Post-transcriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol (original) (raw)
. Author manuscript; available in PMC: 2009 Jul 31.
Summary
Tolerance represents a critical component of addiction. The large conductance calcium-and voltage-activated potassium channel (BK) is a well-established alcohol target, and an important element in behavioral and molecular alcohol tolerance. We tested whether microRNA, a newly-discovered class of gene expression regulators, plays a role in the development of tolerance. We show that in adult mammalian brain alcohol upregulates microRNA (miR-9) and mediates post-transcriptional reorganization in BK mRNA splice variants by miR-9-dependent destabilization of BK mRNAs containing 3’UTRs with a miR-9 Recognition Element (MRE). Different splice variants encode BK isoforms with different alcohol sensitivities. Computational modeling indicates that this miR-9 dependent mechanism contributes to alcohol tolerance. Moreover, this mechanism can be extended to regulation of additional miR-9 targets relevant to alcohol abuse. Our results describe a novel mechanism of multiplex regulation of stability of alternatively spliced mRNA by miRNA in drug adaptation and neuronal plasticity.
Introduction
A challenge to our understanding of addiction is to identify adaptations within individual molecules that underlie tolerance. In this study, we identify dramatic molecular changes attributable to post-transciptional modulation of mRNA stability by miRNA that occurs within minutes of alcohol exposure.
Diversity of mRNA species starts with alternative splicing in the cell nucleus, where different exonal combinations of the gene are selected (Black, 2000; Black, 2003; Smith and Valcarcel, 2000) to form various transcripts of the same gene. These transcripts are exported to the cytoplasm, where their stability is regulated by post-transcriptional processes (Guhaniyogi and Brewer, 2001), and protein isoforms with varying properties are produced from the different transcripts (Coetzee et al., 1999; Dredge et al., 2001; Shipston, 2001). These regulatory processes (Blencowe, 2006; Grabowski and Black, 2001) play essential roles in neuronal plasticity, allowing the neuron to quickly fine-tune its protein composition to adapt to different stimuli. These processes are also involved in neurological diseases (Licatalosi and Darnell, 2006, Guhaniyogi and Brewer, 2001). Here, we explore whether a drug of abuse (alcohol) can affect microRNA-based regulatory mechanisms of expression of alternatively spliced mRNA transcripts.
The miRNAs are powerful post-transcriptional regulators of mRNA expression (Jirtle and Skinner, 2007, Filipowicz et al. 2008). They are small (19–25nt), non-coding RNAs belonging to an important class of endogenous repressors of gene expression (Ambros, 2004; Bartel, 2004). They control expression of target mRNAs by binding to miRNA Response Elements (MREs) located in the 3’UTR region of target mRNA.
We investigated whether alcohol, via miRNA, can regulate expression and stability of alternatively spliced mRNAs encoding the main, pore forming α subunit of the BK channel, a large conductance calcium- and voltage-activated potassium channel (Slowpoke, BK, MaxiK), which is a product of the KCNMA1 gene. The BK channel (Atkinson et al., 1991) is widely expressed in brain (Knaus et al., 1996; Misonou et al., 2006; Wanner et al., 1999), and influences neuronal excitability, firing frequency and transmitter release (Storm, 1990). Alternatively spliced variants of the α subunit, and their assembly into functional tetramers contributes to functional diversity of the BK channel (Adelman et al., 1992;Atkinson et al., 1991;Butler et al., 1993;Lagrutta et al., 1994;Navaratnam et al., 1997;Rosenblatt et al., 1997;Shipston, 2001;Tseng-Crank et al., 1994) in the brain (Ha et al., 2000; MacDonald et al., 2006). Alternative splicing of BK is dynamically regulated, e.g., the inclusion of the STREX exon is determined by neuronal activity or stress (Xie and McCobb, 1998).
The BK channel is one of the best-described targets of alcohol. Typically, the BK channel is potentiated by alcohol (Butler et al., 1993;Chu et al., 1998;Crowley et al., 2003;Dopico et al., 1998;Dopico et al., 1999;Jakab et al., 1997;Knott et al., 2002), although in some tissues, it is inhibited (Dopico, 2003;Walters et al., 2000). In C.elegans, deletion of neuronal BK blocks the action of alcohol, while constitutive activation mimics the presence of the drug (Davies et al., 2003). In D. melanogaster, deletion of BK results in a loss of behavioral rapid alcohol tolerance (Cowmeadow et al., 2005).
Previously, we determined that in two mammalian brain regions important in alcohol abuse and addiction, the supraoptic nucleus (SON), and the striatum, the BK channel develops tolerance to alcohol (Knott et al., 2000;Knott et al., 2002;Pietrzykowski et al., 2004). In neurons of both regions, this BK tolerance is manifested as decreased alcohol sensitivity and reduced BK channel density (Pietrzykowski et al., 2004, Martin et al., 2004).
Here, we illustrate that alcohol causes a rapid increase in miR-9 expression in these neurons. Only BK mRNAs containing a miR-9 binding site in their 3’UTRs are degraded, while others are spared. This reorganization of neuronal BK splice variants leads to a change in BK channel isoform profile consistent with the development of tolerance. This mechanism was found to affect ten additional alcohol-relevant miR-9 targets in the brain, providing a potential mechanism for an integrated response to the drug. The data describe a remarkably fast and elegant mechanism of alcohol multiplex regulation of neuronal alcohol tolerance.
Results
Alcohol rapidly and specifically downregulates a subset of pre-existing BK mRNA variants
First, we determined which pre-exposure BK mRNA variants are expressed in the naïve SON, using end-point polymerase chain reaction (PCR). BK has at least six alternative splice sites residing within the coding sequence (Beisel et al., 2007, Figure 1A). Using primers unique to each splice site in rat brain, we found alternatively spliced exons at only three (X3, X4, and X6) of these sites (Figure 1, and Figure S1 in the Supplemental Materials). Next, using primers bracketing sites X3 through X6, we observed several distinct bands (Figure 1B), suggesting the expression of several distinct variants of BK, possibly with different exonal concatenations. Indeed, subcloning and sequencing revealed that these bands corresponded to splice variants with differing exonal concatenations (Figure 1C). We observed only eight of the possible sixteen variants, indicating that exonal assembly of BK message within SON neurons is not random. Exon 29 located at site X6 was most frequent.
Figure 1. BK mRNA variants containing ALCOREX exon are eliminated by alcohol.
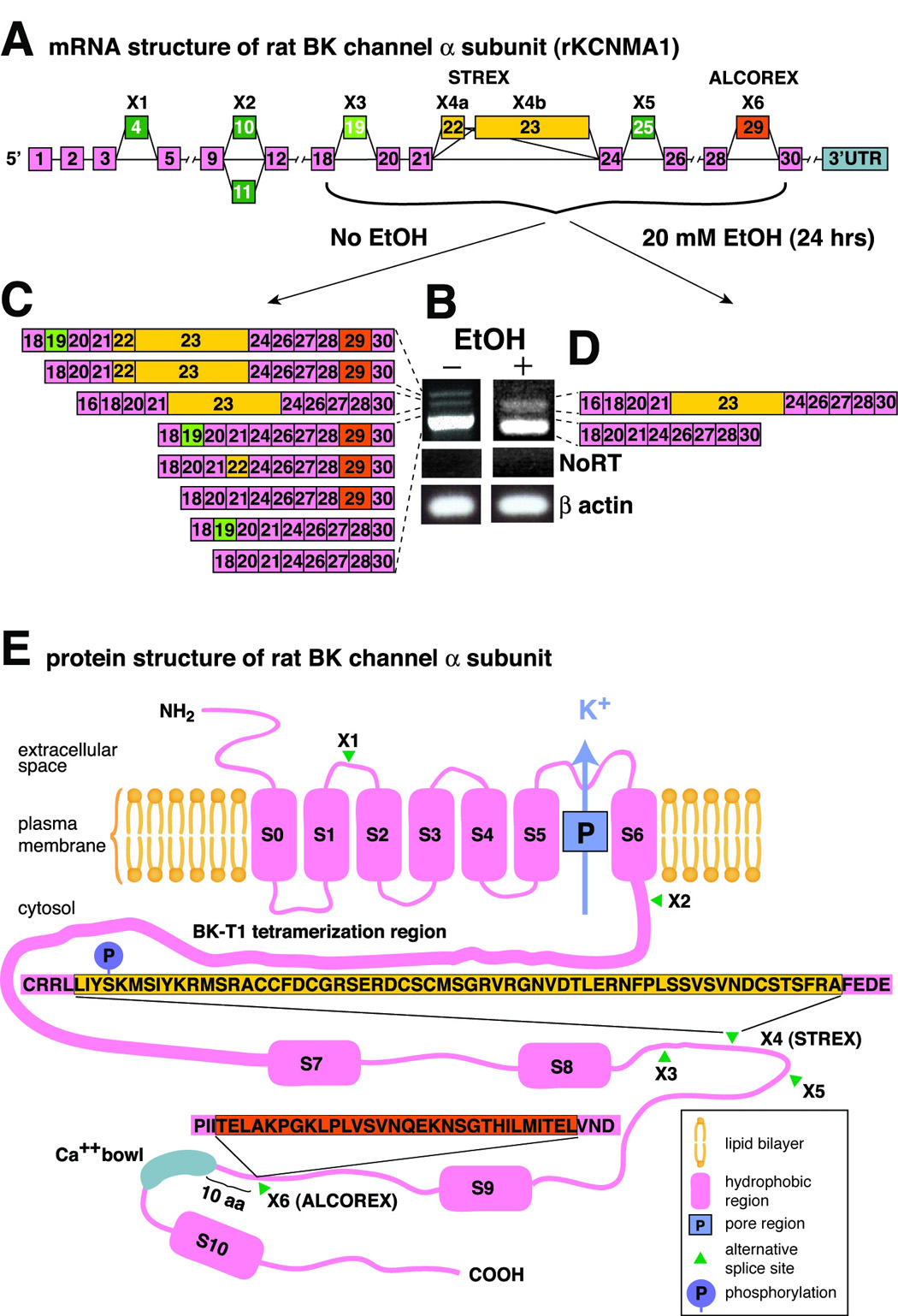
(A) A schematic of BK constitutive and alternatively spliced exons within the coding region. Note multiple sites of alternative splicing (marked with X). Exon X4b (referred to here as STREX) corresponds to Xie’s (Xie et al., 1998) et al. STREX-1, while the combination of X4a and X4b corresponds to Xie et al. STREX-2. Exon # 29 at the alternatively spliced site X6 has been named ALCOREX, due to its regulation by alcohol (see text). 3’UTR - the 3’ untranslated region of mRNA. (B) End point PCR was performed using primers bracketing alternative spliced sites X3 through X6 of BK mRNA. Agarose gel electrophoresis revealed different band patterns of BK mRNA isolated from supraoptic nuclei (SON) harvested from naive and alcohol-exposed (20 mM, 24 hrs) explants. NoRT – no reverse transcriptase control. β-actin – loading control. (C) Subcloning and sequencing of cDNA bands from the naïve SON indicated that they correspond to eight alternatively spliced variants with different exonal concatenations. Incomplete separation of bands is probably caused by heteroduplexing of BK cDNAs (Mahmoud et al., 2002). (D) Subcloning and sequencing of two cDNA bands found in the alcohol-exposed SON revealed that they correspond to splice variants carrying only the STREX exon or Insertless, the completely spliced-out variant. A total of 54 clones (28 – no alcohol exposure, 26 – 24 hrs 20 mM alcohol exposure) was isolated and sequenced. (E) Schematics of BK alpha protein structure (not drawn to scale). S0–S10 – hydrophobic domains, P – pore region, K+ - outward flow of potassium ions, green arrowheads – alternative splicing sites (X1–X6), blown up boxes – polypeptide sequences of exon 22/23 and exon 29 products. Thicker line between S6 and S7 represents BK T1 tetramerization domain (Quirk et al., 2001). Blue “P” circle indicates position of STREX Ser phosphorylation site described by Tian (Tian et al., 2001).
Exposure of SON neurons to alcohol (20 mM, at or just above legal intoxication levels, up to 24h) in HNS organotypic explants changed the BK variant landscape dramatically. End-point PCR, subcloning and sequencing revealed the presence of two bands (Figure 1B) encoding two variants (Figure 1D): 1) the STREX variant (STREX-1 in Xie and McCobb, 1998), and 2) a completely spliced-out variant (INSERTLESS).
Thus, alcohol exposure reduced the number of BK mRNA variants by eliminating several variants, five out of six of them containing exon 29. Due to its regulation by alcohol we refer to exon 29 as ALCOREX (alcohol-regulated exon), by analogy to STREX (stress axis-regulated exon - Xie and McCobb, 1998). Sequences of products of both exons (ALCOREX and STREX) and their positions in the BK polypeptide are shown in Figure 1E.
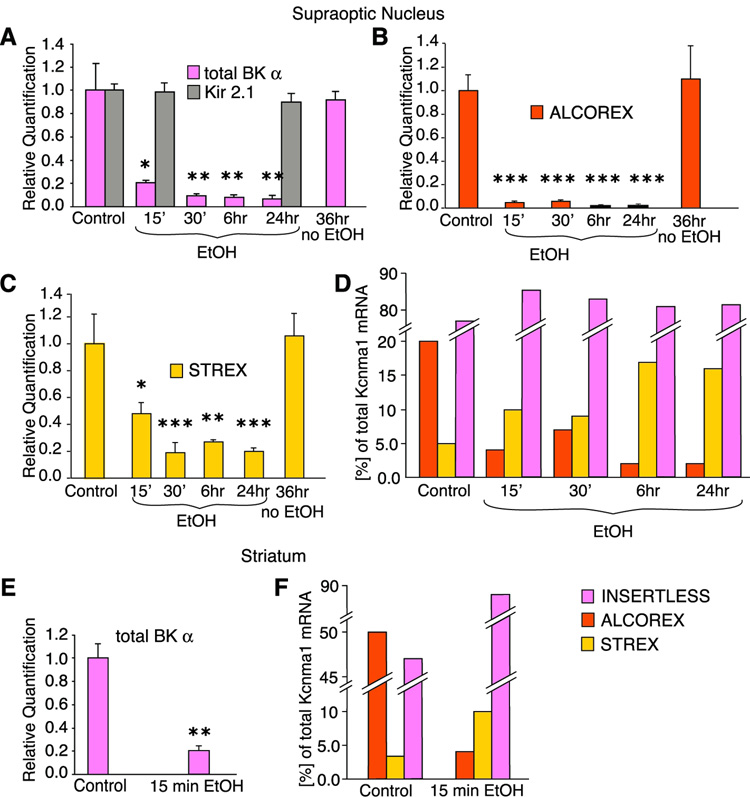
Next, we determined the quantitative and temporal changes in total BK mRNA and individual exons using real-time PCR (qRT-PCR) (Experimental Materials, Figure S2, Bustin et al., 2002). Alcohol rapidly (15 min of alcohol exposure) downregulated total BK mRNA. This effect was BK- and alcohol-specific and augmented by longer exposures (Figure 2A).
Figure 2. Alcohol rapidly and specifically switches alternatively spliced BK mRNA variants.
(A) HNS explants were cultured in regular medium with alcohol for up to 24 hours and without alcohol for up to 36 hours. 20 mM alcohol rapidly downregulates total BK mRNA, but culturing alone does not change its level (n = 9). Moreover, mRNA level of another potassium channel, Kir 2.1, remains unchanged (n = 3). (B) Within 15 min of 20 mM alcohol exposure the ALCOREX exon is downregulated to very low levels. Alcohol-free culturing has no effect (n = 9). (C) STREX exon is also downregulated within 15 min of 20 mM alcohol exposure but to a lesser extent. A longer exposure times, STREX expression levels off. Alcohol-free culturing has no effect (n = 9). (D) Initially, ALCOREX constituted 20%, while STREX constituted 5% of total BK message. Short (15–30 min) exposure to 20 mM alcohol decreased ALCOREX level three- to five-fold, while doubling STREX expression level. Longer exposure to alcohol augmented that effect. The level of the INSERTLESS variant remained almost unchanged. Data calculated from A, B and C using BK plasmids as standards (see Experimental Procedures). (E) In striatal neurons, similarly to the SON neurons, alcohol dramatically decreased total BK message within 15 min of exposure. (F) In naïve striatal neurons BK transcript landscape is different than in SON neurons: ALCOREX expression level also dominates over STREX, but constitutes a larger amount of total BK message (about 50%), while INSERTLESS is less represented. Similarly to SON, alcohol causes a switch in expression of ALCOREX and STREX in striatal neurons. Additionally, the INSERTLESS contribution to the total amount of BK mRNA increased. Data calculated as in D. * P < 0.05, ** P < 0.01, *** P < 0.005 comparing to control.
These results, particularly the rapidity of alcohol’s downregulation of BK message, suggested that alcohol acts on pre-existing, alternatively spliced BK mRNA variants, rather than regulating alternative splicing during production of BK transcripts. Lack of effect of a transcriptional blocker (10 µg/µL, Actinomycin D) confirmed this hypothesis (Figure S3).
Next, we determined that alcohol exposure resulted in a radical change in the expression of individual BK exons. Alcohol rapidly (15 minutes) and profoundly decreased expression of the ALCOREX exon (Figure 2B). Moreover, longer alcohol exposure (6 hr, 24 hr) augmented that decrease, resulting in almost undetectable levels of this exon, corroborating our end-point PCR results. The STREX exon was also decreased, but to a lesser degree (Figure 2C).
Thus, we observed that alcohol changed the BK transcript landscape dramatically. Initially, before alcohol exposure of the HNS explant, ALCOREX constituted 20% of total BK message, STREX comprised a smaller fraction (5%), similar to amounts found in other tissues (Zhu et al., 2005) while the INSERTLESS variant (no ALCOREX or STREX exons) constituted about 75% of total BK message (Figure 2D). Short (15 min) alcohol exposure caused a reorganization of BK alternatively spliced transcript profile, best illustrated as relative amounts: ALCOREX variants decreased four-fold, STREX doubled, while the INSERTLESS variant was changed the least (Figure 2D). Longer exposure times augmented these effects (Figure 2D, 6 hrs, 24 hrs).
To examine the generality of the subtractive mechanism of alcohol reorganization of BK transcript landscape in other brain regions, we extended our study to the striatum - a brain region important in the development of various types of addiction (Hyman et al., 2006;Koob, 1999). Using striatal cell culture we observed similar changes (Figure 2E, F). Interestingly, alcohol reorganization of BK splice variants in medium spiny neurons was even more profound then in the SON, since ALCOREX was found in half of the total BK transcripts in unexposed striatum, and alcohol caused greater changes in relative expression: 10-fold downregulation of ALCOREX, and a larger increase in INSERTLESS (Figure 2F).
These data suggest that, in alcohol-relevant brain regions, alcohol downregulates BK mRNA by a post-transcriptional mechanism.
miR-9 mediates post-transcriptional alcohol regulation of BK mRNA
miRNAs are small (21–26 nt), endogenous, single-stranded RNA molecules that act mainly as post-transcriptional repressors of gene expression (He and Hannon, 2004) in many tissues, including CNS (Kosik, 2006). In addition, miRNAs can downregulate their mRNA targets in mammals (Farh et al., 2005;Lewis et al., 2005;Yekta et al., 2004) by binding to target mRNA resulting in rapid mRNA cleavage (Hutvagner and Zamore, 2002;Valencia-Sanchez et al., 2006;Yekta et al., 2004). These features make them ideal candidates for mediators of the observed alcohol regulation of BK transcripts.
miRNA regulation of its target depends upon: 1) degree of complementarity between the miRNA and its binding site (the MRE - miRNA Recognition Element), typically located in the 3’ untranslated region (3’UTR) of the target (Kiriakidou et al., 2004;Yekta et al., 2004), and 2) number of MREs (Valencia-Sanchez et al., 2006). In contrast to plants, complementarity is usually partial in mammals (He and Hannon, 2004). The highest likelihood of successful miRNA:MRE binding in mammals is determined by a perfect match between miRNA seed sequence (nt #2 through #7–8) and adenosine-flanked MRE (Lewis et al., 2005). It is believed that a single MRE of high complementarity generally results in mRNA cleavage (Valencia-Sanchez et al., 2006;Yekta et al., 2004), while multiple sites result in translational repression (Doench and Sharp, 2004;Kiriakidou et al., 2004).
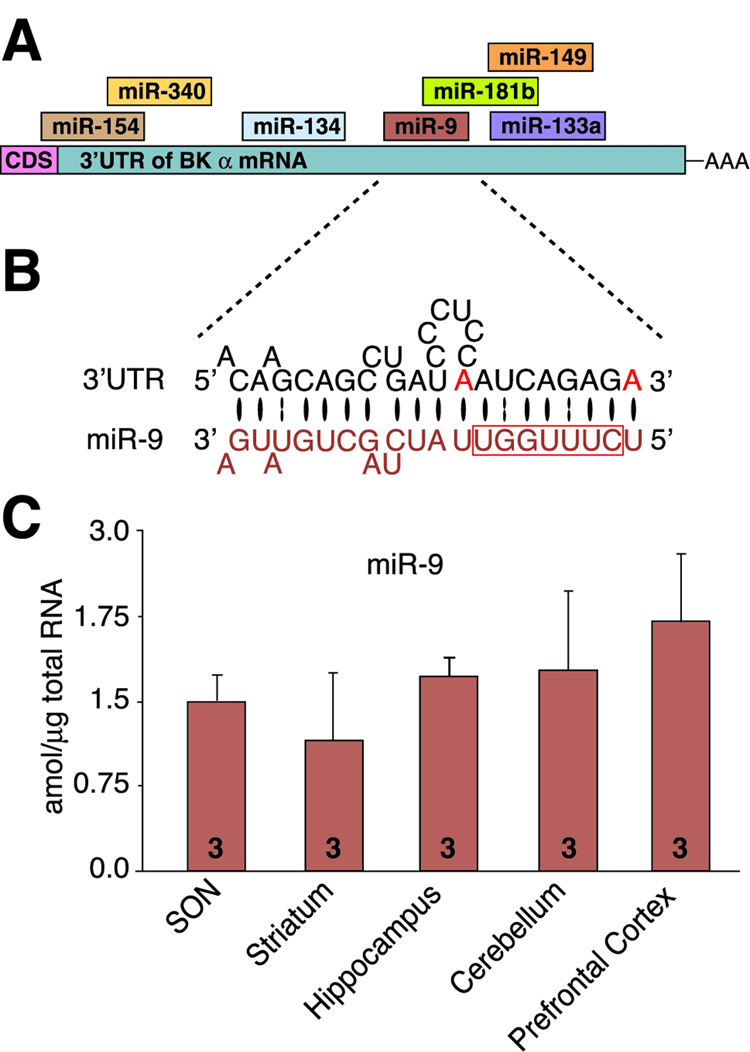
To determine which miRNAs would form the most complementary and stable duplex with the BK mRNA 3’UTR, we base-paired 109 rodent neuronal miRNAs with the known rat BK mRNA 3’UTR (AF135265), using RNAHybrid (Kruger and Rehmsmeier, 2006), and visually inspected each hybridization. Among promising miRNA species (Figure 3A) miR-9 was the best candidate, with high complementarity (Figure 3B), one of the lowest predicted free energy of hybridization (−22.3 kcal/mol), and a single miR-9 MRE (Figure S6). We confirmed our analysis using TargetScan (Lewis et al., 2005;Lewis et al., 2003, Grimson et al., 2007). These characteristics strongly suggested that miR-9 could bind to BK mRNA and cause its cleavage and downregulation.
Figure 3. miR-9 is the best candidate for mediator of alcohol regulation of alternatively spliced BK mRNA variants.
(A) Schematic showing a subset of miRNAs, which can potentially bind to BK 3’UTR. CDS – a coding sequence, AAA – poly(A) tail. (B) The detailed alignment between miR-9 and the best-fitting sequence within the BK 3’UTR. The BK 3’UTR and miR-9 5’ nucleotides #1 through #12 have perfect complementarity (including the miR-9 seed sequence: nucleotides #2–8, depicted within box). Additionally, BK 3’UTR anchoring adenosines (red) flank the seed sequence. Bases paired by Watson-Crick bond are depicted by a large black oval, G:U pairs by two dots. (C) miR-9 is expressed in brain regions important in alcohol effects.
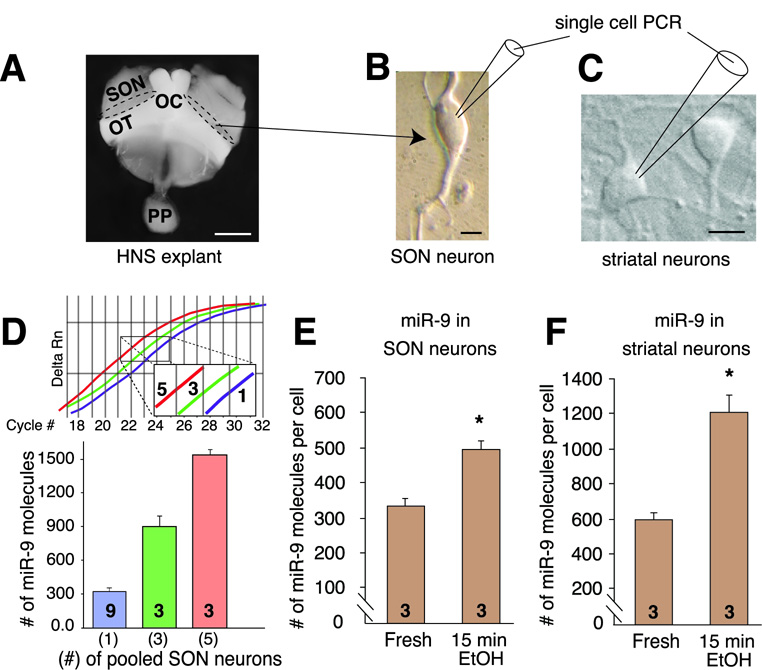
miR-9 is considered a brain-specific miRNA important for neurogenesis (Krichevsky et al., 2003), but there is conflicting evidence for its expression in adult brain (Farh et al., 2005;Wu et al., 2006). We establish, using mature miR-9 specific primers (Figure S4), that miR-9 is expressed in alcohol-relevant brain regions, including SON and striatum (Figure 3C). Using the same primers, serial dilutions of synthetic miR-9 (Figure S4), and individually dispersed SON neurons (Figure 4A, B) and striatal neurons (Figure 4C), we determined that a single SON neuron expressed miR-9 at the level of ~300 mature miR-9 molecules per SON neuron (Figure 4D, E) and ~ 600 per striatal neuron (Figure 4F). Alcohol increased expression of miR-9 in both SON (Figure 4E) and striatal (Figure 4F) neurons on a time scale comparable to the downregulation of BK mRNA (Figure 2). These results suggest that miR-9 can mediate alcohol post-transcriptional regulation of neuronal BK mRNA.
Figure 4. miR-9 is regulated by alcohol on a single neuron level.
(A) Supraoptic nuclei (dotted line) of the HNS explant were the source of the dispersed, single SON neurons. SON – supraoptic nucleus, OC – optic chiasm, OT – optic tract. (B) Single-cell PCR was performed using dispersed SON neurons to determine miR-9 levels in individual SON neurons. (C) Medium spiny neurons were isolated from the striatum, cultured and used for single-cell real-time PCR. For both neurons, the cell body content was aspirated into the micropipette in RNase-free conditions. (D) Single-cell qRT-PCR for miRNA detection indicate miR-9 levels in pooled and individual SON neurons (Inset shows an example of the real-time PCR plot). Both approaches indicate that individual SON neurons contain approximately 300 miR-9 molecules. (E) Alcohol upregulates miR-9 in SON neurons on a time scale similar to BK mRNA downregulation (see Figure 2). (F) Similarly, alcohol rapidly doubles the expression of miR-9 in striatal neurons. Number on each bar represents n value. Numbers in parentheses in D represent number of neurons pooled. Scale bars in A = 1 mm, B = 10 µm, C = 10 µm.
Alcohol downregulates only BK 3’UTRs with miR-9 MREs
Since miRNA targets 3’UTRs, one possible explanation for the selective destruction of transcripts is the presence of multiple 3’UTRs (3’UTR heterogeneity) with only some of them containing miR-9 MRE. 3’UTR heterogeneity is a common feature of many genes (Hughes, 2006), and can result from: 1) alternative polyadenylation within the same 3’UTR, and/or 2) the presence of multiple 3’UTRs. Alternative polyadenylation signal (PAS) sites can produce mRNAs with 3’UTRs of different lengths (Legendre et al., 2006). We observed that the rat BK 3’UTR (AF135265) has two potential PAS sites bracketing miR-9 MRE, which potentially could produce a long 3’UTR with a miR-9 MRE, and a shorter 3’UTR lacking the miR-9 MRE (Figure S6).
3’ rapid amplification of cDNA ends (3’RACE) indicated that this is unlikely. We detected only the full length of this 3’UTR in fresh tissue (Figure 5A, lower band, Figure S6). Surprisingly, we observed two additional larger bands (Figure 5A). Indeed, two novel, longer BK 3’UTRs have recently been cloned (Beisel et al., 2007) in rat inner ear hair cells. We determined that they are expressed in neurons and constructed a schematic summarizing BK α 3’UTR heterogeneity (Figure 5B, S5) using a rat genome database to determine chromosomal position of 3’UTR sequences, and 3’UTR sequence described by us and others (Beisel et al., 2007). The three different 3’UTRs associated with BK α are a result of alternative splicing in the 5’-end of the coding sequence and the 3’UTR region. Despite partial homology of one of the new 3’UTRs (2.2) with the previously known 3’UTR (2.1), only 3’UTR-2.1 has the miR-9 MRE (Figure 5B, C).
Figure 5. miR-9 controls expression of alternatively spliced BK mRNA variants by binding to specific BK 3’UTR (−2.1).
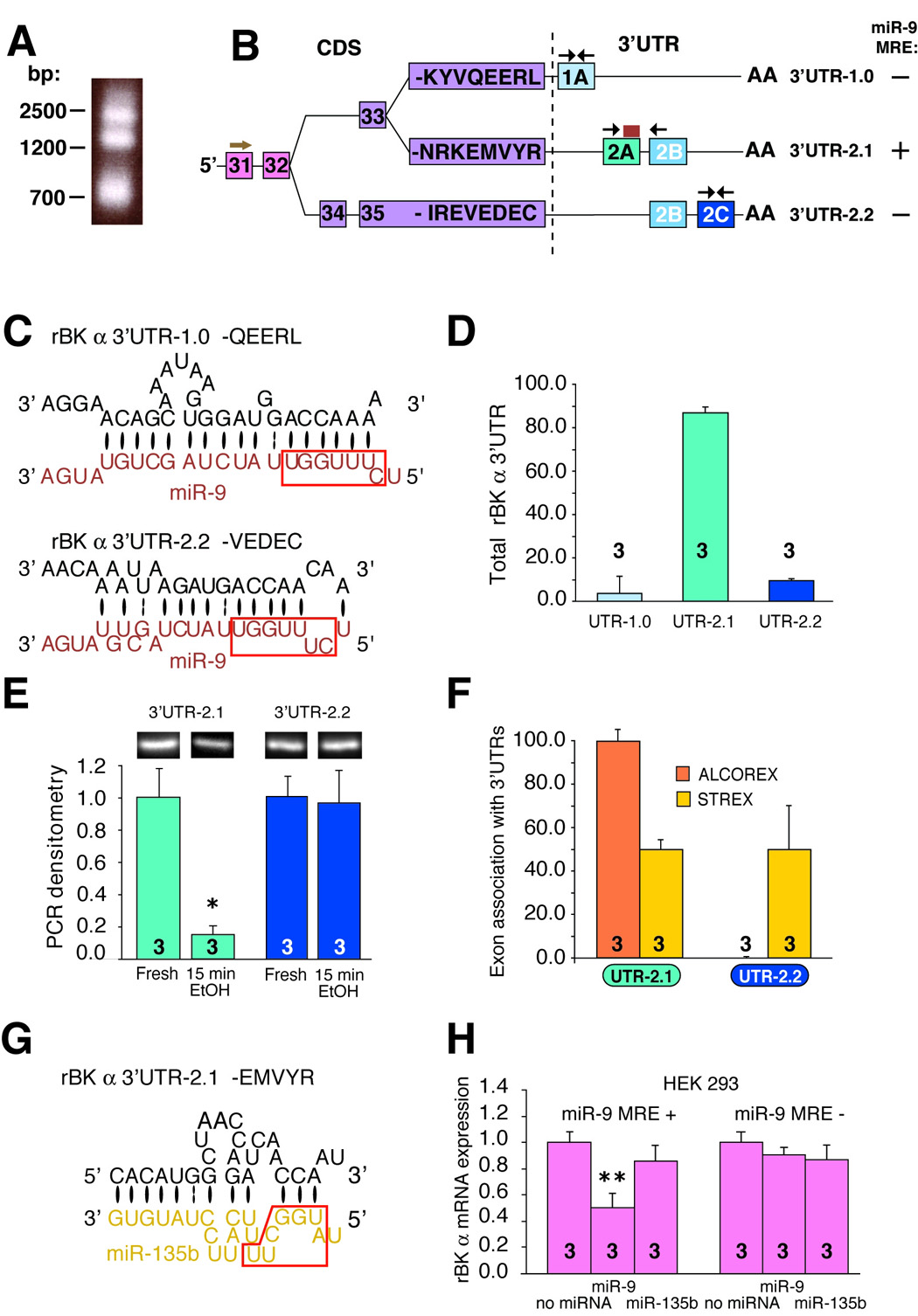
(A) Agarose gel electrophoresis of BK 3’RACE products reveals the presence of three possible 3’UTR regions of BK mRNA. (B) Schematic constructed based on our results, rat BK gene information (rat Ensembl, Hubbard et al., 2007) and data from reference (Beisel et al., 2007) shows options for exonal assembly of the 3’ end of the CDS (coding sequence) and 3’UTR. Numbers in boxes represent exons. Letters represent the eight last amino acid residues characteristic for each BK isoform. The modular structure of 3’UTR is depicted. Note that the latter part of 3’UTR-2.1 and the former part of 3’UTR-2.2 are homologous (see also Figure S5). Note also that among three BK 3’UTR regions a miR-9 MRE is present on 3’UTR-2.1 (yellow box), but not on two others. An arrow above exon 31 indicate position of 3’-RACE forward primer, black arrows in each 3’UTR region show position of pair of qRT-PCR primers used to detect and quantify individual 3’UTRs. (C) A detailed juxtaposition of 3’UTR-1.0 and 3’UTR-2.2 with miR-9 shows poor binding of miR-9 to 3’UTR-1.0 and 3’UTR-2.2. Bases paired by Watson-Crick bond are depicted by a large black oval, G:U pairs by two dots. (D) Quantification of BK 3’UTRs in naïve neurons. (E) End-point PCR and gel densitometry was used to quantify the alcohol effect on expression of 3’UTR-2.1 and -2.2 variants. Alcohol rapidly and profoundly downregulated BK 3’UTR-2.1, but not 3’UTR-2.2. Similar qRT-PCR data not shown. (F) qRT-PCR data revealed that ALCOREX is associated with 3’UTR-2.1, while STREX is associated with both, ALCOREX and STREX. (G) A detailed juxtaposition of BK 3’UTR-2.1 with miR-135b (control miRNA with poor complementarity to BK mRNA) used in (H) miR-9 specifically downregulates only BK mRNA with the miR-9 MRE as determined using real-time PCR. HEK293 cells were transiently transfected with BK constructs with or without the 3’UTR region carrying miR-9 MRE (miR-9 MRE (+), miR-9 MRE (−), respectively) alone or together with one of two miRNAs: miR-9 or miR-135b. BK message is downregulated by miR-9 only when this message contains the 3’UTR+ sequence. miR-135b was unaffected in both cases. P value was determined by independent samples t-test. Number on each bar represents n value. *P < 0.05, **P < 0.01. See also Figure S5–8 for primer positions and Table S1 for their sequences.
We next established, using specific primers to each 3’UTR (Figure S7, 8), that although all three 3’UTRs are present in the SON, their relative amounts vary substantially (Figure 5D). 3’UTR-2.1 was the most abundant (~ 90% of total 3’UTR), 3’UTR-2.2 was less abundant (~ 10% of total 3’UTR), while 3’UTR-1.0 was hardly detectable (Figure 5D). Therefore, we determined alcohol regulation of expression of the two most abundant 3’UTRs: UTR-2.1 and -2.2. Alcohol did not affect the expression of 3’UTR-2.2; however it caused a dramatic downregulation of 3’UTR-2.1 (Figure 5E) corresponding temporally to the upregulation of miR-9 expression (Figure 4E, F) and to the downregulation of specific BK mRNA variants (Figure 2).
Thus, we observed that alcohol differentially affects transcripts with specific 3’UTRs, similar to its differential regulation of transcripts with distinct exonal concatenations (ALCOREX vs STREX) located in the protein coding sequences (CDS). These data led us to hypothesize that association of specific CDS exons with specific 3’UTRs is not coincidental. Indeed, we observed that CDS exons associate with 3’UTRs in a deliberate manner: STREX exon was associated with both 3’UTRs in very similar amounts, while ALCOREX exon was exclusively associated with 3’UTR-2.1 (Figure 5F).
To further test the hypothesis that miR-9 downregulates BK mRNA by binding directly to the BK mRNA 3’UTR-2.1 region, we constructed BK plasmids containing the rat BK 3’UTR region with or without miR-9 MRE (miR-9 MRE+, miR-9 MRE−, respectively; based on the AF135265 sequence) and transfected HEK293 (which lack endogenous miR-9 and BK) with each of these constructs in the presence or absence of miR-9 and miR-135b (a miRNA with poor complementarity to BK mRNA, Figure 5G). This approach revealed that miR-9 MRE in the BK-3’UTR is required for miR-9 specific downregulation of BK mRNA, as determined by qRT-PCR (Figure 5H).
BK mRNA splice variants encode channels with varied alcohol responsiveness
To determine whether reorganization of BK transcript profile can contribute to the development of alcohol tolerance we used heterologous expression to measure alcohol’s effect on activity of individual BK splice variants: INSERTLESS, ALCOREX and STREX.
Ion flux through a channel is determined by 1) conductance of a single channel, 2) its open probability (the fraction of the time the channel remains open), and 3) its density (the number of channels in the plasma membrane). In neurons, alcohol changes open probability of BK channels (sensitivity) and their density, but not their conductance (Pietrzykowski et al., 2004).
The response of BK activity to acute alcohol challenge varied among isoforms (Figure 6A). The presence of the ALCOREX exon produced BK channels with the greatest alcohol sensitivity of those tested: the magnitude of potentiation was largest, and persisted the longest (Figure 6B). INSERTLESS BK channels were also potentiated by alcohol, but to a much smaller degree than ALCOREX and returned to baseline faster (Figure 6A, B). In contrast, the mRNA variant encoding a BK channel with STREX produced channels which were resistant to alcohol (Figure 6B). Measurements made in cell-attached mode at lower Ca2+ levels, designed to provide optimal opportunity for alcohol to potentiate gating by lowering NPo, confirmed the absence of alcohol potentiation in STREX channels (data not shown). Integrated measurement of the magnitude and the duration of alcohol potentiation (area under the curve in Figure 6B, see Methodology), which corresponds to ion flow through the channel, show the differences in acute alcohol sensitivity of BK variants (Figure 6C).
Figure 6. BK channel isoforms encoded by alternatively spliced messages adapt differently to acute alcohol exposure.
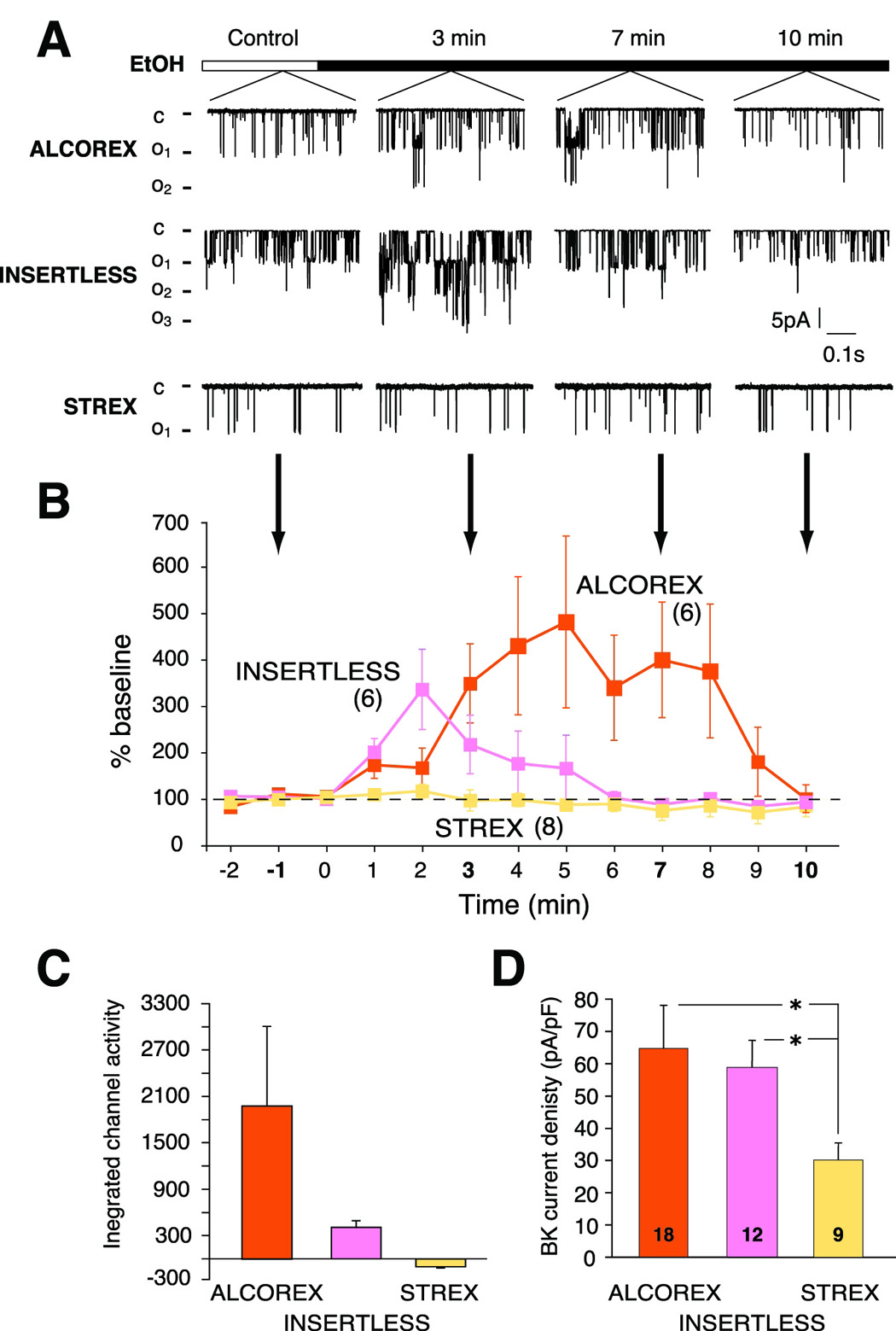
(A) Representative single channel currents from BK-ALCOREX, BK-INSERTLESS and BK-STREX at different time points of a continuous alcohol exposure recorded from transiently transfected HEK 293 cells; inside-out patches, 135 mM symmetrical K+, and 5 µM-free calcium. (B) The presence of ALCOREX produced channels of greatest alcohol sensitivity in both the magnitude of potentiation by alcohol, and persistence of that potentiation. INSERTLESS channels were also potentiated by alcohol, but to lesser extent, and more transiently. STREX-containing channels were completely resistant to alcohol. Baseline activity is defined here as open probability (NPo) before alcohol application. Number in parenthesis represents n value. (C) Shows quantitation of (B) performed by measurement of the integrated area under the curve using the trapezoid formula (see Experimental Procedures). (D) Innate current density of ALCOREX and INSERTLESS isoforms is higher then STREX current density. Current density was calculated as previously (Pietrzykowski et al., 2004) as current/membrane surface area (determined from capacitance measurements). Number on each bar represents n value. *P < 0.05.
Native current density differed among isoforms similarly to acute alcohol sensitivity, which contrasted ALCOREX and INSERTLESS with STREX (Figure 6D, Controls). ALCOREX and INSERTLESS BK channels had similar current density (65 pA/pF ± 13 pA/pF, 59 pA/pF ± 9 pA/pF, respectively), while STREX current density was lower (30 pA/pF ± 8 pA/pF) (Figure 6D).
Modeling the consequences of miR-9 regulation of BK transcripts
Although we observed that alcohol, via miR-9, regulated alternatively spliced BK transcripts encoding channel isoforms exhibiting different alcohol responsiveness it was still unclear how these changes contribute to tolerance. The most abundant isoform (INSERTLESS) had only a mild response to alcohol, and its relative amount changed the least. In contrast, the relative amounts of isoforms with the least (STREX) and the greatest (ALCOREX) alcohol responses changed the most (Figure 2D), but their relative contribution to overall BK channel amount was rather small.
To determine how these changes in various transcripts might contribute to alcohol tolerance we developed a computational model integrating our molecular biological and electrophysiological outcomes. Importantly, BK channels can function only as tetrameric assemblies of four monomers, where each monomer is encoded by individual BK transcript. To assess the functional consequences of alcohol regulation of BK transcripts, we incorporated into the model published data describing differential effects of PKA on different BK tetramers (Tian et al., 2004), and our new data describing the relative functional weighting of monomers in determining alcohol sensitivity of assembled BK channel tetramers.
First, based on the quantities of individual BK transcripts encoding individual splice variant monomers (Figure 2), we calculated the probabilities of all possible combinations of monomers assembled into tetramers in both SON and striatal neurons (Equation 1, Methodology). We determined that in naive SON neurons four types of tetramer were most prevalent: ALCOREX/INSERTLESS heteromers (almost half of the total amount of the channel), INSERTLESS homomers (about one third), INSERTLESS/STREX heteromers and ALCOREX/INSERTLESS/STREX heteromers (each about 10% of total amount) (Figure 7A).
Figure 7. Mathematical modeling indicates that shift in BK transcript landscape changes tetrameric make up of BK channels in neurons, decreases overall BK channels responsiveness to alcohol and causes the development of tolerance.
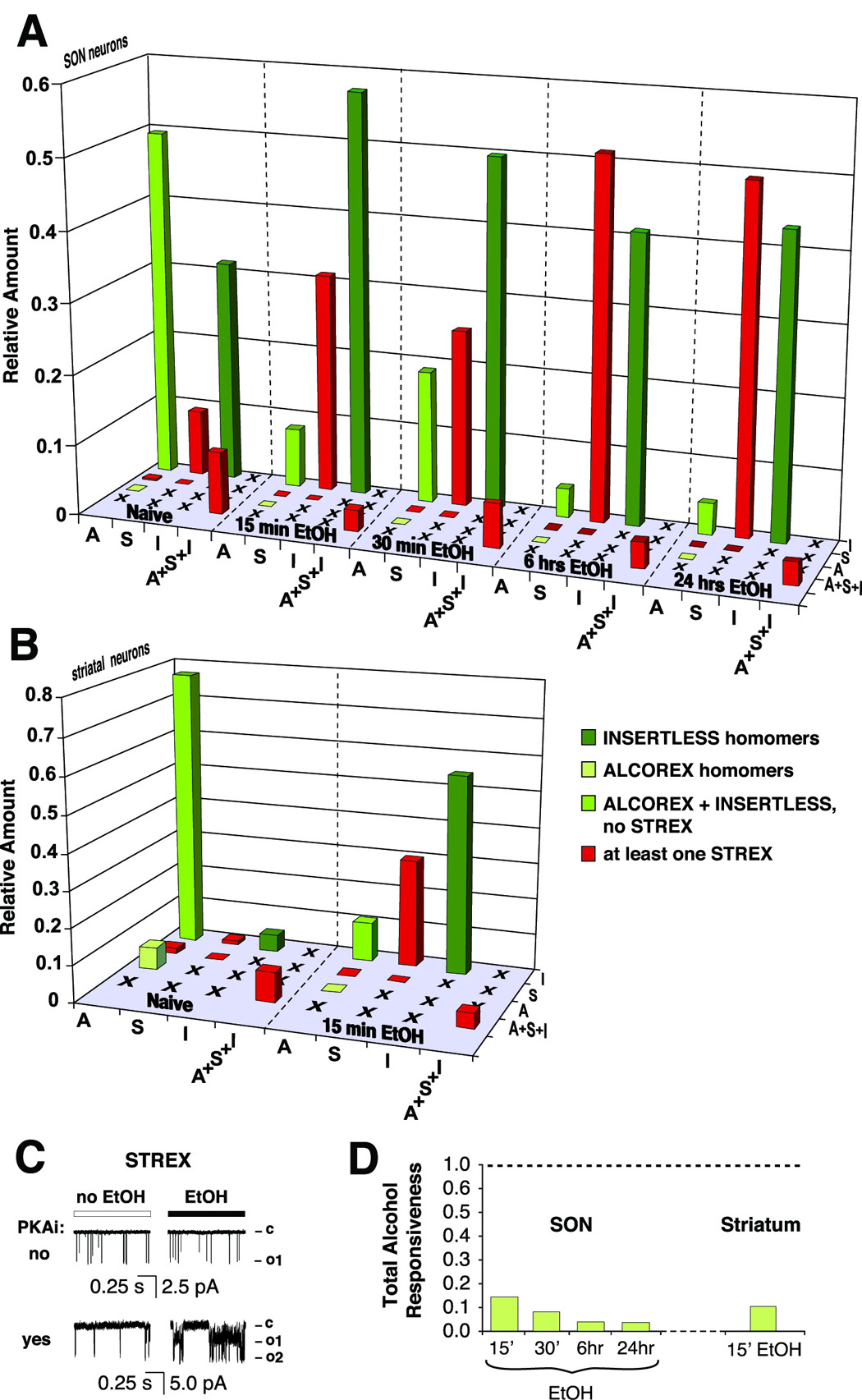
(A) A matrix of BK splice variant monomers assembly created to estimate BK tetrameric assembly and to calculate their alcohol sensitivity in SON neurons. Height of columns corresponds to the amount of a tetramer as a fraction of the total BK channel amount. Color of a column represents level of BK potentiation by alcohol: light green – high potentiation, dark green - low potentiation, medium green –intermediate level of potentiation. In naive SON neurons the most abundant BK tetrameric assemblies were: ALCOREX/INSERTLESS heteromers, INSERTLESS/STREX heteromers, ALCOREX/INSERTLESS/STREX heteromers, and INSERTLESS homomers. Alcohol decreased the amount of ALCOREX containing assemblies (ALCOREX/INSERTLESS, ALCOREX/INSERTLESS/STREX heteromers), and increased the amount of INSERTLESS/STREX heteromers. The amount of INSERTLESS homomers also increased but to a lesser extent. (B) A similar matrix was created for striatal neurons. In naive striatal neurons the vast majority of BK channels consisted of ALCOREX/INSERTLESS heteromers, with very low amounts of ALCOREX/INSERTLESS/STREX heteromers, ALCOREX homomers, and STREX homomers. Alcohol decreased the level of ALCOREX/INSERTLESS heteromers, and increased the level of STREX/INSERTLESS heteromers and INSERTLESS homomers. Color code as in (A). (C) Alcohol inhibition of STREX is PKA-dependent. In the absence of PKA inhibitor (14–22 Amide) alcohol exposure decreased activity of BK channels containing STREX (upper traces). Block of PKA BK-STREX phosphorylation allowed potentiation of the channel by alcohol (lower traces). (D) The development of alcohol tolerance of the entire BK channel population in SON and striatal neurons is expressed as a ratio of BK alcohol responsiveness after alcohol exposure to BK alcohol responsiveness before alcohol exposure (Equation 2, Methodology). Only the outcomes of the most conservative calculation (alcohol sensitivity of ALCOREX/INSERTLESS heteromers equals alcohol sensitivity of the INSERTLESS homomers) are shown. 15–30 min alcohol exposure profoundly decreased total BK alcohol responsiveness in both, SON and striatal neurons. Longer alcohol exposures augmented this effect. Assuming equal alcohol sensitivity of ALCOREX/INSERTLESS heteromers and ALCOREX homomers the decrease in responsiveness is even more profound (data not shown). These changes are fully compatible with the development of alcohol tolerance. A – ALCOREX, S – STREX, I – INSERTLESS.
Alcohol significantly changed the contribution of certain tetrameric assemblies to the total amount of BK channel. The most striking change was a several fold drop in the ALCOREX/INSERTLESS assembly contribution, and a collateral increase in the INSERTLESS/STREX assembly. Modeling also predicted a concurrent moderate increase in INSERTLESS homomers. Interestingly, in naive striatal neurons the calculated distribution of BK channel assemblies differed from SON neurons, with a higher percentage of ALCOREX/INSERTLESS heteromers and ALCOREX homomers, and smaller amounts of STREX-containing assemblies and INSERTLESS homomers (Figure 7B). Nevertheless, similar to SON neurons, the alcohol-dependent shift away from ALCOREX-containing assemblies, and toward STREX-containing assemblies was observed. Additionally, in striatal neurons, alcohol caused a larger increase in the INSERTLESS homomer.
After determining the tetrameric make up of BK channels in neurons, we considered alcohol sensitivity of various assemblies. In Figure 6C we described different alcohol effects on different BK homomers (potentiation of INSERTLESS and ALCOREX by alcohol vs STREX alcohol resistance). Interestingly, recent reports (Tian et al. 2001, Tian et al., 2004) described a similar differential effect, with respect to the action of PKA phosphorylation on BK homomer activity: i.e. INSERTLESS potentiation and STREX inhibition. Moreover, the STREX channel was inhibited even when only a single STREX monomer was present within a heteromer. We reasoned that if STREX alcohol resistance was mediated by PKA, then similar dominance of the STREX insert (i.e. alcohol resistance) within heteromers might occur. Using a specific PKA inhibitor (14–22 Amide) we found that the alcohol resistance of STREX is indeed PKA-dependent (Figure 7C). Thus, we assumed that any BK channel containing a STREX monomer is alcohol resistant to the same degree as a STREX homomer.
Therefore, BK tetrameric groups can be placed into four categories of alcohol sensitivity (color-coded in Figure 7A and 7B): 1) assemblies with at least one STREX, yielding alcohol resistant channels (red), 2) ALCOREX homomers – highly activated by alcohol (light green), 3) INSERTLESS homomers – low potentiation by alcohol (dark green), and 4) ALCOREX/INSERTLESS heteromers – for which the relative alcohol sensitivity is unknown (medium green).
In the final step, we calculated the overall responsiveness to alcohol of the composite BK channel population (Eq. 2, Methodology) as a measure of alcohol tolerance. Since we did not know the level of ALCOREX/INSERTLESS heteromer potentiation by alcohol (category # 4), we ran the model through two extreme variations: alcohol potentiation of the ALCOREX/INSERTLESS heteromer is 1) as low as the INSERTLESS homomer, or 2) as high as the ALCOREX homomer. These two extremes provided upper and lower limits of the possible level of ALCOREX/INSERTLESS potentiation by alcohol, and allowed us to determine the potential range of the decrease in total alcohol responsiveness of the BK channel in neurons. Clearly, even in the most conservative calculation (variation # 1) a 15–30 min exposure to alcohol resulted in BK channels with only 15 % of naive neuron responsiveness, exhibiting almost complete tolerance to alcohol (an even more profound effect was evident in variation # 2, not shown), in both SON and striatal neurons (Figure 7D). Moreover, longer alcohol exposures caused an augmentation of alcohol tolerance (Figure 7D, 6 hrs, 24 hrs).
Additional miR-9 targets important for alcohol actions in the CNS
Since microRNAs are known to affect multiple targets (Ambros 2004, Bartel 2004), we wondered whether alcohol regulated other miR-9 targets. A search by miRNA target prediction software - TargetScan (Release 4.1, Grimson et al., 2007) retrieved 826 predicted miR-9 targets (as of January 18, 2008). From this catalog we selected a list of targets (Table 1) with known roles in alcohol’s actions, and documented expression in the CNS (12 total, see Methodology).
Table 1. Additional, CNS-specific miR-9 targets relevant to alcohol actions.
A total of thirteen targets were determined using TargetScan. For each target its TargetScan’s specific number, symbol, total context score, full name and alcohol effect is shown. A more negative score is associated with a more favorable miR-9 binding. Alcohol effect classification: +/− 25% (no change), 26–50% upregulation (+), 51–75% upregulation (+ +), 76–100% upregulation (+ + +), 26–50% downregulation (−), 51–75% downregulation (− −), 76–100% downregulation (− − −).
| TargetScan miR-9 target genes | Effect of 15 min., 20 mM EtOH exposure | ||||
|---|---|---|---|---|---|
| # | TargetScan # | Symbol | Score | Name | |
| 1 | 4 | KCNJ2 | −0.80 | potassium inwardly-rectifying channel, subfamily J, member 2 | no change |
| 2 | 35 | GABRB2 | −0.50 | gamma-aminobutyric acid (GABA) A receptor, beta 2 | − − |
| 3 | 93 | KCNMB2 | −0.39 | BK channel, subfamily M, beta member 2 | − − |
| 4 | 95 | CLOCK | −0.39 | clock homolog (mouse) | − − |
| 5 | 101 | PPARA | −0.38 | peroxisome proliferator-activated receptor alpha, 2 isoforms | − − |
| 6 | 117 | NPY2R | −0.36 | neuropeptide Y receptor Y2 | no change |
| 7 | 132 | SYNJ1 | −0.35 | synaptojanin 1 | − − |
| 8 | 144 | TGFBR2 | −0.34 | transforming growth factor, beta receptor II (70/80kDa) | + + + |
| 9 | 157 | HDAC5 | −0.33 | histone deacetylase 5 | − |
| 10 | 243 | CACNB1 | −0.26 | calcium channel, voltage-dependent, beta 1 subunit | − − |
| 11 | 273 | NOX4 | −0.24 | NADPH oxidase 4 | + |
| 12 | 346 | DRD2 | −0.20 | dopamine receptor D2 | − − − |
| 13 | 749 | PRKCA | −0.04 | protein kinase C, alpha | no change |
| 14 | not a target | KCNMB1 | X | BK channel, subfamily M, beta member 1 | no change |
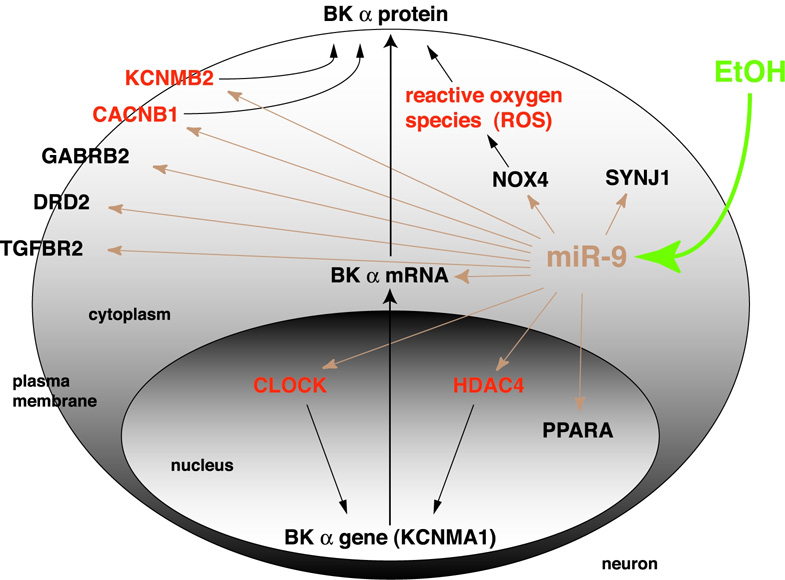
Expression of 8 out of the 12 targets was downregulated by alcohol. Two targets were upregulated, while the expression of 2 other targets was unchanged (Table 1). All ten regulated targets play important roles in different aspects of neuronal physiology (Supplementary Materials), including excitability (GABRB2, KCNMB2, CACNB1, DRD2), gene expression (CLOCK, PPARA, TGFBR2, HDAC5, CACNB1), lipid metabolism (PPARA), and function of presynaptic terminals (GABRB2, SYNJ1, CACNB1, KCNMB2). Moreover, some of these targets are known to directly regulate BK channel expression and/or function, while others could potentially contribute to that regulation (Figure 8, Supplementary Materials).
Figure 8. Additional targets involved in alcohol actions in the CNS are modulated by miR-9.
A schematic depicts a simplified network of eleven miR-9 targets important in alcohol actions in the CNS centered on BK expression pathway. miR-9 targets mRNA molecules in the cytoplasm as shown for BK. For clarity, mRNAs of other targets were omitted. Shown are functional proteins of these targets to indicate their main cellular localization. miR-9 targets plus reactive oxygen species (ROS) known to affect BK gene expression or protein function are in bold. Dotted lines show additional pathways of other miR-9 targets, which could potentially regulate BK. For description of symbols see Table 1.
Discussion
Here, we describe a mechanism of alcohol tolerance involving miRNA. Clinically relevant alcohol concentrations rapidly increase miR-9 levels in central neurons. Only one out of three BK channel 3’UTRs contains a MRE with complementarity to miR-9. Thus, there is a selective degradation of message, resulting in reorganization of BK splice variant profile. Modeling, based upon experimental results indicating the baseline levels of the various transcripts, combined with the differential downregulation of BK transcripts by alcohol, allowed a determination of the post-exposure distribution of tetrameric channels. Further, when the model included data (obtained from expression studies) describing differential alcohol responsiveness within this channel population, the resulting prediction was consistent with the development of tolerance. We show also that alcohol, via miR-9 upregulation, regulates additional targets, suggesting a central role for miR-9 in alcohol’s actions in the CNS.
microRNA post-transcriptional regulation of transcript stability via 3’UTR heterogeneity as a mechanism of alcohol tolerance
In this paper we demonstrate that, in neurons, several different splice variants of the BK channel α subunit are constructed by combining various concatenations of protein coding exons (e.g. STREX alone, STREX and ALCOREX, ALCOREX alone, INSERTLESS) with different 3’UTRs. Since the 3’UTR serves as a major regulatory element of mRNA expression, stability, turnover and translation efficiency (Garneau et al., 2007; Hughes, 2006), this mechanism allows for BK channel plasticity. We showed that, regardless of partial homology of two BK 3’UTRs, only one of them was regulated by alcohol, due to the selective presence of the miR-9 MRE. These results suggest that even subtle differences in miRNA binding profiles can have profound consequences on gene expression regulation. As many mammalian genes express alternative UTRs (Hughes, 2006), this mechanism of neuronal plasticity is likely to be of great importance in adaptation to many exogenous or endogenous factors. This mechanism might also contribute to alcohol’s effects in humans, since the miR-9 MRE in BK mRNA 3’UTR is conserved between rodents and humans (Figure S9).
We observed that the ALCOREX exon is always associated with 3’UTR-2.1 but not 3’UTR-2.2. This is in contrast to the STREX exon, which can associate with either UTR. It will be of interest to understand the mechanisms controlling the association of particular exonal concatenations in the coding sequence of mRNA with particular 3’UTRs.
Both the rise in miR-9 and the concomitant BK mRNA downregulation were well underway within 15 minutes of alcohol exposure. Our data indicate that the fast regulation of BK mRNA by alcohol is likely occurring through the post-transcriptional downregulation of pre- existing mRNA variants, via a miRNA-regulated mechanism, which directs them to a degradation pathway. Once destined for destruction, mRNA is degraded quickly (Garneau et al., 2007), which could explain the rapidity of the process observed here.
The rapid upregulation of mature miR-9 by alcohol may result from an increase in miR-9 gene expression, via production of new primary miR-9 gene transcript (pri-miR-9) in the nucleus, and/or accelerated conversion of immediate pre-miR-9 precursors (pre-miR-9) into mature miR-9, possibly by increased activity of enzymes such as Dicer (Bernstein et al., 2001) and/or Armitage (Cook et al., 2004;Tomari et al., 2004).
Interestingly, the cell-to-cell consistency of our single-cell measurements suggests precise regulation of miR-9 levels in neurons. One siRNA molecule with miRNA-like features can degrade several target molecules in a very short time (Hutvagner and Zamore, 2002). Thus, the cell needs to tightly control levels of miRNA, because even subtle changes in miRNA expression could have profound effects on the expression of miRNA targets. This could also explain why we observe such a massive downregulation of BK mRNA in a very short period of time, with relatively small (two- fold) upregulation of miR-9.
Functional consequences of miR-9 regulation of BK mRNA
Blocking the actions of miR-9 with miR-9 inhibitor, or more specifically, blocking an interaction between miR-9 and 3’UTR-2.1 with microRNA target protector (Choi et al., 2007) could help to establish the consequences of miR-9 regulation of BK mRNA. However, these RNAi approaches would be unlikely to produce interpretable data. There are multiple molecular targets for miR-9 (Figure 8), some directly influencing BK gene expression or protein function, raising the possibility of a complicated and dynamic response pattern. Additionally, we have evidence for multiple forms of BK tolerance, based on several mechanisms (described below), some miR-9-independent. Therefore, in place of the RNAi approach, we used computational modeling. Although, in both, SON and striatal neurons, INSERTLESS is the most abundant transcript, while STREX and ALCOREX are less abundant, a linear extrapolation from the amounts of monomeric transcripts to the profile of the resulting channel population would be misleading. It is necessary to consider the unequal contribution of individual monomers within the tetrameric BK structure to BK channel activity and alcohol sensitivity. For example, although Insertless predominates in amount, its influence on alcohol sensitivity is diminished because of the dominant nature of STREX in a tetramer. Our modeling approach considered this feature, and posits a mechanism by which the reorganization of BK transcripts results in the development of alcohol tolerant BK current. Of course, as with all such modeling, the results are suggestive rather than definitive. Nevertheless, they are consistent with the development of alcohol tolerance previously reported in neuronal cells (Pietrzykowski et al., 2004).
Why does the neuron goes to such remarkable lengths to counteract the consequences of the potentiating effects of alcohol on BK, by both 1) minimizing the potentiation of gating by ethanol, and further, 2) dramatically reducing message level and current density of the channel? BK channels play a central role in the regulation of neuronal excitability, controlling neurotransmitter release and shaping of action potentials in many brain regions. On a cellular level, BK serves as an integrator of regulatory processes, as it is activated by both voltage and intracellular calcium (Salkoff et al., 2006). This channel has a very high conductance, and sustained activation would likely have serious consequences for nervous system function. Because of its large conductance, potentiation by alcohol of even the small population of channels remaining could be undesirable. A related question is how the nervous system retains normal function in the face of such a large reduction of BK. Indeed, BK knockout mice, while able to survive, are seriously impaired (Sausbier et al., 2004). It is possible that the overall, relative shift to the STREX isoform, which has relatively high Ca sensitivity (Xie and McCobb, 1998), helps to maintain adequate levels of BK activity, while minimizing the effects of alcohol.
miR-9 can serve as a master-switch for alcohol effects in the CNS
The miR-9 dependent downregulation of target mRNA and reorganization of its splice variants may represent a general mechanism of neuronal adaptation to alcohol. We determined that miR-9 affects at least ten additional targets besides BK with documented roles in alcohol actions in the CNS (Table 1, Supplementary Materials), some of which can undergo alternative splicing (SYNJ1, DRD2). Moreover, we observed that the expression of these targets is regulated by brief alcohol exposure. These additional downstream targets of miR-9 are involved in major aspects of neuronal physiology (for details see Supplementary Materials) including excitability, regulation of gene expression, metabolism of lipids and function of presynaptic terminals.
miRNA interaction with its mRNA target usually results in down-regulation of that target, or translation repression. Surprisingly, in our study two miR-9 targets (TGFBR2, NOX4) were up-regulated. A recent report gives an example of a miRNA species (Let-7) switching its target expression from repression to activation dependent upon the cell cycle (Vasudevan et al., 2007). Thus, up-regulation of genes by microRNA is possible. Activation of miR-9 by alcohol might cause up-regulation of some targets and concomitant down-regulation of others.
microRNA involvement in mechanisms of tolerance
In addition to the route for adaptation provided by post-transcriptional regulation of ion channel mRNA stability by miR-9, other cellular mechanisms contribute to the development of BK alcohol tolerance. Alcohol tolerance is a complex phenomenon and has several forms, e.g. acute, rapid, or chronic tolerance, depending upon the length of alcohol exposure and the consequent involvement of cellular mechanisms. Our working hypothesis is that acute tolerance occurs via posttranslational effects of the drug directly on the existing channel, which allow rapid alterations that are relatively short-lived. In contrast, longer forms of tolerance can be attributable to other mechanisms acting up-stream of posttranslational changes. They include post-transcriptional regulation of stability of existing transcripts (e.g. via miR-9), followed by protein synthesis and insertion of new functional channels into the plasma membrane. These post-transcriptional mechanisms may result in a slower-developing, but longer-lived adaptation to alcohol exposure. Thus, although the effects of alcohol on upregulation of miR-9 and subsequent subtractive rearrangement of the BK mRNA isoform landscape are surprisingly fast, the functional consequences will be delayed, dependent upon translation of the message, and subsequent delivery and insertion of the channel protein into the neuronal plasma membrane.
Moreover, additional upstream pathways, specifically regulating transcription of BK gene expression (Figure 8) might be involved after longer alcohol exposures. Previous studies of regulation of BK channel alternative splicing by e.g. stress or activity (McCobb et al., 2003;Xie and McCobb, 1998) have focused on splicing decisions during production of pre-mRNA from the gene, with a time frame of weeks. Recently, it has been discovered that BK channel transcription is under epigenetic control (Wang et al., 2007). Could alcohol act via a similar mechanism and could miRNA be involved? For example, alcohol might modulate alternative splicing during transcription and pre-mRNA exonal assembly, skipping the ALCOREX exon and excluding it from the final variants of BK mRNA. Considering the length of the BK gene (~ 690 kb, AC 000083) and the elongation rate of polymerase II (~30 nt/sec, Alberts et al., 1994), production of modified BK transcripts would be expected to take at least 6 hours. Indeed, our data indicate the augmentation of tolerance after at least 6 hours of exposure (Figure 7D). It has recently been shown also that another microRNA species (miR-133) can regulate alternative splicing during gene transcription in muscle (Boutz et al., 2007). We also observed that in HEK cells, which lack miR-9, several hours of alcohol exposure decreases BK current density in the plasma membrane (data not shown). Therefore, it is possible that for longer alcohol exposures, additional mechanisms of gene expression, possibly including other miRNA species, could be regulated by alcohol.
We show here also that INSERTLESS and ALCOREX exhibit different patterns of alcohol sensitivity and channel density. The molecular mechanisms underlying these differences are, at this point, unclear. These two BK isoforms do not have apparent differences in consensus phosphorylation sequence (NetPhos 2.0, data not shown), making this an unlikely explanation for the difference in short-term adaptation. Possibly, the insertion of ALCOREX produces a conformational change in the BK polypeptide, resulting in a change in potentiation by alcohol. The ALCOREX insert is located just 10 residues upstream of the calcium bowl, and this physical proximity could potentially change the interaction of the calcium bowl with calcium ions and subsequently affect channel activity. Indeed, as reported by Ha (Ha et al., 2000) the BK channel with an insert encoded by exon 29 (rSlo27 = ALCOREX) activates faster than BK channels without this insert, dependent upon calcium concentration. Previous data (Dopico et al., 1998) indicate that alcohol acts as a partial agonist of the BK channel, with calcium as full agonist. Another intriguing possibility derives from recent work, which has shown that bilayer thickness can affect alcohol’s potentiation of BK activity (Yuan et al., 2007), allowing speculation that if the different isoforms reside in different membrane domains (e.g. lipid rafts), this could lead to different adaptation patterns.
In summary, our data provide an elegant mechanism of alcohol tolerance involving miRNA regulation of mRNA transcript stability. The process described in this study may represent a general mechanism of neuronal adaptation to alcohol, with miR-9 playing a pivotal role in a complex regulatory network. These miR-9 dependent mechanisms may have roles in neuronal plasticity extending beyond adaptation to drugs of abuse, and present the potential to uncover novel therapeutic targets.
Experimental Procedures
Explant Preparation and Culture
Rat SON explants were prepared as previously described (Pietrzykowski et al., 2004). In transcription block experiments, water-soluble Actinomycin D (10 µg/µL, final concentration, Sigma) was added to the culture medium for the indicated time.
Primary Striatal Cultures
Cultures of dissociated rat striatal neurons were prepared using a modification of a protocol described by Leveque (Leveque et al., 2000) using postnatal day eight Sprague-Dawley rat pups. All experiments were performed on neurons that were 14–21 days in culture.
Alcohol treatment
Alcohol concentration in explant and striatal cultures was obtained as previously (Pietrzykowski et al., 2004). Alcohol levels were measured using a GM7Analyser (Analox Instruments Inc., MA).
Molecular Biology
For a full description of molecular biology methods, including RNA isolation from explants and striatal cultures, end-point PCR, cloning, sequencing, 3’RACE, real-time PCR detection of BK, miRNA and additional miR-9 targets, single-cell real-time PCR, and plasmid preparation see the Supplementary Materials.
HEK cell transfection
HEK293 were cultured in DMEM medium with 10% fetal bovine serum and, for transfection, confluent HEK293 cells were plated on 60 mm Petri dishes and transfected using PolyFect transfection reagent (Qiagen,Balencia, CA) complexed with BK variants (BK-Insertless, BK-ALCOREX, BK-STREX) in pVAX vector (Invitrogen) cDNAs.
In miRNA experiments, BK 3’UTR+/− plasmids (0.0442 fmol/well) were transfected alone, or with one of two miRNA precursors: miR-9 and miR-135b (200 pmol/well). BK mRNA was isolated for quantitation 24 hours after transfection.
For electrophysiological analysis cells were transfected with a BK variant (BKALCOREX, BK-STREX or BK-Insertless in pVAX) together with the expression plasmid (πH3-CD8) for the α subunit of the human CD8 lymphocyte surface antigen (GeneBank M12824), allowing for identification of transfected cells with CD-8 antibody-coated beads (Dynal/Invitrogen) as previously.
Electrophysiology
For a full description of electrophysiological recordings including whole-cell and single-channel recordings, current density and alcohol sensitivity see Supplementary Materials.
Computational modeling of the miR-9 regulation of BK transcripts
For a full description of computational modeling of miR-9 regulation of BK transcripts, including assumptions and equations see Supplementary Materials.
Statistical Analysis
For a full description of statistical analysis see Supplementary Materials. P < 0.05 was defined as statistically significant. n = number of independent biological preparations. Error bars represent standard error of the mean (SEM). Statistical analysis was performed using SPSS.
Supplementary Material
01. Supplemental Materials.
The Supplemental Materials for this article can be found online at … and include: a Word text file, Table S1 (Word), Table S2 (Excel), Supplementary Figures S1–S9 with legends.
02
Acknowledgements
We thank C. Mello, T. Rana, E. Rogaev, S. Akbarian, P, Gardner, A. Tapper, A. Casselman, Y Moliaka, A. Grigorenko, N. Boulghassoul-Pietrzykowska and members of the Treistman laboratory for stimulating discussions and valuable comments, S. Baker for help with statistical analysis, D. Black for providing an unpublished manuscript, S.C. Park and D. McCobb for BK plasmids, O. Loureiro of the Siegelmann laboratory for great help with starting the modeling, and A. Wilson and L. Millard for technical assistance. Supported by the NIAAA grant # AA08003 (S.T.) and the Alcoholic Beverage Medical Research Foundation (A. P.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. RNA Synthesis and RNA Processing. Garland Publishing, Inc.; 1994. Molecular Biology of The Cell; p. 368. [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Beisel KW, Rocha-Sanchez SM, Ziegenbein SJ, Morris KA, Kai C, Kawai J, Carninci P, Hayashizaki Y, Davis RL. Diversity of Ca(2+)-activated K(+) channel transcripts in inner ear hair cells. Gene. 2007;386:11–23. doi: 10.1016/j.gene.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Black DL. Protein Diversity from Alternative Splicing: A Challenge for Bioinformatics and Post-Genome Biology. Cell. 2000;103:367–370. doi: 10.1016/s0092-8674(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Black DL. Mechanisms of alternative pre-messenger rna splicing. Annual Review of Biochemistry. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- Blencowe BJ. Alternative splicing: new insights from global analyses. Cell. 2006;126:37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Boutz PL, Chawla G, Stoilov P, Black DL. MicroRNAs regulate the expression of the alternative splicing factor nPTB during muscle development. Genes and Development. 2007;21:71–84. doi: 10.1101/gad.1500707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J.Mol.Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding "maxi" calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Choi WY, Giraldez AJ, Schier AF. Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science. 2007;318:271–274. doi: 10.1126/science.1147535. [DOI] [PubMed] [Google Scholar]
- Chu B, Dopico AM, Lemos JR, Treistman SN. Ethanol potentiation of calcium-activated potassium channels reconstituted into planar lipid bilayers. Mol.Pharmacol. 1998;54:397–406. doi: 10.1124/mol.54.2.397. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz dM, Rudy B. Molecular diversity of K+ channels. Ann.N.Y.Acad.Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Cook HA, Koppetsch BS, Wu J, Theurkauf WE. The Drosophila SDE3 homolog armitage is required for oskar mRNA silencing and embryonic axis specification. Cell. 2004;116:817–829. doi: 10.1016/s0092-8674(04)00250-8. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcohol Clin.Exp.Res. 2005;29:1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Treistman SN, Dopico AM. Cholesterol antagonizes ethanol potentiation of human brain BKCa channels reconstituted into phospholipid bilayers. Mol.Pharmacol. 2003;64:365–372. doi: 10.1124/mol.64.2.365. [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes and Development. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM. Ethanol sensitivity of BK(Ca) channels from arterial smooth muscle does not require the presence of the beta 1-subunit. Am.J.Physiol Cell Physiol. 2003;284:C1468–C1480. doi: 10.1152/ajpcell.00421.2002. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Anantharam V, Treistman SN. Ethanol increases the activity of Ca(++)-dependent K+ (mslo) channels: functional interaction with cytosolic Ca++ J.Pharmacol.Exp.Ther. 1998;284:258–268. [PubMed] [Google Scholar]
- Dopico AM, Chu B, Lemos JR, Treistman SN. Alcohol modulation of calcium-activated potassium channels. Neurochem.Int. 1999;35:103–106. doi: 10.1016/s0197-0186(99)00051-0. [DOI] [PubMed] [Google Scholar]
- Dredge BK, Polydorides AD, Darnell RB. The splice of life: alternative splicing and neurological disease. Nat.Rev.Neurosci. 2001;2:43–50. doi: 10.1038/35049061. [DOI] [PubMed] [Google Scholar]
- Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;2:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nature Reviews Molecular Cell Biology. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- Grabowski PJ, Black DL. Alternative RNA splicing in the nervous system. Prog.Neurobiol. 2001;65:289–308. doi: 10.1016/s0301-0082(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- Ha TS, Jeong SY, Cho SW, Jeon H, Roh GS, Choi WS, Park CS. Functional characteristics of two BKCa channel variants differentially expressed in rat brain tissues. Eur J Biochem. 2000;267:910–918. doi: 10.1046/j.1432-1327.2000.01076.x. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. microRNAs: small RNAs with a big role in gene regulation. Nature Reviews Genetics. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Hubbard, et al. Ensembl. Nucleic Acids Res. 2007;35:D610–D617. doi: 10.1093/nar/gkl996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TA. Regulation of gene expression by alternative untranslated regions. Trends in Genetics. 2006;22:119–122. doi: 10.1016/j.tig.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu.Rev.Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jakab M, Weiger TM, Hermann A. Ethanol activates maxi Ca2+-activated K+ channels of clonal pituitary (GH3) cells. J.Membr.Biol. 1997;157:237–245. doi: 10.1007/pl00005895. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat.Rev.Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriakidou M, Nelson PT, Kouranov A, Fitziev P, Bouyioukos C, Mourelatos Z, Hatzigeorgiou A. A combined computational-experimental approach predicts human microRNA targets. Genes and Development. 2004;18:1165–1178. doi: 10.1101/gad.1184704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca(2+)-activated K+ channels in rat brain: targeting to axons and nerve terminals. Journal of Neuroscience. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott TK, Dayanithi G, Coccia V, Custer EE, Lemos JR, Treistman SN. Tolerance to acute ethanol inhibition of peptide hormone release in the isolated neurohypophysis. Alcohol Clin.Exp.Res. 2000;24:1077–1083. [PubMed] [Google Scholar]
- Knott TK, Dopico AM, Dayanithi G, Lemos J, Treistman SN. Integrated channel plasticity contributes to alcohol tolerance in neurohypophysial terminals. Mol.Pharmacol. 2002;62:135–142. doi: 10.1124/mol.62.1.135. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann.N.Y.Acad.Sci. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat.Rev.Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34:W451–W454. doi: 10.1093/nar/gkl243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrutta A, Shen KZ, North RA, Adelman JP. Functional differences among alternatively spliced variants of Slowpoke, a Drosophila calcium-activated potassium channel. Journal of Biological Chemistry. 1994;269:20347–20351. [PubMed] [Google Scholar]
- Legendre M, Ritchie W, Lopez F, Gautheret D. Differential Repression of Alternative Transcripts: A Screen for miRNA Targets. PLoS Computational Biology. 2006;2:e43. doi: 10.1371/journal.pcbi.0020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of Mammalian MicroRNA Targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Leveque JC, Macías W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, Li XM, Coyle JT, Huganir RL, Heckers S, Konradi C. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci. 2003;11:4011–4020. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Darnell RB. Splicing regulation in neurologic disease. Neuron. 2006;52:93–101. doi: 10.1016/j.neuron.2006.09.017. [DOI] [PubMed] [Google Scholar]
- MacDonald SH, Ruth P, Knaus HG, Shipston MJ. Increased large conductance calcium-activated potassium (BK) channel expression accompanied by STREX variant downregulation in the developing mouse CNS. BMC.Dev.Biol. 2006;6:37. doi: 10.1186/1471-213X-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud SF, Bezzerides AL, Riba R, Lai GJ, Lovell PV, Hara Y, McCobb DP. Accurate quantitative RT-PCR for relative expression of Slo splice variants. Journal of Neuroscience Methods. 2002;115:189–198. doi: 10.1016/s0165-0270(02)00015-8. [DOI] [PubMed] [Google Scholar]
- Martin G, Puig S, Pietrzykowski A, Zadek P, Emery P, Treistman S. Somatic localization of a specific large-conductance calcium-activated potassium channel subtype controls compartmentalized ethanol sensitivity in the nucleus accumbens. J Neurosci. 2004;24:6563–6572. doi: 10.1523/JNEUROSCI.0684-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb DP, Hara Y, Lai GJ, Mahmoud SF, Flugge G. Subordination stress alters alternative splicing of the Slo gene in tree shrew adrenals. Horm.Behav. 2003;43:180–186. doi: 10.1016/s0018-506x(02)00010-7. [DOI] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca(2+)-activated K(+) channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnam DS, Bell TJ, Tu TD, Cohen EL, Oberholtzer JC. Differential distribution of Ca2+-activated K+ channel splice variants among hair cells along the tonotopic axis of the chick cochlea. Neuron. 1997;19:1077–1085. doi: 10.1016/s0896-6273(00)80398-0. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Martin GE, Puig SI, Knott TK, Lemos JR, Treistman SN. Alcohol Tolerance in Large-Conductance, Calcium-Activated Potassium Channels of CNS Terminals Is Intrinsic and Includes Two Components: Decreased Ethanol Potentiation and Decreased Channel Density. Journal of Neuroscience. 2004;24:8322–8332. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk JC, Reinhart PH. Identification of a novel tetramerization domain in large conductance K(ca) channels. Neuron. 2001;32:13–23. doi: 10.1016/s0896-6273(01)00444-5. [DOI] [PubMed] [Google Scholar]
- Rosenblatt KP, Sun ZP, Heller S, Hudspeth AJ. Distribution of Ca2+-activated K+ channel isoforms along the tonotopic gradient of the chicken's cochlea. Neuron. 1997;19:1061–1075. doi: 10.1016/s0896-6273(00)80397-9. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nature Reviews Neuroscience. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proceedings of the National Academy of Sciences. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am.J.Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Shipston MJ. Alternative splicing of potassium channels: a dynamic switch of cellular excitability. Trends Cell Biol. 2001;11:353–358. doi: 10.1016/s0962-8924(01)02068-2. [DOI] [PubMed] [Google Scholar]
- Smith CW, Valcarcel J. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem.Sci. 2000;25:381–388. doi: 10.1016/s0968-0004(00)01604-2. [DOI] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog.Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, Ruth P, Knaus HG, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 2004;32:11897–11902. doi: 10.1073/pnas.0402590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. Journal of Biological Chemistry. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- Tomari Y, Du T, Haley B, Schwarz DS, Bennett R, Cook HA, Koppetsch BS, Theurkauf WE, Zamore PD. RISC assembly defects in the Drosophila RNAi mutant armitage. Cell. 2004;116:831–841. doi: 10.1016/s0092-8674(04)00218-1. [DOI] [PubMed] [Google Scholar]
- Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes and Development. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Walters FS, Covarrubias M, Ellingson JS. Potent inhibition of the aortic smooth muscle maxi-K channel by clinical doses of ethanol. Am.J.Physiol Cell Physiol. 2000;279:C1107–C1115. doi: 10.1152/ajpcell.2000.279.4.C1107. [DOI] [PubMed] [Google Scholar]
- Wang Y, Krishnan HR, Ghezzi A, Yin JC, Atkinson NS. Drug-induced epigenetic changes produce drug tolerance. PLoS Biol. 2007;5:2342–2353. doi: 10.1371/journal.pbio.0050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner SG, Koch RO, Koschak A, Trieb M, Garcia ML, Kaczorowski GJ, Knaus HG. High-conductance calcium-activated potassium channels in rat brain: pharmacology, distribution, and subunit composition. Biochemistry. 1999;38:5392–5400. doi: 10.1021/bi983040c. [DOI] [PubMed] [Google Scholar]
- Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc.Natl.Acad Sci U.S.A. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- Yekta S, Shih Ih, Bartel DP. MicroRNA-Directed Cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Yuan C, O'Connell RJ, Jacob RF, Mason RP, Treistman SN. Regulation of the gating of BKCa channel by lipid bilayer thickness. J Biol Chem. 2007;282:7276–7286. doi: 10.1074/jbc.M607593200. [DOI] [PubMed] [Google Scholar]
- Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L. Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Letters. 2005;579:4856–4860. doi: 10.1016/j.febslet.2005.07.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01. Supplemental Materials.
The Supplemental Materials for this article can be found online at … and include: a Word text file, Table S1 (Word), Table S2 (Excel), Supplementary Figures S1–S9 with legends.
02