Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice (original) (raw)
Abstract
CD20 antibody depletion of B lymphocytes effectively ameliorates multiple T cell-mediated autoimmune diseases through mechanisms that remain unclear. To address this, a mouse CD20 antibody that depletes >95% of mature B cells in mice with otherwise intact immune systems was used to assess the role of B cells in CD4+ and CD8+ T cell activation and expansion in vivo. B cell depletion had no direct effect on T cell subsets or the activation status of CD4+ and CD8+ T cells in naive mice. However, B cell depletion impaired CD4+ T cell activation and clonal expansion in response to protein antigens and pathogen challenge, whereas CD8+ T cell activation was not affected. In vivo dendritic cell ablation, along with CD20 immunotherapy, revealed that optimal antigen-specific CD4+ T cell priming required both B cells and dendritic cells. Most importantly, B cell depletion inhibited antigen-specific CD4+ T cell expansion in both collagen-induced arthritis and autoimmune diabetes mouse models. These results provide direct evidence that B cells contribute to T cell activation and expansion in vivo and offer insights into the mechanism of action for B cell depletion therapy in the treatment of autoimmunity.
Keywords: autoimmune disease, B lymphocyte, immunotherapy, antigen presentation
Central defects in T lymphocyte regulation contribute to most autoimmune diseases (1). Surprisingly however, mAb therapy directed against the CD20 cell surface molecule of human B cells (rituximab) has shown varying degrees of efficacy in rheumatoid arthritis, idiopathic thrombocytopenic purpura, hemolytic anemia, lupus erythematosus, and pemphigus vulgaris treatment (2, 3). Despite the benefits of this immunotherapy, the cellular and molecular basis for the protective effect mediated by B cell depletion is unknown (4). Understanding the mechanisms underlying the therapeutic benefit of B cell depletion is complicated by the fact that B cells not only produce autoantibodies (5) but also release inflammatory or immunomodulatory cytokines (6), regulate lymphoid tissue neogenesis and structure, provide costimulatory signals, alter dendritic cell (DC) function and homeostasis (7), can function as antigen-presenting cells, promote naïve CD4+ T cell differentiation into Th1 or Th2 subsets, and may influence regulatory T cell numbers and function (8).
Examining the effects of B cell depletion in mice with intact immune systems is possible by using mouse anti-mouse CD20 mAbs in which B cells are depleted in vivo by monocyte-mediated antibody-dependent cellular cytotoxicity (9, 10). More than 95% of mature B cells in blood and primary lymphoid organs are depleted after 2 days by a single dose of CD20 mAb (MB20-11, 250 μg per mouse), with the effect lasting 8 weeks (11). B cell depletion prevents autoimmunity in mouse models of collagen-induced arthritis (CIA) (12), scleroderma (13), Sjogren's syndrome (14), and diabetes in nonobese diabetic (NOD) mice (Y. Xiu, C. P. Wong, J.-D.B., Y. Hamaguchi, Y.W., S. M. Pop, R.M.T., and T.F.T., unpublished work). In these models, B cell depletion after disease onset is less beneficial, indicating a possible role for B cells in the early stages of autoimmunity during autoreactive T cell activation and/or expansion. The effect of B cell depletion on T cell activation in vivo was therefore assessed to identify mechanisms by which B cell depletion ameliorates T cell-dependent autoimmune disease.
Results
CD20 Expression Is B Cell-Restricted in Mice.
CD20 mAb treatment depleted the majority of B220+ B cells on days 7 and 28, whereas absolute numbers of other leukocyte subsets in all primary lymphoid organs were not affected (Table 1). Although a small subset of human T and natural killer cells has been reported to be depleted after CD20 mAb therapy in rheumatoid arthritis patients as a result of low-level CD20 expression (15, 16), immunofluorescence staining of all leukocytes subsets revealed that CD20 expression is B cell restricted in mice [supporting information (SI) Fig. 5], as in humans (17). Thereby, CD20 mAb treatment was unlikely to modify autoimmune responses by depleting functionally important non B cell leukocyte subsets.
Table 1.
Lymphocyte populations after B cell depletion
| Tissue and lymphocyte subset | 7 days | 28 days | ||
|---|---|---|---|---|
| Control mAb | CD20 mAb | Control mAb | CD20 mAb | |
| Blood | ||||
| B220+ | 4.0 ± 0.9 | 0.013 ± 0.002* | 3.49 ± 0.75 | 0.010 ± 0.003* |
| CD4+ | 1.7 ± 0.4 | 1.2 ± 0.2 | 1.2 ± 0.1 | 1.3 ± 0.2 |
| CD4+CD25+FoxP3+ | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.75 ± 0.08 | 0.9 ± 0.2 |
| CD8+ | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.07 | 0.7 ± 0.1 |
| NK1.1+CD3− | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.05 |
| Spleen | ||||
| B220+ | 52.6 ± 13.3 | 0.7 ± 0.2* | 70.4 ± 10.6 | 0.27 ± 0.01* |
| CD4+ | 14.9 ± 3.5 | 13.1 ± 2.3 | 20.6 ± 2.3 | 19.0 ± 1.5 |
| CD4+CD44+CD62L− | 3.4 ± 0.8 | 2.9 ± 1.1 | 3.1 ± 0.3 | 3.1 ± 0.4 |
| CD4+CD25+FoxP3+ | 1.6 ± 0.2 | 1.4 ± 0.1 | 2.1 ± 0.3 | 1.9 ± 0.1 |
| CD8+ | 7.8 ± 1.6 | 6.8 ± 1.6 | 9.7 ± 1.3 | 9.0 ± 1.1 |
| CD8+CD44+CD62L− | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.22 ± 0.04 | 0.18 ± 0.01 |
| CD11chighCD8α− | 3.4 ± 0.1 | 3.8 ± 0.1 | 3.5 ± 0.1 | 3.6 ± 0.1 |
| CD11chighCD8α+ | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 |
| CD11cintPDCA-1+B220+ | 0.31 ± 0.04 | 0.27 ± 0.10 | 0.51 ± 0.12 | 0.65 ± 0.10 |
| Peripheral LN | ||||
| B220+ | 8.6 ± 1.2 | 0.09 ± 0.01* | 10.7 ± 1.8 | 0.03 ± 0.01* |
| CD4+ | 12.3 ± 1.5 | 11.6 ± 2.3 | 15.4 ± 0.9 | 16.8 ± 2.2 |
| CD4+CD44+CD62L− | 0.71 ± 0.01 | 0.63 ± 0.02 | 0.85 ± 0.09 | 0.76 ± 0.08 |
| CD4+CD25+FoxP3+ | 1.2 ± 0.1 | 1.1 ± 0.2 | 1.6 ± 0.1 | 1.5 ± 0.2 |
| CD8+ | 8.4 ± 1.4 | 7.9 ± 0.9 | 10.5 ± 0.4 | 9.8 ± 1.6 |
| CD8+CD44+CD62L− | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 |
| Thymus | ||||
| B220+ | 0.17 ± 0.01 | 0.05 ± 0.01* | 0.17 ± 0.02 | 0.07 ± 0.02* |
| CD4−CD8− | 2.8 ± 0.5 | 2.5 ± 0.4 | 3.7 ± 0.4 | 4.1 ± 0.5 |
| CD4+CD8+ | 96 ± 24 | 76 ± 12 | 137 ± 20 | 175 ± 10 |
| CD4+CD8− | 13 ± 4 | 13 ± 3 | 24 ± 4 | 36 ± 4 |
| CD4−CD8+ | 3.3 ± 0.5 | 2.4 ± 0.3 | 4.8 ± 0.6 | 5.6 ± 0.6 |
B Cell Depletion Does Not Affect Serum Cytokine Levels and T Cell Phenotypes or Functional Capacity.
Whether B cell depletion and/or monocyte activation could influence serum cytokines that in turn may modify T cell function was examined. CD20 mAb treatment did not significantly alter serum IL-1, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17, TNF-α, IFN-γ, GM-CSF, or TGF-β1 levels over the course of a week (SI Fig. 6_A_ and data not shown). Therefore, B cell depletion was unlikely to induce a systemic cytokine release that altered T cell function.
To assess possible indirect effects of CD20 mAb treatment on T cells, expression of activation markers by CD4+ and CD8+ T cells was examined. Expression of CD25, CD28, CD18, CD69, CD40L, OX40, or CTLA-4 by spleen or lymph node CD4+ or CD8+ T cells was unaffected by B cell depletion over 7 and 28 days (SI Fig. 6_B_ and data not shown). Likewise, B cell depletion did not impair the capacity of spleen or lymph node CD4+ T cells to proliferate and produce cytokines in vitro after polyclonal stimulation (SI Fig. 6_C_ and data not shown). To rule out more subtle effects on T cells, monocytes, and DCs after B cell depletion, gene expression profiles were assessed by quantitative real-time PCR analysis. Transcripts for 84 genes related to T cell activation and differentiation were analyzed by using B220− splenocytes from mice treated 4 days earlier with CD20 or control mAb. B cell depletion did not alter transcript levels for any of the 84 genes analyzed (SI Fig. 6_D_ and data not shown). Thus, in vivo B cell depletion did not significantly affect CD4+ T cell phenotypes, functional capacity, or gene expression profiles.
B Cell Depletion Inhibits Antigen-Specific CD4+ T Cell Expansion and Activation in Vivo.
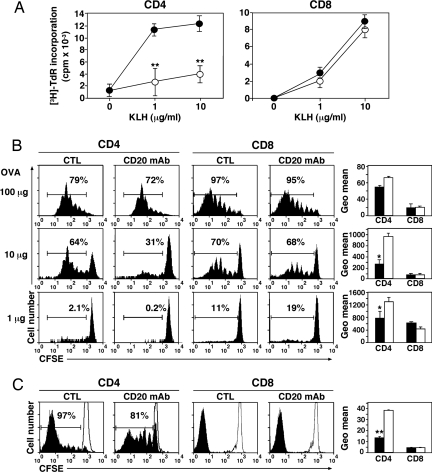
To evaluate the impact of B cell depletion on T cell responses to protein antigen challenge, B cell-depleted mice were immunized with keyhole limpet hemocyanin (KLH), with draining lymph node CD4+ and CD8+ T cells being purified 7 days later. Antigen-specific T cell proliferation was quantified in vitro by using purified mitomycin C-treated B cells from control mAb-treated littermates as antigen-presenting cells. CD4+ T cell recall responses to KLH in B cell-depleted mice were reduced by 71 ± 13% (P < 0.01) at 1 μg/ml KLH and 63 ± 8% (P < 0.01) at 10 μg/ml KLH compared with control mAb-treated littermates (Fig. 1A), whereas antigen-specific CD8+ T cell proliferation was unaffected.
Fig. 1.
B cell depletion inhibits antigen-specific CD4+ but not CD8+ T cell expansion. (A) Impaired CD4+ T cell proliferation in B cell-depleted mice after KLH restimulation in vitro. CD20 (open circles) or control (CTL) (filled circles) mAb-treated mice were immunized with KLH. Seven days later, CD4+ (Left) or CD8+ (Right) T cells were purified from draining lymph nodes and cultured in vitro with KLH and mitomycin C-treated B cells from control mice. The values represent mean (±SEM) [3H]thymidine (TdR) uptake from triplicate cultures. (B) Impaired CD4+ T cell proliferation in vivo in B cell-depleted mice after low-dose OVA immunization. CD4+ cells from OT-II Thy1.1+ mice or CD8+ cells from OT-I Thy1.1+ mice were CFSE-labeled and transferred into Thy1.2+ recipients that had been treated with CD20 or control mAb 7 days earlier. After adoptive transfers, the mice were immunized with graded OVA doses (1, 10, and 100 μg). Three days later, splenocytes were harvested and stained to analyze divisions of donor cells. The bar graphs show CFSE geometric mean fluorescence of the whole histogram, which is inversely proportional to cell divisions (CD20 mAb, open bars; control mAb, closed bars). (C) Impaired CD4+ T cell proliferation in B cell-depleted mice after Listeria infection. The same experimental protocols were used as in B except that mice were infected 1 day after T cell adoptive transfers with Listeria that secretes OVA peptide and that splenocytes were harvested 7 days after infection. All data are representative of two independent experiments with at least three mice in each group. Significant differences between sample means are indicated: *, P < 0.05; **, P < 0.01.
The effect of B cell depletion on CD4+ and CD8+ T cell responses in vivo was directly investigated by using ovalbumin (OVA)-specific Thy1.1+ CD4+ and CD8+ T cells prepared from OT-II and OT-I transgenic mice, respectively (18, 19). 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled OT-II CD4+ and OT-I CD8+ T cells were adoptively transferred into CD20 or control mAb-treated littermates before immunization with OVA. CD4+ T cells proliferated similarly in B cell-depleted and control mAb-treated littermates after immunization with high-dose OVA (100 μg; Fig. 1B). By contrast, CD4+ T cell expansion was significantly reduced in B cell-depleted mice compared with control mAb treated littermates immunized with low doses of OVA (at 10 μg, 40 ± 14% reduction, P < 0.01; at 1 μg, 75 ± 33% reduction, P < 0.05). Heterogeneity in the frequency and extent of T cell proliferation is likely to result from heterogeneity among T cells, heterogeneous frequencies of T cells interacting with appropriate antigen-presenting DCs or B cells in vivo, and/or the strength/duration of antigen-specific interactions. CD8+ T cell proliferation was identical in B cell-depleted and control mAb-treated littermates at all OVA concentrations.
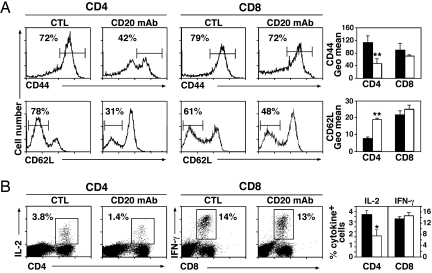
Whether B cells also influence T cell responses to pathogens was assessed by infecting mice with a strain of Listeria monocytogenes modified to secrete soluble OVA (20). Listeria replicate in macrophages and induce both CD4+ and CD8+ T cell responses (21). Listeria infection induced robust CD4+ and CD8+ T cell reactivity in control mAb-treated littermates, whereas B cell depletion significantly reduced the frequency of dividing CD4+ T cell proliferation (14 ± 1% reduction; P < 0.01) (Fig. 1C Left), and the extent of CD4+ T cell proliferation (evaluated by DFSE geometric mean fluorescence decrease; Fig. 1C Right) but not CD8+ T cell proliferation. B cell depletion had no effect on mouse survival, consistent with the rapid expansion of CD8+ T cells after Listeria infection. Moreover, characteristic of T effector cell generation, most CD4+ T cells exhibited a CD62lowCD44high phenotype by day 7 after Listeria infection in control mAb-treated mice (Fig. 2A). By contrast, CD44 up-regulation and CD62L down-regulation were significantly reduced in B cell-depleted littermates (35 ± 5% reduction and 54 ± 4% reduction, P < 0.01, for CD44 and CD62L, respectively) (Fig. 2A). IL-2 and IFN-γ production by CD4+ T cells was also significantly reduced in B cell-depleted littermates after Listeria infection (for IL-2: 52 ± 15% reduction, P < 0.05; for IFN-γ: 82 ± 6% reduction, P < 0.01) (Fig. 2B and data not shown). Despite this, B cell depletion did not significantly alter CD8+ T cell phenotypes or cytokine production after Listeria infection. Thus, short-term B cell depletion significantly affects antigen-specific CD4+ T cell expansion, activation, and effector cell differentiation in vivo.
Fig. 2.
Impaired CD4+ T cell activation after B cell depletion. WT mice treated with CD20 or control (CTL) mAb were infected with Listeria 7 days later. Splenocytes were harvested 7 days after infection. (A) CD44 and CD62L expression by CD4+ and CD8+ T cells. Flow cytometric histograms from a representative mouse in each group are shown. (Left and Center) Numbers denote the percentage of CD4+ (Left) and CD8+ (Center) T cells that were CD44high or CD62Llow. (Right) Bar graphs show CD44 and CD62L geometric mean fluorescence intensities for CD4+ and CD8+ T cells (CD20 mAb, open bars; control mAb, closed bars). (B) (Left and Center) Intracellular IL-2 production by CD4+ T cells (Left) and IFN-γ production by CD8+ T cells (Center) after Listeria infection. Flow cytometric histograms from a representative mouse in each group are shown. (Right) The bar graph denotes the percentage of CD4+ and CD8+ T cells that produce IL-2 and IFN-γ, respectively (CD20 mAb, open bars; CTL mAb, closed bars). All data are representative of two independent experiments with three mice in each group. Significant differences between sample means are indicated: *, P < 0.05; **, P < 0.01.
B Cell and DC Interactions Regulate CD4+ T Cell Expansion in Vivo.
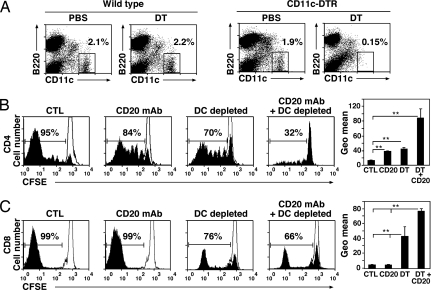
The relative contributions of B cells and DCs to CD4+ T cell priming in vivo were compared by using B6.CD11c–DTR transgenic mice, a mouse model for inducible DC ablation in vivo (22). A single injection of diphtheria toxin (DT) (100 ng) into CD11c–DTR mice induced a selective >10-fold depletion of conventional splenic DCs after 10 h that persisted for 3 days (Fig. 3A). CD20 mAb-mediated B cell depletion was similar in WT and B6.CD11c–DTR littermates treated with DT (data not shown). B cell depletion significantly reduced the frequency of dividing OT-II CD4+ T cells after adoptive transfer (14 ± 1% reduction; P < 0.01) and the extent of divisions of OT-II CD4+ T cells in response to Listeria infection (Fig. 3B). The absence of DCs in B6.CD11c–DTR mice also reduced the frequency of proliferating OT-II CD4+ T cells in response to Listeria infection (33 ± 4% reduction; P < 0.01), and also dramatically reduced the extent of CD4+ T cell proliferation. Combined B cell and DC depletion further inhibited the frequency of proliferating OT-II CD4+ T cells (76 ± 9%; P < 0.01) and almost eliminated their proliferation extent after Listeria infection. By contrast, only DC depletion significantly affected the proliferation of adoptively transferred OT-I CD8+ T cells in response to Listeria infection (34 ± 11% reduction; P < 0.01) (Fig. 3C). Thus, DCs alone were not capable of inducing optimal CD4+ T cell responses to a pathogen challenge.
Fig. 3.
Both B cells and DCs contribute to CD4+ T cell expansion in vivo. (A) DT depletion of CD11c+ DCs in CD11c–DTR mice. WT or CD11c–DTR mice were given DT or PBS. Splenocytes were harvested 10 h later and stained for CD11c and B220 expression. Frequencies of DCs are indicated by the gated subpopulations. (B and C) CD4+ and CD8+ T cell proliferation in vivo after B cell and/or DC depletion and Listeria infection. OT-II Thy1.1+ CD4+ cells or OT-I Thy1.1+ CD8+ cells were CFSE-labeled and transferred into Thy1.2+ recipients. WT recipients were treated with control or CD20 mAb, whereas CD11c–DTR mice were treated with DT (DC-depleted) or DT and CD20 mAb (DC- and B cell-depleted). One day after T cell adoptive transfer, mice were infected with Listeria. At day 7 after infection, splenocytes were harvested to analyze divisions of donor cells. The thin lines represent adoptively transferred T cells without Listeria infection. The bar graphs show CFSE geometric (Geo) mean fluorescence of the whole histogram, which is inversely proportional to cell divisions. The data are representative of two experiments with three mice in each group. Significant differences between means are indicated: *, P < 0.05; **, P < 0.01.
B Cell Depletion Inhibits Autoantigen-Specific CD4+ T Cell Proliferation.
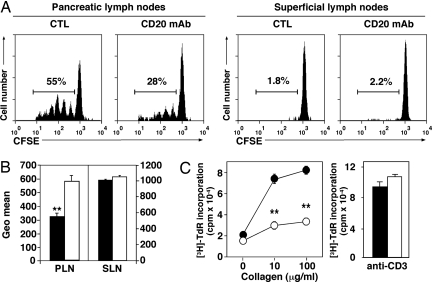
The effect of CD20 mAb treatment on autoreactive CD4+ T cell expansion was evaluated in murine models of autoimmune diabetes (NOD mice) and arthritis, two T cell-dependent autoimmune diseases in which B cell-depletion significantly reduces disease severity (ref. 12 and Y. Xiu, C. P. Wong, J.-D.B., Y. Hamaguchi, Y.W., S. M. Pop, R.M.T., and T.F.T., unpublished work). Five-week-old female NOD mice were treated with CD20 or control mAb and then received an injection of CFSE-labeled CD4+ T cells from transgenic BDC2.5 NOD mice. BDC2.5 T cells proliferate in response to β cell autoantigens including glutamic acid decarboxylase within the pancreatic lymph nodes of NOD mice without any immunization (23). Four days after adoptive transfer, CD20 mAb treatment had no effect on the numbers of BDC2.5 CD4+ T cells migrating to pancreatic and superficial lymph nodes (data not shown). However, B cell depletion significantly reduced the frequency of proliferating BDC2.5 CD4+ T cells (42 ± 6%; P < 0.05; Fig. 4A Left) and the total relative extent of labeled CD4+ T cell proliferation within pancreatic lymph nodes (Fig. 4B), whereas BDC2.5 CD4+ T cell proliferation was not detected in superficial lymph nodes or spleen of either group of recipients (Fig. 4A Right and data not shown). Thus, B cell depletion reduced autoreactive T cell proliferation in response to endogenous autoantigens.
Fig. 4.
B cell depletion inhibits autoantigen-driven CD4+ T cell expansion in vivo. (A) B cell depletion impairs β cell-specific CD4+ T cell expansion in pancreatic lymph nodes of NOD mice. CFSE-labeled BDC2.5 CD4+ cells were transferred into NOD recipients that had been treated with CD20 or control (CTL) mAb 7 days earlier. Four days later, pancreatic and superficial lymph node cells were stained for Vβ4/CD4 expression and analyzed for CFSE flourescence intensity by flow cytometric analysis. Representative CFSE profiles are shown. (B) Mean CFSE geometric mean fluorescence intensities (± SEM) for CD20 (open bars) and control (closed bars) mAb-treated mice shown in A. (C) B cell depletion impairs CD4+ T cell proliferation in response to collagen immunization. CD20 (open circles) or control (filled circles) mAb-treated DBA/1J mice were immunized with collagen to induce arthritis. Fourteen days later, CD4+ T cells were purified from draining lymph nodes and incubated with collagen plus mitomycin C-treated B cells from control mice (Left) or stimulated with CD3 mAb (Right). Values represent mean (± SEM) [3H]thymidine uptake from triplicate cultures. (A–C) All data represent results obtained in at least four mice for each group. Significant differences between samplemeans are indicated: **, P < 0.01.
The role of B cells in CIA was assessed in DBA/1J mice treated with CD20 or control mAb before collagen immunization as described in ref. 24. DBA/1J mice have a high penetrance of arthritis after heterologous collagen immunizations (25). CD4+ T cells were purified from draining lymph nodes 14 days after immunization, with collagen-specific T cell proliferation being quantified in vitro. Responses of CD4+ T cells to recall collagen challenge at 10 and 100 μg/ml were reduced by 61 ± 8% and 60 ± 2%, respectively, in B cell-depleted mice when compared with control mAb-treated littermates (Fig. 4C Left), whereas polyclonal mitogen-induced proliferation remained intact (Fig. 4C Right). Thus, B cell depletion inhibited collagen-specific CD4+ T cell proliferation in response to autoantigen challenge.
Discussion
This study demonstrates that B cell depletion in mice with otherwise intact immune systems significantly reduced CD4+ T cell responses to foreign and self antigens, whereas CD8+ T cell reactivity was unaffected (Figs. 1 and 4). B cell depletion also reduced the conversion of CD4+ T cells from a naive CD44lowCD62Lhigh phenotype to a memory CD44highCD62Llow phenotype in response to Listeria challenge, whereas CD8+ T cell phenotypes were only modestly affected (Fig. 2). These effects likely result from the absence of B cell and antigen-specific CD4+ T cell interactions because CD20 expression and mAb-mediated depletion were restricted to mature B cells (SI Fig. 5 and Table 1). Moreover, circulating and tissue T cell numbers, subsets, and phenotypes were not affected by short-term B cell depletion (SI Fig. 6 B–D and Table 1), and ex vivo T cell proliferation in response to polyclonal stimuli remained intact in the absence of B cells (SI Fig. 6_C_). Consistent with the established role of DCs in T cell activation, both B cells and DCs were required for optimal CD4+ T cell activation (Fig. 3). This suggests that CD20 mAb-induced changes in lymphoid tissue architecture (11) do not eliminate DC and CD4+ T cell interactions, their respective functions, or their migration. Moreover, CD20 mAb-induced B cell depletion and monocyte activation did not result in the release of inflammatory cytokines into the serum that could affect T cell responses (SI Fig. 6_A_). Therefore, B cell depletion had specific and selective effects on CD4+ T cell responses in vivo.
Inhibiting autoantigen-specific CD4+ T cell activation may elucidate the therapeutic benefit of B cell depletion in both the CIA and NOD mouse models. Arthritogenic collagen-specific CD4+ T cells are essential for CIA induction, perpetuation, and exacerbation (26). CD20 mAb-mediated B cell depletion significantly delays CIA development whether B cells are depleted before collagen immunizations, with modest effects once arthritis first appears (12). Therefore, therapeutic B cell depletion may prevent disease by reducing collagen-specific CD4+ T cell activation (Fig. 4C) in addition to inhibiting autoantibody production. Likewise, B cell depletion delayed autoreactive CD4+ T cell expansion in response to islet β cell autoantigens in NOD mice (Fig. 4B). Inhibiting autoantigen-specific T cell activation by B cell depletion may explain diabetes prevention in mature NOD mice with otherwise intact immune systems (Y. Xiu, C. P. Wong, J.-D.B., Y. Hamaguchi, Y.W., S. M. Pop, R.M.T., and T.F.T., unpublished work).
In both the CIA and NOD mouse models, B cell depletion from birth ameliorates disease (27–29). Studies demonstrating a role for B cells in CD4+ T cell priming have also predominantly used mice given anti-IgM antibody since birth (30–33) or mice with genetic defects in B cell development (34). In some studies, the absence of B cells can impair CD4+ T cell priming (28, 31, 32, 35, 36), whereas in other studies, CD4+ T cell priming was not affected (7, 37–40). However, the absence of B cells during mouse development results in significant quantitative and qualitative abnormalities within the immune system, including a remarkable decrease in thymocyte numbers and diversity (41), significant defects within spleen DC and T cell compartments (7, 42, 43), an absence of Peyer's patch organogenesis and follicular DC networks (44, 45), and an absence of marginal zone and metallophilic macrophages, with decreased chemokine expression (43, 45). That T cell numbers and function appear intact in mature mice after CD20 mAb-mediated B cell depletion demonstrates that B cells are critical for normal immune system development but not its maintenance. Thus, induced B cell depletion in mice with intact immune systems may dampen some cell-mediated immune responses without the pleiotropic effects induced by congenital B cell depletion.
The current B cell depletion studies demonstrate that both B cells and DCs are essential for optimal CD4+ T cell responses (Fig. 3). Although an intact B cell compartment was unable to support optimal CD4+ T cell activation when DCs were depleted, B cells were not required for CD4+ T cell proliferation in vivo after high-dose OVA challenge (100 μg; Fig. 1B). However, low-dose OVA challenge (1–10 μg; Fig. 1B) and Listeria infection (Fig. 1C) required B cells for optimal CD4+ T cell activation in vivo. B cells were also required for optimal CD4+ T cell activation in response to high-dose KLH challenge in vivo (100 μg) when assessed in vitro by using restimulation assays with exogenous B cells as antigen-presenting cells (Fig. 1A). That B cells may be required for effective low-dose antigen recognition in vivo may reflect a functional balance between DCs, B cells, and other antigen-presenting cell populations. This balance is most likely influenced by the molecular nature of the antigen being presented, its concentration and route of exposure, and the adjuvants present. Additionally, DCs may prime naïve CD4+ T cells in vivo, whereas B cells may have specific roles in stimulating antigen-specific CD4+ T cell proliferation after activation by DCs (40).
B cell depletion therapy most likely affects autoimmune disease onset by regulating CD4+ T cell expansion, thereby delaying the inflammatory phase of disease that leads to tissue destruction and pathology. That B cells provided extra and essential functions over and above those provided by DCs for CD4+ T cell activation in response to low-level antigen may be important during early autoimmune disease development, when continuous waves of low-level self-antigen stimulation occur. The effects of B cell depletion on CD4+ T cell activation may explain rare infections in lymphoma patients receiving rituximab with microorganisms generally associated with T cell immunosuppression such as Jamestown Canyon (JC) papovavirus, CMV, or parvovirus B19 (46). B cell depletion may also have other unanticipated effects on T cell effector functions including the growth regulation of non-B cell malignancies. An optimal therapy for disease quiescence may not only require the effective elimination of mature B cells but may also require continuous B cell depletion because environmental triggers are likely to persist indefinitely. Nonetheless, it must be taken into account that autoimmune disease patients are normally given CD20 mAb in combination with immunosuppressive drugs (47). In rheumatoid arthritis, rituximab is often administered in combination with methotrexate. Therefore, the synergistic combination of B cell depletion and immunosuppression of T cell activation may lead to more significant clinical effects than B cell depletion alone. For example, B cell depletion may block nascent autoantigen-specific T cell activation and autoantibody development, whereas immunosuppression may arrest the clonal expansion of existing autoreactive lymphocytes, thereby ameliorating disease progression. Although multiple factors contribute to autoimmune disease pathogenesis, this insight into the potential mechanism of action for CD20 immunotherapy may lead to the development of additional therapeutic strategies for autoimmune disease management.
Materials and Methods
Mice and Immunotherapy.
C57BL/6, B6.PL THy1a/Cy (B6.Thy1.1+), DBA/1J, NOD/LtJ, C57BL/6-Tg(TcraTcrb)425Cbn/JB6 (OT-II), and C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I) were from The Jackson Laboratory. OT-II and OT-I transgenic mice generate CD4+ and CD8+ T cells that respectively respond to peptide 323–339 and 257–264 of OVA (18, 19). OT-II and OT-I mice (Thy1.2+) were crossed to B6.Thy1.1+ mice to generate Thy1.1-expressing T cells for adoptive transfer experiments. CD20−/− mice were as described in ref. 48. NOD.Cg-Tg(TcraBDC2.5)1Doi Tg(TcrbBDC2.5)2Doi/DoiJ mice (BDC2.5) (49) were housed and bred at the University of North Carolina at Chapel Hill. CDB6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J (B6.CD11c DT receptor transgenic mice, CD11c–DTR) as originally described were provided by M. D. Gunn (Duke University). To deplete CD11c+ DCs in vivo, CD11c–DTR transgenic mice were treated i.p. with 100 ng DT (Sigma) in 200 μl of PBS as described in ref. 22. To induce in vivo B cell depletion, sterile CD20 (MB20-11, IgG2c) or isotype-matched control mAbs (250 μg) were injected in 200 μl of PBS through lateral tail veins (9). All mice were bred in a specific pathogen-free barrier facility and used at 8–12 weeks of age unless otherwise noted. Duke University and University of North Carolina, Chapel Hill, Animal Care and Use Committees approved all studies.
Immunizations and in Vitro T Cell Proliferation Assays.
For KLH assays, mice were injected s.c. with KLH (100 μg; endotoxin free; Sigma) in complete Freund's adjuvant (Sigma). After 7 days, 3 × 105 purified CD4+ or CD8+ T cells harvested from draining lymph nodes were cultured in 96-well plates in 200 μl of complete RPMI 1640 culture medium with 1.5 × 105 mitomycin C-treated (Sigma) B cells and KLH (0, 1, and 10 μg/ml). Collagen immunizations in male DBA1/J mice used type II chicken collagen (Chondrex) dissolved in 10 mM acetic acid solution (5 mg/ml) overnight at 4°C. Dissolved collagen (100 μg) was emulsified with an equal volume of complete Freund's adjuvant, with 100 μl being injected s.c. into the base of the tail. For collagen-specific CD4+ T cell proliferation, draining lymph nodes were harvested 14 days after collagen immunization, with 5 × 105 T cells being cultured with 1 × 106 mitomycin C-treated (Sigma) B cells and T cell proliferation grade type II collagen (0, 10, and 100 μg/ml; Chondrex), as recommended by the manufacturer. Proliferation was measured by [3H]thymidine incorporation during the final 12-h of 4-day cultures, followed by scintillation counting. In some experiments, thymidine incorporation was measured after 48 h of CD4+ T cell stimulation with tissue culture plate-bound CD3 mAb (5 μg/ml; BD PharMingen).
Adoptive Transfer Experiments.
Donor Thy1.1+ OT-II CD4+ or OT-I CD8+ T cells from pooled spleens and lymph nodes were labeled with CFSE fluorescent dye (1 μM; Vybrant CFDA SE; Invitrogen–Molecular Probes) according to the instructions of the manufacturer. Labeled Thy1.1+ cells (5 × 106) were given i.v. to Thy1.2+ congenic recipients. Recipient mice were immunized i.p. with alum precipitated OVA (Sigma) or were given Listeria monocytogenes producing OVA peptide (amino acids 134–387) capable of activating both OT-II CD4+ and OT-I CD8+ T cells (20). Bacteria were grown in brain–heart infusion media supplemented with 5 μg/ml erythromycin (Sigma), with 5 × 105 colony-forming units (0.1 LD50)/mouse in 200 μl of PBS injected i.v. (lateral tail vein). The proliferation of transferred cells was visualized by flow cytometric analysis of 1 × 104 CFSE-labeled Thy1.1+ cells. Transferred OT-II CD4+ or OT-I CD8+ T cells were identified by Thy1.1 and CD4 or CD8 mAb staining, respectively. For adoptive transfer experiments involving BDC2.5 CD4+ T cells, 2 × 106 CFSE-labeled cells were injected i.v. into female NOD recipients, and T cell proliferation was analyzed after staining with CD4 and Vβ4 mAbs.
Supplementary Material
Supporting Information
ACKNOWLEDGMENTS.
We thank Charlene Prazma and David DiLillo for help with this research. This research was supported by National Institutes of Health Grants CA105001, CA81776, CA96547, AI56363 (all to T.F.T.), and AI058014 (to R.M.T.) and the Arthritis Foundation. J.D.B. is supported by grants from Association pour la Recherche contre le Cancer, the Fondation René Touraine, and the Philippe Foundation.
Footnotes
Conflict of interest statement: T.F.T. is a paid consultant for MedImmune, Inc. and a consultant and shareholder for Angelica Therapeutics, Inc. J.C.P is a paid consultant for Angelica Therapeutics, Inc.
This article is a PNAS Direct Submission.
References
- 1.Ermann J, Fathman CG. Nat Immunol. 2001;2:759–761. doi: 10.1038/ni0901-759. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JCW, Cambridge G. Rheumatology. 2001;40:205–211. doi: 10.1093/rheumatology/40.2.205. [DOI] [PubMed] [Google Scholar]
- 3.Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, Amadori S. Blood. 2007;110:2924–2930. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 4.Edwards JCW, Cambridge G. Rheumatology. 2005;44:151–156. doi: 10.1093/rheumatology/keh446. [DOI] [PubMed] [Google Scholar]
- 5.Lipsky PE. Nat Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 6.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 7.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. J Exp Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu S, Maiti PK, Dyson M, Jain R, Braley-Mullen H. J Exp Med. 2006;203:349–358. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. J Exp Med. 2006;203:743–753. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamaguchi Y, Uchida J, Cain DW, Venturi GM, Poe JC, Haas KM, Tedder TF. J Immunol. 2005;7:4389–4399. doi: 10.4049/jimmunol.174.7.4389. [DOI] [PubMed] [Google Scholar]
- 12.Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St. Clair EW, Tedder TF. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa H, Hamaguchi Y, Yanaba K, Bouaziz J-D, Uchida J, Fujimoto M, Matsushita T, Matsushita Y, Horikawa M, Komura K, et al. Am J Pathol. 2006;169:954–966. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayakawa I, Tedder TF, Zhuang Y. Immunology. 2007;122:73–79. doi: 10.1111/j.1365-2567.2007.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 16.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. Cytometry. 1993;14:196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L-J, Tedder TF. In: Leukocyte Typing V: White Cell Differentiation Antigens. Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto C, Ritz J, Shaw S, Silverstein R, Springer T, et al., editors. Oxford: Oxford Univ Press; 1995. pp. 511–514. [Google Scholar]
- 18.Barnden MJ, Allison J, Heath WR, Carbone FR. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 19.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 20.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 21.Kursar M, Bonhagen K, Kohler A, Kamradt T, Kaufmann SH, Mittrucker HW. J Immunol. 2002;168:6382–6387. doi: 10.4049/jimmunol.168.12.6382. [DOI] [PubMed] [Google Scholar]
- 22.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tada Y, Ho A, Koh DR, Mak TW. J Immunol. 1996;156:4520–4526. [PubMed] [Google Scholar]
- 25.Holmdahl R, Jansson L, Gullberg D, Rubin K, Forsberg PO, Klareskog L. Clin Exp Immunol. 1985;62:639–646. [PMC free article] [PubMed] [Google Scholar]
- 26.Seki N, Sudo Y, Yoshioka T, Sugihara S, Fujitsu T, Sakuma S, Ogawa T, Hamaoka T, Senoh H, Fujiwara H. J Immunol. 1988;140:1477–1484. [PubMed] [Google Scholar]
- 27.Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 29.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. J Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 30.Ron Y, De Baetselier P, Gordon J, Feldman M, Segal S. Eur J Immunol. 1981;11:964–968. doi: 10.1002/eji.1830111203. [DOI] [PubMed] [Google Scholar]
- 31.Ron Y, Sprent J. J Immunol. 1987;138:2848–2856. [PubMed] [Google Scholar]
- 32.Janeway CA, Jr, Ron J, Katz ME. J Immunol. 1987;138:1051–1055. [PubMed] [Google Scholar]
- 33.Kurt-Jones EA, Liano D, HayGlass KA, Benacerraf B, Sy MS, Abbas AK. J Immunol. 1988;140:3773–3778. [PubMed] [Google Scholar]
- 34.Kitamura D, Roes J, Kuhn R, Rajewsky K. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, Zhao M. Int Immunol. 1995;7:1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- 36.Linton PJ, Harbertson J, Bradley LM. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 37.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 39.Lassila O, Vainio O, Matzinger P. Nature. 1988;334:253–255. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- 40.Ronchese F, Hausmann B. J Exp Med. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joao C, Ogle BM, Gay-Rabinstein C, Platt JL, Cascalho M. J Immunol. 2004;172:4709–4716. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 42.AbuAttieh M, Rebrovich M, Wettstein PJ, Vuk-Pavlovic Z, Limper AH, Platt JL, Cascalho M. J Immunol. 2007;178:2950–2960. doi: 10.4049/jimmunol.178.5.2950. [DOI] [PubMed] [Google Scholar]
- 43.Ngo VN, Cornall RJ, Cyster JG. J Exp Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Science. 1999;286:1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 45.Crowley MT, Reilly CR, Lo D. J Immunol. 1999;163:4894–4900. [PubMed] [Google Scholar]
- 46.Goldberg SL, Pecora AL, Alter RS, Kroll MS, Rowley SD, Waintraub SE, Imrit K, Preti RA. Blood. 2002;99:1486–1488. doi: 10.1182/blood.v99.4.1486. [DOI] [PubMed] [Google Scholar]
- 47.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 48.Uchida J, Lee Y, Hasegawa M, Liang Y, Bradney A, Oliver JA, Bowen K, Steeber DA, Haas KM, Poe JC, et al. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 49.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information