Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts (original) (raw)
Abstract
Given their self-renewing and pluripotent capabilities, human embryonic stem cells (hESCs) are well poised as a cellular source for tissue regeneration therapy. However, the host immune response against transplanted hESCs is not well characterized. In fact, controversy remains as to whether hESCs have immune-privileged properties. To address this issue, we used in vivo bioluminescent imaging to track the fate of transplanted hESCs stably transduced with a double-fusion reporter gene consisting of firefly luciferase and enhanced GFP. We show that survival after transplant is significantly limited in immunocompetent as opposed to immunodeficient mice. Repeated transplantation of hESCs into immunocompetent hosts results in accelerated hESC death, suggesting an adaptive donor-specific immune response. Our data demonstrate that transplanted hESCs trigger robust cellular and humoral immune responses, resulting in intragraft infiltration of inflammatory cells and subsequent hESC rejection. Moreover, we have found CD4+ T cells to be an important modulator of hESC immune-mediated rejection. Finally, we show that immunosuppressive drug regimens can mitigate the anti-hESC immune response and that a regimen of combined tacrolimus and sirolimus therapies significantly prolongs survival of hESCs for up to 28 days. Taken together, these data suggest that hESCs are immunogenic, trigger both cellular and humoral-mediated pathways, and, as a result, are rapidly rejected in xenogeneic hosts. This process can be mitigated by a combined immunosuppressive regimen as assessed by molecular imaging approaches.
Keywords: molecular imaging, immunological response, immunosuppression
Human embryonic stem cells (hESCs) have generated great interest given their pluripotency and capacity to self-renew. Specifically, hESCs can be cultured indefinitely in vitro and can differentiate into virtually any cell type in the adult body (1). For these reasons, hESCs are an attractive source for tissue regeneration and repair therapies. There is a growing number of reports showing the therapeutic benefit of hESC derivatives after transplantation into animal models of disease, such as myocardial infarction (2) and Parkinson's disease (3). Although such data are encouraging, significant hurdles remain before hESC-based treatments can be safely and successfully translated into clinical therapies (4).
An important obstacle facing in vivo engraftment and function of hESCs is the potential immunologic barrier (5). hESCs express low levels of Class I human leukocyte antigen (HLA), which increases as these cells differentiate (6). The presence of distinct major histocompatibility complex (MHC) antigens suggests that hESCs may elicit an immune response and be at risk for rejection when introduced in vivo across histocompatibility barriers (5). At the same time, hESCs theoretically represent an immune-privileged cell population, because embryos consisting of 50% foreign paternal material are usually not rejected by the maternal host. Recent reports have indeed shown that both mouse embryonic stem cells (mESCs) and hESCs seem to have the capability to evade immune recognition in allogeneic as well as in xenogeneic hosts. mESCs have been shown to survive in immunocompetent mice (7), as well as in rats (8) and sheep (9) for many weeks after transplantation. Similarly, rat ESC-like cells were demonstrated to permanently engraft in allogeneic recipients leading to allospecific down-regulation of the host immune response (10). In addition, not only have hESCs been reported to inhibit allogeneic T cell proliferation in vitro, but also to evade immune recognition in xenogeneic immunocompetent mice (11).
Nevertheless, our group and others have found that after transplantation into allogeneic recipients, mESCs triggered progressive immune cell infiltration and were subsequently rejected (12, 13). Others have concluded that hESC grafts are infiltrated by inflammatory cells (14) and do not form teratomas in immunocompetent mice (15), suggesting rejection. Clearly, questions of whether hESCs have immune-privileged properties and whether immunological rejection of transplanted hESCs and hESC derivatives is something that must be addressed remains to be clarified (16).
In this study, we used noninvasive molecular imaging techniques to longitudinally track hESC fate after transplantation. We present evidence of an adaptive donor-specific xenogeneic immune response that is launched against hESCs shortly after transplantation into immunocompetent mice, resulting in rejection. We further delineate the role of T lymphocyte subsets in mediation of the murine anti-hESC immune response. Finally, we compared the efficacy of various combinations of clinically available immunosuppressive regimens for enhancing survival of transplanted hESCs in vivo.
Results
Characterization of hESCs Expressing a Double Fusion (DF) Reporter Gene.
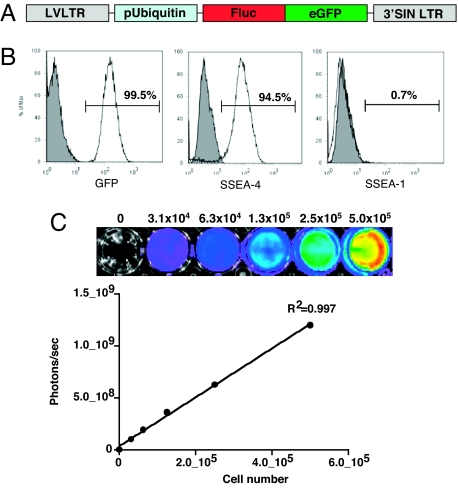
To date, most studies on hESC therapy have relied on conventional reporter gene technology such as GFP (11) and β-galactosidase (LacZ) (17) to monitor cell survival and behavior after transplantation. These reporter genes are typically identified by immunohistochemical staining techniques, which provide only a “snapshot” representation rather than a comprehensive picture of cell survival over time (18). Such limited techniques may, in part, contribute to the conflicting observations of hESC survival in xenogeneic hosts. Results from previous studies range from no signs of rejection (11) to complete rejection of hESCs (15) after transplantation into mice. To circumvent these issues, a DF reporter gene construct carrying firefly luciferase (fLuc) and eGFP driven by a constitutive human ubiquitin promoter (pUB) was successfully transduced into undifferentiated hESCs (H9 line), using a self-inactivating (SIN) lentiviral vector (Fig. 1A). This enabled us to track the hESCs in vivo by bioluminescent imaging (fLuc) as well as ex vivo by immunohistochemistry (eGFP). After two or three passages of feeder-free culture in mTersh culture medium, FACS analysis of H9DF hESCs revealed robust expression of eGFP concomitant with expression of pluripotent hESC markers (SSEA-4+ and SSEA-1−) (Fig. 1B). The cells exhibited a robust correlation between fLuc expression and hESC number (_r_2 = 0.99) (Fig. 1C). In vitro analysis showed that H9DF hESCs were able to proliferate and differentiate into cells of all three germ layers at a frequency similar to control H9 hESCs (data not shown).
Fig. 1.
Characterization of the DF fLuc and enhanced eGFP transduced hESCs. (A) Schema of the DF reporter gene containing fLuc and eGFP driven by a human ubiquitin promoter. (B) Flow cytometric analysis of H9DF hESCs shows robust expression of eGFP. Transduced hESCs are largely positive for SSEA-4, and negative for SSEA-1, confirming their pluripotent state. (C) Stably transduced hESCs show robust correlation between cell number and reporter gene activity. BLI of a 24-well plate containing increasing numbers of H9DF hESCs are shown above the corresponding graph depicting correlation between cell number and fLuc activity.
The major system of alloantigens responsible for cell incompatibility is the major histocompatibility complex (MHC) (19). In agreement with previous reports (6, 20), we found low expression levels of both MHC-I and β2-microglobulin proteins and no expression of MHC-II on both H1 and H9 hESCs, compared with a positive control (human lymphocytes). Importantly, these profiles were not altered by the introduction of our reporter genes [supporting information (SI) Fig. S1_A_]. Also, lentiviral transduction did not result increased autocrine secretion of IFN-γ, a cytokine known to induce MHC expression (6) (Fig. S1 B and C).
Monitoring of Transplanted hESCs in Immunocompetent and Immunodeficient Mice.
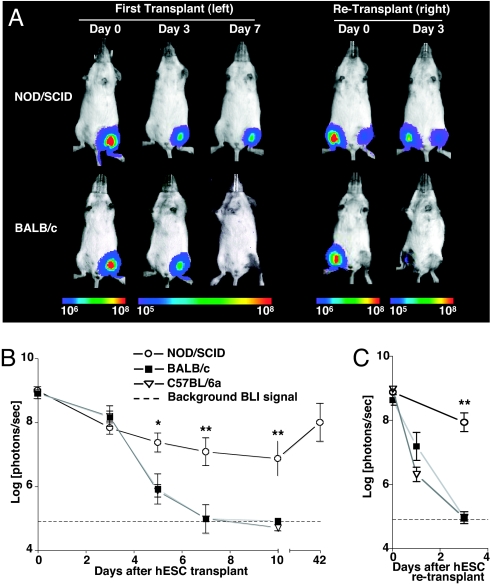
We investigated longitudinal hESC survival after intramuscular (gastrocnemius muscle) transplantation of 1 × 106 H9DF hESCs into immunodeficient (NOD/SCID, n = 5) vs. two strains of immunocompetent mice (BALB/c and C57BL/6a, n = 5 per group) by in vivo bioluminescent imaging (BLI). hESC survival was significantly limited in immunocompetent animals compared with NOD/SCID mice. (day 5 BLI signal: NOD/SCID, 7.37 ± 0.3; BALB/c, 5.91 ± 0.47; C57BL/6a, 6.1 ± 0.19 log[photons per second]; P < 0.05 immunodeficient vs. immunocompetent). BLI signal completely disappeared in immunocompetent animals between 7 and 10 days after transplant (Fig. 2 A and B). Repeated transplantation of H9DF hESCs in the contralateral gastocnemius muscle at two weeks after primary injection resulted in accelerated hESC death in immunocompetent animals, with BLI signal reaching background levels by day 3 after transplant (NOD/SCID, 7.95 ± 0.29; BALB/c, 4.97 ± 0.10; C57BL/6a, 4.97 ± 0.19 log[photons per second]; P < 0.001 immunodeficient vs. immunocompetent), suggesting an adaptive, donor-specific immune response (Fig. 2 A and C). hESC death after transplant in immunocompetent mice was confirmed in a control experiment, in which 1 × 106 H1 hESCs were transplanted into an additional group of BALB/c animals (n = 5). Consistent with BLI data, histological evaluation of the graft site at 10 days revealed no evidence of hESC survival (Fig. S2 A and B). By contrast, H9DF hESC survived well in NOD/SCID animals with progressively increasing BLI signal intensity starting at the 10th day after transplant, suggesting hESC proliferation (Fig. 2B). At 42 days after primary transplantation, intramuscular teratomas were found in transplanted NOD/SCID animals (Fig. S2 C and D), whereas neither teratomas nor persistent hESCs were seen in immunocompetent animals (data not shown).
Fig. 2.
In vivo visualization of hESC survival. (A) Representative BLI images of H9DF hESC transplanted animals show a rapid decrease in BLI signal in immunocompetent animals (BALB/c), as opposed to immunodeficient (NOD/SCID) mice, reaching background levels at day 7 after transplant. Accelerated BLI signal loss in BALB/c animals was seen after repeated hESC transplantation into the contralateral gastrocnemius muscle. Color scale bar values are in photons per second per square centimeter per steradian (sr). (B and C) Graphical representation of longitudinal BLI after primary (B) and secondary (C) hESC transplantation into immunodeficient (NOD/SCID, n = 5) and two immunocompetent (BALB/c and C57BL/6a, n = 5 per group) mouse strains. Note that in NOD/SCID animals, starting at the 10th day after transplant, BLI intensity increases progressively, suggesting hESC proliferation. *, P < 0.05, **, P < 0.01.
To exclude the possibility that the adaptive immune reaction was launched against xenoantigens produced by the reporter genes introduced into the cells, rather than against hESC xenoantigens, we next transplanted 1 × 106 nontransduced H9 hESCs into a second group of BALB/c mice (n = 3), followed by retransplantation of 1 × 106 H9DF hESCs into the contralateral leg at 2 weeks after primary injection. BLI after retransplantation showed a similar loss of signal as compared with animals that were primarily stimulated with H9DF hESCs (Fig. S3 A and B), indicating that the adaptive immune response was in fact directed toward the hESCs.
To determine whether hESC differentiation would influence their capacity to escape immunological rejection, we next injected 1 × 106 H9DF hESC-derivatives after spontaneous in vitro differentiation during 14 days before transplantation into BALB/c mice (n = 5). Overall, our results did not show a significant difference in their survival compared with undifferentiated hESCs (Fig. S3_C_).
Transplantation of hESCs Triggers Severe Graft Infiltration by a Variety of Immune Cells.
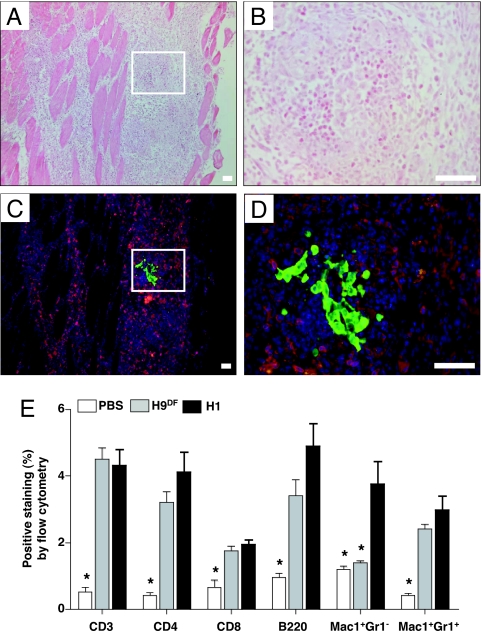
Five days after transplantation of either 1 × 106 H9DF or H1 hESCs (n = 6) or PBS (n = 3) as a control, gastrocnemius muscles of BALB/c animals were analyzed for graft infiltrating cells. Histological analysis demonstrated severe intramuscular infiltration of inflammatory cells (Fig. 3 A and B). Immunofluorescent staining showed that a large percentage of infiltrating cells stained positive for the T lymphocyte surface marker CD3 (Fig. 3 C and D). Quantification and further characterization of graft infiltrating cells was carried out by enzymatic digestion of the explanted muscles followed by FACS analysis. Comparison of the control PBS to the hESC injected muscles confirmed that both H9DF and H1 hESC transplantation elicited severe infiltration of various types of immune cells involved in both adaptive and innate types of immunity (Fig. 3E). Interestingly, both CD3+ T cells (H9DF, 4.5 ± 0.3%; H1, 4.3 ± 0.5% vs. PBS control: 0.5 ± 0.1%; P < 0.01) and B220+ B cells (H9DF, 3.4 ± 0.5%; H1, 4.9 ± 0.7% vs. PBS control: 1.0 ± 0.1%; P < 0.01) were present at a high frequency, suggesting a prominent role for adaptive immunity in hESC rejection. Furthermore, CD4+ T cells, CD8+ T cells and Mac-1+Gr-1+ neutrophils, and Mac-1+Gr-1− macrophages (the latter only in the H1 group) infiltrated into the hESC graft at a significantly higher frequency compared with PBS controls (Fig. 3E).
Fig. 3.
Robust inflammatory cell infiltration after intramuscular hESC transplantation. (A and B) Histopathological evaluation by H&E staining of muscle sections of BALB/c animals, obtained at 5 days after H9DF hESC transplantation, demonstrates robust intramuscular inflammatory cell infiltration at low power (A) and high power (B) view. (C and D) Immunofluorescent staining on corresponding sections reveals abundant presence of CD3+ T cells (red) surrounding eGFP+ hESCs (green). Counterstaining was performed with 4,6-diamidino-2-phenylindole (DAPI, blue). (Scale bars, 50 μm.) (E) FACS analysis of enzymatically digested muscles revealed intra- H9DF and H1 hESC graft infiltration of CD3+ T cells, CD4+ Th cells, CD8+ cytotoxic T cells, B220+ B cells, and Mac-1+Gr-1+ neutrophils at significantly higher intensities compared with PBS injections. Mac-1+Gr-1− (macrophages) cells had a significantly higher presence only in the H1 group. *, P < 0.05.
hESC Transplantation Triggers Systemic Cellular and Humoral Murine Immune Responses.
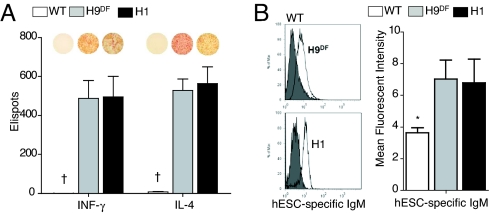
To investigate the cellular immune response, we next performed ELISPOT assays using splenocytes of both H9DF and H1 hESC recipient animals. Cytokine release was abundant in these animals. At 5 days after transplantation, splenocytes from hESC recipients secreted significant amounts of both IFN-γ and IL-4, compared with wild-type animals (H9DF: IFN-γ, 488 ± 91 and IL-4, 529 ± 57; H1: IFN-γ, 495 ± 106 and IL-4, 563 ± 87 vs. WT group: IFN-γ, 0.5 ± 0.3 and IL-4, 8.5 ± 2; P < 0.001) (Fig. 4A). IFN-γ is produced by T-helper (Th)-1 cells and induces cellular immune activity, whereas IL-4 produced by Th-2 cells activates humoral immune pathways. Thus, our data suggest the involvement of an antibody-mediated B cell response. Indeed, FACS analysis showed a significantly higher presence of circulating xeno-reactive antibodies in hESC recipient sera, compared with wild-type animals (mean fluorescent intensity: H9DF, 7.0 ± 1.2; H1, 6.8 ± 1.5 vs. WT group: 3.8 ± 0.6; P < 0.05) (Fig. 4B).
Fig. 4.
hESC transplantation triggers cellular and humoral murine immune responses. (A) ELISPOT assay revealed significantly higher production of both INF-γ and IL-4 by both H1 and H9DF hESC recipient BALB/c splenocytes (n = 6) compared with wild-type animals (n = 3). Representative images of ELISPOT wells are shown above the corresponding bars. †P < 0.001 (B) Representative flow cytometry histograms (Left) and graphical representation (Right) of hESC-specific xeno-reactive IgM antibodies detected at significantly higher rate in H1 and H9DF hESC recipient BALB/c sera (n = 6) compared with WT animals (n = 3). *, P < 0.05.
Prominent Role for CD4+ T-Cells in Mouse Anti-hESC Rejection.
The phylogenetic disparity between mice and humans leads to a lower affinity of mouse TCRs for human MHC molecules (21). Therefore, the indirect pathway of immune recognition, whereby the recipient's antigen presenting cells (APCs) process and present xenoantigens to recipient CD4+ T cells, plays a major role in discordant cellular xenorejection (21). For these reasons, combined with the fact that hESCs lack expression of MHC-II antigens (Fig. 1D) necessary for direct xenograft recognition by recipient CD4+ T cells, we hypothesized that indirect immune recognition by CD4+ T cells could play an important role in mouse anti-hESC rejection. To further delineate the role of T cell subsets in hESC rejection, we transplanted 1 × 106 H9DF hESCs into T cell deficient BALB/c Nude, CD4+ T cell knockout (CD4-KO), and CD8+ T cell knockout (CD8-KO) animals (n = 4 or 5 per group) and followed hESC survival by BLI. In agreement with prior data (15), hESCs survived in Nude mice over the 42-day study course (Fig. 5 A and B), and were able to form teratomas. Interestingly, hESCs survived significantly longer in CD4-KO compared with CD8-KO animals (BLI signal at the fifth day after transplant: CD4-KO: 6.5 ± 0.6 vs. CD8-KO: 5.0 ± 0.3 log[photons per second]; P < 0.05). However, in both groups, hESC xenografts were eventually rejected (Fig. S4 A and B).
Fig. 5.
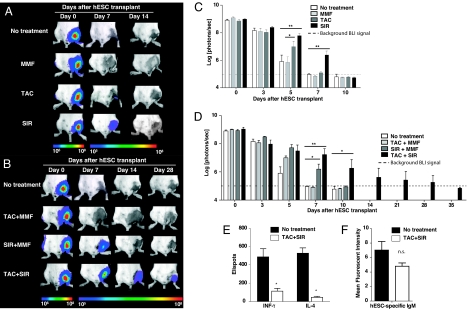
Immunosuppressive drug treatment prolongs survival of transplanted hESCs and mitigates adaptive immune response. (A and B) Representative BLI images of H9DF hESCs-transplanted mice receiving no treatment compared with those receiving immunosuppressive monotherapy (MMF, TAC, or SIR) (A) or combined therapy (TAC+MMF, SIR+MMF, TAC+SIR) (B). Although SIR as monotherapy extended hESC survival significantly, TAC+SIR combination therapy proved to be optimal and extended survival of the cells up to day 28 after transplant. Color scale bar values are in photons per second per squared centimeter per sr. (C and D) Graphical representation of single (C) or combined (D) drug treatment efficacy on hESC survival after transplant (n = 5 per group). *, P < 0.05, **, P < 0.01. (E and F) Combined TAC+SIR treatment effectively suppressed INF-γ and IL-4 production by hESC recipient splenocytes (**, P < 0.01) (E) and reduced production of donor-specific xeno-reactive antibodies (F) (P = 0.14; n.s. = not significant).
Immunosuppressive Therapy Prolongs Survival of hESCs After Transplantation.
Because hESC death after transplant appears largely due to T cell mediated donor-specific immune response, we next investigated the efficacy of single and combined immunosuppressive drug regimens for preventing hESC rejection after transplant. Clinically available immunosuppressants were chosen based on different mechanism of action: (i) calcineurin inhibitors [tacrolimus (TAC)], (ii) target of rapamycin (TOR) inhibitors [sirolimus (SIR)], and (iii) antiproliferatives [mycophenolate mofetil (MMF)] (22). A group of BALB/c mice (n = 30) were randomized to receive daily TAC, SIR, MMF, TAC+MMF, SIR+MMF, or TAC+SIR (n = 5 per group) treatment after transplantation of 1 × 106 H9DF hESCs into the gastrocnemius muscle. The therapeutic dose range was confirmed by serum drug trough level measurements (Table S1).
As monotherapy, SIR extended hESC survival to the greatest degree. Significantly higher BLI signals from the SIR treated animals were seen up to 7 days after transplantation compared with the nontreated (NT) group (BLI signal at day 7, SIR: 6.4 ± 0.29 vs. NT, 4.98 ± 0.04 log[photons per second]; P < 0.05). However, the signal in all single drug treatment groups (TAC, SIR, MMF) had decreased to background levels by day 10 after transplant (Fig. 5 A and C), emphasizing the strong anti-hESC immune response despite high dose immunosuppressive treatment. In our model, addition of MMF did not result in significant improvement of hESC survival over single TAC and/or SIR treatment (Fig. 5 B and D). Combined TAC+SIR treatment, however, markedly improved survival of hESCs. BLI signals from the TAC+SIR-treated animals were significantly higher starting at 7 days after transplantation and could be followed out to day 28 (Fig. 5 B and D). Finally, the efficacy of combined TAC+SIR treatment for effective suppression of the recipient anti-hESC immune response was confirmed by in vitro analysis. ELISPOT assay showed a significant inhibition of effector cytokine production (TAC+SIR: INF-γ, 113 ± 32 and IL-4: 45 ± 12 vs. NT: INF-γ, 488 ± 91 and IL-4, 529 ± 57; P < 0.01) (Fig. 5E) and FACS analysis revealed a strong trend in reduction of circulating xeno-reactive antibodies (TAC+SIR, 4.8 ± 0.5 vs. NT, 7.0 ± 1.2; P = 0.14) (Fig. 5F).
Discussion
The field of hESC-based therapy is advancing rapidly. Although federal regulations still restrict the generation of new hESC lines in the United States, regional funding institutions such as the California Institute of Regenerative Medicine foresee hESC-based therapies to go into phase-I clinical trails within the next 10 years (23). To accomplish such goals, several significant hurdles that preclude clinical translation of such therapy need to be overcome, of which hESC immunogenicity is a major concern (16).
This study was designed to characterize hESC immunogenicity in a human-to-mouse transplantation model, and to evaluate the efficacy of different immunosuppressive drug regimens to improve transplanted hESC survival. Specifically, we have demonstrated that: (i) molecular imaging can be used to quantify hESC survival and noninvasively follow donor cell fate; (ii) hESCs can trigger potent cellular and humoral immune responses after transplantation into immunocompetent mice, resulting in intragraft infiltration of a variety of inflammatory cells, leading to rejection; (iii) CD4+ T lymphocytes play an important role in mouse anti-hESC rejection; and (iv) an immunosuppressive drug regimen consisting of TAC and SIR significantly mitigates the host immune response to prolong hESC survival.
Specific studies evaluating immunogenicity of hESCs in vivo are few and have yielded mixed conclusions regarding hESC's potential to induce immune response and/or survive in xenogeneic hosts (11, 15, 16). In these studies, results were based on histological techniques to evaluate hESC survival. To allow noninvasive cell tracking, our group has developed and validated reporter gene-based molecular imaging techniques. In particular, fLuc-based optical BLI has proven to be a reliable technique for assessing engraftment and survival of stem cells after transplantation (24). An important advantage in using BLI is that the expression of the fLuc reporter gene, which is integrated into the DNA of the transplanted cells, is expressed only by living cells, making it a highly accurate tool for tracking cell graft rejection in the living subject (25). Using this approach in this report, we have clearly shown impaired survival of hESCs in immunocompetent versus immunodeficient mice, a phenomenon which was even more pronounced after repeated transplantation of the hESCs.
Xenotransplantation of cells or organs is usually complicated by severe immunological responses (21). Previous studies have addressed murine xenogeneic immune responses to adult human cells or tissues. For example, human-to-mouse pancreatic islet transplants trigger progressive infiltration of lymphocytes leading to rejection within 5–6 days (26). Human skin transplants are rejected by immunocompetent mice within 10 days, and a delay of rejection is seen when skin is transplanted onto mice lacking CD4+ T cells, but not on those lacking CD8+ T cells (27). A comparison of these data to the results of our study, in which we show a similar time course of rejection of hESCs (7–10 days) that seems largely mediated by CD4+ T cells, suggests that hESCs are recognized by the murine immune system in a comparable way as adult human cells. This leads us to conclude that, in a discordant xenotransplant model, hESCs do not retain immune-privileged and/or immunosuppressive properties. During the first 10 days after transplantation, spontaneous nonimmune related hESC death also occurred in immunodeficient mice (Fig. 2). In immunocompetent mice, spontaneous hESC death could have lead to activation of the adaptive immune system through the indirect pathway, in which intracellular antigens shed by hESC debris are phagocytosed by host APCs and presented to CD4+ T lymphocytes. This would explain the major role of CD4+ cells that we found in our study.
Studies addressing the character and intensity of immune responses toward hESCs in a human allogeneic setting in vivo raises ethical considerations and thus are currently not feasible. However, the results of this study emphasize that solutions which can reduce or eliminate potential immune responses need to be evaluated. Strategies that could prevent hESC immune recognition include: (i) forming MHC isotyped hES cell-line banks; (ii) creating a universal donor cell by genetic modification; (iii) inducing tolerance by hematopoietic chimerism; (iv) generating isogeneic hESC lines by somatic nuclear transfer; and/or (v) using immunosuppressive medication (28, 29). In the near future, successful clinical application of hESC-based transplantation will most likely rely on immunosuppressive therapy based in part on the experience learned from organ transplantation. Thus, the significance of evaluating the effects of immunosuppressive drugs upon hESC survival in our animal model is twofold: (i) to investigate the efficacy of various compounds that may be used in conjunction with clinical hESC-based therapies in the future and (ii) to develop an immunosuppressive drug regimen that optimizes hESC survival in animal models. Our results show that, in a xenogeneic murine setting, a combined immunosuppressive drug regimen consisting of TAC and SIR optimally suppressed anti-hESC immune response and prolonged their survival to 28 days after transplantation. TAC and SIR are a potential combination for an immunosuppressive strategy because of their different mechanisms of action, side-effect profiles, and apparent synergism when used together (30). TAC and SIR are structurally similar macrolide immunosuppressants. Both drugs bind to a common family of immunophilins called FK506 binding proteins (FKBPs). SIR binds to FKBP, thereby blocking signal transduction by inhibiting two kinases late in the G1 cell cycle progression. These kinases have been designated TOR-1 and -2, targets of rapamycin. TAC exerts its effect through the inhibition of calcineurin, by the FK506/FKBP complex. Calcineurin plays a critical role in interleukin-2 promoter induction after T cell activation (22). Although this combination is used with caution in clinical transplantation because of potential adverse drug effects, we recommend applying this treatment protocol to studies in preclinical animal models that address the biology and therapeutic efficacy of hESC-derivatives.
In summary, our data show that hESC xenografts are effectively recognized and rejected by the adaptive murine immune system after transplantation. We also show that standard immunosuppressive drugs have the potency to prolong survival of the transplanted cells but cannot completely prevent rejection. Finally, the integration of molecular imaging techniques for development and validation of different strategies to improve posttransplant survival of hESC-derivatives should accelerate progress in this field.
Methods
Lentiviral Production and Generation of Stable hESC Line.
SIN lentivirus (LV) was prepared by transient transfection of 293T cells (31). H9 hESCs (Wicell) were transduced with LV-pUB-fLuc-eGFP DF reporter gene at a multiplicity of infection of 10. The infectivity was determined by eGFP expression as analyzed on FACScan (BD Bioscience). The eGFP positive cell populations (≈20%) were isolated by fluorescence activated cell sorting (FACS) Vantage SE cell sorter (Becton Dickinson Immunocytometry Systems) followed by plating on the feeder layer cells for culturing.
Culture and Transplantation of hESCs.
H1, H9 and H9DF hESCs were initially maintained on top of murine embryonic fibroblasts feeder (MEF) layers as detailed in SI Methods. To prevent contamination of the transplanted hESC population with MEF, hESC colonies were separated from MEF by incubation with dispase (Invitrogen) and subcultured on feeder-free matrigel (hESC qualified, BD Biosciences) coated six-well plates in mTeSR1 maintenance medium (Stem Cell Technologies) for two to five passages. MHC expression on hESCs was evaluated by flow cytometry as detailed in SI Methods. Shortly before transplantation, hESCs were trypsinated, and resuspended in sterile PBS at 1 × 106 cells per 20 μl. hESC viability was >95% as determined by flow cytometry using 7-amino-actinomycin D (7-AAD) cell viability solution (eBioscience). hESC transplantation was performed by direct injection into gastrocnemius muscles of recipient mice using a 29.5-gauge insulin syringe.
Animal Experiments.
All animal procedures were approved by the Animal Care and Use Committee of Stanford University. Mouse stains are detailed in SI Methods.
Optical Bioluminescent Imaging of hESC Transplanted Animals.
BLI was performed using the Xenogen in vivo imaging system as previously described (32). Briefly, mice were anesthetized with isoflurane and d-luciferin was administered i.p. at a dose of 375 mg per kilogram of body weight. At the time of imaging, animals were placed in a light-tight chamber, and photons emitted from luciferase expressing hESCs transplanted into the animals were collected with integration times of 5 sec to 5 min, depending on the intensity of the bioluminescence emission. The same mice were scanned repetitively as per the study design. BLI signal was quantified in units of photons per second (total flux) and presented as log[photons per second].
Quantification of Graft-Infiltrating Cells.
Intra-hESC graft-infiltrating cells were measured by FACS analysis of enzymatically digested gastrocnemius muscles as detailed in SI Methods.
Quantification of Cellular Immune Response.
Animals were killed on day 5, and the spleens were harvested and splenocytes were isolated. ELISPOT assays using 1 × 105 γ-irradiated hESCs (1,500 rad) as stimulator cells and 1 × 106 recipient splenocytes as responder cells were performed according to the manufacturer's protocol (BD Bioscience) using IFN-γ and IL-4-coated plates. Spots were automatically enumerated using an ELISPOT plate reader (CTL) for scanning and analyzing.
Quantification of Humoral Immune Response.
Donor-specific xenoreactive antibodies were detected by FACS analysis of target hESCs after incubation with recipient mouse serum as detailed in SI Methods.
Immunosuppressive Therapy.
Tacrolimus (TAC, 4 mg·kg−1·d−1; Sigma-Aldrich), sirolimus (SIR, 3 mg·kg−1·d−1; Rapamune oral solution; Sigma-Aldrich), and mycophenolat mofetil (MMF, 30 mg·kg−1·d−1; Roche) were administered once daily as detailed in SI Methods.
Statistical Analysis.
Data are presented as mean ± SEM. Comparisons between groups were done by independent sample t tests or ANOVA with LSD post-hoc tests, where appropriate. Differences were considered significant for P < 0.05. Statistical analysis was performed using SPSS statistical software for Windows (SPSS).
Supplementary Material
Supporting Information
Acknowledgments.
We thank Dr. Hannes Vogel for histopathological analysis. This work was supported in part by National Institutes of Health Grants HL074883 and HL089027 (to J.C.W), a Burroughs Wellcome Foundation Career Award in Biomedical Sciences (to J.C.W), the California Institute of Regenerative Medicine Grant RS1-00322 (J.C.W), the Howard Hughes Medical Institute (M.M.D.), an International Society for Heart and Lung Transplantation research grant (to S.S.), and the European Society for Organ Transplantation–Astellas Study and Research Grant (to R.J.S.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Hur T, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 4.Swijnenburg RJ, van der Bogt KE, Sheikh AY, Cao F, Wu JC. Clinical hurdles for the transplantation of cardiomyocytes derived from human embryonic stem cells: Role of molecular imaging. Curr Opin Biotechnol. 2007;18:38–45. doi: 10.1016/j.copbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2:859–871. doi: 10.1038/nri934. [DOI] [PubMed] [Google Scholar]
- 6.Drukker M, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells. 2008;26:89–98. doi: 10.1634/stemcells.2007-0151. [DOI] [PubMed] [Google Scholar]
- 8.Min JY, et al. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003;125:361–369. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 9.Menard C, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: A preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 10.Fandrich F, et al. Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nat Med. 2002;8:171–178. doi: 10.1038/nm0202-171. [DOI] [PubMed] [Google Scholar]
- 11.Li L, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448–456. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 12.Swijnenburg RJ, et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 13.Robertson NJ, et al. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc Natl Acad Sci USA. 2007;104:20920–20925. doi: 10.1073/pnas.0710265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grinnemo KH, et al. Human embryonic stem cells are immunogenic in allogeneic and xenogeneic settings. Reprod Biomed Online. 2006;13:712–724. doi: 10.1016/s1472-6483(10)60663-3. [DOI] [PubMed] [Google Scholar]
- 15.Drukker M, et al. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 16.Grinnemo KH, Sylven C, Hovatta O, Dellgren G, Corbascio M. Immunogenicity of human embryonic stem cells. Cell Tissue Res. 2007;331:67–78. doi: 10.1007/s00441-007-0486-3. [DOI] [PubMed] [Google Scholar]
- 17.Caspi O, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 18.van der Bogt KE, Swijnenburg RJ, Cao F, Wu JC. Molecular imaging of human embryonic stem cells: Keeping an eye on differentiation, tumorigenicity and immunogenicity. Cell Cycle. 2006;5:2748–2752. doi: 10.4161/cc.5.23.3533. [DOI] [PubMed] [Google Scholar]
- 19.Game DS, Lechler RI. Pathways of allorecognition: Implications for transplantation tolerance. Transpl Immunol. 2002;10:101–108. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 20.Draper JS, Pigott C, Thomson JA, Andrews PW. Surface antigens of human embryonic stem cells: Changes upon differentiation in culture. J Anat. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auchincloss H, Jr, Sachs DH. Xenogeneic transplantation. Annu Rev Immunol. 1998;16:433–470. doi: 10.1146/annurev.immunol.16.1.433. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Lomax GP, Hall ZW, Lo B. Responsible oversight of human stem cell research: The California Institute for Regenerative Medicine's medical and ethical standards. PLoS Med. 2007;4:e114. doi: 10.1371/journal.pmed.0040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao YA, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao YA, et al. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation. 2005;80:134–139. doi: 10.1097/01.tp.0000164347.50559.a3. [DOI] [PubMed] [Google Scholar]
- 26.Lenschow DJ, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 27.Uchida T, et al. Roles of CD4+ and CD8+ T cells in discordant skin xenograft rejection. Transplantation. 1999;68:1721–1727. doi: 10.1097/00007890-199912150-00016. [DOI] [PubMed] [Google Scholar]
- 28.Drukker M. Immunogenicity of human embryonic stem cells: Can we achieve tolerance? Springer Semin Immunopathol. 2004;26:201–213. doi: 10.1007/s00281-004-0163-5. [DOI] [PubMed] [Google Scholar]
- 29.Boyd AS, Higashi Y, Wood KJ. Transplanting stem cells: Potential targets for immune attack. Modulating the immune response against embryonic stem cell transplantation. Adv Drug Deliv Rev. 2005;57:1944–1969. doi: 10.1016/j.addr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 31.De A, Lewis XZ, Gambhir SS. Noninvasive imaging of lentiviral-mediated reporter gene expression in living mice. Mol Ther. 2003;7:681–691. doi: 10.1016/s1525-0016(03)00070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao F, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information