A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker (original) (raw)
. Author manuscript; available in PMC: 2011 Mar 5.
Abstract
Ion channels on the surface membrane of sinoatrial nodal pacemaker cells (SANC) are the proximal cause of an action potential. Each individual channel type has been thoroughly characterized under voltage clamp, and the ensemble of the ion channel currents reconstructed in silico generates rhythmic action potentials. Thus, this ensemble can be envisioned as a surface “membrane clock” (M clock). Localized subsarcolemmal Ca2+ releases are generated by the sarcoplasmic reticulum via ryanodine receptors during late diastolic depolarization and are referred to as an intracellular “Ca2+ clock”, because their spontaneous occurrence is periodic during voltage clamp or in detergent-permeabilized SANC, and in silico as well. In spontaneously firing SANC, the M and Ca2+ clocks do not operate in isolation, but work together via numerous interactions modulated by membrane voltage, subsarcolemmal Ca2+, and PKA and CaMKII-dependent protein phosphorylation. Through these interactions the two subsystem clocks become mutually entrained to form a robust, stable, coupled-clock system that drives normal cardiac pacemaker cell automaticity. G-protein coupled-receptors signaling creates pacemaker flexibility, i.e. effects changes in the rhythmic action potential firing rate, by impacting on these very same factors that regulate robust basal coupled-clock system function. This review examines evidence that forms the basis of this coupled-clock system concept in cardiac SANC.
Keywords: Sinoatrial node, cardiac pacemaker cells, ion channels, local Ca2+ releases, β-adrenergic stimulation, cholinergic stimulation, protein kinase A, CaMKII, sarcoplasmic reticulum, phospholamban, ryanodine receptors
Introduction
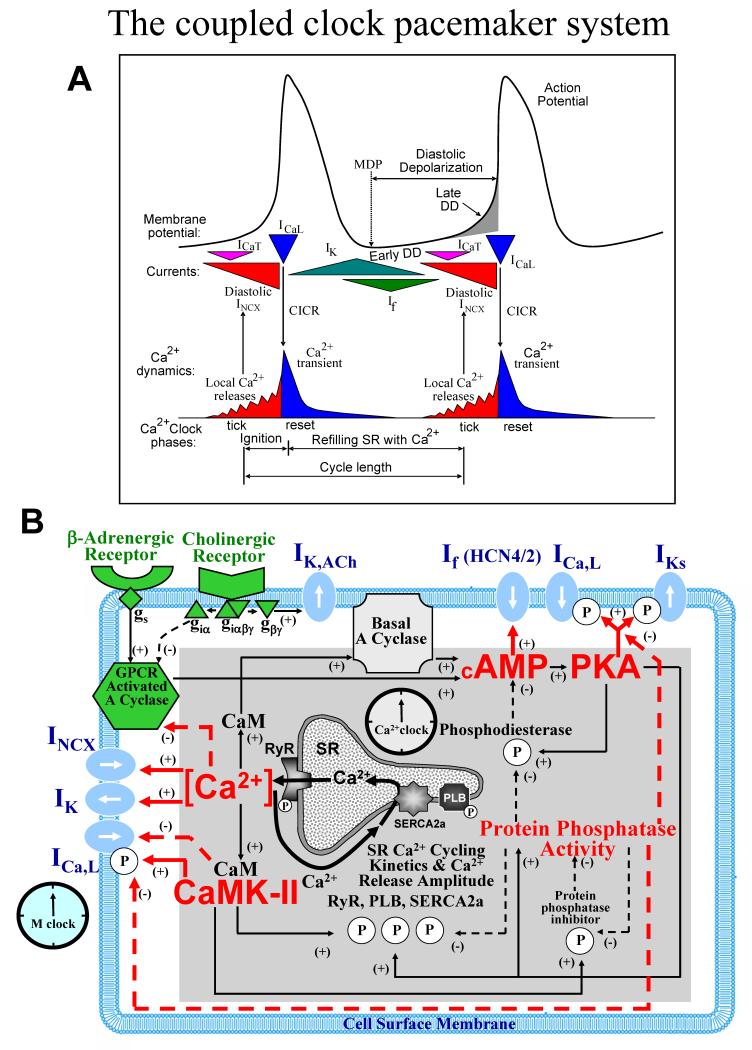
The major electrogenic surface membrane molecules (and their ion currents) in sinoatrial nodal pacemaker cells (SANC) include ion channels and transporters: L-type Ca2+ channels (ICaL), T-type Ca2+ channels (ICaT), delayed rectifier K+ channels (IK), hyperpolarization-actiavted, funny channels (If), Na+/Ca2+exchanger (NCX, INCX), and Na+/K+ATPase (INaK). The structure and function of these, and of other electrogenic molecules, have been extensively studied and comprehensively described in numerous reviews (e.g.1-3). Figure 1A presents a graphic scheme of the coupled-clock system concept: temporal profiles of sarcolemmal ion currents, membrane potential (Vm), and intracellular Ca2+ cycling during SANC duty cycles. When voltage and time-dependent properties of each individual ion channel derived from voltage clamp studies are entered into numerical models, the ensemble of ion channels in silico can generate rhythmic spontaneous AP’s4, and thus the ensemble can be envisioned as a surface “membrane clock” (M clock). But direct experimental characterization of the ensemble function of the M clock cannot be made during the normal SANC duty cycle, i.e. in the course of normal interactions with intracellular processes.
Fig.1. The coupled-clock pacemaker system.
A. Schematic illustration of key phases of the functional interactions between M clock and Ca2+ clocks. Modified from103. B. Schematic illustration of interactions of molecules comprising the full coupled-pacemaker clock. Note that the same regulatory factors (red lettering) of the SR Ca2+ clock (gray intracellular area, black lettering) couple the Ca2+ clock to the M clock (blue membrane area, blue lettering). G protein-coupled receptors (green lettering) regulate both the Ca2+ clock and membrane clock via those same factors (red lettering) and other coupling factors (green shapes). See text for numerous additional details. Modified from67.
Numerous studies over the last two decades have investigated the role of intracellular Ca2+ cycling in cardiac pacemaker function5-12. Specific, detailed mechanisms of Ca2+ cycling contributions have become available in more recent studies (reviews13-15 and numerical modeling16). The sarcoplasmic reticulum (SR) is wired to oscillate Ca2+ via its Ca2+ pumps (SERCA-2) and Ca2+ release channels, ryanodine receptors (RyR)(Fig.2). Because Ca2+ oscillations generated by the SR during experiments in the absence of sarcolemmal function17,18, and in silico16 are rhythmic, the SR has been referred to as an intracellular “Ca2+ clock” (Fig.1B). But in nature, neither clock functions in the absence of the other. Abundant evidence indicates that functional interactions that are critical for normal automaticity occur between the two clocks (reviews13,14). Specific surface membrane proteins not only effect changes in membrane potential, but also directly or indirectly regulate intracellular Ca2+ cycling; and conversely, intracellular Ca2+ cycling proteins also regulate Vm via Ca2+-modulation of surface membrane electrogenic molecules. Furthermore, other coupling factors, in addition to Ca2+, i.e. protein phosphorylation by protein kinase A (PKA) or Ca2+-calmodulin-dependent protein kinase II (CaMKII), that affect function of proteins of both clocks (Fig.1B), are critical for regulation of normal automaticity by the coupled-clock system.
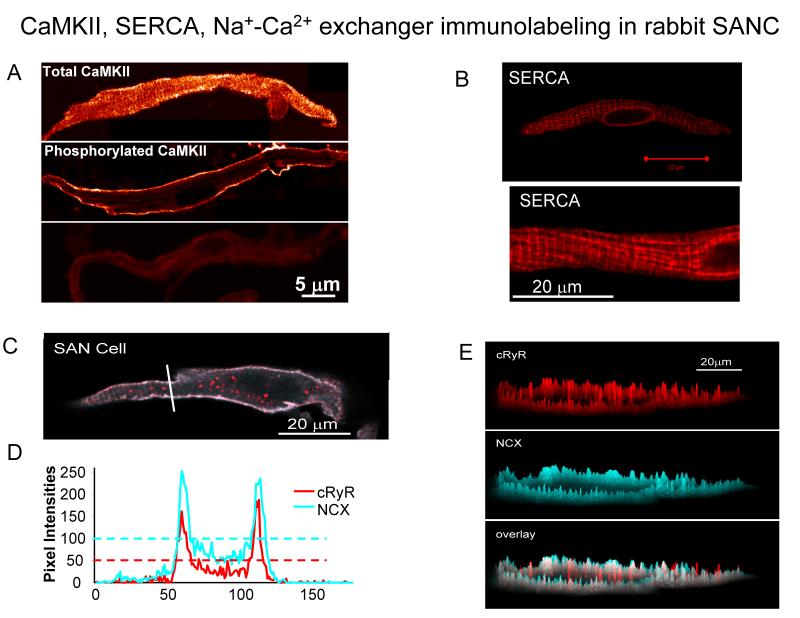
Fig.2. CaMKII, SERCA, RyR and NCX immunolabeling in rabbit SANC.
A. The intracellular distribution of total and active CaMKII in SANC. A uniform distribution of the total CaMKII immunolabeling (top); the localization of active CaMKII beneath the sarcolemmal membrane (middle); the negative control (bottom), i.e., in the absence of the primary antibodies (from49). B SERCA labeling in the single rabbit SANC. C, Confocal image of SANC double immunolabeled for NCX and RyR. D, pixel-by-pixel fluorescence intensities of labeling along an arbitrary (white) line in panel C. The horizontal dashed lines show the average pixel intensity. E, Topographical profiles of the pixel intensity levels of each antibody labeling and overlay in the small SANC in panel C. The maximum height represents the brightest possible pixel in the source image. B-E from20.
Specific voltage- time- Ca2+- dependent coupling within and between surface membrane and intracellular Ca2+ cycling proteins during the SANC AP duty cycle
M and Ca2+ clock events and coupling during late diastolic depolarization
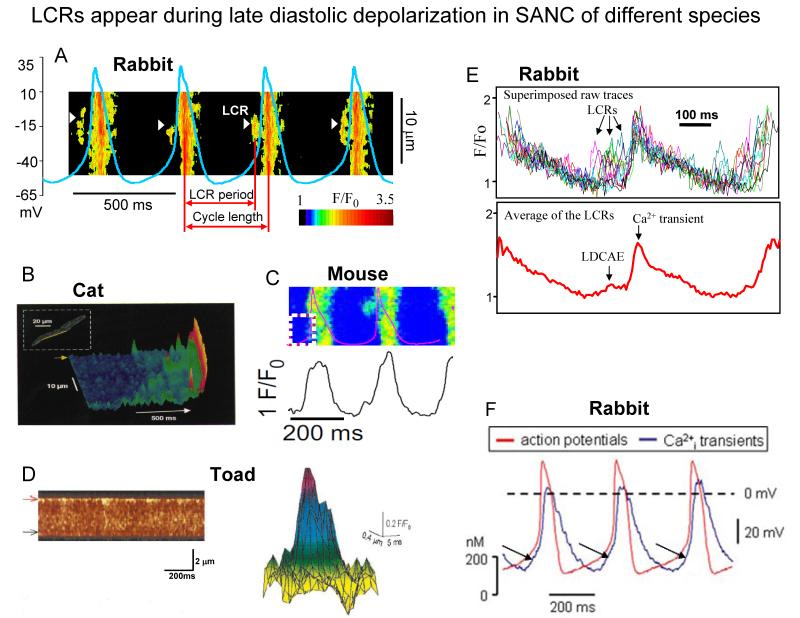
Spontaneous diastolic depolarization (DD) is the essence of cardiac pacemaker cell automaticity. The DD occurs in two phases: the early and late DD (Fig.1A). Confocal imaging of Ca2+ in mammalian SANC and atrial subsidiary pacemaker cells combined with non-invasive perforated patch-clamp electrophysiology8,19 and imaging of toad sinus venosus cells7, has documented the occurrence of subsarcolemmal Local Ca2+ Releases (LCR’s) during the late DD (Fig.1A and Fig.3). SANC exhibit robust SERCA2 and RyR immunolabeling11,20 (but see also21). SERCA2 immunolabeling in SANC is located diffusely throughout the cytoplasm and per nuclear area, whilst RyR immunolabeling is most intense in the subsarcolemmal space (Fig.2)11,20,21. In spontaneously firing rabbit SANC, LCRs emanate from SR via RyRs and in confocal line-scan images appear as 4-10 μm Ca2+ wavelets; they emerge following the dissipation of the global systolic transient effected by the prior AP, and crescendo during the DD, peaking during the late DD, as they merge into the global cytosolic Ca2+ transient triggered by the next AP (Fig.3)17,19. LCRs (Fig.3A-D), or the integral of LCR’s (Fig.3E), i.e., late diastolic Ca2+ elevations (LDCAE) (Fig.3F), have now been documented in numerous species7,8,19,22-24.
Fig. 3. LCR appear during late diastolic depolarization in SANC of different species.
A. Line-scan image of LCRs with superimposed spontaneous APs in rabbit SANC. White arrowheads show LCRs. The LCR period is defined as indicated. Modified from27. B. local Ca2+ releases in cat latent pacemaker cells (from8, with permission). C, A line-scan image of LCRs with superimposed spontaneous APs in a mouse SANC (from22, with permission). D. Ca2+ sparks in toad pacemaker cells: (left) a line-scan image showing regions of localised, transient increase in fluorescence along the upper border of the toad pacemaker cell; (right) intensity map of fluorescence of a composite spark obtained by superimposing 11 sparks (from7, with permission). E. Confocally measured subsarcolemmal LCRs (upper panel) in rabbit SANC. The temporal average of the individual LCRs within each cycle (lower panel) generates Late Diastolic Ca2+ Elevation (LDCAE) that precedes Ca2+ transient induced by action potential. Modified from104. F. Superimposed APs (red trace) and associated Ca2+ transients (blue trace) recorded in rabbit SANC (LDCAE are indicated by arrows) (from24, with permission).
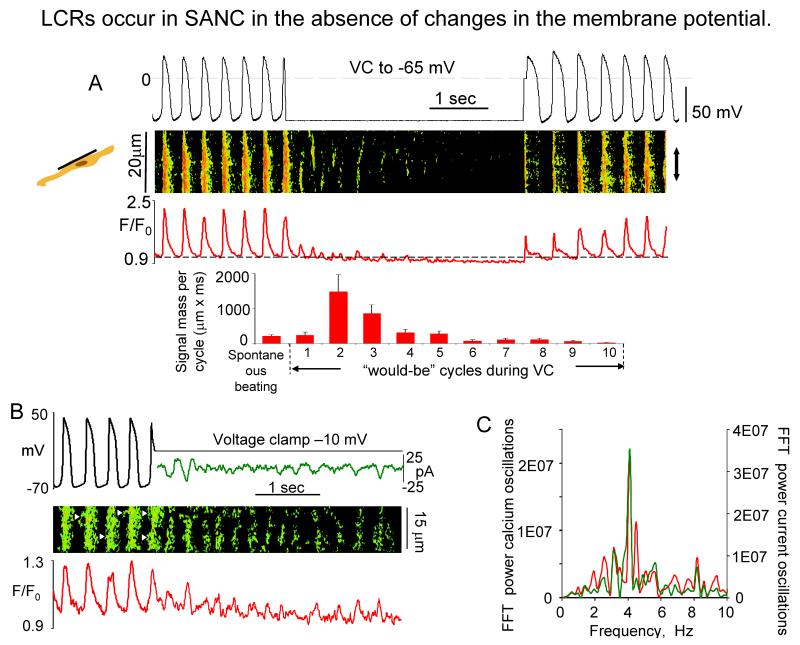
While there is some evidence to indicate that ICaT may activate LCR’s in cat latent pacemaker cells8, voltage clamp studies in rabbit SANC indicate that LCR’s are not appreciably affected by ICaT inhibition25, do not require membrane depolarization (Fig.4A), but occur spontaneously17,18. In Fig.4A, note that following acute voltage clamp at the MDP of spontaneously firing SANC, several oscillatory cycles of Ca2+ release occur prior to their damping out, due to SR and cytosolic Ca2+ depletion17. Rhythmic or periodic spontaneous Ca2+ oscillations persist during acute voltage clamp at a potential that prevents Ca2+ loss from the cell and are accompanied by membrane current fluctuations of the same periodicity (Fig.4B). Similar perspectives regarding the characteristics of spontaneous LCRs are gleaned from studies in saponin-skined rabbit SANC, in which intracellular [Ca2+] is buffered at a constant physiological level17,18. LCR-initiated, late DD inward current in rabbit SANC varies from 0.3 pA/pF26 to 1.6 pA/pF19, yielding an INCX range from 9 to 48 pA for a 30 pF SANC. This range of inward INCX is sufficient to broadly modulate the DD16,26,27, because the SANC membrane resistance is high, and extremely small net ion current change (a few pA in rabbit SANC28) has marked effects on Vm. At the resolution of the confocal microscope20 NCX and RyRs molecules colocalize across the ~12 nm subsarcolemmal gap between RyR’s on the SR and NCX molecules on the sarcolemma (Fig.2). While INCX is voltage dependent, it does not exhibit its own time-dependent gating mechanisms, as do most surface membrane ion channels; rather time-dependent changes in [Ca2+] in the subsarcolemmal space regulate the timing of INCX activation. Subsarcolemmal Ca2+ release induces an inward INCX, as each Ca2+ that is transported out of the cell is exchanged for 3Na+. The rate of Na+/Ca2+ exchange by NCX, and hence the instant value of INCX, is determined by Vm and the instant Na+/Ca2+ gradient across the sarcolemma (Fig.1A).
Fig. 4. LCR occur in SANC the absence of changes in membrane potential.
A. (top) Simultaneous recordings of APs, linescan image and normalized subsarcolemmal fluorescence averaged spatially over the band indicated by doubleheaded arrow in a representative SANC prior to, during, and following acute voltage clamp at the maximum diastolic potential (from17); (bottom) Average total LCR signal mass in 9 cells in control during spontaneous beating, and during each “would-be” cycle of voltage clamp. B, Simultaneous recordings of membrane potentials or current (top), confocal line-scan image (middle), and normalized fluo-3 fluorescence (bottom) averaged over the line-scan image, in a representative spontaneously beating SANC before and during voltage clamp to −10 mV. Fast Fourier transform (FFT) of Ca2+ and membrane current fluctuations during voltage clamp to −10 mV (from18).
The membrane current fluctuations generated by LCRs during late DD (Fig.4B) drive miniature Vm oscillations (Fig.5A). When these are suppressed by disabling RyRs with ryanodine (Fig.5A inset), the exponential character of the late DD is abolished13,27 (Fig.5B). (Of note, ryanodine blocks neither If12, nor ICaL25). The membrane current and voltage responses to the LCR’s, and, most importantly, AP generation by SANC, require extracellular Na+, because when Na+ is acutely removed from the bathing milieu of rabbit19 (Fig.5C) or guinea pig29 SANC (Fig.5D) following a prior AP, generation of the subsequent AP acutely fails (Fig.5C,D), while LCR’s persist (Fig.5C). This indicates that forward mode NCX, by generating inward INCX, couples LCRs to the late DD acceleration19. Activation of ICaL during the late DD (activation threshold is about −50 mV to −40 mV) results in the generation of the AP rapid upstroke, which triggers a global SR Ca2+ release (see next section for detailed interactions during the AP).
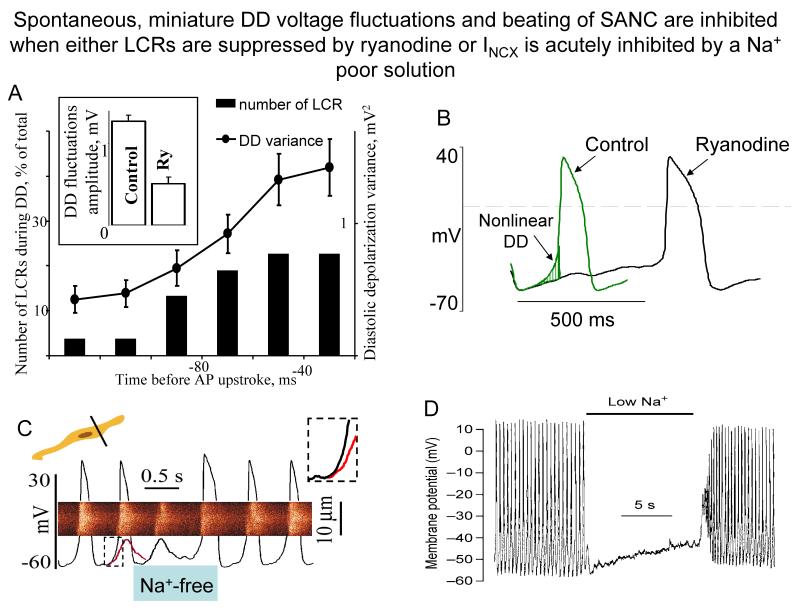
Figure 5. Spontaneous miniature DD voltage fluctuations and beating of SANC are inhibited when either LCRs are suppressed by ryanodine or INCX is acutely inhibited by a Na+ poor solution.
A. Time course of DD variance (dots) and of the relative LCRs occurrence (bars) observed at different times during the measurement period (24 SANC). Inset shows that the average amplitude of DD fluctuations, calculated for the 50-ms segment preceding AP upstroke, is suppressed by ryanodine (from27). B. Superimposed APs of representative rabbit SANC before and after treatment with 3 μmol/L ryanodine (from13). C. Linescan image of Ca2+ release with superimposed AP records during rapid sprits with Na+ free solution. Red curve superimposed on the last AP preceding spritz of Na+ free solution is a copy of the residual membrane potential oscillation observed during Na+-free solution spritz. Note that this maneuver suppressed late DD (see inset) and blocked the subsequent AP firing. (from19). D. A rapid switch to low-Na+ solution (during the time indicated by the bar) caused immediate cessation of spontaneous action potentials in guinea-pig SANC. Action potentials speedily reappeared following rapid switch back to normal Na+ solution (with permission from29).
L-type Ca channels are a multi-subunit complex, and α1C represents the major isoform of the channel central pore subunit in the cardiovascular system. However, recently α1D has been discovered in the mouse SA node30,31. In comparison to α1C subunit as a central pore, L-type Ca2+ current with α1D subunit is activated at lower voltages, suggesting that this channel might be involved in the generation of DD. A substantial contribution of Cav1.3 channel in total ICaL in mouse SANC was confirmed in Cav1.3 knockout mice in which the density of ICaL compared with wild-type mice was reduced by 79%30. Low-voltage-activated ICaL has also been shown in rabbit SANC, but the contribution of this current to the total ICaL in rabbit SANC is still unknown32.
The combined perspectives about interactions between the Ca2+ and M clocks gleaned thus far are that: 1) there is a rhythmic spontaneous Ca2+ oscillator or clock that generates LCRs within SANC; 2) during spontaneous AP firing, LCRs, generated by the Ca2+ clock emerge during late DD; 3) the intracellular Ca2+ clock requires recurring APs to provide Ca2+ to sustain its rhythmic oscillator function; 4) LCRs generated by the Ca2+ clock interact with the surface membrane and, via activation of INCX, generate miniature current and voltage oscillations that confer the exponential increase to the late phase of DD; 5) This late DD acceleration by INCX is required for sufficient ICaL activation to generate the subsequent timely rapid AP upstroke. In the absence of this LCR-activated INCX “prompt” to the membrane, rhythmic AP’s do not occur.
M - Ca2+ clock events and coupling during the AP
Ca2+ influx via L-type channels during the AP induces Ca2+ release from SR via RyRs (i.e. Ca2+ -induced Ca2+ release, CICR)33,34. SANC do not have T-tubules, so that AP-triggered Ca2+ release, resulting in a global subsarcolemmal and cytosolic Ca2+ transient, occurs via subsarcolemmal RyR’s19,34. Ca2+ that accumulates within the subsarcolemmal space binds to calmodulin to inactivate ICaL (Fig.1B) and modulates the NCX throughout the AP, contributing to the Ca2+ transient decay by causing Ca2+ efflux from the cell. SR pumps Ca2+ continuously throughout the AP cycle, at a rate modulated by the amount of Ca2+ to be pumped. A substantial component of Ca2+ influx via the L-type Ca2+ channels is pumped directly into the SR35, and replenishes cell Ca2+ load to balance Ca2+ efflux via NCX exchange. Ca2+ influx may also occur via Ca2+ store-operated via TRPC channels that have been identified in mouse SANC36.
The occurrence of AP-triggered Ca2+ release from the SR (via CICR) synchronizes the global SR in a relatively Ca2+ depleted state that temporarily suspends generation of spontaneous LCRs. In Figure 4A, note that in the absence of the timely occurrence of APs during voltage clamp, the integrated LCR signal mass (i.e. LCR number, amplitude and width) generated by the Ca2+ clock continues to grow in magnitude, and passes through a maximum prior to its damping out (Fig.4A, lower panel). This transient exaggeration of the LCR signal mass can be construed as a fail-safe “prompt” for the membrane in the event that restitution processes of M clock (e.g. ICaL) becomes sluggish and cannot respond to the initial LCR signal.
Membrane depolarization during the AP activates K+ channels which effect AP repolarization. Intracellular Ca2+ also modulates K+ channel gating37,38 (Fig.1B). As NCX flux is both voltage- and Ca2+-dependent, K+ channels, via AP repolarization, modulate the activation of forward mode NCX exchange. Since NCX assists in reducing the cytosolic and subsarcolemmal Ca2+, K+ channels, by activating NCX, indirectly modulate cell Ca2+ balance. The membrane repolarization effected by K+ channel activation also inactivates L-type Ca2+ channels, limiting Ca2+ influx and thus modulates Ca2+ balance via this effect.
M and Ca2+clock events and coupling following the AP in early and mid DD
The [Ca2+] within the cytosolic and subsarcolemmal compartments decays (due to Na+-Ca2+ exchange and SR Ca2+ pumping) to a nadir near the end of early DD (Fig.1A). Inward INCX concomitantly decays and also reaches its nadir near the end of early DD (just before LCRs emerge) (Fig.1A).
Following achievement of the maximum diastolic potential (MDP), IK conductance (gK) decreases (Fig.1A) and this is one mechanism of the early DD in pacemaker cells. This “gK decay” is thought to unmask an inward current, initially described as a background current (IbNa) (presumably of Na+ selectivity)39. A large amplitude background current is a critical component of all numerical SANC models: IbNa is not only a partner in gK mechanism, but also balances the outward INaK. It was speculated that window currents of ICaL and ICaT and INa (when present in SANC)40, a chloride current41, or a Ca2+-activated nonselective (TRPM4) cation channel42 contribute to the background currents in SANC. It has also been speculated that a Na+-H+ mechanism contributes to background current because it is reduced by amiloride43. An interesting idea is that background current is generated by a non-transported Na+-Ca2+ leak of NCX44. Additionally, since IbNa is a Na+ current that is present during early and mid DD, it could be postulated that a substantial inward NCX Na+ current driven by the decaying Ca2+ transient (as predicted by many numerical SANC models4), also contributes to background current during this period. IbNa may also embrace the Na+ current of Ist (see below). A radical idea is that a background current within the DD range does not exist, but is an artifact of the seal leak that always accompanies the whole-cell patch clamp recording28. In short, despite extensive electrophysiological and pharmacological studies28,40,43, the molecular identity of the underlying “background channel” remains unknown.
A non-selective, sustained or steady current (Ist)45 has also been suggested as a DD mechanism. Ist has some characteristics of ICaL, e.g. it is sensitive to dihydropyridines and has a reversal potential close to +37 mV. However, Ist is activated at more negative potentials than ICaL and achieves peak amplitude at about −50 mV46. Since Ist is mainly carried by Na+, increased by lowering extracellular [Ca2+], and reduced by lowering extracellular [Na]45, it behaves as INCX and, hence, might, in part at least, reflect INCX activation by LCRs during mid DD (because LCRs just begin to occur also at about −50 mV). The reported Ist sensitivity to dihydropyridines is not surprising, then, because a reduction of ICaL reduces Ca2+ influx, the cell Ca2+ and SR Ca2+ loads, and hence reduces LCRs. Thus, it is possible that Ist reflects combined (direct and indirect) effects of INCX and ICaL.
The later repolarization phase of the AP also activates If (Fig.1A). If is a non-selective current (carried by both Na+ and K+). Since it has a reversal potential of about −25 mV, it generates an inward current during DD, and is yet another early-mid DD mechanism (Fig.1A)28,47. Cytosolic Ca2+ does not directly regulate If48.
The mid DD eventually gives way to late DD, a new cycle begins, and the events during late DD, as described above, recur. The numerous interactions between the M and Ca2+ clock subsystems, as illustrated in Fig.1, and discussed above, are summarized in Online Table I. These numerous interactions result in a mutual entrainment of the molecular function of both clocks that confers robustness to coupled-clock pacemaker system (a numerical study16 and review14). Indeed, by releasing Ca2+ into the subsarcolemmal space with precise timing during late DD, the spontaneous Ca2+ clock interacts with membrane proteins to cause an inward INCX current that facilitates the “on-time” occurrence of the next AP. By “igniting” the membrane depolarization that facilitates the production of a timely AP, the Ca2+ clock guarantees its own existence during future cycles: the AP (that the Ca2+ clock facilitates) resets the Ca2+ clock via CICR-mediated SR Ca2+ depletion, and refuels the Ca2+ clock with Ca2+, via Ca2+ influx through L-type Ca2+ channels. The M clock, in turn, not only generates the AP, the sine-qua-non of SANC function, but, by ensuring the continual function of the Ca2+ clock, the M clock also insures its future normal function to generate on-time rhythmic APs via surface membrane ignitions by its partner, the Ca2+ clock.
The crucial role of phosphorylation of Ca2+ cycling and membrane proteins in subsystem clock functions and coupling
The voltage- time- Ca2+ - interactions in Fig 1A are modulated by phosphorylation of surface membrane and SR proteins by both PKA and CaMKII (Fig.1B). This protein phosphorylation is required for normal coupled-clock basal function, because spontaneous AP firing ceases when either kinase is inhibited18,49 (see below for further details).
Phosphorylation-dependent regulation of intracellular Ca2+ cycling and surface membrane proteins of the coupled-clock system is complex, with numerous functional redundancies (Fig.1B). Ca2+ ion begets protein kinase activation, and the latter leads to both an increase in Ca2+ influx, via modulation of surface membrane molecular functions, and acceleration of SR Ca2+ cycling, via modulation of SR Ca2+ cycling proteins. SANC have a high constitutive activation of adenylyl cyclase (AC) that results in a high level of basal cAMP18. Although SANC, like ventricular myocytes, express high levels of Ca2+-inhibited AC types 5 and 650, the discoveries of Ca2+-activated AC types, i.e. AC1 and AC8, in rabbit and guinea-pig SANC48,51,52, and localization of the basal Ca2+-activated AC activity within lipid raft microdomains52, link Ca2+ to localized cAMP production (Fig.1B). Ca2+ binds to calmodulin to activate AC, leading to a high basal level of cAMP-mediated, PKA-dependent phosphorylation of surface membrane and intracellular proteins involved in cell Ca2+ balance and SR Ca2+ cycling18,52 (Fig.1B).
Basal PKA-dependent phospholamban phosphorylation modulates kinetics of SR Ca2+ pumping, and phosphorylation of RyRs alters threshold of spontaneous activation of RyRs by Ca2+ within the SR. PKA-dependent mechanisms (Fig.1B, red; Online Table I) also regulate function of surface electrogenic proteins (Fig.1B, blue; Online Table I) and couple M and Ca2+ clocks. PKA is targeted to the L-type Ca2+ channel through the membrane associated anchoring protein, AKAP15/1853,54. In rabbit SANC, there appears to be a high basal PKA-dependent phosphorylaton of L-type Ca2+ channels, as a specific PKA inhibitor peptide, PKI, suppresses ICaL by ~80%55. IKs is also modulated by cAMP-mediated, PKA-dependent phosphorylation56 (Fig.1B). Augmented Ca2+ influx and accelerated Ca2+ cycling via PKA-dependent protein phosphorylation enhances basal CaMKII activity57 which further promotes Ca2+ influx and intracellular Ca2+ cycling. Key roles of Ca2+-CaMKII-dependent phosphorylation on surface membrane proteins in SANC are the facilitation of ICaL amplitude58 and the acceleration of the removal of ICaL inactivation49. Ca2+-CaMKII dependent phosphorylation of SERCA stimulates Ca2+ pumping59, and phosphorylation of RyR modulates Ca2+ release characteristics. In isolated rabbit SANC activated CaMKII is localized beneath the cell membrane, whereas the total CaMKII is uniformly present throughout the cell49(Fig.2A). This restricted localization of active CaMKII to the subsarcolemmal space of SANC is consistent with the idea that CaMKII targets sarcolemmal and subsarcolemmal compartments, and that CaMKII activity is likely regulated by local Ca2+ gradients in submembrane microdomains49,60. Note that unlike PKA or CaMKII-dependent protein phosphorylation, cAMP, per se, does not directly regulate SR Ca2+ cycling, but it indirectly does so via regulation of PKA-dependent phosphorylation of SR and surface membrane proteins.
This “feed-forward” Ca2+ signaling (Fig.1B), i.e., Ca2+ release begets Ca2+ release via both Ca2+-activated AC-cAMP-dependent PKA activity and CaMKII activity of both surface membrane and SR Ca2+ cycling proteins, is kept in check by factors that regulate Ca2+ and kinase activates, so that, basal state Ca2+ influx and Ca2+ cycling kinetics remain stable. High constitutive basal phosphodiesterase (PDE) activity within SANC26, which degrades cAMP, is one such control check point (Fig.1B). Indeed, inhibition of basal PDE within SANC markedly elevates cAMP-mediated, PKA-dependent phosphorylation of SR proteins (c.f. Fig.7A) and surface membrane proteins (that generate ICaL and IK) (Fig.1B), resulting in an acceleration the spontaneous AP firing rate of rabbit SANC26. Phosphoprotein phosphatase activity is likely another control point (Fig.1B)61.
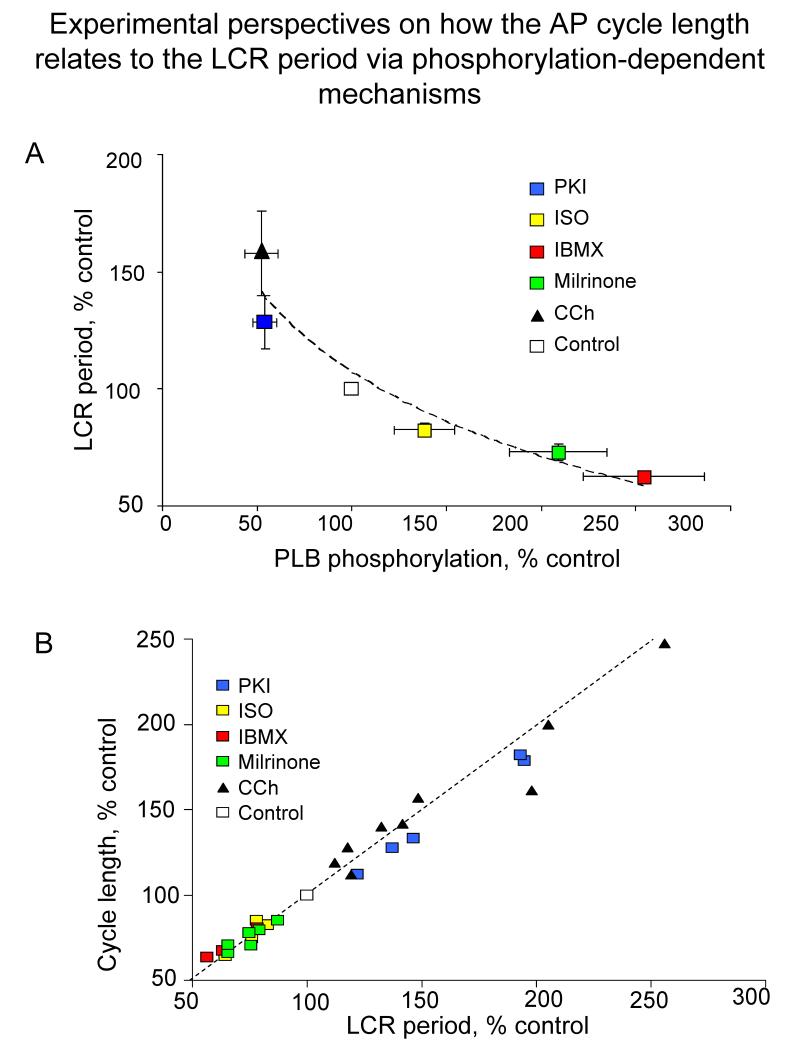
Figure 7. Experimental perspectives on how the AP cycle length relates to the LCR period via phosphorylation-dependent mechanisms.
A. The relative effects of a PKA inhibitor PKI, carbachol (CCh), PDE inhibition (IBMX, milrinone) and isoproterenol (ISO) to alter the LCR period are linked to their effects to alter phospholamban (PLB) phosphorylation. The dashed line is the best fit least squares logarithmic function through the points: Y= −52.01 ln(X) + 107.11. B. The relative effects of PKI, CCh, PDE inhibition and ISO to alter the spontaneous cycle length over a wide range are linked to their effects on the LCR period. The best fit least squares linear functions (dashed line) through the points is: cycle length = 0.89 LCR period + 11.43 msec.
How the coupled pacemaker clock keeps time
The delay between the onset of the global SR Ca2+ depletion triggered by an AP (via CICR) and the spontaneous emergence of an LCR during the subsequent DD is the LCR period (Fig.3A). The LCR period is the master integrated function of coupled-clock system, the LCR period determines the “ticking speed” of the coupled-clock SYSTEM. The LCR period does not report Ca2+ clock function, per se, but reports the function of the coupled-clock system, because of intimate interactions of the electrogenic sarcolemmal molecules with the intracellular Ca2+ cycling apparatus described above and depicted in Fig.1B. For example, the Ca2+ available for SR pumping, which is regulated by PKA and CaMKII dependent phosphorylation of SR Ca2+ cycling proteins (Fig.1), is critically dependent on beat to beat Ca2+ influx via L-type Ca2+ channels. While an occurrence of an AP-induced global SR Ca2+ depletion causes the spontaneous LCRs to stop, the Ca2+ clock does not stop: after being synchronized in the Ca2+ depleted state, it continues to measure time from the onset of its Ca2+ depletion and when threshold conditions for spontaneous Ca2+ release are achieved begins to generate LCRs again. Thus, the LCR period is determined by the duration of this restitution process.
The schematic in Figure 6A depicts the concept that the restitution process that determines the LCR period is regulated (i) by the kinetics of SR Ca2+ cycling, i.e., by the rate of Ca2+ pumping into the SR, and (ii) the threshold of SR Ca2+ load required for spontaneous RyR activation. Ca2+ and PKA- and CaMKII-dependent phosphorylation by controlling cell Ca2+ balance and SR Ca2+ cycling (Fig.1B) regulates the restitution process. This concept is supported by numerical model simulations of the SANC coupled-clock system16 (Fig.6B). Ca2+, cAMP-mediated, PKA-dependent and CaMKII-dependent phosphorylation (Fig.1B, red) are crucial nodes within the coupled-clock system because their joint action on Ca2+ and M clock proteins determines the LCR period in a given steady-state.
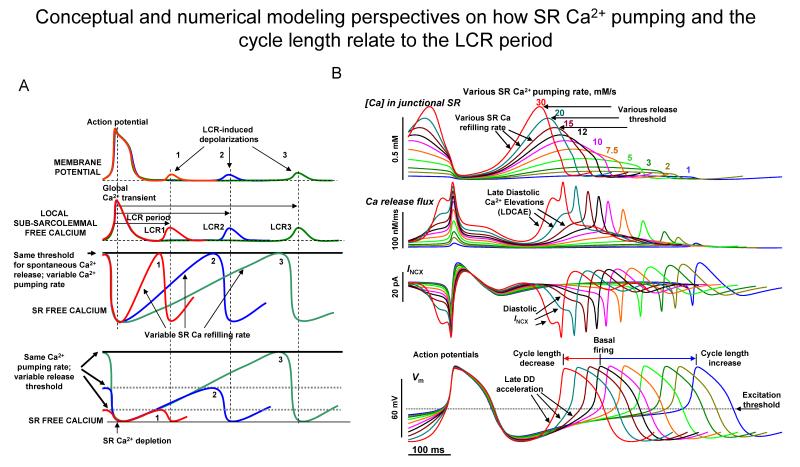
Figure 6. Conceptual and numerical modeling perspectives on how SR Ca2+ pumping and the cycle length relate to the LCR period.
A. Schematic illustration of the concept that the rate of SR Ca2+ refilling and Ca2+ release threshold determine the LCR period, and the timing of the LCR induced diastolic depolarization (Modified from105). B. A novel numerical SANC model16 of a dynamically integrated system of Ca2+ and membrane clocks predicts the wide range of pacemaker rate modulation via variations in SR Ca2+ pumping rate (color coded, 1 to 30 mmol/s), mimicking various degrees of PKA-dependent phospholamban phosphorylation. Shown are simulations of SR [Ca2+], SR Ca2+ release flux, INCX, and Vm.
Manipulation of the coupled-clock systems critical nodes alters its ticking speed and spontaneous AP firing rate
Disabling the major coupled-system nodes, i.e. the regulatory factors of the subsystem clocks and their coupling to each other (Fig.1B red) has marked effects on the basal LCR period and AP cycle length. As noted above, INCX-Ca2+ coupling (Fig.1) is clearly required for spontaneous AP firing (Fig.5C,D)19,29. Since acute augmentation of INCX during DD requires an acute increase in subsarcolemmal [Ca2+], it would be expected that intracellular Ca2+ buffering, by disrupting numerous Ca2+ dependent functions in Fig.1B that regulates the LCR period, would lead to severe disregulation of the coupled-clock system. Indeed, buffering intracellular Ca2+ in rabbit SANC by BAPTA (but not the slower Ca2+ chelater, EGTA) severely impairs or blocks spontaneous AP firing, i.e. normal automaticity20,49. Since subsarcolemmal Ca2+ regulation during DD depends upon SR Ca2+ pumping, it would be also expected that impairment of the SR Ca2+ pumping or release mechanisms would impair the LCR period and impair normal automaticity11,29. As expected, SERCA2 inhibition, by cyclopiazonic acid, prolongs the LCR period and reduces the SANC beating rate11,29.
The plant alkaloid ryanodine, which locks RyR’s in an open sub-conductance state, effecting depletion of SR Ca2+ content, impairs Ca2+ release, also prolongs the LCR period (and can abolish LCRs in concentration-dependent manner), flatters the late DD (Fig.5B), and results in impairment of normal automaticity (see Online Table II for data and references).
Although ryanodine significantly reduced the spontaneous beating rate in all 20 studies in isolated SA node tissue or isolated SANC or the intact heart reviewed in Online Table II, the extent of ryanodine-induced suppression varies from 12% to 100%, average ~40 % refer to Online Table II. The response to ryanodine varies with the [ryanodine], the time of ryanodine exposure, and species. A small suppression of the SAN beating rate by ryanodine in some studies may be due to an insufficient ryanodine concentration, or insufficient time of exposure. The kinetics of the ryanodine effect are especially important because a rapid application of ryanodine to isolated SANC or SAN tissue initially increases RyR Ca2+ release, and the spontaneous beating rate concomitantly increases; then as the SR becomes depleted with time, the spontaneous beating slows5,62. This poses problems if sampling of the ryanodine effect on beating rate among different cells within or among studies is made at different relative times of the evolution of the ryanodine response. For example, a study that failed to detect any average change in the spontaneous beating of small SANC, thought to be isolated from the central area of the SA node63, reported a large variation around the null average change in the beating rate of small SANC, indicating that in half of the cells ryanodine increased beating rate, whilst in the other half it decreased the beating rate63. A subsequent study reported the ryanodine effect to suppress spontaneous AP firing was independent of cell size20. Finally, a long exposure to a high ryanodine concentration that causes a severe SR Ca2+ depletion, can lead to a compensatory increase in Ca2+ influx64.
Manipulation of PKA or CaMKII-dependent phosphorylation of coupled-clock proteins via basal PDE or PKA inhibition, have marked impacts on the LCR period and AP cycle length. But direct experimental elucidation of the specific role of any single coupling factor is not possible, because of the complex interactions within the coupled system (Fig.1B). Figure 7A illustrates the dependence of the LCR period in spontaneously beating SANC upon PKA-dependent phosphorylation of phospholamban across a broad range of phospholamban phosphorylation at serine 16. Shifts in the phosphorylation status of proteins other than phospholamban, e.g. RyR or of surface membrane molecules, e.g. ICaL, IK (Fig.1B) are also involved in regulation of the LCR period, as noted above. But phosphorylation of these proteins has not yet been measured directly. Thus, phospholamban phosphorylation presently serves as a general index of phosphorylation of proteins of the coupled pacemaker clock systems. Changes in phosphorylation status, reported by phospholamban phosphorylation, and changes in LCR period are closely correlated with changes in the spontaneous SANC cycle length (Fig 7B).
Simulations of a novel numerical model that predicts the LCR period of the coupled-clock system (LDCAE in Fig.6B) can isolate and explore coupling factor contributions16,65. Model simulations support the concept that the LCR period, in its role as the master integrated function of numerous tightly coupled processes in SANC (Fig.1), regulates the pacemaker system “ticking speed” (Note the tight correlation between the timing of LDCAE emergence, the timing of DD acceleration and the cycle length in Fig.6B)16,65.
Pacemaker rate modulation via G protein-coupled receptor (GPCR) signaling
In addition to robustness or “fail-safe”, stable basal operation, the heart’s pacemaker clock must tick over a wide range of frequencies that encompass the physiologic range of heart rates. This flexibility of the pacemaker clock’s “throttle” is achieved via modulation of the LCR period by GPCR signaling (reviews13-15). G protein-coupled receptors modulate the coupled system of M and Ca2+ clocks (Fig.1, green) via same factors (Ca2+ and protein phosphorylation) that regulate the basal state clock function (Fig.1 red). Specifically, β adrenergic receptor (β-AR) stimulation further increases, and Cholinergic receptor (ChR) stimulation reduces basal levels of phosphorylation of coupled-clock proteins (Fig.7A), leading to an increase and a reduction, respectively, in LCR period. Note in Fig.7B, modulation of LCR period and cycle length by experimental maneuvers that interrupt basal PKA-dependent signaling and those effected by GPCR stimulation form a continuum. Novel numerical modeling simulations of coupled-clock function predict this continuum on the basis of phosphorylation-dependent gradations in the SR Ca2+ cycling rate (Fig.6B)16.
β-adrenergic receptor stimulation effects on the LCR period and AP cycle length
β-AR stimulation (or PDE inhibition) reduces the LCR period (Fig. 7A), shifting LCR occurrence to an earlier time during DD, i.e., a time occupied solely by early DD mechanisms during basal state beating18. In addition to the phase shift, the integrated LCR Ca2+ release signal mass also increases as RyR activation becomes more synchronized via local recruitment25,26. This earlier and stronger Ca2+ release results in earlier and stronger INCX and DD acceleration, resulting in a reduction in cycle length. Thus pacemaker rate acceleration by β-AR stimulation is linked to both the early and late DD phases27,66,67. The resultant cycle length modulation, effected by β-AR signaling is due to phosphorylation- dependent effects on both ion channels and SR Ca2+ cycling, as discussed above.
Numerous recent studies also have shown that in order to increase the beating rate, β-AR stimulation (or PDE inhibition) requires a link between β-AR-induced increases in PKA or CaMKII modulation of surface membrane molecules and SR Ca2+ cycling molecules within the coupled-clock system (Fig.8)9,18,25. In rabbit SANC, when the function of intracellular RyRs is disabled by ryanodine, neither β-AR stimulation (nor PDE inhibition) augments INCX or accelerates the DD25,27, or produces the expected increase in the spontaneous SANC firing rate (Fig.8A). In the presence of ryanodine there is an approximate three-fold reduction in the ability of stimulation of β-ARs with isoproterenol to accelerate the firing rate, and about a six-fold increase in the isoproterenol EC5025, even though the effect of these maneuvers to increase ICaL amplitude remains intact25,26 (Fig.8B). Thus, β-AR stimulation (or PDE inhibition) effects to augment the M clock function alone, via ICaL augmentation (or IK activation26,56), are not sufficient to effect the normal increase in AP firing rate. In other words, SERCA2 Ca2+ pumping and Ca2+ release within the coupled-clock system is a required “switchboard” that links Ca2+ influx via M clock proteins in response to β-AR stimulation (or PDE inhibition) to an increase in the SANC firing rate. The specific link function of the switchboard is to generate an increased LCR signal mass, that occurs at an earlier time during DD. The requirement of intact RyR function within the coupled-clock system for β-AR stimulation-induced positive chronotropic effect has also been confirmed in both single SANC and intact SA node of numerous species (Fig. 8) (the results of 8 studies are summarized in Online Table VA and VB, respectively).
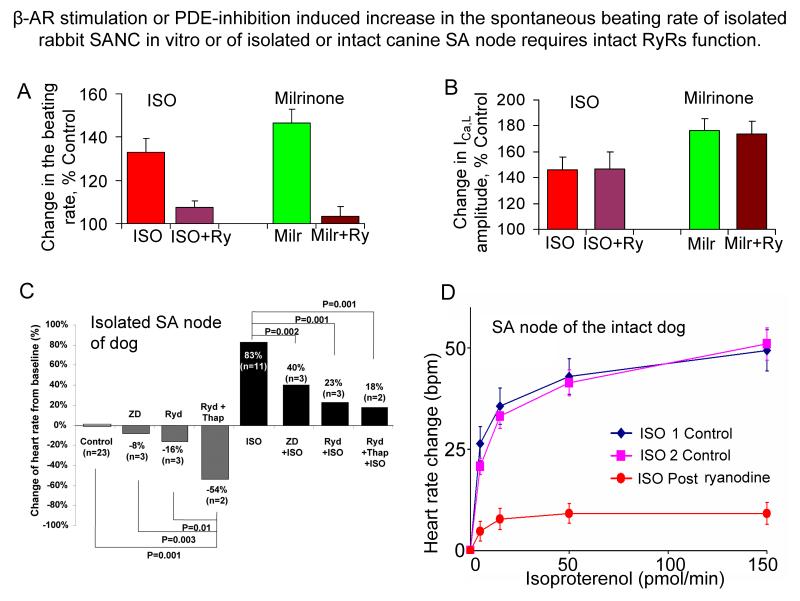
Figure 8. β-AR stimulation or PDE-inhibition induced increase in spontaneous beating rate of isolated rabbit SANC in vitro or of isolated or intact canine SA node requires intact RyRs function.
A. Changes of rabbit SANC firing rate by β-AR stimulation or PDE inhibition in the presence and absence of ryanodine. B. The increase in L-type Ca2+ current by β-AR stimulation or PDE inhibition is not affected when RyR Ca2+ release in rabbit SANC is inhibited by ryanodine. C. Changes in the canine heart rate (percent from baseline) induced by different pharmacological interventions. The gray bars show the changes during 3 μmol/L ZD 7288 (If channel inhibitor, ZD), 3 μmol/L ryanodine (Ryd), and 10 μmol/L ryanodine plus 200 nmol/L thapsigargin (Ryd+Thap) infusion without isoproterenol (ISO), and black bars during 1 μmol/L ISO infusion (with permission from23). D. The increase of the heart rate of intact canines in response to sequential ISO infusions (two control infusions followed by a third graded-dose infusion in the presence of ryanodine) delivered by microdialysis into the SA nodal artery: ISO produces a brisk, reproducible dose-dependent tachycardia, and sequential dose responses in control are superimposable. Disabling of RyRs with ryanodine (5 nmol/min) following the second control ISO infusion dramatically reduces the effects of the next ISO infusion to increase the in vivo heart rate. Local nodal dialysis with ryanodine prior to the third ISO infusion reduced resting heart rate by 12% (from 108 ± 5 to 96 ± 6 bpm; P < 0.05), and suppressed the subsequent response to ISO by 75% (from18).
In the presence of a sub-maximal [ryanodine] the ability of β-AR stimulation to increase the spontaneous SANC beating rate in most species is strikingly suppressed (up to 100%), Online Table VA. In contrast, in the presence of a very high concentration of ryanodine (30 μmol/L), i.e. a concentration that exceeds that used in the most studies in conjunction with β-AR stimulation (Online Table V), a high SANC firing rate is observed during β-AR stimulation68,69. This may be attributable to the idea that high [ryanodine] locks RyRs in a closed state, preventing SR Ca2+ depletion68. An additional issue that complicates the interpretation of a failure of ryanodine to suppress the isoproterenol induced increase in AP firing rate in69 is that the AP cycle length prior to β-AR stimulation was not reported.
Importantly, the chronotropic response to β-AR stimulation in vivo, requires intact SR Ca2+ cycling within the coupled-clock system: in open chest dogs, a robust, dose-dependent increase in the spontaneous beating rate effected by microdialysis of the SA node artery with isoproterenol is virtually abolished when RyR is inhibited by ryanodine (Fig.8D, Online Table VB).
In spite of the robust evidence detailed above, an alternative view of the mechanism of β-AR-modulated increase in AP firing rate denies the requirement for a change in PKA-dependent Ca2+ regulation of the complex coupled-clock for a normal chronotropic response to β-AR stimulation. This alternative view point attributes β-AR-induced chronotropy to a direct cAMP-dependent facilitation of If channel opening, via a cAMP-dependent shift of the voltage dependence of its activation curve70,71. Numerous studies, however, employing pharmacologic (Online Table III), or genetic (Online Figure I) manipulations have demonstrated that when If current is suppressed or deleted, the resting heart rate or cell beating rate is modestly decreased (~16% in SA node or isolated heart and ~18% in SANC, Online Table III), and a full or major part of the positive chronotropic response to β-AR stimulation still remains intact (Online Table IV and Online Figure I, c.f.15,67 for review). While the If current does not appear to have a major effect to accelerate the beating rate of SANC during β-AR stimulation15,67, it may have a key role in stabilizing the pacemaker rhythm, particularly during transition states16,67,72-75.
Cholinergic receptor stimulation effects on the LCR period and AP cycle length
Two recent studies in rabbit SANC24,61 have demonstrated that ChR stimulation suppresses LCR’s (or LDCAE), and that LCR or LDCAE suppression contributes substantially (35%-75%) to the slowing of the beating rate. In isolated rabbit SANC, the ChR stimulation-induced beating rate reduction is entirely dependent on Gi activation61 (Fig.1 green; Online Table I). At low [carbachol], IKACh activation is not evident, and the prolongation of the LCR period and cycle are attributable to a suppression of cAMP-mediated, PKA-dependent Ca2+ signaling: a 50% reduction in phospholamban phosphorylation is accompanied by ~60% prolongation of LCR period (Fig.7A) that is linked to prolongation in the cycle length (Fig.7B). Phosphatase inhibition reverses the effect of carbachol on SANC LCR period and cycle length61. At higher [carbachol] IKACh activation underlies a more pronounced reduction in the beating rate61.
It had previously been thought that ChR stimulation induced chronotropic effect (similar to that of β-AR stimulation) is due to an impact on sarcolemmal membrane ion channels, specifically IKACh, If, and ICaL70,76-79. In rabbit SANC under voltage clamp, a ChR stimulation-induced suppression of ICaL in the absence of prior β-AR stimulation55,61,80 has been ascribed to a reduction of basal PKA-dependent L-type Ca2+ channel phosphorylation55. (Note that such an effect would be expected to have a marked effect on intacellular Ca2+ cycling, vide supra.) If suppression has specifically been considered to be an important mechanism in the ChR stimulation-induced decrease of the spontaneous SA node beating rate, particularly at low ChR agonist concentrations76 (but see61,79,81). Studies performed either in the isolated Langendorff hearts or in isolated SANC using tertiapin Q to block IKACh24,61,82,83 provide different perspectives on the contribution of IKACh and If to the bradycardic response to carbachol. In one study in isolated rabbit SANC, If blockade did not affect the beating rate reduction at any level of stimulation with carbachol61; in another study the combined contribution of If and of IK,ACh currents has been estimated to be about 25% of ACh-induced decrease in SANC beating rate24.
To summarize this section, graded changes in the steady-state phosphorylation status of proteins that regulate Ca2+ within the coupled-clock system lead to gradations in the LCR period by GPCR signaling (Fig.7A), cause graded changes in the timing of the onset and amplitude of the late DD exponential increase, and thus cause concomitant gradations of the steady-state AP cycle length18,26,27,61 (Fig.7B). It is necessary to emphasize, however, that modulation of the LCR period and SANC beating rate by coupled-clock functions in response to GPCR stimulation depends not only on the direct modulation of protein phosphorylation, per se, that is produced by GPCR stimulation (Fig.1), but also depends upon the secondary modulation via rate and rhythm of AP occurrence that results from GPCR stimulation: changes in the AP firing rate or AP shape, per se, due to any cause, indirectly effect changes in intracellular Ca2+cycling characteristics via an effect on steady state cell Ca2+ levels, i.e., via the Bowditch treppe effect84.
A coupled-clock system also regulates the automaticity of embryonic heart
Ca2+ cycling proteins (RyR2, SERCA2a, NCX) are abundantly expressed very early in development of cardiomyocytes and embryonic stem cell-derived cardiocytes (ESC’s). SR Ca2+ cycling is crucially important in the developmental regulation of Ca2+ transients and contraction in these cells85, which are presently considered to be a prime substrate for engineering biological pacemakers86. Numerous studies indicate that spontaneous intracellular Ca2+ releases via RyRs drive automaticity (likely via NCX) and contraction of embryonic cardiomyocytes87,88 and ESC’s85,89-94, In addition to RyR, Ca2+ release channels activated by IP3, are also involved in spontaneous Ca2+ release in embryonic cardiomyocytes and ESCs87,91,92. Genetic manipulation of Ca2+ cycling proteins (see below), or pharmacologic inhibition of Ca2+ cycling (ryanodine, thapsigargin, cyclopiazonic acid, BAPTA-AM), or of NCX function, substantially slows or halts automaticity of embryonic cardiomyocytes87,88 and ESCs94.
Genetic manipulation of RyR, NCX, CaMKII or ankyrin B
Genetic manipulation of RyR or NCX, like pharmacologic maneuvers discussed above, also markedly impairs normal automaticity and markedly impairs the chronotropic response to β-AR stimulation. RyR2 knockout in mice results in embryonic lethality, which was initially interpreted to result from generally perturbed Ca2+ homeostasis, due to a Ca2+ overloaded SR, that resulted in contractile impairment and cell death95. A more specific explanation of this lethality was later gleaned from studies of mouse ESCs90, in which RyR2 knock-out not only causes a failure of spontaneous LCR’s, markedly suppresses basal spontaneous cell beating and the response to β-AR stimulation, but also leads to a marked depression of the obligatory developmental increases in heart rate and cardiac output required to support continued cardiac embryonic differentiation90. Recent data also demonstrate that RyR mutations can initiate severe abnormalities in human cardiac pacemaker function: genomic deletion of RyR2 exon-3 leads to sinoatrial and atrioventricular node dysfunctions, atrial fibrillation and atrial standstill96.
The importance of NCX in automaticity of embryonic cardiomyocytes is supported by observations that NCX knockout mice are embryonic lethals, without evidence that the heart ever having a single beat97,98. Interestingly, mice with NCX knockout only in ventricular myocytes (with NCX in SANC remaining intact) live to adulthood with only modestly reduced cardiac function99
CaMKII has been genetically manipulated, via conditional expression of CaMKII peptide inhibitor, AC3-I. These mice have the same basal heart beating rate as wild type mice23, but during stress exhibit significantly less heart rate acceleration than their wild type counterparts. Ryanodine significantly and equivalently reduces the beating rate in SANC isolated from either wild type or AC3-I mice. In the AC3-I mouse SANC, the positive chronotropic effect of β-AR stimulation are severely reduced. Thus, the β-AR chronortopic response in mouse SANC requires CaMKII-dependent activation and its concomitant effects to increase SR Ca2+ load and to increase diastolic RyR Ca2+ release in the context of the coupled-clock system22. (Of note, the results of this study also strongly suggest that the reduced response to β-AR stimulation in AC3-I mice is independent of If, since this current, as well as its response to isoproterenol, is fully preserved22.)
Reduced expression of AnkB in mice (heterozygous AnkB mice) results in SA node dysfunction, severe bradycardia and heart rate variability, associated with a substantial decrease in INCX and ICaL and abnormal Ca2+ cycling in SANC, while ICaT and If remain unchanged100.
In contrast to knockout of RyR2, NCX or CaMKII function, or Ankyrin-B, or mutations in human RyRs, genetic manipulation in mice including HCN2 or HCN4 (general or cardiac-specific SA node and AV node) knock outs, or inhibition of cAMP binding to HCN4 subunits, have minimal or no effects on resting heart rate, or on increases in heart rate during exercise, or chronotropic effect of β-AR stimulation (Online Figure I). Conditional, global deletion of HCN4 isoform in adult mice (Online Figure IA) results in a significantly augmented response to ChR stimulation by carbachol73. But when such conditional HCN4 deletion only occurred within the SA and atrio-ventricular nodes (Online Figure IB), carbachol markedly and equally decreased the heart rate in both genotypes. This strongly suggests that a full response to ChR stimulation does not require If74.
Summary
Over 30 years ago, Rapp and Berridge101, in a theoretical treatise on biological rhythms, in the absence of experimental data, made an intriguing prediction about the heart’s rhythm:
“The heart is a complicated organ containing different regions of specialized tissue. Unfortunately, the region of greatest interest form the point of view of oscillations, the sino-atrial node, is least accessible experimentally. Accordingly, anything said about the heart beat must be stated circumspectly. However, the case for a central role for calcium and cyclic AMP is now very strong, indeed. We wish to carry the argument one stage further and suggest that oscillation in cyclic AMP and calcium constitutes the basic driving signal for the initiation of heart beat. Should this theory be correct, it would resolve what must be one of the oldest and most intriguing scientific questions.”
The material reviewed herein indicates that the Rapp-Berridge prediction is accurate, in large measure. Prolific experimental evidence, supported by novel numerical modeling, permits the conclusion that SANC normal automaticity is regulated by constitutive Ca2+ activation of AC, and that the resultant increase in cAMP activates PKA- , and that PKA/CaMKII-dependent protein phosphorylation govern a complex clock system comprised of intracellular SR and surface membrane molecules that regulate intracellular Ca2+ cycling (Fig.1B). This coupled pacemaker clock system is robust, because the same factors that regulate SR Ca2+ cycling and sarcolemmal molecular function, also couple SR Ca2+ cycling to sarcolemmal protein function to effect normal basal automaticity. G protein-coupled receptor signaling insures pacemaker flexibility, effecting rate regulation by impacting on these very same factors that regulate coupled-clock Ca2+ cycling to guarantee basal state pacemaker stability and robustness. Thus, intimately intertwined properties of robustness and flexibility of the heart’s coupled pacemaker system insure stable heart rates of a wide range that is required for peaceful rest or flight or fight.
Where do we go from here?
It has become clear (to us) that normal SANC function results from a complex integration of its component subsystem clocks. In this context, issues, such as, “what is the most important mechanism that underlies pacemaker cell function”, or references to a specific ion channel as “THE PACEMAKER CHANNEL”2,102 cease to have substance. Still, numerous experimental gaps exist with respect to a complete understanding of the molecular functions of the individual components of this complex clock system, and in the complete conceptualization of how these components interact with each other. In our opinion, for this field to move forward, novel data derived from reductionist approaches must be integrated, via implementation of multifaceted protocols, and via numerical modeling of a complex pacemaker clock systems such as that as illustrated in Fig.1B and summarized in Online Table I. A few specific items for a future research agenda that relates to specific subsystem components and their interactions are listed below.
- What are the molecular identities of IbNa and Ist? Is it NCX?
- Do TRPC channels exist in SANC of other species besides mouse36; and, if so, what is their function?
- Are low-voltage activation L-type Ca2+ channels functional in SANC of larger mammals?
- What is the molecular mechanism of spontaneous RyR Ca2+ release during DD? How does local control of RyR’s work in SANC? How do stochastic RyR openings become partially synchronized to generate periodic LCRs?
- Are there “orphan” RyR’s in SANC, as in ventricular cells?
- Are there functional RyR3′s in SANC?
- What is the precise spatial arrangement of L-type Ca2+ channels, RyRs, and NCX molecules across the ~12 nm subsarcolemmal gap? What is the precise interplay of the different system components, i.e. Ca2+ release via RyRs, Ca2+ influx via Ca2+ channels and Ca2+ removal via NCX to regulate [Ca2+] in the subsarcolemmal space during very late DD and the onset of the rapid AP upstroke,
- Do periodic cAMP oscillations occur within SANC, and is their steady-sate period the same as the LCR period.
- Can specific actions of phospholamban phosphorylation to modulate SR Ca2+ pumping and SR Ca2+ load be distinguished from phosphorylation dependence of RyR-mediated Ca2+ release?
- How do Ca2+-calmodulin-activated and Ca2+-inhibited AC types interact in cAMP generation, e.g. with respect to GPCR receptor stimulation? Specifically, how does PKA-signaling interact with Ca2+-CaMKII signaling (in time and space)?
- In addition to activating PKA in SANC, does cAMP activate Exchange Proteins directly Activated by Cyclic AMP (EPAC), as in some cell types?
- To what extent does basal phosphatase activity regulate basal PKA and CaMKII-dependent protein phosphorylation in SANC?
- What is the geometrical arrangement of micro-scaffold complexes within SANC that link function of specific subtypes of AC’s, PDE’s, PKA, CaMKII, and phosphatases? How are these micro-scaffolds compartmentalized within SANC.
- Experiments that define transient state kinetics of cAMP-, PKA-, CamKII- signaling in spontaneously firing SANC, are required for more definitive systems modeling of biochemistry kinetics to match Vm and Ca2+ transient state kinetics of the AP firing rate.
- In addition to GPCR, how do naturally occurring biochemical/biophysical stimuli that are external to the cell, i.e. mechanical deformation (stretch) and temperature, regulate SANC pacemaker function?
- How is the Na+ equilibrium potential (ENa) regulated in SANC? What are the roles of Na+/K+ ATPase and Na+/H+ exchanger? What is the phosphorylation status of NCX, or of Na+/K+ ATPase, and its accessory proteins?
- Mitochondria cycle both Ca2+ and Na+: Is there a mitochondrial Ca2+ clock? How does it interact with the SR Ca2+ clock?
- Ditto for myofilament Ca2+ clock.
- Since SANC have a prominent mitochondrial density, but a sparse myofilament density, why are ATP production and consumption so high? Is this required for continual formation and degradation of cAMP by constitutively active AC’s and PDE’s?
- Will cultured rabbit SANC models enable genetic manipulation of coupled-clock system proteins to progress beyond the mouse?
- Are there resident precursor cells within the SA node cell system to replenish damaged cells?
- Will those who aspire to design biologic pacemakers begin to address the complex pacemaker clock system (Fig.1 and Online Table.I)?
- Given the wide range of literature values for parameters of a given ion current and Ca2+ cycling, don’t numerical models need to explore the entire range of all parameters, including those of local late diastolic subsarcolemmal Ca2+ releases, by performing extensive parametric sensitivity analyses?
- Can stochastic, locally propagating LCRs be numerically modeled?
- Can theoretical modeling of the intact SA node function incorporate the concept of the interacting Ca2+ and M clocks within individual SANC comprising the node?
Supplementary Material
Supp1
Acknowledgments
Sources of Funding This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
List of non-standard abbreviations and acronyms
SANC
sinoatrial nodal cell
LCR
subsarcolemmal Local Ca2+ Releases
SR
sarcoplasmic reticulum
‘Ca2+ clock’
SR generating rhythmic, spontaneous Ca2+ releases
Surface “membrane clock” or ‘M clock’
the ensemble of sarcolemmal ion currents generating spontaneous action potentials
DD
diastolic depolarization.
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- 2.Baruscotti M, Barbuti A, Bucchi A. The cardiac pacemaker current. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.06.019. (in print) [DOI] [PubMed] [Google Scholar]
- 3.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–982. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- 4.Wilders R. Computer modelling of the sinoatrial node. Med Biol Eng Comput. 2007;45:189–207. doi: 10.1007/s11517-006-0127-0. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein DS, Lipsius SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989;64:648–657. doi: 10.1161/01.res.64.4.648. [DOI] [PubMed] [Google Scholar]
- 6.Ju YK, Allen DG. Intracellular calcium and Na+-Ca2+ exchange current in isolated toad pacemaker cells. J Physiol. 1998;508(Pt 1):153–166. doi: 10.1111/j.1469-7793.1998.153br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju YK, Allen DG. The distribution of calcium in toad cardiac pacemaker cells during spontaneous firing. Pflugers Arch. 2000;441:219–227. doi: 10.1007/s004240000418. [DOI] [PubMed] [Google Scholar]
- 8.Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000;524(Pt 2):415–422. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Lipsius SL. Na+-Ca2+ exchange current in latent pacemaker cells isolated from cat right atrium. J Physiol. 1993;466:263–285. [PMC free article] [PubMed] [Google Scholar]
- 10.Rigg L, Terrar DA. Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Exp Physiol. 1996;81:877–880. doi: 10.1113/expphysiol.1996.sp003983. [DOI] [PubMed] [Google Scholar]
- 11.Rigg L, Heath BM, Cui Y, Terrar DA. Localisation and functional significance of ryanodine receptors during beta-adrenoceptor stimulation in the guinea-pig sino-atrial node. Cardiovasc Res. 2000;48:254–264. doi: 10.1016/s0008-6363(00)00153-x. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Qu J, Nathan RD. Ionic basis of ryanodine’s negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am J Physiol. 1997;273:H2481–2489. doi: 10.1152/ajpheart.1997.273.5.H2481. [DOI] [PubMed] [Google Scholar]
- 13.Lakatta EG, Vinogradova T, Lyashkov A, Sirenko S, Zhu W, Ruknudin A, Maltsev VA. The integration of spontaneous intracellular Ca2+ cycling and surface membrane ion channel activation entrains normal automaticity in cells of the heart’s pacemaker. Ann N Y Acad Sci. 2006;1080:178–206. doi: 10.1196/annals.1380.016. [DOI] [PubMed] [Google Scholar]
- 14.Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res. 2008;77:274–284. doi: 10.1093/cvr/cvm058. [DOI] [PubMed] [Google Scholar]
- 15.Vinogradova TM, Lakatta EG. Regulation of basal and reserve cardiac pacemaker function by interactions of cAMP mediated PKA-dependent Ca2+ cycling with surface membrane channels. Journal of Molecular and Cellular Cardiology. 2009;47:456–474. doi: 10.1016/j.yjmcc.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol. 2009;296:H594–H615. doi: 10.1152/ajpheart.01118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res. 2004;94:802–809. doi: 10.1161/01.RES.0000122045.55331.0F. [DOI] [PubMed] [Google Scholar]
- 18.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 19.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 20.Lyashkov AE, Juhaszova M, Dobrzynski H, Vinogradova TM, Maltsev VA, Juhasz O, Spurgeon HA, Sollott SJ, Lakatta EG. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ Res. 2007;100:1723–1731. doi: 10.1161/CIRCRESAHA.107.153676. [DOI] [PubMed] [Google Scholar]
- 21.Musa H, Lei M, Honjo H, Jones SA, Dobrzynski H, Lancaster MK, Takagishi Y, Henderson Z, Kodama I, Boyett MR. Heterogeneous expression of Ca2+ handling proteins in rabbit sinoatrial node. J Histochem Cytochem. 2002;50:311–324. doi: 10.1177/002215540205000303. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, Hund TJ, Kutschke W, Sarma S, Grumbach IM, Wehrens XH, Mohler PJ, Song LS, Anderson ME. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci U S A. 2009;106:5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joung B, Tang L, Maruyama M, Han S, Chen Z, Stucky M, Jones LR, Fishbein MC, Weiss JN, Chen PS, Lin SF. Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation. 2009;119:788–796. doi: 10.1161/CIRCULATIONAHA.108.817379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Borren MM, Verkerk AO, Wilders R, Hajji N, Zegers JG, Bourier J, Tan HL, Verheijck EE, Peters SL, Alewijnse AE, Ravesloot JH. Effects of muscarinic receptor stimulation on Ca2+ transient, cAMP production and pacemaker frequency of rabbit sinoatrial node cells. Basic Res Cardiol. 2009 doi: 10.1007/s00395-009-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinogradova TM, Bogdanov KY, Lakatta EG. beta-Adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- 26.Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, Yang D, Spurgeon HA, Lakatta EG. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res. 2008;102:761–769. doi: 10.1161/CIRCRESAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- 27.Bogdanov KY, Maltsev VA, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD, Lakatta EG. Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res. 2006;99:979–987. doi: 10.1161/01.RES.0000247933.66532.0b. [DOI] [PubMed] [Google Scholar]
- 28.DiFrancesco D. The contribution of the ‘pacemaker’ current (if) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J Physiol. 1991;434:23–40. doi: 10.1113/jphysiol.1991.sp018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders L, Rakovic S, Lowe M, Mattick PA, Terrar DA. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol. 2006;571:639–649. doi: 10.1113/jphysiol.2005.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangoni ME, Couette B, Marger L, Bourinet E, Striessnig J, Nargeot J. Voltage-dependent calcium channels and cardiac pacemaker activity: from ionic currents to genes. Prog Biophys Mol Biol. 2006;90:38–63. doi: 10.1016/j.pbiomolbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N. Functional Roles of Cav1.3 (α1D) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res. 2002;90:981–987. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- 32.Verheijck EE, van Ginneken AC, Wilders R, Bouman LN. Contribution of L-type Ca2+ current to electrical activity in sinoatrial nodal myocytes of rabbits. Am J Physiol. 1999;276:H1064–1077. doi: 10.1152/ajpheart.1999.276.3.H1064. [DOI] [PubMed] [Google Scholar]
- 33.Bers DM. Excitation-contraction coupling and cardiac contractile force. 2nd ed Kluwer Academic Publishers; Norwell, Mass: 2001. [Google Scholar]
- 34.Chen B, Wu Y, Mohler PJ, Anderson ME, Song LS. Local control of Ca2+-induced Ca2+ release in mouse sinoatrial node cells. J Mol Cell Cardiol. 2009;47:706–715. doi: 10.1016/j.yjmcc.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janczewski AM, Lakatta EG. Buffering of calcium influx by sarcoplasmic reticulum during the action potential in guinea-pig ventricular myocytes. J Physiol. 1993;471:343–363. doi: 10.1113/jphysiol.1993.sp019904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ju YK, Chu Y, Chaulet H, Lai D, Gervasio OL, Graham RM, Cannell MB, Allen DG. Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circ Res. 2007;100:1605–1614. doi: 10.1161/CIRCRESAHA.107.152181. [DOI] [PubMed] [Google Scholar]
- 37.Tohse N. Calcium-sensitive delayed rectifier potassium current in guinea pig ventricular cells. Am J Physiol. 1990;258:H1200–1207. doi: 10.1152/ajpheart.1990.258.4.H1200. [DOI] [PubMed] [Google Scholar]
- 38.Heath BM, Terrar DA. Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J Physiol. 2000;522(Pt 3):391–402. doi: 10.1111/j.1469-7793.2000.t01-2-00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noble D. Cardiac action and pacemaker potentials based on the Hodgkin-Huxley equations. Nature. 1960;188:495–497. doi: 10.1038/188495b0. [DOI] [PubMed] [Google Scholar]
- 40.Denyer JC, Brown HF. Pacemaking in rabbit isolated sino-atrial node cells during Cs+ block of the hyperpolarization-activated current if. J Physiol. 1990;429:401–409. doi: 10.1113/jphysiol.1990.sp018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bescond J, Bois P, Petit-Jacques J, Lenfant J. Characterization of an angiotensin-II-activated chloride current in rabbit sino-atrial cells. J Membr Biol. 1994;140:153–161. doi: 10.1007/BF00232903. [DOI] [PubMed] [Google Scholar]
- 42.Demion M, Bois P, Launay P, Guinamard R. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res. 2007;73:531–538. doi: 10.1016/j.cardiores.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Hagiwara N, Irisawa H, Kasanuki H, Hosoda S. Background current in sino-atrial node cells of the rabbit heart. J Physiol. 1992;448:53–72. doi: 10.1113/jphysiol.1992.sp019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilgemann DW. New insights into the molecular and cellular workings of the cardiac Na+/Ca2+ exchanger. Am J Physiol Cell Physiol. 2004;287:C1167–1172. doi: 10.1152/ajpcell.00288.2004. [DOI] [PubMed] [Google Scholar]
- 45.Guo J, Ono K, Noma A. A sustained inward current activated at the diastolic potential range in rabbit sino-atrial node cells. J Physiol. 1995;483(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitsuiye T, Shinagawa Y, Noma A. Sustained inward current during pacemaker depolarization in mammalian sinoatrial node cells. Circ Res. 2000;87:88–91. doi: 10.1161/01.res.87.2.88. [DOI] [PubMed] [Google Scholar]
- 47.Maylie J, Morad M, Weiss J. A study of pace-maker potential in rabbit sino-atrial node: measurement of potassium activity under voltage-clamp conditions. J Physiol. 1981;311:161–178. doi: 10.1113/jphysiol.1981.sp013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the If pacemaker current. J Physiol. 2007;582:1195–1203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, Cheng H, Xiao RP. Sinoatrial node pacemaker activity requires Ca2+/calmodulin-dependent protein kinase II activation. Circ Res. 2000;87:760–767. doi: 10.1161/01.res.87.9.760. [DOI] [PubMed] [Google Scholar]
- 50.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 51.Vinogradova TM, Ruknudin AM, Zhu W, Lyashkov AE, Volkova M, Boheler KR, Xiao RP, Spurgeon H, Lakatta EG. High basal cAMP content markedly elevates PKA-dependent protein phosphorylation and sustains spontaneous beating in rabbit sinoatrial nodal pacemaker cells (SANC) Biophys J. 2006;90:155a. (Abstract) [Google Scholar]
- 52.Younes A, Lyashkov AE, Graham D, Sheydina A, Volkova MV, Mitsak M, Vinogradova TM, Lukyanenko YO, Li Y, Ruknudin AM, Boheler KR, van Eyk J, Lakatta EG. Ca2+-stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem. 2008;283:14461–14468. doi: 10.1074/jbc.M707540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP-responsive membrane events. Embo J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 55.Petit-Jacques J, Bois P, Bescond J, Lenfant J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflugers Arch. 1993;423:21–27. doi: 10.1007/BF00374956. [DOI] [PubMed] [Google Scholar]
- 56.Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242:67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Sirenko SG, Vinogradova TM, Lyashkov AE, Zhu W, Juhaszova M, Ziman B, Wang S, Lakatta EG. High basal Ca2+/calmodulin kinase II activity modulates spontaneous sarcoplasmic reticulum Ca2+ cycling that drives normal automaticity in sinoatrial nodal cells. Circulation. 2007;116(part II):86–87. (Abstract) [Google Scholar]
- 58.Mangoni ME, Fontanaud P, Noble PJ, Noble D, Benkemoun H, Nargeot J, Richard S. Facilitation of the L-type calcium current in rabbit sino-atrial cells: effect on cardiac automaticity. Cardiovasc Res. 2000;48:375–392. doi: 10.1016/s0008-6363(00)00182-6. [DOI] [PubMed] [Google Scholar]
- 59.Narayanan N, Xu A. Phosphorylation and regulation of the Ca(2+)-pumping ATPase in cardiac sarcoplasmic reticulum by calcium/calmodulin-dependent protein kinase. Basic Res Cardiol. 1997;92(Suppl 1):25–35. doi: 10.1007/BF00794065. [DOI] [PubMed] [Google Scholar]
- 60.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 61.Lyashkov AE, Vinogradova TM, Zahanich I, Li Y, Younes A, Nuss HB, Spurgeon HA, Maltsev VA, Lakatta EG. Cholinergic receptor signaling modulates spontaneous firing of sinoatrial nodal cells via integrated effects on PKA-dependent Ca2+ cycling and IKACh. Am J Physiol Heart Circ Physiol. 2009:H949–H959. doi: 10.1152/ajpheart.01340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D. If-dependent modulation of pacemaker rate mediated by cAMP in the presence of ryanodine in rabbit sino-atrial node cells. J Mol Cell Cardiol. 2003;35:905–913. doi: 10.1016/s0022-2828(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 63.Lancaster MK, Jones SA, Harrison SM, Boyett MR. Intracellular Ca2+ and pacemaking within the rabbit sinoatrial node: heterogeneity of role and control. J Physiol. 2004;556:481–494. doi: 10.1113/jphysiol.2003.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewartowski B, Hansford RG, Langer GA, Lakatta EG. Contraction and sarcoplasmic reticulum Ca2+ content in single myocytes of guinea pig heart: effect of ryanodine. Am J Physiol. 1990;259:H1222–1229. doi: 10.1152/ajpheart.1990.259.4.H1222. [DOI] [PubMed] [Google Scholar]
- 65.Maltsev VA, Lakatta EG. Novel perspectives in cardiac pacemaker regulation based on numerical modeling of coupled subcellular Ca and membrane voltage oscillators. Journal of Physiological Sciences. 2009;59(Suppl1):66. (Abstract) [Google Scholar]
- 66.Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D. Modulation of rate by autonomic agonists in SAN cells involves changes in diastolic depolarization and the pacemaker current. J Mol Cell Cardiol. 2007;43:39–48. doi: 10.1016/j.yjmcc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 67.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009;47:157–170. doi: 10.1016/j.yjmcc.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ju YK, Allen DG. How does beta-adrenergic stimulation increase the heart rate? The role of intracellular Ca2+ release in amphibian pacemaker cells. J Physiol. 1999;516(Pt 3):793–804. doi: 10.1111/j.1469-7793.1999.0793u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Honjo H, Inada S, Lancaster MK, Yamamoto M, Niwa R, Jones SA, Shibata N, Mitsui K, Horiuchi T, Kamiya K, Kodama I, Boyett MR. Sarcoplasmic reticulum Ca2+ release is not a dominating factor in sinoatrial node pacemaker activity. Circ Res. 2003;92:e41–44. doi: 10.1161/01.res.0000055904.21974.be. [DOI] [PubMed] [Google Scholar]
- 70.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 71.DiFrancesco D, Mangoni M. Modulation of single hyperpolarization-activated channels (if) by cAMP in the rabbit sino-atrial node. J Physiol. 1994;474:473–482. doi: 10.1113/jphysiol.1994.sp020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. Embo J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herrmann S, Stieber J, Stockl G, Hofmann F, Ludwig A. HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. Embo J. 2007;26:4423–4432. doi: 10.1038/sj.emboj.7601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoesl E, Stieber J, Herrmann S, Feil S, Tybl E, Hofmann F, Feil R, Ludwig A. Tamoxifen-inducible gene deletion in the cardiac conduction system. J Mol Cell Cardiol. 2008;45:62–69. doi: 10.1016/j.yjmcc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Harzheim D, Pfeiffer KH, Fabritz L, Kremmer E, Buch T, Waisman A, Kirchhof P, Kaupp UB, Seifert R. Cardiac pacemaker function of HCN4 channels in mice is confined to embryonic development and requires cyclic AMP. Embo J. 2008;27:692–703. doi: 10.1038/emboj.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DiFrancesco D, Ducouret P, Robinson RB. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science. 1989;243:669–671. doi: 10.1126/science.2916119. [DOI] [PubMed] [Google Scholar]
- 77.Dokos S, Celler BG, Lovell NH. Vagal control of sinoatrial rhythm: a mathematical model. J Theor Biol. 1996;182:21–44. doi: 10.1006/jtbi.1996.0141. [DOI] [PubMed] [Google Scholar]
- 78.Demir SS, Clark JW, Giles WR. Parasympathetic modulation of sinoatrial node pacemaker activity in rabbit heart: a unifying model. Am J Physiol. 1999;276:H2221–2244. doi: 10.1152/ajpheart.1999.276.6.H2221. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H, Holden AV, Noble D, Boyett MR. Analysis of the chronotropic effect of acetylcholine on sinoatrial node cells. J Cardiovasc Electrophysiol. 2002;13:465–474. doi: 10.1046/j.1540-8167.2002.00465.x. [DOI] [PubMed] [Google Scholar]
- 80.Zaza A, Robinson RB, DiFrancesco D. Basal responses of the L-type Ca2+ and hyperpolarization-activated currents to autonomic agonists in the rabbit sino-atrial node. J Physiol. 1996;491(Pt 2):347–355. doi: 10.1113/jphysiol.1996.sp021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyett MR, Kodama I, Honjo H, Arai A, Suzuki R, Toyama J. Ionic basis of the chronotropic effect of acetylcholine on the rabbit sinoatrial node. Cardiovasc Res. 1995;29:867–878. [PubMed] [Google Scholar]
- 82.Yamada M. The role of muscarinic K+ channels in the negative chronotropic effect of a muscarinic agonist. J Pharmacol Exp Ther. 2002;300:681–687. doi: 10.1124/jpet.300.2.681. [DOI] [PubMed] [Google Scholar]
- 83.Bolter CP, English DJ. The effects of tertiapin-Q on responses of the sinoatrial pacemaker of the guinea-pig heart to vagal nerve stimulation and muscarinic agonists. Exp Physiol. 2008;93:53–63. doi: 10.1113/expphysiol.2007.038901. [DOI] [PubMed] [Google Scholar]
- 84.Lakatta EG. Beyond Bowditch: the convergence of cardiac chronotropy and inotropy. Cell Calcium. 2004;35:629–642. doi: 10.1016/j.ceca.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 85.Fu JD, Li J, Tweedie D, Yu HM, Chen L, Wang R, Riordon DR, Brugh SA, Wang SQ, Boheler KR, Yang HT. Crucial role of the sarcoplasmic reticulum in the developmental regulation of Ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. Faseb J. 2006;20:181–183. doi: 10.1096/fj.05-4501fje. [DOI] [PubMed] [Google Scholar]
- 86.Rosen MR. Conference report: building a biologic pacemaker. J Electrocardiol. 2007;40:S197–198. doi: 10.1016/j.jelectrocard.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sasse P, Zhang J, Cleemann L, Morad M, Hescheler J, Fleischmann BK. Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells. J Gen Physiol. 2007;130:133–144. doi: 10.1085/jgp.200609575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rapila R, Korhonen T, Tavi P. Excitation-contraction coupling of the mouse embryonic cardiomyocyte. J Gen Physiol. 2008;132:397–405. doi: 10.1085/jgp.200809960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viatchenko-Karpinski S, Fleischmann BK, Liu Q, Sauer H, Gryshchenko O, Ji GJ, Hescheler J. Intracellular Ca2+ oscillations drive spontaneous contractions in cardiomyocytes during early development. Proc Natl Acad Sci U S A. 1999;96:8259–8264. doi: 10.1073/pnas.96.14.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang HT, Tweedie D, Wang S, Guia A, Vinogradova T, Bogdanov K, Allen PD, Stern MD, Lakatta EG, Boheler KR. The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci U S A. 2002;99:9225–9230. doi: 10.1073/pnas.142651999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mery A, Aimond F, Menard C, Mikoshiba K, Michalak M, Puceat M. Initiation of embryonic cardiac pacemaker activity by inositol 1,4,5-trisphosphate-dependent calcium signaling. Mol Biol Cell. 2005;16:2414–2423. doi: 10.1091/mbc.E04-10-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kapur N, Banach K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. J Physiol. 2007;581:1113–1127. doi: 10.1113/jphysiol.2006.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Satin J, Itzhaki I, Rapoport S, Schroder EA, Izu L, Arbel G, Beyar R, Balke CW, Schiller J, Gepstein L. Calcium Handling in Human Embryonic Stem Cell Derived Cardiomyocytes. Stem Cells. 2008 doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 94.Zahanich I, Sirenko SG, Maltseva LA, Tarasova YS, Spurgeon HA, Boheler KR, Stern MD, Lakatta EG, Maltsev VA. Robust pacemakers derived from embryonic stem cells require dynamic interactions of sarcolemma and subcellular Ca2+ cycling driven by high basal cAMP/PKA activity. Journal of Molecular and Cellular Cardiology. 2009;46:S9. (Abstract) [Google Scholar]
- 95.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. Embo J. 1998;17:3309–3316. doi: 10.1093/emboj/17.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders M, Postma AV, van Langen I, Mannens MM, Wilde AA. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007;116:1569–1576. doi: 10.1161/CIRCULATIONAHA.107.711606. [DOI] [PubMed] [Google Scholar]
- 97.Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. Faseb J. 2001;15:1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- 98.Cho CH, Lee SY, Shin HS, Philipson KD, Lee CO. Partial rescue of the Na+-Ca2+ exchanger (NCX1) knock-out mouse by transgenic expression of NCX1. Exp Mol Med. 2003;35:125–135. doi: 10.1038/emm.2003.18. [DOI] [PubMed] [Google Scholar]
- 99.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res. 2004;95:604–611. doi: 10.1161/01.RES.0000142316.08250.68. [DOI] [PubMed] [Google Scholar]
- 100.Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008;105:15617–15622. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rapp PE, Berridge MJ. Oscillations in calcium-cyclic AMP control loops form the basis of pacemaker activity and other high frequency biological rhythms. J Theor Biol. 1977;66:497–525. doi: 10.1016/0022-5193(77)90299-5. [DOI] [PubMed] [Google Scholar]
- 102.Verkerk AO, Wilders R, van Borren MM, Peters RJ, Broekhuis E, Lam K, Coronel R, de Bakker JM, Tan HL. Pacemaker current (If) in the human sinoatrial node. Eur Heart J. 2007;28:2472–2478. doi: 10.1093/eurheartj/ehm339. [DOI] [PubMed] [Google Scholar]
- 103.Maltsev VA, Lakatta EG. Cardiac pacemaker cell failure with preserved If, ICaL, and IKr: A lesson about pacemaker function learned from ischemia-induced bradycardia. J Mol Cell Cardiol. 2007;42:289–294. doi: 10.1016/j.yjmcc.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maltsev VA, Vinogradova TM, Bogdanov KY, Lakatta EG, Stern MD. Diastolic calcium release controls the beating rate of rabbit sinoatrial node cells: numerical modeling of the coupling process. Biophys J. 2004;86:2596–2605. doi: 10.1016/S0006-3495(04)74314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lakatta EG, Vinogradova TM, Maltsev VA. The missing link in the mystery of normal automaticity of cardiac pacemaker cells. Ann N Y Acad Sci. 2008;1123:41–57. doi: 10.1196/annals.1420.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp1