present at higher concentrations within the cell body compared with axons or dendrites. However, this difference may not be as dramatic as it appears in the micrographs because even the thinnest optical section of the cell body is thicker than the width of a neuronal process. Entirely similar results were obtained in cultures fixed with aldehydes rather than cold methanol. No detectable staining was observed in cultures prepared with the secondary antibody but not the primary antibody.
It has been our experience that immunofluorescence microscopy is not well suited to visualizing the centrosome in the near-spherical cell body of the neuron, even with the use of optical sectioning. For example, gamma tubulin is localized to the centrosome in neurons, but we have found that with immunofluorescence staining for gamma tubulin the centrosome appears as a small speckle that is difficult to distinguish from background speckles. Therefore, we have come to rely on immunoelectron microscopy as the best approach to determine with confidence whether a protein is associated with the centrosome (for example, see Baas and Joshi, 1992). To investigate whether a portion of the katanin in the neuron is associated with the centrosome (even though the protein does not appear to be specifically concentrated at the centrosome), we analyzed the neurons with immunoelectron microscopy. In all neurons examined (n = 18), katanin immunoreactivity was detected within or around the pericentriolar material (Fig. 2, A–C). In addition, katanin immunoreactivity was sometimes associated with the clusters of amorphous cytoplasmic material that persist extraction (see Fig. 2 A). Occasionally, immunoreactivity was found at a discrete site along the length of a microtubule (for example, see Fig. 2 A, thin arrow). Immunoreactivity was also found in axons (Fig. 2 D) and dendrites (not shown). Only an extremely rare gold particle was ever detected in cultures prepared in a similar fashion in the absence of the primary antibody, indicating the specificity of the results obtained with the primary antibody. Taken together, these immunological analyses demonstrate that katanin is expressed in neurons, that it has a widespread distribution throughout the cytoplasm, that it can associate with microtubules, and that a portion of the katanin is associated with the centrosome.
Figure 2.
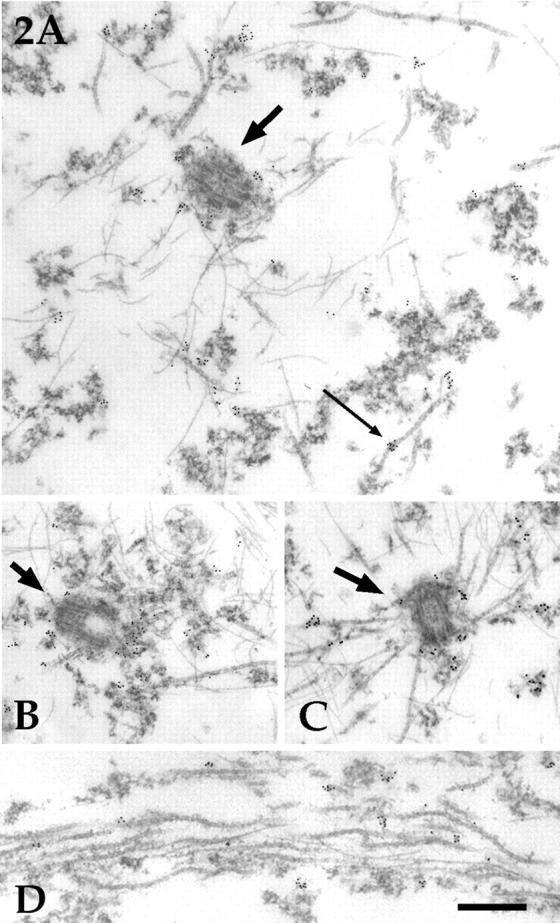
Immunoelectron microscopic analyses on katanin distribution in rat sympathetic neurons. Shown are immunoelectron micrographs from neurons stained with the antibody against the 60-kD katanin subunit and a second antibody conjugated to 5-nm colloidal gold particles. In all neurons examined, katanin immunoreactivity was detected within or around the pericentriolar material (A–C, thicker arrows point to the centrosome). In addition, katanin immunoreactivity was typically associated with the clusters of amorphous cytoplasmic material that persist extraction (A). Occasionally, immunoreactivity was found at a discrete site along the length of a microtubule (A, thin arrow). There was no correlation between the number of microtubules attached to any given centrosome and the number of gold particles associated with it. C is a rare centrosome with several attached microtubules, but the number of gold particles is no higher than with other centrosomes. Immunoreactivity for katanin was also detected in axons (D) and dendrites (not shown). D is shown at slightly higher magnification than A–C. Bar (A–C), 0.40 μm. Bar (D), 0.30 μm.
It has been suggested that the presence of katanin at the centrosome might depend on having an array of attached microtubules (McNally and Vale, 1996). On this basis, it seems reasonable that a portion of the katanin might move to the centrosome shortly after a burst of microtubule nucleation, and then dissipate from the centrosome after the microtubules are released (Baas, 1997). To investigate this possibility, we compared the levels of katanin present at centrosomes with few or no attached microtubules with the levels present at centrosomes with higher numbers of attached microtubules. The vast majority of the centrosomes that we observed had few or no microtubules attached to them, and these centrosomes displayed roughly 30–50 gold particles (see Materials and Methods). A small fraction of the centrosomes we examined (2 of 18) showed roughly 15–20 attached microtubules, but these centrosomes did not display any higher immunoreactivity for katanin than centrosomes with few or no attached microtubules (compare Fig. 2 C with A and B). Thus, the present observations do not support the idea that the levels of katanin at the neuronal centrosome vary as a result of the number of microtubules attached to it.
Effects of Microinjection of the Function-blocking Katanin Antibody
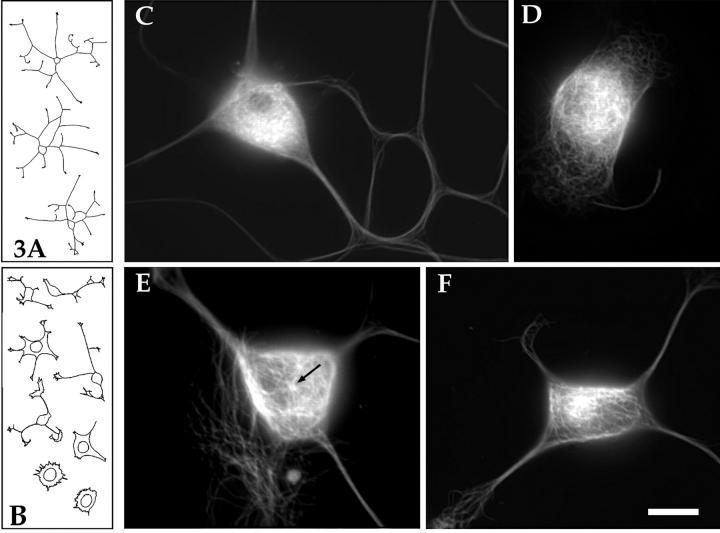
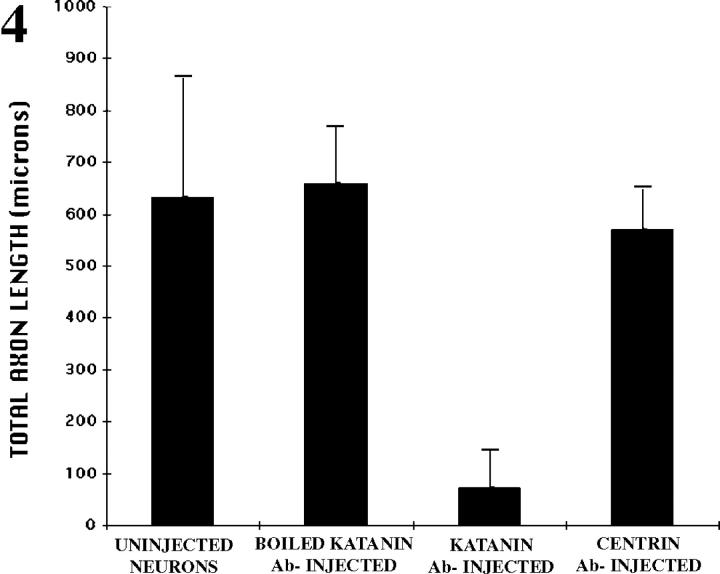
In a first set of experimental analyses, freshly plated neurons were microinjected with the polyclonal antibody against the 60-kD subunit of katanin. This antibody has been shown by in vitro analyses to interfere with the microtubule-severing activity of katanin (McNally and Thomas, 1998). Typical uninjected neurons grew an average of 633 ± 229 μm of total axon length during the first 6 h of plating. 4 of 50 neurons observed showed little or no outgrowth, presumably due to damage during plating. Neurons injected with katanin antibody that had been boiled before injection showed an entirely similar pattern of axon outgrowth, with 23 of 27 neurons each displaying levels of axon outgrowth comparable to controls (659 ± 108 μm total axon length). In sharp contrast, neurons injected with the viable katanin antibody were severely stunted with regard to axon outgrowth. A total of 45 such neurons were examined. The average total length of axons extended by these cells was 73.1 ± 73 μm. Fig. 3 A shows tracings of typical control neurons, while B shows tracings of typical katanin-antibody–injected neurons. Notably, neurons injected with a function-blocking antibody to centrin (another protein that has been suggested as a mediator of microtubule-release from the centrosome) showed no dramatic inhibition of process outgrowth. 20 cells injected with the centrin antibody grew an average of 569 ± 79 μm of total axon length. These data are summarized in Fig. 4.
Figure 3.
Effects of microinjecting neurons with the function-blocking katanin antibody. A shows tracings of typical control neurons roughly 6 h after plating, while B shows tracings of typical antibody-injected neurons (injected roughly 45 min after plating and photographed 5–6 h later). The levels of axon outgrowth were markedly reduced in the antibody-injected cells compared with the uninjected cells. C shows an immunofluorescence micrograph of the microtubule array within a typical control (uninjected) neuron. There is a widespread and relatively even distribution of microtubules throughout the cell body. D shows a neuron that grew no processes after injection with the viable katanin antibody. Microtubules appear throughout the cell body, but, as with uninjected cells, it is impossible to discern whether or not the microtubules are attached to the centrosome. E shows a neuron that extended short processes after injection of the viable katanin antibody. The microtubules have reorganized such that it is clear that several microtubules are attached to a “point source” in the cell body (arrow). In addition, these microtubules are unusually long, extending to the periphery of the cell body. F shows a neuron that grew somewhat longer processes after injection of the viable katanin antibody. In this cell, it is not possible to discern the attachment of microtubules to a point source, but it is clear that many of the microtubules are clustered together near the center of the cell body. There is a particularly bright region of staining within the cluster of microtubules that may correspond to the centrosome. In addition, some of the individual microtubules that can be resolved appear to be unusually long (note the long microtubules extending from the bright cluster toward the right). Bar (A and B), 66 μm. Bar (C–F), 10 μm.
Figure 4.
Bar graph showing the total microns of axon growth that occurred over a 5–6-h period of time for uninjected neurons and neurons injected with either boiled katanin antibody, viable katanin antibody, or viable centrin antibody.
Fig. 3 C shows an immunofluorescence micrograph of the microtubule array within a typical control (uninjected) neuron. There is a widespread and relatively even distribution of microtubules throughout the cell body. Neurons injected with the boiled katanin antibody or the centrin antibody were indistinguishable from uninjected neurons (not shown). Fig. 3 D shows a neuron that grew no processes after injection with the viable katanin antibody. Microtubules appear throughout the cell body, but, as with the uninjected cells, it is impossible to discern whether or not the microtubules are attached to the centrosome (even with optical sectioning at the confocal microscope; data not shown). Fig. 3 E shows a neuron that has extended short processes after injection of the viable katanin antibody. The microtubules have reorganized such that it is clear that several microtubules are attached to a “point source” in the cell body, presumably the centrosome. In addition, these microtubules are unusually long, extending to the periphery of the cell body. Fig. 3 F shows a neuron that has grown somewhat longer processes after injection of the viable katanin antibody. In this cell, it is not possible to discern the attachment of microtubules to a point source, but it is clear that many of the microtubules are clustered together near the center of the cell body. In addition, some of the individual microtubules appear to be unusually long.
As noted above, immunofluorescence labeling is not well suited to identifying the centrosome of neuronal cells with confidence. The centrosome is very small and usually difficult to distinguish from background speckles, and there is usually insufficient resolution to visualize whether or not microtubules are actually attached to it. Therefore, electron microscopy was used to better evaluate the relationship between the microtubules and the centrosome within these control and experimental neurons. As previously reported (Yu et al., 1993), few or no microtubules are attached to the centrosome in control neurons (Fig. 5, A and B). We previously speculated that this was due to the rapid release of microtubules after their nucleation (Yu et al., 1993). Indeed, neurons injected with the viable katanin antibody showed numerous microtubules attached to the centrosome. Fig. 5, C and D show one such neuron (4–5 h after injection of the antibody). There are 18 microtubules attached to the centrosome in the thin section showed in the figure, and similar numbers in each of the other thin sections. We estimate that roughly 50–75 microtubules accumulated at this centrosome over the 4–5-h period of time. Similar results were obtained in all six such cells examined by electron microscopy. In the most dramatic case, 75–100 microtubules accumulated at the centrosome over the 4–5-h period of time (not shown).
Figure 5.
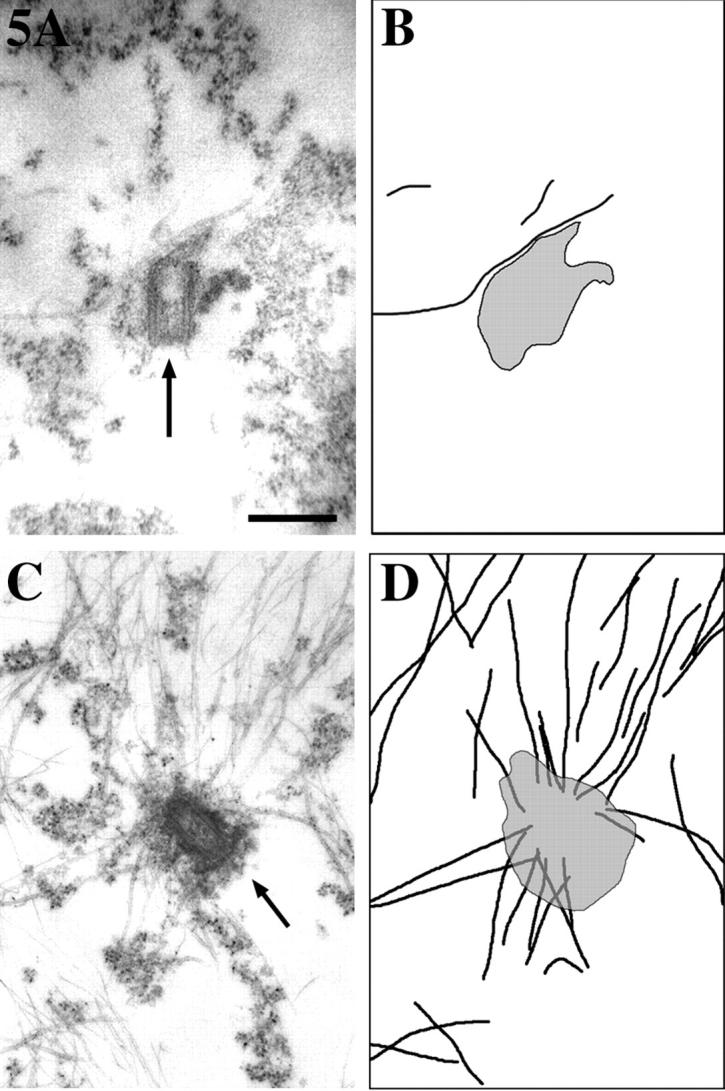
Electron micrographs of centrosomes from control and katanin antibody-injected neurons. Neurons treated as described in Fig. 3 were examined by electron microscopy. Shown in this figure are the electron micrographs (A and C) and corresponding tracings to make clearer the microtubules and centrosome, shown in black and gray, respectively (B and D). Control neurons show few or no microtubules attached to the centrosome (A and B). By contrast, antibody-injected neurons show many attached microtubules (C and D). Bar, 0.5 μm.
Quantification of Microtubule Levels and Free Ends in the Presence of the Katanin Antibody
The fact that process outgrowth was compromised in the presence of the katanin antibody is consistent with the view that the released microtubules are essential for process outgrowth. However, the immediacy of the response was somewhat surprising given that the neuronal cell body contains a large number of microtubules that are not attached to the centrosome, presumably because they have already been released. Thus there is a “storage supply” of microtubules that can be transported into developing processes, and must be depleted from the cell body before axon outgrowth would be notably compromised by inhibiting the manufacture of new microtubules at the centrosome (Ahmad et al., 1994). For this reason, the immediacy of the inhibition of axon outgrowth by the katanin antibody suggests that katanin serves another important function in the neuron besides releasing microtubules from the centrosome. One possibility is that released microtubules must be continuously severed by katanin to keep them sufficiently short that they can effectively funnel into developing processes. If this is correct, we would expect the microtubules to be substantially longer in the antibody-injected cells compared with the control cells. On the basis of the immunofluorescence images shown in Fig. 3, it was already our impression that some of the microtubules in the antibody-injected cells were unusually long, but it was impossible to draw a conclusion on this basis alone given that we could not discern individual microtubules in the control neurons.
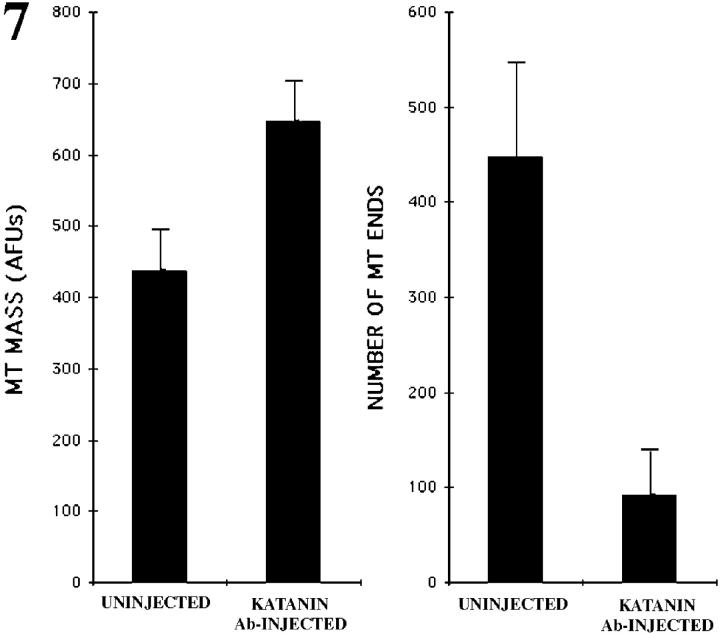
Unfortunately, the near-spherical geometry of the neuronal cell body precluded our ability to directly measure microtubule lengths using the same kind of serial reconstruction electron microscopy that has previously proved effective in such analyses on developing axons (Yu and Baas, 1994). For this reason, we used an indirect method to compare microtubule lengths. Specifically, we reasoned that we could indirectly compare microtubule lengths by determining the total microtubule mass in the cell body and the number of free microtubule ends. To quantify the total microtubule mass, we prepared cultures for immunofluorescence visualization of microtubules 4–5 h after a portion of the neurons had been injected with the katanin antibody. We found that the cell bodies of antibody-injected cells contained 647 ± 54 AFUs (4–5 h after antibody injection), while the cell bodies of uninjected cells contained 438 ± 55 AFUs (n = 32 in each case). These data indicate that there is roughly a 48% increase in the levels of microtubule polymer in the cell bodies of the antibody-injected neurons compared with control neurons. Such an increase is consistent with an inhibition of microtubule severing, given that microtubule severing probably results in the depolymerization of a portion of the polymer (McNally and Vale, 1993). Fig. 6, A and B, shows immunofluorescence images of a control and injected neuron, respectively, in a standard pseudocolor range to illustrate the fact that the cell bodies of antibody-injected neurons contained more polymer than control neurons.
Figure 6.
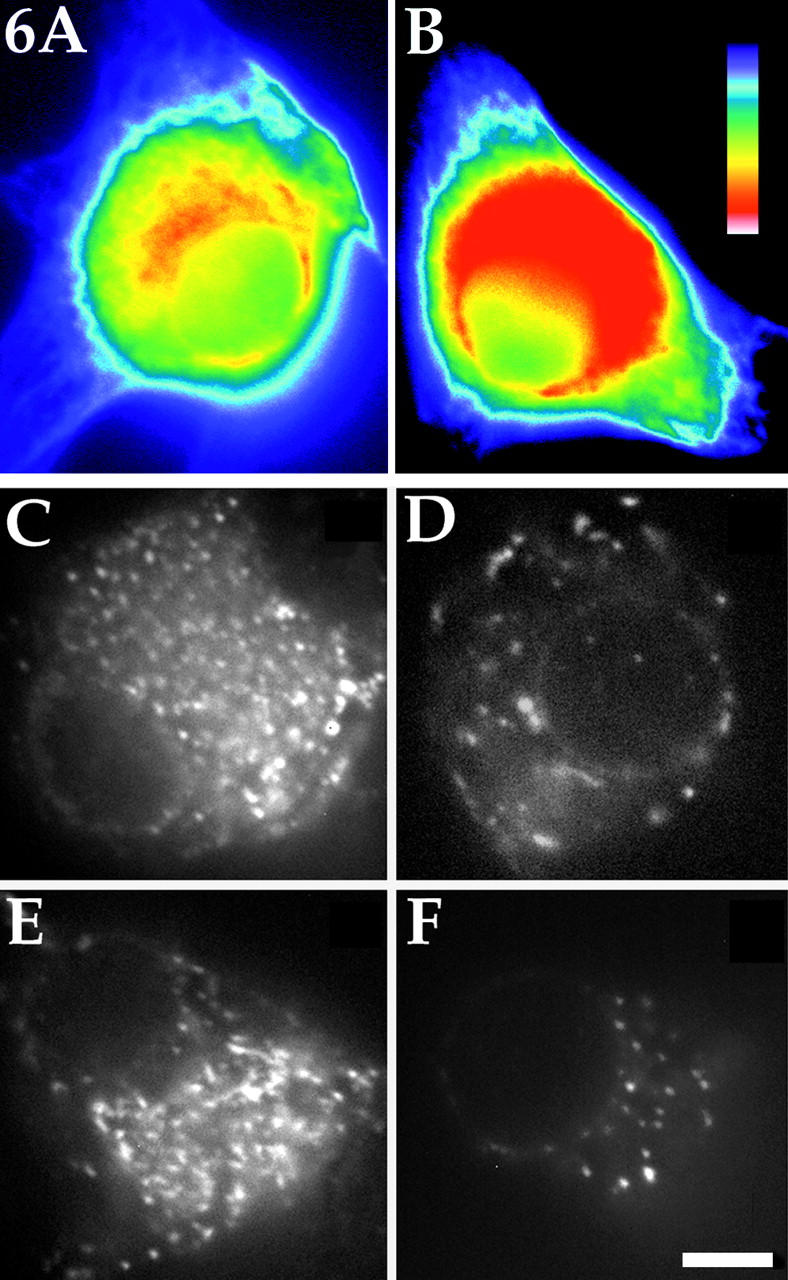
Quantification of microtubule mass and microtubule ends in control and katanin antibody-injected neuronal cell bodies. To quantify total microtubule mass in the cell body of the neuron, cultures were prepared for immunofluorescence visualization of microtubules 4–5 h after a portion of the neurons had been injected with the function-blocking katanin antibody. There was an increase of roughly 48% in the total microtubule mass in the cell bodies of the antibody-injected neurons compared with the cell bodies of the control neurons. Examples of a cell body of a control and injected neuron displayed in a standard pseudocolor scale are shown in A and B, respectively. The pseudocolor scale is shown in B, with red indicating the most and purple the least intense levels of staining. To quantify microtubule ends, rhodamine-labeled tubulin was injected into the cell bodies of uninjected neurons and neurons that had been injected with the katanin antibody 4–5 h before the injection of the tubulin. 1 min was permitted for the incorporation of fluorescent tubulin onto the free ends of the microtubules, after which the cells were extracted in a microtubule-stabilizing buffer and rapidly imaged. In control cells (C and E), there were four to five times fewer microtubule ends as in antibody-injected cells (D and F). Bar, 5 μm.
To quantify the number of microtubule ends, we microinjected rhodamine-labeled tubulin into the cell bodies of uninjected neurons and neurons that had been injected with the katanin antibody 4–5 h before the injection of the tubulin. We then waited 1 min to permit the incorporation of fluorescent tubulin onto the free ends of the microtubules, after which the cells were extracted in a microtubule-stabilizing buffer and rapidly imaged with a cooled CCD (see Materials and Methods). 30 s proved to be too short of a time to reveal much detectable incorporation of fluorescent tubulin, while 2 and 3 min proved to be too long, resulting in incorporation along substantial lengths of the microtubules. The 1-min time point was chosen because it produced a “speckled” appearance of free microtubule ends that could be quantified by counting the speckles on the computer monitor. In 10 control cells, we observed 448.3 ± 97 free microtubule ends (see Fig. 6, C and E), while in 10 antibody-injected cells we observed 96.1 ± 42 free microtubule ends (see Fig. 6, D and F). These results indicate that the average microtubule length was increased by at least four to five times (and probably even more given the greater microtubule mass) in the cell bodies of neurons in which katanin function had been inhibited for 4–5 h. The data for these studies are summarized in Fig. 7.
Figure 7.
Bar graph showing both the total microtubule (MT) mass measured in AFUs and the number of MT ends in the cell bodies of uninjected neurons and neurons that had been injected with the katanin antibody.
Pharmacologic Analyses
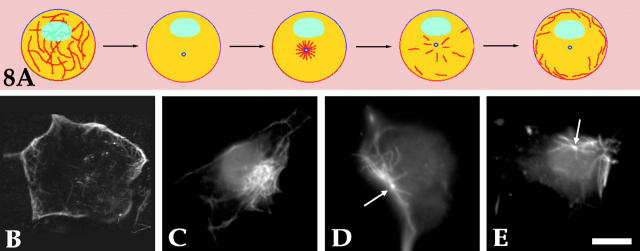
Another set of experimental analyses was aimed at more directly visualizing the effects of inhibiting microtubule release from the centrosome with the katanin antibody. These analyses were based on a pharmacologic assay that we used in earlier studies in which we documented that cytoplasmic dynein is the motor protein that conveys microtubules from the centrosome into the axon (Ahmad and Baas, 1995; Ahmad et al., 1998). For this assay, freshly plated neurons are treated with 10 μg/ml nocodazole for 6 h to depolymerize most of the microtubule polymer, after which the drug is removed for 3–5 min to permit a burst of synchronized microtubule assembly from the centrosome. Then, to prevent further microtubule assembly from occurring, 50 nM vinblastine is added. Under these conditions, showed schematically in Fig. 8 A, the microtubules are rapidly released from the centrosome and conveyed to the cell periphery in a dynein-dependent manner. Thus, this regime is useful for studying the outward transport of microtubules from the centrosome. Here, we injected the katanin antibody shortly after the addition of nocodazole. Fig. 8 B shows an uninjected cell; microtubules are concentrated around the periphery of the cell body 1 h after the addition of vinblastine. The same result was obtained with cells injected with the boiled katanin antibody (not shown). Fig. 8, C–E, shows three examples of cells that were injected with the viable antibody. 1 h after addition of the vinblastine, in all cases the microtubules remained clustered within a discrete region of the cell body, and did not distribute around its periphery. In some cases, a clear point of attachment could not be clearly discerned (Fig. 8 C), but in most cases the attachment of the microtubules to the centrosome could be discerned even at the immunofluorescence level (see Fig. 8, D and E, arrows). Interestingly, under these experimental conditions, the entire centrosome/microtubule complex often relocated from cell center to cell periphery. This relocation, which occurred in 16 of 29 total neurons examined, is presumably the result of the same motor-driven forces that normally convey individual microtubules to the cell periphery. Entirely similar results were obtained in a small number of experiments in which the katanin antibody was introduced during the first hour in nocodazole rather than the last hour (data not shown).
Figure 8.
Studies using a pharmacologic regime to test the role of katanin in releasing microtubules from the neuronal centrosome. A schematically illustrates the pharmacologic regime for revealing the transport of microtubules from the centrosome to the periphery of the neuronal cell body. Under these conditions, a synchronized burst of microtubules is assembled at the centrosome, and then rapidly released and conveyed to the cell periphery. Here, the katanin antibody was injected shortly after the addition of nocodazole. Shown in B–E are cells prepared for immunofluorescence visualization of microtubules. B shows a cell that had not been injected, with microtubules concentrated beneath the periphery of the cell body after completion of the pharmacologic regime. C–E show three examples of cells that had been injected with the antibody. In all cases, the microtubules remained clustered within a discrete region of the cell body, and did not distribute beneath its periphery. In some cases, a clear point of attachment could not be clearly discerned (C), but in most cases the attachment of the microtubules to the centrosome could be discerned (D and E, arrows). In many cases, the entire centrosome/microtubule complex tended to relocate from cell center to cell periphery. Bar, 8 μm.
Discussion
The mechanisms that establish the neuronal microtubule arrays have been intensely studied for many years, but remain controversial. Studies from our laboratory support a model in which microtubules destined for axons and dendrites arise within the cell body of the neuron. In this model, the microtubules are nucleated by the centrosome and are oriented relative to their polarity by the motor proteins that convey them into axons and dendrites. In previous studies, we documented that gamma tubulin within the centrosome is essential for nucleating these microtubules (Baas and Joshi, 1992; Ahmad et al., 1994), and that cytoplasmic dynein and CHO1/MKLP1 are the motor proteins that transport microtubules with their plus- or minus-ends leading, respectively, into neuronal processes (Sharp et al., 1997; Ahmad et al., 1998). In the present study, we sought to test whether katanin is the protein responsible for severing (and thereby releasing) microtubules from the neuronal centrosome. We originally proposed that katanin might play this role because it is an ATPase with potent microtubule-severing properties that has been localized to the centrosome in other cell types (see Baas, 1997). In the present study, we have documented that katanin is expressed in developing neurons, and that it is present throughout all regions of the neuronal cytoplasm, including the centrosome. In addition, we have performed functional studies in which we microinjected into cultured neurons an antibody that inhibits the capacity of katanin to sever microtubules. These studies demonstrated that katanin is essential for releasing microtubules from the neuronal centrosome, and for regulating the lengths of microtubules throughout the cell body of the neuron. Injection of the antibody also inhibited process outgrowth, suggesting that katanin-mediated events are also crucial for the normal deployment of microtubules into developing neuronal processes.
Our initial expectation was that katanin would be concentrated more highly at the centrosome than in other locations in the neuron, or that the katanin that was not localized at the centrosome would be unbounded and lost during extraction. However, neither of these expectations proved to be the case. While katanin was always found at the centrosome, the amounts of the protein were not appreciably higher than those found elsewhere in the cell body. The fact that the noncentrosomal katanin is not detergent-extractable might suggest that it is associated with insoluble cytoplasmic structures. However, the identity of these structures is unclear from our micrographs, and there is always the possibility that the detergent extraction itself could have altered the normal association of katanin with specific cytoplasmic structures. In our preparations, katanin was typically found in association with amorphous cytoplasmic material, and was sometimes (but not often) observed on microtubules. It is possible that the katanin that was observed on microtubules might have been in the process of severing those microtubules at the time of fixation. However, the widespread distribution of the protein (even into the microtubule-free regions of the cell) indicates that much of the katanin is not microtubule associated. Previous studies suggested that the presence of microtubules at the centrosome might be required to target katanin to the pericentriolar material (McNally and Vale, 1996). However, in neurons, we found no relationship between the number of microtubules attached to the centrosome and the levels of katanin associated with it. Instead, the neuronal centrosome contains a fairly consistent amount of katanin at all times, which suggests that additional regulatory factors (rather than the absolute levels of katanin) probably determine the rate and degree of microtubule severing that occur at the centrosome at any moment in time.
The fact that katanin is present throughout the neuron was our first indication that this protein may serve other functions in addition to releasing microtubules from the centrosome. It is well documented that the lengths of individual microtubules are tightly regulated within different regions of the neuron (Yu and Baas, 1994), but it has been assumed that microtubule length is principally regulated by dynamic assembly and disassembly events. One exception to this assumption was a study in which we showed that microtubule fragmentation occurs during collateral branch formation to transform a smaller number of long microtubules into a larger number of short microtubules (Yu et al., 1994). We hypothesized that a microtubule-severing protein such as katanin might be responsible for this fragmentation. Our present results are consistent with this hypothesis, but also show that microtubule severing by katanin might be a critical factor in regulating microtubule lengths throughout all regions of the neuron. Indeed, microinjection of the katanin antibody caused an inhibition of axon outgrowth that was far too rapid and dramatic to be attributed entirely to cessation of microtubule release from the centrosome. Neuronal cell bodies contain a “storage supply” of already-released microtubules that can support process outgrowth for several hours even in the absence of the production of new microtubules from the centrosome (see Ahmad et al., 1994). On the basis of these results, it seems reasonable that microtubule severing by katanin might be necessary to keep microtubules in the cell body sufficiently short that they can funnel effectively into axons and dendrites. Indeed, analyses on the number of free microtubule ends confirmed that, after 4–5 h in the katanin antibody, the average length of the microtubules in the neuronal cell body was at least four to five times greater than in control neurons.
On the basis of these very different microtubule arrays, we propose the following model for the effects of katanin inhibition on process outgrowth. In the control cell, microtubules are nucleated from the centrosome and are rapidly released by katanin after having obtained a length of no more than a few microns. At this point, motor proteins transport the microtubules outward toward the cell periphery. The microtubules undergo dynamic assembly and disassembly events, but they also undergo severing events, and these severing events are essential for ensuring that the microtubules remain relatively short. The transport machinery is able to effectively direct the ends of these short microtubules into the hillock regions of developing processes. By contrast, the experimental cells with inactive katanin do not release microtubules from the centrosome and hence do not have a continuous supply of new microtubules to support process outgrowth. In addition, unlike in control cells, the number of individual microtubules cannot be increased by severing the microtubules that had already been released. Moreover, without functional katanin, the already-released microtubules in the cell body rapidly obtain lengths that are simply too great to be effectively directed into developing processes by motor proteins. For all of these reasons, there is a rapid and dramatic inhibition of process outgrowth when katanin is experimentally inactivated.
It should be acknowledged that there are other models for process outgrowth that do not invoke the transport of microtubules from the cell body. Such models might also be consistent with a crucial role for the severing of microtubules by katanin. For example, if one believes a model in which microtubules cannot be transported into processes from the cell body, microtubule severing by katanin could be invoked as the sole means to generate new microtubules for axons and dendrites (by severing preexisting microtubules within the processes). However, in our opinion, there is now unequivocal evidence indicating that microtubules are indeed transported into developing processes from the cell body (Slaughter et al., 1997). Thus we feel that the most reasonable interpretation of the present observations is that katanin releases microtubules from the centrosome, and then regulates their lengths after release so that they can be effectively transported into developing processes. However, given its widespread distribution, it seems reasonable to suggest that microtubule severing by katanin may also help to regulate microtubule number and length within the processes themselves, especially during the formation of branches.
As previously noted, katanin is expressed in a wide variety of cells and is presumably also responsible for microtubule severing at the centrosome in these cells. However, most interphase cells do not release microtubules from the centrosome as rapidly or as completely as neurons. Even interphase epithelial cells, which establish microtubule arrays at sites distal to the centrosome, show significantly less active microtubule release than we have observed in neurons (see Keating et al., 1997). It has been proposed that katanin may be particularly active during mitosis, during which time the minus ends of many of the microtubules that comprise the spindle must be released from the duplicated centrosomes for there to be a flux of tubulin subunit through these microtubules. At present, the factors that regulate katanin activity during the cell cycle are unknown, but our results suggest that the activity of katanin in terminally postmitotic neurons may be more similar to that in mitosis than during interphase. This is not a surprising conclusion, however, given that several recent studies from our laboratory indicate that many of the mechanisms that establish the microtubule arrays of the neuron are variations on analogous mechanisms that organize microtubules during mitosis (for review, see Baas, 1999).
The widespread distribution of katanin at all stages of neuronal development suggests that the protein is not continuously active. As mentioned earlier, katanin is probably under the regulation of factors that locally activate or deactivate its microtubule-severing properties. It may be that microtubule release from the centrosome occurs in “pulses” determined by such activation and deactivation. If this is true, the frequency and/or timing of these pulses might be an important factor in targeting the appropriate number of microtubules to their appropriate locations during neuronal development. Whatever the mechanisms are that regulate katanin activity in the neuron, it seems reasonable that they would be similar to the factors that regulate the activity of the protein during the stages of the cell cycle of dividing cell types.
Acknowledgments
We thank Crist Cook for technical assistance and Dr. Jeffrey Salisbury for providing the centrin antibody.
This work was supported by grants from the National Institutes of Health to P.W. Baas and F.J. McNally.
Footnotes
1. Abbreviation used in this paper: AFUs, arbitrary fluorescence units.
References
- Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. J Cell Sci. 1995;108:2761–2769. doi: 10.1242/jcs.108.8.2761. [DOI] [PubMed] [Google Scholar]
- Ahmad FJ, Joshi HC, Frohlich V, Baas PW. Inhibition of microtubule nucleation at the centrosome compromises axon growth. Neuron. 1994;12:271–280. doi: 10.1016/0896-6273(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Ahmad FJ, Echeverri CJ, Vallee RB, Baas PW. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J Cell Biol. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW. Microtubules and axonal growth. Curr Opin Cell Biol. 1997;9:29–36. doi: 10.1016/s0955-0674(97)80148-2. [DOI] [PubMed] [Google Scholar]
- Baas PW. Microtubules and neuronal polarity: lessons from mitosis. Neuron. 1999;22:23–31. doi: 10.1016/s0896-6273(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Baas PW, Joshi HC. Gamma-tubulin distribution in the neuron: implications for the origins of neuritic microtubules. J Cell Biol. 1992;119:171–178. doi: 10.1083/jcb.119.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. The plus ends of stable microtubules are the exclusive microtubule nucleating structures in the axon. J Cell Biol. 1992;116:1231–1241. doi: 10.1083/jcb.116.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Yu W. A composite model for establishing the microtubule arrays of the neuron. Mol Neurobiol. 1996;12:145–161. doi: 10.1007/BF02740651. [DOI] [PubMed] [Google Scholar]
- Black MM, Keyser P, Sobel E. Interval between the synthesis and assembly of cytoskeletal proteins in cultured neurons. J Neurosci. 1986;6:1004–1012. doi: 10.1523/JNEUROSCI.06-04-01004.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman JJ, Mahr J, McNally K, Okawa K, Iwamatsu A, Thomas S, Cheesman S, Heuser J, Vale RD, McNally FJ. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93:277–287. doi: 10.1016/s0092-8674(00)81578-0. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Momcilovic D, Rodionov VI, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- McNally FJ, Okawa K, Iwamatsu A, Vale RD. Katanin, the microtubule-severing protein, is concentrated at centrosomes. J Cell Sci. 1996;109:561–567. doi: 10.1242/jcs.109.3.561. [DOI] [PubMed] [Google Scholar]
- McNally FJ, Thomas S. Katanin is responsible for the M-phase microtubule-severing activity in Xenopuseggs. Mol Biol Cell. 1998;9:1847–1861. doi: 10.1091/mbc.9.7.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MA, Salisbury JL. Centrin plays an essential role in microtubule severing during flagellar excision in Chlamydomonas reinhardtii. . J Cell Biol. 1994;124:795–805. doi: 10.1083/jcb.124.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Yu W, Baas PW. Transport of dendritic microtubules establishes their nonuniform polarity orientation. J Cell Biol. 1995;130:93–104. doi: 10.1083/jcb.130.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Yu W, Ferhat L, Kuriyama R, Rueger DC, Baas PW. Identification of a microtubule-associated motor protein essential for dendritic differentiation. J Cell Biol. 1997;138:833–843. doi: 10.1083/jcb.138.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter TS, Wang J, Black MM. Transport of microtubules from the cell body into the axons of cultured neurons. J Neurosci. 1997;17:5807–5819. doi: 10.1523/JNEUROSCI.17-15-05807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Baas PW. Changes in microtubule number and length during axon differentiation. J Neurosci. 1994;14:2818–2829. doi: 10.1523/JNEUROSCI.14-05-02818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Centonze VE, Ahmad FJ, Baas PW. Microtubule nucleation and release from the neuronal centrosome. J Cell Biol. 1993;122:349–359. doi: 10.1083/jcb.122.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Ahmad FJ, Baas PW. Microtubule fragmentation and partitioning in the axon during collateral branch formation. J Neurosci. 1994;14:5872–5884. doi: 10.1523/JNEUROSCI.14-10-05872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]