RANKL-induced DC-STAMP Is Essential for Osteoclastogenesis (original) (raw)
Abstract
Osteoclasts are bone-resorbing, multinucleated giant cells that are essential for bone remodeling and are formed through cell fusion of mononuclear precursor cells. Although receptor activator of nuclear factor–κB ligand (RANKL) has been demonstrated to be an important osteoclastogenic cytokine, the cell surface molecules involved in osteoclastogenesis are mostly unknown. Here, we report that the seven-transmembrane receptor-like molecule, dendritic cell–specific transmembrane protein (DC-STAMP) is involved in osteoclastogenesis. Expression of DC-STAMP is rapidly induced in osteoclast precursor cells by RANKL and other osteoclastogenic stimulations. Targeted inhibition of DC-STAMP by small interfering RNAs and specific antibody markedly suppressed the formation of multinucleated osteoclast-like cells. Overexpression of DC-STAMP enhanced osteoclastogenesis in the presence of RANKL. Furthermore, DC-STAMP directly induced the expression of the osteoclast marker tartrate-resistant acid phosphatase. These data demonstrate for the first time that DC-STAMP has an essential role in osteoclastogenesis.
Keywords: osteoclast, cell fusion, adhesion, seven-transmembrane receptor, TRAP
Introduction
Osteoclasts are the multinucleated cells (MNCs) generated from mononuclear osteoclast precursor cells of the monocyte/macrophage lineage upon osteoclastogenic stimulations (1–4). Receptor activator of NF-κB ligand (RANKL) is now known as an essential cytokine for the osteoclastogenesis, and downstream signaling events mediated by its receptor RANK have been studied extensively (3, 4). However, the molecular mechanisms regulating the polykaryon formation still remain mostly unknown, although several cell surface molecules are considered to play a role (5–8). From a mouse macrophage-like cell line, RAW264, we have recently isolated an osteoclast precursor clone termed RAW-D, which efficiently differentiates into osteoclast-like MNCs upon treatment with RANKL, and a control clone termed RAW-N, which does not differentiate into MNCs even in the presence of RANKL (9). To identify molecules involved in the osteoclastogenesis, we compared the profiles of mRNA expression between RAW-D and RAW-N by using a cDNA subtraction technique and identified mRNA species that were highly expressed in RAW-D but not in RAW-N upon stimulation with RANKL (unpublished data). Dendritic cell–specific transmembrane protein (DC-STAMP; reference 10), also known as IL-4–induced gene (FIND; reference 11), is among the identified genes markedly induced in RANKL-stimulated RAW-D. DC-STAMP was originally isolated from a cDNA library of human monocyte–derived dendritic cells and encodes a protein with putative seven-transmembrane domains. However, it has no strong sequence homology with any other proteins and little is known about its role in the dendritic cell function. Here, we show for the first time that DC-STAMP is critically involved in the MNC formation and tartrate-resistant acid phosphatase (TRAP) gene expression in RAW-D cells and mouse BM osteoclast precursor cells.
Materials and Methods
Cell Culture.
RAW-D and RAW-N cells were cultured as described previously (9). These cells were stimulated with 20 ng/ml of soluble human RANKL (PeproTech) and 1 ng/ml of human TNF-α (PeproTech). Mouse BM cells were cultured in the presence of 10−8 M 1α,25 dihydroxyvitamin D3 (1α,25(OH)2D3) to form osteoclast-like MNCs as described previously (12). Isolation of osteoclasts and pit formation assays were performed as described previously using osteoclast-like cells formed in the rat BM culture system (13). Mouse L1.2 pre–B cell line was provided by E. Butcher (Stanford University, Stanford, CA).
Northern Blot Analysis and RT-PCR.
For Northern blot analysis, total cellular RNAs were separated, blotted onto membrane filters, and hybridized with cRNA probes. The probes were labeled and detected with DIG RNA labeling and detection kit (Roche Diagnostics). RT-PCR was performed as described previously (14). Nucleotide sequences of PCR primers are shown in Table S1 (available at http://www.jem.org/cgi/content/full/jem.20040518/DC1).
siRNA Preparation and Transfection.
Small interfering RNA (siRNA) oligonucleotides were prepared and transfected using Silencer siRNA construction and transfection kits (Ambion). The sequences of oligonucleotides synthesized for templates are shown in Table S1. After transfection, cells were cultured with RANKL and TNF-α for 3 d.
Expression Vectors for DC-STAMP and DC-STAMPΔT7.
DC-STAMP and DC-STAMPΔT7 cDNAs were amplified by RT-PCR and inserted into pCI-neo (Promega) or pEGFP-N1 expression vector (CLONTECH Laboratories, Inc.). The PCR primers used are shown in Table S1. For transient expression of DC-STAMP and DC-STAMPΔT7, 3 × 106 RAW-D cells were transfected with 3 μg of expression vectors by using the DEAE-dextran method. Cells were cultured for 3 d without or with RANKL and TNF-α. For stable expression of DC-STAMP and DC-STAMPΔT7, we used a retrovirus expression vector pMX-IRES-EGFP II (a gift from T. Kitamura, Tokyo University, Tokyo, Japan). The cDNAs were ligated into the SalI–NotI sites of the vector. Production of recombinant retroviruses and infection of mouse L1.2 cells with the recombinant viruses were performed as described previously (15).
Preparation of Rabbit Anti–DC-STAMP Antibody.
A synthetic DC-STAMP peptide corresponding to the fourth extracellular domain (SLPGLEVHLKLRGE; Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20040518/DC1) was conjugated to KLH and immunized to rabbits using a standard protocol. The antibody was affinity purified from pooled antiserum.
Immunostaining.
10-μm cryosections of the mandibular tissue were prepared from newborn mice. Isolated osteoclasts or stimulated RAW-D cells were fixed and stained with 4 μg/ml of anti–DC-STAMP antibody. Immunoreactivity was detected with ABC kit (Vector Laboratories).
Paraformaldehyde Fixation.
Mouse L1.2 cells (2 × 106) stably expressing DC-STAMP or DC-STAMPΔT7 were suspended in 4% paraformaldehyde-PBS and incubated at room temperature for 20 min. After washing, cells were resuspended in 1 ml of αMEM containing 10% FBS.
Statistical Analysis.
Each data point represents mean ± SEM from quadruplicate cultures. Statistical significance was determined using Student's t test or post–analysis of variance test.
Online Supplemental Material.
Table S1 contains oligonucleotide sequences. Fig. S1 depicts the alignment of amino acid sequences of mouse DC-STAMP and DC-STAMPΔT7 with that of human DC-STAMP. Fig. S2 shows surface immunostaining of differentiated RAW-D cells with anti–DC-STAMP. Fig. S3 depicts localization of the COOH terminals of DC-STAMP and DC-STAMPΔT7 tagged with EGFP. Fig. S4 shows enhanced polykaryon formation in RANKL-stimulated RAW-D cells transfected with DC-STAMP expression vector. Fig. S5 shows how overexpression of DC-STAMP-EGFP induces TRAP positive cells in RAW-D cells. Fig. S6 depicts the postulated signaling pathways for osteoclastogenesis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20040518/DC1.
Results and Discussion
Based on the sequence of a cDNA fragment identified through subtraction between RANKL-stimulated RAW-D and RAW-N, we prepared specific primers for mouse DC-STAMP and performed RT-PCR using RANKL-stimulated RAW-D. We consistently observed two bands on the gel (see Fig. 1 C). We isolated both cDNAs by RT-PCR using primers based on the cDNA sequence of mouse DC-STAMP reported in the mouse full-length cDNA sequencing project (GenBank/EMBL/DDBJ accession no. AK014697). One cDNA clone was found to encode the mouse full-length DC-STAMP, which showed 74% identity to the human counterpart, whereas the other, apparently derived from an alternative splicing, encoded a protein lacking the putative seven-transmembrane domain, which we designated DC-STAMPΔT7 (Fig. 1 A and Fig. S1).
Figure 1.
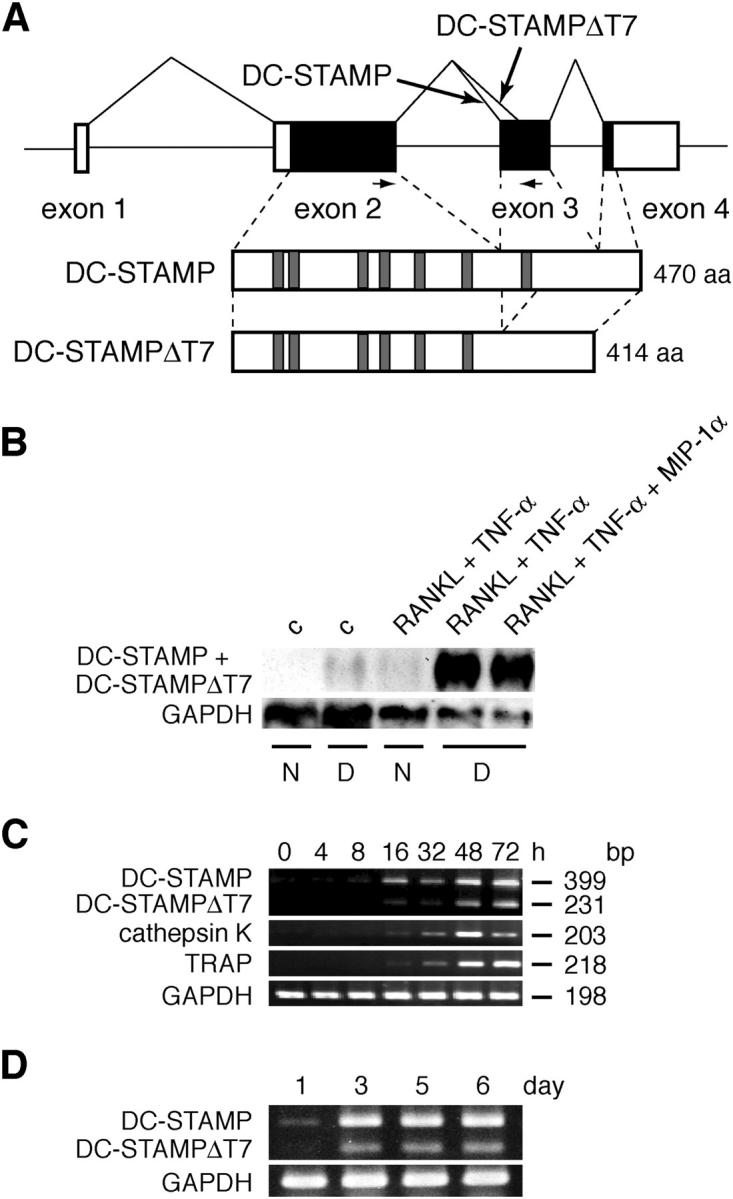
Induction of DC-STAMP and its splicing variant DC-STAMPΔT7 in osteoclastogenesis. (A) Structures of DC-STAMP and DC-STAMPΔT7. Noncoding and coding sequences are shown in unshaded and shaded boxes, respectively. PCR primers used in C and D are indicated by arrows. Schematic protein structures are shown below the gene. Shaded regions indicate the putative transmembrane domains. (B) Northern blot analysis for induction of DC-STAMP and DC-STAMPΔT7 in RAW-D cells. RAW-D and RAW-N cells were stimulated for 72 h as indicated. c, control; N, RAW-N; D, RAW-D. (C) RT-PCR analysis on the time course of DC-STAMP and DC-STAMPΔT7 induction in RAW-D cells. RAW-D cells were stimulated with RANKL + TNF-α for indicated periods of time. Expression of DC-STAMP, DC-STAMPΔT7, and osteoclast marker genes (cathepsin K and TRAP) was analyzed. (D) RT-PCR analysis on the time course of DC-STAMP and DC-STAMPΔT7 induction in mouse BM cells. Mouse BM cells were stimulated with 1α,25(OH)2D3. Expression of DC-STAMP and DC-STAMPΔT7 was analyzed. The GenBank/EMBL/DDBJ accession nos. of mouse DC-STAMP and DC-STAMPΔT7 are AB109560 and AB109561, respectively.
By Northern blot analysis, we examined the expression of DC-STAMP mRNA in RAW-N and RAW-D cells treated without or with RANKL + TNF-α. TNF-α was included because it enhances RANKL-induced osteoclastogenesis (16). As shown in Fig. 1 B, treatment with RANKL + TNF-α for 3 d strongly induced DC-STAMP mRNA (∼2 kb in size) in RAW-D but not in RAW-N. The formation of osteoclast-like MNCs in RAW-D was also at its peak on the third day of culture. Even though MIP-1α/CCL3 was known to enhance osteoclastogenesis (17, 18), it did not further up-regulate DC-STAMP expression in RAW-D cells. By semi-quantitative RT-PCR analysis, both DC-STAMP and DC-STAMPΔT7 mRNAs were induced in RAW-D cells within 16 h after stimulation with RANKL + TNF-α and further up-regulated during the period of 72 h (Fig. 1 C). It was also evident that DC-STAMP mRNA was much more abundant than DC-STAMPΔT7 mRNA. Furthermore, DC-STAMP and DC-STAMPΔT7 were induced well ahead of the osteoclast marker enzymes TRAP and cathepsin K (Fig. 1 C). We also confirmed strong induction of DC-STAMP and DC-STAMPΔT7 in mouse BM cultures treated with a potent osteoclastogenesis inducer 1α,25(OH)2D3 (Fig. 1 D and reference 19). In this system, the formation of osteoclasts reached at its peak on the sixth day of culture. Again, DC-STAMP mRNA was dominant over DC-STAMPΔT7 mRNA. Collectively, these data clearly demonstrated that both DC-STAMP and DC-STAMPΔT7 are strongly induced in RAW-D and mouse BM culture upon osteoclastogenic stimulations. Consistent with our findings, Rho et al. reported previously that osteoclasts expressed DC-STAMP mRNA at levels much higher than dendritic cells (20).
Next, we performed immunological staining of DC-STAMP proteins in RANKL-stimulated RAW-D cells and osteoclasts derived from mouse BM. We prepared affinity purified anti–DC-STAMP from pooled sera of rabbits immunized with a polypeptide common to both DC-STAMP and DC-STAMPΔT7. By immunoblotting, we confirmed that anti–DC-STAMP specifically reacted with a broad band of ∼50 kD, probably the mixture of DC-STAMP (54 kD) and its ΔT7 variant (48 kD), in the protein extract of RANKL-stimulated RAW-D cells (unpublished data). As shown in Fig. 2 A, anti–DC-STAMP stained osteoclast-like MNCs and some mononuclear cells in RAW-D cells stimulated with RANKL + TNF-α. Anti–DC-STAMP also stained the basolateral membrane of osteoclasts in the cryosections of mouse bone tissues (Fig. 2 B), osteoclasts isolated from tibiae of newborn mice (Fig. 2 C), and rat osteoclasts (not depicted). Furthermore, living RANKL-stimulated RAW-D cells, but not RAW-N cells, strongly reacted with anti–DC-STAMP (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20040518/DC1). These results clearly demonstrated that DC-STAMP is highly expressed on the surface of osteoclasts.
Figure 2.
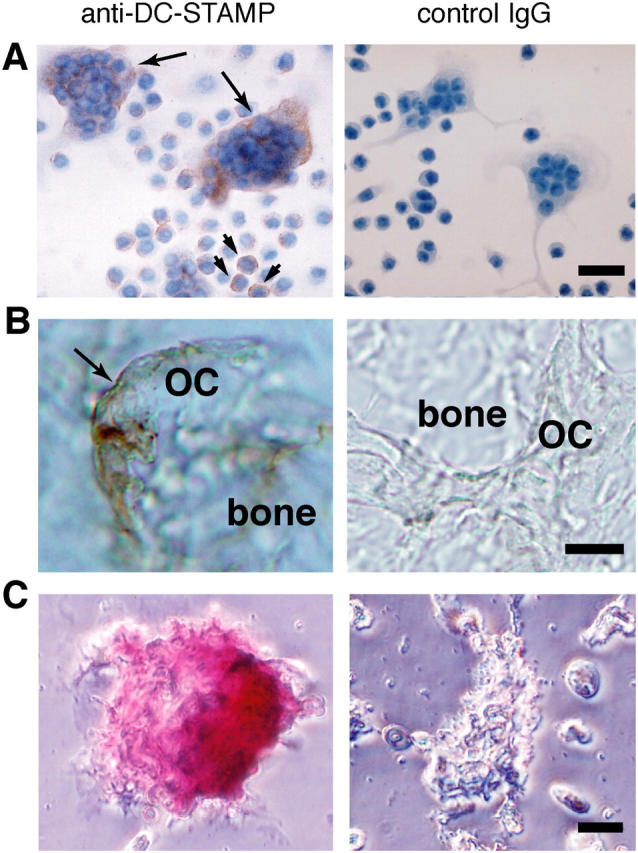
Immunological staining of DC-STAMP in osteoclasts. Cells and tissues were stained with anti–DC-STAMP (left) or control preimmune IgG (right). (A) RAW-D cells stimulated with RANKL + TNF-α for 3 d. Nuclei were visualized by staining with hematoxylin. DC-STAMP positive osteoclast-like MNCs and mononuclear cells are indicated by arrows and arrowheads, respectively. (B) Osteoclasts present in the mandibular tissue of newborn mice. (C) Osteoclasts isolated from the tibia of newborn mice. OC, osteoclast. Bars: 25 μm (A), 10 μm (B), and 20 μm (C).
The COOH terminal of human DC-STAMP was reported to be located within the cytoplasm of dendritic cells (10). Alternately, the COOH terminal of the DC-STAMPΔT7 lacking the seven-transmembrane domain might be extracellular. To test this hypothesis, we generated plasmids encoding DC-STAMP and DC-STAMPΔT7 tagged at their COOH terminals with enhanced GFP (EGFP) and transfected these plasmids into HEK293T cells. We confirmed the expression of DC-STAMP proteins in transfected HEK293T cells by immunocytochemical staining with anti–DC-STAMP (Fig. S3 A, available at http://www.jem.org/cgi/content/full/jem.20040518/DC1). As expected, we observed that the EGFP-tagged COOH terminals of DC-STAMP and DC-STAMPΔT7 are indeed cytoplasmic and extracellular, respectively (Fig. S3 B).
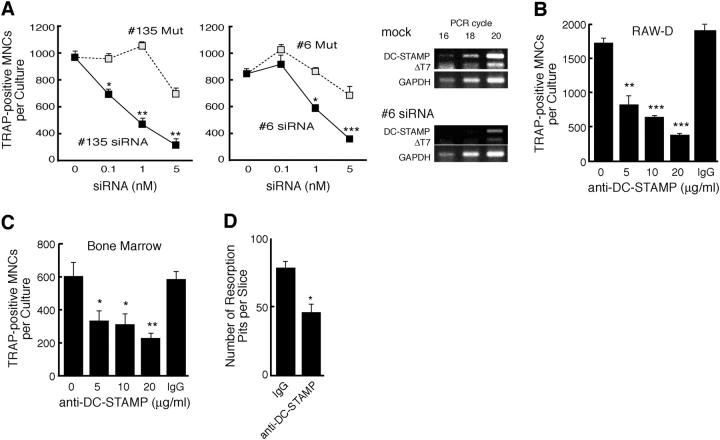
To investigate the role of DC-STAMP in osteoclastogenesis, we first performed experiments using siRNAs; one siRNA (no. 135) was designed to target the seven-transmembrane domain, which is present only in DC-STAMP, and the other (no. 6) was designed to suppress the expression of both DC-STAMP and DC-STAMPΔT7. A mutant siRNA with two nucleotide changes in the middle of the sequence was also synthesized for each siRNA. RAW-D cells were transfected with these siRNAs and cultured for 3 d in the presence of RANKL + TNF-α. After that, TRAP positive MNCs were counted. As shown in Fig. 3 A, both nos. 135 and 6 siRNAs dose dependently suppressed the formation of TRAP positive MNCs in RAW-D cells stimulated with RANKL + TNF-α, whereas mutant siRNAs were much less effective. We observed no significant difference between the inhibitory effects of nos. 135 and 6 siRNAs. This was most probably due to the dominant expression of DC-STAMP over DC-STAMPΔT7. By RT-PCR, we confirmed that no. 6 siRNA efficiently inhibited the expression of both DC-STAMP and DC-STAMPΔT7 (Fig. 3 A, right). These results strongly suggested that DC-STAMP plays an essential role in osteoclastogenesis of RAW-D cells.
Figure 3.
Inhibition of osteoclastogenesis by DC-STAMP siRNAs and by anti–DC-STAMP. (A) Effects of the siRNAs on the formation of osteoclast-like TRAP positive MNCs in RAW-D cells stimulated with RANKL and TNF-α for 3 d. Specific reduction of DC-STAMP mRNA and DC-STAMPΔT7mRNA by #6 siRNA was evaluated by RT-PCR (right). *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Inhibition of osteoclast-like TRAP positive MNC formation in RAW-D cells by anti–DC-STAMP. RAW-D cells were treated with RANKL and TNF-α for 3 d without or with indicated concentrations of anti–DC-STAMP. **, P < 0.01; ***, P < 0.001. IgG, control IgG (20 μg/ml). (C) Inhibition of osteoclastogenesis in mouse BM cells by anti–DC-STAMP. BM cells were cultured in the presence of 1α,25(OH)2D3 for 6 d without or with indicated concentrations of anti–DC-STAMP. *, P < 0.05; **, P < 0.01. (D) Anti–DC-STAMP inhibits osteoclast function. Rat osteoclast-like cells formed in BM cultures were seeded on dentin slices and cultured for 3 d in the presence of 10 μg/ml of control IgG or anti–DC-STAMP. *, P < 0.05.
To further investigate the role of DC-STAMP in osteoclastogenesis, next we examined the effect of anti–DC-STAMP. As shown in Fig. 3 B, anti–DC-STAMP significantly reduced the formation of TRAP positive MNCs in RAW-D cells stimulated for 3 d with RANKL + TNF-α. Anti–DC-STAMP also significantly suppressed osteoclastogenesis in 1α,25(OH)2D3-stimulated mouse BM cultures (Fig. 3 C). We also examined the effects of anti–DC-STAMP on the pit formation by osteoclasts generated from rat BM cultures. As shown in Fig. 3 D, anti–DC-STAMP significantly suppressed the formation of resorption pits on dentin slices in contrast to control IgG. These results further demonstrated that DC-STAMP is critically involved in the generation and function of osteoclasts.
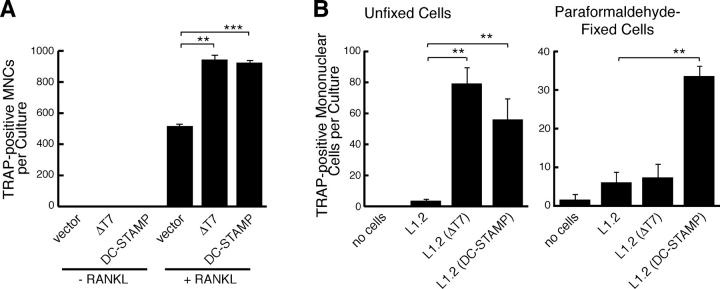
Next, we examined forced expression of DC-STAMP and DC-STAMPΔT7 in RAW-D cells. The expression vectors were transiently transfected into RAW-D cells. The transfection efficiency was ∼10%. As shown in Fig. 4 A, forced expression of either DC-STAMP or DC-STAMPΔT7 in RAW-D cells did not induce the formation of TRAP positive MNCs but significantly enhanced their formation upon further stimulation with RANKL. Enhanced fusion process was evident from the increased multinuclearity of TRAP positive MNCs (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20040518/DC1). Considering the relatively low transfection efficiency of RAW-D cells, the enhancing effects of DC-STAMP proteins on the formation of TRAP positive MNCs might be quite high. We confirmed that anti–DC-STAMP effectively blocked the formation of TRAP positive MNCs in transfected RAW-D cells (unpublished data).
Figure 4.
Promotion of osteoclastogenesis in RAW-D cells by DC-STAMP and DC-STAMPΔT7. (A) Transfection of RAW-D cells with control, DC-STAMP, or DC-STAMPΔT7 expression vectors. Cells were treated without or with RANKL + TNF-α for 3 d. Osteoclast-like TRAP positive MNCs were counted. **, P < 0.01; ***, P < 0.001. (B) Induction of TRAP in RAW-D cells by coculture with mouse pre–B L1.2 cells stably expressing DC-STAMP or DC-STAMPΔT7. RAW-D cells were cocultured with live (left) or fixed (right) L1.2 cells or L1.2 cells stably expressing DC-STAMP or DC-STAMPΔT7 in the absence of osteoclastogenic factors for 3 d. Osteoclast-like TRAP positive MNCs were counted. **, P < 0.01.
To further investigate how the forced expression of DC-STAMP proteins enhanced the formation of TRAP positive MNCs in RANKL-stimulated RAW-D cells, we transiently transfected RAW-D cells with an expression vector encoding DC-STAMP fused with EGFP and cultured the cells without any differentiation stimuli. We consistently observed that mononuclear cells in close contact with GFP positive MNCs were TRAP positive (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20040518/DC1). In contrast, forced expression of DC-STAMP–EGFP did not induce TRAP positive cells in RAW-N cells. Collectively, these results suggested that DC-STAMP on the cell surface are capable of inducing TRAP expression in neighboring RAW-D cells probably via interacting with a putative DC-STAMP ligand on the cell surface. Furthermore, RAW-N cells may also be defective in the expression of this DC-STAMP ligand.
To further investigate whether DC-STAMP interacts with a membrane-bound ligand expressed on RAW-D cells, we generated mouse L1.2 preB cells stably expressing DC-STAMP or DC-STAMPΔT7 by using a retrovirus vector polycistronically encoding EGFP, and tested whether these cells were able to adhere and/or fuse with RAW-D cells. When L1.2 cells expressing DC-STAMP or DC-STAMPΔT7 were cocultured with RAW-D cells without any differentiation stimuli, no firm adhesion strong enough to resist gentle washing or cell fusion was observed under fluorescence microscopy (unpublished data). However, marked induction of TRAP positive mononuclear cells was observed in RAW-D cells (Fig. 4 B). Both DC-STAMP and DC-STAMPΔT7 were similarly effective in inducing TRAP expression in RAW-D cells. To eliminate a possible involvement of cytokines secreted by L1.2 cells, the cells were prefixed with paraformaldehyde and added to RAW-D cells. Fixed DC-STAMP–expressing L1.2 cells still significantly induced TRAP positive mononuclear cells in RAW-D, whereas fixed DC-STAMPΔT7–expressing L1.2 cells failed to do so (Fig. 4 B). This might be due to structural instability of DC-STAMPΔT7 upon fixation. We also confirmed that anti–DC-STAMP effectively suppressed induction of TRAP in RAW-D cells by fixed DC-STAMP–expressing L1.2 cells (unpublished data). These data supported that a putative DC-STAMP ligand expressed on the cell surface of RAW-D mediates induction of TRAP expression upon interaction with DC-STAMP proteins, although additional cell surface molecules induced and/or activated by RANKL may be required for the efficient formation of MNCs.
Cell surface molecules such as macrophage fusion receptor, CD47, and CD44 have been demonstrated to be involved in the osteoclastogenesis (5–8). Compared with these molecules, DC-STAMP has several unique features. First, DC-STAMP has a distinct structure that these proteins do not have. DC-STAMP has been predicted to have a seven-transmembrane domain structure similar to the members of the G protein–coupled receptor (GPCR) superfamily. Does DC-STAMP function as a cell adhesion molecule in osteoclastogenesis? Among the GPCR superfamily, two chemokine receptors are known to function as direct cell adhesion molecules through interactions with their transmembrane-type ligands (14, 21). Given such examples, DC-STAMP may also function as a direct cell adhesion molecule by interacting with its putative membrane-bound ligand. The osteoclast fusion process may be initiated upon this adhesive interaction, which may be too weak to be seen in the present experimental conditions. Second, TRAP induction in RAW-D cells by DC-STAMP and DC-STAMPΔT7 suggests signaling through the putative cell surface ligand of DC-STAMP. Third, DC-STAMP and DC-STAMPΔT7 have similar activities so far, indicating that the seven-transmembrane domain and the intracellular COOH terminus may not be essential for its function. The splicing variant identical with DC-STAMPΔT7 has not been identified in human dendritic cells, but a larger deletion mutant that also lacks the seven-transmembrane domain has been reported previously (22). Because the domains in GPCRs, which are critical for the interaction with the G proteins, are localized at the second and third cytoplasmic loops and the COOH terminal (23), DC-STAMPΔT7 may not be properly associated with the G proteins. Thus, DC-STAMP and DC-STAMPΔT7 may behave differently upon binding with putative DC-STAMP ligand. It remains to be seen whether DC-STAMP ΔT7 has any function different from DC-STAMP.
In conclusion, we have demonstrated for the first time that DC-STAMP plays an important role in the osteoclastogenesis (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20040518/DC1). DC-STAMP induces TRAP expression in osteoclast precursors via its putative cell surface ligand. However, aside from DC-STAMP and its cell surface ligand, other cell surface molecules may also be needed to initiate efficient MNC formation. Such cell surface factors may be induced and/or activated by signaling via DC-STAMP and/or its putative ligand. Thus, identification of DC-STAMP ligand will further elucidate the molecular mechanisms of the osteoclastogenesis.
Acknowledgments
This work is supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan and by Solution Oriented Research for Science and Technology of Japan Science and Technology Agency.
The authors have no conflicting financial interests.
References
- 1.Roodman, G.D. 1999. Cell biology of the osteoclast. Exp. Hematol. 27:1229–1241. [DOI] [PubMed] [Google Scholar]
- 2.Suda, T., N. Takahashi, N. Udagawa, E. Jimi, M.T. Gillespie, and T.J. Martin. 1999. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20:345–357. [DOI] [PubMed] [Google Scholar]
- 3.Boyle, W.J., W.S. Simonet, and D.L. Lacey. 2003. Osteoclast differentiation and activation. Nature. 423:337–342. [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum, S.L., and F.P. Ross. 2003. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4:638–649. [DOI] [PubMed] [Google Scholar]
- 5.Han, X., H. Sterling, Y. Chen, C. Saginario, E.J. Brown, W.A. Frazier, F.P. Lindberg, and A. Vignery. 2000. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J. Biol. Chem. 275:37984–37992. [DOI] [PubMed] [Google Scholar]
- 6.Vignery, A. 2000. Osteoclasts and giant cells: macrophage-macrophage fusion mechanism. Int. J. Exp. Pathol. 81:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higuchi, S., N. Tabata, M. Tajima, M. Ito, M. Tsurudome, A. Sudo, A. Uchida, and Y. Ito. 1998. Induction of human osteoclast-like cells by treatment of blood monocytes with anti-fusion regulatory protein-1/CD98 monoclonal antibodies. J. Bone Miner. Res. 13:44–49. [DOI] [PubMed] [Google Scholar]
- 8.Mbalaviele, G., H. Chen, B.F. Boyce, G.R. Mundy, and T. Yoneda. 1995. The role of cadherin in the generation of multinucleated osteoclasts from mononuclear precursors in murine marrow. J. Clin. Invest. 95:2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe, T., T. Kukita, A. Kukita, N. Wada, K. Toh, K. Nagata, H. Nomiyama, and T. Iijima. 2004. Direct stimulation of osteoclastogenesis by MIP-1alpha: evidence obtained from studies using RAW264 cell clone highly responsive to RANKL. J. Endocrinol. 180:193–201. [DOI] [PubMed] [Google Scholar]
- 10.Hartgers, F.C., J.L. Vissers, M.W. Looman, C. van Zoelen, C. Huffine, C.G. Figdor, and G.J. Adema. 2000. DC-STAMP, a novel multimembrane-spanning molecule preferentially expressed by dendritic cells. Eur. J. Immunol. 30:3585–3590. [DOI] [PubMed] [Google Scholar]
- 11.Staege, H., A. Brauchlin, G. Schoedon, and A. Schaffner. 2001. Two novel genes FIND and LIND differentially expressed in deactivated and Listeria-infected human macrophages. Immunogenetics. 53:105–113. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi, N., N. Udagawa, S. Tanaka, and T. Suda. 2003. Generating murine osteoclasts from bone marrow. Methods Mol. Med. 80:129–144. [DOI] [PubMed] [Google Scholar]
- 13.Kukita, T., A. Kukita, K. Nagata, H. Maeda, K. Kurisu, T. Watanabe, and T. Iijima. 1994. Novel cell-surface Ag expressed on rat osteoclasts regulating the function of the calcitonin receptor. J. Immunol. 153:5265–5273. [PubMed] [Google Scholar]
- 14.Nakayama, T., K. Hieshima, D. Izawa, Y. Tatsumi, A. Kanamaru, and O. Yoshie. 2003. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J. Immunol. 170:1136–1140. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida, T., D. Izawa, T. Nakayama, K. Nakahara, M. Kakizaki, T. Imai, R. Suzuki, M. Miyasaka, and O. Yoshie. 1999. Molecular cloning of mXCR1, the murine SCM-1/lymphotactin receptor. FEBS Lett. 458:37–40. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, K., N. Takahashi, E. Jimi, N. Udagawa, M. Takami, S. Kotake, N. Nakagawa, M. Kinosaki, K. Yamaguchi, N. Shima, et al. 2000. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL–RANK interaction. J. Exp. Med. 191:275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi, S.J., J.C. Cruz, F. Craig, H. Chung, R.D. Devlin, G.D. Roodman, and M. Alsina. 2000. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 96:671–675. [PubMed] [Google Scholar]
- 18.Kukita, T., H. Nomiyama, Y. Ohmoto, A. Kukita, T. Shuto, T. Hotokebuchi, Y. Sugioka, R. Miura, and T. Iijima. 1997. Macrophage inflammatory protein-1 alpha (LD78) expressed in human bone marrow: its role in regulation of hematopoiesis and osteoclast recruitment. Lab. Invest. 76:399–406. [PubMed] [Google Scholar]
- 19.Ibbotson, K.J., G.D. Roodman, L.M. McManus, and G.R. Mundy. 1984. Identification and characterization of osteoclast-like cells and their progenitors in cultures of feline marrow mononuclear cells. J. Cell Biol. 99:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rho, J., C.R. Altmann, N.D. Socci, L. Merkov, N. Kim, H. So, O. Lee, M. Takami, A.H. Brivanlou, and Y. Choi. 2002. Gene expression profiling of osteoclast differentiation by combined suppression subtractive hybridization (SSH) and cDNA microarray analysis. DNA Cell Biol. 21:541–549. [DOI] [PubMed] [Google Scholar]
- 21.Imai, T., K. Hieshima, C. Haskell, M. Baba, M. Nagira, M. Nishimura, M. Kakizaki, S. Takagi, H. Nomiyama, T.J. Schall, and O. Yoshie. 1997. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 91:521–530. [DOI] [PubMed] [Google Scholar]
- 22.Hartgers, F.C., M.W. Looman, B. van der Woning, G.F. Merkx, C.G. Figdor, and G.J. Adema. 2001. Genomic organization, chromosomal localization, and 5′ upstream region of the human DC-STAMP gene. Immunogenetics. 53:145–149. [DOI] [PubMed] [Google Scholar]
- 23.Hall, R.A., R.T. Premont, and R.J. Lefkowitz. 1999. Heptahelical receptor signaling: beyond the G protein paradigm. J. Cell Biol. 145:927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]