Chemical and Morphological Alterations of Spines Within the Hippocampus and Entorhinal Cortex Precede the Onset of Alzheimer’s Disease Pathology in Double Knock-In Mice (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 23.
Published in final edited form as: J Comp Neurol. 2007 Dec 1;505(4):352–362. doi: 10.1002/cne.21485
Abstract
Mice with knock-in of two mutations that affect beta amyloid processing and levels (2xKI) exhibit impaired spatial memory by 9–12 months of age, together with synaptic plasticity dysfunction in the hippocampus. The goal of this study was to identify changes in the molecular and structural characteristics of synapses that precede and thus could exert constraints upon cellular mechanisms underlying synaptic plasticity. Drebrin A is one protein reported to modulate spine sizes and trafficking of proteins to and from excitatory synapses. Thus, we examined levels of drebrin A within postsynaptic spines in the hippocampus and entorhinal cortex. Our electron microscopic immunocytochemical analyses reveal that, by 6 months, the proportion of hippocampal spines containing drebrin A is reduced and this change is accompanied by an increase in the mean size of spines and decreased density of spines. In the entorhinal cortex of 2xKI brains, we detected no decrement in the proportion of spines labeled for drebrin A and no significant change in spine density at 6 months, but rather a highly significant reduction in the level of drebrin A immunoreactivity within each spine. These changes are unlike those observed for the somatosensory cortex of 2xKI mice, in which synapse density and drebrin A immunoreactivity levels remain unchanged at 6 months and older. These results indicate that brains of 2xKI mice, like those of humans, exhibit regional differences of vulnerability, with the hippocampus exhibiting the first signatures of structural changes that, in turn, may underlie the emergent inability to update spatial memory in later months.
Indexing terms: amyloid precursor protein, presenilin 1, drebrin, excitatory synapses, electron microscopic immunocytochemistry, ultrastructure
Acquisition, retention, and updating of memory rely on cellular mechanisms in the CNS that couple experience-evoked synaptic activities to modifications of synaptic strengths (Malinow and Malenka, 2002; Malenka and Bear, 2004). Conversely, there are strong indications that memory impairment associated with Alzheimer’s disease (AD) is caused by the slow but progressive changes in synaptic physiology that precedes neuronal loss (Selkoe, 2002; Selkoe and Schenk, 2003). Recently, we showed that an animal model of AD exhibits reduced levels of AMPA receptors within hippocampal spines, together with deficits in spatial memory, impairments in synaptic physiology, and deposition of amyloid beta (Ab) plaques (Chang et al., 2006). The AD pathology, including the reduction of AMPA receptor subunits within postsynaptic spines of the hippocampus, is clearly evident by the 14th month, but some aspects of the pathology are likely to emerge several months earlier. We sought to identify constraints imposed upon synaptic plasticity at the onset of the disease. To this end, we looked for chemical and molecular changes within postsynaptic spines at ages preceding the emergence of synaptic and behavioral deficits associated with AD pathology.
The animal model of familial AD (FAD) we used in the previous study and again in this study was generated by targeted knock-in of two genes (2xKI)—one encoding the mutated amyloid precursor protein (APP; K670N/M671L, Swedish mutation with humanized amyloid beta sequence) and another encoding the mutated presenilin-1 (P264L; Flood et al., 2002). This gene-targeting technique ensures that the mutant genes’ expressions are controlled by endogenous promoters and therefore are not over-expressed and also that the endogenous wildtype (WT) genes are absent. Accordingly, it has been shown that Ab plaques increase linearly, starting from 6 months (Chang et al., 2006). The same study has characterized the emergence and progression of the AD-like pathology in this 2xKI model. Based on this study, we now know that soluble forms of Ab (sAb, namely, sAb42 and sAb40) become detectable within brain homogenate supernatants by the second postnatal month and increase fourfold by 6–9 months. Deficiency in LTP expression does not become manifest until 7–9 months, whereas spatial memory deficits and reduction of AMPA receptor mEPSCs in the hippocampus are not measurable until 9–12 months. We reasoned that 6 months would be an ideal age for identifying synaptic changes, caused by the rise of sAb, that are beginning to impose constraints upon synaptic physiology.
A growing body of work indicates that synaptic plasticity is closely linked to structural changes in postsynaptic spines. Kasai et al. have shown that larger spines are morphologically stable, whereas smaller spines have a greater propensity to enlarge following LTP induction. Based on these observations, Kasai et al. suggest that the smaller spines are sites recruited for memory acquisition, whereas larger spines are sites for memory storage (Matsuzaki et al., 2001, 2004; Kasai et al., 2003). These ideas led us to hypothesize that synapses of the 2xKI mice may be impaired in the mechanism linking synaptic activity to structural changes in postsynaptic spines.
One protein proposed to be involved in the link between synaptic activity and spine morphology is an F-actin binding protein, drebrin (Shirao and Sekino, 2001; Shiraishi et al., 2003; Takahashi et al., 2003, 2005; Fujisawa et al., 2006; Kojima, 2007; Sekino et al., 2007). This protein is enriched at postsynaptic sides of excitatory synapses (Aoki et al., 2005). There are two isoforms of drebrin, drebrin E, which is expressed across multiple cell types from embryonic stages, and drebrin A, which is neuron specific and becomes the dominant isoform in adulthood. When drebrin A is expressed in non-neuronal cells, neurite-like processes appear (Shirao et al., 1992), whereas down-regulation of the drebrin A gene within hippocampal neurons grown in culture causes reduction in spine density, decreased width of spines, and suppression of the NMDA receptor targeting to spines (Takahashi et al., 2005). Drebrin (either the A or E isoform) is reduced within cortices and hippocampi of AD patients (Harigaya et al., 1996; Hatanpaa et al., 1999; Shim and Lubec, 2002; Calon et al., 2004) and begins to show regionally specific changes in levels at the stage at which patients are diagnosed with mild cognitive impairment (Counts et al., 2006). In the neocortex of rats and limbic cortex of mice, spines containing drebrin A are larger (Fujisawa et al., 2006; Kobayashi et al., 2007). These ideas and previous observations led us to predict that the loss of drebrin A may precede the deficits in spatial memory, impaired synaptic plasticity, and reduction in AMPA currents measurable in the hippocampus of 2xKI mice by 9 months. Thus, we compared the proportion of spines within samples of 2xKI brains that contain drebrin A with those of WT samples matched for brain region and age.
In brains of patients diagnosed with AD, degeneration and chemical changes occur earlier in the limbic cortex and the hippocampus, relative to the primary motor and primary sensory cortices (Braak and Braak, 1991; Counts et al., 2006). Our previous ultrastructural examination of the primary somatosensory cortex of 2xKI brains indicated that there are no signs of excitatory synapse loss or of decrement in drebrin A within spines, even at age 18 months or older (Mahadomrongkul, 2005). In this study, we analyzed the synaptic neuropil of the hippocampus, so that we could relate the ultrastructural data to the electrophysiological data previously collected from the hippocampus of 2xKI mice. In order to determine whether the cortex of FAD model mice also exhibits regional differences in pathology related to AD, we extended our analysis to the entorhinal cortex, one of the limbic areas of the cortex.
Our measurements in these two regions agree with our prediction regarding the spinous levels of drebrin A within 2xKI brains. We also observed subtle differences in the way drebrin A level and spine sizes change across the two brain regions. We discuss how these changes may lead to the reduction in activity-dependent fine-tuning of synaptic receptor levels and memory dysfunction.
MATERIALS AND METHODS
Animals
All the mice used in the experiment were male. The FAD mouse model used in this study were homozygous 2xKI mice expressing the genes for APP and presenilin-1 (PS1) carrying familial AD mutations (APPNLh and PS1P264L). For comparisons with WT mice, we used mice of identical background strain (129/CD-1). Cephalon (West Chester, PA) generously provided us with the 2xKI mutant mice and the WT mice matched in age and background. The animals were housed for 1 day to >18 months at the New York University (NYU) animal facility. Before and after arrival at the facility, animals were maintained on a 12:12-hour light/dark cycle, fed ad libitum, and cared for according to the NIH Guide for the Care and Use of Laboratory Animals and also the guidelines approved by NYU’s Animal Care and Use Committee.
We collected brain samples from three age groups. The number and ages of 2xKI mice were as follows: four aged 3–4 months, two aged 6 months, and three aged 18 months and older (>18 months). Two of the WT animals were 3–4 months old, two were 6 months old, and three were older than 18 months (>18 months). Brain samples from these six age-genotype groups were prepared by first anesthetizing the animals deeply with Nembutal (70 mg/ kg) and then perfusing them transcardially with a mixture of 4% paraformaldehyde and 1% glutaraldehyde.
The drebrin A antibody
The rabbit anti-drebrin A antibody, DAS2, was generated against the peptide unique to the adult form of drebrin (residues 325–336) that is identical among mouse, rat, and human. This antibody was affinity-purified by the antigen peptide; it was previously shown by Western blotting to recognize a single band corresponding to drebrin A but not the band corresponding to the embryonic isoform, drebrin E (Aoki et al., 2005). Specific labeling of drebrin A within aldehyde-fixed tissue was demonstrated by elimination of immunoreactivity in rat brain tissue, when drebrin A was preadsorbed by the synthetic peptide used to generate the antiserum, and by the absence of immunolabeling when it was applied to brain tissue from drebrin A knock-out animals (Aoki et al., 2005).
Immunocytochemical detection of drebrin A and electron microscopy
Preparation of tissue
A Vibratome was used to prepare coronal sections of the hippocampus and entorhinal cortex at Bregma −2 mm, determined by the Franklin and Paxinos (2001) mouse atlas.
Details of tissue preparation for drebrin A immunocytochemistry and electron microscopy were exactly as described previously (Aoki et al., 2005; Mahadomrongkul, 2005). Two immunolabels were used: HRP-DAB, which allowed for optimal detection of drebrin A’s presence within spines, and silver-intensified colloidal gold (SIG), which allowed for quantification of drebrin A levels within individual spines. Following the immunocytochemical steps, the HRP-DAB-labeled sections were postfixed by using osmium tetroxide and embedded by using Epon 812; the SIG-labeled sections were postfixed by using Phend’s osmium-free method (Phend et al., 1995), so as to avoid the loss of SIG particles due to strong oxidation by osmium tetroxide. The SIG-labeled tissues were also embedded by using Epon 812.
Procedures for sampling postsynaptic spines
Ultrathin sections were collected from surface-most regions of Vibratome sections located in the stratum radiatum of the CA1 field of the hippocampus or layer 1 of entorhinal cortex. The stratum radiatum was the area chosen for this study, because this region in aged 2xKI mouse brains was shown in an earlier study to exhibit reduced immunoreactivity for AMPA receptors and AMPA receptor currents (Chang et al., 2006). There are four reasons we chose to analyze layer 1 of the entorhinal cortex. Axons and dendrites forming excitatory synapses are the most heterogeneous and of highest density here (Marin-Padilla, 1984), thereby allowing us to generalize our findings to excitatory synapses formed by multiple pathways most efficiently. Because excitatory synapses are formed the earliest in this layer and are maintained throughout development (Chun and Shatz, 1988), we reasoned that we would have the best chance of detecting cumulative effects of excitatory synaptic transmission here. Lastly, a preceding study of the somatosensory cortex of 2xKI mice analyzed layer 1 (Mahadomrongkul et al., 2005). We wanted to analyze a corresponding region of the entorhinal cortex, so that we could make comparisons between the two cortical regions most directly.
Images were captured by using a JEOL 1200EXII electron microscope (Peabody, MA) and a digital acquisition system from AMT (Danvers, MA) equipped with Hamamatsu’s CCD camera (Bridgewater, NJ). The magnifications for capturing images were 40,000× for SIG-labeled tissue and 25,000× for the HRP-DAB-immunolabeled tissues. Spines were identified by the presence of asymmetric synapses, i.e., a thick postsynaptic density apposed to a profile identified as an axon terminal, based on the presence of multiple vesicles. The absence of mitochondria and microtubules served as additional cues to differentiate dendritic spines from dendritic shafts and mushroom spines.
Assessment of the proportion of spines containing drebrin A
For each age-genotype group, the proportion of spines containing drebrin A in the hippocampus was determined. This value was obtained in the following way. A minimum of 30 micrographs of the hippocampus was collected from samples of each brain that were immunolabeled for drebrin A, by using HRP-DAB as the marker. Each micrograph spanned 29 μm2 of synaptic neuropil (i.e., gray matter that excludes cell bodies and blood vessels). For every group of 25 randomly encountered spines, the proportion containing HRP-DAB labeling was calculated. From these repeated measurements, the mean percent and SEM values were determined.
Analysis of the entorhinal cortex was restricted to the 6-month-old group. The proportion of spines containing drebrin A was assessed by using two sources of tissue: that immunolabeled by the HRP-DAB procedure and that immunolabeled by the SIG procedure. The analysis of spines labeled by the HRP-DAB procedure was identical to that described above for the hippocampus. For analysis of SIG-labeled tissue, a minimum of 35 digital images was captured, and the proportion of every group of 10 randomly encountered spines containing SIG labeling was calculated. From these repeated measurements, the mean percent and SEM values were determined.
Assessment of spine density in the synaptic neuropil
The same set of micrographs captured from the HRP-DAB-labeled tissue was used to calculate the density of drebrin A-labeled versus drebrin A-unlabeled spines per micrograph. For the analysis of spine density in the entorhinal cortex at 6 months of age, we also used tissue that had not undergone drebrin A immunolabeling.
Areas of spine profiles of the hippocampus and entorhinal cortex
These areas were also measured, by using the same set of micrographs from HRP-DAB-labeled tissue used to determine spine density (per unit area), and Image J software (NIH).
Correlative assessment of spine profile area and drebrin A immunoreactivity level in the entorhinal cortex
To compare drebrin A levels within spines with spine size, we used a set of images captured from SIG-labeled tissue. The area, in nm2, occupied by drebrin A-labeled and -unlabeled spines, all randomly encountered from the surface-most zone of Vibratome sections, was measured. The area occupied by all of the SIG particles within each spine cytoplasm was also measured, by using Image J.
Statistical analyses
All statistical analyses were performed by using the software Statistica (Statsoft, Tulsa, OK). When we were comparing data across the two genotypes, Student’s t-test was used. When we were comparing multiple groups of data, one- or two-way ANOVA was performed, followed by post hoc Fisher’s least squares design (LSD) test. Significance was accepted for P < 0.05. The Spearman rank order correlation analysis was performed to determine the degree of correlation between drebrin A immunoreactivity within spines (quantified as the area occupied by SIG particles) and spine area in the entorhinal cortex at the age of 6 months. Significance was accepted for P < 0.05.
Preparation of figures from electron microscopic digital images
The digital electron microscopic images, first captured by using a Hamamatsu CCD camera and software produced by AMT, were adjusted with Adobe Photoshop (version 7.0; Adobe Systems, San Jose, CA). Adjustments performed were for the purpose of matching the contrast and brightness of one digital image to another within a single figure. Adobe Photoshop was also used for cropping the image and for adding text and arrows. No dodging or copy-pasting of digital images was performed.
RESULTS
Morphological and chemical characteristics of spines in the hippocampus at 3, 6, and greater than 18 months
Drebrin A immunoreactivity in the hippocampus
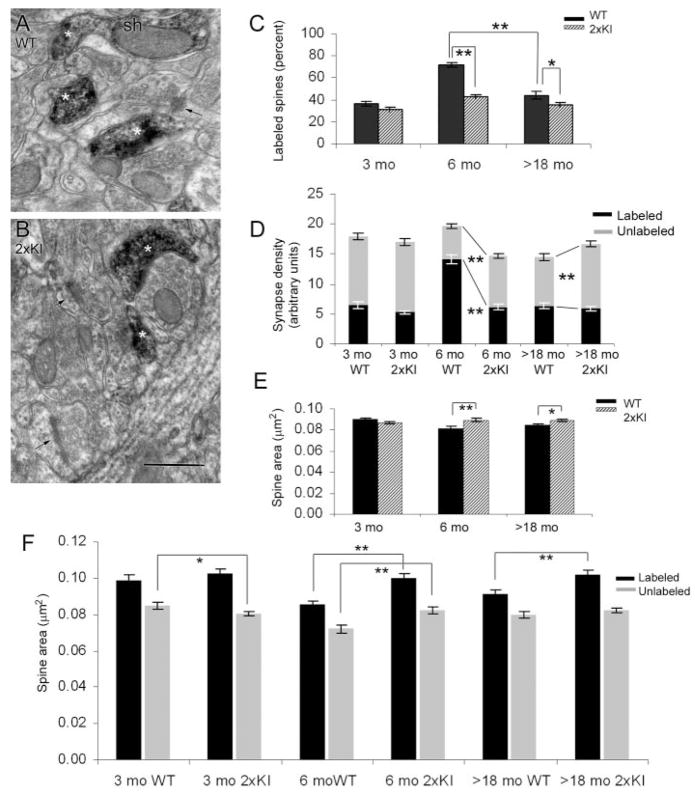
In the hippocampus, the presence of drebrin A was assessed by using an anti-drebrin A antibody and HRP-DAB as the immunolabel. As was observed previously for regions elsewhere within mouse brains, drebrin A immunolabeling in both the WT and 2xKI hippocampi occurred within the cytoplasm of spines forming asymmetric, presumably excitatory synapses (Fig. 1A,B). Drebrin A immunolabeling also occurred in the dendritic shafts (e.g., Fig. 1A). Notably, for both the 2xKI and WT tissues, not all spines were immunoreactive for drebrin A. Thus, for each age and genotype, the proportion of postsynaptic spines in the CA1 field of the hippocampus exhibiting drebrin A immunolabeling was assessed. The outcome of the analysis revealed a significant main effect of genotype (F(1,474) = 45.328; P < 0.0001). The proportion of drebrin A-labeled spines was smaller for the 2xKI brains, compared with the WT brains (Fig. 1C). Post hoc Fisher’s LSD analysis revealed that this difference across genotypes was most prevalent for the 6-month group (_P_ < 0.0001). The proportion of drebrin A-containing spines in WT brains was less at ages 18 months and older (>18 months), compared with the values observed for the WT at 6 months of age. However, the difference across genotypes persisted at >18 months (P < 0.001). No difference was detected across the genotypes at 3 months.
Fig. 1.
Dendritic spines of the hippocampus immunolabeled for drebrin A. A,B: Examples of digitally captured electron micrographs from the stratum radiatum of the CA1 of a WT and a 2xKI mouse, respectively, at the age of 6 months. The tissues were processed to immunolabel for drebrin A, by using HRP-DAB as the electron-dense tag. Asterisks indicate examples of spines that are immunolabeled for drebrin A, and arrows point to unlabeled spines. Sh, example of a dendritic shaft that is contiguous with the labeled spine and is also immunoreactive. C: The proportion of synapses labeled for drebrin A is markedly different at 6 months. The synapses encountered from each age-genotype were divided into multiple groups (at least 60), each consisting of 25 synapses. For each group, the proportion of synapses immunolabeled for drebrin was determined. The graph shows the mean and SEM values of the percent labeled. **, P < 0.0001; *, _P_ < 0.001 (two-way ANOVA). **D:** Spine density difference across genotypes is most prevalent at 6 months. The same sets of synapses analyzed in C were subjected to synapse density measurements. The number of labeled and unlabeled synapses appearing in each micrograph equal to 29 μm2 (arbitrary unit) was determined. The graph represents the mean and SEM values obtained per age-genotype (minimum of 60 micrographs). The dark bars represent the density of labeled synapses per unit area, and the light bars represent the unlabeled synapses per unit area. **, _P_ < 0.0001. **E:** The areas of spines differ the most at 6 months. The same sets of synapses analyzed for data shown in C and D were subjected to spine profile area comparisons across genotypes. The solid bars represent the mean values of spines in the WT brains, and the striped bars represent the mean values of spine sizes in the 2xKI brains. **, _P_ < 0.0001; *, _P_ < 0.05. **F:** Both the labeled and unlabeled spines are larger in the hippocampus of 2xKI mice at 6 months. The same sets of synapses analyzed in C–E were first categorized as labeled or unlabeled spines and then subjected to area comparisons across genotypes. Both the labeled and unlabeled spines exhibit size differences at 6 months. (_P_ < 0.0001). At >18 months, only the drebrin A-labeled spines are larger in the hippocampus of 2xKI mice (P < 0.0001). At 3 months, the unlabeled spines of 2xKI hippocampi are slightly smaller (_P_ < 0.05). The numbers of observations were 1,078 and 2,042 for 3-month WT and 2xKI mice, respectively; 1,177 and 879 for 6-month WT and 2xKI; and 1,306 and 1,507 for >18-month WT and 2xKI mice. For abbreviations, see list. Scale bar = 500 nm in B (applies to A,B).
Density of labeled and unlabeled spines in the hippocampus
A smaller proportion of drebrin A-immunoreactive synapses in the 2xKI hippocampus can result from an increase in spines lacking drebrin A and/or a decrease in the spines containing drebrin A. To identify and differentiate between these two contributing factors, we analyzed the density of labeled and unlabeled spines per unit area of synaptic neuropil separately. A genotype × immunoreactivity two-way ANOVA was performed for each age group. At 3 months, there was no significant difference in the density of spines, labeled or unlabeled, across the two genotypes (Fig. 1D; F(1,356) = 1.75; P >0.05). In the 6-month group, there was a main effect of genotype (F(1,236) = 22.31; P < 0.001), showing a decrease in synapse density for the 2xKI tissue relative to that of age-matched WT tissue. This difference across genotypes was due to a decrease in the density of drebrin A-labeled spines within the 2xKI brains (−56%, _P_ < 0.001, Fisher’s LSD) and a concomitant increase in the density of unlabeled spines in the same synaptic neuropil, which also was significant (+54%, _P_ < 0.001, Fisher’s LSD). In comparison, the older group (>18 months) showed less difference in the density of spines across genotypes, even though the difference was statistically significant (F(1,356) = 4.86; P < 0.05), and the change was in the opposite direction (greater synapse density in the 2xKI neuropil, compared with the WT neuropil). The increased synapse density in 2xKI tissue was brought about by the increase in the density of drebrin-negative spines (+32%, P < 0.0001).
For comparisons of the density of labeled spines across ages, one-way ANOVA with age-genotype condition as the independent factor was performed. This test verified an interaction of age-genotype upon the density of labeled spines (F(5,474) = 47.39; P < 0.0001). Post hoc analysis using Fisher’s LSD showed a 117% difference for the WT animals between 3 and 6 months (_P_ < 0.001), reflecting an increase as the animal continues to mature (Fig. 1D). This chronological pattern was greatly reduced among the 2xKI brains, which showed only a small (17%) increase from 3 to 6 months, a magnitude that did not reach statistical significance (_P_ = 0.14). The densities of labeled spines in the synaptic neuropil of 2xKI hippocampi at 6 months were already like those observed for the >18-month neuropil of the WT and 2xKI genotypes (P = 0.73 and 0.71, respectively), indicating signs of accelerated degeneration or failure to thrive from 3 to 6 months.
Areas of spine profiles in the hippocampus
Assessment of synapse density from two-dimensional (2D) images can be influenced by the sizes of the spines and synapses, because larger spines are captured more readily in single ultrathin sections (Mouton, 2002). We wondered whether the lower rate of encounter with drebrin A-immunoreactive spines within the 6-month 2xKI hippocampus might be due to spine size reduction. This possibility was tested by measuring the area occupied by each spine profile encountered within the same neuropil (i.e., same set of micrographs) used to assess synapse density. This analysis revealed a difference in spine profile area across genotypes for the 6-month group (t = −3.56; P < 0.0001) and a less marked, although still significant difference in the >18-month group (t = −2.30; P < 0.05). However, the reduced density of spines in the 2xKI tissue could not be due to reduction of spine sizes, because for both age groups, the spine profile area was relatively larger within the 2xKI neuropil than within the WT neuropil (Fig. 1E). These findings indicated that the decreased encounter with spines in the 6-month 2xKI neuropil, relative to the 6-month WT neuropil, did reflect a reduction in spine density rather than an increased failure to detect spine profiles. No difference was detectable across the genotypes of the 3-month group.
Area of spines containing and lacking drebrin A
The observed difference in the size of spines was an unexpected result. Earlier work on the cortex had shown that spines containing drebrin A are, on average, larger than those lacking drebrin A (Fujisawa et al., 2006; Kobayashi et al., 2007). Based on the observation that drebrin A-containing spines were less numerous in the hippocampus of 2xKI mice, we had predicted that spines contained within these tissues would be smaller. In order to determine whether there might be a reduction in the size of spines specific to those lacking drebrin A, we next examined areas of drebrin A-labeled and drebrin A-unlabeled spines separately (Fig. 1F). We first confirmed earlier observations, namely, that for each age-genotype group, the drebrin A-immunoreactive spines are larger than the spines lacking drebrin A immunoreactivity (two-way ANOVA, (F(1,5) = 183.75; P < 0.0001). More importantly, post hoc analysis using Fisher’s LSD showed that in the 6-month 2xKI neuropil, both drebrin A-immunoreactive and -negative spines were larger than those in the 6-month WT neuropil (_P_ < 0.0001 for both). Such differences in spine size across genotypes were also observed for the drebrin A-immunoreactive spines of the >18-month group (P < 0.0001). In contrast, the 3-month group of the 2xKI hippocampus showed a significant but relatively smaller decrease in the area of unlabeled (but not the labeled) spines, relative to those of WT hippocampus (P < 0.05 for the unlabeled group of spines). Together, these observations indicated that spines of both varieties—those with and those without drebrin A— began to exhibit subtle differences in the synaptic neuropil by 6 months, with the 2xKI hippocampi containing fewer but larger spines.
Morphological and chemical characteristics in the 6-month entorhinal cortex
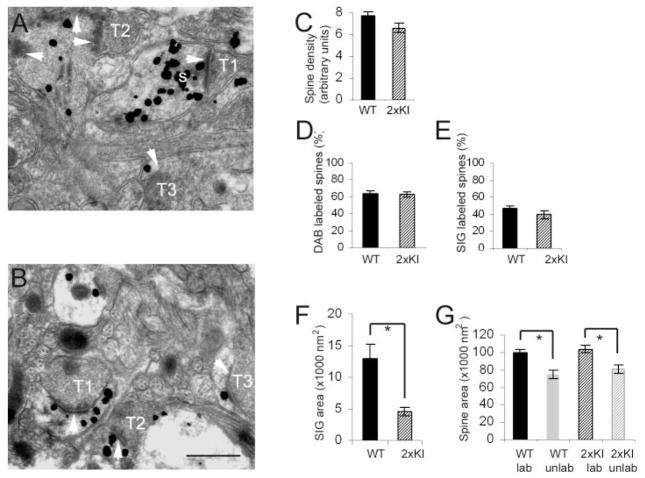
All of the analyses described above pointed to 6 months as the approximate age at which the effect of double knock-in begin to emerge. To address whether these changes are also evident in the entorhinal cortex by 6 months, we first assessed whether spines in the entorhinal cortex contain drebrin A. Electron microscopic visualization of the entorhinal cortex at 6 months revealed robust drebrin A labeling within spines and dendritic shafts of both the WT and 2xKI brains (Fig. 2A,B). Thus, we proceeded with analyses of spine density, spine size, and drebrin A immunoreactivity within postsynaptic spines.
Fig. 2.
At 6 months, spines of the WT entorhinal cortex contain greater amounts of drebrin A immunoreactivity compared with 2xKI spines. A,B: Examples of spine labeling in layer 1 of the entorhinal cortex of a WT and a 2xKI animal, respectively. The large maximally electron-dense particles, such as the one tagged with a white letter s in A, represent silver-intensified gold (SIG) immunolabeling for drebrin A. White arrows point to the postsynaptic densities (PSD). T1, T2, and T3 are presynaptic axon terminals. C: Mean and SEM values of the number of postsynaptic spines per unit area (spine density). The samples were taken from Vibratome sections that were semi-adjacent to the ones shown in A and B. These tissues were not immunolabeled for drebrin A and were postfixed by using the osmium procedure. Altogether, 514 spines from WT tissue and 484 spines from 2xKI tissue were encountered within fields of equal total area (30 micrographs × 29 μm2 per micrograph shot at a magnification of 25,000× = 870 μm2) for each animal. We detected no difference across the genotypes (t = 1.09, P >0.05). D: Mean and SEM values of the proportion of spines encountered from tissue that underwent drebrin A immunolabeling by the HRP-DAB procedure. For each animal, a minimum of 30 micrographs at a magnification of 25,000× were captured to sample 15–17 groups of 25 spines (375 spines per animal), for assessing the proportion of spines immunolabeled for drebrin A. We detected no difference across the genotypes (t = 0.21, P >0.05). E–G: Mean and SEM values of three types of measurements made from Vibratome sections that underwent the SIG immunolabeling procedure to detect drebrin A. E: Proportion of postsynaptic spines with drebrin A immunoreactivity. Sixteen groups of 10 synapses were sampled from the WT tissue, and 17 groups of 10 synapses were sampled from the 2xKI tissue. F: Mean and SEM values of area occupied by SIG particles within each labeled spine encountered. G: Cytoplasmic area captured within individual spine profiles within single 2D images that were labeled and unlabeled. Asterisks indicate statistical significance (P < 0.001) by Student’s t-test in F and two-way ANOVA in G. For abbreviations, see list. Scale bar = 500 nm in B (applies to A,B).
Spine density in the entorhinal cortex
Ultrastructural analysis of spine density was performed as described for the hippocampus, by using two sources of Vibratome sections; one set had undergone drebrin A immunocytochemistry, and a second set was not immunolabeled. Regardless of the tissue source, the entorhinal cortex exhibited no difference in the density of spines across the two genotypes at 6 months (t = 1.09; P >0.05 for tissue that had not undergone drebrin A immunocytochemistry [Fig. 2C]; t = −0.001; P >0.05, for tissue that had undergone drebrin A immunocytochemistry).
Drebrin A immunoreactivity within spines of the entorhinal cortex
For the analysis of the proportion of spines containing drebrin A, we first used HRP-DAB as the immunolabel, so as to optimize detection of drebrin A. We detected no difference in the percent of spines immunolabeled for drebrin A (Fig. 2D, t = 0.21; P >0.05). This assessment was confirmed by using SIG as the label (t = 1.35; P >0.05 [Fig. 2E]). As was observed for the hippocampus of both the WT and 2xKI brains, the spines of the entorhinal cortex of both genotypes containing drebrin A were consistently larger than those lacking drebrin A, whether we used SIG (Fig. 2G, main effect for drebrin A labeling, F(1,347) = 54.31; P < 0.0001) or HRP-DAB (_F_(1, 855) = 31.395; _P_ < 0.0001) as the immunolabel. However, unlike the hippocampus, the mean area of drebrin A-immunoreactive spines of entorhinal cortex from 2xKI brains was not significantly different from the that of the labeled spines of the WT brains, whether we used SIG or HRP-DAB to detect drebrin A (_P_ >0.05, Fisher’s LSD).
Drebrin A immunoreactivity within spines of the entorhinal cortex
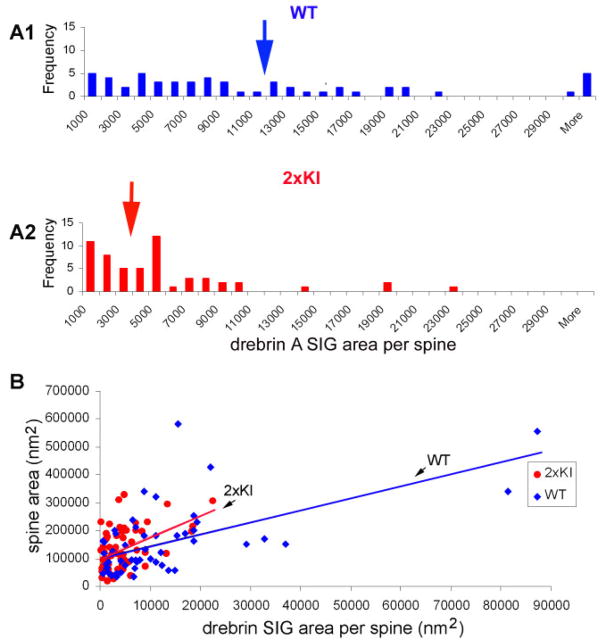
We used SIG area to quantify the relative level of drebrin A immunoreactivity within individual spines. For both genotypes, drebrin A immunoreactivity within spines varied by more than 40-fold. Specifically, the SIG area occupying the spine cytoplasm ranged from 187 nm2 to 87,282 nm2. We detected a large difference across genotypes in drebrin A immunoreactivity within the labeled spines (Fig. 2F, t = 3.46; P < 0.001, Student’s t-test). Analysis of the frequency of spines containing varying levels of drebrin A immunoreactivity indicated that the differences in drebrin A immunoreactivity across the genotypes was due to a shift in the population of spines from the ones with extremely high levels of drebrin A immunoreactivity (>12,000 nm2 within a single spine) to those with lower levels of drebrin A immunoreactivity (< 6,000 nm2; Fig. 3A1,A2). The entorhinal cortex of both genotypes exhibited wide variation in spine profile areas as well as drebrin A immunoreactivity (Fig. 3B). These two parameters were positively correlated for both WT and 2xKI (r = 0.5249 and 0.4071, respectively; P < 0.05 for both).
Fig. 3.
Spines with low levels of drebrin A immunoreactivity are more numerous in the entorhinal cortex of 2xKI brains. SIG immunolabeling for drebrin A indicates that the levels of drebrin A can vary by more than 40-fold. A1,A2: Outcome of a frequency analysis performed to compare the relative number of spines containing varying levels of drebrin A immunoreactivity. Levels of drebrin A within spines was quantified by measuring the area occupied by SIG particles, using Image J software. This analysis indicates that, in the 2xKI entorhinal cortex, spines containing low levels of drebrin A immunoreactivity are more numerous than those containing high levels of drebrin A immunoreactivity. In contrast, the entorhinal cortices of WT brains contain a more even distribution of spines across the range of drebrin A immunoreactivity. B: Drebrin A immunoreactivity correlates weakly with dendritic spine size (r = 0.524887 for WT, 0.407118 for 2xKI, P < 0.05). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Our previous studies of 2xKI mice indicated that their inability to update spatial memory becomes evident at around 9–12 months, whereas the earliest signs of decrement in synaptic plasticity are detectable around 7–8 months (Chang et al., 2006). This study sought to determine whether these decrements in synaptic and behavioral plasticity may be dictated by limitations in the synaptic structure within two brain regions recognized to be of particular importance for learning and memory—the hippocampus and the entorhinal cortex. By 6 months, changes in the levels of drebrin A immunoreactivity (and presumably of drebrin A protein levels) become detectable specifically within postsynaptic spines of these two brain regions. The significance of these changes is discussed below.
Regional differences in drebrin A distribution among spines
Although both the hippocampus and entorhinal cortex exhibit lower levels of drebrin A immunoreactivity within spines of 2xKI animals relative to those of age-matched WT animals, closer inspection by using an electron microscope revealed that the two regions differ subtly in their pattern of drebrin A decrement. In the hippocampus, the decrease of drebrin A manifested as a decrease in the proportion of spines that are drebrin A immunoreactive. In contrast, the entorhinal cortex showed no detectable difference in the proportion of spines immunoreactive for drebrin A. However, the use of discrete silver-intensified gold particles (SIG) allowed us to detect lower levels of drebrin A immunoreactivity within individual spines. The more subtle differences across the genotypes in the entorhinal cortex at 6 months suggest that the change in spinous drebrin A emerges first as a decline (or a failure to increase in level) but not a complete loss. In the hippocampus, the loss of drebrin A may be more drastic, leading to the emergence of spines that have become “emptied” of drebrin A to a level that is undetectable, even by the most sensitive labeling method currently available for immunodetection, i.e., the avidin-biotin complex with HRP-DAB as the tag. The increased density of unlabeled spines that we measured for the hippocampus from 3 to 6 months supports this idea. The lower density of labeled spines in the 2xKI hippocampus suggests that spines are not only losing drebrin A but may be disappearing altogether. Alternatively, it is possible that sAb is beginning to have its effect as early as 3 months, by suppressing the program that normally increases spines with drebrin A between 3 and 6 months. Future studies that correlate drebrin A level to the half-life and motility of spines would be able to test whether drebrin A contributes to the structural stability of spines.
The SIG-based labeling procedure that we used for the entorhinal cortex revealed that the reduction in the average level of drebrin A within spines of 2xKI mice reflects the loss of spines with extremely high levels of drebrin A (i.e., more than 40-fold above the threshold level for detection) and a concomitant increase in the number of smaller spines with lower levels of drebrin A. The results obtained from spine density measurements of the entorhinal cortex indicate that spines here are unlike those in the hippocampus in that they are not yet disappearing. However, it is possible that these spines are exhibiting signs that lead to their disappearance as the animal attains the age at which the inability to update spatial memory becomes apparent. As was proposed for the hippocampus, it is possible that sAb is beginning to manifest its effect in the entorhinal cortex by 3 months by suppressing the normal program to increase drebrin A level within individual spines.
Although both the hippocampus and entorhinal cortex of 2xKI mice exhibit decreases of drebrin A within spinous portions of dendrites by 6 months, this is not the pattern observed globally. In our hands, the somatosensory cortex at 6 months shows no decrease in the density of spines containing drebrin A or of the levels of drebrin A immunoreactivity within individual spines (Mahadomrongkul, 2005). Such differences across brain regions in drebrin A levels were noted recently by Counts et al. (2006), who compared drebrin A levels across brain regions of patients diagnosed with mild cognitive impairment (MCI) as well as at the end stages of AD. Previous studies have also noted that limbic areas are prone to earlier and more severe degeneration, when compared with the somatosensory and motor regions of cortex (Braak and Braak, 1991). Our analysis of the 2xKI model indicates that the changes in drebrin A levels that were measured previously from homogenates relate to levels that are specific to postsynaptic spines and can predate the loss of spines.
It is not yet known why some regions degenerate more or earlier than others, but one hypothesis is that the culprit of the disease—sAb—binds to and then accumulates near nicotinic acetylcholine receptors containing the α7 subunits, which are more abundant in the limbic cortex and hippocampus than other cortical regions (D’Andrea and Nagele, 2006). It is possible that, even in the healthy stage, these receptors tend to occur near excitatory synapses more often within the limbic cortex and hippocampus, relative to regions that are spared degeneration until later ages. An electron microscopic immunocytochemical study that systematically compares the localization of nicotinic receptors in relation to excitatory synapses across the brain regions would be one way to test the validity of this idea. It would also be important to learn whether sAb occurs extracellularly or intracellularly.
The hippocampus but not the cortex exhibits changes in spine size
Although smaller profile sizes can cause underestimation in the counting of profiles (Mouton, 2002), the decreased density of spines we observed within the neuropil of 2xKI hippocampi cannot be explained by a reduction in spine sizes. On the contrary, the mean spine size was greater for the 2xKI hippocampi, when compared with age-matched WT hippocampi.
A number of studies, including ours, have documented a trend that is the opposite of what we have just described here. Specifically, what had been observed previously is that spines containing drebrin A tend to be larger. For example, the overexpression of drebrin A within cultured neurons causes spines to become unusually large (Hayashi and Shirao, 1999). Ultrastructural analysis of the rat somatosensory cortex and mouse entorhinal cortex also showed that spines containing drebrin A are, on average, larger than those without (Fujisawa et al., 2006; Kobayashi et al., 2007). Our current observation agrees, in part, with these previous studies, because we observed a weak correlation between drebrin A levels and spine sizes (in the entorhinal cortex), and the average areas of spines containing drebrin A were greater than those without (for both regions). Thus, what we observed for the 2xKI hippocampus, namely, a reduced proportion of spines with drebrin A, accompanied by an increase in the average area of individual spine profiles, was contrary to our expectation. Moreover, the prevalence of larger spine sizes was detected for spines lacking drebrin A as well as those containing drebrin A. These patterns indicate that, in adult hippocampus, drebrin A level influences but does not dictate spine size.
What might be the factor(s) influencing spine sizes? One possibility is sAb, whose presence becomes detectable within homogenates of 2xKI brains by 2 months and will have risen fourfold by 6–9 months. However, a recent in vitro study reports on a decrease (not an increase) in the size of dendritic spine, following exposure of hippocampal neurons to picomolar concentrations of sAb for 1–2 hours (Calabrese et al., 2007; Shankar et al., 2007). The same study also reports on this decrease being transient: another and perhaps opposite response might be expected following chronic exposure to sAb, as the sAb causes desensitization of nicotinic receptors containing α7 subunits.
Besides this link of drebrin A to spine size, drebrin A has also been proposed to play a role in regulating the trafficking of synaptic molecules. Downregulation of drebrin A within cultured hippocampal neurons leads to the loss of activity-dependent trafficking of NMDAR subunits into spines (Takahashi et al., 2005). Conversely, an acute pharmacological manipulation that induces an increase of NR2A subunits into spines (Aoki et al., 2003) also leads to an increase in the proportion of spines containing drebrin A, without inducing measurable increases in spine size or lengths of postsynaptic densities (Aoki et al., 2003; Fujisawa et al., 2006). Studies that determine the robustness of activity-dependent trafficking of synaptic molecules within spines of 2xKI mice with decreased levels of drebrin A would be helpful for testing this idea.
Measurement of the area of individual spine profiles also had the utility of allowing us to speculate about the abundance of glutamate receptors within spines. Earlier results obtained from electron microscopic immunocytochemistry have shown that the diameter of the active zone of axospinous junctions correlates well with the abundance of AMPA type of glutamate receptors (Matsubara et al., 1996; Kharazia and Weinberg, 1999; Matsuzaki et al., 2001). Within in vitro slices, synapse strengthening that follows LTP induction is achieved by an increased delivery of AMPA receptor subunits to the synaptic membrane (Shi et al., 1999; Rumpel et al., 2005), and this change is accompanied by increased volume of spines (Matsuzaki et al., 2004; Park et al., 2006). It has been noted that smaller spines are more amenable to LTP and spine enlargement, whereas larger spines are morphologically more stable (Trachtenberg et al., 2002; Matsuzaki et al., 2004; Zuo et al., 2005). When these observations have been put together (for review, see Hayashi and Majewska, 2005; Segal, 2005), it has been hypothesized that larger spines are sites for memory storage, whereas smaller spines are sites for acquisition of new memory (Kasai et al., 2003).
Prompted by these ideas, we reasoned that the cumulative, activity-dependent modifications of synaptic strengths may be reflected in the size of individual postsynaptic spine profiles that we measured from 2D images of spines. The relative scarcity in the number of small spines in the hippocampus of 2xKI mice indicates that fewer of their synapses may be available to become engaged in the formation of new, labile memory. Synapse density in the hippocampus of 2xKI mice at 6 months is lower than the densities observed in age-matched WT hippocampi and is similar to that observed by 18 months in both genotypes. Similarly, the average size of spines that we observed for the hippocampus of 2xKI mice at 6 months is greater than that of age-matched WT hippocampi but is similar to those of the 18-month hippocampi of both genotypes. What we have observed in the 2xKI hippocampi at 6 months may be the cellular signatures and structural constraints that are accelerated by the presence of sAb, leading to excessive stability of spines and the animals’ inability to update spatial memory, as was noted from the behavioral tests of the 2xKI mice (Chang et al., 2006), other animal models of FAD (Moolman et al., 2004; Trinchese et al., 2004; Ryman and Lamb, 2006; Shen and Kelleher, 2007), and AD patients (Braak and Braak, 1991; Selkoe, 2002).
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: 1P30EY13079, R01-NS41091, and R01-EY13145 (all to C.A.); Grant sponsor: Research Challenge Fund of New York University (to C.A.).
We thank Cephalon (West Chester, PA) for providing us with the 2xKI mutant mice and the WT mice matched in age and background. We thank Hilda Pena Fernandez for her technical assistance and intellectual input in the data analyses.
Abbreviations
2xKI
double knock-in
Ab
amyloid beta
AD
Alzheimer’s disease
AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
APP
amyloid precursor protein
CNS
central nervous system
HRP-DAB
horseradish peroxidase-3,3′-diaminobenzidine HCl
LSD
least squares design
LTP
long-term potentiation
MCI
mild cognitive impairment
mEPSCs
miniature excitatory postsynaptic currents
NMDA
_N_-methyl-D-aspartate
sAb
soluble amyloid beta
PS1
presenilin 1
SIG
silver-intensified colloidal gold
WT
wildtype
LITERATURE CITED
- Aoki C, Fujisawa S, Mahadomrongkul V, Shah PJ, Nader K, Erisir A. NMDA receptor blockade in intact adult cortex increases trafficking of NR2A subunits into spines, postsynaptic densities, and axon terminals. Brain Res. 2003;963:139–149. doi: 10.1016/s0006-8993(02)03962-8. [DOI] [PubMed] [Google Scholar]
- Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, Shirao T. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol. 2005;483:383–402. doi: 10.1002/cne.20449. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Shaked GM, Tabarean IV, Braga J, Koo EH, Halpain S. Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-beta protein. Mol Cell Neurosci. 2007;35:183–193. doi: 10.1016/j.mcn.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. AMPA receptor downscaling at the onset of Alzheimer’s disease pathology in double knockin mice. Proc Natl Acad Sci U S A. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JJ, Shatz CJ. Redistribution of synaptic vesicle antigens is correlated with the disappearance of a transient synaptic zone in the developing cerebral cortex. Neuron. 1988;1:297–310. doi: 10.1016/0896-6273(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- D’Andrea MR, Nagele RG. Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer’s disease pyramidal neurons. Curr Pharm Des. 2006;12:677–684. doi: 10.2174/138161206775474224. [DOI] [PubMed] [Google Scholar]
- Flood DG, Reaume AG, Dorfman KS, Lin YG, Lang DM, Trusko SP, Savage MJ, Annaert WG, De Strooper B, Siman R, Scott RW. FAD mutant PS-1 gene-targeted mice: increased A beta 42 and A beta deposition without APP overproduction. Neurobiol Aging. 2002;23:335–348. doi: 10.1016/s0197-4580(01)00330-x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; 1996. [Google Scholar]
- Fujisawa S, Shirao T, Aoki C. In vivo, competitive blockade of N-methyl-D-aspartate receptors induces rapid changes in filamentous actin and drebrin A distributions within dendritic spines of adult rat cortex. Neuroscience. 2006;140:1177–1187. doi: 10.1016/j.neuroscience.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya Y, Shoji M, Shirao T, Hirai S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer’s disease. J Neurosci Res. 1996;43:87–92. doi: 10.1002/jnr.490430111. [DOI] [PubMed] [Google Scholar]
- Hatanpaa K, Isaacs KR, Shirao T, Brady DR, Rapoport SI. Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:637–643. doi: 10.1097/00005072-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Shirao T. Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci. 1999;19:3918–3925. doi: 10.1523/JNEUROSCI.19-10-03918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Majewska AK. Dendritic spine geometry: functional implication and regulation. Neuron. 2005;46:529–532. doi: 10.1016/j.neuron.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J Comp Neurol. 1999;412:292–302. doi: 10.1002/(sici)1096-9861(19990920)412:2<292::aid-cne8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kobayashi CA, Aoki C, Kojima N, Yamazaki H, Shirao T. Drebrin A content correlates with spine head size in the adult mouse cerebral cortex. J Comp Neurol. 2007;503:618–26. doi: 10.1002/cne.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima NaST. Synaptic dysfunction and disruption of postsynaptic drebrin-actin complex: a study of neurological disorders accompanied by cognitive deficits. J Neurosci Res. 2007;58:1–5. doi: 10.1016/j.neures.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Mahadomrongkul V, Huerta PT, Shirao T, Aoki C. Altered distribution of drebrin A in spines of sensory cortex layer I of mice expressing mutated APP and PS1 genes. Brain Res. 2005;1064:66–74. doi: 10.1016/j.brainres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Neurons of layer I: a developmental analysis. In: Peters A, Jones EG, editors. Cerebral cortex, vol. 1: Cellular components of the cerebral cortex. New York: Plenum; 1984. pp. 447–478. [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in Alzheimer models. J Neurocytol. 2004;33:377–387. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology: an introduction for bioscientists. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phend KD, Rustioni A, Weinberg RJ. An osmium-free method of Epon embedment that preserves both ultrastructure and antigenicity for post-embedding immunocytochemistry. J Histochem Cytochem. 1995;43:283–292. doi: 10.1177/43.3.7532656. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Ryman D, Lamb BT. Genetic and environmental modifiers of Alzheimer’s disease phenotypes in the mouse. Curr Alzheimer Res. 2006;3:465–473. doi: 10.2174/156720506779025198. [DOI] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shim KS, Lubec G. Drebrin, a dendritic spine protein, is manifold decreased in brains of patients with Alzheimer’s disease and Down syndrome. Neurosci Lett. 2002;324:209–212. doi: 10.1016/s0304-3940(02)00210-0. [DOI] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Mikoshiba K, Furuichi T. Coincidence in dendritic clustering and synaptic targeting of homer proteins and NMDA receptor complex proteins NR2B and PSD95 during development of cultured hippocampal neurons. Mol Cell Neurosci. 2003;22:188–201. doi: 10.1016/s1044-7431(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Shirao T, Sekino Y. Clustering and anchoring mechanisms of molecular constituents of postsynaptic scaffolds in dendritic spines. Neurosci Res. 2001;40:1–7. doi: 10.1016/s0168-0102(01)00209-7. [DOI] [PubMed] [Google Scholar]
- Shirao T, Kojima N, Obata K. Cloning of drebrin A and induction of neurite-like processes in drebrin-transfected cells. Neuroreport. 1992;3:109–112. doi: 10.1097/00001756-199201000-00029. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Sekino Y, Tanaka S, Mizui T, Kishi S, Shirao T. Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J Neurosci. 2003;23:6586–6595. doi: 10.1523/JNEUROSCI.23-16-06586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Mizui T, Shirao T. Down-regulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurones. J Neurochem. 2005;97(Suppl 1):110–5. doi: 10.1111/j.1471-4159.2005.03536.x. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]