The rate of protein digestion affects protein gain differently during aging in humans (original) (raw)
Abstract
In young men ingesting protein meals, slowly digested proteins (caseins: CAS) induce a higher protein gain than those that are rapidly digested (whey proteins: WP). Our aim was to assess whether or not this is true in elderly men receiving mixed meals. The effects of meals containing either CAS or two different amounts of WP (WP-iN: isonitrogenous with CAS, or WP-iL: providing the same amount of leucine as CAS) on protein metabolism (assessed by combining oral and intravenous leucine tracers) were compared in nine healthy, elderly (mean ±s.e.m. age 72 ± 1 years) and six young men (24 ± 1 years). In both age groups, WP-iL and WP-iN were digested faster than CAS (P < 0.001, ANOVA). Proteolysis was inhibited similarly whatever the meal and age groups (P = NS). Protein synthesis was higher with WP-iN than with CAS or WP-iL (P < 0.01), irrespective of age (P = NS). An age-related effect (P < 0.05) was found with postprandial leucine balance. Leucine balance was higher with CAS than with WP-iL (P < 0.01) in young men, but not in elderly subjects (P = NS). In isonitrogenous conditions, leucine balance was higher with WP-iN than with CAS (P < 0.001) in both age groups, but the magnitude of the differences was higher in the elderly men (P = 0.05). In conclusion, during aging, protein gain was greater with WP (rapidly digested protein), and lower with CAS (slowly digested protein). This suggests that a ‘fast’ protein might be more beneficial than a ‘slow’ one to limit protein losses during aging.
The decline of lean body mass is a deleterious effect of aging. It has direct and indirect consequences (e.g. on the reduction of physical performance, loss of autonomy and increased susceptibility to illness; Roubenoff, 2000). An adequate dietary strategy could help to limit these losses.
In the postprandial period, the main regulators of protein metabolism are insulin and amino acid (AA) availability. Insulin acts through inhibition of proteolysis (Boirie et al. 2001) and stimulation of muscle protein synthesis (Biolo et al. 1999). The availability of AAs, which is reflected by plasma aminoacidaemia, is the major regulating factor of protein synthesis and oxidation (Gibson et al. 1996; Volpi et al. 1998). Since AA availability is affected by the protein digestion rate, this might explain the effects of nitrogen sources differing by their kinetics on postprandial protein gain (Boirie et al. 1997_a_; Dangin et al. 2001). Indeed, we have shown recently that in young men, proteins that are digested slowly, such as casein (CAS), induce a lower but more prolonged hyperaminoacidaemia and a higher postprandial leucine deposition than a rapidly digested protein fraction, such as whey protein (WP). This effect was independent of the AA composition of the dietary proteins (Dangin et al. 2001).
However, the relevance of these results for elderly nutrition is unclear. Indeed, in elderly subjects, indirect evidence suggests that the response of protein metabolism to AA availability is disturbed. First, the alteration of muscle protein synthesis in response to feeding, which has been detected both in old rats (Mosoni et al. 1995; Dardevet et al. 2002) and in elderly subjects (Welle et al. 1994), can be reversed by strong hyperaminoacidaemia (Volpi et al. 1998). Second, the specific anabolic response of muscle to leucine, observed in young rats, is blunted in older animals (Dardevet et al. 2000, 2002). Third, in elderly women, a ‘pulse feeding’ pattern (i.e. 80 % of the daily dietary protein consumed at noon) induces a higher nitrogen balance than a ‘spread feeding’ pattern (i.e. a daily protein intake evenly distributed over four meals; Arnal et al. 1999). In contrast, in young women, the spread diet tends to induce a better balance (Arnal et al. 2000). Since the pulse-feeding pattern very probably induces a higher hyperaminoacidaemia than the spread-feeding pattern (Wolever, 1994; Dangin et al. 2001), a different sensitivity of elderly and young subjects to AAs might partly explain these results. Taken together, these data suggest that in elderly subjects, protein synthesis could be resistant to AA availability and that high hyperaminoacidaemia (or leucinaemia) improves postprandial protein gain.
Thus, with respect to the kinetics of digestion of dietary proteins, a ‘fast’ protein might induce higher postprandial protein retention than a ‘slow’ one in elderly subjects by increasing AA availability. To verify this hypothesis, the effect of the protein digestion rate on whole-body postprandial protein metabolism was assessed in healthy elderly men and compared with the responses obtained in young adults. In both age groups, postprandial leucine kinetics were compared after ingestion of a mixed meal containing either CAS or an isonitrogenous amount of WP (WP-iN) or an amount of WP providing the same quantity of leucine (WP-iL) as CAS. This design was selected because both nitrogen and leucine have been shown to modulate protein retention (Pannemans et al. 1998; Dardevet et al. 2002).
METHODS
Materials
l-[1-13C]Leucine (99 mol percent excess, MPE), l-[5,5,5-2H3] leucine (97 MPE) and sodium [13C]bicarbonate (99 MPE) were obtained from Eurisotop (Gif-sur-Yvette, France). The isotopic and chemical purity of leucine was checked by gas chromatography-mass spectrometry (GCMS) and was tested for sterility and pyrogenicity before use.
l-[5,5,5-2H3]Leucine was used to produce intrinsically labelled CAS and WP. Labelled proteins were obtained by infusing a cow with the deuterated tracer, collecting milk and purifying CAS and WP as described previously (Boirie et al. 1995). The leucine enrichments, checked by GCMS after hydrolysis, were 8.28 and 8.16 MPE for CAS and WP, respectively. Labelled protein fractions were mixed with their respective unlabelled fraction in order to obtain 10 μmol (kg body mass)−1 of l-[5,5,5-2H3] leucine. The final enrichments were 3.37 MPE for CAS and WP-iL and 2.27 MPE for WP-iN. The proteins met chemical and bacteriological specifications for human consumption.
Subjects
Nine elderly (mean ±s.e.m. age, 72 ± 1 years; body mass (BM), 73.5 ± 3.1 kg; body mass index (BMI), 25.3 ± 1.0 kg m−2; fat-free mass (FFM), 53.6 ± 1.5 kg; fat mass, 27.2 ± 1.2 % of BM) and six young healthy male volunteers (age, 24 ± 2 years; BM, 66.4 ± 1.6 kg; BMI, 21.1 ± 0.6 kg m−2; FFM, 57.7 ± 1.9 kg; fat mass, 13.4 ± 1.3 % of BM) participated in the study. Subjects had a normal blood biochemical profile and physical condition and had no medical history of renal, cardiovascular, gastrointestinal or endocrine disease. The study was approved by our ethical committee (CCPPRB-Auvergne) and informed written consent was obtained from each participant after an explanation of the purpose, methodology and potential risks of the study. The study was in accordance with the Declaration of Helsinki.
Experimental design
Each subject was studied on three separate occasions differing only by the protein composition of the mixed meal. (1) Meal CAS contained 0.48 g CAS (kg BM)−1 (∼34 g), and provided 296 μmol leucine (kg BM)−1. (2) Meal WP-iL contained 0.31 g WP (kg BM)−1 (∼22 g) and provided identical amounts of leucine as CAS (296 μmol leucine (kg BM)−1). (3) Meal WP-iN was isonitrogenous with CAS (0.48 g WP (kg BM)−1 (∼34 g)) and provided 449 μmol leucine (kg BM)−1. Thus, the amount of protein ingested was lower with WP-iL than with CAS and WP-iN. Conversely, the leucine intake was higher with WP-iN than with CAS and WP-iL. The CAS, WP-iL and WP-iN provided 0.20, 0.12 and 0.19 g (kg BM)−1 of essential AAs, respectively. The volumes ingested (5.6 ml (kg BM)−1) and the composition of the meals were otherwise identical: 0.75 g (kg BM)−1 of carbohydrates (54 % sucrose, 46 % maltodextrins) and 0.13 g (kg BM)−1 of fat (91 % sunflower oil, 9 % monoglycerides).
The meals were administered in a random order. Between two test meals, a wash-out period of at least 3 weeks was observed. During the 4 days preceding a test meal, the volunteers received a balanced diet (∼30 kcal kg−1 day−1; 16 % protein), and maintained their usual physical activity. The evening preceding the test meal, they consumed a standard meal providing 850 kcal and 16 % protein in the laboratory at 19.00 h. Thereafter, no food was allowed. The following day, at 07.30 h, a catheter was inserted retrogradely into a dorsal hand vein and used for arterialized blood sampling after introduction of the hand into a 60 °C heated, ventilated box. A second catheter was inserted into a vein of the contralateral arm for tracer infusion. After a priming dose of [13C]bicarbonate (6 mg), a primed (4.2 μmol (kg BM)−1) continuous intravenous (i.v.) infusion of l-[1-13C]leucine (0.06 μmol (kg BM)−1 min−1) was started and was continued for 590 min (Fig. 1). After 170 min of infusion (t = 0 min), the test meal was ingested within 5 min. Each meal provided 10 μmol (kg BM)−1 of l-[5,5,5-2H3]leucine.
Figure 1. Experimental design.
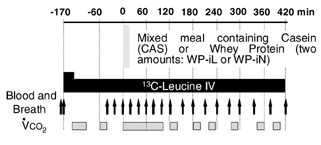
Young (n = 6) and elderly men (n = 9) ingested mixed meals containing different types of protein: casein (CAS: 0.48 g CAS (kg BM)−1 and 296 μmol leucine (kg BM)−1) or two amounts of whey protein (WP): one was isonitrogenous with CAS (WP-iN) and the other provided the same amount of leucine as CAS (WP-iL). CAS and WP were intrinsically labelled with [5,5,5,2H3]leucine. i.v., intravenous.
Blood and breath samples were collected (Fig. 1) before any infusion (−180, −170 min), before the meal when the i.v. tracer had reached an isotopic plateau (−40, −20 and 0 min), and after meal ingestion, at 20 min intervals until 120 min, then at 30 min intervals until 300 min and finally at 40 min intervals until 420 min. After centrifugation, the plasma samples were mixed with an internal standard (Norleucine), and analysed. Breath samples were collected in 10 ml Vacutainers (Becton Dickinson, Grenoble, France) for [13C]CO2 enrichment analysis. Total CO2 production rates (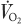 ) were measured by open-circuit indirect calorimetry (Deltatrac, Datex Ohmeda, Lyon, France) at regular intervals (Fig. 1).
) were measured by open-circuit indirect calorimetry (Deltatrac, Datex Ohmeda, Lyon, France) at regular intervals (Fig. 1).
Plasma insulin, glucagon and AA concentrations were measured before (−20 min) and after the meal (+20, +40, +60, +80, +120 and +300 min for hormones and +60, +120 and +240 min for AAs).
Analytical methods
Body composition was assessed by dual-energy X-ray absorptiometry (Hologic QDR-4500A, Waltham, MA, USA). Plasma l-[1-13C]leucine, l-[5,5,5-2H3]leucine and ketoisocaproate (KIC) enrichments (MPE) were measured by GCMS (Hewlett-Packard 5971A) using tertiary-butyldimethylsilyl derivatives, as described previously (Boirie et al. 1996). Corrections for the 13C and 2H3 enrichments were applied according to Biolo et al. (1992). Leucine concentrations were measured by GCMS using norleucine as the internal standard. [13C]CO2 enrichments (Atom Percent Excess) were measured on a gas chromatography isotope ratio mass spectrometer (μGas system, Fisons Instruments, VG Isotech, Middlewich, UK). Plasma insulin and glucagon concentrations were measured by radioimmunoassay (CIS bio international, Gif-sur-Yvette, France). Total and essential plasma AA concentrations were measured by the ninhydrin method combined with ion-exchange chromatography (Biotech-Kontron, Saint-Quentin, France). Meals were analysed for leucine content and enrichment by GCMS, using norleucine as the internal standard and nitrogen content by Kjeldahl analysis.
Calculations
Protein metabolism parameters were estimated in non-steady-state conditions using oral and i.v. administration of leucine tracers. Leucine oxidation was calculated using KIC as the precursor pool because KIC is the immediate precursor of leucine decarboxylation (Matthews et al. 1981). For the other fluxes, calculations were performed using both plasma leucine MPE and KIC MPE as precursors. Although KIC MPE was probably more representative of the intracellular leucine enrichment, we present as the main results, calculations performed with leucine MPE because: (1) the conclusions were identical whatever the precursor pool used, (2) for some authors (Yu et al. 1990; Collin-Vidal et al. 1994), but not all (Matthews et al. 1993), dietary leucine is weakly transaminated on its first pass and thus it may be more appropriate to estimate dietary leucine rate of appearance, (3) it is the most classical approach used both in steady-state and non-steady-state conditions and (4) this allowed easier comparisons with our previous single-meals studies (Boirie et al. 1996, 1997_a_; Dangin et al. 2001) in which the same approach was selected. Results obtained with KIC are shown in the electronic archives.
The total leucine rate of appearance into the circulation (Total Leu Ra) is the sum of: (1) the rate of entry of exogenous (i.e. dietary) leucine (Exo Leu Ra), which is taken as an index of protein digestion rate, (2) the intravenously infused labelled leucine (ir) and (3) the rate of entry of endogenous leucine derived from protein breakdown (Endo Leu Ra). These parameters and splanchnic extraction of leucine (Sp; i.e. the fraction of dietary leucine taken up by the gut and the liver during its first pass) were calculated as follows:
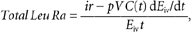 |
(1) |
|---|
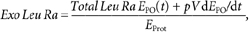 |
(2) |
|---|
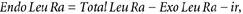 |
(3) |
|---|
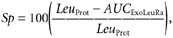 |
(4) |
|---|
where, pV (0.125) is the leucine pool size corrected for instant mixing. This constant is the same as used previously (Tessari et al. 1988; Boirie et al. 1996; Dangin et al. 2001). C(t) represents the mean plasma leucine concentration between two sampling points. d_E_iv/d_t_ corresponds to time-dependent variations of plasma leucine or KIC MPE of the intravenous tracer (13C tracer), and _E_iv(t) is the mean plasma leucine or KIC MPE derived from the intravenous tracer between two consecutive time points. E_PO(t) represents the mean plasma leucine or KIC MPE of the oral tracer between two time points, d_E_PO/d_t is the time-dependent evolution of plasma leucine or KIC MPE of the oral tracer, and _E_Prot corresponds to l-[5,5,5-2H3]leucine enrichment in dietary proteins. Exo Leu Ra is calculated according to Proietto's transposition of Steele's equations (Proietto et al. 1987). _Leu_Prot is the amount of dietary leucine ingested. _AUC_ExoLeuRa represents the area under the curve (AUC) of Exo Leu Ra (calculated by the trapezoidal method). This corresponds to the amount of dietary leucine that appeared in the peripheral blood over 7 h after meal ingestion.
The total leucine rate of disappearance from the plasma (Total Leu Rd) corresponds to the sum of the fluxes of leucine oxidized (Leu Ox) and that utilized for protein synthesis (non-oxidative leucine disposal, NOLD). These parameters are calculated as follows:
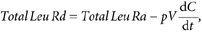 |
(5) |
|---|
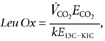 |
(6) |
|---|
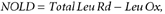 |
(7) |
|---|
where, d_C_/d_t_ corresponds to the time-dependent variation of plasma leucine concentration. _E_CO2 and _E_13C-KIC correspond to [13C]CO2 and l-[1-13C]KIC MPE, respectively. k is a correcting factor for the incomplete recovery of CO2 in the breath (0.8), as described previously (Boirie et al. 1997_a_; Raguso et al. 1999; Dangin et al. 2001).
Postprandial leucine balance was calculated over a 420 min period as follows:
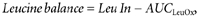 |
(8) |
|---|
where Leu In and _AUC_LeuOx correspond to the leucine intake (ingested + infused) and the amount of leucine oxidized over 7 h, respectively. The efficiency of postprandial protein utilization (PPUN) was estimated according to Millward et al. (2000, 2002). PPUN was the ratio between a predicted nitrogen balance, based on the difference between postprandial and postabsorptive leucine balance, assuming a constant body protein leucine content of 625 mg (mg N)−1 and nitrogen intake (mg N).
Each individual curve was characterized by its zenith or nadir (_Y_max), by its AUC and by the time at which half the amount of AUC was obtained (_t_1/2).
Statistical analysis
Results are expressed as means ±s.e.m. Statistical analyses (Statview, 5.0, Abacus Concepts, Berkeley, CA, USA) were performed in order to: (1) characterize the changes induced by meal ingestion (i.e. modifications from the postabsorptive state after every test meal); (2) assess the effect of the type of meal; and (3) determine the effect of aging.
Changes induced by meal ingestion
Individual curves were standardized by subtracting the average of the baseline values from each individual time point. A confidence interval (α= 0.05) was calculated from the standard deviation of the n standardized baseline individual values within each experiment, and from the critical value of Student's distribution for (n - 1) degrees of freedom. The changes were declared significantly different from the baseline when all of the individual values fell outside this interval.
Effect of the type of meal within each age-group
_Y_max, AUC and _t_1/2 were compared by repeated-measures ANOVA. When an effect of the type of meal was detected (P < 0.05), the Least Significant Difference method was used to define which meal was different from the others.
Effect of age
The difference between CAS and WP-iL (CAS vs. WP-iL, identical amount of leucine) and between CAS and WP-iN (CAS vs. WP-IN, isonitrogenous meals) were first calculated. The effect of age on these differences was then assessed using ANOVA.
RESULTS
Tracer enrichments
Figures and details concerning leucine, KIC and CO2 enrichments are provided as Supplementary material with the online version of this paper, and can be found at:http://www.jphysiol.org/cgi/content/full/549/2/635.
Plasma concentrations of hormones and amino acids
Glucagon and insulin increased after meals ingestion (Fig. 2_A_ and B). For glucagon, the increase and the shape of the curves were similar among meals and age groups (Fig. 2_A_). In contrast, for insulin (Fig. 2_B_), age affected the differences between WP meals and CAS. In young subjects, insulin concentrations were higher (P < 0.01) 20 and 40 min after WP-iL and WP-iN than after CAS. This was not the case in elderly men, for whom the shape of the curve was similar among meals.
Figure 2. Effect of ingestion of CAS, WP-iL and WP-iN on plasma concentrations of glucagon and insulin.
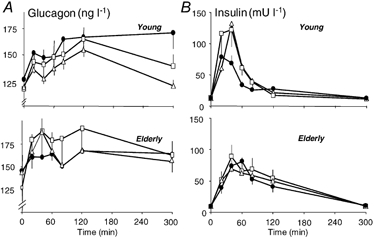
Plasma glucagon (A) and insulin concentrations (B) after ingestion of CAS (•), WP-iL (△) and WP-iN (□) by young (n = 6, upper graphs) or elderly men (n = 9, lower graphs). Values are means ±s.e.m.
Before meal ingestion, leucine, essential and total AA concentrations were similar between groups (Table 1, Fig. 3_A, B_ and C). In the postprandial period, these parameters increased differently with the different meals (P < 0.05). WP-iL induced a higher leucine _Y_max (P < 0.001), a shorter _t_1/2 (P < 0.001) and a faster return to baseline of leucine concentration than CAS (Fig. 3_A_, Table 1) despite the identical leucine content of these meals. Also, and as expected, leucine concentrations were higher after WP-iN than after CAS, since WP contained more leucine than CAS (Fig. 3_A_, Table 1).
Table 1.
Baseline and kinetics of leucine concentrations and fluxes after meal ingestion
| Young (n =6) | Elderly (n =9) | |||||||
|---|---|---|---|---|---|---|---|---|
| WP-iN | CAS | WP-iL | WP-iN | CAS | WP-iL | Age-effect Δ WP vs. CAS | ||
| [Leu] | Basal | 140 ± 7 | 129 ± 4 | 140 ± 7 | 134 ± 8 | 146 ± 8 | 138 ± 8 | |
| _Y_max | 380 ± 14* | 226 ± 8 | 314 ± 10* | 401 ± 20* | 270 ± 18 | 336 ± 24* | ||
| _t_½ | 153 ± 3* | 217 ± 3 | 161 ± 3* | 160 ± 5* | 207 ± 6 | 163 ± 5* | ||
| Exo Leu Ra | _Y_max | 2.11 ± 0.19* | 0.92 ± 0.07 | 1.78 ± 0.13* | 2.01 ± 0.14* | 1.15 ± 0.10 | 1.37 ± 0.07 | † |
| _t_½ | 115 ± 5* | 187 ± 7 | 92 ± 4* | 131 ± 7* | 159 ± 12 | 123 ± 10* | † | |
| AUC | 336 ± 23* | 206 ± 10 | 211 ± 4 | 355 ± 18* | 218 ± 4 | 219 ± 6 | ||
| Endo Leu Ra | Basal | 1.67 ± 0.08 | 1.72 ± 0.07 | 1.63 ± 0.06 | 1.65 ± 0.09 | 1.61 ± 0.09 | 1.62 ± 0.11 | |
| _Y_max | 1.11 ± 0.05 | 1.23 ± 0.07 | 1.15 ± 0.04 | 1.05 ± 0.07 | 1.05 ± 0.09 | 1.12 ± 0.09 | ||
| _t_½ | 221 ± 6 | 213 ± 3 | 216 ± 4 | 211 ± 6 | 219 ± 3 | 216 ± 2 | ||
| AUC | 599 ± 19 | 609 ± 33 | 600 ± 27 | 561 ± 36 | 557 ± 28 | 568 ± 36 | ||
| NOLD | Basal | 1.33 ± 0.06 | 1.37 ± 0.05 | 1.27 ± 0.02 | 1.35 ± 0.09 | 1.27 ± 0.08 | 1.22 ± 0.08 | |
| _Y_max | 2.46 ± 0.27* | 1.86 ± 0.16 | 2.01 ± 0.06 | 2.69 ± 0.39* | 1.76 ± 0.11 | 1.87 ± 0.10 | ||
| _t_½ | 179 ± 7* | 210 ± 2 | 184 ± 4* | 168 ± 4* | 199 ± 5 | 182 ± 6* | † | |
| AUC | 616 ± 51 | 579 ± 35 | 535 ± 20 | 621 ± 56* | 521 ± 24 | 522 ± 22 | ||
| Leu Ox | Basal | 0.39 ± 0.02 | 0.39 ± 0.03 | 0.41 ± 0.03 | 0.35 ± 0.02 | 0.40 ± 0.02 | 0.37 ± 0.03 | |
| _Y_max | 1.88 ± 0.10* | 0.89 ± 0.05 | 1.50 ± 0.11* | 1.73 ± 0.11* | 1.18 ± 0.12 | 1.50 ± 0.19* | † | |
| _t_½ | 160 ± 4* | 195 ± 7 | 147 ± 2* | 172 ± 7* | 188 ± 7 | 168 ± 9* | † | |
| AUC | 440 ± 19* | 289 ± 15 | 340 ± 12* | 424 ± 18* | 347 ± 15 | 361 ± 17 | † |
Figure 3. Effect of ingestion of CAS, WP-iL and WP-iN on plasma concentrations of leucine, amino acids (AAs) and the rate of appearance of exogenous leucine.
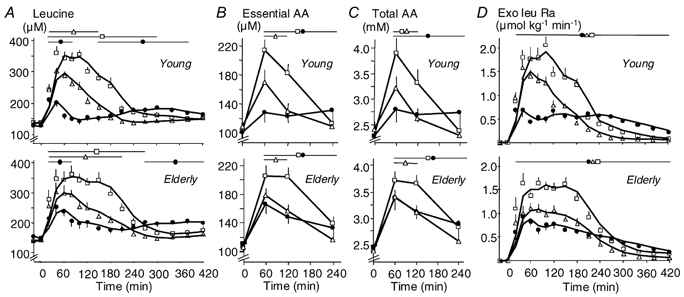
Plasma concentrations of leucine (A), the sum of essential (B) and total AAs (C) and the rate of appearance of exogenous leucine (Exo Leu Ra, D) after ingestion of CAS (•), WP-iL (△) and WP-iN (□) by young (n = 6, upper graphs) or elderly men (n = 9, lower graphs). Values are means ±s.e.m. The lines at the top of the graphs indicate time-points where values are different (P < 0.05) from baseline within each study.
Essential and total AA concentrations were higher (P < 0.01) 60 min after WP-iN than after CAS, despite similar AA intakes (Fig. 3_B_ and C). Then, from 120 to 240 min after the meal, AA concentrations decreased more rapidly after WP-iN than after CAS (Fig. 3_B_ and C). In young men, essential AA concentrations were higher 60 min after WP-iL than after CAS, despite a lower AA intake (Fig. 3_B_). No significant effect of age was detected, but the shapes of the curves were slightly different (Fig. 3_A_, B and C). The slope of the decrease seemed to be more rapid with CAS and slower with WP-iL and WP-iN in elderly men than in young men.
Protein digestion rate
Leucine derived from the meals appeared rapidly in the plasma, with differences related to the type of meal ingested (Fig. 3_D_, Table 1). With identical leucine intakes, WP-iL had faster absorption than CAS in both age groups (_t_1/2: P < 0.01) and a higher peak leucine appearance (P < 0.001 in the young; P = NS in the elderly). With WP-iN (higher leucine intake), the _Y_max (P < 0.001) and _t_1/2 (P < 0.05) of the exogenous leucine rate of appearance were again higher and shorter, respectively, than with CAS (Table 1). There was an effect of age, since the differences between the WP meals and CAS (WP-iL - CAS and WP-iN - CAS) were smaller in the old group (_Y_max: P < 0.05; t_1/2: P < 0.01) than in the young one. That effect of age was apparently related to both a slower rate of digestion of WP and a faster digestion rate of CAS in the elderly population. As expected, the total dietary leucine appearing in the peripheral circulation over 7 h (AUC, Table 1) was higher with WP-iN than with the two other meals. When normalized for leucine intake, there was no effect of age or the meal. Indeed, no difference was found on the percentage of ingested leucine taken up by the splanchnic area during its first pass (Young: WP-iN, 31 ± 4 %; WP-iL, 34 ± 1 %; CAS, 36 ± 3 %; Elderly: WP-iN, 25 ± 4 %; WP-iL, 31 ± 2 %; CAS, 32 ± 1 %). The conclusions drawn were similar when the rate of appearance of exogenous leucine was calculated using KIC MPE (Supplementary material, Fig. 8_A and Table 2).
Whole-body protein metabolism
In both the postabsorptive and postprandial states, Endo Leu Ra, an index of whole-body proteolysis, was similar whatever the meal and age group (Table 1, Fig. 4_A_, P = NS). All the meals induced a persistent inhibition of Endo Leu Ra, with a maximal decrease at 30–40 % below baseline. Again, similar results were observed when calculations were performed with KIC MPE (Supplementary material, Fig. 8_B_ and Table 2).
Figure 4. Effect of ingestion of CAS, WP-iL and WP-iN on the rate of appearance of endogenous leucine, non-oxidative leucine disposal (NOLD) and leucine oxidation.
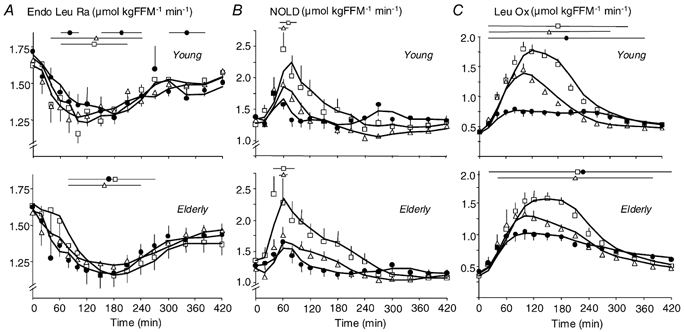
A, rate of appearance of endogenous leucine (Endo Leu Ra; i.e. proteolysis). B, NOLD (i.e. protein synthesis) and C, leucine oxidation (Leu Ox) after ingestion of CAS (•), WP-iL (△) and WP-iN (□) by young (n = 6, upper graphs) or elderly men (n = 9, lower graphs). Results are means ±s.e.m. FFM: fat free mass (kg). The lines at the top of the graphs indicate time-points where values are different from baseline within each study (P < 0.05).
In the postabsorptive period, NOLD, an index of whole-body protein synthesis, was similar in all groups (Table 1). However, in the postprandial period, the type of meal affected NOLD (_Y_max and _t_1/2, P < 0.01): WP-iN induced a higher _Y_max and a shorter _t_1/2 than CAS (P < 0.01). Although WP-iL induced shorter _t_1/2 than CAS (P < 0.01), _Y_max values were not different (P = NS, Fig. 4_B_ and Table 1). There was no effect of age on _Y_max and AUC (P = NS), but t_1/2 values were shorter in elderly men than in young men (P < 0.01). Similar effects were detected when NOLD was calculated using KIC MPE (Supplementary material, Fig. 8_C and Table 2).
Total leucine oxidation responded differently to meals (P < 0.01, Table 1, Fig. 4_C_). WP-iL and WP-iN induced a higher _Y_max (P < 0.05), a shorter _t_1/2 (P < 0.01) and a greater AUC than CAS (P < 0.05, except WP-iL vs. CAS in the older group, P = NS). Age affected the differences between WP meals and CAS on _Y_max, _t_1/2 and AUC (Table 1). The differences of AUC between WP-iN and CAS (Elderly: 77 ± 22 μmol (kg FFM)−1; Young: 151 ± 26 μmol (kg FFM)−1) and between WP-iL and CAS (Elderly: 14 ± 17 μmol (kg FFM)−1; Young: 50 ± 12 μmol (kg FFM)−1) were smaller in elderly subjects than in young men (P < 0.05).
Postprandial leucine balance was also modulated by the type of meal (P < 0.001). In addition, age (P < 0.05) affected the differences between WP meals and CAS (Fig. 5). Indeed, when considering the meals that provided identical amounts of leucine (WP-iL vs. CAS, Fig. 5_A_), CAS (88 ± 15 μmol (kg FFM)−1) induced a higher leucine balance than WP-iL (39 ± 9 μmol (kg FFM)−1, P < 0.01) in young adults (n = 6). In contrast in the older group (n = 9), CAS (70 ± 17 μmol (kg FFM)−1) was not more efficient than WP-iL (53 ± 18 μmol (kg FFM)−1, P = NS).
Figure 5. Effect of ingestion of CAS and WP on leucine balance in elderly and younger men.
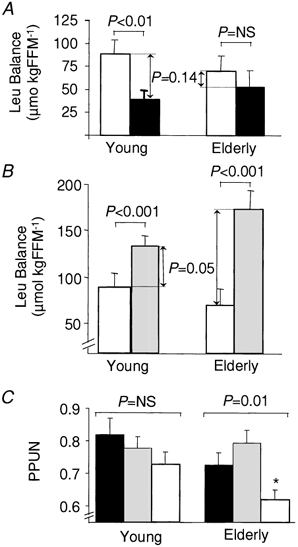
Leucine (Leu) balance over 420 min after ingestion of CAS (white bars) and of WP providing either the same amount of leucine as CAS (black bars: WP-iL; A) or the same quantity of protein as CAS (grey bars: WP-iN; B), and efficiency of posptrandial protein utilization (PPUN; C). Meals were ingested by young (n = 6; left) and elderly men (n = 9; right). FFM, fat-free mass (kg). The results are presented as means ±s.e.m. The lines at the top of the graphs indicate differences between CAS and WP within the age group. *Significant differences between age groups within each meal (P < 0.05).
When considering the isonitrogenous condition (WP-iN vs. CAS, Fig. 5_B_), postprandial leucine balance was higher (P < 0.001) with WP-iN (Young: 133 ± 11 μmol (kg FFM)−1, n = 6; Elderly: 174 ± 20 μmol (kg FFM)−1, n = 9) than with CAS (see values above), in both populations. However, the magnitude of the difference between WP and CAS was smaller for the young (+44 ± 9 μmol (kg FFM)−1) than for the elderly subjects (+104 ± 19 μmol (kg FFM)−1, P = 0.05).
Finally, when the results are expressed as PPUN, no significant difference was detected between meals in the younger group (n = 6, P = NS), while in the older group WP meals were more efficient than CAS (n = 9, P = 0.01). Age did not affect PPUN of the WP meals (no age effect), while in elderly men, CAS was less efficient than in the young (CAS: Young vs. Elderly, P < 0.05).
Discussion
The present study was designed to assess the impact of dietary protein digestion rate on protein metabolism in elderly subjects. For this purpose, we compared the effects induced by a mixed liquid meal containing either CAS or WP, taken as protocols for ‘slow’ and ‘fast’ digested proteins, respectively (Mahé_et al._ 1991; Boirie et al. 1997_a_; Dangin et al. 2001). We show that the effects of protein digestion rate on postprandial protein gain change with age. Indeed, in elderly subjects, in contrast to young adults, a fast protein induced a higher postprandial leucine balance than a slow protein.
The methodological approach was similar to that reported previously (Boirie et al. 1997_a_), but with adaptations. In our former studies, each volunteer had to be studied on two separate occasions to test the effect of a given meal. On one occasion, l-[1-13C]leucine-labelled protein was given orally, while on another occasion, that tracer was infused intravenously to assess whole-body leucine oxidation. In the present study, intrinsically l-[5,5,5-2H3]leucine-bound proteins were produced and administered with the meal, and l-[1-13C]leucine was infused. Using this approach it is possible to assess whole-body protein metabolism parameters and the metabolic fate of dietary proteins in a single experiment, thereby reducing the number of studies carried out on an individual volunteer and producing more homogeneous results.
A major change from our previous studies was the addition of carbohydrate and fat to the meal (Boirie et al. 1997_a_; Dangin et al. 2001). Non-protein energy sources (fat or carbohydrates) are known to decrease the rate of gastric emptying (Jian et al. 1986; Calbet & MacLean, 1997) and might have suppressed the differences in digestion rate between CAS and WP. This was not the case: WP was still more rapidly absorbed and induced higher and more transient hyperaminoacidaemia than CAS. However, in young subjects the differences between these two proteins were less marked than when the protein was given alone (Boirie et al. 1997_a_; Dangin et al. 2001). This was mostly due to a slower WP digestion rate in the presence of carbohydrate or fat (Jian et al. 1986; Calbet & MacLean, 1997). The differences between WP and CAS were attenuated by age: the rate of digestion of WP tended to be slower and that of CAS faster in elderly than in young subjects. The slowing down of the WP digestion rate may be explained by an age-related decrease of the gastric emptying rate (Clarkston et al. 1997; Cook et al. 1997). The acceleration of the CAS digestion rate may be due to an age-related reduction in gastric acid secretions (Korkushko et al. 1992; Morihara et al. 2001); this decrease might alter the clotting of CAS, maintaining it in a liquid form, which is emptied more rapidly than in the solid form (Achour et al. 2001).
In elderly subjects, leucine splanchnic extraction was not higher than in the young subjects, and was much lower than reported previously in steady-state conditions in a similar population (Boirie et al. 1997_b_). It is unlikely that this discrepancy is due to potential errors in the calculations and/or to the use of a non-steady-state approach. The calculations depend on the variations in size of the truly active metabolic pool of leucine (i.e. pV), which may be not accurate and may fluctuate over the time course of the study. However, as discussed by Boirie et al. (1996), variations of pV over a wide range do not affect the amount of dietary leucine appearing in the peripheral blood. In addition, splanchnic extractions of our previous studies with slowly digested meals (Boirie et al. 1997_a_; Dangin et al. 2001) as well as the present results obtained in young men are consistent with those observed in a similar population in a steady-state condition (Matthews et al. 1993; Boirie et al. 1997_b_). This discrepancy is more likely to be due to differences in the BMI of elderly subjects between the two studies. Indeed, because it has been reported that high splanchnic extractions are found only in elderly subjects with a large BMI (Boirie et al. 1997_b_), it may be possible that the lower BMI of our older group would result in lower leucine splanchnic extractions.
We confirm a nitrogen-sparing effect of energy (Pellet & Young, 1992), since postprandial leucine balance was higher with the mixed meals (this study) than with proteins alone (Boirie et al. 1997_a_; Dangin et al. 2001). However, the apparent AA-sparing effect of energy was more pronounced with WP (results of WP alone were derived from Dangin et al. (2001) and are expressed in μmol kg−1: 6 ± 19; WP + energy (this study): 115 ± 10) than with CAS (CAS alone: 38 ± 19; CAS + energy: 76 ± 13). The more beneficial effect of CAS was thus reversed with energy. This effect was mediated by a better inhibition of proteolysis (probably due to carbohydrate-induced hyperinsulinaemia) rather than by a greater stimulation of protein synthesis (Boirie et al. 2001). Indeed, energy added to WP did not affect the protein synthesis, but induced a strong and persistent inhibition of proteolysis that was not present with WP alone (Boirie et al. 1997_a_). By contrast, energy added to CAS had lower impact on proteolysis because CAS alone had already decreased that parameter (Boirie et al. 1997_a_) and, as previously, did not modify protein synthesis.
The most important result was that postprandial protein gain induced by CAS and WP, differed with age. In young men, and in agreement with our previous results (Boirie et al. 1997_a_), CAS induced a higher postprandial leucine balance than WP, with meals providing identical amount of leucine (CAS vs. WP-iL, Fig. 4_A_). This was not the case in the elderly subjects since CAS and WP-iL resulted in similar balances. When considering isonitrogenous meals (Fig. 4_B_), leucine balance was higher with WP-iN than with CAS in both age groups. This was foreseeable because WP-iN provided more leucine than CAS, a factor known to improve leucine balance (Dardevet et al. 2002). The key point is that an effect of age was again detected; the magnitude of the difference between WP-iN and CAS was 58 % larger in elderly than in young subjects. This age-related effect was also confirmed when expressing the data as PPUN, as proposed by Millward et al. (2002). This mode of expression compensates for the difference in AA composition between proteins. In young men, PPUN was similar with every meal, while in elderly subjects, PPUN was higher with WP-iL and WP-iN than with CAS. In fact, this difference was due to a decreased efficiency of CAS with age, rather than to an increased efficiency of WP. Therefore, whatever the pair of studies examined and the way of expression, there was an age-related effect of the rate of digestion on postprandial protein gain. WP, a fast protein, seemed to be more efficient than CAS, a slow one, in elderly subjects, at least in the short term.
The modifications responsible for a lower efficiency of CAS and/or a greater utilization of WP during aging are unclear. In the absence of clear-cut differences in the rates of whole-body synthesis or breakdown, it is difficult to ascribe the final differences of leucine balance to an alteration of one of these two parameters. It is tempting to incriminate protein synthesis in particular at the muscle level (the main tissue affected by aging). Indeed, it has been shown that impairment of protein synthesis of old muscle (Welle et al. 1994; Mosoni et al. 1995) after meal ingestion could be normalized by high levels of AA (Mosoni et al. 1993; Volpi et al. 1998). This suggests that AA availability becomes critical in elderly subjects. If so, it is possible that the slow rate of delivery of AA from CAS may be rate limiting for muscle protein synthesis with aging. This hypothesis was also proposed to explain the specific age-related effect of the pulse diet pattern (Arnal et al. 1999).
Another possibility is a resistance of old muscle to leucine-induced stimulation of mRNA translation initiation (Kimball & Jefferson, 2002). Indeed, stimulation of protein synthesis by a meal was blunted in muscle from old rats, but a normal response could be obtained with leucine supplementation that provoked a higher increment in leucine availability (Dardevet et al. 2002). In contrast, in young rats, stimulation of muscle protein synthesis was submaximal after meal ingestion, and leucine supplementation did not have additive effects. Therefore, in elderly subjects, high leucine concentrations, such as those induced by WP meals, might possibly be required to overcome a ‘leucine resistance’ that could exist with CAS. However, we failed to observe an age-specific impairment of protein synthesis in response to CAS (i.e. to low variations in AA and/or leucine concentrations). This is not surprising since protein synthesis was measured at the whole-body level and since muscle proteins only represent approximately 27 % of whole-body protein synthesis (Nair et al. 1988).
In conclusion, we have demonstrated that whey protein and casein affect protein gain differently during aging. In elderly men, when consuming mixed meals, there was a greater utilization of WP, a rapidly digested protein, than CAS, a slowly digested protein, compared with the younger men. Variation of AA and/or leucine availability is probably responsible for this difference. Therefore, a fast protein might be more beneficial than a slow protein to improve postprandial protein gain and consequently to limit the body protein losses of elderly subjects. Studies examining the long-term effects of protein digestion rate on nitrogen balance are needed.
Acknowledgments
We wish to thank P. Rousset, D. Troulier, C. Giraudet and L. Morin for their technical and nursing assistance, S. Corny for tracer preparations, C. Hager, M. Brandolini, Y. Boirie and K. Acheson for their valuable assistance, and H. Derumeaux for medical examination of the volunteers. This study was supported by Nestlé Research Centre, INRA, Région Auvergne, and by the French Ministry of Research.
Supplementary material
The online version of this paper can be found at:http://www.jphysiol.org/cgi/content/full/549/2/635DOI: 10.1113/jphysiol.2002.036897 and contains material entitled:Postprandial protein metabolism during aging: raw data and calculations using KIC as a precursor
Supplementary material
References
- Achour L, Meance S, Briend A. Comparison of gastric emptying of a solid and a liquid nutritional rehabilitation food. Eur J Clin Nutr. 2001;55:769–772. doi: 10.1038/sj.ejcn.1601221. [DOI] [PubMed] [Google Scholar]
- Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrère B, Mirand PP. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69:1202–1208. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrère B, Mirand PP. Protein feeding pattern does not affect protein retention in young women. J Nutr. 2000;130:1700–1704. doi: 10.1093/jn/130.7.1700. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tessari P, Inchiostro S, Bruttomesso D, Fongher C, Sabadin L, Fratton MG, Valerio A, Tiengo A. Leucine and phenylalanine kinetics during mixed meal ingestion: a multiple tracer approach. Am J Physiol. 1992;262:E455–463. doi: 10.1152/ajpendo.1992.262.4.E455. [DOI] [PubMed] [Google Scholar]
- Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997a;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie Y, Fauquant J, Rulquin H, Maubois JL, Beaufrère B. Production of large amounts of [13C]leucine-enriched milk proteins by lactating cows. J Nutr. 1995;125:92–98. doi: 10.1093/jn/125.1.92. [DOI] [PubMed] [Google Scholar]
- Boirie Y, Gachon P, Beaufrère B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997b;65:489–495. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- Boirie Y, Gachon P, Cordat N, Ritz P, Beaufrère B. Differential insulin sensitivities of glucose, amino acid, and albumin metabolism in elderly men and women. J Clin Endocrinol Metab. 2001;86:638–644. doi: 10.1210/jcem.86.2.7193. [DOI] [PubMed] [Google Scholar]
- Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrère B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol. 1996;271:E1083–1091. doi: 10.1152/ajpendo.1996.271.6.E1083. [DOI] [PubMed] [Google Scholar]
- Calbet JA, MacLean DA. Role of caloric content on gastric emptying in humans. J Physiol. 1997;498:553–559. doi: 10.1113/jphysiol.1997.sp021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkston WK, Pantano MM, Morley JE, Horowitz M, Littlefield JM, Burton FR. Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults. Am J Physiol. 1997;272:R243–248. doi: 10.1152/ajpregu.1997.272.1.R243. [DOI] [PubMed] [Google Scholar]
- Collin-Vidal C, Cayol M, Obled C, Ziegler F, Bommelaer G, Beaufrère B. Leucine kinetics are different during feeding with whole protein or oligopeptides. Am J Physiol. 1994;267:E907–914. doi: 10.1152/ajpendo.1994.267.6.E907. [DOI] [PubMed] [Google Scholar]
- Cook CG, Andrews JM, Jones KL, Wittert GA, Chapman IM, Morley JE, Horowitz M. Effects of small intestinal nutrient infusion on appetite and pyloric motility are modified by age. Am J Physiol. 1997;273:R755–761. doi: 10.1152/ajpregu.1997.273.2.R755. [DOI] [PubMed] [Google Scholar]
- Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballèvre O, Beaufrère B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340–348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- Gibson NR, Fereday A, Cox M, Halliday D, Pacy PJ, Millward DJ. Influences of dietary energy and protein on leucine kinetics during feeding in healthy adults. Am J Physiol. 1996;270:E282–291. doi: 10.1152/ajpendo.1996.270.2.E282. [DOI] [PubMed] [Google Scholar]
- Jian R, Ruskone A, Filali A, Ducrot F, Rain JD, Bernier JJ. Effect of the increase of the caloric load of a meal on gastric emptying of its solid and liquid phases. Gastroenterol Clin Biol. 1986;10:831–836. [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Control of protein synthesis by amino acid availability. Curr Opin Clin Nutr Metab Care. 2002;5:63–67. doi: 10.1097/00075197-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Korkushko OB, Hrihorov IUH, Dzizins'ka OO. Aging-related characteristics of the secretory response of the stomach to stimulation of its mechanoreceptors. Fiziol Zh. 1992;38:53–59. [PubMed] [Google Scholar]
- Mahé S, Messing B, Thuillier F, Tomé D. Digestion of bovine milk proteins in patients with a high jejunostomy. Am J Clin Nutr. 1991;54:534–538. doi: 10.1093/ajcn/54.3.534. [DOI] [PubMed] [Google Scholar]
- Matthews DE, Bier DM, Rennie MJ, Edwards RH, Halliday D, Millward DJ, Clugston GA. Regulation of leucine metabolism in man: a stable isotope study. Science. 1981;214:1129–1131. doi: 10.1126/science.7302583. [DOI] [PubMed] [Google Scholar]
- Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol. 1993;264:E109–118. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- Millward DJ, Fereday A, Gibson NR, Cox MC, Pacy PJ. Efficiency of utilization of wheat and milk protein in healthy adults and apparent lysine requirements determined by a single-meal [1–13C]leucine balance protocol. Am J Clin Nutr. 2002;76:1326–1334. doi: 10.1093/ajcn/76.6.1326. [DOI] [PubMed] [Google Scholar]
- Millward DJ, Fereday A, Gibson NR, Pacy PJ. Human adult amino acid requirements: [113C]leucine balance evaluation of the efficiency of utilization and apparent requirements for wheat protein and lysine compared with those for milk protein in healthy adults. Am J Clin Nutr. 2000;72:112–121. doi: 10.1093/ajcn/72.1.112. [DOI] [PubMed] [Google Scholar]
- Morihara M, Aoyagi N, Kaniwa N, Kojima S, Ogata H. Assessment of gastric acidity of Japanese subjects over the last 15 years. Biol Pharm Bull. 2001;24:313–315. doi: 10.1248/bpb.24.313. [DOI] [PubMed] [Google Scholar]
- Mosoni L, Houlier ML, Mirand PP, Bayle G, Grizard J. Effect of amino acids alone or with insulin on muscle and liver protein synthesis in adult and old rats. Am J Physiol. 1993;264:E614–620. doi: 10.1152/ajpendo.1993.264.4.E614. [DOI] [PubMed] [Google Scholar]
- Mosoni L, Valluy MC, Serrurier B, Prugnaud J, Obled C, Guezennec CY, Mirand PP. Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol. 1995;268:E328–335. doi: 10.1152/ajpendo.1995.268.2.E328. [DOI] [PubMed] [Google Scholar]
- Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol. 1988;254:E208–213. doi: 10.1152/ajpendo.1988.254.2.E208. [DOI] [PubMed] [Google Scholar]
- Pannemans DL, Wagenmakers AJ, Westerterp KR, Schaafsma G, Halliday D. Effect of protein source and quantity on protein metabolism in elderly women. Am J Clin Nutr. 1998;68:1228–1235. doi: 10.1093/ajcn/68.6.1228. [DOI] [PubMed] [Google Scholar]
- Pellet PL, Young VR. The effects of different levels of energy intake on protein metabolism and of different levels of protein intake on energy metabolism: a statistical evaluation from the published literature. In: Scrimshaw N, Schürch B, editors. Protein and Energy Interactions. Waterville Valley, USA: I.D.E.C.G.; 1992. pp. 81–121. [Google Scholar]
- Proietto J, Rohner-Jeanrenaud F, Ionescu E, Terrettaz J, Sauter JF, Jeanrenaud B. Non-steady-state measurement of glucose turnover in rats by using a one-compartment model. Am J Physiol. 1987;252:E77–84. doi: 10.1152/ajpendo.1987.252.1.E77. [DOI] [PubMed] [Google Scholar]
- Raguso CA, El-Khoury AE, Young VR. Leucine kinetics in reference to the effect of the feeding mode as three discrete meals. Metabolism. 1999;48:1378–1386. doi: 10.1016/s0026-0495(99)90147-6. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54:S40–47. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- Tessari P, Pehling G, Nissen SL, Gerich JE, Service FJ, Rizza RA, Haymond MW. Regulation of whole-body leucine metabolism with insulin during mixed-meal absorption in normal and diabetic humans. Diabetes. 1988;37:512–519. doi: 10.2337/diab.37.5.512. [DOI] [PubMed] [Google Scholar]
- Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M, McHenry B. Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. Am J Physiol. 1994;267:E599–604. doi: 10.1152/ajpendo.1994.267.4.E599. [DOI] [PubMed] [Google Scholar]
- Wolever TM. Effect of meal frequency on serum amino acids and creatinine clearance in young men. Am J Med Sci. 1994;307:97–101. doi: 10.1097/00000441-199402000-00005. [DOI] [PubMed] [Google Scholar]
- Yu YM, Wagner DA, Tredget EE, Walaszewski JA, Burke JF, Young VR. Quantitative role of splanchnic region in leucine metabolism: L-[1–13C, 15N]leucine and substrate balance studies. Am J Physiol. 1990;259:E36–51. doi: 10.1152/ajpendo.1990.259.1.E36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be found at:http://www.jphysiol.org/cgi/content/full/549/2/635DOI: 10.1113/jphysiol.2002.036897 and contains material entitled:Postprandial protein metabolism during aging: raw data and calculations using KIC as a precursor
Supplementary material