Inclusion of a Nitric Oxide Congener in the Insufflation Gas Repletes S‐Nitrosohemoglobin and Stabilizes Physiologic Status During Prolonged Carbon Dioxide Pneumoperitoneum (original) (raw)
Abstract
A method to maintain organ blood flow during laparoscopic surgery has not been developed. Here we determined if ethyl nitrite, an _S_‐nitrosylating agent that would maintain nitric oxide bioactivity (the major regulator of tissue perfusion), might be an effective intervention to preserve physiologic status during prolonged pneumoperitoneum. The study was conducted on appropriately anesthetized adult swine; the period of pneumoperitoneum was 240 minutes. Cohorts consisted of an anesthesia control group and groups insufflated with CO2 alone or CO2 containing fixed amounts of ethyl nitrite (1–300 ppm). Insufflation with CO2 alone produced declines in splanchnic organ blood flows and it reduced circulating levels of _S‐_nitrosohemoglobin (i.e., nitric oxide bioactivity); these reductions were obviated by ethyl nitrite. In a specific example, preservation of kidney blood flow with ethyl nitrite kept serum creatinine and blood urea nitrogen concentrations constant whereas in the CO2 alone group both increased as kidney blood flow declined. The data indicate ethyl nitrite can effectively attenuate insufflation‐induced decreases in organ blood flow and nitric oxide bioactivity leading to reductions in markers of acute tissue injury. This simple intervention provides a method for controlling a major source of laparoscopic‐related morbidity and mortality: tissue ischemia and altered postoperative organ function.
Keywords: surgery, nitric oxide, regional blood flow
Introduction
Recent advances in operative procedures and surgical instrumentation have enabled the application of laparoscopic techniques to more complex surgeries. However, complex procedures require extended insufflation times (typically greater than 3 hours) 1 , 2 , 3 , 4 , 5 , 6 and consequently patients undergo prolonged periods of elevated intra‐abdominal pressure. And while the benefits of using minimally invasive techniques for longer procedures are presumed to be similar to those obtained for “simple” laparoscopic surgeries, it is now recognized that carbon dioxide (CO2) pneumoperitoneum and the resultant rise in intra‐abdominal pressure can produce reductions in organ blood flow, acidosis, and alterations in cardiovascular and respiratory status, 5 , 6 , 7 , 8 , 9 , 10 all of which can impact postoperative organ function and patient recovery; the severity of these effects correlates to the duration of insufflation. 11
To some extent, the acidosis and systemic effects of pneumoperitoneum can be controlled by altering ventilation rates and/or administration of vasoactive agents but such interventions have minimal ability to preserve end‐organ blood flow and oxygen delivery. This is because local tissue blood flow, rather than blood oxygen content, is the primary determinant of oxygen delivery. Local tissue perfusion is regulated by a physiological response termed hypoxic vasodilation in which tissue oxygen requirements are directly coupled to blood flow, the domain of nitric oxide (NO) bioactivity. 12 Second to second changes in microcirculatory flow are controlled by complex interactions between oxygen, NO, and hemoglobin (Hb) within the red blood cell with Hb serving as an oxygen sensor and as a hypoxia‐responsive transducer of NO signals. 13 , 14 Vasodilation by _S_‐nitrosoHb (SNO‐Hb; i.e., release of NO bioactivity) is linked to Hb desaturation and provides a regulated mechanism for matching blood flow and oxygen delivery with local metabolic demand. 15
Decreased levels and/or impaired processing of SNO‐Hb have been observed in disparate diseases characterized by tissue hypoxemia; 16 , 17 , 18 , 19 , 20 , 21 , 22 where examined, red blood cells from these patients exhibited impaired vasodilatory capacity. Such data suggest that red blood cell derived NO bioactivity plays an important role in the respiratory cycle and that impairment of this activity might contribute to the pathophysiology of ischemic conditions. Based on these findings, we reasoned that insufflation‐induced reductions in splanchnic blood flow may be due, atleast in part, to alterations (reductions) in SNO‐Hb homeostasis. By extension, an intervention directed toward increasing NO bioactivity could potentially ameliorate pneumoperitoneum‐induced reductions in organ blood flow. Theoretical support for this hypothesis comes from the observations that reductions in blood pH, as occur during CO2 pneumoperitoneum, accelerate SNO‐Hb decay 23 , 24 and increased mechanical ventilation (as may be initiated to control hybercarbia) increases the concentration of exhaled NO 25 both of which could decrease circulating SNO‐Hb levels. Empiric support for this hypothesis comes from our previous proof‐of‐concept study where we determined that inclusion of the _S_‐nitrosylating agent ethyl nitrite (ENO) within the insufflating gas maintained liver blood flow during 60 minutes of CO2 insufflation; in the CO2 alone group, liver blood flow decreased by >30%. 26
The goals of the present study were to monitor circulating SNO‐Hb levels during insufflation and then extend our initial positive finding with ENO to the setting of prolonged pneumoperitoneum. Using swine, we characterized the organ blood flow, hemodynamic, and humoral responses to 4 hours of CO2 insufflation and then repeated these assessments in separate cohorts insufflated with a wide range of ENO concentrations (from 1 to 300 ppm) to identify the optimal ENO dose for preserving physiologic status.
Methods
Overview
All aspects of the study design were approved by the Institutional Animal Care and Use Committee of Duke University. Experiments were conducted on young adult swine of both genders weighing between 35 and 40 kg. Animals were housed individually and were allowed ad libitum access to food and water except for a 12‐to 14‐hour fasting period prior to the surgical procedure; water access was not restricted. On the day of experimentation, swine were serially assigned to one of the following experimental groups: anesthesia alone (basal control group, no insufflation); CO2 alone (active control group); or CO2 gas containing 1, 10, 50, 100, 200, or 300 ppm ENO. Pressurized gas cylinders containing these fixed concentrations of ENO blended with CO2 were prepared and supplied by Custom Gas Solutions (Durham, NC, USA).
Surgical instrumentation
Animals were sedated with intramuscular injections of acepromazine (0.8 mg/kg) and ketamine (20 mg/kg); atropine (0.04 mg/kg) was given to reduce mucosal secretions. Venous access was established (ear vein) and then surgical anesthesia was induced in each pig with an intravenous injection of thiopental (10 mg/kg). After endotracheal intubation, surgical anesthesia was maintained with isoflurane (1.5 to 2%) in oxygen delivered by a Narkomed 2B ventilation system (North American Dräger, Telford, PA, USA). Tidal volume was set between 10 and 15 mL/kg and the ventilation rate (15–20 per minute) adjusted to maintain EtCO2 below 35 mmHg throughout the experiment. Isoflurane and EtCO2 concentrations were continually measured by an airway gas monitor (Datex Instrumentation Corporation, Helsinki, Finland). Body temperature was monitored with an esophageal probe and maintained between 37.5 and 38.5 °C with the use of a Bair Hugger body warming system (Arizant Healthcare Incorporated, Eden Prairie, MN, USA). Supportive fluid therapy was provided throughout the study (intravenous lactated ringers at 10 mL × kg−1× hour−1).
Both femoral arteries were revealed and catheterized with 8.5 Fr catheters and one of the femoral veins was also catheterized for obtainment of venous blood samples. The right femoral catheter was advanced into the left ventricle (position verified by typical pressure tracing) for subsequent microsphere injections whereas the left femoral catheter was advanced into the abdominal aorta and used for withdrawal of arterial blood reference samples. The right brachial artery was isolated and catheterized with a 16 G catheter; this was used to continually record heart rate and blood pressure. For the cohort used to quantitate changes in SNO‐Hb concentration, only single site arterial and venous access was obtained as microspheres were not administered.
Experimental procedure
After the monitoring instrumentation was completed, four 12 mm laparoscopic ports were placed under direct vision into the peritoneal cavity and the animals were allowed to stabilize before insufflation. The experiment consisted of a 30‐minute baseline period (without pneumoperitoneum) in which basal blood flow was determined. With the exception of the anesthesia control group, the peritoneal cavity was next insufflated to a final intra peritoneal pressure of 15 mmHg with CO2 in the presence or absence of ENO at a rate of 1.0 L/min using a high‐flow clinical insufflator (Stryker Endoscopy, Santa Clara, CA, USA). Pneumoperitoneum was maintained for 4 hours during which gas was constantly vented from the peritoneal cavity to ensure continual CO2 and ENO exposure. after 4 hours, insufflation was discontinued and the abdomen manually deflated. Monitoring was continued under anesthesia for an additional 2 hours.
Tissue blood flow
Regional tissue blood flow was quantitated at eight experimental time points using stable isotope‐labeled microspheres (15 μm in diameter; BioPhysics Assay Laboratory, Worcester, MA, USA). To increase accuracy, basal tissue blood flow (i.e., prior to insufflation) was determined twice (10 minutes apart) while the other determinations were done at hourly intervals. For each time point, approximately 10 million microspheres were administered. Each microsphere solution was sonicated for at least 1 minute and then quickly injected into the left ventricle followed by a 4 mL saline flush. Using an accurate syringe pump (Harvard Apparatus, South Natick, MA, USA), blood was withdrawn from the abdominal aorta at a fixed rate of 6 mL/min. The withdrawal started 10 seconds before each microsphere injection and continued for 100 seconds. These reference blood samples were then prepared following the manufacturer's guidelines. After the final microsphere injection, the animal was euthanized, exsanguinated, and tissue samples were obtained from the organs of interest (brain, heart, liver, kidney, stomach, small intestine, colon, spleen, pancreas). All samples are weighed at the time of extraction and then dried via a warming oven (70˚C, overnight). The neutron activation analysis to quantitate the microsphere concentrations in tissue and blood was performed by BioPhysics Assay Laboratory.
Additional physiologic analyses
Serial arterial and venous blood samples were collected 5 minutes prior to each microsphere injection. Blood gas parameters were measured using a Gem Premier 3000 (Instrumentation Laboratory, Lexington, MA, USA) and/or a CCX8 blood gas analyzer (Nova Biomedical, Waltham, MA, USA) while mercury‐coupled photolysis‐chemiluminescence was used to quantitate red blood cell SNO‐Hb concentrations. 23 , 26 Additional venous blood samples (10 mL) were collected at baseline, 2 and 4 hours into the insufflation, and 2 hours after desufflation for complete clinical chemistry analyses (Super Chem/CBC, Antech Diagnostic, Cary, NC, USA).
Data presentation and statistical analyses
Blood gases and serum chemistry data are presented as means ± standard deviations (SD). Analysis of variance was used to assess for changes over time within each treatment group (to preserve statistical power, we did not test for inter‐group differences); when the _F_‐statistic was significant, post hoc testing used Dunnet's test to compare change from baseline. For continually recorded parameters, median values were calculated for each animal at 1‐minute intervals and then averaged to obtain group data. Area under the curve calculations were conducted at 60‐minute intervals and then compared for both treatment and time effects using analysis of variance. Tissue blood flow (mL × min−1× g−1) and cardiac output (mL/min) were calculated per the manufacturer's guidelines based on the number of disintegrations recorded in the tissues and reference samples for each type of microsphere. For most organs, flow was compared between the average blood flow before and during pneumoperitoneum using paired _t_‐tests. Additional assessments of change in kidney blood flow and cardiac output over time utilized analysis of variance. For all endpoints, p values of < 0.05 were considered significant.
Results
SNO‐Hb and insufflation
Changes in venous red blood cell SNO‐Hb concentration in response to peritoneal insufflation with CO2 alone (_n_= 6) or CO2 with 50 ppm ENO (_n_= 7) are presented in Figure 1 . The SNO‐Hb concentrations were similar between the two groups prior to insufflation (127 ± 145 and 143 ± 120 nM, respectively; as with humans there was significant interanimal variation in the circulating SNO‐Hb concentrations) but declined in the CO2 alone group as the experiment progressed such that by the 240 minute mark SNO‐Hb concentration had decreased by approximately 50%. In contrast, inclusion of 50 ppm ENO in the insufflating gas produced an increase in SNO‐Hb that was sustained for the duration of the experiment.
Figure 1.
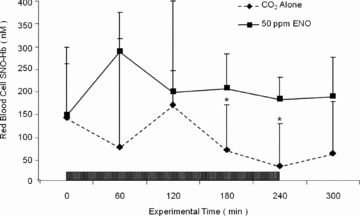
Time course of red blood cell _S‐_nitrosohemoglobin (SNO‐Hb) concentrations during and after insufflation with CO2 (_n_= 6; hatched line) or CO2 plus 50 ppm ethyl nitrite (ENO; _n_= 7; solid line). Intra‐abdominal pressure was kept at 15 mmHg during the 240‐minute period of insufflation (box). Data are expressed as mean ± standard deviation. SNO‐Hb concentrations in the CO2 alone group were significantly different from those in the 50 ppm ENO cohort at the 180 and 240 minute marks (p < 0.05).
Blood gas status
Arterial and venous blood gas values for the anesthesia control group, CO2 alone, and CO2 with 1 or 10 ppm ENO are presented in Figure 2 . As noted in the methods, ventilation was actively regulated to keep EtCO2 below 35 mmHg. Despite this intervention, reductions in pH and increases in pCO2 were recorded for all of the insufflation groups. Similar degrees of acidosis and hypercarbia were observed in the cohorts insufflated with higher concentrations of ENO (50–300 ppm; data not shown). In all experimental groups, arterial and venous pH and pCO2 returned to baseline levels within 60 minutes of desufflation. No group differences in arterial or venous blood oxygen saturation were observed.
Figure 2.
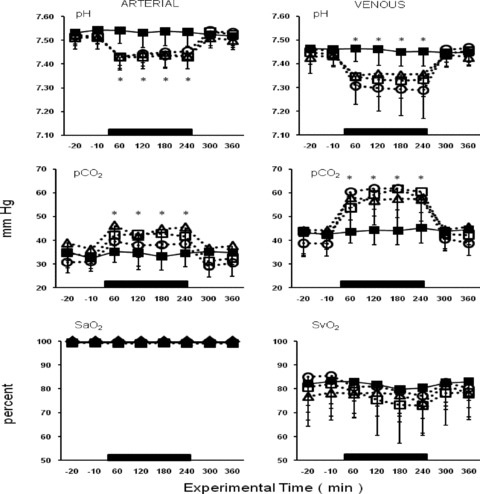
Arterial and venous blood gas values for pH, pCO2, and oxygen saturation recorded during the course of the study for the anesthesia control group (solid square, _n_= 11), the CO2 alone group (open triangle, _n_= 11), and the animals insufflated with either 1 (open square, _n_= 10) or 10 (open circle, _n_= 10) ppm ethyl nitrite; parameters are presented in standard units. The period of insufflation is demarcated by the black rectangle. Animals were well oxygenated during the course of the study but similar degrees of acidosis and hybercarbia occurred in all groups that were insufflated despite maintaining end‐tidal CO2 below 35 mmHg. Time‐points marked with an asterisk (*) indicate where the values for the insufflation groups are significantly different from their respective baseline values (p < 0.05).
Hemodynamics
The time course for three parameters of hemodynamic activity (heart rate, mean arterial pressure, and cardiac output) before, during, and after insufflation are presented in Figure 3 . For the anesthesia control group, all three variables were essentially constant for the duration of the experiment (the fluctuations in cardiac output were not significant). Area under the curve comparisons (taken at 60‐minute intervals) between treatment groups and the anesthesia controls determined that heart rate was increased during and for the first 60 minutes after CO2 insufflation (p < 0.01); insufflation with ENO at all concentrations did not significantly alter heart rate. With respect to mean arterial pressure, the CO2 and anesthesia groups were similar despite the transient decline in pressure that occurred immediately after desufflation (_p_ > 0.05). In contrast, insufflation with 1 or 10 ppm ENO increased mean arterial pressure for the 4‐hour period (p < 0.01) and pressure remained elevated in the 10 ppm group during the 2‐hour postpneumoperitoneum monitoring period. An increase in mean arterial pressure was not observed with higher concentrations of ENO (data not shown). Cardiac output fluctuated in all treatment groups during the course of the study but only in the 10 ppm ENO group was cardiac output significantly higher compared to both its own baseline value and to the anesthesia control group (p < 0.05).
Figure 3.
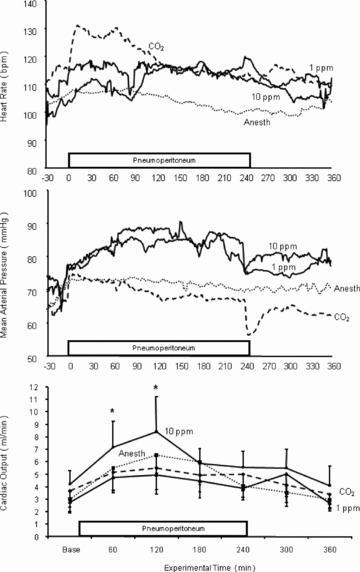
Heart rate, mean arterial pressure, and cardiac output changes during the course of the study for the anesthesia alone group (_n_= 11), the CO2 alone group (_n_= 11), and the animals insufflated with either 1 or 10 ppm ethyl nitrite (both, _n_= 10). Insufflation with CO2 alone produced an initial rise in heart rate that resolved after about 90 minuets; the heart rate changes observed with the other experimental groups were not significant. Mean arterial pressure was elevated for the duration of the study in the 1 and 10 ppm ethyl nitrite treatment groups. In addition, cardiac output increased during insufflation with 10 ppm ethyl nitrite. (See text for a description of the statistical comparisons.)
Organ blood flow
Blood flow values before and during insufflation for several different splanchnic organs are presented in Table 1 . For the anesthesia control group, the only areas where blood flow changed were the GI tract (total gut) and pancreas, which increased as the experiment progressed. For the CO2 insufflation group, blood flow declined within the liver, kidneys, spleen, and adrenals; blood flow was constant in the pancreas; and, similar to the anesthesia cohort, blood flow increased in the GI tract. Overall, the presence of ENO in the insufflating gas obviated these pneumoperitoneum‐induced reductions. Organ blood flow was consistently maintained (kidney, liver, adrenals, spleen) or increased (GI tract, pancreas) by inclusion of 10 ppm ENO in the insufflating gas. For the high doses of ENO (50 ppm and above) this improvement was not observed for all tissue.
Table 1.
Blood flow changes in swine splanchnic organs/tissues before and during insufflation.
| Treatment | Organ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pancreas | Kidney | Liver | |||||||
| Before | During | p | Before | During | p | Before | During | p | |
| Anesthesia | 0.39 ± 0.12 | 0.69 ± 0.45 | 0.021 | 5.16 ± 1.30 | 5.77 ± 1.77 | 0.097 | 1.54 ± 0.75 | 1.56 ± 0.80 | 0.867 |
| CO2 | 0.38 ± 0.14 | 0.44 ± 0.22 | 0.137 | 5.61 ± 1.82 | 4.42 ± 1.85 | 0.019 | 1.32 ± 0.92 | 0.75 ± 0.43 | 0.047 |
| 1 ppm | 0.34 ± 0.09 | 0.40 ± 0.13 | 0.048 | 4.55 ± 1.59 | 4.27 ± 1.28 | 0.349 | 1.68 ± 1.07 | 1.25 ± 0.53 | 0.299 |
| 10 ppm | 0.44 ± 0.23 | 0.59 ± 0.21 | 0.012 | 5.95 ± 2.03 | 6.36 ± 1.46 | 0.458 | 1.50 ± 1.09 | 1.29 ± 0.46 | 0.489 |
| 50 ppm | 0.48 ± 0.53 | 0.61 ± 0.46 | 0.080 | 4.52 ± 0.86 | 3.65 ± 0.98 | 0.001 | 1.02 ± 0.38 | 1.23 ± 0.88 | 0.293 |
| 100 ppm | 0.33 ± 0.16 | 0.46 ± 0.18 | 0.005 | 4.58 ± 1.23 | 4.29 ± 1.12 | 0.377 | 0.85 ± 0.31 | 1.02 ± 0.36 | 0.174 |
| 200 ppm | 0.34 ± 0.43 | 0.43 ± 0.66 | 0.003 | 4.33 ± 3.56 | 3.56 ± 3.67 | 0.003 | 1.09 ± 0.93 | 0.93 ± 0.98 | 0.249 |
| 300 ppm | 0.31 ± 0.15 | 0.39 ± 0.13 | 0.002 | 4.75 ± 1.42 | 4.05 ± 1.12 | 0.035 | 1.28 ± 0.66 | 1.25 ± 0.65 | 0.793 |
| Treatment | Total gut | Spleen | Adrenals | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | During | p | Before | During | p | Before | During | p | |
| Anesthesia | 2.20 ± 0.79 | 2.72 ± 0.85 | 0.015 | 2.42 ± 1.14 | 2.32 ± 1.15 | 0.535 | 2.29 ± 1.08 | 1.96 ± 0.34 | 0.309 |
| CO2 | 2.24 ± 0.37 | 2.86 ± 0.94 | 0.019 | 1.58 ± 0.81 | 0.85 ± 0.52 | 0.001 | 2.53 ± 0.84 | 1.63 ± 0.44 | 0.001 |
| 1 ppm | 1.90 ± 0.75 | 2.19 ± 0.71 | 0.037 | 2.89 ± 1.31 | 2.16 ± 1.39 | 0.019 | 2.54 ± 1.10 | 1.77 ± 0.47 | 0.074 |
| 10 ppm | 2.73 ± 1.02 | 3.41 ± 0.76 | 0.028 | 3.79 ± 2.58 | 3.43 ± 1.64 | 0.532 | 2.43 ± 1.25 | 2.38 ± 0.96 | 0.887 |
| 50 ppm | 1.76 ± 0.43 | 2.75 ± 0.95 | 0.004 | 1.35 ± 0.89 | 1.30 ± 1.45 | 0.879 | 1.98 ± 0.73 | 2.02 ± 1.40 | 0.905 |
| 100 ppm | 1.96 ± 0.54 | 2.62 ± 0.70 | 0.005 | 2.19 ± 1.43 | 1.39 ± 0.88 | 0.025 | 1.92 ± 0.99 | 1.85 ± 0.96 | 0.738 |
| 200 ppm | 1.87 ± 2.63 | 2.63 ± 2.38 | 0.002 | 0.83 ± 0.65 | 0.65 ± 0.55 | 0.102 | 2.29 ± 1.88 | 1.88 ± 1.31 | 0.196 |
| 300 ppm | 1.93 ± 0.57 | 2.43 ± 0.51 | 0.006 | 1.22 ± 0.64 | 0.98 ± 0.49 | 0.041 | 1.90 ± 0.71 | 1.61 ± 0.22 | 0.150 |
Clinical chemistry
Serum clinical chemistry data for a number of different variables before, during, and after insufflation are presented in Table 2 . Baseline values (T 0) for most parameters are within the published normal range for this species. 27 The one exception is cortisol where levels for all animals were elevated at the start of the procedure and then declined during the 6‐hour experimental period although only levels in the anesthesia and 10 ppm ENO group became significantly different from baseline. We attribute this initial elevation as part of the residual stress response associated with preparing the animals for the experimental procedure. Values for another stress mediator (adrenocorticotropin hormone) were highly variable with group values ranging between 87 ± 75 and 225 ± 157 pg/mL; no time or treatment effects were identified (data not shown). The principal difference between the anesthesia and CO2 alone groups was the latter's rise in creatinine. Compared to baseline, serum creatinine was elevated after 4 hours of CO2 insufflation (T 240) and it remained elevated 2 hours after desufflation (T 360). Furthermore, serum creatinine concentrations did not increase with concentrations of ENO that preserved kidney blood flow (1, 10, and 100 ppm; Table 1 ) but were increased at doses of ENO for which kidney blood flow declined (50, 200, and 300 ppm). An increase in blood urea nitrogen (BUN) concentration was observed in all experimental groups except the 10 ppm ENO cohort. No other treatment‐specific changes in serum chemistry were identified. The intraoperative increases in aspartate aminotransferase and creatin phosphokinase are attributed to isoflurane and/or the skeletal muscle injury produced during animal instrumentation.
Table 2.
Swine clinical chemistry data before, during, and after insufflation.
| Variable | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Anesthesia | CO2 | 1 ppm | 10 ppm | 50 ppm | 100 ppm | 200 ppm | 300 ppm | |
| Cre (mg/dL) | _n_= 11 | _n_= 11 | _n_= 10 | _n_= 10 | _n_= 10 | _n_= 10 | _n_= 10 | _n_= 10 |
| T 0 | 1.5 ± 0.1 | 1.4 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.3 | 1.9 ± 0.2 | 1.7 ± 0.3 | 1.8 ± 0.3 | 1.6 ± 0.2 |
| T 120 | 1.6 ± 0.2 | 1.6 ± 0.4 | 1.8 ± 0.2 | 1.6 ± 0.3 | 2.1 ± 0.3 | 1.9 ± 0.4 | 2.0 ± 0.2 | 1.9 ± 0.3 |
| T 240 | 1.7 ± 0.2 | 2.0 ± 0.4* | 1.9 ± 0.2 | 1.7 ± 0.3 | 2.4 ± 0.4* | 2.2 ± 0.6 | 2.3 ± 0.3* | 2.0 ± 0.4* |
| T 360 | 1.6 ± 0.3 | 1.8 ± 0.4* | 1.9 ± 0.3 | 1.6 ± 0.3 | 2.4 ± 0.5* | 2.2 ± 0.8 | 2.3 ± 0.3* | 2.1 ± 0.4* |
| BUN (mg/dL) | ||||||||
| T 0 | 9.8 ± 2.5 | 10.1 ± 3.2 | 9.8 ± 3.2 | 10.8 ± 4.1 | 9.4 ± 2.3 | 10.7 ± 3.5 | 9.9 ± 3.2 | 9.7 ± 2.9 |
| T 120 | 11.5 ± 2.1 | 13.7 ± 5.1 | 11.4 ± 3.6 | 11.8 ± 4.6 | 11.9 ± 2.0 | 12.9 ± 3.5 | 13.8 ± 5.2 | 12.5 ± 3.8 |
| T 240 | 13.6 ± 2.4* | 15.7 ± 4.4* | 12.4 ± 3.4 | 12.0 ± 3.6 | 14.9 ± 3.0* | 16.6 ± 3.9* | 14.4 ± 3.3* | 14.6 ± 4.1* |
| T 360 | 14.9 ± 2.9* | 18.2 ± 5.3* | 13.9 ± 3.6* | 13.3 ± 3.1 | 17.6 ± 3.2* | 18.5 ± 4.8* | 17.0 ± 4.8* | 16.9 ± 4.3* |
| T‐Bil (mg/dL) | ||||||||
| T 0 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.3 | 0.3 ± 0.2 | 0.4 ± 0.2 |
| T 120 | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| T 240 | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| T 360 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| ALP (U/L) | ||||||||
| T 0 | 199 ± 42 | 181 ± 40 | 164 ± 21 | 131 ± 31 | 193 ± 71 | 187 ± 76 | 221 ± 81 | 245 ± 90 |
| T 120 | 190 ± 37 | 183 ± 38 | 167 ± 18 | 126 ± 28 | 185 ± 66 | 190 ± 72 | 210 ± 64 | 256 ± 102 |
| T 240 | 186 ± 30 | 192 ± 41 | 160 ± 13 | 123 ± 26 | 190 ± 65 | 189 ± 77 | 217 ± 76 | 251 ± 92 |
| T 360 | 187 ± 32 | 183 ± 37 | 145 ± 13 | 116 ± 28 | 178 ± 61 | 178 ± 69 | 204 ± 63 | 233 ± 84 |
| ALT (U/L) | ||||||||
| T 0 | 31.9 ± 8.9 | 26.9 ± 7.8 | 31.0 ± 7.3 | 32.4 ± 10.4 | 24.8 ± 7.1 | 31.6 ± 6.6 | 39.2 ± 15.7 | 37.5 ± 15.7 |
| T 120 | 29.3 ± 8.8 | 27.4 ± 9.7 | 30.1 ± 7.4 | 31.8 ± 10.4 | 23.8 ± 6.0 | 30.1 ± 6.6 | 35.8 ± 15.2 | 36.6 ± 15.1 |
| T 240 | 27.7 ± 8.1 | 26.6 ± 8.1 | 25.7 ± 9.1 | 30.8 ± 10.3 | 22.8 ± 6.0 | 31.4 ± 8.0 | 33.9 ± 14.9 | 36.0 ± 14.7 |
| T 360 | 28.6 ± 8.3 | 27.6 ± 8.0 | 25.1 ± 7.8 | 28.2 ± 9.2 | 20.2 ± 5.5 | 27.1 ± 7.5 | 31.4 ± 14.0 | 33.1 ± 13.0 |
| AST (U/L) | ||||||||
| T 0 | 33.4 ± 11.8 | 35.8 ± 17.7 | 34.1 ± 8.5 | 25.6 ± 7.2 | 26.6 ± 5.4 | 32.7 ± 5.7 | 36.0 ± 11.7 | 29.2 ± 7.8 |
| T 120 | 34.0 ± 10.3 | 42.2 ± 20.8 | 34.7 ± 8.5 | 28.3 ± 12.7 | 29.6 ± 5.9 | 41.6 ± 21.3 | 32.5 ± 11.4 | 31.9 ± 7.7 |
| T 240 | 39.2 ± 12.2 | 53.4 ± 20.6 | 40.3 ± 14.4 | 26.7 ± 7.0 | 35.3 ± 6.9* | 55.7 ± 23.3* | 44.6 ± 13.5 | 43.1 ± 14.5 |
| T 360 | 51.3 ± 19.4* | 67.1 ± 39.9* | 51.1 ± 25.6* | 37.2 ± 13.7* | 46.9 ± 7.9* | 62.5 ± 25.8* | 61.1 ± 19.2* | 56.6 ± 22.4* |
| CPK (U/L) | ||||||||
| T 0 | 1401 ± 947 | 1426 ± 319 | 1853 ± 681 | 1360 ± 696 | 1038 ± 561 | 1515 ± 1095 | 1413 ± 558 | 1198 ± 471 |
| T 120 | 1455 ± 944 | 1763 ± 465 | 2311 ± 1160 | 1560 ± 816 | 1353 ± 698 | 2050 ± 1184 | 1665 ± 557 | 1543 ± 676 |
| T 240 | 1856 ± 887 | 2722 ± 805 | 2707 ± 1160 | 1848 ± 886 | 1763 ± 825 | 3031 ± 1626 | 2283 ± 1126 | 2459 ± 1377 |
| T 360 | 2777 ± 1359* | 3797 ± 1522* | 3308 ± 1856* | 2550 ± 1093* | 2519 ± 888* | 4011 ± 2259* | 3555 ± 1496* | 3899 ± 2056* |
| Cortisol (ug/dL) | ||||||||
| T 0 | 17.1 ± 4.0 | 15.0 ± 6.3 | 13.9 ± 3.0 | 16.3 ± 5.3 | 15.7 ± 4.1 | 16.6 ± 4.0 | 16.8 ± 2.3 | 16.4 ± 5.0 |
| T 120 | 17.3 ± 3.4 | 14.3 ± 5.1 | 16.5 ± 4.9 | 14.2 ± 3.3 | 15.0 ± 6.2 | 17.8 ± 5.4 | 15.9 ± 4.4 | 16.3 ± 4.7 |
| T 240 | 14.0 ± 5.2 | 13.9 ± 4.2 | 14.0 ± 2.1 | 14.5 ± 3.2 | 16.1 ± 4.8 | 17.0 ± 4.1 | 14.2 ± 2.8 | 15.9 ± 3.7 |
| T 360 | 12.5 ± 5.1* | 13.1 ± 3.6 | 11.8 ± 1.7 | 12.1 ± 2.0* | 15.3 ± 4.2 | 14.8 ± 3.6 | 14.8 ± 2.8 | 14.5 ± 2.4 |
The complete temporal relationships between kidney blood flow and serum creatinine for the anesthesia, CO2 alone, 1 ppm ENO, and 10 ppm ENO groups are presented in Figure 4 . For the CO2 alone group, the decline in kidney blood flow occurred within the first hour of insufflation and remained low up to 60 minutes after desufflation. This 30% reduction in flow resulted in almost a 50% increase in serum creatinine. When kidney blood flow did not change (anesthesia, 1 ppm ENO, and 10 ppm ENO) serum creatinine also remained constant.
Figure 4.
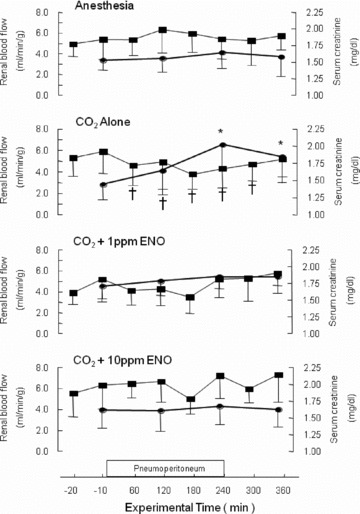
Time course and temporal relationship between kidney blood flow and serum creatinine. Neither parameter changed in the anesthesia control group (_n_= 11); they also remained constant in the animals insufflated with 1 or 10 ppm ethyl nitrite (both, _n_= 10). In contrast, insufflation with CO2 alone (_n_= 11) significantly reduced kidney blood flow (time points marked with a †) and significantly increased serum creatinine (marked with an *), with the latter remaining elevated 2 hours after desufflation (p < 0.05).
Discussion
Tissue perfusion is regulated by a physiological response termed hypoxic vasodilation in which local oxygen requirements are directly coupled to blood flow, the domain of NO bioactivity. 12 _S_‐nitrosothiols (SNOs) in the forms of SNO‐Hb and low molecular weight moieties such as _S‐_nitrosoglutathione are a central component of this regulation in normal tissues. Altered levels of SNO‐Hb and impaired vasodilatory activity of red blood cells have been observed in several disease states characterized by disorders in local blood flow and tissue oxygenation. Results from the present study show that a common medical manipulation (peritoneal insufflation) can also reduce SNO‐Hb concentration and impair organ blood flow. The results also suggest that CO2 levels and blood pH can influence NO bioactivity. Furthermore, just as administration of the _S_‐nitrosylating agent ENO will correct disease‐mediated SNO‐Hb deficits (and thus improve tissue oxygenation) 16 we found that inclusion of ENO in the insufflating gas has similar benefits. At its most efficacious dose (10 ppm), ENO increased cardiac output and improved or maintained blood flow to a number of splanchnic organs, which in turn obviated increases in serum markers of acute tissue injury.
The concepts for laparoscopic surgery were put forth over 100 years ago but its acceptance as a useful procedure for gastrointestinal surgery has only occurred within the last 20 years; 28 , 29 despite the belated application, it has subsequently flourished. Currently in the United States, greater than 95% of cholecystectomies and the vast majority of appendectomies are performed using laparoscopic techniques. As minimally invasive surgery becomes more established and surgeons acquire more experience, laparoscopy is being used for increasingly complex surgeries (e.g., nephrectomy, 30 liver resection 4 ) and in higher‐risk patients (e.g., morbidly obese, 31 pregnant, 32 elderly 33 ). Technical improvements, such as better cameras and light sources along with finer gripping and cutting instruments, have greatly enhanced the field so that insufflation of the peritoneal cavity is considered a routine event. However, effective methods to control the vascular and respiratory changes produced by elevations in intra‐abdominal pressure in combination with the presence of CO2 in the gut have not been developed. As a result, tissue ischemia and altered postoperative organ function remain major causes of laparoscopic‐associated morbidity and mortality and these are accentuated as the duration of insufflation increases. 5 , 6 , 7 , 8 , 9 , 10 , 11
Most interventions designed to improve organ blood flow during pneumoperitoneum have met with limited success. Systemic intravenous administration of vasoactive agents (dombutamine, dopamine, sodium nitroprusside) 34 , 35 are ineffectual presumably because their concentrations would be higher in well‐perfused tissues and their resultant vasorelaxation at these sites could actually result in further decreases in flow to poorly perfused regions (i.e., the steal effect). Direct application of vasodilators can improve local perfusion 36 but this type of intervention is impracticable for the whole peritoneal cavity. And none of these interventions is designed to correct pneumoperitoneum‐induced decreases in SNO‐Hb concentrations. Inclusion of ENO in the insufflating gas appears to overcome all of these concerns. By increasing NO bioactivity, ENO acts to augment the innate ability of red blood cells to deliver oxygen to hypoxic tissues, 16 and this is further enhanced through an increase in cardiac output ( Figure 2 ). Finally, since ENO can directly dilate vessels (unpublished observation), its application within the peritoneal cavity (as apposed to inhaling ENO during surgery) offers an additional means for maintaining adequate flow to splanchnic organs.
The breakdown product of ENO following the nitrosylation reaction is ethanol so the risk of toxicity appears low at the efficacious dose of 10 ppm. And even at the highest doses where organ blood flow was not always preserved there were no indications of ENO‐induced toxicity since the serum chemistry data did not suggest an exacerbation of the insufflation‐mediated changes. One possible explanation for the lack of efficacy at doses of 50 ppm ENO and higher is that the treatment acted to increase blood flow in other vascular beds (most probably, skeletal muscle) at the expense of benefit to organs within the abdominal cavity.
The present study builds upon our previous assessment of the physiologic effects of CO2 insufflation with and without ENO. 26 One major difference is that the earlier study did not demonstrate a decline in kidney blood flow, which we attribute to the monitoring method: use of laser Doppler flow probes. Such probes allow for continual measurement of surface flow but lack the quantitative sensitivity of the microsphere technique for determining overall organ perfusion. In this regard, our current finding that kidney blood flow decreases during CO2 insufflation is consistent with the results from various animals and human studies. 37 Similarly, our observation that gut blood flow is not decreased during insufflation is also consistent with other recent reports 38 and suggests that pneumoperitoneum‐induced GI perfusion changes are not a major concern.
Our inclusion of an isoflurane control group allowed for the identification of anesthesia‐mediated effects. These include increases in pancreatic and gut blood flow and increases in BUN, aspartame aminotransferase, and creatine phosphokinase. A number of clinical studies have reported similar serum chemistry changes following inhalational anesthesia with halogenated agents. 39 , 40 This indicates that such markers by themselves may not be useful for identifying pneumoperitoneum‐specific effects although the lack of change in BUN concentration with the 10 ppm ENO group does support the general conclusion that this dose stabilizes overall physiologic status. The one pneumoperitoneum specific marker was serum creatinine, the levels of which showed a temporal relationship to kidney blood flow. In humans, a greater than 20% increase in serum creatinine has been linked to postoperative kidney impairment. 41 By extension, it is tempting to speculate that obviating the intraoperative increase in creatinine with 10 ppm ENO would yield postoperative benefits but this remains to be determined.
Conclusion
In summary, the inclusion of the _S_‐nitrosylating agent ENO in the insufflating gas is an effective means to attenuate pneumoperitoneum induced reductions in SNO‐Hb concentrations and splanchnic organ blood flow leading to reductions in markers of acute tissue injury. At its most efficacious dose (10 ppm), these benefits were consistently observed throughout 4 hours of insufflation, an interval that would encompass the vast majority of laparoscopic procedures currently conducted on humans. As a result, clinical assessments of the utility and benefits of this intervention are warranted. More generally, the present findings support a heretofore under‐appreciated role for CO2 in the control of NO bioactivity and the regulation of tissue oxygen delivery. The findings also provide an example of a common medical manipulation that acutely alters SNO‐Hb, which further supports the idea that unintended reductions in organ blood flow associated with other medical procedures may also result from reductions in nitric oxide bioactivity (e.g., blood transfusion 42 ).
Acknowledgments
The authors thank Dr. Taijiro Sueda of the Department of Surgery at Hiroshima University, School of Medicine for his invaluable support and Dr. Hector J. Lacassie (now at the Pontificia Universidad Catolica de Chile, Santiago, Chile) for his assistance with establishing the experimental preparation. This work was supported in part by the National Institutes of Health (grant HD042471, the Duke Anesthesiology Research Fund, and by an unrestricted educational grant from N30 (formerly Nitrox, LLC) a company that is developing strategies for treating disorders of oxygen delivery. JSS and JDR have consulting and/or equity relationships with this company. In addition, RJM was the recipient of a Howard Hughes Medical Institute Medical Student Fellowship.
References
- 1.Cherqui D, Husson E, Hammoud R, Malassagne B, Stéphan F, Bensaid S, Rotman N, Fagniez PL. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000; 232: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown SL, Bieh TR, Rawlins MC, Hefty TR. Laparoscopic live donor nephrectomy: a comparison with the conventional open approach. J Urol. 2001; 165: 766–769. [PubMed] [Google Scholar]
- 3.Marcello PW, Milsom JW, Wong SK, Brady K, Goormastic M, Fazio VW. 2001. Laparoscopic total colectomy for acute colitis: a case‐control study. Dis Colon Rectum. 2001; 44: 1441–1445. [DOI] [PubMed] [Google Scholar]
- 4.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006; 93: 67–72. [DOI] [PubMed] [Google Scholar]
- 5.Meininger D, Byhahn C, Bueck M, Binder J, Kramer W, Kessler P, Westphal K. Effects of prolonged pneumoperitoneum on hemodynamics and acid‐base balance during totally endoscopic robot‐assisted radical prostatectomies. World J Surg. 2002; 26: 1423–1427. [DOI] [PubMed] [Google Scholar]
- 6.Andrei VE, Schein M, Wise L. Small bowel ischemia following laparoscopic cholecystectomy. Dig Surg. 1999; 16: 522–524. [DOI] [PubMed] [Google Scholar]
- 7.Richmond BK, Lucente FC, Boland JP. Laparoscopy‐associated mesenteric vascular events: description of an evolving clinical syndrome. J Laparoendosc Adv Surg Tech. 1997; 7: 363–367. [DOI] [PubMed] [Google Scholar]
- 8.Razvi HA, Fields D, Vargas JC, Vaughan ED, Vukasin A, Sosa RE. Oliguria during laparoscopic surgery: evidence for direct renal parenchymal compression as an etiologic factor. J Endourol. 1996; 10: 1–4. [DOI] [PubMed] [Google Scholar]
- 9.Taura P, Lopez A, Lacy AM, Anglada T, Beltran J, Fernandez‐Cruz L, Targarona E, Garcia‐Valdeca‐sas JC, Marin JL. Prolonged pneumoperitoneum at 15 mmHg causes lactic acidosis. Surg Endosc. 1998; 12: 198–201. [DOI] [PubMed] [Google Scholar]
- 10.O’Malley C, Cunninghamm AJ. Physiologic changes during laparoscopy. Anesthesiol Clin North America. 2001; 19: 1–19. [DOI] [PubMed] [Google Scholar]
- 11.Schachtrupp A, Toens C, Hoer J, Klosterhalfen B, Lawong AG, Schumpelick V. A 24‐h pneu‐moperitoneum leads to multiple organ impairment in a porcine model. J Surg Res. 2002; 106: 37–45. [DOI] [PubMed] [Google Scholar]
- 12.Allen BW, Piantadosi CA. How do red cells cause hypoxic vasodilation? The SNO‐hemoglobin paradigm. Am J Physiol Heart Circ Physiol. 2006; 291: H1507–H1512. [DOI] [PubMed] [Google Scholar]
- 13.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernet K, Piantadosi CA. Blood flow regulation by _S‐_nitrosohemoglobin in the physiological oxygen gradient. Science 1997; 276: 2034–2037. [DOI] [PubMed] [Google Scholar]
- 14.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med. 2002; 8: 711–717. [DOI] [PubMed] [Google Scholar]
- 15.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells; role of nitric and SNO‐hemoglobin. Ann Rev Physiol. 2005; 67: 99–145. [DOI] [PubMed] [Google Scholar]
- 16.McMahon TJ, Ahearn GS, Moya MP, Gow AJ, Huang YCT, Luchsinger BP, Nudelman R, Yan Y, Krichman AD, Bashore TM, Califf RM, Singel DJ, Piantadosi CA, Tapson VF, Stamler JS. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of _S‐_nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci USA. 2005; 102: 14801–14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci USA. 2005; 102: 2531–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James PE, Lang D, Tufnell‐Barret T, Milson AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004; 94: 976–983. [DOI] [PubMed] [Google Scholar]
- 19.Padron J, Peiro C, Cercas E, Llergo JL, Sanchez‐Ferrer CF. Enhancement of _S‐_nitrosylation in glycosylated hemoglobin. Biochem Biophys Res Commun. 2000; 271: 217–221. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Yan Y, Zeng M, Zhang J, Hames MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of _S‐_nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004; 116: 617–628. [DOI] [PubMed] [Google Scholar]
- 21.Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP. Transduction of NO‐bioac‐tivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004; 104: 1375–1382. [DOI] [PubMed] [Google Scholar]
- 22.Datta B, Tufnell‐Barrett T, Bleasdale RA, Jones CJH, Beeton I, Paul V, Frenneaux M, James P. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004; 109: 1339–1342. [DOI] [PubMed] [Google Scholar]
- 23.Hausladen A, Rafikov R, Angelo M, Singel DJ, Nudler E, Stamler JS. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc Natl Acad Sci USA. 2007; 104: 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon TJ, Stamler JS. Concerted nitric oxide/oxygen delivery by hemoglobin. Methods Enzymol. 1999; 301: 99–114. [DOI] [PubMed] [Google Scholar]
- 25.Forsberg S, Ludwigs U, Hedenstierna G. Effects of ventilatory patern on exhaled nitric oxide in mechanically ventilated rabbits. Acta Anaesth Scand. 1999; 43: 464–469. [DOI] [PubMed] [Google Scholar]
- 26.Ali NA, Eubanks WS, Stamler JS, Gow AJ, Lagoo‐Deenadayalan SA, Villegas L, El‐Moalem HE, Reynolds JD. A method to attenuate pneumoperitoneum‐induced reductions in splanchnic blood flow. Ann Surg. 2005; 241: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swindle MM.Swine in the laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. 2nd ed Boca Raton : CRC Press, 2007. [Google Scholar]
- 28.Dubois F, Berthelot G, Levard H. Laparoscopic cholecystectomy: historic perspective and personal experience. Surg Laparosc Endosc Percut Tech. 1991; 1: 52–57. [PubMed] [Google Scholar]
- 29.Vierra M. Minimally invasive surgery. Annu Rev Med. 1995; 46: 147–158. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigue JR, Cross NJ, Newman RC, Widows MR, Guenther RT, Kaplan B, Morgan MA, Howard RJ. Patient‐reported outcomes for open versus laparoscopic living donor nephrectomy. Prog Transplant. 2006; 16: 162–169. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen NT, Wolfe BM. The physiologic effects of pneumoperitoneum in the morbidly obese. Ann Surg. 2005; 241: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds JD, Booth JV, De La Fuente S, McMahon R, Eubanks WS. A review of laparoscopy for non‐obstetric–related surgery during pregnancy. Curr Surg. 2003; 60: 164–173. [DOI] [PubMed] [Google Scholar]
- 33.Weber DM. Laparoscopic surgery: an excellent approach in elderly patients. Arch Surg. 2003; 138: 1083–1088. [DOI] [PubMed] [Google Scholar]
- 34.Agusti M, Elizalde JI, Adalia R, Martinez‐Palli G, Garcia‐Valdecasas JC, Pique JM, Taura P. The effects of vasoactive drugs on hepatic blood flow changes induced by CO2 laparoscopy: an animal study. Anesth Analg. 2001; 93: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 35.Junghans T, Neudecker J, Dorner F, Wieland R, Haasa O, Schwenk W. Effect of increasing cardiac preload, sympathetic antagonism, or vasodilation on visceral blood flow during pneumo‐peritoneum. Langenbecks Arch Surg. 2005; 390: 538–543. [DOI] [PubMed] [Google Scholar]
- 36.Zacherl J, Thein E, Stangl M, Feussner H, Bock S, Mittlbock M, Erhardt W, Siewert JR. The influence of periarterial papaverine application on intraoperative renal function and blood flow during laparoscopic donor nephrectomy in a pig model. Surg Endosc. 2003; 17: 1231–1236. [DOI] [PubMed] [Google Scholar]
- 37.Demyttenaere S, Feldman LS, Fried GM. Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surg Endosc. 2007; 21: 152–160. [DOI] [PubMed] [Google Scholar]
- 38.Goitein D, Papasavas P, Yeaney W, Gagne D, Hayetian F, Caushaj P, Keenan R, Landreneau R. 2005. Microsphere intestinal blood flow analysis during pneumoperitoneum using carbon dioxide and helium. Surg Endosc. 2005; 19: 541–545. [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama T, Yokoyama T, Hanaoka K. Liver function after sevoflurane or isoflurane anaesthesia in neurosurgical patients. Can J Anaesth. 1998; 45: 753–756. [DOI] [PubMed] [Google Scholar]
- 40.Kharasch ED, Frink EJ, Artru A, Michalowski P, Rooke GA, Nogami W. Long‐duration low‐flow sevoflurane and isoflurane effects on postoperative renal and hepatic function. Anesth Analg. 2001; 93: 1511–1520. [DOI] [PubMed] [Google Scholar]
- 41.Charlson ME, MacKenzie CR, Gold JP, Shires TG. Postoperative changes in serum creatinine: when do they occur and how much is important? Ann Surg. 1989; 209: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. _S‐_nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci USA. 2007; 104: 17058–17062. [DOI] [PMC free article] [PubMed] [Google Scholar]