Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells (original) (raw)
Abstract
- Exocytosis and endocytosis were measured following single, or trains of, simulated action potentials (sAP) in bovine adrenal chromaffin cells. Catecholamine secretion was measured by oxidative amperometry and cell membrane turnover was measured by voltage clamp cell capacitance measurements.
- The sAPs evoked inward Na+ and Ca2+ currents that were statistically identical to those evoked by native action potential waveforms. On average, a single secretory granule underwent fusion following sAP stimulation. An equivalent amount of membrane was then quickly internalised (τ = 560 ms).
- Stimulation with sAP trains revealed a biphasic relationship between cell firing rate and endocytic activity. At basal stimulus frequencies (single to 0.5 Hz) cells exhibited a robust membrane internalisation that then diminished as firing increased to intermediate levels (1.9 and 6 Hz). However at the higher stimulation rates (10 and 16 Hz) endocytic activity rebounded and was again able to effectively maintain cell surface near pre-stimulus levels.
- Treatment with cyclosporin A and FK506, inhibitors of the phosphatase calcineurin, left endocytosis characteristics unaltered at the lower basal stimulus levels, but blocked the resurgence in endocytosis seen in control cells at higher sAP frequencies.
- Based on these findings we propose that, under physiological electrical stimulation, chromaffin cells internalise membrane via two distinct pathways that are separable. One is prevalent at basal stimulus frequencies, is lessened with increased firing, and is insensitive to cyclosporin A and FK506. A second endocytic form is activated by increased firing frequencies, and is selectively blocked by cyclosporin A and FK506.
Over recent years, many studies have been completed which greatly improve the understanding of the biochemistry and kinetics of exocytosis-coupled endocytosis (De Camilli & Takei, 1996). In secretory cells, differing stimulation intensities can activate multiple endocytic mechanisms (Koenig & Ikeda, 1996; Cousin & Robinson, 2000). Biochemical studies have identified an array of intracellular proteins that are likely to be involved in the endocytic processes. Examples include cytoskeletal elements (Hamm-Alvarez & Sheetz, 1998), kinase/phosphatase enzymes (Turner et al. 1999), adaptor complexes (Robinson, 1994; Gonzalez-Gaitan & Jackle, 1997) and nucleotide binding proteins (De Camilli & Takei, 1996; Artalejo et al. 1997). Other studies have provided evidence that at least one route of membrane internalisation depends upon the activity of the protein phosphatase calcineurin (Bauerfeind et al. 1997; Marks & McMahon, 1998; Lai et al. 1999).
Differing cell activity has been shown to play a modulatory role in the endocytic process. In experimental systems as diverse as the motor nerve terminal, pancreatic β-cells, pituitary cells and cultured hippocampal neurons, elevated activity enhances endocytosis (Ramaswami et al. 1994; Koenig & Ikeda, 1996; Klingauf et al. 1998; Lee & Tse, 2001). In the frog neuromuscular junction, increased cell firing results in a decreased endocytic response, but seems to be independent of cytosolic Ca2+ levels (Wu & Betz, 1996). In contrast, in several systems including pituitary neurohypophyseal terminals (Hsu & Jackson, 1996), brain synaptosomes (Cousin & Robinson, 2000) and retinal bipolar neurons (von Gersdorff & Matthews, 1994; Neves & Lagnado, 1999), heightened cell activity inhibits the endocytic response, perhaps acting through elevated Ca2+.
Quantitative descriptions of multiple kinetic modes of endocytosis have been published for the neuroendocrine chromaffin cell system. Electrical square pulse stimuli, leading to subplasmalemmal Ca2+ elevations measuring up to a few tens of micromolar trigger exocytosis that is followed by graded membrane internalisation, so recovering a surface area roughly equal to that added immediately prior (Burgoyne, 1995; Smith & Neher, 1997; Engisch & Nowycky, 1998). More intense stimulation, leading to local [Ca2+] i measuring several tens of micromolar or higher causes cells to overinternalise membrane by retrieving much more membrane than added through the coupled exocytic event (Artalejo et al. 1995; 1996; Nucifora & Fox, 1999). These two retrieval modes have been shown to be the result of separate mechanisms and have been termed ‘compensatory’ and ‘excess’ endocytosis, respectively (Engisch & Nowycky, 1998). The control of transition between compensatory and excess endocytosis is commonly attributed to differences in Ca2+ affinity. Even within the compensatory endocytic mechanism, Ca2+ acts as a modulator, with increasing concentrations resulting in a more rapid kinetic and more complete retrieval (Engisch & Nowycky, 1998). Finally, endocytosis in chromaffin cells has also been shown to be modulated by divalent cations other than Ca2+ (Nucifora & Fox, 1998) as well as multiple kinase/phosphatase pathways (Artalejo et al. 1996; Engisch & Nowycky, 1998). However, given such a variety of endocytic behaviour, it is not yet clear which kinetic mode(s) or molecular cascade(s) is responsible for membrane recycling following physiological stimulation.
Due to their accessibility for quantitative electrophysiological and electrochemical techniques, the chromaffin cell has proven a fertile system for the study of the highly convolved processes of stimulus-evoked exo- and endocytosis. From the literature, it appears likely that in chromaffin cells, Ca2+ influx either directly or indirectly provides a modulatory influence over the kinetics, and possibly affects mode transitions of exocytosis-coupled endocytosis. Previous studies of electrically evoked endocytosis utilised square pulse stimulation with pulse durations ranging from 10 ms (Engisch & Nowycky, 1998) up to 1 s (Smith & Betz, 1996). These pulses were commonly separated by long inter-pulse periods measuring several tens of seconds to minutes (Artalejo et al. 1995; Smith & Neher, 1997; Engisch & Nowycky, 1998). Such stimulation protocols are expected to cause influx of up to a few hundred picocoulombs Ca2+ charge, which is likely to lead to transient highly localised subplasmalemmal Ca2+ concentrations measuring several tens of micromolar. Within a few hundred microseconds these large Ca2+ gradients collapse to equilibrium within the cytosol (Chow et al. 1994; Monck et al. 1994) and are eventually cleared from the cell (Neher & Augustine, 1992; Xu et al. 1997). In essence, the square pulse protocol leads to brief, discrete periods of very high [Ca2+] i at the site of endocytosis.
Action potential trains, on the other hand, form a very different Ca2+ profile from that seen for square pulse depolarisation. Due to their brief duration, Ca2+ influx is low. On average, a single action potential leads to the influx of approximately 1 pC Ca2+ charge (Giovannucci et al. 1999) (see also Figs 5 and 8). Action potential trains therefore do not lead to the transient, high calcium concentration gradients that are elicited by square pulse depolarisation, but rather lead to a sustained tonic elevation in [Ca2+] i whose magnitude depends on firing frequency. Given that endocytosis may be dependent upon, and modulated by, Ca2+, the differences in Ca2+ influx and clearance expected between square pulse and action potential stimulation are likely to result in different endocytic behaviours. Therefore, it is not certain to what extent our current understanding of endocytosis in chromaffin cells, which is based on square-pulse depolarisation, reflects physiological behaviour. Given the multiplicity of endocytic modes described in the literature, we wondered which, if any, of these kinetically or molecularly defined endocytic mechanisms were active in chromaffin cells following physiological action potential stimulation. We set out to determine which, if any, of the described endocytic modes are found to occur following physiological electrical stimulation, a condition that had not yet been measured.
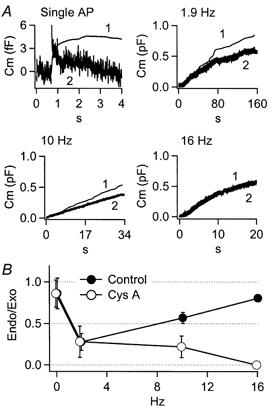
Figure 5. Cell capacitance and integrated amperometry vary with cell activity.
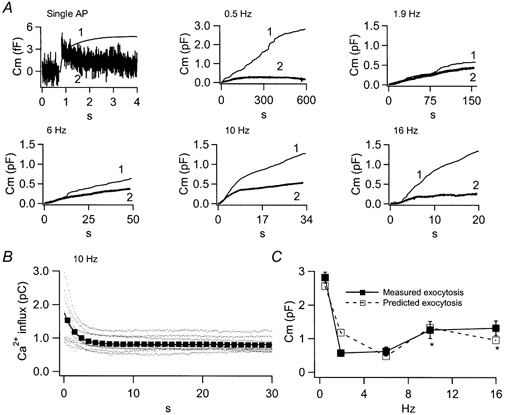
A, the scaled integrated amperometric response (traces 1) and measured cell capacitance (traces 2) are plotted for 6 different stimulation conditions. For the single AP, 16 cells were stimulated with a total of 1534 sAPs. Trains contained 300 sAPs each and were recorded at the following frequencies: 0.5 Hz, n = 9 cells; 1.9 Hz, n = 9 cells; 6 Hz, n = 7 cells; 10 Hz, n = 12 cells; and 16 Hz, n = 9 cells. B, the calcium influx for each sAP of the stimulus trains was measured (10 Hz data plotted as an example, dots). The data were then binned into groups of 10 and averaged (squares). The binned averages were fitted with a mono-exponential decay (continuous line), reflecting activity-dependent current rundown. The fitted line from each frequency then served as the input Ca2+ function for a kinetic simulation of exocytosis. C, the magnitude of total evoked exocytosis from A was simulated with a kinetic model for secretion based on the ‘two step model for secretion control’ (see Results for references). The results of the simulation and the measured exocytosis values are plotted for comparison. Conditions in which Ca2+ activation of protein kinase C is expected, and is so modelled are marked with an asterisk.
Figure 8. Cyclosporin A does not alter endocytosis by lowering Ca2+ influx.
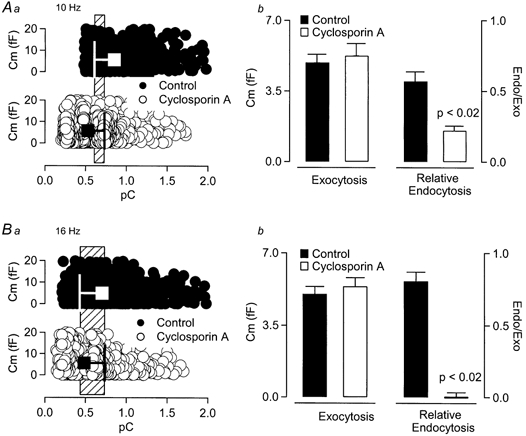
Aa, data measured from individual sAP stimuli in control and cyclosporin A-treated cells are plotted against evoked Ca2+ influx. Large square symbols represent the mean ±s.d. The range representing overlap of mean plus or minus 1 standard deviation (common range) is highlighted by the hatched box in the background. b, the average exocytosis and relative endocytosis values within the common range (hatched region from a) are presented for comparison. While, at comparable Ca2+, control and treated cells displayed similar exocytic activity, cyclosporin A decreased relative endocytosis from control (Student's t test, P < 0.02). B, the same analysis as that represented in A was repeated for cells stimulated at 16 Hz. Again, at comparable Ca2+ influx, cyclosporin A treatment did not significantly affect exocytic activity, but blocked endocytosis (Student's t test, P < 0.02).
In this study we employed the electrophysiological perforated patch clamp technique with concurrent electrochemical detection of catecholamine release to determine simultaneous exo- and endocytic activity. Simulated action potential voltage templates were developed to investigate exocytosis-coupled endocytosis at basal firing rates and under physiological electrical activation. Here we have reported for the first time that at low firing intensity, representing basal sympathetic activity (Brandt et al. 1976; Kidokoro, 1980; Wakade, 1981), chromaffin cells exhibited a robust and active membrane retrieval which we term ‘phase I’ endocytosis. Phase I endocytosis quickly diminished in response to increased cell firing. However, at the elevated frequencies, a second activity-enhanced form of endocytosis (termed phase II endocytosis) emerged. Cyclosporin A and FK506 selectively blocked phase II endocytosis.
METHODS
Overview
Chromaffin cells of the adult bovine adrenal medulla were used in this study. Cells were voltage clamped in the perforated patch configuration (Horn & Marty, 1988; Gillis et al. 1991). After patch perforation cells were stimulated by electrical depolarisation, causing Ca2+ influx, fusion of secretory granules and subsequent catecholamine exocytosis. In this study, exo- and endocytosis were measured by monitoring changes in cell capacitance (Neher & Marty, 1982), an index of granule-plasma membrane fusion, and by the direct electrochemical detection of secreted catecholamine (Wightmann et al. 1991; Chow et al. 1992). Endocytosis was measured as the difference between scaled integrated amperometric current and cell capacitance, a method discussed in detail below.
Cell culture and recording solutions
Adult bovine adrenal glands were acquired fresh from a slaughterhouse, rapidly cooled to 4 °C and transported to the laboratory. Cells were isolated and kept in primary culture for 1-4 days in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Gaithersburg, MD, USA) as previously described (Smith, 1999). The DMEM growth medium was supplemented with penicillin (20 units ml−1), streptomycin (20 μg ml−1) and ITS-X (a defined serum substitute; Life Technologies).
During recording cells were constantly superfused with Ringer solution, of the following composition (mm): 150 NaCl, 10 Hepes-H, 10 glucose, 2.5 CaCl2, 2.8 KCl, 2 MgCl2. The osmolarity was adjusted to 320 mosmol l−1 with mannitol, and pH was adjusted to 7.2. For experiments performed in current clamp configuration, the pipette solution contained (mm): 145 potassium glutamate, 10 Hepes-H, 8 NaCl, 1 MgCl2, 0.53 amphotericin B; pH was adjusted to 7.2 and osmolarity to 320 mosmol l−1. The standard voltage clamp perforated patch pipette solution contained (mm): 135 caesium glutamate, 10 Hepes-H, 9.5 NaCl, 0.5 TEA-Cl, 0.53 amphotericin B; pH was adjusted to 7.2 and osmolarity to 320 mosmol l−1.
For patching the cells, pipettes of approximately 2-3 MΩ resistance were pulled from borosilicate glass, partially coated with molten dental wax (Orbis Dental, Offenbach, Germany), and lightly fire polished. Patch pipette had their tips dipped in amphotericin-free solution for 2-10 s, and were back-filled with freshly mixed amphotericin-containing solution. Amphotericin B stock solution was prepared as previously described (Smith & Neher, 1997). The liquid junction potential between the extracellular Ringer solution and the intracellular solution was measured to be approximately 13 mV for the caesium glutamate-based solution and all potentials reported are adjusted accordingly. Only cells that showed patch perforation in response to less than 50 MΩ pipette series resistance were used in the analyses reported (mean ±s.d. series resistance was 21.5 ± 1.23 MΩ). Reagents and chemicals were obtained from Sigma Chemical Co. (St Louis, MO, USA), with the exception of CsOH (Aldrich, Milwaukee, WI, USA), and amphotericin B (Calbiochem, La Jolla, CA, USA), or as otherwise noted.
Electrochemical and electrophysiological measurements
Commercially available 5 μm diameter carbon-fibre electrodes were utilised for amperometric catecholamine detection (ALA Scientific, Longneck, NY, USA). Fibre tips were freshly cut daily with a scalpel blade, and whenever debris accumulated on their tip. For recording, a +650 mV potential was placed on the carbon fibre, the fibre was lowered into the bath, and the background current was allowed to relax to a steady value. If the background current was greater than 10 pA, the fibre was re-cut or discarded. Under recording conditions, the fibre was placed as close to the cell as possible without mechanically perturbing the cell. The +650 mV tip potential causes electrical oxidation of catecholamine molecules as they diffuse to the carbon tip. The oxidative amperometric current was recorded using a VA-10 dedicated amperometry amplifier (ALA Scientific). Signals were passed through an analog 1 kHz filter and sampled at 12 kHz through an EPC-9 patch clamp amplifier in the ‘continuous’ mode (HEKA Elektronik, Lambrecht, Germany). Headstages for the VA-10 and EPC-9 shared a common bath ground. In an attempt to minimise cross-talk noise between headstages, we inserted a 10 Ω resistor into the ground wire of the VA-10 to separate the ground planes of the two amplifiers. Basal amperometric current, a 500 ms average prior to the first sAP stimulus, was subtracted prior to integration. Amperometric signals were further digitally notch filtered off-line in their Fourier transform to specifically remove contributions of 60 Hz line-noise from electronic devices as well as a 393 Hz cross-talk signal from the voltage clamp sine wave (see below) of the EPC-9 headstage.
Electrophysiological data were acquired through an EPC-9 amplifier under the control of ‘Pulse’ software (HEKA Elektronik). Cell capacitance (_C_m) was estimated by the Lindau-Neher technique (Gillis, 1995) implemented as the ‘Sine + DC’ feature of the Pulse lock-in module. A 393 Hz, 35 mV peak amplitude sine wave was applied to a holding potential of -83 mV and the reversal potential of the lock-in module was set to 0 mV. Membrane current was sampled at 12 kHz and _C_m was calculated at 393 Hz as the average value of 12 points per sine cycle. Only cells with less than 10 pA resting leak current, and stable access resistance consistent with the perforated patch configuration were included in analysis. Experiments were carried out at 22-25 °C. All data analysis and simulation was performed with IGOR Pro software (WaveMetrics Inc., Lake Oswego, OR, USA).
RESULTS
Native and simulated action potentials result in equivalent Ca2+ influx
As part of the sympathetic nervous system, chromaffin cells receive input from cholinergic neurons, fire action potentials upon stimulation and release catecholamines (Kidokoro & Ritchie, 1979, 1980). It was the goal of this study to measure the exocytosis-coupled endocytosis evoked under physiological electrical action potential stimulation. We first measured native action potentials in whole-cell current clamp, and used the measured voltage response as a stimulus template in perforated voltage clamp records (Fig. 1_A_). However, we found that our computer software, when using native action potential stimulus templates, did not deliver regular and reproducible stimulus frequencies as necessary for this study. Additionally, the time required by the control computer to transition between stimulus and capacitance measurements interfered with capacitance estimates immediately before and after the stimulation. For these reasons we designed a simulated action potential (sAP, Fig. 1_B_) stimulus wave form consisting of a simple three-segment series of voltage ramps embedded in a sine wave voltage template. This stimulus allowed for regular train frequencies as well as capacitance estimates immediately prior to and following stimulation.
Figure 1. Native action potentials are approximated by ramp voltage protocols.
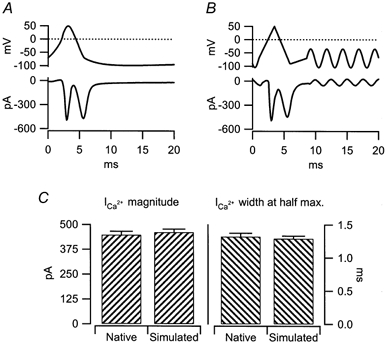
A, isolated chromaffin cells were held in the whole cell current clamp configuration and injected with 100 μs, 100 pA currents. The resulting action potential was measured and then utilised as a stimulus template in perforated patch voltage clamp recordings. The stimulus template (upper trace) and resulting evoked currents (lower trace) were measured and averaged from 100 stimuli in 4 cells (0.02 Hz). B, a 3-component ramp protocol was designed to mimic as closely as possible the native stimulus wave form presented in A. The ramp segments were as follows (start potential, end potential, duration): -70 mV, 50 mV, 2.5 ms; 50 mV, -90 mV, 2.5 ms; -90 mV, -70 mV, 2.52 ms. Again, evoked currents were measured and averaged in response to 100 stimuli in 4 cells (0.02 Hz). As in A, the stimulus template (upper trace) and evoked currents (lower trace) are plotted. C, the magnitude (left) and width at half-maximum current (right) of the Ca2+ influx measured in response to the native and simulated action potentials were quantified. Both measures were statistically identical between stimulus groups (Student's t test, P < 0.02).
In a set of control experiments, the evoked current influx from native and sAP waveforms was compared. Both stimulus templates resulted in an initial inward Na+ current (as pharmacologically identified by block with 1 μm tetrodotoxin, data not shown). Following the decay in the Na+ current, both native and simulated action potential stimulus templates triggered a strong inward Ca2+ current. The Na+ and Ca2+ components of the evoked current influx were readily temporally separable, so further experiments were not conducted in the presence of tetrodotoxin. Quantitative analysis of the current profiles showed that both the native, and sAP voltage templates resulted in a Ca2+ current statistically indiscernible in either magnitude or width at half-maximal magnitude (Fig. 1_C_). From these results it was concluded that both the native and sAP protocols resulted in a functionally identical Ca2+ influx. Since the sAP stimulus template does not suffer from the technical limitations associated with the recorded native action potential template, all further experiments were conducted with the sAP stimulus protocol.
Single sAP stimulation is followed by a robust membrane internalisation
In order to describe the fundamental exocytosis and endocytosis evoked by a physiological electrical stimulus we set out to measure membrane turnover triggered by single simulated action potentials.
Figure 2 shows the result of a set of experiments in which we stimulated chromaffin cells with very low frequency train of sAPs (n = 335 at 0.02 Hz from 16 cells). The cell capacitance (lower trace) and amperometric current (middle trace) were signal averaged with respect to each sAP stimulus. The amperometric trace was then integrated to give an index of total evoked exocytosis (upper trace). The sAP stimulation resulted in a very slight average capacitance increase (Δ_C_m) of approximately 3.5 fF. Exact determination of the secretion-dependent Δ_C_m component is somewhat complicated by the possible presence of rapid non-secretory _C_m artefacts due to Na+ channel gating charge movements sometimes detected in chromaffin cells (termed Δ_C_mt, τ = 16 ms for bovine; Chow et al. 1996). However, in our data the Δ_C_m value at 3τΔ_C_mt (48 ms) is approximately 2.9 fF, slightly larger than a single secretory granule (Chow et al. 1992). The capacitance trace fell rapidly back to pre-pulse levels (τ = 560 ms). Since little or no post-pulse secretion was detected in the integrated amperometry trace, the falling post-pulse _C_m trace reflects endocytosis.
Figure 2. Single sAP stimuli evoke exocytosis followed by a robust membrane internalisation.
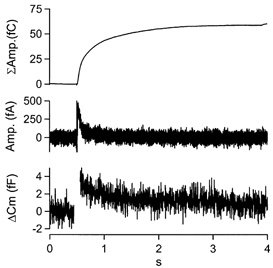
Cells (n = 16) were stimulated with a total of 1534 sAPs in the perforated patch voltage clamp configuration at 0.02 Hz. Cell capacitance (lower trace) and amperometric current (middle trace) were measured. The traces were signal averaged with respect to the stimulus. sAP stimulation resulted in a rapid jump in cell capacitance, followed by a decrease. The averaged amperometric signal was integrated (upper trace) to generate an estimate of total exocytosis.
Separation of endocytosis from exocytosis
Given the complete and rapid endocytosis observed following single sAP stimulation, we set out to measure exocytosis-coupled endocytosis in response to a train of stimuli. However, the use of sAP waveforms in a stimulus train presents a challenge in the separation of exocytosis from endocytosis. For this reason, we offer a detailed description of the technique used to achieve this separation.
It is expected that exocytosis and endocytosis will overlap during the trains. For this reason it was necessary to develop a method to separate exocytosis from endocytosis during the stimuli. As outlined in Fig. 3, a simple protocol was designed in which chromaffin cells were stimulated with a train of 300 sAPs, allowed to rest, and again stimulated with a 200 ms depolarisation pulse to +7 mV (‘DP’ pulse). Each cell was stimulated with this protocol only once. Cell capacitance (the sum of exocytosis plus endocytosis; Fig. 3, lower trace), and amperometric current (time-resolved exocytosis; Fig. 3, middle trace) were measured during the sAP train and DP square-pulse. The amperometric trace was integrated off-line (summed exocytosis; Fig. 3, upper trace).
Figure 3. sAP trains incorporate a calibration square pulse depolarisation.
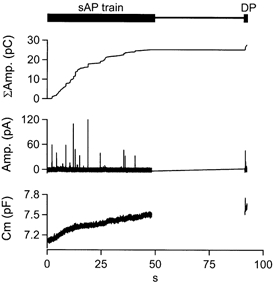
A single chromaffin cell was stimulated with a train of 300 sAPs at 6 Hz. Cell capacitance (lower trace) and amperometric current (middle trace) were measured during the train (sAP train) and a delayed 200 ms depolarising pulse (DP). The amperometric current was then integrated off-line (upper trace) in order to give an index of cumulative catecholamine release.
The ‘DP’-depolarisation was incorporated at the end of the stimulation protocol in order to provide a large, temporally isolated and high ‘signal-to-noise ratio’ exocytic signal in both the cell capacitance and amperometric traces. We did not incorporate the ‘DP’ pulse prior to the sAP train since the evoked, large Ca2+ influx might alter the exo-/endocytic properties of the cell by saturating Ca2+ sequestration processes such as endoplasmic reticulum or mitochondrial Ca2+ sinks, or invoking Ca2+-modulated biochemical cascades such as kinase activation. Additionally, we allowed cells to rest for approximately 45 s prior to the DP pulse (Fig. 4_A_) in order to maximize the amplitude of the capacitance and amperometric responses, resulting in a larger signal for scaling. The DP pulses resulted in robust, highly resolvable deflections in both cell capacitance (Fig. 4_B_, ‘Δ_C_m‘, lower trace) as well as integrated amperometric signals (Fig. 4_B_, ‘ΔΣAmp.‘, upper trace). Δ_C_m and ΔΣamperometric responses were measured as the difference between pre- and post-pulse values. The peak deflection in the _C_m trace and the ΔΣamp. traces were offset in time, with the peak amperometric signal delayed with respect to the capacitance. Such temporal discrepancies in amperometric and capacitance signals have been previously reported in chromaffin cells (Haller et al. 1998) and are probably due to diffusion of the released catecholamine molecules to the carbon fibre.
Figure 4. Integrated amperometric currents can be scaled to the capacitance trace.
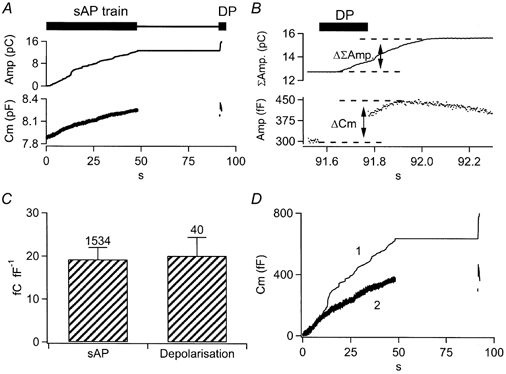
A, chromaffin cells were stimulated with the sAP-DP protocol presented in Fig. 3. Cell capacitance (lower trace) and integrated amperometric current (upper trace) were averaged from a total of 7 cells at 6 Hz. B, the same capacitance and integrated amperometry traces from A are expanded for the time frame encompassing the DP calibration pulse. The signals evoked by the 200 ms depolarisation are highlighted and the offsets used for scaling are labelled (ΔΣamp. and Δ_C_m). C, amperometric charge is plotted as a function of capacitance increase for sAP and DP stimuli. There was no statistical difference between sAP and DP values (Student's t test, P > 0.02). Numbers indicate the events averaged in each case. D, following scaling to the DP, integrated amperometric charge (trace 1) and cell capacitance (trace 2) are plotted for the same data set shown in A and B.
The integrated amperometry was scaled to the _C_m trace by dividing the DP-evoked ΔΣamperometry value by the Δ_C_m value, and then dividing the entire integrated amperometry trace by the resulting quotient. This scaling then allows for the subtraction of the cell capacitance trace from the integrated amperometry trace, resulting in an estimate of endocytosis during the train. However, there are several assumptions made that need to be addressed: (1) Δ_C_m is not influenced by non-secretory artefacts, (2) DP-evoked Δ_C_m is not contaminated by endocytosis, (3) catecholamine is uniformly released over the surface of the cell and (4) amperometric measures are equally sensitive during sAP and DP stimulation.
The four assumptions are addressed in detail here. (1) Δ_C_m and ΔΣamperometry values in the first 50 ms following the depolarisation were not considered in order to allow for the decay of tail conductances and Na+ channel gating artefacts (τ = 16 ms; Chow et al. 1996). This delay also allows for the diffusion of the catecholamine to the amperometric carbon fibre. (2) It seems very unlikely that the Δ_C_m and ΔΣamperometry values measured in response to the DP scaling pulse were contaminated by endocytosis. The mode of endocytosis expected after the 200 ms depolarisation falls into the ‘compensatory’ category. On average, the 200 ms DP stimuli evoked an influx of 34.24 ± 2.858 pC Ca2+ (mean ±s.e.m., n = 41). Compensatory endocytosis has been well quantified in the literature and is predicted to retrieve membrane with a time constant of approximately 20 s following Ca2+ influx of this magnitude (Smith & Neher, 1997; Engisch & Nowycky, 1998). Such an endocytic kinetic is far slower than the time in which Δ_C_m and ΔΣamperometry were determined and therefore would not be expected to influence Δ_C_m values. It must be considered that despite the quantitative support provided by the literature, it remains a possibility that the scaling factor determined through the DP pulse is compromised by a contaminating degree of unexpected endocytosis, leading to a slight overestimation of total exocytosis. However, this error would be present in all experimental protocols and would not alter comparisons of endocytosis under differing stimulus frequencies or pharmacological conditions. (3) It has been demonstrated that under certain circumstances, chromaffin cells may not release catecholamine uniformly over their entire surface (Monck et al. 1994; Schroeder et al. 1994). The amperometric fibre, however, samples catecholamine released from a limited region of the cell. Any non-uniform ‘zonal’ release could therefore introduce significant variability in the scaling factor if applied to single cells. For this reason, responses from all cells stimulated at like frequencies were averaged prior to scaling. The pooling of data acts to randomise any potential zonal catecholamine release. (4) Correlation of ‘DP’ pulse-evoked amperometric signals to those recorded during sAP trains requires that both stimuli result in equal catecholamine release for a given capacitance increase, as is shown to be true in Fig. 4_C_. This may not always be the case. Possible exceptions would occur if sAP and DP pulses access separate populations of granules that may contain different catecholamine loads. Vesicular capacitance increases that are not associated with transmitter release have been shown to be evoked in varied cells such as chromaffin cells (Xu et al. 1998), fibroblasts and CHO cells (Coorssen et al. 1996; Ninomiya et al. 1996). These occurred when cells were challenged with very high Ca2+ elevations. Xu et al. (1998) found that such non-catecholamine secreting capacitance increases were observed only at Ca2+ concentrations of at least 70 μm in chromaffin cells, and that they were insensitive to pre-treatment with clostridial neurotoxins, a marker for SNARE-dependent exocytosis that is commonly associated with regulated secretory granule or vesicle fusion. One interpretation for such non-secretory capacitance increases is that they represent a patho-physiological wound-healing response (Miyake & McNeil, 1995; Terasaki et al. 1997) and that they only occur at very high Ca2+ elevations that are not expected to occur under normal circumstances, but rather following membrane rupture. Such high elevations (at least 70 μm in the chromaffin cell) are clearly not expected to be reached with action-potential stimulation as used in this study. Otherwise, the measured secretory rates would have been much higher than the few tens of femtofarads per second reported in Fig. 5 and 7, but rather many thousands of femtofarads per second expected at such Ca2+ levels (Chow et al. 1994; Heinemann et al. 1994). A second possible scenario under which the scaling factor would result in inaccuracies would be if the ‘DP’ and sAP stimulation protocols access different sized or differentially loaded granules. To test this assumption, the relationship between amperometric and capacitance responses was compared between 200 ms depolarisations and the sAP voltage template (Fig. 4_C_). This test reported an average amperometric efficiency of 20.09 ± 4.297 fC fF−1 for square pulses and 19.24 ± 2.764 fC fF−1 for sAPs (values are means ±s.d. and were judged not to be statistically significant using Student's t test, P > 0.02). This demonstrates that the use of 200 ms depolarisations at the end of experiments provides an internal calibration for the amperometric data, and allows for the scaling and comparison of the amperometric data to cell capacitance during stimulus trains (Fig. 4_D_). This point is further supported in that, following scaling, cell capacitance never rose significantly above the scaled integrated amperometry. Additionally, we were not able to guard against contamination of the _C_m trace by non-secretory Δ_C_mt artefacts within the trains as we did following the DP pulse. Therefore the potential that our cell capacitance values were slightly over-reported during trains exists. This overestimation would be most prominent at higher frequency stimulations as the interpulse interval approaches the duration of the Δ_C_mt. However, the magnitude of this error would be very small relative to the total capacitance increase measured (less than 1 % at 16 Hz). If present, such an error introduced by Δ_C_mt would have led to a slight underestimation of the endocytic efficiencies at the upper frequency range.
Figure 7. Cyclosporin A pre-treatment selectively blocks endocytosis at higher frequencies.
Cells were treated for 10 min in extracellular Ringer solution containing 1 μm cyclosporin A prior to recording. A, integrated scaled amperometry (trace 1) and cell capacitance (2) are presented for single sAP stimuli (n = 14 cells, 328 sAPs), 1.9 Hz (n = 6 cells), 10 Hz (n = 15 cells) and 16 Hz (n = 8 cells). B, endocytosis was quantified as reported for control cells in Fig. 6, and is presented for the cyclosporin A-treated and control cells for comparison. These data show that cyclosporin A treatment significantly lessens endocytosis at 10 Hz, and completely blocks endocytosis at 16 Hz.
Varied cell activity reflects expected vesicle depletion and mobilisation
With a method for the separate measure of train-evoked exo- and endocytosis, we set out to test the endocytic behaviour in response to a range of stimulus frequencies. The sAP frequency range tested in this study, single pulse to 16 Hz, was chosen because it covers the normal firing rate measured for sympathetic nervous system fibres as well as resting and activated chromaffin cells (Brandt et al. 1976; Kidokoro & Ritchie, 1980; Wakade, 1981). In fact, above approximately 15 Hz firing, sympathetic nerve impulses fail to produce action potentials on a one-to-one basis in guinea-pig chromaffin cells (Holman et al. 1994). Additionally, we measured the absolute refractory period in cultured chromaffin cells in current clamp and found that it limited AP firing to approximately 25 Hz (data not shown), a number consistent with the maximal firing rate measured with optical techniques (Iijima et al. 1992).
Delivering trains of 300 sAPs at increasing frequencies (0.5, 1.9, 6, 10 and 16 Hz) resulted in complex amperometric and capacitance signals (Fig. 5_A_). Analysis of the data presented in Fig. 5 revealed that the greatest increase in scaled integrated amperometric records was evoked with the 0.5 Hz stimulus train, becoming smaller with increasing stimulus intensity. Additionally, the rates of exocytosis often seemed to diminish during trains (see 10 and 16 Hz data). In order to understand the source of this behaviour better, we simulated the stimulus protocols with the ‘two step’ secretion model described previously (Heinemann et al. 1993) and with kinetic parameters as defined under similar conditions (Smith et al. 1998; Smith, 1999). The experimental protocol was simulated as vesicle recruitment and release in a Ca2+-dependent manner, and was driven by the Ca2+ influx measured from recordings at each frequency (see Fig. 5_B_ for an example recorded during 10 Hz sAP trains). We found that the exocytosis measured in our stimulation range could be well matched assuming initial granule depletion (0.5-1.9 Hz), subsequent Ca2+-dependent vesicle recruitment and Ca2+-dependent activation of protein kinase C (1.9-16 Hz), a behaviour that is expected under these stimulation conditions (Smith, 1999; Fig. 2_C_). Additionally, the decrease in exocytic rate observed within stimulus trains (especially prominent at higher frequencies) was mirrored in the simulations and was due to vesicle depletion. With an understanding of the exocytic behaviour, we moved on to investigating the evoked endocytosis.
Physiological stimuli evoke a biphasic endocytic response
Endocytosis did not occur to a similar extent at all frequencies tested, as indicated by the divergence of the integrated amperometry from the cell capacitance traces. Surprisingly, a robust and nearly complete endocytosis of added membrane was seen following single sAP stimuli and at 0.5 Hz. Additionally, it was unexpected to see that endocytosis seemed to slow with increased firing, allowing cell surface area to significantly increase during intermediate frequency trains (Fig. 5_A_, 1.9 and 6 Hz). Although demonstrated in several other secretory cell types (von Gersdorff & Matthews, 1994; Hsu & Jackson, 1996; Cousin & Robinson, 2000), such an activity-dependent decrease in endocytosis had not been reported in chromaffin cells before. However, further increases in firing rate seemed to invoke a more familiar endocytic behaviour for chromaffin cells in that endocytic rate and efficacy increased with stimulus intensity (10 and 16 Hz).
We examined the endocytic activity in closer detail by quantifying the endocytic efficiency at all tested frequencies. The endocytic efficiency was determined by dividing the endocytosis activity (the difference between scaled integrated amperometry and cell capacitance) by the exocytosis (scaled integrated amperometry). A value of 1.0 is equal to complete retrieval and 0 equal to no retrieval. This analysis was performed on the data collected from all the stimulus frequencies tested (Fig. 6). At all frequencies, the endocytic efficiency profiles approached a steady value by the end of the stimulus train. Evoked endocytic efficiency was determined as the average value over the final 20 % of the endocytosis ratio curve, and not a time-resolved estimate of absolute membrane internalisation; rather it is representative of the sustained relative rate at which cells internalised membrane to maintain a constant cell surface. The results from this analysis are presented in Fig. 6_B_, and show that the endocytic efficiencies relate to stimulus frequency in a biphasic manner. The lowest (single AP) and highest frequency (16 Hz) sAP train showed the highest degree of membrane retrieval, while the 1.9 and 6 Hz sAP stimulations showed less than 50 % retrieval during the train. The data collected at 1.9 and 6 Hz are consistent with previous studies that employed relatively light stimulation protocols (Smith & Neher, 1997; Engisch & Nowycky, 1998), in that the endocytosis measured during the sAP trains resulted in incomplete return of membrane capacitance to prestimulation levels. Recognising that at most stimulus level, there was a significant amount excess membrane ‘stranded’ after the trains, we attempted to hold cells for prolonged periods of time to track the eventual expected retrieval of the added membrane. However, we were not able to maintain stable recordings long enough for quantitative analysis of the endocytosis of this ‘stranded’ membrane. Additionally, we noted that there seemed to be a delay in the onset of the endocytic activity that varied with stimulus frequency (see Fig. 5_A_). However, the efficiency curves presented in Fig. 6_A_ are essentially ratiometric measures and are therefore noisy when signals are small, as is the case when endocytosis first results in a divergence of the scaled amperometric from the cell capacitance traces. Due to this noise, attempts to quantify this behaviour were inconclusive.
Figure 6. Endocytosis efficiency follows a biphasic frequency dependency.
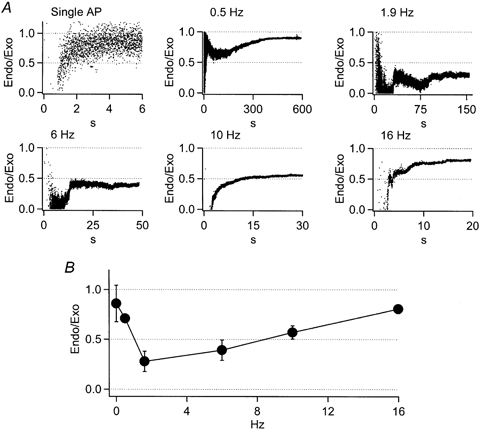
A, endocytic efficiency was calculated for each stimulation condition as:
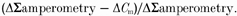
The points in each plot of A represent the proportion of membrane retrieved to that added at a given time point, or the time resolved endocytic efficiency measured during the train. B, the steady-state endocytic efficiency for each stimulation condition was determined as the mean value over the final 20 % of the efficiency curve. The endocytosis efficiency is plotted against stimulus frequency as mean ±s.d.
Cyclosporin A selectively blocks endocytosis at higher stimulation frequencies
It seemed clear that chromaffin cell endocytosis in response to simulated action potential stimulation was complex, with the most efficient maintenance of cell surface area occurring at the lowest and highest frequencies. We noted that the biphasic behaviour could be well explained by the co-existence of two endocytic mechanisms. Under this scenario one form of endocytosis would prevail at low stimulation intensities, but would rapidly wane as stimulation increased. A second endocytic mechanism would in turn be activated at sAP frequencies near 6 Hz, and be positively modulated by further increases in stimulus firing rates. In order to test this hypothesis, we searched for an experimental condition to separate the complex endocytic behaviours. Several reports have indicated an inhibitory action of the immunosuppressants cyclosporin A and FK506 on endocytic activity (Bauerfeind et al. 1997; Marks & McMahon, 1998; Lai et al. 1999). Cyclosporin A and FK506 have been shown to inhibit the calcium- calmodulin-dependent protein phosphatase calcineurin (Liu et al. 1991), and block endocytosis by dephosphorylating dynamin (Bauerfeind et al. 1997). For example, it has been reported in bovine chromaffin cells under moderate stimulation that treatment with cyclosporin A diminishes the Ca2+ modulation of exocytosis-coupled endocytosis (Engisch & Nowycky, 1998). We tested cyclosporin A and FK506 for their possible effects on the biphasic frequency dependence of endocytosis reported here (data shown are for cyclosporin A). As demonstrated in Fig. 7_A_, a 10 min pretreatment of cells with 1 μm cyclosporin A left endocytosis unaffected at lower frequency stimulation (sAP, 1.9 Hz) but selectively and progressively blocked the endocytic activity at higher frequencies (10 and 16 Hz). This effect was quantified and is presented in Fig. 7_B_. At lower stimulus frequencies the endocytosis efficiencies of control and cyclosporin A-treated cells were identical (single AP and 1.9 Hz). However the endocytosis efficiency at 10 Hz was significantly reduced from control after the cyclosporin A treatment (Student's t test, P < 0.02). The most dramatic cyclosporin A-dependent effect on the evoked endocytic process occurred at 16 Hz, decreasing the efficiency from near-complete retrieval in control cells to undetectable levels in pretreated cells.
The cyclosporin A effect is independent of exocytosis
Cyclosporin A pre-treatment results in a lower evoked Ca2+ influx (Engisch & Nowycky, 1998). It remained a concern that the specific effects observed in cyclosporin A-treated cells was a consequence of lowered exocytic activity, and not a specific effect on the endocytic mechanisms at higher stimulus frequencies. In order to test for this possibility, we identified a Ca2+ influx range common to both control and cyclosporin A-treated cells, and asked if there was a difference in endocytic activity at this common stimulus level. Data from individual sAP stimuli that were delivered at 10 and 16 Hz are plotted in Fig. 8, revealing that cyclosporin A treatment did indeed diminish evoked Ca2+ influx by approximately 35 %. A common range of Ca2+ influx in both control and cyclosporin A-treated cells was defined as falling within 1 standard deviation of the mean of both data sets, and is represented as a hatched box in Fig. 8_Aa_ and Ba. Average exocytic and endocytic efficiencies of the data points that fall within the common range of Ca2+ influx are plotted (Fig. 8_Ab_ and Bb). These data show that, following comparable stimulus intensity, cyclosporin A does not alter evoked exocytosis, but does significantly decrease (Fig. 8_A_, 10 Hz stimuli) or even block endocytosis (Fig. 8_B_, 16 Hz stimuli).
The data presented here demonstrate that cyclosporin A selectively inhibits endocytosis in the higher range of sAP train frequencies. Because these experiments were performed in perforated patch configuration, there was no way to quickly remove the cyclosporin A from the cell cytosol to measure recovery from the cyclosporin A treatment. Cells pretreated with 2 μm FK506, another calcineurin inhibitor, also showed an inhibition of endocytic activity at higher stimulation frequencies similar to cyclosporin A-treated cells (data not shown).
DISCUSSION
Based on the data presented here, we propose that in response to a physiological range of exocytic activity, bovine adrenal chromaffin cells alternate between at least two stimulus-modulated forms of endocytosis, one of which is prevalent at basal firing rates but gives way to the other with cell activation. This is consistent with evidence that continues to mount showing that exocytosis-coupled membrane retrieval may be accomplished by several morphologically (Heuser & Reese, 1973; Ceccarelli & Hurlbut, 1980; Koenig & Ikeda, 1996), kinetically (Wu & Betz, 1996; Thomas & Waring, 1997; Artalejo et al. 1998; Klingauf et al. 1998) and pharmacologically (Artalejo et al. 1995; Engisch & Nowycky, 1998) differentiable mechanisms.
Here we report, for the first time, the use of action potential wave forms to measure exocytosis and endocytosis in adrenal chromaffin cells with the use of cell capacitance and amperometric catecholamine measurements. We introduce an electrical stimulation technique that, while quantitatively identical to native action potentials, allows for the highly regular stimulation of cells while measuring both electrochemical and cell capacitance changes with a minimal lag time. This technique mimics the unique Ca2+ entry and clearance dynamics expected from native action potential current influx. As outlined in the Introduction, square pulse and action potential stimuli are likely to result in very different cytosolic Ca2+ profiles. Because chromaffin cell secretory granule exocytosis has been shown to be dependent on Ca2+ influx (Burgoyne, 1991; Aunis, 1998), the exocytosis-coupled endocytosis may differ significantly following action potential and square pulse stimuli. These differences would be expected to be smallest with the highest rates of action potential firing, when evoked Ca2+ concentrations are the greatest. In fact, that is the general behaviour observed. However, in cells stimulated with lower frequency action potential trains, the evoked Ca2+ influx and metabolism from action potential stimulation is expected to be unique and not resemble that following square pulse. It is under these conditions that we report endocytic behaviour different from that observed in studies utilizing short square pulse stimuli (Figs 5_A_ and 7_A_).
Our results show that sAP stimuli resulted in very little Ca2+ influx (about 1 pC in Fig. 5_B_) and caused a small increase in _C_m (∼3 fF). Using this technique we demonstrate that at lower activity levels bovine adrenal chromaffin cells exhibit a robust endocytosis (referred to here as ‘phase I’). We were surprised by this initial finding in that endocytosis had been shown to be either absent or moderate in magnitude at low stimulation intensities, then increase with greater Ca2+ influx; we therefore expected sAP stimuli to result in very little exocytosis-coupled endocytosis. Indeed, following square pulse-evoked capacitance increases of less than 4 fF, endocytosis was often not detected in chromaffin cells (Engisch & Nowycky, 1998). Stronger stimulation, triggering capacitance jumps greater than 100 fF, resulted in an increase in both the magnitude and rate of exocytosis-coupled endocytosis, but did not result in subsecond time constants until a mode transition to ‘excess endocytosis’ achieved following elevation of cytosolic Ca2+ to at least 50 μm (Burgoyne, 1995; Smith & Neher, 1997; Engisch & Nowycky, 1998).
As with the endocytic mechanisms previously studied (Bauerfeind et al. 1997; Cousin & Robinson, 2000), pretreatment of the cells with cyclosporin A and FK506, resulted in a block of endocytosis under certain conditions (leading to ‘phase II’ in this case). Previous studies in chromaffin cells reported mixed effects of cyclosporin A treatment (Artalejo et al. 1996; Engisch & Nowycky, 1998). These seemingly contradictory results may be explained by the fact that earlier investigators utilised vastly different stimulus protocols and were accessing separate endocytic mechanisms that differ in their cyclosporin A sensitivity. For example, Engisch & Nowycky (1998) reported that in chromaffin cells under moderate stimulation in the perforated patch configuration, cyclosporin A selectively removes a calcium sensitivity from the ‘compensatory’ form of endocytosis. Artalejo et al. (1996) stimulated cells at a much greater intensity in the whole-cell configuration. They found cyclosporin A to activate a kinetic component of an evoked ‘excess’ endocytosis (see Engisch & Nowycky, 1998, for a definition of ‘compensatory’_versus_‘excess’ endocytosis). Our finding that cyclosporin A and FK506 leave evoked phase I endocytosis unaltered at low frequency stimulation, but block phase II endocytosis at higher stimulation, is more consistent with Engisch & Nowycky (1998).
Despite that our results being similar, Engisch & Nowycky (1998) found that cyclosporin A pretreatment did not abolish endocytic activity as we report for phase II, but seemed to remove a Ca2+-sensitive component of evoked endocytosis. This could be due to the fact that their square pulse stimuli were longer in duration than those employed here, and that they were probably probing endocytosis at stimulus levels higher than our minimal stimulation (single sAP). As introduced earlier, square pulse stimuli are likely to result in much greater transient subplasmalemmal Ca2+ concentrations than action potential stimuli. For this reason it seems possible that they were measuring a mix of phase I and phase II endocytosis and that the cyclosporin A treatment acted to selectively block the activity-enhanced phase II component, leaving behind the cyclosporin A-insensitive phase I. This would result in a seeming loss of the Ca2+ enhancement as they report.
Our data show an inhibition of endocytic behaviour between single sAP stimulation and 1.9 Hz train stimulation protocols (phase I endocytosis). Such an activity-dependent inhibition of endocytosis had not previously been described in bovine adrenal chromaffin cells. However, it is not a novel behaviour among other secretory cell types. Previously, rapid inhibition or delay of endocytosis following cell activity has been observed in other neuroendocrine cell types (Hsu & Jackson, 1996), the giant ribbon synapse of the goldfish retinal bipolar neuron (von Gersdorff & Matthews, 1994), and rat brain synaptosomes (Cousin & Robinson, 2000). It is certainly possible that phase I endocytosis had not yet been observed in chromaffin cells due to its sensitivity to even modest cell activity. It is likely that most previous experimental protocols in chromaffin cell studies delivered stimuli that resulted in much higher Ca2+ concentrations than those expected during the trains in this study, such that they had already inhibited the phase I mechanism, leaving only the activity-dependent phase II mode remaining. This is particularly likely if one considers the differences in stimulus methods utilised in this versus previous studies. Previously, endocytosis had been evoked with square pulses. As described above, such square pulses will result in very different cytosolic calcium environments when compared with action potential waveform stimulation. If phase I endocytosis is blocked by elevated cytosolic Ca2+, then it would be particularly susceptible to inhibition by square pulse stimuli.
The phase II endocytic mechanism we describe shares an activity-evoked up-regulation with several already well-described membrane retrieval behaviours measured in adrenal chromaffin cells (Smith & Neher, 1997; Engisch & Nowycky, 1998), cultured hippocampal autapses (Klingauf et al. 1998; Sankaranarayanan & Ryan, 2001), and pancreatic β-cells (Eliasson et al. 1996). A possible messenger for the evoked increase in phase II efficiency again could be cytosolic Ca2+, already shown to be a positive modulator of several other activity-dependent forms of endocytosis. Ca2+ also acts as a key activator for the calcium-calmodulin-calcineurin second messenger pathway. Interestingly, phase II endocytosis is eliminated by pre-treatment with cyclosporin A and FK506, both of which have been shown to block the calcium- calmodulin- calcineurin cascade by immunophilin activation. It remains to be determined whether phase II endocytosis is dependent on dynamin, which would be consistent with the calcium-calmodulin-calcineurin cascade, and as has been demonstrated in cortical synapse preparations (Bauerfeind et al. 1997; Lai et al. 1999; Cousin & Robinson, 2000). Further experiments and kinetic analysis will be required to determine whether compensatory endocytosis, as described in the literature, represents the same mechanism as the phase II endocytosis reported here.
What purpose multiple endocytic mechanisms might serve remains a puzzle, especially when they are differentially modulated by cell activity. Studies have indicated that granule and plasma membrane components are kept separate during rounds of exocytosis and endocytosis (Patzak & Winkler, 1986; Valtorta et al. 1988; Sankaranarayanan & Ryan, 2000; Smith et al. 2000). Therefore it seems questionable that the two forms of endocytosis play a role in differential membrane sorting. Another possibility is that both mechanisms retrieve the same membrane, but lead to different internal trafficking and recycling pathways. For example, it may be that the robust phase I endocytosis observed following single sAPs and at lower frequency stimulation results in a local recycling of membrane while the activity- and cyclosporin A-sensitive phase II endocytosis leads to the slower endocytic recycling observed following heavier cell stimulation (Kobayashi et al. 1978; Lingg et al. 1983). Experiments combining measures of both surface and intracellular membrane trafficking will be required to test this possibility.
Lastly, we have shown that while endocytosis can retrieve added membrane with quite high efficiency under certain circumstances, this is not always the case. For example, cells stimulated at 1.9 Hz only retrieved approximately 30 % of the membrane added during the stimulus train. We did attempt to measure the expected eventual internalisation of this membrane, but did not succeeded in maintaining conditions necessary for an accurate critical evaluation of these retrieval kinetics. This baits the questions: what is the eventual fate of this added membrane, and why is it left behind? Perhaps the observed under-retrieval during trains is the source of the membrane eventually internalised via the high Ca2+ threshold excess retrieval observed in several cell types (Thomas et al. 1994; Artalejo et al. 1995; Coorssen et al. 1996; Ninomiya et al. 1996). Two potential mechanistic scenarios for the under-retrieval exist: (1) of all the granules that fused in response to 1.9 Hz stimulation, 30 % of them were retrieved in their entirety, or (2) only 30 % of the surface area of each and all of the granules that fused in response to 1.9 Hz stimulation was retrieved. The difference between these options is important; if option 1 were true, it would imply that there were two functionally different types of granules undergoing fusion. Under this scenario, a more retrievable granule subtype would be preferentially released at low frequency levels (granules retrieved by phase I, while at higher stimulation, less retrievable vesicles fuse (granules retrieved by phase II). The phase II granules are then internalised to a greater degree as cell firing rates increase. Alternatively, if option 2 were found to be the case, it would argue that all membrane is equally retrievable, and includes the machinery for two differentially regulated endocytic mechanisms (separate machinery responsible for phase I and phase II). Again, it is likely that cross-disciplinary experimentation will be required to answer this fundamental question.
Acknowledgments
Portions of this work were completed at the Medical College of Georgia, Augusta, GA, USA. We wish to thank Dr Neil Williams of the Shapiro Packing Company Inc. and Mahan Packing Company Inc. for providing adrenal tissues utilised in this study. We would also like to thank Drs Stephan Jones for valuable critical comment of this manuscript and Nevin Lambert and Steven Vogel for helpful discussions. This work was supported by a grant from the National Science Foundation (IBN-0196136) and was also supported by the Alfred P. Sloan Foundation.
References
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC. Secretion: dense-core vesicles can kiss-and-run too. Current Biology. 1998;8:R62–65. doi: 10.1016/s0960-9822(98)70036-3. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proceedings of the National Academy of Sciences of the USA. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Lemmon MA, Schlessinger J, Palfrey HC. Specific role for the pH domain of dynamin-1 in the regulation of rapid endocytosis in adrenal chromaffin cells. EMBO Journal. 1997;16:1565–1574. doi: 10.1093/emboj/16.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunis D. Exocytosis in chromaffin cells of the adrenal medulla. International Review of Cytology. 1998;181:213–320. doi: 10.1016/s0074-7696(08)60419-2. [DOI] [PubMed] [Google Scholar]
- Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. Journal of Biological Chemistry. 1997;272:30984–30992. doi: 10.1074/jbc.272.49.30984. [DOI] [PubMed] [Google Scholar]
- Brandt BL, Hagiwara S, Kidokoro Y, Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. Journal of Physiology. 1976;263:417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. Control of exocytosis in adrenal chromaffin cells. Biochimica et Biophysica Acta. 1991;1071:174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. Fast exocytosis and endocytosis triggered by depolarisation in single adrenal chromaffin cells before rapid Ca2+ current run-down. Pflügers Archiv. 1995;430:213–219. doi: 10.1007/BF00374652. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. Journal of Cell Biology. 1980;87:297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, Klingauf J, Heinemann C, Zucker RS, Neher E. Mechanisms determining the time course of secretion in neuroendocrine cells. Neuron. 1996;16:369–376. doi: 10.1016/s0896-6273(00)80054-9. [DOI] [PubMed] [Google Scholar]
- Chow RH, Klingauf J, Neher E. Time course of Ca2+ concentration triggering exocytosis in neuroendocrine cells. Proceedings of the National Academy of Sciences of the USA. 1994;91:12765–12769. doi: 10.1073/pnas.91.26.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, Von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Coorssen JR, Schmitt H, Almers W. Ca2+ triggers massive exocytosis in Chinese hamster ovary cells. EMBO Journal. 1996;15:3787–3791. [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Ca2+ Influx inhibits dynamin and arrests synaptic vesicle endocytosis at the active zone. Journal of Neuroscience. 2000;20:949–957. doi: 10.1523/JNEUROSCI.20-03-00949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Takei K. Molecular mechanisms in synaptic vesicle endocytosis and recycling. Neuron. 1996;16:481–486. doi: 10.1016/s0896-6273(00)80068-9. [DOI] [PubMed] [Google Scholar]
- Eliasson L, Proks P, Ämmälä C, Ashcroft FM, Bokvist K, Renström E, Rorsman P, Smith PA. Endocytosis of secretory granules in mouse pancreatic β-cells evoked by transient elevation of cytosolic calcium. Journal of Physiology. 1996;493:755–767. doi: 10.1113/jphysiol.1996.sp021420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch KL, Nowycky MC. Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. Journal of Physiology. 1998;506:591–608. doi: 10.1111/j.1469-7793.1998.591bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis K, Pun R, Misler S. Single cell assay of exocytosis from adrenal chromaffin cells using ‘perforated patch recording’. Pflügers Archiv. 1991;418:611–613. doi: 10.1007/BF00370579. [DOI] [PubMed] [Google Scholar]
- Gillis KD. Techniques for membrane capacitance measurements. In: Sakmann B, Neher E, editors. Single-Channel Recording. 2. New York: Plenum Press; 1995. pp. 155–198. chap. 7. [Google Scholar]
- Giovannucci DR, Hlubek MD, Stuenkel EL. Mitochondria regulate the Ca2+-exocytosis relationship of bovine adrenal chromaffin cells. Journal of Neuroscience. 1999;19:9261–9270. doi: 10.1523/JNEUROSCI.19-21-09261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M, Jackle H. Role of Drosophila alpha-adaptin in presynaptic vesicle recycling. Cell. 1997;88:767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]
- Haller M, Heinemann C, Chow RH, Heidelberger R, Neher E. Comparison of secretory responses as measured by membrane capacitance and by amperometry. Biophysical Journal. 1998;74:2100–2113. doi: 10.1016/S0006-3495(98)77917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm-Alvarez SF, Sheetz MP. Microtubule-dependent vesicle transport: modulation of channel and transporter activity in liver and kidney. Physiological Reviews. 1998;78:1109–1129. doi: 10.1152/physrev.1998.78.4.1109. [DOI] [PubMed] [Google Scholar]
- Heinemann C, Chow RH, Neher E, Zucker RS. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+ Biophysical Journal. 1994;67:2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann C, Von Rüden L, Chow RH, Neher E. A two-step model of secretion control in neuroendocrine cells. Pflügers Archiv. 1993;424:105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. Journal of Cell Biology. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman ME, Coleman HA, Tonta MA, Parkington HC. Synaptic transmission from splanchnic nerves to the adrenal medulla of guinea-pigs. Journal of Physiology. 1994;478:115–124. doi: 10.1113/jphysiol.1994.sp020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. Journal of General Physiology. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SF, Jackson MB. Rapid exocytosis and endocytosis in nerve terminals of the rat posterior pituitary. Journal of Physiology. 1996;494:539–553. doi: 10.1113/jphysiol.1996.sp021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T, Matsumoto G, Kidokoro Y. Synaptic activation of rat adrenal medulla examined with a large photodiode array in combination with a voltage-sensitive dye. Neuroscience. 1992;51:211–219. doi: 10.1016/0306-4522(92)90486-l. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Role of action potentials in hormone secretion. Biochemical Research. 1980;1:117–123. [Google Scholar]
- Kidokoro Y, Ritchie A. Effect of tetrodotoxin on adrenaline secretion in the perfused rat adrenal medulla. Nature. 1979;278:63–65. doi: 10.1038/278063a0. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y, Ritchie AK. Chromaffin cell action potentials and their possible role in adrenalin secretion from the rat adrenal medulla. Journal of Physiology. 1980;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Kavalali ET, Tsien RW. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature. 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Kent C, Coupland R. Observations on the localization of labelled amino acid in mouse adrenal chromaffin cells after the injection of l-[4,5-3H] leucine. Journal of Endocrinology. 1978;78:21–29. doi: 10.1677/joe.0.0780021. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. Journal of Cell Biology. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MM, Hong JJ, Ruggiero AM, Burnett PE, Slepnev VI, De Camilli P, Snyder SH. The calcineurin-dynamin 1 complex as a calcium sensor for synaptic vesicle endocytosis. Journal of Biological Chemistry. 1999;274:25963–25966. doi: 10.1074/jbc.274.37.25963. [DOI] [PubMed] [Google Scholar]
- Lee AK, Tse A. Endocytosis in identified rat corticotrophs. Journal of Physiology. 2001;533:389–405. doi: 10.1111/j.1469-7793.2001.0389a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingg G, Fischer CR, Schmidt W, Winkler H. Exposure of an antigen of chromaffin granules on cell surface during exocytosis. Nature. 1983;301:610–611. doi: 10.1038/301610a0. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Current Biology. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Miyake K, McNeil PL. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. Journal of Cell Biology. 1995;131:1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monck JR, Robinson IM, Escobar AL, Vergara JL, Fernandez JM. Pulsed laser imaging of rapid Ca2+ gradients in excitable cells. Biophysical Journal. 1994;67:505–514. doi: 10.1016/S0006-3495(94)80554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Augustine GJ. Calcium gradients and buffers in bovine chromaffin cells. Journal of Physiology. 1992;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proceedings of the National Academy of Sciences of the USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Lagnado L. The kinetics of exocytosis and endocytosis in the synaptic terminal of goldfish retinal bipolar cells. Journal of Physiology. 1999;515:181–202. doi: 10.1111/j.1469-7793.1999.181ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Kishimoto T, Miyashita Y, Kasai H. Ca2+-dependent exocytotic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+ compound. Journal of Biological Chemistry. 1996;271:17751–17754. doi: 10.1074/jbc.271.30.17751. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Fox AP. Barium triggers rapid endocytosis in calf adrenal chromaffin cells. Journal of Physiology. 1998;508:483–494. doi: 10.1111/j.1469-7793.1998.483bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora PG, Fox AP. Tyrosine phosphorylation regulates rapid endocytosis in adrenal chromaffin cells. Journal of Neuroscience. 1999;19:9739–9746. doi: 10.1523/JNEUROSCI.19-22-09739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzak A, Winkler H. Exocytotic exposure and recycling of membrane antigens of chromaffin granules: Ultrastructural evaluation after immunolabeling. Journal of Cell Biology. 1986;102:510–515. doi: 10.1083/jcb.102.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M, Krishnan KS, Kelly RB. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994;13:363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Current Opinion in Cell Biology. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nature Cell Biology. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nature Neuroscience. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Schroeder TJ, Jankowski JA, Senyshyn J, Holz RW, Wightman RM. Zones of exocytotic release on bovine adrenal medullary cells in culture. Journal of Biological Chemistry. 1994;269:17215–17220. [PubMed] [Google Scholar]
- Smith C. A persistent activity-dependent facilitation in chromaffin cells is caused by Ca2+ activation of protein kinase C. Journal of Neuroscience. 1999;19:589–598. doi: 10.1523/JNEUROSCI.19-02-00589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Moser T, Xu T, Neher E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron. 1998;20:1243–1253. doi: 10.1016/s0896-6273(00)80504-8. [DOI] [PubMed] [Google Scholar]
- Smith C, Neher E. Multiple forms of endocytosis in bovine adrenal chromaffin cells. Journal of Cell Biology. 1997;139:885–894. doi: 10.1083/jcb.139.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CB, Betz WJ. Simultaneous independent measurement of endocytosis and exocytosis. Nature. 1996;380:531–534. doi: 10.1038/380531a0. [DOI] [PubMed] [Google Scholar]
- Smith RM, Baibakov B, Ikebuchi Y, White BH, Lambert NA, Kaczmarek LK, Vogel SS. Exocytotic insertion of calcium channels constrains compensatory endocytosis to sites of exocytosis. Journal of Cell Biology. 2000;148:755–767. doi: 10.1083/jcb.148.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. Journal of Cell Biology. 1997;139:63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Lee AK, Wong JG, Almers W. A triggered mechanism retrieves membrane in seconds after Ca2+-stimulated exocytosis in single pituitary cells. Journal of Cell Biology. 1994;124:667–675. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Waring DW. Modulation of stimulus- secretion coupling in single rat gonadotrophs. Journal of Physiology. 1997;504:705–719. doi: 10.1111/j.1469-7793.1997.705bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KM, Burgoyne RD, Morgan A. Protein phosphorylation and the regulation of synaptic membrane traffic. Trends in Neurosciences. 1999;22:459–464. doi: 10.1016/s0166-2236(99)01436-8. [DOI] [PubMed] [Google Scholar]
- Valtorta F, Jahn R, Fesce R, Greengard P, Ceccarelli B. Synaptophysin (p38) at the frog neuromuscular junction: its incorporation into the axolemma and recycling after intense quantal secretion. Journal of Cell Biology. 1988;107:2717–2727. doi: 10.1083/jcb.107.6.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. Journal of Physiology. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightmann RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Dilberto EJJ, Viveros OH. Temorally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proceedings of the National Academy of Sciences of the USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Betz WJ. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 1996;17:769–779. doi: 10.1016/s0896-6273(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Xu T, Binz T, Niemann H, Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nature Neuroscience. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- Xu T, Naraghi M, Kang H, Neher E. Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytosol of adrenal chromaffin cells. Biophysical Journal. 1997;73:532–545. doi: 10.1016/S0006-3495(97)78091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]