IMAGING ENDOCYTIC CLATHRIN STRUCTURES IN LIVING CELLS (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 1.
Published in final edited form as: Trends Cell Biol. 2009 Nov;19(11):596–605. doi: 10.1016/j.tcb.2009.09.002
Abstract
Our understanding of the clathrin-dependent endocytic pathway owes much to new visualization techniques. Budding coated pits and clathrin-coated structures are transient molecular machines with distinctive morphological characteristics, and fluorescently labeled versions of a variety of marker proteins have given us a tantalizing glimpse of the dynamics of the system in living cells. Recent live-cell imaging studies reveal unexpected modes of coat assembly, with distinct kinetics, recruitment of associated proteins, requirements for the participation of actin and its accessory proteins, and apparently, distinct mechanisms of membrane deformation. A crucial issue is to connect the events detected by light microscopy with the structures and properties of the molecular constituents. Here, I outline descriptions of coat assembly in different circumstances that are consistent with what is known from x-ray crystallography and electron microscopy.
The need to analyze endocytosis in live cells
Cells require ordered movement of proteins and lipids from one membrane-bound compartment to another. The most common mechanism for such transport is formation and budding of a vesicle from the membrane of a donor compartment and fusion of the vesicle with the membrane of an acceptor compartment. This process maintains the organization, function and heterogeneity of the donor and acceptor membranes. The molecular machinery scaffolded by clathrin is a particularly well-defined and physiologically important example of vesicle formation. Assembly of a clathrin coat at a donor membrane deforms and invaginates a membrane patch, which, after pinching and scission, becomes a carrier of membrane traffic. Clathrin-coated vesicles are the most prominent form of traffic from the plasma membrane to endosomes (endocytosis), a pathway by which ligands, such as hormones and other signaling molecules, transferrin, immunoglobulins, low-density lipoproteins (LDLs), viruses and their receptors, enter cells. Clathrin coats are also important for traffic between endosomes and the _trans_-Golgi network (TGN) 1–9.
The ‘life cycle’ of a clathrin-coated vesicle, from coat assembly and cargo loading to coat disassembly and cargo delivery, involves a large number of structural and regulatory proteins and lipids. These components are often present in small amounts. They participate in a series of rapid, regulated events (Fig. 1) (Box 1), and their study therefore poses particular challenges, both for the strategies required to trap a defined state of a dynamic assembly and for the technologies needed to follow its normal cycle in cells.
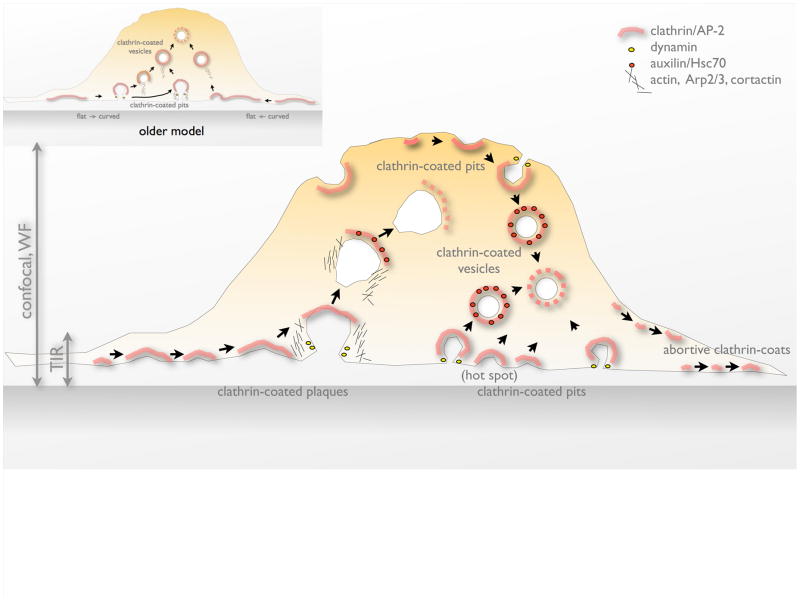
Figure 1.
Formation of endocytic clathrin-coated structures on the plasma membrane of mammalian cells. The continuous line represents the plasma membrane of a cell grown in culture; a thick, ‘pink stripe’ represents the clathrin coat (clathrin plus the AP-2 adaptor); yellow dots represent dynamin; red dots, the uncoating ATP Hsc70 and its cofactor auxilin; short thin lines, short-branched actin polymers plus the Arp2/3 complex, cortactin and N-Wasp. The stippled thick pink stripe represents the ATP-dependent dissolution of the clathrin coat mediated by Hsc70 and auxilin. TIR, WF and spinning disk confocal microscopy have all been used to obtain live-cell imaging data; the vertical arrows indicate the _z_-positions accessible to each imaging method. Stable and transient expression of recombinant, fluorescently tagged constructs have been used to follow the dynamic behavior of plasma membrane structures containing different combinations of clathrin, AP-2, auxilin, Arp2/3 complex, cortactin and dynamin. Canonical clathrin-coated pits form at both free and attached cell surfaces; clathrin-coated plaques form only at the attached surface. Coated-pit formation proceeds by sequential addition of clathrin triskelions to an initial nucleus, generating a sharply curved coat; adaptor-mediated interactions with membrane-bound proteins (and lipids) deform the underlying membrane; dynamin mediates scission when the deformation has created a suitably narrow neck; auxilin, which arrives immediately following scission, recruits the uncoating ATPase, Hsc70. The relatively long-lasting clathrin-coated plaques probably initiate in a fashion similar to coated pits, but subsequent addition of clathrin triskelions maintains a relatively flat structure with mainly hexagonal facets. The number of clathrin triskelions and AP-2 complexes associated with a clathrin-coated plaque can fluctuate during its lifetime. Coated plaques drive membrane invagination when the entire coat moves rapidly inwards, in a step that requires formation of short-branched actin filaments; by contrast, actin polymerization does not generally accompany assembly or budding of coated pits. Abortive clathrin coats are incomplete, short-lived structures that fail to associate with cargo. Inset: diagram illustrating an older model, in which a flat coated plaque deforms into a sharply invaginated coated pit. Such a transformation is structurally implausible, as discussed in the text and illustrated by the exercise in Fig. 5.
Box 1. Clathrin and its partners.
Abortive clathrin coat: a short-lived clathrin assembly that dissociates without internalizing membrane.
Actin, cortactin, Arp2/3 complex, N-Wasp: the Arp2/3 complex nucleates actin polymerization into short-branched filaments. Proteins that regulate this process include cortactin, N-Wasp and Cdc42.
AP-2: the main endocytic clathrin adaptor. It comprises four subunits, the large - and -adaptins, the medium μ2 adaptin, and the small σ2-adaptin. AP-2 has a key role in coat assembly under physiological conditions, a process mediated by its interactions with clathrin. AP-2 selectively binds to the phosphoinositide phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5) _P_2] and to short peptide motifs present in the cytosolic tail of membrane-bound cargo receptors. It also binds to a number of other proteins, some of which can also interact with cargo receptors. The function AP-2 is therefore essential for the specific entrapment and sorting of cargo into clathrin-coated structures.
Auxilin, Hsc70: auxilin is a J-domain-containing protein that binds to the chaperone Hsc70, clathrin, AP-2 and phosphoinositides. Recruitment of auxilin to clathrin coats occurs after membrane scission; it in turn recruits Hsc70 to the clathrin coat, so that an ATP-dependent uncoating reaction can ensue.
Clathrin: a trimer of three ~190-kDa heavy chains, each with an associated 23–26 kDa light chain. The heavy chain has a very elongated structure, approximately -- Å in contour length, with 42 -helical zig-zags connecting a globular N-terminal domain with a C-terminal trimer hub. The light chains bind near the hub. The overall shape resembles the three-legged ‘triskelion’ in various traditional emblems. A clathrin triskelion is the assembly unit of a vesicular coat. It forms a curved, polygonal lattice, with open hexagonal and pentagonal facets and the hub of a triskelion centered at each vertex. Each leg of a clathrin triskelion extends around three lattice edges.
Clathrin-coated pit: a sharp membrane invagination produced by assembly of a clathrin coat. The radius of membrane curvature can range from 200 Å to more than 800 Å, and the radius of the polymerized coat, from approximately 350 Å to more than 1000 Å. The coat ultimately closes in on itself, driving formation of a narrow membrane neck.
Clathrin-coated plaque: an extended, relatively flat clathrin array, often found at an adherent surface of a cell in culture.
Dynamin: a large GTPase recruited to clathrin coats when the lattice is nearly complete; its function is essential for membrane scission.
Dynasore: the term originates from combining ‘dyna’ (dynamin) and ‘sore’ (pain). A cell-permeable small molecule that rapidly (and reversibly) inhibits dynamin function. It is widely used to interfere acutely with the cellular function of dynamin.
Hot spot: a more-or-less diffraction-limited region on a cell surface from which repeated clathrin-coated vesicles are seen to emanate. The hot-spot area can also contain a more stationary, long-lived clathrin assembly.
Cellular, biochemical and high-resolution structural approaches have defined the molecular properties of clathrin and many of its associated proteins 1, 10–17. Biochemical and structural approaches, however powerful, can only provide snapshots or ensemble-averaged information about the properties of objects within a heterogeneous population. They are not sufficient to resolve important steps in vesicle formation and uncoating. Advanced imaging methods can now achieve high temporal resolution in the context of a living cell 18, 19, allowing one to localize components during a given step, to determine the order in which components are incorporated or released, and to analyze the way the composition of an assembling vesicle affects its behavior 20–34. So far, most studies have focused on endocytosis. This is a particularly favorable membrane-traffic pathway for such studies, because labeled cargo can be prepared and followed, thus identifying functional uptake, and because events at the cell surface are particularly accessible to live-cell imaging methods.
This review focuses on the interpretation of results from live-cell imaging, rather than on data obtained using fixed samples that in principle could provide improved spatial resolution 35, 36 but at the cost of losing the temporal component. Gathering data over time is essential for analyzing highly dynamic systems such as those that direct membrane traffic. A central issue in developing models to describe the dynamics of clathrin coat formation is how to relate the data from live-cell imaging to the static morphological information obtained from cells by electron microscopy and to the high-resolution structures obtained from x-ray crystallography and advanced electron cryo-microscopy (cryoEM). Correlation between distinguishable classes of clathrin-based endocytic events as detected by fluorescence microscopy with their corresponding morphologies as seen by electron microscopy resolves a number of previous conflicts and misconceptions. These distinctions make our picture of clathrin dynamics consistent with the molecular structures defined by high-resolution methods.
Live-cell imaging of clathrin-coated structures
The advent of GFP combined with time-lapse wide-field epifluorescence microscopy (Box 2) provided the first glimpse of assembling clathrin-coated structures in cells expressing the clathrin light chain LCa fused to GFP and demonstrated their transient character 29. Although the time series were obtained from an optical plane close to the cell surface attached to the coverslip, thus providing images of endocytic clathrin structures assembling at the plasma membrane, fluorescent objects located further inside the cell, probably clathrin coats on endosomal membranes, also contributed significantly to the data sets, leading to ambiguities in the interpretation. This limitation was soon overcome by use of total-internal-reflection fluorescence (TIRF) microscopy, a powerful approach that is naturally suited to observe fluorescent events at the cell surface in contact with the glass coverslip 25.
Box 2. A close-up view of microscopy terms.
Diffraction limit: the resolution of an optical system, as defined by the classical Rayleigh criterion. In practical terms, the diffraction limit for fluorescence microscopy is ~250 nm in the _xy_-plane and ~500–800 nm in z plane.
DiNa: ‘differential evanescence nanometry’, a method based on TIR and WF illumination to determine the axial separation of the centroids of two different fluorophore distributions with a precision of ~10 nm. This method was used in real time for the differential localization of AP-2 and epsin with respect to clathrin during the formation of clathrin-coated pits and plaques 22.
Laser-scanning confocal microscopy: in a confocal microscope, the illuminating beam converges on the focal plane; fluorescent radiation from the point of illumination will then converge on the conjugate point in the image plane, and a pinhole can be used to eliminate fluorescence from out-of-focus planes. Scanning the beam on an _xy_-raster over a succession of suitably spaced focal planes allows one to build up a three-dimensional image. The data are collected with photomultiplier detector and the image reconstructed computationally.
Spinning disk confocal microscopy: by arranging a set of microlenses and pinholes in an appropriate array on a rapidly rotating wheel, a confocal microscope can be constructed that repeatedly scans many points in parallel, thus greatly increasing the rate of data acquisition, while decreasing phototoxicity. An image for each focal plane is collected on a standard CCD camera. With currently available laser illumination, it is possible to acquire single frame images in a few milliseconds. Spinning-disk confocal microscopy is thus a relatively straightforward way to achieve high temporal and spatial resolution for dynamic structures.
Total internal reflection fluorescence (TIRF) microscopy: total internal reflection (TIR) occurs when a beam of light is incident at a suitably small angle from a medium of higher refractive index onto an interface with a medium of lower refractive index. In particular, light is totally reflected when incident from a glass coverslip (_n_~1.5) onto the aqueous medium of a cell in culture (_n_~1.35). There is nonetheless an ‘evanescent wave’ produced in the lower refractive index medium, with an intensity that falls off exponentially with distance from the interface. Thus, if the excitation illumination in a fluorescence microscope is so directed that it is totally reflected from the interface between coverslip and adherent cell, fluorophores located within 100–200 nm of the coverslip are strongly excited, but those further away are not. The images of the layer at or near the cell surface thus contain little or no interference from fluorescent molecules elsewhere in the cytosol.
Wide-field (WF) illumination fluorescence microscopy: visualization using excitation illumination propagating parallel to the _z_-axis. The intensity of fluorescent molecules is directly proportional to their number within the illuminated volume. Images generated in this way have significant contributions from objects located above or below the focal plane. Spatial deconvolution is required for accurate representation of the image.
Time-lapse TIRF of cells expressing clathrin light chain fused to dsRED confirmed the dynamic character of endocytic structures and revealed their inward movement before dissolution of the clathrin coat 25, 26. To obtain temporal information regarding the time of membrane scission from the donor plasma membrane of the budding clathrin-coated membrane, a visual method was used to detect exposure or protection from the incubation medium of the ectodomain of the transferrin receptor (TfR)26. A chimeric protein was constructed from TfR, fused in its ectodomain with pHluorin, a GFP-based fluorescent pH-sensor. With this probe, the fluorescence of pHluorin-tagged TfRs located at the cell surface is sensitive to the pH of the incubating medium, while the fluorescence of those present in completely sealed intracellular vesicles is not. By cyclically alternating the pH of the incubating medium between pH 7.4 and pH 5.5, it was possible to detect a sudden loss in pH-dependence in the fluorescence signal of a subset of chimeric TfR clusters associated with clathrin coats. The combined used of TIR and wide-field fluorescence imaging revealed that a pH-independent spot of the TfR chimera appeared just as its associated fluorescent clathrin patch underwent abrupt inward movement. These observations provided the first direct link between the invagination step of a clathrin-coated structure and membrane scission 26.
Other studies based on TIRF or spinning disk confocal microscopy followed the temporal recruitment of dynamin, the large GTPase required for scission of the membrane neck connecting invaginating clathrin-coated pits from the plasma membrane 25, 30, 37, 38. The live-cell imaging data obtained alone or in combination with dynasore, a cell-permeable chemical compound that acutely inhibits the function of dynamin, revealed both the expected burst of dynamin recruitment upon completion of coat assembly and an unexpected recruitment of a small amount of dynamin during the clathrin-coat assembly process 37. While it is clear that the function of dynamin recruitment at the end of coat assembly is directly linked to scission of the neck between the plasma membrane and the underlying coated membrane, the role of dynamin recruited prior to membrane scission remains to be determined.
Motivated by the observation that, in yeast cells, endocytosis requires both clathrin- and actin- based components (reviewed in 39, 40), several studies used TIRF, mainly in HeLa cells and fibroblasts, to explore the temporal recruitment of actin and a subset of regulators of short-branch actin assembly, including Arp2/3, cortactin and N-Wasp 26, 27, 41–43. In general, most of the clathrin-containing, fluorescent objects tracked in these experiments were relatively long-lived, with life spans of 2 minutes or more, and they seldom showed a steady increase in fluorescence during the lifetime of the clathrin-coated structure; the remaining clathrin objects were either shorter-lived, with life spans of ~1 minute, or appeared as small (diffraction limited) structures detaching from larger ones. Many of these structures recruited actin, Arp2/3 complex or cortactin at or near the time of membrane scission, but others did not 21, 25, 26. These studies led to the conclusion that, in mammalian cells, as in yeast, actin is a key participant in clathrin-based endocytosis and that its assembly is required to drive the inward movement of the budding clathrin-coated vesicle 21, 26.
The observations summarized above were at odds with the variable dependence of clathrin traffic on actin polymerization, as defined by pharmacological criteria (sensitivity to actin depolymerizers, etc.) 44. In some cases, for example the formation of clathrin-coated vesicles in presynaptic terminals of giant synapses from lampreys 45, 46, or in the apical surface of polarized epithelial cells 47, 48, and in the internalization of vesicular stomatitis virus (VSV) by clathrin-coated structures 49, actin polymerization is indeed linked to membrane deformation in clathrin-coated pits. However, in many other cases – for example, endocytosis in the presynapse of rat hypocampal neurons 50 or transferrin uptake by coated pits of non-polarized BSC1 fibroblastic cells in culture 34, 51, actin polymerization is clearly not essential. We can now distinguish between at least two modes of clathrin coat formation at the plasma membrane, with quite different mechanisms for coat internalization (Fig. 1). At one end of the spectrum, the actin cytoskeleton is not required for normal formation and budding of the rapidly growing pits, whereas, at the other, it is essential for the formation, inward movement and dissolution of longer-lived clathrin structures. That is, clathrin is a scaffold for at least two kinds of membrane-associated processes. This distinction allows resolution of apparent contradictions between the high-resolution structural data and some of the models put forward to explain how invagination of clathrin-coated structures might occur (Fig. 1, inset).
Distinct forms of endocytic clathrin-coated structures
Spinning disk confocal microscopy, another sensitive and rapid imaging approach, has been used to observe the assembly and disassembly dynamics of fluorescent clathrin structures as they form at the free plasma membrane at the free (top) and the attached (bottom) surfaces of cells in culture (Fig. 2a). These studies began with BSC1 cells, taking advantage of the observation that more than 95% of the plasma-membrane clathrin-coated pits in these cells are rapidly forming, diffraction-limited structures 30. The experiments were conducted in cells stably expressing fluorescently tagged forms of clathrin light chain or 2-adaptin, the small subunit of the endocytic clathrin adaptor AP-2. They were later extended to other cell types 22, 34. Regardless of cell type, the clathrin and AP-2 signals always colocalize.
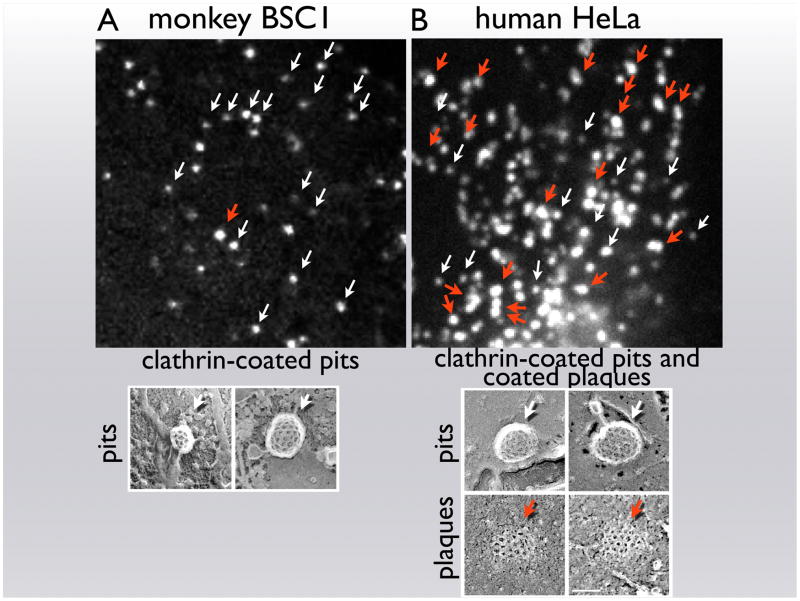
Figure 2.
Different types of clathrin-coated structures detected on the attached surface of cells. The upper panels are single frames from time series recorded from: (a) a monkey BSC1 cell expressing σ2 EGFP of the adaptor AP-2; (b) a human HeLa cell expressing Tomato–LCa light chain of clathrin. The live-cell images are from optical sections, acquired with a spinning disk confocal microscope, at the level at which plasma membrane adheres to the glass coverslip. The white and red arrows single out short- and long-lived fluorescent objects, whose dynamic behavior corresponds to coated pits and coated plaques, respectively. The bottom panels are electron micrographs obtained from the attached surfaces of ‘unroofed’ preparations of the corresponding cell type. Whereas BSC1 cells display only deeply curved clathrin-coated structures (pits), HeLa cells contain both deeply curved and relatively flat clathrin lattices (pits and plaques, respectively). The clathrin lattices in coated pits contain both hexagonal and pentagonal facets; the coated plaques contain mostly hexagonal facets.
It is particularly important in imaging studies of cells growing on a coverslip to distinguish the properties of clathrin-related processes at the free surface of a cell from those of processes at the coverslip-attached surface. Clathrin-containing fluorescent structures detected at the free surface are, with rare exception, diffraction limited (below the ~ 250 nm optical resolution of the imaging setup). They show a characteristic steady protein accumulation, typically for a period of 40–70 s. They disappear quite suddenly at the end of the accumulation period, as coat disassembly rapidly follows membrane budding. These events correspond to ‘canonical’ coated pits, in which sequential recruitment of clathrin and its adaptors results in a coat that assembles progressively into a curved lattice, deforming the membrane as it grows (Fig. 1). In canonical coated pits, adaptors selectively capture membrane-bound proteins destined for endocytosis; membrane pinching, mediated by dynamin, separates the fully formed coated vesicle from its parent membrane, and an ATP-dependent Hsc70 chaperone uncoats the released vesicle. A subset of these coated pits forms sequentially at preferred sites or ‘hot spots’ 29 (Fig. 1).
Clathrin and AP-2 structures at the coverslip-attached surface have more-diverse characteristics (Fig. 2b). One set comprises diffraction-limited objects with the same dynamic properties as the canonical coated pits observed on the top surface. Members of a second set are of variable size, sometimes slightly larger than diffraction limited, and they have relatively long lifetimes, usually greater than 2 minutes and sometimes longer than 15 minutes 21, 25, 28–30. Just prior to dissolution, the full coat moves inwards towards the cell interior, bringing with it a portion of the underlying membrane, which then buds off in a dynamin-dependent manner 26, 34. Classical images from electron microscopy (Fig. 3) have shown extended clathrin lattices, approximately planar or moderately domed and with mostly hexagonal facets 52, 53, called in one paper ‘coated plaques’ 54. Recent work shows that the long-lived clathrin structures studied by live-cell imaging correspond, at least in many cases, to coated plaques (Fig. 2) 34. The relative abundance of coated plaques and canonical coated pits depends strongly on cell type. For example, coated plaques are hard to observe in freshly plated BSC1 cells, whereas plaques lasting more than 15 minutes are dominant features on the adherent surface of HeLa cells.
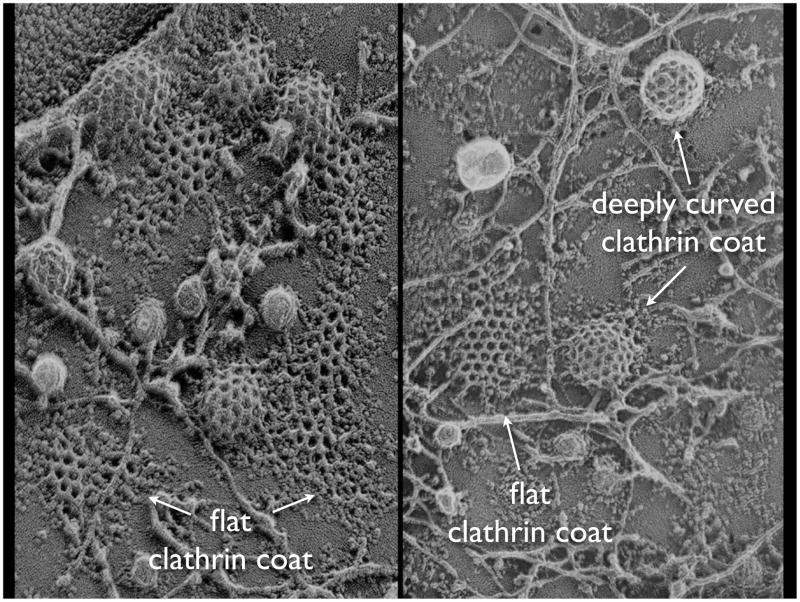
Figure 3.
Morphology of clathrin-coated structures on the attached surface of cells. Electron micrograph obtained from an ‘unroofed’ preparation of human A4321 cells imaged by electron microscopy after quick-freeze deep etching, to show a range of different shapes of deeply curved and relatively flat clathrin structures on the adherent plasma membrane. (Reproduced, with permission, from 52.)
‘Non-terminal events’ 25 is a phrase that has been used to describe those cases in which a long-lived fluorescent clathrin structure remains in place, while a small drop in clathrin fluorescence in the overall object coincides with formation and inward movement of a vesicular carrier marked (for example) by a fluorescent transferrin receptor. The departing carrier contains only a small fraction of total transferrin receptor associated with the long-lived clathrin structure 26. This type of event probably represents formation and budding of a small clathrin-coated structure that is not spatially resolved from the coated plaque (i.e., separated from it by less than the 250 nm resolution limit of the light microscope). Indeed, electron micrographs of ‘unroofed’ cells often show deeply invaginated pits close to flat clathrin arrays (Fig. 3).
Further experimental distinction between clathrin-coated pits and plaques comes from use of differential nanometry (DiNa), a high-_z_-precision (~10 nm) imaging technique that correlates the time dependence of fluorescence, recorded both by total-internal-reflection (TIR) and wide-field (WF) illumination, from two distinct components in an assembling structure 22. DiNa, when applied to cells expressing fluorescently tagged clathrin, AP-2 and epsin, has allowed determination, with a temporal resolution of ~1 s, of the relative displacement of AP-2 or epsin with respect to clathrin in endocytic structures forming on the attached membrane of mammalian cells 22, 34.
DiNa measurements show that a canonical coated pit grows continuously as an invaginating shell, which pinches off immediately upon completion. The entire process takes typically 30–60 seconds. AP-2 concentrates in the older part of the curved coat, away from the plasma membrane, whereas epsin resides primarily at the growing rim of the pit, closer to the plasma membrane. This polarized distribution of AP-2 corresponds to the asymmetric distribution of electron density in cryoEM tomographic reconstructions of coated vesicles isolated from brain 55. The polarized location of AP-2 with respect to clathrin in a budding coated pit explains why, under TIR conditions of illumination, the relative fluorescence of AP-2 decreases more rapidly than the corresponding fluorescence of clathrin, as the coated pit moves in the evanescent field away from the coverslip and towards the cell interior. Clathrin and AP-2 signals always disappear together when determined by spinning disk confocal or wide-field fluorescence microscopy. Thus, earlier loss of the AP-2 signal determined by TIR does not imply a proposed alternative interpretation, in which AP-2 dissociates from the coat prior to disassembly of the clathrin lattice 23, 56. The well-established presence of both clathrin and AP-2 in coated vesicles isolated from tissues or cells also contradicts this alternative model.
DiNa measurements show that a coated plaque, by contrast, grows initially at about the same rate as a coated pit, but without displacement from the cell surface. Its growth reaches a fluctuating plateau of fluorescence intensity, generally 2–3 times that of a typical pit, and the plaque remains in place for up to several minutes before moving uniformly inward, a few seconds before membrane pinching. The fluctuation in the fluorescence signal might represent partial addition and/or removal of clathrin and AP-2 from the coat, a behavior that is fully consistent with the partial fluorescence recovery after photobleaching (FRAP) of clathrin assemblies located at the bottom surface of HeLa cells 57. In contrast to the relative displacement (with respect to clathrin) of AP-2 and epsin in coated pits, AP-2 and epsin have the same mean _z_-position as the bulk of the clathrin during the whole process of coated plaque formation 34.
Sharply curved pits and relatively flat, extended arrays have both been seen by electron microscopy (Fig. 3), but there was no direct way to link the shape of the clathrin coats with their dynamic characteristics. The distinction between coated pits and coated plaques, derived from live-cell imaging, correlates with two kinds of lattices seen by standard transmission electron microscopy of thin sections or by freeze-etch electron microscopy on the inward-facing surface of adherent plasma membranes from ‘unroofed’ cells 53, 54, 58, 59. Recent work shows that the relative frequency of the two kinds of structures, as detected by electron microscopy, correlates well with their relative frequencies determined by live-cell imaging criteria. Specifically, BSC1 cells, that display primarily short-lived clathrin events at the cell surface, show only sharply curved pits when prepared for electron microscopy (Fig. 2a, white arrows), whereas HeLa cells, which display both short- and long-lived coated structures (Fig. 2b white and red arrows, respectively), show both sharply curved pits and extended clathrin lattices 34. This correlation between clathrin lattice morphology and clathrin coat dynamics is fully consistent with the clathrin assembly model put forward more than 16 years ago 60; this model also conforms to the structural constraints imposed by high-resolution cryoEM reconstructions of clathrin coats 17.
Many investigators appear to assume that the flat clathrin sheets can be an initial stage for coated pit formation and that curved ‘domes’ can emerge by transforming hexagons into pentagons in the middle of an array. But the introduction of the proper number of pentagons (12) or combinations of heptagon–pentagons for curvature requires major molecular rearrangements. The intricate and extended association of legs from six different clathrin triskelions along any one edge makes introduction of curvature (pentagons) into a flat (hexagonal) sheet exceptionally unlikely (see Fig. 4 for an illustration of how clathrin triskelions are packed into the lattice and Fig. 5 for an ‘origami’ exercise showing why such rearrangement from hexagons to pentagons is implausible). Acquisition of a pentagon would require the stepwise removal of a 60° clathrin ‘pie slice’ (Fig. 5), an operation that would have to be repeated 12 times. Not only are there no live-cell experimental data supporting such events, but also, none of the necessary intermediates has ever been observed by electron microscopy. Internalization of a membrane coated by an extended, flat clathrin array, as occurs with coated plaques, involves an abrupt inward movement into the cell of the entire coat 25, 31, 32, 34, 61. Membrane invagination by a curved clathrin lattice, by contrast, involves continuous deformation linked to sequential addition of soluble triskelions 22, 62.
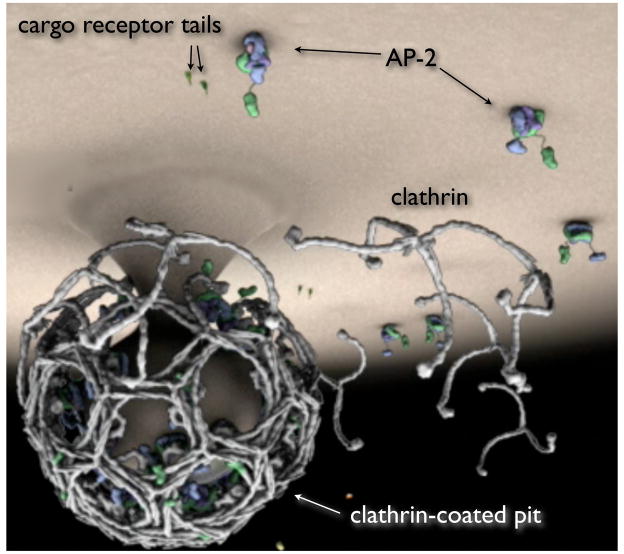
Figure 4.
Receptor sorting and clathrin-coated pit formation. Schematic representation of a clathrin lattice during the assembly of a deeply invaginated coated pit, obtained from the accompanying animation, Movie 1. The shape and location of the triskelions are based on the map, of resolution 0.8 nm, of a clathrin coat obtained by cryoEM 17. The position of AP-2 within the coat is approximate. The shape of AP-2 is based on X-ray crystallographic data 10. AP-2 adaptor complexes bind selectively to the plasma membrane by interacting both with PtdIns(4,5)_P_2 in the membrane bilayer and with sorting motifs in the cytosolic tails of membrane-bound cargo receptors.
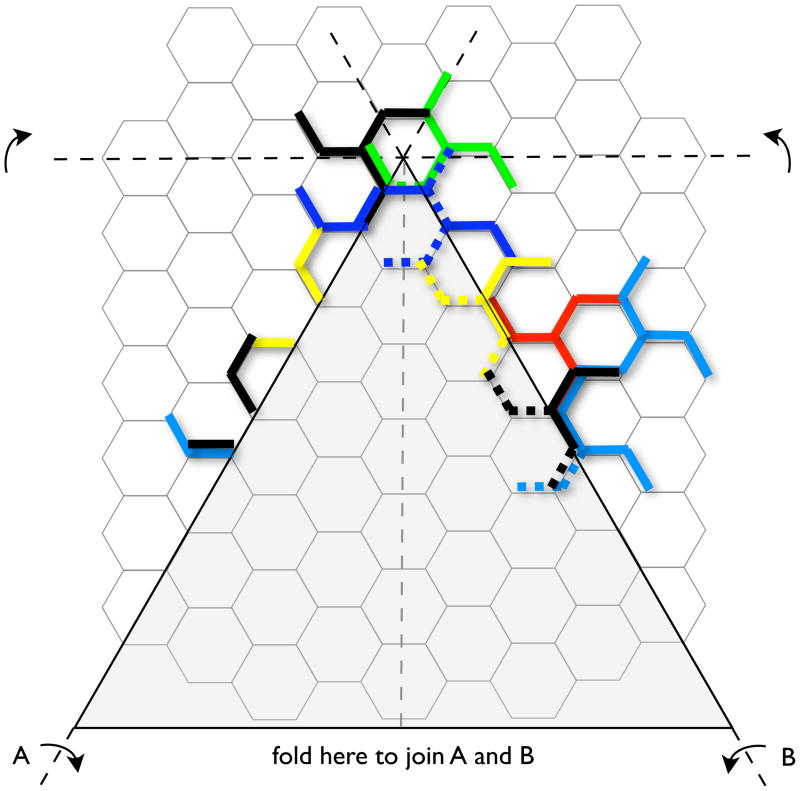
Figure 5.
Rearrangements of clathrin triskelions in a flat hexagonal lattice that would be required to transform a single hexagon into a pentagon. This figure is adapted from 60. If hexagonal sheets are indeed intermediates in the budding of coated pits, major rearrangements of this kind would be necessary. High-resolution structural data show that a triskelion is centered at every vertex, with each of its three legs spanning three consecutive edges 17, 79. To simplify the drawing, only a subset of color-coded triskelions is shown, depicting just their proximal and distal legs (those spanning the two edges closest to the vertex). Thick lines highlight a subset of triskelions that would need to refold into the new lattice; the color-coded stippled lines indicate the new positions adopted by the legs after the rearrangement. The reader is invited to print the diagram (adapted from 60), to cut out the gray triangular region and to fold it along the lines indicated to generate a curved sheet. To make an approximately spherical clathrin lattice, 12 such rearrangements are required at precise locations within the lattice. This lattice contains 154 triskelions, and 72 would need to be removed (under the gray pie section) in order to transform a single hexagon into a pentagon. Eleven additional reductions (of different amounts, depending on the location of the new pentagons) are required to generate a completely enclosed structure. No such reductions have ever been detected during the formation of clathrin-coated structures. Clathrin is a relatively stiff protein (especially considering its contour length), with legs disposed at orientations relatively close to those adopted in the final coat 17. Overall, coat formation can readily be understood by sequential assembly of triskelions into a curved lattice. There is no need to postulate a flat, hexagonal intermediate, most of which would have to disassemble in order to generate curvature.
Another distinction among different clathrin-based endocytic structures is their substantially different dependence on actin for invagination and internalization of membrane, as described. Assembly, budding and uncoating of canonical clathrin-coated pits in non-polarized cells such as BSC1 or HeLa are all insensitive to pharmacological interference with actin dynamics, at both the free and attached surfaces 51. Canonical pits do not recruit actin-assembly regulators such as the Arp2/3 complex and cortactin 49. By contrast, formation and internalization of clathrin-coated plaques do involve recruitment of these regulators and require normal actin dynamics 34.
While coated pits and coated plaques appear to represent distinct assembly modes, there might be ‘hybrid’ modes as well. VSV entry seems to be based on such a hybrid form of clathrin-mediated uptake. In that case, coat assembly proceeds normally in the absence of actin dynamics, as in canonical coated pit formation, but budding does not occur until actin activity is restored 49. Electron microscopy of endocytic VSV uptake suggests that curved coat formation ‘stalls’ when the coated pit curves back against the shaft of the bullet-shaped virus particle 49. The findings with VSV have parallels with the clathrin-dependent uptake of the bacterium Listeria monocytogenes 63, 64. Specifically, clathrin, dynamin, auxilin and actin are recruited during entry of both pathogens, and actin polymerization is crucial for their endocytosis but not for initial clathrin assembly. In addition, localization of the actin machinery to sites of L. monocytogenes invasion requires the prior recruitment of clathrin and dynamin 64.
In summary, canonical coated pits are invaginating structures, in which assembly of a curved clathrin lattice, linked by adaptors and other proteins to deformation of the underlying membrane, gives rise, by constriction and (dynamin-catalyzed) scission, to a coated vesicle (Fig. 1b). The early electron micrographs of yolk and LDL uptake 65, 66, much subsequent work on a great variety of other cargo 67 and countless studies of membrane reuptake at presynaptic nerve endings 68 all illustrate this process. Extended clathrin lattices of low curvature (enriched in hexagonal arrays), now called clathrin-coated plaques, have also been observed for many years at the attached surface of cells 53, 54, 58, 59, and their relationship to the canonical, curved assembly has now been resolved. The coated plaques internalize, but by a mechanism requiring actin remodeling, probably similar to one proposed for clathrin-mediated membrane uptake in yeast cells 39. In contrast to events in yeast cells, however, membrane scission in metazoan cells is catalyzed by dynamin.
Abortive clathrin coats
Clathrin assemblies that fail to bud, referred to as ‘abortive clathrin coats’, are also seen (Fig. 1). First detected by global analysis of data obtained by spinning disk confocal microscopy 30, and later confirmed, also by global analysis of TIRF time series 69, abortive clathrin coats have typical lifetimes of less than 20 s. They contain less clathrin and AP-2 than do canonical coated pits at completion, and they are in some cases even more abundant than coated pits and plaques. It is currently believed that abortive clathrin coats represent nucleation and/or initiation events of coat components that start to assemble but then dissolve and fail to progress to invaginating structures because they do not associate with cargo. Evidence supporting this model comes from absence of LDL, VSV or reovirus colocalization with abortive structures 30, 49 and from the decrease in the number of abortive coats upon conditions of extreme overexpression of TfR 69, a membrane cargo actively internalized by clathrin-coated pits and plaques.
Role of actin
The relationship between clathrin and actin in endocytic membrane traffic appears to depend on the cell type and on the kinds of clathrin structures involved. The relevant recruitment and switching mechanisms have not yet been fully worked out. Genetic and imaging data clearly show a strong link between clathrin and actin dynamics for endocytosis in yeast cells 31, 32 (see also review by Kaksonen and colleagues39). Live-cell imaging experiments that follow the recruitment of a large number of fluorescently tagged proteins at locations marked by the transient appearance of clathrin demonstrate early transient accumulation of Las17, followed by recruitment of clathrin and associated proteins such as Sla1, Pan1, End3, Sla2 or myosin V, and of actin and regulators of its assembly such as the Arp2/3 complex, Abp1, Cap1/2 and Sac6 39. These observations have been extended to metazoan cells, leading to the assumption that actin dynamics are essential, both during early stages of clathrin coat formation and for budding. Assembly of endocytic clathrin coats at the free surface of non-polarized cells is actin-independent, however, just as for canonical coated pits at the attached surface of the same cells. Formation of coated plaques at the attached surface does require actin dynamics 34, as does coat formation in some polarized cells 48, 70. Actin and clathrin dynamics are also linked during internalization of VSV, even though the clathrin coat surrounding the virus is highly curved, and in the AP-2 adaptor-independent mode of clathrin array formation induced during phagocytic uptake of L. monocytogenes 64.
Hip1R is a regulator of actin dynamics that associates with clathrin light chains, cortactin and F-actin 71–74. Like the loss of clathrin endocytic activity in yeast cells, interference with clathrin light chains or with Hip1R association with clathrin light chains in (nonpolarized) mammalian cells minimally affects the clathrin-mediated uptake of transferrin 74 and has no effect on the dynamics of canonical coated pits but prevents assembly of plaques 34. These observations, that actin-dependent and actin-independent modes of clathrin assembly co-exist in the same cells, suggest that there are molecular signals that determine which mechanism of membrane deformation and budding will prevail. In VSV entry, there seems to be a switch, in which clathrin coat assembly proceeds normally in the absence of actin dynamics, but budding followed by coat disassembly does not 49; in the same cells, canonical coated pits forms and pinch normally. Thus, the actin cytoskeleton has a variable role in clathrin-based endocytosis, and we expect that future analysis will reveal the mechanism(s) required to switch from one form to the other. Live-cell imaging designed to monitor single endocytic events has been instrumental in uncovering these relationships, which would otherwise have been confounded by the results of ensemble biochemical methodologies or by the unconnected snapshots obtained from fixed cells.
Future directions
The availability of improved imaging hardware, including fast, reliable and sensitive digital cameras for data acquisition, automated motorized microscopes, correction of spherical aberration, strong light sources, in situ expression of chimeric fluorescent proteins, increased computational power, improved spatial resolution during live-cell imaging 22, 75–77 and new analytical tools for unbiased detection of weak fluorescent events 78, should now allow investigators to gather information on early events in the initiation of coat formation, on the regulation of assembly and disassembly, on the relationships between the content of coat components and properties of different classes of clathrin-coated structures and on whether the characteristics of endocytic clathrin coats in cells within tissues is the same or different as in cultured cells. Perhaps it will be possible to work out in detail how receptors are captured by adaptors, when SNAREs are recruited to a forming coat, and what are the functions of the various ‘flavors’ of a clathrin coat. The availability of more-robust data sets will also permit the generation of detailed kinetic and/or stochastic mathematical models that can then be used to test ideas and hypotheses of how clathrin coats form.
Unbiased methods are crucial for the proper analysis of a large population of relatively heterogeneous, spatio-temporal events, especially those that give relatively weak fluorescent signals. These approaches are particularly crucial when monitoring the kinds of single events detected by live-cell imaging. The first application of such an approach to clathrin-coated structures was based on computational selection of events that satisfied the combined criteria of having a fluorescence intensity statistically higher than that of the surrounding background and a lifetime longer than 15 s 30. This method allowed detection of all clathrin-based endocytic events and detected the presence of abortive structures, but it was not sufficiently robust to track them. A more recent improved particle-tracking approach, also designed for unbiased detection, now allows both detection and tracking of all clathrin-based endocytic processes69, 78.
Concluding remarks
High-resolution fluorescence imaging techniques have made it possible to classify distinct kinds of clathrin-containing endocytic objects: abortive, non-invaginating events; relatively shorter-lived, continuously invaginating, canonical coated pits; and longer-lived, generally larger, non-curved structures (coated plaques). The coated plaques appear to be similar to the clathrin-containing, endocytic structures found in yeast cells. Different roles for actin dynamics have been identified for each of these modes of clathrin assembly, and there is a regulated interplay between clathrin assembly and actin dynamics. We now have an internally consistent mechanistic picture of how clathrin coats form, derived from the spatial and temporal characteristics of assembling clathrin objects obtained by live-cell imaging and from the structural constraints imposed by high-resolution descriptions of the clathrin lattice.
Acknowledgments
I thank the members of my laboratory, current and past, for helping create and sustain a stimulating environment. I thank my colleagues, and Stephen C. Harrison in particular, for the opportunity to share good science and for many enlightening discussions. Our visualization efforts have been supported by NIH grants GM075252 and NERCE U54 A1057159. Finally, I apologize to colleagues whose work I have inadvertently failed to quote.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhausen T. Three ways to make a vesicle [Review] Nature Reviews Molecular Cell Biology. 2000;1:187–198. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- 3.Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacino JS, Traub LM. Signals for Sorting of Transmembrane Proteins to Endosomes and Lysosomes. Annu Rev Biochem. 2003 doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 5.Hirst J, Robinson MS. Clathrin and adaptors. Biochem Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky FM, et al. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 7.Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Duncan MC, Payne GS. Cell biology: protein choreography. Nature. 2005;438:571–573. doi: 10.1038/438571a. [DOI] [PubMed] [Google Scholar]
- 9.Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochimica et biophysica acta. 2005;1744:415–437. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Owen DJ, et al. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 11.Motley AM, et al. Functional analysis of AP-2 alpha and mu2 subunits. Mol Biol Cell. 2006;17:5298–5308. doi: 10.1091/mbc.E06-05-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keyel PA, et al. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol Biol Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaidarov I, Keen JH. Membrane targeting of endocytic adaptors: cargo and lipid do it together. Dev Cell. 2005;8:801–802. doi: 10.1016/j.devcel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Edeling MA, et al. Life of a clathrin coat: insights from clathrin and AP structures. Nat Rev Mol Cell Biol. 2006;7:32–44. doi: 10.1038/nrm1786. [DOI] [PubMed] [Google Scholar]
- 15.Brett TJ, Traub LM. Molecular structures of coat and coat-associated proteins: function follows form. Curr Opin Cell Biol. 2006;18:395–406. doi: 10.1016/j.ceb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Miwako I, Schmid SL. A cell-free biochemical complementation assay reveals complex and redundant cytosolic requirements for LRP endocytosis. Exp Cell Res. 2006;312:1335–1344. doi: 10.1016/j.yexcr.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Fotin A, et al. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature. 2004;432:573–579. doi: 10.1038/nature03079. [DOI] [PubMed] [Google Scholar]
- 18.Murray JM, et al. Evaluating performance in three-dimensional fluorescence microscopy. Journal of microscopy. 2007;228:390–405. doi: 10.1111/j.1365-2818.2007.01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters JC. Accuracy and precision in quantitative fluorescence microscopy. J Cell Biol. 2009;185:1135–1148. doi: 10.1083/jcb.200903097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoncu R, et al. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarar D, et al. A Dynamic Actin Cytoskeleton Functions at Multiple Stages of Clathrin-mediated Endocytosis. Mol Biol Cell. 2005;16:964–975. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saffarian S, Kirchhausen T. Differential evanescence nanometry: live-cell fluorescence measurements with 10-nm axial resolution on the plasma membrane. Biophys J. 2008;94:2333–2342. doi: 10.1529/biophysj.107.117234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rappoport JZ, et al. Analysis of the AP-2 Adaptor Complex and Cargo During Clathrin-Mediated Endocytosis. Traffic. 2005;6:539–547. doi: 10.1111/j.1600-0854.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rappoport JZ, et al. Dynamics of clathrin and adaptor proteins during endocytosis. Am J Physiol Cell Physiol. 2006;291:C1072–1081. doi: 10.1152/ajpcell.00160.2006. [DOI] [PubMed] [Google Scholar]
- 25.Merrifield CJ, et al. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 26.Merrifield CJ, et al. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Le Clainche C, et al. A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. EMBO J. 2007;26:1199–1210. doi: 10.1038/sj.emboj.7601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keyel PA, et al. Endocytic Adaptor Molecules Reveal an Endosomal Population of Clathrin by Total Internal Reflection Fluorescence Microscopy. J Biol Chem. 2004;279:13190–13204. doi: 10.1074/jbc.M312717200. [DOI] [PubMed] [Google Scholar]
- 29.Gaidarov I, et al. Spatial control of coated-pit dynamics in living cells. Nature Cell Biology. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- 30.Ehrlich M, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Kaksonen M, et al. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Newpher TM, et al. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Developmental Cell. 2005;9:87–98. doi: 10.1016/j.devcel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Mettlen M, et al. Endocytic Accessory Proteins Are Functionally Distinguished by Their Differential Effects on the Maturation of Clathrin Coated Pits. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saffarian S, et al. Distinct Dynamics of Endocytic Clathrin-Coated Pits and Coated Plaques. PLoS biology. 2009 doi: 10.1371/journal.pbio.1000191. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann M, et al. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc Natl Acad Sci U S A. 2005;102:17565–17569. doi: 10.1073/pnas.0506010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang B, et al. Whole-cell 3D STORM reveals interactions between cellular structures with nanometer-scale resolution. Nature methods. 2008;5:1047–1052. doi: 10.1038/nmeth.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Rappoport JZ, et al. Dynamics of dynamin during clathrin mediated endocytosis in PC12 cells. PLoS ONE. 2008;3:e2416. doi: 10.1371/journal.pone.0002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaksonen M, et al. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 40.Engqvist-Goldstein AEY, Drubin DG. Actin assembly and endocytosis: From Yeast to Mammals. Annual Review of Cell and Developmental Biology. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- 41.Merrifield CJ, et al. Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur J Cell Biol. 2004;83:13–18. doi: 10.1078/0171-9335-00356. [DOI] [PubMed] [Google Scholar]
- 42.Benesch S, et al. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. Journal of cell science. 2005;118:3103–3115. doi: 10.1242/jcs.02444. [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, et al. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. Journal of cell science. 2005;118:807–817. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]
- 44.Lamaze C, et al. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 45.Bourne J, et al. Actin polymerization regulates clathrin coat maturation during early stages of synaptic vesicle recycling at lamprey synapses. J Comp Neurol. 2006;497:600–609. doi: 10.1002/cne.21006. [DOI] [PubMed] [Google Scholar]
- 46.Shupliakov O, et al. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci USA. 2002;99:14476–14481. doi: 10.1073/pnas.212381799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X, Whittaker GR. Role of the actin cytoskeleton during influenza virus internalization into polarized epithelial cells. Cellular microbiology. 2007;9:1672–1682. doi: 10.1111/j.1462-5822.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 48.Gottlieb TA, et al. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. The Journal of Cell Biology. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cureton DK, et al. Vesicular Stomatitis Virus Enters Cells through Vesicles Incompletely Coated with Clathrin That Depend upon Actin for Internalization. PLoS Pathog. 2009;5:e1000394. doi: 10.1371/journal.ppat.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sankaranarayanan S, et al. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nature neuroscience. 2003;6:127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- 51.Boucrot E, et al. Role of lipids and actin in the formation of clathrin-coated pits. Exp Cell Res. 2006;312:4036–4048. doi: 10.1016/j.yexcr.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujimoto LM, et al. Actin Assembly Plays a Variable, but not Obligatory Role in Receptor-Mediated Endocytosis. Traffic. 2000;1:161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- 53.Heuser J. Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol. 1980;84:560–583. doi: 10.1083/jcb.84.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maupin P, Pollard T. Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J Cell Biol. 1983;96:51–62. doi: 10.1083/jcb.96.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng Y, et al. Cryo-electron tomography of clathrin-coated vesicles: structural implications for coat assembly. J Mol Biol. 2007;365:892–899. doi: 10.1016/j.jmb.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rappoport JZ, et al. The AP-2 Complex Is Excluded from the Dynamic Population of Plasma Membrane-associated Clathrin. J Biol Chem. 2003;278:47357–47360. doi: 10.1074/jbc.C300390200. [DOI] [PubMed] [Google Scholar]
- 57.Wu X, et al. Clathrin exchange during clathrin-mediated endocytosis. J Cell Biol. 2001;155:291–300. doi: 10.1083/jcb.200104085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aggeler J, et al. High-resolution three-dimensional views of membrane-associated clathrin and cytoskeleton in critical-point-dried macrophages. J Cell Biol. 1983;97:1452–1458. doi: 10.1083/jcb.97.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aggeler J, Werb Z. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J Cell Biol. 1982;94:613–623. doi: 10.1083/jcb.94.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirchhausen T. Coated pits and coated vesicles - sorting it all out. Current Opinion in Structural Biology. 1993;3:182–188. [Google Scholar]
- 61.Idrissi FZ, et al. Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. J Cell Biol. 2008;180:1219–1232. doi: 10.1083/jcb.200708060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perera RM, et al. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci USA. 2006;103:19332–19337. doi: 10.1073/pnas.0609795104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veiga E, Cossart P. The role of clathrin-dependent endocytosis in bacterial internalization. Trends Cell Biol. 2006;16:499–504. doi: 10.1016/j.tcb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veiga E, et al. Invasive and Adherent Bacterial Pathogens Co-Opt Host Clathrin for Infection. Cell Host & Microbe. 2007;2:340–351. doi: 10.1016/j.chom.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson RG, et al. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977;10:351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- 66.Roth TF, Porter KR. Yolk protein uptake in the oocyte of the mosquito aedes aegypti. L. J Cell Biol. 1964;20:313–331. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanover JA, et al. Transit of receptors for epidermal growth factor and transferrin through clathrin-coated pits. Analysis of the kinetics of receptor entry. J Biol Chem. 1985;260:15938–15945. [PubMed] [Google Scholar]
- 68.Newton AJ, et al. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loerke D, et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS biology. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buss F, et al. Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J. 2001;20:3676–3684. doi: 10.1093/emboj/20.14.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engqvist-Goldstein AEY, et al. The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. Journal of Cell Biology. 2001;154:1209–1223. doi: 10.1083/jcb.200106089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen CY, Brodsky FM. Huntingtin-interacting Protein 1 (Hip1) and Hip1-related Protein (Hip1R) Bind the Conserved Sequence of Clathrin Light Chains and Thereby Influence Clathrin Assembly in Vitro and Actin Distribution in Vivo. J Biol Chem. 2005;280:6109–6117. doi: 10.1074/jbc.M408454200. [DOI] [PubMed] [Google Scholar]
- 73.Legendre-Guillemin V, et al. Huntingtin interacting protein 1 (HIP1) regulates clathrin assembly through direct binding to the regulatory region of the clathrin light chain. J Biol Chem. 2005;280:6101–6108. doi: 10.1074/jbc.M408430200. [DOI] [PubMed] [Google Scholar]
- 74.Poupon V, et al. Clathrin light chains function in mannose phosphate receptor trafficking via regulation of actin assembly. Proc Natl Acad Sci USA. 2008;105:168–173. doi: 10.1073/pnas.0707269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hein B, et al. Stimulated emission depletion (STED) nanoscopy of a fluorescent protein-labeled organelle inside a living cell. Proc Natl Acad Sci U S A. 2008;105:14271–14276. doi: 10.1073/pnas.0807705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eggeling C, et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–1162. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- 77.Manley S, et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nature methods. 2008;5:155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 78.Jaqaman K, et al. Robust single-particle tracking in live-cell time-lapse sequences. Nature methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Musacchio A, et al. Functional organization of clathrin in coats: Combining electron cryomicroscopy and x-ray crystallography. Molecular Cell. 1999;3:761–770. doi: 10.1016/s1097-2765(01)80008-3. [DOI] [PubMed] [Google Scholar]