Transient frictional slip between integrin and the ECM in focal adhesions under myosin-II tension (original) (raw)
. Author manuscript; available in PMC: 2011 Jul 13.
Published in final edited form as: Curr Biol. 2010 Jun 10;20(13):1145–1153. doi: 10.1016/j.cub.2010.05.049
SUMMARY
Background
The spatiotemporal regulation of adhesion to the extracellular matrix is important in metazoan cell migration and mechanosensation. While adhesion assembly depends on intracellular and extracellular tension, the biophysical regulation of force transmission between the actin cytoskeleton and extracellular matrix during this process remains largely unknown.
Results
To elucidate the nature of force transmission as myosin-II tension is applied to focal adhesions, we correlated the dynamics of focal adhesion proteins and the actin cytoskeleton to local traction stresses. We find that, under low extracellular tension, newly formed adhesions near the cell periphery undergo a transient retrograde displacement preceding elongation. We identify that myosin-II generated tension drives this mobility and determine the interface of differential motion, or ‘slip’, to be between the integrin and the ECM. We found that the magnitude and duration of both adhesion slip and associated F-actin dynamics is strongly modulated by the ECM compliance. Furthermore, traction forces are generated throughout the slip period with adhesion immobilization occurring at a constant tension.
Conclusions
We have identified a tension-dependent, extracellular ‘clutch’ between integrins and the extracellular matrix that is utilized to stabilize adhesions under myosin-II driven tension. This work signifies that modulating adhesion alters force transmission during focal adhesion maturation.
Keywords: focal adhesion assembly, F-actin cytoskeleton, traction force, cellular mechanosensation
Introduction
Focal adhesions (FAs) form hierarchical connections between the F-actin cytoskeleton and the extracellular matrix (ECM) to transmit mechanical forces across the plasma membrane. Forces generated within the F-actin cytoskeleton exert traction on the ECM, and are important in cell migration and ECM remodeling (1, 2). In turn, the mechanical properties of the ECM are sensed by adherent cells and directly affect the morphology of FAs and the F-actin cytoskeleton (3). Cellular force sensing is thought to be dominated through the regulation of FA assembly and growth by both intracellular and extracellular forces (4–7). Thus, an intricate feedback exists between the F-actin cytoskeleton, ECM mechanics, traction force generation and FA assembly.
FA assembly is thought to modulate force transmission by regulating the coupling of F-actin motion to the underlying ECM. Near the cell periphery, F-actin polymerization drives a rapid retrograde flow of a branched, dendritic network, termed the lamellipodium. Proximal to the lamellipodium, the F-actin cytoskeleton transitions into a contractile organelle, termed the lamella, where retrograde flow is mediated by myosin-II. In the absence of FA formation, lamellar retrograde flow is quite rapid and uniform throughout the cell body (8, 9). During FA assembly, F-actin retrograde flow slows (8) and traction stress builds on the ECM (10). This process is consistent with FAs serving as a molecular “clutch” between dynamic F-actin and the immobile ECM (11–13).
Identification of the molecular interactions that regulate the molecular clutch during FA assembly is critical to further understanding of cellular force transduction. Generally, clutch regulation could occur intracellularly, by FA proteins between the F-actin and transmembrane integrins, and/or extracellularly, between integrins and ECM proteins. During FA initiation, talin plays an important role in regulating this linkage and talin-deficient cell lines have impaired integrin-ligand binding, enhanced retrograde F-actin flow and reduced traction stress (9, 14). Likewise, in elongated FAs, differential motion between retrograde F-actin and immobilized FAs occurs at the interfaces between F-actin and α-actinin (15) and vinculin (16). The extent to which these intracellular proteins modulate intracellular clutch engagement as tension is built during FA assembly and growth is unknown. Furthermore, while extracellular clutch regulation between the integrin and the ECM has been suggested (12), no direct observations have been made. Thus, the components of the molecular clutch during tension dependent adhesion assembly remain unknown.
In this study, we identify a tension-dependent clutch at the integrin-ECM interface on physiologically flexible substrates. At low levels of tension, FAs undergo rapid, micron-scale retrograde displacement relative to the ECM while transmitting traction force; we term this behavior “frictional slip” (11). When the ECM stiffness decreased, the magnitude and duration of frictional slip is enhanced but adhesions immobilize at similar levels of tension. Once this extracellular clutch is engaged, F-actin moves relative to FA components. Thus, actomyosin retrograde flow and substrate mechanics are coordinated to regulate an extracellular clutch during the initial stages of tension-dependent stabilization of FAs.
Results
ECM compliance regulates the mobility of nascent FAs
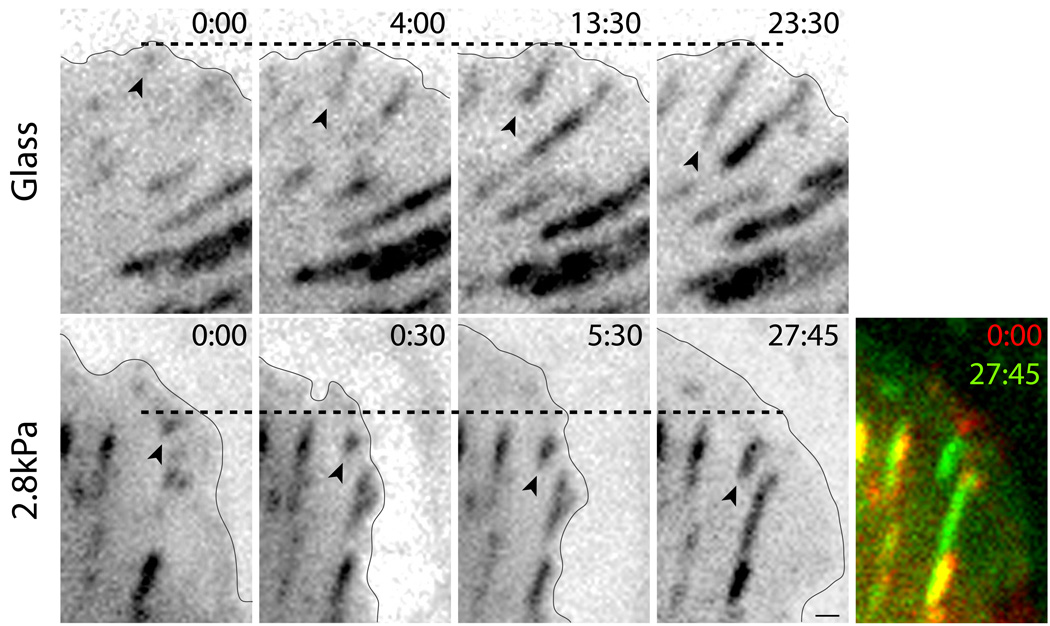
To confirm that the morphological changes associated with FA assembly in human osteosarcoma (U2OS) cells are similar to that observed in other cell types, we used time-lapse microscopy to image the assembly of GFP-paxillin rich adhesions near the periphery of cells plated on fibronectin-coated glass coverslips. We observed the appearance of newly-formed, or nascent adhesions, near the leading cell edge as small (~0.5 µm diameter) fluorescent spots (Figure 1, upper panel). These nascent adhesions appeared stationary with respect to the underlying coverslip and, over the course of 15–20 minutes, elongated rearward into larger plaques with a length of 3–5 microns. These morphological events are in accordance with published studies of FA maturation on rigid, glass substrates in numerous cell types (2, 17, 18).
FIGURE 1. FA maturation on rigid and compliant substrata.
(Upper panel) On glass, GFP-paxillin puncta appears near the cell edge, remains immobilized and elongates within minutes. (Lower panel) On a compliant substrate (2.8kPa gel), GFP-paxillin puncta appears near the cell edge and undergoes retrograde displacement prior to immobilization and elongation. Dashed lines highlight initial location of distal edge of FA puncta. Arrowheads indicate proximal end of FA. Solid black line indicates cell boundary. Color combined image of FA at 0:00 (red) and 27:45 (greed). Time stamp in min:sec. Scale bar = 1 µm.
To explore how FA assembly is impacted by ECM stiffness, we visualized FA dynamics in U2OS cells plated on fibronectin-coated polyacrylamide gels with a shear elastic modulus of 2.8 kPa, which resembles the stiffness of physiological tissue (3). On compliant gels, cells spread and formed elongated FA plaques of similar size to those found in cells plated on fibronectin-coated glass coverslips; FA maturation consisted of the appearance of small (~0.5 µm) fluorescent spots near the cell periphery and subsequent elongation over a period of 15–20 minutes. However, on compliant gels, nascent adhesions underwent a rapid, retrograde displacement from their origin of appearance prior to immobilization to the ECM (Figure 1, lower panel). This retrograde movement was only seen in nascent adhesions, within 30–90 seconds after appearance and occurred in a majority of nascent adhesions observed (65%, nFA=39, ncell=6). The magnitude of retrograde displacement was 0.67±0.33 µm (nFA=39, ncell=6) and, once immobilized, a majority (77%, nFA=39, ncell=6) of adhesions elongated proximally. Thus, on compliant matrices, a rapid, transient rearward translocation of newly formed, adhesion punctae usually precedes growth to an elongated FA plaque.
Blebbistatin treatment and removal as a methodology to study myosin-II driven FA maturation
The retrograde movement of nascent adhesions observed on compliant matrices suggests a probable role for myosin-II contractility. To investigate this, we designed an experimental approach to isolate and synchronize myosin-II driven adhesion maturation by perfusion and removal of the myosin-II ATPase inhibitor, blebbistatin (19). In combination with traction force and high resolution confocal microscopy of GFP-actin and mApple-paxillin (Methods), this enabled a characterization of both cytoskeletal dynamics and force transmission at adhesions during the first steps of myosin-II impinged tension.
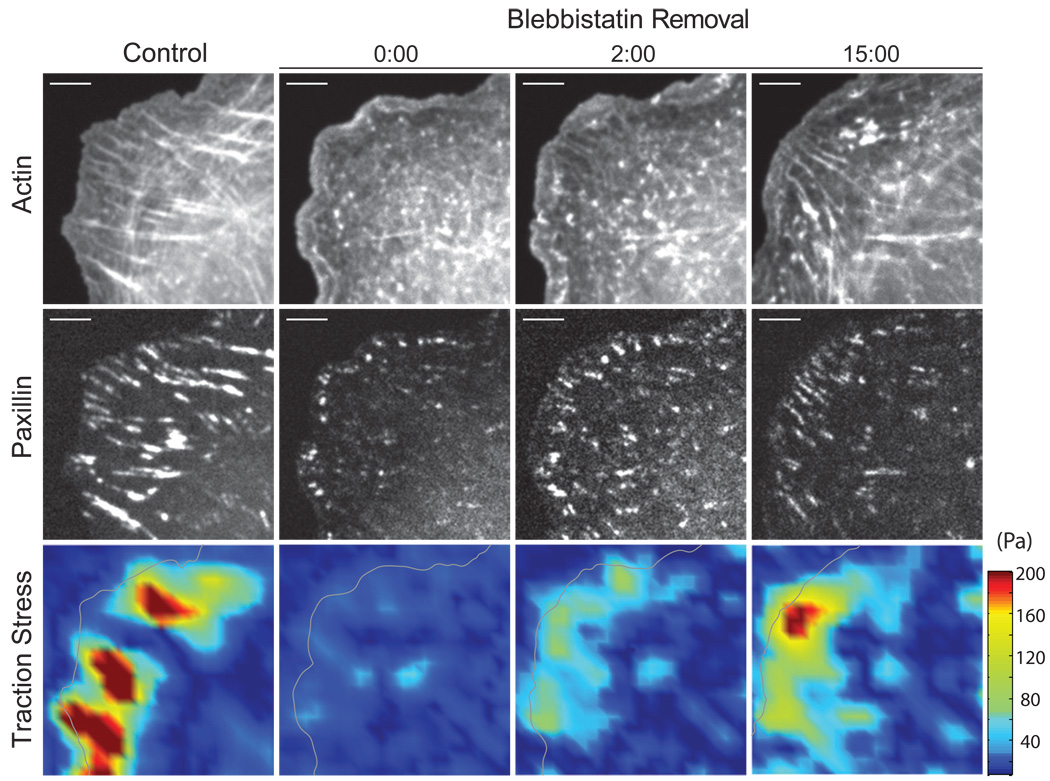
In control conditions, condensed actin bundles ranging from 5–70 µm in length terminated in elongated adhesions with an average length of 3 µm (Figures 2; Figure S1 and S3C); the average traction stress exerted at peripheral FAs was 150 Pa (Figures 2 and Figure S3B). After treatment with 25–50 µM of blebbistatin for 30 min, only small paxillin- and vinculin-rich punctae which resembled nascent adhesions remained (Figure 2; Figures S1–S3). Under blebbistatin treatment, actomyosin bundles disassembled and dense “patches” of actin colocalized with adhesions at the lamellipodial/lamellar border (LLB; Figure 2; Figures S1–S3). Immunostained samples revealed an F-actin meshwork with a dense ribbon of F-actin at the LLB and myosin-II punctae, presumably minifilaments (20), across the lamellar region (Figure S1). Traction stresses were constrained to the lamellipodial base and were reduced to 20–30 Pa (Figures 2 and S3).
FIGURE 2. Cytoskeletal remodeling and traction stress recovery upon myosin reactivation by blebbistatin removal.
Images of GFP-actin (Top), mApple-paxillin (Middle) and traction stress magnitude at times prior to blebbistatin treatment (Control) and at times indicated after blebbistatin removal. Time is indicated in min:sec. Gray line indicates cell boundary. Scale bar = 5µm.
We synchronized myosin-II driven adhesion maturation across the entire cell by deactivating blebbistatin, by means of drug removal and imaging with 488nm light (21) (Movies S1 and S2). Within 15 sec, the traction stress at paxillin-rich adhesions increased to 57±13 Pa and a marginal increase in the average adhesion was resolved (Figures S3). Two minutes after removing blebbistatin, the traction stress exerted at individual adhesions increased by approximately 4-fold, adhesions elongated by roughly twice their myosin-II independent length, and short actin bundles were resolved (Figure 2 and Figure S3). Fifteen minutes after blebbistatin removal, adhesions elongated to 80% of their control length, condensed myosin-rich actin bundles were observed to span the cell body and traction stresses recovered to the same order of magnitude as pre-blebbistatin levels (Figures 2; Figure S1 and S3). Thus, blebbistatin treatment and removal has a reversible effect on cytoskeletal organization and can be used as a means to study the process of myosin-II driven maturation of FAs (6, 22).
F-actin and FA dynamics reveal frictional slip at the FA-ECM interface
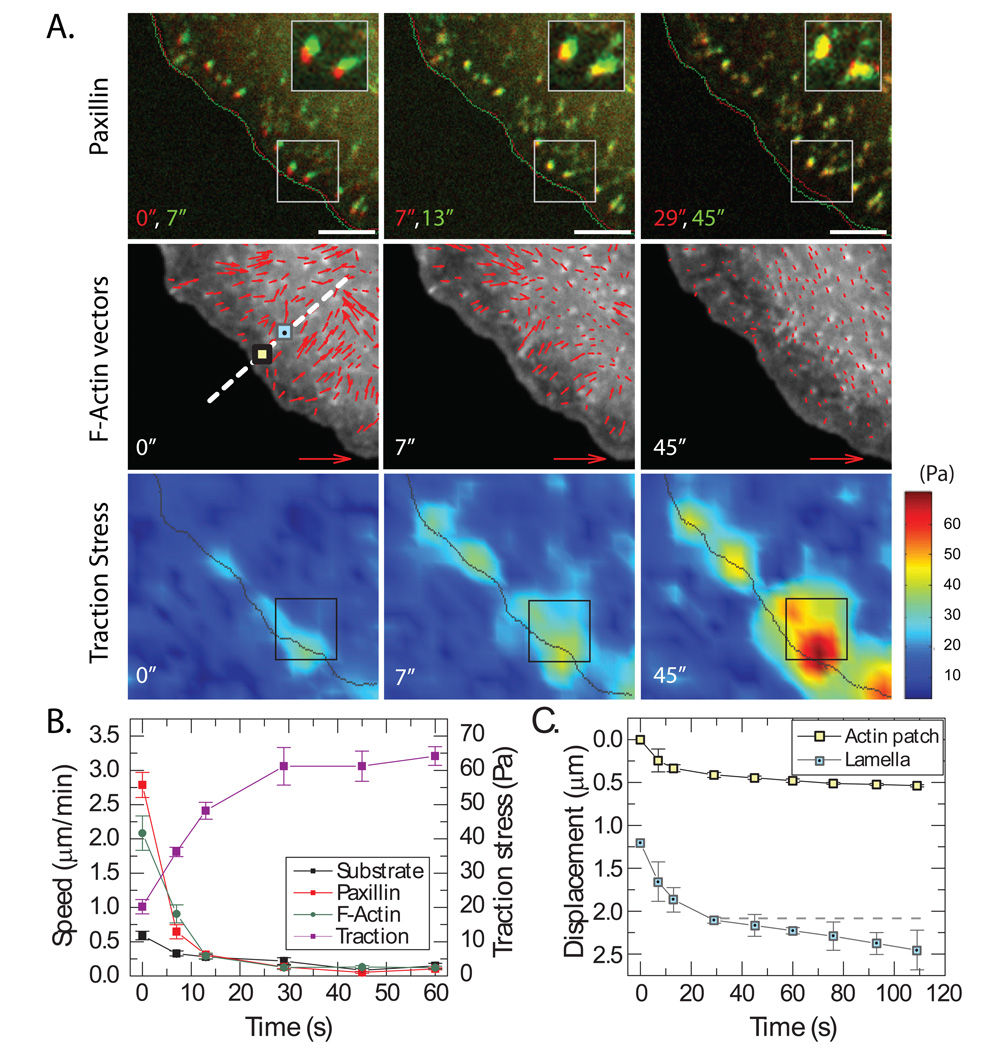
To examine the mobility of adhesions upon blebbistatin removal, we compared pairs of mApple-paxillin images in an aligned time-lapse sequence (Figure 3A, Movie S3). Similar to newly formed adhesions in drug-free conditions, adhesion puncta moved retrogradely upon removal of blebbistatin. Comparing images obtained immediately (0 seconds) and 7 seconds post blebbistatin-removal indicated that the mApple-paxillin puncta displaced towards the cell center, while the location of the cell edge did not change significantly (indicated by line traces Figure 3A). Between 7 and 13 seconds post removal, retrograde movement of adhesions was substantially reduced. At later times, the distal tip of adhesions did not translocate (Figure 3A).
FIGURE 3. F-actin and FAs move retrogradely as myosin-II is reactivated.
(A) (Top) Color combined images of mApple-paxillin at successive times post blebbistatin removal, early (red) and late (green) times. Insets show magnification of outlined regions. Scale bar = 5 µm. (Middle) GFP-actin images overlaid with actin flow vectors (red arrows). Time indicates the initial time point used in velocity calculation. Red arrow indicates trajectory scale = 4.5 µm/min. Dashed white line indicates typical linescan across cell front used in analysis with location of adhesion and actin patche (yellow square with black outline) and lamellar actin (light blue square). (Bottom) Heat-scale map of traction stress with scale bar indicated at right (from blue to red). Times are indicated in seconds. (B) Traction stress (violet), F-actin flow (green), paxillin speed (red), and substrate speed (black) over time post blebbistatin removal. F-actin flow was tracked at actin patches associated with FA. Data reflect mean plus standard error for nFA=26 and ncell=5. (C) Displacement of actin patches colocalized with adhesions (yellow square, black outline), and at 1.2 µm proximal to adhesion puncta within the lamella (blue square). Approximate locations of these data points indicated in A, middle panel. Horizontal dashed line is a line of zero slope.
To quantify this motion, the centroids of the mApple-paxillin puncta were identified and rates of displacement were calculated. Line scans across individual adhesions during frictional slip revealed a Gaussian intensity profile with a full-width half maximum of ~300nm and a significant signal-to-noise ratio (Figure S4), indicating these features were nearly diffraction-limited and amenable to computational tracking algorithms that track the centroid with sub-pixel resolution, providing a resolution of mobility on the order of 0.02 µm/min (23, 24). Initially, between 0 and 7 seconds, the adhesion moved retrogradely with a speed of 2.7 µm/min (Figure 3B). Within 15 seconds of blebbistatin removal, the apparent movement of paxillin-rich puncta subsided to values near our resolution limit. Since cells were adhered to compliant substrates, it is possible that some retrograde movement could be accommodated by substrate deformation. However, fiducial markers in the top surface of the substrate underlying adhesion puncta moved less than 1 µm/min, with a rapid decay thereafter (Figure 3B); movement of fibronectin adhered to the top surface of the substrate moved at rates similar to the substrate (Figure S4). Thus, by 15 seconds after blebbistatin removal, the apparent retrograde motion of the paxillin is abrogated with respect to the underlying fibronectin-coated substrate and defines FA immobilization.
To observe F-actin dynamics during blebbistatin-removal, fiducial marks of GFP-actin were monitored over time. Immediately after blebbistatin removal, actin patches co-localized with adhesion puncta at the LLB (yellow square, Figure 3A) and underwent a rapid, coherent retrograde movement, decreasing from 2.1 µm/min to <0.1 µm/min within 15 seconds of blebbistatin removal (Figures 3A and 3B; Figure S5A). Thus, actin patches stabilized ~0.5 µm proximal from their starting location (Figure 3C). Lamellar actin, located approximately 1.2 µm proximal to these patches (blue square, Figure 3A), exhibited similar, but slightly enhanced, retrograde flow dynamics and stabilized at roughly 30 seconds to a steady state retrograde flow of 0.25 µm/min, the value observed in compact stress fibers (25) (Figure S5A). Within 15 seconds after blebbistatin removal, lamellar F-actin moved retrogradely ~1 µm, and continued to move towards the cell center at later times (Figure 3C). Thus, the distance between the actin patches colocalized with adhesions and fiduciary marks in lamellar actin increased under myosin-driven dynamics.
The traction stress exerted on the extracellular matrix increased monotonically from about 20 Pa to 50 Pa as mApple-paxillin underwent retrograde movement (Figures 3A and 3B). Interestingly, during this time, we observed an inverse relationship between the traction stress and F-actin retrograde flow rate, such that traction stress increased while F-actin retrograde flow speed decayed (Figure S5B), a relationship consistent with known mechanochemistry of individual myosin-II motors (Supplementary Text; Figures S5). Thus, even while FAs move relative to the underlying matrix, mechanical forces are transmitted to the extracellular matrix. We refer to this transient state as myosin-II driven “frictional slip” of adhesion puncta, a phenomenon previously observed in filopodial adhesions (11).
Frictional slip in nascent adhesions occurs between integrin and ECM
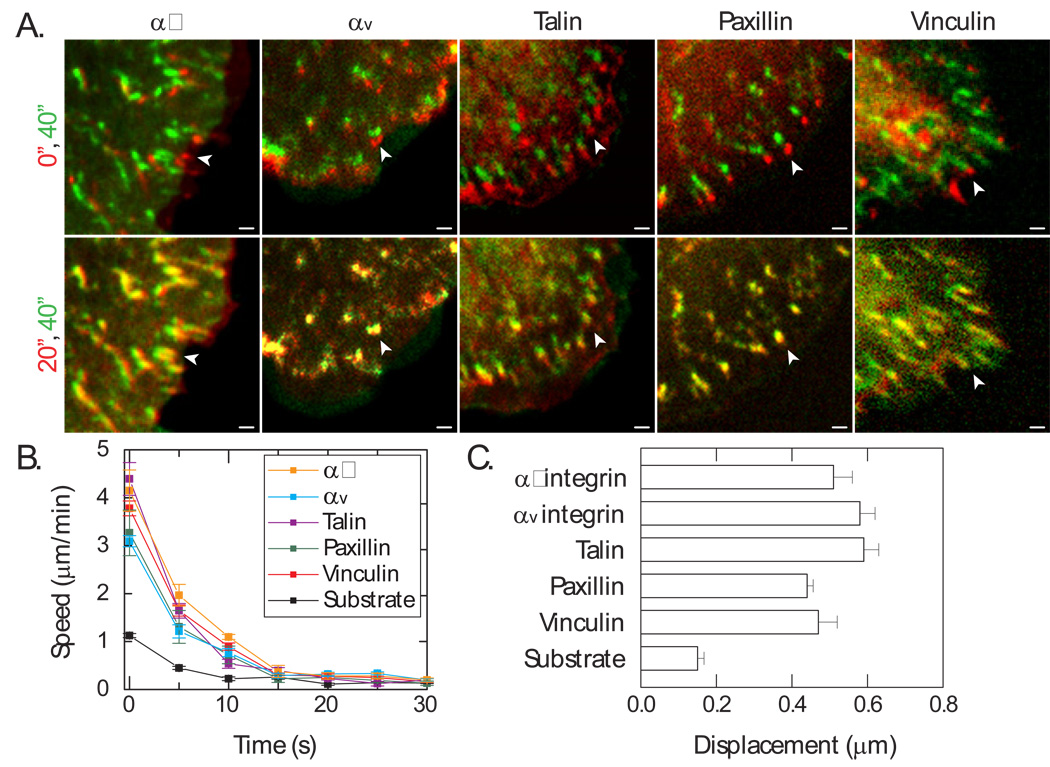
Since FAs are multi-component ensembles that contain a diverse group of actin and ECM binding proteins (26, 27), we sought to determine if varying retrograde flow dynamics occur at different components during frictional slip. We investigated two classes of integrins, αv and α5, known to bind fibronectin, as well as two FA proteins, talin (9, 14) and vinculin (15, 16, 28), which establish a molecular link between integrin and actin. We found that all of these proteins underwent retrograde motion comparable to paxillin within the first 20 seconds post blebbistatin removal and were immobilized thereafter (Figure 4A, Movies S4 and S5). The retrograde dynamics of different FA proteins were highly correlated, as measured by Pearson’s correlation coefficient (0.98), and all proteins became immobilized to the substrate after approximately 15 seconds (Figure 4B). Furthermore, the total displacement of each protein population prior to substrate engagement was similar, with an average of 0.5 µm, approximately four-fold greater than the underlying substrate (Figure 4C). These data indicate a diverse set of FA associated proteins, ranging from associated actin to integrin, displace as a collective unit prior to their immobilization, and that a predominant slip occurs at the interface between the integrin and ECM.
FIGURE 4. FA displacement occurs at the integrin-ECM interface.
(A) Color combined images in time of FA proteins upon blebbistatin inactivation, (top) 0 sec (red) and 40 sec (green) and (bottom) 20 sec (red) and 40 sec (green). Scale bar = 1µm. Arrowheads indicate adhesion puncta. (B) Speeds of FA proteins and beads in substrate over time after blebbistatin inactivation. (C) Total displacement of adhesion proteins and substrate prior to adhesion immobilization. No statistical significant difference between FA proteins; substrate displacement is statistically significantly different from FAs (p<0.0001). Data reflect the mean and standard error for all samples, with sample sizes: α5 (nFA=8; ncell=6), αv (nFA=9; ncell=4), talin (nFA=6; ncell=3), paxillin (nFA=26; ncell=16), and vinculin (nFA=12; ncell=3).
FRAP reveals stable association of αv integrin to adhesion puncta during slip
Micron-sized movements of integrin relative to the underlying substratum occur simultaneously with increases in traction. Since these movements are too large to be accommodated by individual bond deformation or stretching, we hypothesize this motion could result from either polarized remodeling (29) or the nature of binding kinetics between the integrin and ECM (11, 30). For polarized remodeling, dissociation of integrin from the distal tip is balanced by incorporation of new integrin at proximal end to result in apparent motion (31). In this scenario, we would expect nearly complete exchange of integrins with the diffuse population during frictional slip, as has been observed in polarized remodeling of mature FAs over long time scales (29). By contrast, if the integrins are stably bound to intracellular FA components, integrin exchange may not occur.
We utilized fluorescence recovery after photobleaching (FRAP) to determine if integrins are stably associated within the adhesion puncta during frictional slip. GFP-α5 and GFP-αv were bleached upon blebbistatin removal and changes in fluorescence intensity were measured. To ensure that FRAP measurements of GFP-tagged proteins were performed on adhesions undergoing frictional slip, we confirmed adhesion retrograde movement by visualization with mApple-paxillin and traction stress increase (data not shown). Both integrins underwent marginal fluorescence recovery (~20–25%) within the first 20 seconds after blebbistatin removal (Figures 5A and 5B). At longer times, α5 underwent partial exchange while αv underwent negligible turnover.
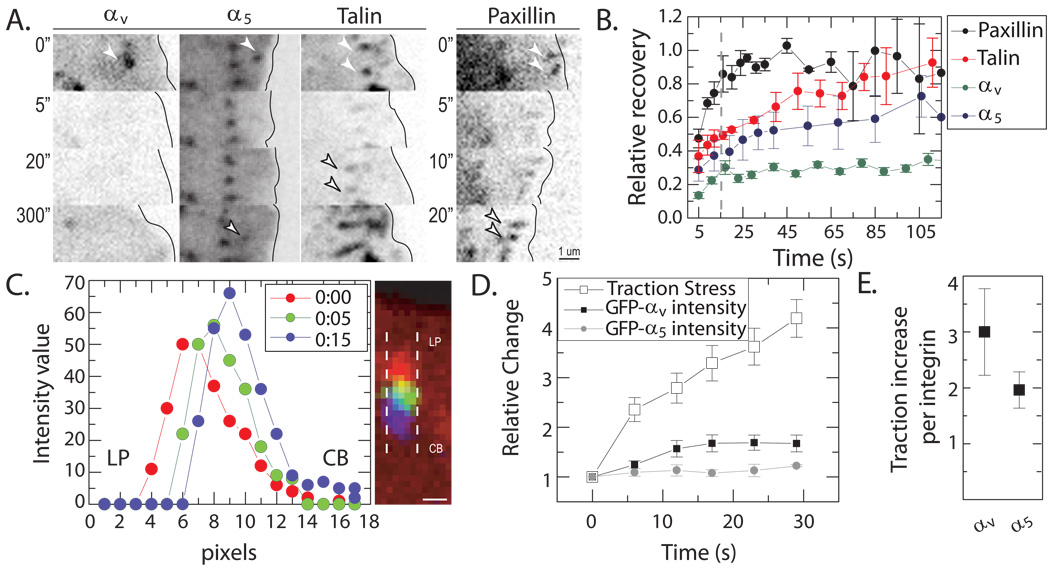
FIGURE 5. Minimal Exchange of Integrin within FA during frictional slip.
(A) Vertical montage of images of GFP-tagged αv, α5, talin, and paxillin. Prebleached adhesions shown at t=0 sec (top, white solid arrows), photobleaching occurred between 0 and 5 seconds. Post-bleach images for αv, α5, talin and paxillin at times indicated at left. Times indicated in seconds. Reappearance of adhesions post-bleach in α5, talin and paxillin images are indicated by outlined arrows. (B) Recovery profiles for FA proteins in (A) over time. Dashed line demarcates termination of FA slip (t~15 sec). Data shown for 5 FA per protein. (C) Intensity profiles of GFP-αv from line scans subtending an individual adhesion at t=0 sec (red), t=5 sec (green) and t=15 sec (blue). Color-combined image of GFP-αv at times indicated in plot. Dashed lines indicate FA width. Scale bar = 0.5 µm. LP indicates lamellipodium, while CB marks the cell body. (D) Traction stress and integrin fluorescence were normalized to data at t=0 sec and plotted over time. Data reflect average values taken from 18 FAs from 5 cells. (E) Ratios of relative increase in traction stress to relative change in integrin intensity at adhesion immobilization.
As a control, we photobleached GFP-talin and GFP-paxillin. For talin, complete recovery was observed over long time scales, on the order of 2 min. In stark contrast, paxillin underwent rapid turnover, which was nearly complete by the end of the slip period (Figures 5A and 5B). Interestingly, the degree of dynamic exchange observed for integrin, talin, and paxillin was similar to that measured in large FAs in U2OS cells under no drug treatment (data not shown) and in other cell types (32). These results show that integrins remain stably associated to adhesion puncta during frictional slip and do not support polarized remodeling as the predominate mechanism of adhesion translocation.
Force per available integrin increases during frictional slip
Our FRAP data indicates stable association of integrin within the adhesion during retrograde slip, suggesting that motion is likely accommodated by a population of integrins which undergo cycles of bond association and dissociation to the ECM while facilitating force transmission (11, 30). If the apparent adhesion mobility reflects a time scale determined by competing effects of bond association and dissociation, decreased adhesion mobility would be associated with enhanced bond association and diminished bond dissociation for a constant population. Enhanced bond association may result from factors such as changes in integrin activation, a conformational change of integrin required for integrin-ECM binding. However, our data show that enhanced integrin activation via Mn2+ treatment (33, 34), did not perturb the dynamics of frictional slip (Figure S6).
Alternatively, if bond dissociation is enhanced under increased force, then increasing the total number of integrins, while maintaining a constant binding kinetics, would provide a lower force per integrin and, thus, decrease the rate of integrin unbinding (11). To probe the changes in the total number of integrins contained within the adhesion during frictional slip, we measured the total GFP-αv or GFP-α5 integrin fluorescence intensity within FA. During the slip period (t<15 sec), GFP-αv intensity increased by approximately 1.5-fold and attained a steady state shortly thereafter (Figures 5C and 5D). By contrast, GFP-α5 intensity did not undergo appreciable increase during slip or after adhesions engaged the substrate. Since the traction stress increased by three-fold during this time, the average tension per available integrin increased during frictional slip by approximately 2 to 3-fold for both αv and α5 integrins (Figure 5E). These data suggest that changes in adhesion strength during frictional slip are not mediated entirely by increases in the total number of integrins available for ligand-binding.
Frictional slip of FAs is modulated by the elastic stiffness of the ECM
The contrasting mobility of nascent adhesions on soft and rigid substrates during the process of FA maturation suggests that frictional slip may be intimately coupled to ECM stiffness. We sought to determine how changing ECM stiffness modifies frictional slip of small adhesions near the cell periphery by plating cells on matrices with varied elastic stiffness and glass coverslips. Consistent with previous reports, cells plated on soft (0.6 kPa) gels displayed smaller adhesions and fewer compact F-actin bundles, as compared to cells plated on rigid (2.8 kPa and glass) substrates (Figure S7A).
In the presence of blebbistatin, the differences in cytoskeletal morphology between cells plated on soft and stiff matrices were largely eliminated. Cells plated on soft matrices contained an isotropic network of F-actin throughout the lamella with a band of small (0.5 µm) adhesions near the LLB (Figure S7B, Movie S6). For matrices with a stiffness less than 2.8 kPa, retrograde frictional slip of adhesion puncta was also observed upon blebbistatin removal (Figure 6A; Figures S7B and S7C; Movies S6 and S7). By contrast, retrograde displacement of adhesions was not resolved on glass (~106 kPa) coverslips (Figure 6A;Figure S7D). Perturbations to fibronectin concentration or changing the ECM ligand to collagen did not alter the magnitude or duration of retrograde displacement of adhesions (Figure S6). Thus, ECM stiffness was the predominant regulator of frictional slip. As the ECM stiffness decreased, the total retrograde displacement of the adhesion increased from < 200 nm to 0.8 µm and the time before adhesion immobilization increased from 0 to 25 sec (Figure 6A). Interestingly, during frictional slip, the FAs of cells in different mechanical environments are indistinguishable in size; elongation of FA plaques occurs only after engagement to the ECM.
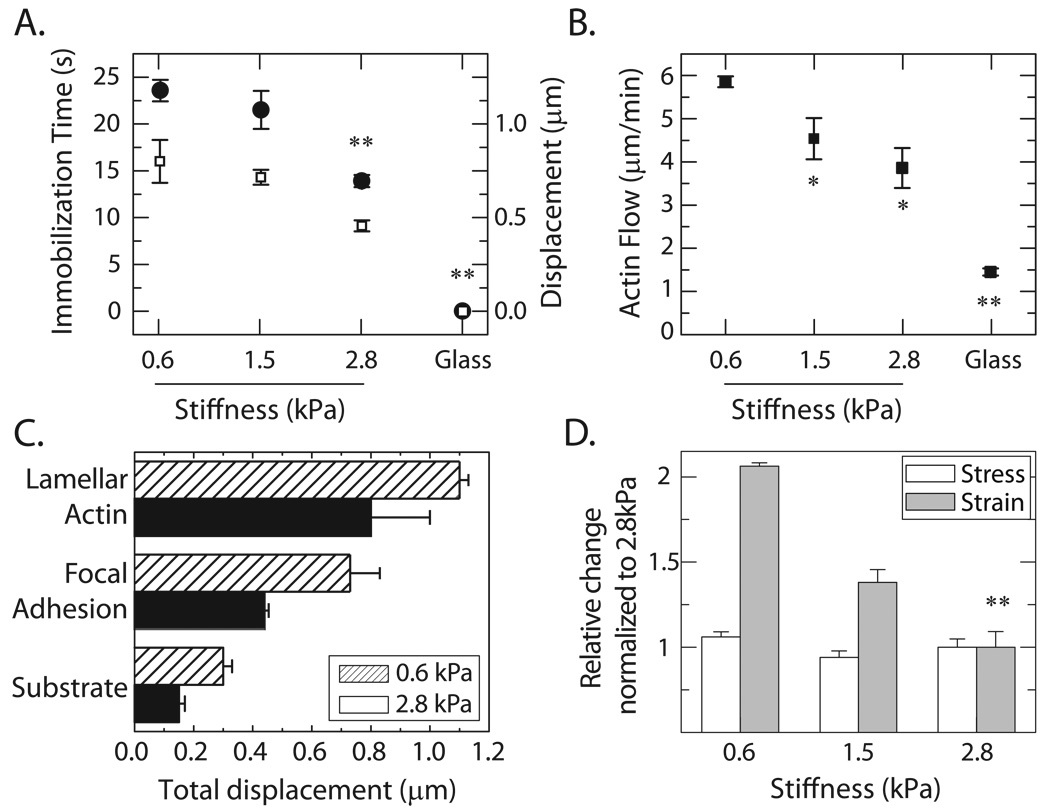
FIGURE 6. FA slip modulated by ECM stiffness.
(A) Adhesion immobilization time (open squares, left) and total displacement during adhesion slip (solid circles, right) plotted against substrate stiffness. (B) Lamellar F-actin retrograde flow at the onset of blebbistatin removal plotted against substrate stiffness. (C) Total displacement during adhesion slip plotted for F-actin, adhesions, and substrate on 2.8kPa and 0.6kPa gels. (D) Relative (normalized to the 2.8 kPa case) changes in substrate strain (deformation) and traction stress (force) for different substrate stiffnesses. Sample size distribution: Glass (nFA=8, ncell=2), 2.8kPa (nFA=36, ncell=5), 1.5kPa (nFA= 8, ncell=3), 0.6 kPa (nFA=8, ncell=3). P-values for data compared to 0.6kPa case; * p<0.01, ** p<0.001.
The initial lamellar F-actin flow rate upon release from blebbistatin also depended on the stiffness of the underlying ECM, increasing from 1.5 µm/min on glass to nearly 6 µm/min on 0.6 kPa gels (Figure 6B). Furthermore, the extent of lamellar F-actin retrograde displacement prior to adhesion engagement also varied on matrices of different stiffness, increasing from 0.8µm on 2.8 kPa gels to 1.1 µm on 0.6 kPa gels (Figure 6C). The displacement of lamellar F-actin always exceeded the retrograde displacement of the nascent adhesion which, in turn, was larger than the substrate deformation (Figure 6C). Collectively, these data indicate that the absolute retrograde displacement of the F-actin, adhesions and ECM during frictional slip depend on the ECM stiffness.
Frictional slip of adhesion is abrogated at a constant tension
Since the abrogation of adhesion frictional slip occurred concomitantly with increased tension and deformation of the extracellular matrix, we sought to determine whether the immobilization of adhesions to the ECM under myosin-II driven tension was a stress (e.g. tension)- or strain (e.g. deformation)-limited process. To explore this, we examined the stress and strain incurred in the substrate at the time of FA engagement for cells plated on matrices with varied elastic stiffness. Modifications to the substrate stiffness change the relationship between the amount of substrate deformation and the magnitude of tension stored within the substrate. As the substrate stiffness is decreased, the amount of deformation required to attain a certain amount of tension increases proportionally. We observed that the magnitude of substrate deformation incurred at the time of FA engagement decreased monotonically as its stiffness increased from 0.6 to 2.8 kPa (Figure 6D). By contrast, the traction stress exerted on the substrate at the time of adhesion engagement was similar for all three substrate stiffnesses, and amounted to a ~30 Pa increase over the traction stress exerted during blebbistatin treatment (Figure 6D). This indicates that the myosin-II driven transition between frictional slip and immobilization to ECM occurs at a constant tension, rather than a constant deformation.
Discussion
We have identified a key biophysical transition that occurs as myosin II tension is applied to adhesions. In the absence of myosin-II activity, polymerization-dependent nascent adhesions form at the base of the lamellipodium (Figure 7A). When myosin-II tension is applied to the lamellar F-actin network, adhesions undergo a transient, rapid, micron-scale rearward translocation while exerting traction against the underlying substrate in a process we term “frictional slip”. During frictional slip, traction stress builds and the retrograde F-actin speed slows. Frictional slip is abrogated once a certain level of force is attained, thereby providing a stable adhesion for further force increase and adhesion elongation.
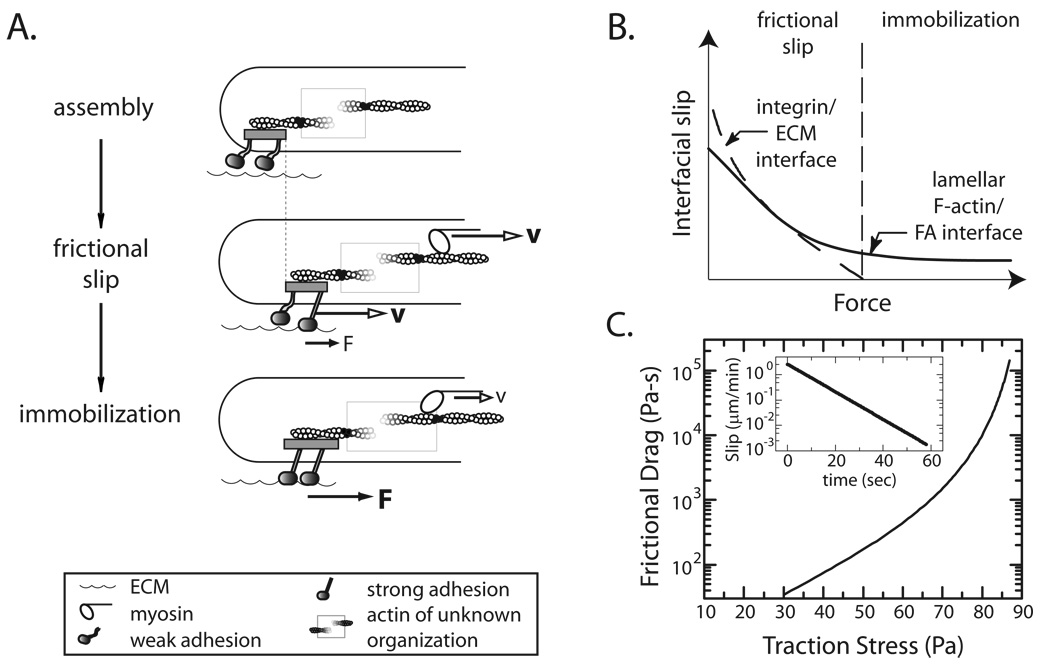
FIGURE 7. Force dependent ‘clutch’ between the integrin and ECM.
(A) Schematic illustrating mobility of cytoskeletal components at different levels of force. Adhesion assembly: integrins are weakly coupled to the ECM. Frictional slip: Myosin-II drives fast retrograde velocity of F-actin and associated integrins (v, open arrow); traction force magnitude is low (small F, solid arrow). Adhesion Immobilization: At a critical tension, integrin-ECM immobilization occurs, traction force increases, myosin-dependent actin flow slows and FA elongation commences. (B) Schematic showing that interfacial slip between the integrin/ECM (dashed line) dominates in early adhesions under low force while slip at the actin/FA interface (solid line) dominates and persists in large adhesions under high force. (C) Semi-log plot of frictional drag coefficient versus traction stress, determined by calculation based on interpolated data points shown in Figure S7C. Inset: a semi-log plot of integrin/ECM slip rate versus time after blebbistatin removal, calculated from interpolated data from Figure S7C.
The rigidity of the ECM regulates the spatiotemporal properties of adhesion frictional slip, such that adhesions displace over longer times and greater distances on softer matrices. With increasing substrate rigidity, the magnitude of rearward translocation decreases such that, for matrices stiffer than ~10 kPa, we estimate that the magnitude of adhesion translocation would not be resolved by light microscopy. This is consistent with observations of FA assembly dynamics on rigid glass coverslips, where nascent FAs appear immobilized immediately upon their appearance (Figure 1) (35).
During the transient frictional slip, the integrins move with multiple FA components and co-localized actin. Thus, the retrograde motion of the F-actin is efficiently transmitted to the integrin. After integrins stabilize relative to the ECM, lamellar F-actin retrograde flow persists. This is consistent with previous experiments, which have found movement between actin and FA proteins within mature FAs (15, 16, 36, 37). This suggests that the initial steps of myosin-II applied tension to FAs are characterized by a transition from a state dominated by slip between integrins and the ECM to one dominated by slip between intracellular FA proteins and F-actin (Figure 7B). We speculate that the location of slip is reflective of the “weakest link” in the actin-FA-ECM connection, and that the locations of such ”weak links” change during adhesion maturation.
The micron-scale movement of adhesions occurs simultaneously with force exertion onto the ECM. This indicates that bonds between the actin cytoskeleton and ECM exist, but the scale of motion is much larger than could be accommodated by deformation of individual molecular bonds. The strong correlation observed between the motion dynamics of numerous adhesion proteins and the FRAP data demonstrating minimal integrin turnover both suggest a stable association of integrins to the FAs during frictional slip. This leads us to speculate that binding kinetics between integrin and the ECM are regulating frictional slip such that detachment and reattachment at bonds between integrins and the ECM would explain the simultaneous force transduction and retrograde movement. A population of such integrins, constituting an adhesion, could allow some to detach, relieve stress, and reattach, while others temporarily transfer the force. Quantitative models incorporating binding kinetics have been successfully used to model the differential motion observed between F-actin and FA proteins (11, 30) and other types of adhesive interactions (38).
Increasing amounts of force occurs concomitantly with decreased retrograde movement, indicating that the strength of the integrin/ECM interface increases during frictional slip. This strength can be estimated by the frictional drag coefficient, σTR/[A(vFA−vS)], where σTR is the traction stress exerted to the underlying matrix, vFA−vS is the difference in flow speed between FA and the substrate (Figure 7C, inset), and A is the approximate area of nascent adhesions (_A_~0.1µm2). A calculation of the frictional drag coefficient from interpolation of our experimental data yields a frictional drag coefficient of the integrin/ECM interface that rapidly rises nearly 100-fold as the applied traction increases from 30 to 70 Pa (Figure 7C). Since little to no accumulation of new integrin is observed within adhesions during this time, we hypothesize that this aggregate bond strengthening occurs predominately through bond reorganization via integrin clustering (39, 40) or through force-induced changes in individual bond strength between the integrin and ECM (41, 42). Future experiments are required to dissect the underlying mechanisms of this strengthening as well as explore the impact of different integrin/ECM interactions.
In summary, we have found that bonds between the integrin and extracellular matrix function as a extracellular “clutch” to modulate the degree of force transmission from the F-actin cytoskeleton to the extracellular matrix in early-stage myosin-II mediated FA maturation. The rapid strengthening of the integrin/ECM interface at small loads would enable protrusions at the leading cell edge to be weakly adherent and mechanically sense the external matrix without rigid attachment. During this initial stage of substrate sensing, the retrograde flow dynamics of the actomyosin cytoskeleton play a prominent role in rapidly building tension to stabilize the integrin binding to ECMs with varied mechanical compliance. However, under sufficient tension generated by actomyosin contraction, this adhesion would rapidly stabilize to impede the retrograde motion of the F-actin and immobilize FA plaques to mediate further FA elongation and growth. This underscores the importance of mechanical feedback between the F-actin cytoskeleton and the ECM in building adhesions that control cell motility and morphology.
Experimental Procedures
Cell culture and transfection
Human osteosarcoma (U2OS) cells were obtained from American Type Culture Collection (ATCC) and maintained in McCoy’s medium (HyClone), supplemented with 10% fetal bovine serum (HyClone), penicillin, and streptomycin (Gibco). Transient transfections of GFP-actin (C. Waterman, NIH), mApple-paxillin, GFP-vinculin (M. Davidson, U. of Florida), GFP-α5 integrin (R. Horwitz, U. of Virginia), GFP-αv integrin and GFP-talin (K. Hu, U. of Indiana) were performed with FuGENE6 Transfection Reagent (Roche) per manufacturer’s instructions.
Immunofluorescence
Immunofluorescence of myosin light chain (monoclonal, Cell Signaling), paxillin (polyclonal, Santa Cruz), vinculin (monoclonal, Sigma), fibronectin (polyclonal, Sigma) and phalloidin (Molecular Probes) staining of F-actin was performed as previously described (18)
Preparation of polyacrylamide (PAA) substrates for traction microscopy
Fibronectin-coated PAA substrates containing 40nm fluorescent spheres were prepared on glass coverslips (10, 43) with varying acrylamide/bis-acrylamide ratios to obtain gels with varying elastic moduli: 7.5%/0.1% for 2.8 kPa, 7.5%/0.05% for 1.5kPa, and 5%/0.075% for 0.6 kPa, as characterized previously (3). Fibronectin or collagen was covalently attached to the top surface of the PAA gel by utilizing the bifunctional cross-linker sulfo-SANPAH (Pierce).
Live Cell Microscopy
Coverslips containing transfected cells bound to PAA substrates were mounted in a perfusion chamber (Warner Instruments) in imaging media consisting of media supplemented with 30 µL/1 mL Oxyrase (Oxyrase Enzyme system, EC0050) and 10mM Hepes, pH 7.5. Cells were imaged at 37C, 24–48 hrs post transfection, on a multispectral spinning disk confocal microscope consisting of a Nikon Ti with a 60× 1.2 NA plan Apo WI objective (Nikon) and a CSUX scanner (Yokogawa) using a CCD camera (Coolsnap HQ2, Photometrics) controlled with Metamorph acquisition software (MDS Analytical Technologies). FAs and beads were monitored at the same confocal section; F-actin was imaged 0.2 microns into the cell interior.
Cells were treated with 25–50µM blebbistatin (Sigma) for 30 min (25). To inactivate blebbistatin, cells were washed with imaging media and visualized with 488nm light (21).
Image Analysis
Particle Imaging Velocimetry code in MATLAB (mpiv, developed by N. Mori and K-A Chang) was used to identify, with sub-pixel accuracy, the movement of beads embedded in polyacrylamide substrate. Fourier transform traction cytometry methods were used to determine traction stress from bead displacements (43). Traction stresses documented in text were calculated as an average of the maximum values across individual FAs.
Fiducial marks of fluorescent F-actin, centroids of FAs and substrate-embedded beads were tracked in separate image channels using computational tracking software developed by the lab of Gaudenz Danuser (24). Manual-tracking, using the point tracking function in Metamorph, of F-actin patches associated with FA punctae was used as an alternative method.
Velocity vector fields were interpolated onto linescans subtending individual FA puncta (perpendicular to cell edge) using a Gaussian-weighted interpolation, with a full-width half maximum of 10 pixels (1 micron). All vector fields exhibited a high degree of directional coupling (10). Lamellar F-actin flow vectors were measured 1.2 µm proximal to the FA centroids (Figure S3A).
FA engagement time was defined as the time at which the displacement rate of FA puncta converged with that of beads within the PAA gel (error of 0.02 µm/min).
Length changes in FAs and actin bundles were quantified in Metamorph. Fluorescence intensity of GFP-α5 and GFP-αv was background-subtracted and integrated across FA area, and normalized to first image plane.
Data plotted in figures reflect the mean plus standard error about the mean.
FRAP
Photobleaching experiments were performed with a 405-nm laser coupled through a micro-mirror array to control the spatial location of illumination (Mosaic, Photonics Instruments) with a 200msec exposure time using a rectangular region of ~ 5 µm2. The photobleaching event took place after acquiring the first image post blebbistatin removal and postbleach images were recorded thereafter. Analysis was performed as in (44).
Highlights
- Under low extracellular tension, new adhesions slip at the integrin-ECM interface
- Traction forces are generated throughout the adhesion slip period
- The magnitude of adhesion slip is regulated by extracellular matrix compliance
- Adhesion immobilization to the extracellular matrix occurs at a constant tension
Supplementary Material
01
02
03
04
05
06
07
08
Acknowledgements
The authors wish to acknowledge use of computational analysis algorithms to analyze cytoskeletal dynamics and traction forces developed in the labs of Gaudenz Danuser and Ulrich Schwarz, respectively, as well as generous gifts of cDNA constructs used in this paper: GFP-actin (C. Waterman, NIH), mApple-paxillin (M. Davidson, U. of Florida), GFP-α5 integrin (R. Horwitz, U. of Virginia), GFP-αv integrin and GFP-talin (Ke Hu, U. of Indiana). Experiments visualizing fibronectin mobility during adhesion slip were conducted by S.P. Winter. The authors would like to thank Y. Beckham, P. Oakes, and T. Schaus for helpful comments and careful reading of the manuscript. This work was supported by a Burroughs Wellcome Career Award and NIH Director's Pioneer Award (DP10D00354) to M.L. Gardel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 2.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. J Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 6.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 8.Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister JJ, Bershadsky AD, Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE. 2008;3:e3234. doi: 10.1371/journal.pone.0003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10:1062–1068. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, Waterman CM. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol. 2008;183:999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- 12.Jurado C, Haserick JR, Lee J. Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol Biol Cell. 2005;16:507–518. doi: 10.1091/mbc.E04-10-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 14.Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–337. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- 15.Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, Horwitz AR, Wiseman PW. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J Cell Sci. 2006;119:5204–5214. doi: 10.1242/jcs.03321. [DOI] [PubMed] [Google Scholar]
- 16.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- 17.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 18.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 20.Verkhovsky AB, Svitkina TM, Borisy GG. Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J Cell Biol. 1995;131:989–1002. doi: 10.1083/jcb.131.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto T, Limouze J, Combs CA, Straight AF, Sellers JR. Blebbistatin, a Myosin II Inhibitor, Is Photoinactivated by Blue Light. Biochemistry. 2005;44:584–588. doi: 10.1021/bi0483357. [DOI] [PubMed] [Google Scholar]
- 22.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danuser G, Waterman-Storer CM. Quantitative fluorescent speckle microscopy of cytoskeleton dynamics. Annu Rev Biophys Biomol Struct. 2006;35:361–387. doi: 10.1146/annurev.biophys.35.040405.102114. [DOI] [PubMed] [Google Scholar]
- 24.Ji L, Danuser G. Tracking quasi-stationary flow of weak fluorescent signals by adaptive multi-frame correlation. J Microsc. 2005;220:150–167. doi: 10.1111/j.1365-2818.2005.01522.x. [DOI] [PubMed] [Google Scholar]
- 25.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 27.Le Clainche C, Carlier M-F. Regulation of Actin Assembly Associated With Protrusion and Adhesion in Cell Migration. Physiol. Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 28.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 2001;155:1319–1332. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macdonald A, Horwitz AR, Lauffenburger DA. Kinetic model for lamellipodal actin-integrin 'clutch' dynamics. Cell Adh Migr. 2008;2:95–105. doi: 10.4161/cam.2.2.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehrle-Haller B, Imhof B. The inner lives of focal adhesions. Trends Cell Biol. 2002;12:382–389. doi: 10.1016/s0962-8924(02)02321-8. [DOI] [PubMed] [Google Scholar]
- 32.Lele TP, Thodeti CK, Pendse J, Ingber DE. Investigating complexity of protein-protein interactions in focal adhesions. Biochem Biophys Res Commun. 2008;369:929–934. doi: 10.1016/j.bbrc.2008.02.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mould AP, Askari JA, Barton S, Kline AD, McEwan PA, Craig SE, Humphries MJ. Integrin activation involves a conformational change in the alpha 1 helix of the beta subunit A-domain. J Biol Chem. 2002;277:19800–19805. doi: 10.1074/jbc.M201571200. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Salas A, Springer TA. Bistable regulation of integrin adhesiveness by a bipolar metal ion cluster. Nat Struct Biol. 2003;10:995–1001. doi: 10.1038/nsb1011. [DOI] [PubMed] [Google Scholar]
- 35.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008 doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo W-h, Wang Y-l. Retrograde Fluxes of Focal Adhesion Proteins in Response to Cell Migration and Mechanical Signals. Mol. Biol. Cell. 2007;18:4519–4527. doi: 10.1091/mbc.E07-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YL. Flux at focal adhesions: slippage clutch, mechanical gauge, or signal depot. Sci STKE. 2007;2007:pe10. doi: 10.1126/stke.3772007pe10. [DOI] [PubMed] [Google Scholar]
- 38.Thomas W. Catch bonds in adhesion. Annu Rev Biomed Eng. 2008;10:39–57. doi: 10.1146/annurev.bioeng.10.061807.160427. [DOI] [PubMed] [Google Scholar]
- 39.Selhuber-Unkel C, López-García M, Kessler H, Spatz JP. Cooperativity in Adhesion Cluster Formation during Initial Cell Adhesion. 2008;95:5424–5431. doi: 10.1529/biophysj.108.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roca-Cusachs P, Gauthier NC, del Rio A, Sheetz MP. Clustering of α5Î21 integrins determines adhesion strength whereas αvÎ23 and talin enable mechanotransduction. Proceedings of the National Academy of Sciences. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 43.Sabass B, Gardel ML, Waterman CM, Schwarz US. High resolution traction force microscopy based on experimental and computational advances. Biophys J. 2008;94:207–220. doi: 10.1529/biophysj.107.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aratyn YS, Schaus TE, Taylor EW, Borisy GG. Intrinsic dynamic behavior of fascin in filopodia. Mol Biol Cell. 2007;18:3928–3940. doi: 10.1091/mbc.E07-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03
04
05
06
07
08