VEGF-C induced angiogenesis preferentially occurs at a distance from lymphangiogenesis (original) (raw)
. Author manuscript; available in PMC: 2009 Jan 2.
Published in final edited form as: Cardiovasc Res. 2007 Dec 7;78(2):315–323. doi: 10.1093/cvr/cvm094
Abstract
Aims
Vascular endothelial growth factor-C (VEGF-C) has been shown to stimulate both angiogenesis and lymphangiogenesis in some but not all models where VEGF-C is over-expressed. Our aim was to investigate the interaction between lymphangiogenesis and angiogenesis in adult tissues regulated by VEGF-C and identify evidence of polarized growth of lymphatics driven by specialized cells at the tip of the growing sprout.
Methods and results
We used an adult model of lymphangiogenesis in the rat mesentery. The angiogenic effect of VEGF-C was markedly attenuated in the presence of a growing lymphatic network. Furthermore, we show that this growth of lymphatic vessels can occur both by recruitment of isolated lymphatic islands to a connected network and by filopodial sprouting. The latter is independent of polarized tip cell differentiation that can be generated all along lymphatic capillaries, independently of the proliferation status of the lymphatic endothelial cells.
Conclusion
These results both demonstrate a dependence of VEGF-C-mediated angiogenesis on lymphatic vascular networks and indicate that the mechanism of VEGF-C-mediated lymphangiogenesis is different from that of classical angiogenic mechanisms.
Keywords: Angiogenesis, Lymphangiogenesis, VEGF-C, Sprouting
1. Introduction
Unlike the network of blood vessels, the lymphatic vessels form a blind-ended transit route, carrying the protein rich lymph from the interstitium back into the blood circulation, and enabling immune screening by extravascular circulation of leucocytes.1 The remodelling of a lymphatic system in adult tissues is required during episodes of tissue damage (such as during wound healing) and also appears to be a major contributor to metastasis of tumours. The development and remodelling of the lymphatic system appears to be primarily dependent on expression of vascular endothelial growth factor-C (VEGF-C) in surrounding tissues, which stimulates growth of lymphatics into the areas of high interstitial flow and transport. The mechanisms by which lymphatic endothelial cells (LEC) migrate into tissue in response to VEGF-C up-regulation in adult tissues are not well understood, although the use of developmental models of VEGF-C overexpression have given many insights into the molecular and physiological mechanism of embryonic lymphangiogenesis.
Adenovirally expressed VEGF-C (Ad-VEGF-C) has been shown to induce an increase in lymphatic density and increased proliferation of LEC in the mouse skin2 and ear.3 Ad-VEGF-C delivered to the mucosal and tracheal membranes4 and systemic administration of recombinant VEGF-C5 induced a pronounced lymphangiogenic response. Transgenic overexpression of VEGF-C in the mouse skin6 and tail7 results in increased proliferation and radial growth of lymphatic vessels, although no increase in lymphatic density was reported in the tail.7 It has been reported that in the mouse tail, VEGF-C is critical for the proliferation of LEC and initial sprout formation,8 and other interstitial forces act to remodel the sprouts into functional lymphatic vessels.9 The physiological mechanisms by which VEGF-C induces lymphangiogenesis are therefore not yet determined.
VEGF-C overexpression has been shown to result in sprouting lymphangiogenesis, and many of the models of lymphangiogenesis have been based on the mechanism of blood vessel growth.10 In particular, blood vessels are thought to grow principally through either polarized sprout formation, whereby specialized cells at the tip of the growing sprout (tip cells) differentiate to guide and drive sprout formation11,12 in response to gradients of VEGF,13 or intussusception of the vessel driven by pericyte mediated focused pressure resulting in elongation of cellular columns through blood vessels to result in splitting of the vessel.14 However, the presence of either specialized tip cells or intussusceptive growth have not been demonstrated in lymphatics in vivo.
VEGF-C has also been reported to induce angiogenesis in the absence of lymphatic tissue,15 but when lymphatic and blood vessels were present there was a preferential lymphangiogenic response.3,16,17 Consequently, it is unknown if the presence of lymphatics inhibits VEGF-C driven angiogenesis or if there is any interaction between the blood endothelium and lymphatic endothelium. The rat mesenteric angiogenesis model permits detailed examination of the phenotype of angiogenesis, including the types of vessels generated, their functional effectiveness, detailed analysis of which cells are proliferating, discrimination between hypertrophic and hyperplastic vessels and so on.19,20 By adapting this model to investigate lymphangiogenesis such that we can quantitatively analyse both blood and lymphatic vessels we have been able to determine the role of the lymphatic system in the regulation of the angiogenic effects of VEGF-C, as well as determining lymphangiogenic physiological mechanisms.
2. Methods
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The rat mesenteric angiogenesis assay was used as previously described. In brief, Male Wistar rats (∼350 g) were anaesthetized by inhalation of halothane and body temperature maintained at 37°C. A laparotomy was performed and part of the intestine exposed. A mesenteric panel was exposed under an intravital microscope (Leica DMIL). The panel was imaged using a Leica DC350F (Leica, Bucks, UK) digital camera and 25 μL of virus (108 plaque forming units) injected into the nearby fat pad with a Hamilton syringe fitted with a 30-gauge needle. Ad-VEGF-C18 or adenovirus expressing enhanced green fluorescent protein (EGFP), Ad-EGFP (a kind gift of J Uney, University of Bristol), was used. Ten microlitres of Monastral blue (0.6%, diluted in saline) was injected into the fat pads on either side of the virus-injected panel. The intestine was gently put back into the cavity and the animal sutured and recovered. After 14 days (day 14), the animal was re-anaesthetized with halothane, and a laparotomy performed. The mesentery was exposed and the virus injected panel located from Monastral blue injection sites. The mesenteric panel was imaged as described above.
Mesenteric panels with a clear lymphatic vessel as identified by intravital microscopy were chosen for the lymphangiogenesis model. If these panels were injected with Ad-VEGF-C they were termed VEGF-C (L+). To determine any diverse effects of VEGF-C on angiogenesis in the absence of lymphatic vessels, panels not containing pre-existing lymphatics were injected and these were termed VEGF-C (L−). EGFP was used as a control. Animals were injected on day 1, and the mesentery excised and the animal sacrificed on day 14. Five random images were taken of lymphatic vessels, and of regions of the mesentery that had no lymphatic drainage. To calculate lymphatic density low power immunofluorescent images were taken.
The panels were washed with 0.5% Triton-X100 in PBS (0.5% PBS-T), for 1 h, changing solutions every 10 min at room temperature. The panels were blocked in 1% BSA-0.5% PBS-T 1 h. The mesentery was incubated overnight at 4°C, with biotinylated Griffonia simplicifolia isolectin B4 (IB4, Molecular Probes, Cambridge, UK) 10 μg/ml and mouse monoclonal antibodies to Ki-67 (Novocastra Lab, Newcastle upon Tyne, UK, NCL-L-Ki67-MM1), rabbit anti-mouse lymphatic vessel endothelial hyaluronic acid receptor (LYVE-1) (10 μg/μl, a kind gift from Dr David Jackson, University of Oxford), or goat-anti-mouse VEGFR-3 (1 μg/mL, R&D systems).
Panels were washed as before, and TRITC labelled streptavidin (1 μg/mL, S-870, Molecular Probes) and Alexa Fluor 488, goat-anti-mouse IgG (2 μg/mL, Molecular Probes) were used as secondary detection antibodies to Lectin and Ki67, respectively. A donkey-anti-goat Alexa Fluor 555 (2 μg/ml, Molecular Probes) was used to detect VEGFR-3, and sheep anti-rabbit IgG FITC conjugate (product F7515, 1:160, Sigma) was used to detect LYVE-1. Primary antibodies and lectin were incubated overnight, on a rocker at 4°C. The panels were washed for 1 h, changing the PBS-T solution six times and secondary incubation lasted 2 h, at room temperature on a rocker. The panels were washed a further six times and if appropriate Hoechst 33324 (1 μM, Molecular Probes) was added to stain mesenteric nuclei.
The panels were carefully manipulated using fine forceps and flattened on a glass slide before being mounted in Vectashield (Vector Lab, Peterborough) and a coverslip was carefully placed on the tissue. The tissue was imaged using Leica Confocal Microscope (Leica Confocal TCS-NT DMIRBE). Five random sections of each panel were imaged and analysed off line at ×40 magnification with oil immersion. Overlaying the images permitted the quantification of vessel phenotype and the presence of proliferating cells in either vessel type. Intravital microscopy was used to image the increase in functional blood vessel area (FVA). ELISA using antibodies raised against human VEGF-C (R&D Systems, DVEC00) was used to determine effective gene transfer by Ad-VEGF-C.
Vessel parameters were measured as previously described.19,20 Briefly, for each mesentery, 8-12 views were selected randomly using a ×40 objective and Openlab software (Improvision, Coventry, UK) used to measure vessel parameters. The total blood (isolectin positive, LYVE-1 negative) or lymphatic vessels (Lyve-1 positive) were counted and labelled, and branch points, proliferating endothelial cells, and sprouts in each image counted. The diameter and length of each vessel were measured. Branch point density, sprout density, and proliferating endothelial cell density were calculated as the number per unit area within five randomly selected fields of view (×40 objective) containing vessels as previously described.19,20 Blood vessels were classified into two groups: <16 μm (exchange vessels) and >16 μm (conduit or resistance vessels-generally arterioles and venules).
All data are presented as mean ± SEM. EGFP blood vessel data n = 12, VEGF-C (L−) n = 11 and VEGF-C (L+) n = 7 except Ki67 data where EGFP n = 8, VEGF-C n = 4. All lymphatic EGFP n = 9, VEGF-C n = 9 except for Ki67 data where EGFP n = 6. All data were analysed by either Student’s _t_-test or student Newman-Keuls where appropriate. P < 0.05 was considered statistically significant.
3. Results
3.1 Vascular endothelial growth factor-C induces angiogenesis
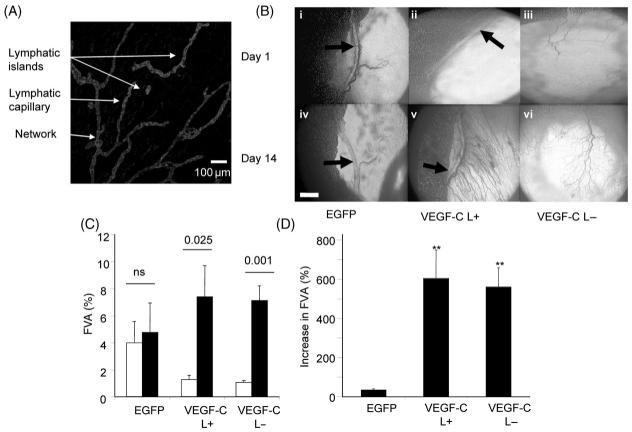
Ad-VEGF-C transduction resulted in significant up-regulation of VEGF-C concentration in the mesenteric fat pad from undetectable in EGFP transduced mesenteries to 5.7 ± 1.9 pg/mg. LYVE-1 staining resulted in initial lymphatics being visualized (Figure 1A) in panels where clear lymphatic collecting ducts were seen by intravital microscopy (Figure 1Bi and ii, arrows). There was a network of lymphatics in the mesentery. This network consisted of blind ending lymphatic capillaries draining into second order lymphatics, which coalesced as a draining network. Of note there were many lymphatics that showed no connection to the pre-existing lymphatic network either by staining with podoplanin, by LYVE 1 or by a pan-endothelial stain IB4. These ‘lymphatic islands’ were seen in most mesenteries and averaged 5 ± 2 islands per panel (Figure 1A). In most mesenteric panels, however, no clear lymphatic collectors were seen (e.g. Figure 1Biii). In these panels, no lymphatics were seen by staining either with LYVE-1 or podoplanin.
Figure 1.
(A) LYVE-1 staining of lymphatic vessels in the mesenteric panel containing a major lymph vessel. (B) Vascular endothelial growth factor-C increased the functional vessel area (FVA) 14 days post-injection irrespective of the presence of lymphatic collecting vessels. (C) Quantification of the increase in functional vessel area. EGFP did not induce an increase in functional vessel area, but vascular endothelial growth factor-C in the presence or absence of lymphatics did. Open bars = day 1, black bars = day 14. (D) The vascular endothelial growth factor-C induced increase in functional vessel area was significantly higher than EGFP. **P < 0.01 vs. EGFP.
Panels in which a clear lymphatic vessel was present were injected by adenovirus containing either GFP or VEGF-C and analysed for their effect on angiogenesis and lymphangiogenesis. Very few vessels were visualized on day 14 that were not present before injection with Ad-EGFP (Figure 1Biv compared with Bi). In contrast, vessels were seen 14 days after injection with Ad-VEGF-C that were not visualized before injection both in the presence (Figure 1Bv) and absence (Figure 1Bvi) of pre-existing collecting lymphatics. EGFP administration did not significantly increase the functional blood vessel area (FVA) 14 days following injection (Figure 1C and D), but VEGF-C induced an increase in FVA irrespective of pre-existing lymphatics (Figure 1C and D, one way ANOVA). There was no difference between the FVA before injection in the groups.
VEGF-A has been demonstrated to be capable of inducing hyperplasia as well as hypertrophy of blood vessels.20 To determine whether the VEGF-C induced increase in FVA was due to angiogenesis, mesenteric panels were stained with IB4 for endothelial cells, LYVE-1 for lymphatics and Ki67 for dividing cells (Figure 2A). VEGF-C induced increased blood vessel density (Figure 2B), sprouting (Figure 2C), and branching (Figure 2D) and reduced blood vessel length (Figure 2E) irrespective of the presence of lymphatics elsewhere in the panel (not shown on figure), but did not affect mean blood vessel diameter (Figure 2F). VEGF-C also increased the number of proliferating blood endothelial cells in the absence of lymphatics, but not in their presence (Figure 2G). To determine whether the effect of VEGF-C was a uniform effect on all blood vessels, the mean blood vessel density and proliferating vascular endothelial density were measured separately in exchange vessels and conduit vessels. While VEGF-C increased the density of both types of blood vessels irrespective of the presence of lymphatics (Figure 3A and B), the proliferating vascular endothelial cell density was only increased in the absence of lymphatics (Figure 3C and D). The frequency distribution of blood vessel diameters shows that VEGF-C increased frequency of all vessel types (Figure 3E).
Figure 2.
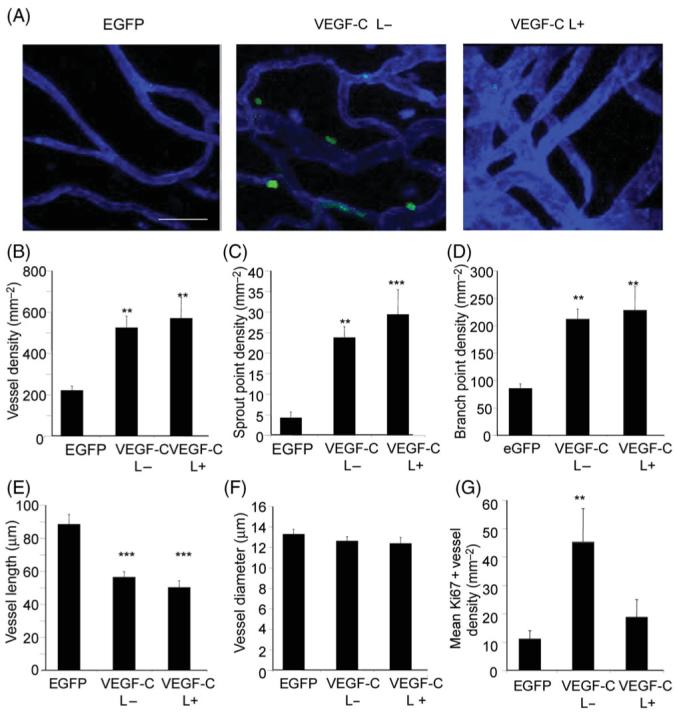
Vascular endothelial growth factor-C (VEGF-C) induced angiogenesis. (A) Confocal stack images of blood vessels induced in the presence (VEGF-C L+) or absence (VEGF-C L−) of lymphatics elsewhere in the mesenteric panel. Blood vessels are stained with isolectin B4 (blue), and dividing cells with Ki67 (green). In the fields of view shown, no lymphatics were visualized. Vascular endothelial growth factor-C increased vessel (B), sprout (C), and branch point (D) density, thus reducing vessel length (E) but not diameter (G). (F) Vascular endothelial growth factor-C only increased Ki67 positive vessel density in the absence of lymphatics (VEGF-C L−). **P < 0.01, ***P < 0.001 vs. EGFP.
Figure 3.
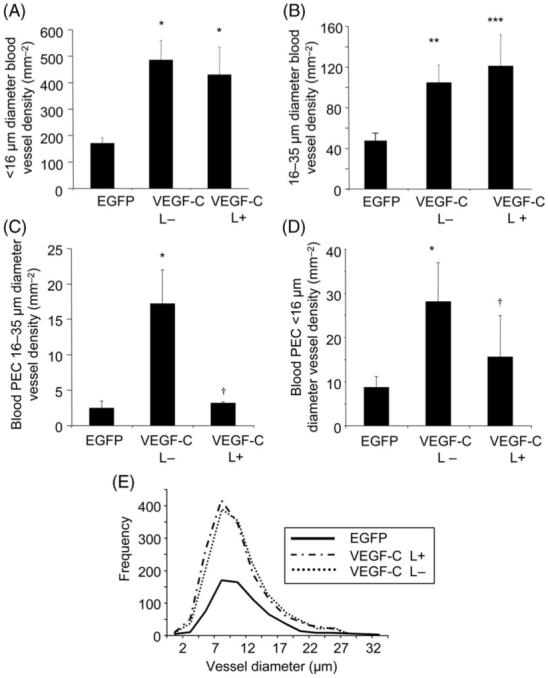
Vascular endothelial growth factor-C induced hyperplasia (A) and hypertrophy (B) correspondingly, but increased proliferation in both vessel subtypes only in the absence of lymphatics (C, D). Frequency distribution of the vessel diameters formed revealed vascular endothelial growth factor-C induced a significantly different distribution of microvessel diameters from that of EGFP irrespective of the presence of lymphatics (P = 0.0015). *P < 0.05. **P < 0.01, ***P < 0.001 vs. EGFP. †P < 0.05 vs. VEGF-C L−.
3.2 Adenovirally expressed-vascular endothelial growth factor-C induced lymphangiogenesis in vivo
Epifluorescence of day 14 mesenteric panels stained for the lymphatic specific marker LYVE-121 revealed the extent of the lymphatic system in the mesenteric panel. EGFP transfected panels had a small but clear lymphatic network (Figure 4A), consisting of lymphatic islands (isolated lymphatic vessels, arrowhead) and a network of blind ending lymphatic vessels. VEGF-C (Figure 4B) dramatically increased the density and extent of the lymphatic bed, and no lymphatic islands were subsequently seen. The total lymphatic density was significantly increased by VEGF-C compared with control (Figure 4C, P < 0.01). Further analysis revealed that the number of primary order lymphatic vessels was also significantly increased by VEGF-C (Figure 4D, P < 0.03). These vessels were defined as those that only connected with another vessel at one end (i.e. blind-ending). Moreover, VEGF-C also increased the number of higher order lymphatic vessels (i.e. the connecting/draining vessels, P < 0.05, Figure 4E).
Figure 4.
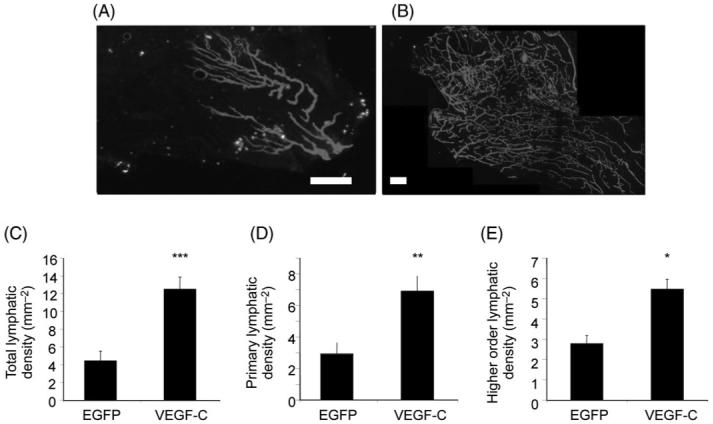
Low power microscopy of EGFP treated (A) and vascular endothelial growth factor-C treated (B) panels. Vascular endothelial growth factor-C induces growth of primary (C) and higher order lymphatics (D), resulting in a total increase in lymphatic density (E). *P < 0.05, **P < 0.03, ***P ≤ 0.01, scale bar 500 μm.
3.3 VEGF-C induces alterations in the lymphatic endothelium
Randomly selected high-power confocal imaging of lymphatic vessels stained for LYVE-1, lectin and Ki67 (Figure 5A-D) were used to quantify measurable parameters of lymphatic vessel morphology. VEGF-C resulted in lymphatic vessel sprouting (Figure 5E). The sprouts contained multiple fine filopodia extending out into the tissue. Filopodia were not present solely on ‘tip cells’ as seen in angiogenic vessels,11 but were present both on sprouts (Figure 5E) and along the length of the vessels (Figure 5F). Proliferating endothelial cells were seen throughout the lymphatic vessels, including at the terminal ends of the sprouts, and in cells extending filopodia (Figure 5G). VEGF-C induced a significantly higher degree of Ki67 positive vessels (Figure 5H, P < 0.05) compared with EGFP. Furthermore, VEGF-C increased the density of filopodial projections compared with EGFP (Figure 5I, P < 0.05). Lymphatic vessel segment length was reduced by VEGF-C (Figure 5K, P < 0.001) and consistently, lymphatic branching was significantly increased (Figure 5J, P < 0.05) compared with EGFP. There was no significant difference in vessel diameter, however, between EGFP and VEGF-C (Figure 5L, P = 0.8) indicating a lack of vessel hypertrophy. The sprouting all along the lymphatic vessels was in contrast with the sprouting in blood vessels induced by VEGF-C where single tips cell appeared to extend one filopodia, and stalk cells did not appear to send out filopodia (Figure 5M). In summary, VEGF-C also altered the lymphatic vessels, and suggests a filopodia-based mechanism of how lymphatic growth might occur.
Figure 5.
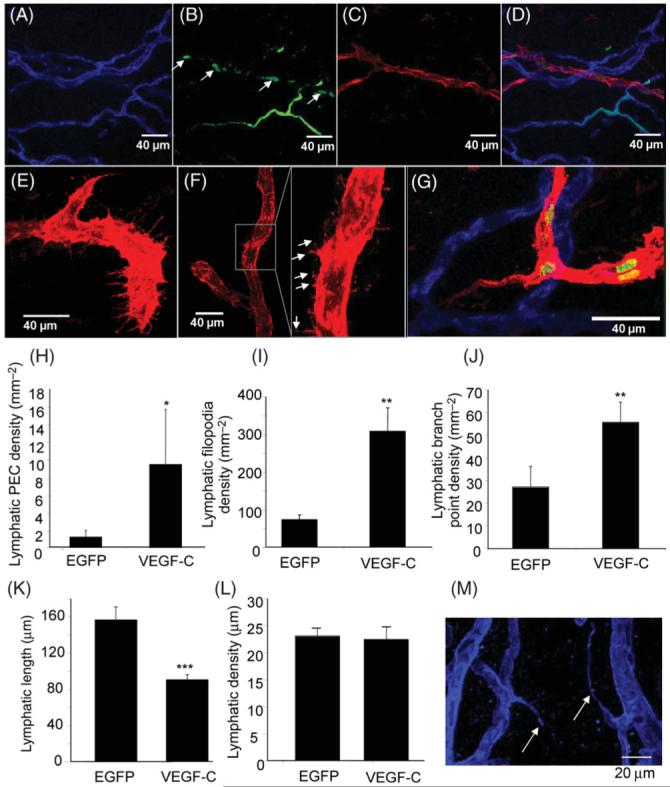
Vascular endothelial growth factor-C treated mesenteries stained with IB4 (A), Ki67 (B) and LYVE 1 (C). The overlay (D) shows that vascular endothelial growth factor-C induced proliferation of lymphatic vessels as well as blood vessels. Vascular endothelial growth factor-C induced filopodial extensions both at the blind ends of the lymphatic system (E), and along the length of the vessel (F, inset). Proliferation was seen both in the cells at the end of the sprouts and in the cells behind (G). Vascular endothelial growth factor-C induced proliferation (H) filopodia (I) and increased branching (J) and reduced lymphatic vessel length (K) without changing the diameter of the vessels (L). Blood vessels had sprouting from tip cells, not stalk cells (M, scale bar 20 μm). *P < 0.05, ** P < 0.002, ***P < 0.001.
3.4 Vascular networks lying near to lymphatic vessels are phenotypically different to vascular endothelial growth factor-C induced networks in the absence of lymphatics
The presence of lymphatic tissue appeared to reduce endothelial proliferation in blood vessels when mesenteric panels with and without lymphatics were compared (Figure 2G). Therefore, to determine whether local association of lymphatic vessels affects the blood microvasculature, the blood vessel phenotype in vessels adjacent to lymphatics was assessed and compared with vessels obtained from areas of mesenteric panels with no lymphatic tissue. VEGF-C did not statistically increase blood vessel density adjacent to the lymphatics (Figure 6A, P > 0.05), whereas VEGF-C induced a significant increase in vessel density compared with EGFP in areas not associated with lymphatics (Figure 6A, P < 0.001). VEGF-C induced sprouting in adjacent vessels was significantly greater than control (Figure 6B, _P_ < 0.01), but significantly less than VEGF-C induced vessels in the absence of lymphatic (Figure 6B, _P_ < 0.01), which was also significantly greater than control (Figure 6B, _P_ < 0.001). Branch point density followed a similar pattern; although VEGF-C increased branching proximally compared with control this did not reach significance (Figure 6C, _P_ > 0.05). In the absence of lymphatics there was a significantly greater branching compared with control (P < 0.001) and with proximal vessel growth (_P_ < 0.01, Figure 6C). In keeping with the increased branch point/sprout point formation, VEGF-C in the absence of lymphatics significantly reduced vessel length compared with control and proximal to the lymphatics (_P_ < 0.01, Figure 6D), whereas VEGF-C adjacent to lymphatics did not induce a significant change in proximal vessel length. Mean vessel diameter was not changed by any VEGF-C treatment compared with control or compared with each other (_P_ > 0.05, Figure 6E). To assess further whether there was a correlation between lymphatic density and the density of blood vessels, a scatter plot of lymphatic density against the density of blood vessels near the lymphatics demonstrated that lymphatic density was inversely correlated with the blood vessel density following VEGF-C treatment (Figure 6F, P < 0.05, Pearson). VEGFR-3 staining co-localized with LYVE-1 (Figure 6G). In the absence of lymphatic vessels, VEGF-C induced a significantly larger number of both exchange capillaries (Figure 6H) and conduit vessels (Figure 6I) compared with EGFP (P < 0.001) or VEGF-C adjacent to lymphatics (P < 0.01).
Figure 6.
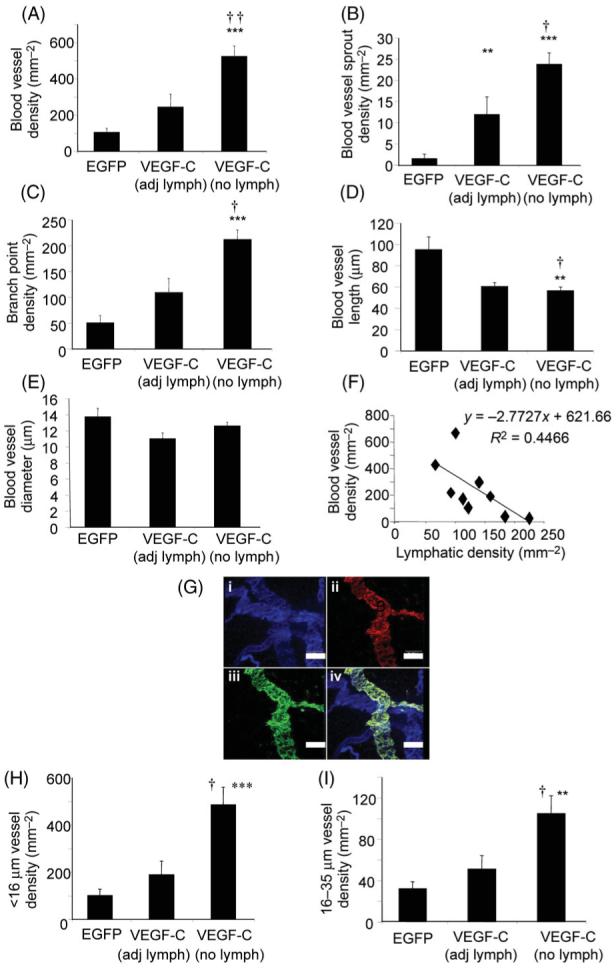
Analysis of blood vessels near lymphatic vessels. Vessels in areas distal to lymphatic vessels are more numerous (A) and with a higher sprout point and branch point density (B, C). Vessel length was reduced by vascular endothelial growth factor-C (D) but vessel diameter was not changed (E). Scatterplot of lymphatic density against blood vessel density (F) revealed an inverse correlation. (G) Confocal image of (i) IB4 (blue), (ii) VEGFR-3 (red), and (iii) LYVE-1 (green). The overlay (iv) shows that VEGFR3 expression was greater in the lymphatic than in the blood vessels. Microvascular hyperplasia (H) and hypertrophy (I) is induced by vascular endothelial growth factor-C is inhibited in the presence of lymphatic vessels. †P < 0.01, ††P < 0.001 vs. vascular endothelial growth factor-C adj lymph **P < 0.01, ***P < 0.001 vs. EGFP.
4. Discussion
4.1 Vascular endothelial growth factor-C induced angiogenesis
VEGF-C induced an increase in functional vessel area and also induced an angiogenic response (increase proliferation and increased vessel density). This angiogenic response, however, appeared to depend upon the presence of local lymphatics. Although VEGF-C has classically been considered as a lymphangiogenic agent, conflicting reports exist on VEGF-C inducing angiogenesis. Overexpression of VEGF-C in the mouse cornea,15,22 chorioallantoic membrane,15,16 mouse skin,3 or mouse ischaemic hindlimb23 induced angiogenesis. In addition to a lymphangiogenic response, all of these studies confirmed an angiogenic response as increased blood vessel density. However, some VEGF-C overexpression studies reported a lack of angiogenesis: in the mature CAM,16 in the mouse skin, where blood vessel morphological changes were reported but no angiogenesis3 and a similar finding in the trachea.4 Furthermore, inhibition of VEGF-C by a soluble VEGFR-3 had no effect on the blood vessels.24
4.2 Vascular endothelial growth factor-C induced lymphangiogenesis
VEGF-C overexpression induced an increase in primary lymphatics and higher order lymphatics. One interpretation of this could be that VEGF-C induces the growth of a new lymphatic tree; however, our model does not permit analysis of lymphatic function, i.e. it is not possible to say if the lymphatic vessels are functional. Injection of a modified VEGF-C virus, which only activates VEGFR-3 was sufficient to induce a pronounced lymphangiogenic response in the subcutaneous tissue of mouse skin,25 and inhibition of VEGF-C (by overexpression of a soluble VEGFR-3) was sufficient to prevent foetal lymphangiogenesis.24 Systemic administration of a neutralizing antibody raised against VEGFR-3 prevented lymphangiogenesis but had no effect upon existing lymphatic vessels in a regenerating lymphatic model,26 and VEGF-C has been demonstrated to be critical for the initial proliferation and initial migration of LECs,8 whereas lymphatic vessel formation can also be maintained by interstitial fluid flow, which synergises with VEGF-C.9
Isolated lymphatic islands were identified in the mesentery, which were not present following VEGF-C treatment. Isolated lymphatic islands have previously been identified in the skin by serial section reconstruction from electron micrograph images, probably the only way to ensure that they are truly lymphatic islands.27 In the skin, it was speculated that they are either regressing lymphatics or they form components of an incomplete network, waiting for a stimulus to reform.27 Therefore, our mesenteric model might rejoin lymphatic islands following VEGF-C treatment in addition to growth of new vessels.
4.3 Vascular endothelial growth factor-C induced angiogenesis is context dependent
Mesenteric panels that did not have a lymphatic vessel observable by intravital microscopy were still LYVE-1 negative after 14 days of VEGF-C treatment. Consequently, VEGF-C was not able to induce spontaneous lymphatic vessel formation. VEGF-C has been reported to induce angiogenesis in the absence of lymphatic tissue15 but in models that have a functional lymphatic system only lymphangiogenesis was observed.3,16,17 In the experiments described here, VEGF-C resulted in less angiogenesis when lymphatics were close by, as evident by reduced blood endothelial proliferation, and reduced blood vessel density in these areas. This was in contrast to the results when an entire panel was investigated (Figure 2), indicating that the local lack of angiogenesis was obscured when the whole area was assessed independent of the presence of lymphatics. This indicates that the local presence of lymphatics needs to be taken into account when the effect of VEGF-C is investigated. The effect of VEGF-C therefore appears to be context dependent.1 Blood and lymphatic vessel growth occurs during development consecutively (lymphatic development lags behind blood vascular development). Given that there were was an inverse correlation between lymphatic and blood vessel density near the lymphatics, and these blood vessels displayed less parameters of vessel activation (branching, sprouting), and VEGF-C binds to VEGFR-3 with a greater affinity than to VEGFR-2,28-30 it is likely that VEGF-C preferentially activates the lymphatic endothelium thus reducing the bioavailability of VEGF-C to the blood vessel endothelium. The finding that vascular density, but not proliferation occurred in panels that did contain lymphatics indicates that VEGF-C did have some effect away from the lymphatics, but that it favoured branching over cell division. Although this supports the concept that VEGF-C biodistribution may be altered by lymphatic vessel presence, it is also possible that VEGF-C stimulates lymphatic cells to release anti-angiogenic agents.
4.4 Vascular endothelial growth factor-C induced lymphatic sprouting and proliferation occurs throughout the lymphatic vessel
VEGF-C induced filopodia along the entire length of lymphatic vessels, as well as at the sprouting tips. This filopodial extension of lymphatic vessels appears to be dependent on VEGFR-3 activation as VEGFR-3 inhibition during inflammation prevented filopodial growth.4 In blood vessels, at least in the retina, filopodial projection is a determinant of sprout point formation,11,31 which is speculated to lead to neovessel formation. However, in blood vessels, the tip cells appear to be critical, and the ability of tip cells to form new sprouts through filopodial extension has been shown to be dependent on the Delta like ligand-4/Notch pathway,12,32 Although we did see filopodial projections on the lymphatic tips, we also saw filopodia along the length of the vessel.
Consequently, it appears that lymphatic growth could originate from anywhere along the lymphatic vessel length. Furthermore, in blood vessels the stalk cells behind the tip cells have been proposed to be proliferative and do not possess filopodia, whereas the tip cells do not proliferate. In contrast, proliferating endothelial cells in lymphatic vessels appeared at the tips of growing sprouts, and these cells also had filopodia. It therefore appears that the molecular mechanisms in growing blood vessels that repress cell cycle in tip cells and filopodia in stalk cells may be different in the lymphatics.
Acknowledgments
Funding
British Heart Foundation (FS/04/22, BB2000003).
Footnotes
Conflict of interest: Professor K.A. is chairman of the scientific board of VeGenics Ltd.
For permissions please email: journals.permissions@oxfordjournals.org.
The online version of this article has been published under an open access model. Users are entitled to use, reproduce, disseminate, or display the open access version of this article for non-commercial purposes provided that the original authorship is properly and fully attributed; the Journal, Learned Society and Oxford University Press are attributed as the original place of publication with correct citation details given; if an article is subsequently reproduced or disseminated not in its entirety but only in part or as a derivative work this must be clearly indicated.
For commercial re-use, please contact journals.permissions@oxfordjournals.org.
References
- 1.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82:673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- 2.Enholm B, Karpanen T, Jeltsch M, Kubo H, Stenback F, Prevo R, et al. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res. 2001;88:623–629. doi: 10.1161/01.res.88.6.623. [DOI] [PubMed] [Google Scholar]
- 3.Saaristo A, Veikkola T, Enholm B, Hytonen M, Arola J, Pajusola K, et al. Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J. 2002;16:1041–1049. doi: 10.1096/fj.01-1042com. [DOI] [PubMed] [Google Scholar]
- 4.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest. 2003;111:717–725. doi: 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirzenius M, Tammela T, Uutela M, He Y, Odorisio T, Zambruno G, et al. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J Exp Med. 2007;204:1431–1440. doi: 10.1084/jem.20062642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 8.Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ. 2006;291:H1402–H1410. doi: 10.1152/ajpheart.00038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ Res. 2003;92:801–808. doi: 10.1161/01.RES.0000065621.69843.49. [DOI] [PubMed] [Google Scholar]
- 10.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 11.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 13.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107–117. doi: 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, et al. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, et al. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- 17.Saaristo A, Karpanen T, Alitalo K. Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene. 2000;19:6122–6129. doi: 10.1038/sj.onc.1203969. [DOI] [PubMed] [Google Scholar]
- 18.Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 19.Benest AV, Salmon AHJ, Wang W-Y, Glover CP, Uney J, Harper SJ, Bates DO. Vascular Endothelial Growth Factor and Angiopoietin-1 stimulate different angiogenic phenotypes that synergise to form a functional vasculature. Microcirculation. 2006;13:423–437. doi: 10.1080/10739680600775940. [DOI] [PubMed] [Google Scholar]
- 20.Wang WY, Whittles CE, Harper SJ, Bates DO. An adenovirus-mediated gene-transfer model of angiogenesis in rat mesentery. Microcirculation. 2004;11:361–375. doi: 10.1080/10739680490437568. [DOI] [PubMed] [Google Scholar]
- 21.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res. 2004;94:664–670. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- 23.Witzenbichler B, Asahara T, Murohara T, Silver M, Spyridopoulos I, Magner M, et al. Vascular Endothelial Growth Factor-C (VEGF-C/VEGF-2) Promotes Angiogenesis in the Setting of Tissue Ischemia. Am J Pathol. 1998;153:381–394. doi: 10.1016/S0002-9440(10)65582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 25.Saaristo A, Veikkola T, Tammela T, Enholm B, Karkkainen MJ, Pajusola K, et al. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med. 2002;196:719–730. doi: 10.1084/jem.20020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 27.Braverman IM, Yen A. Microcirculation in psoriatic skin. J Invest Dermatol. 1974;62:493–502. doi: 10.1111/1523-1747.ep12681007. [DOI] [PubMed] [Google Scholar]
- 28.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 29.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, et al. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol. 2002;160:1683–1693. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 32.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]