O-GlcNAc Signaling in the Cardiovascular System (original) (raw)
. Author manuscript; available in PMC: 2011 Jul 23.
Abstract
Cardiovascular function is regulated at multiple levels. Some of the most important aspects of such regulation involve alterations in an ever-growing list of post-translational modifications. One such modification orchestrates input from numerous metabolic cues to modify proteins and alter their localization and/or function. Known as the beta-O-linkage of N-acetylglucosamine (i.e. O-GlcNAc) to cellular proteins, this unique monosaccharide is involved in a diverse array of physiologic and pathologic functions. This Review will introduce readers to the general concepts related to O-GlcNAc, the regulation of this modification, and its role in primary pathophysiology. Much of the existing literature regarding the role of O-GlcNAcylation in disease addresses the protracted elevations in O-GlcNAcylation observed during diabetes. In this Review, we will focus on the emerging evidence of its involvement in the cardiovascular system. In particular, we will highlight evidence of protein O-GlcNAcylation as an autoprotective alarm or stress response. We will discuss recent literature supporting the idea that promoting O-GlcNAcylation improves cell survival during acute stress (e.g. hypoxia, ischemia, oxidative stress), whereas limiting O-GlcNAcylation exacerbates cell damage in similar models. In addition to addressing the potential mechanisms of O-GlcNAc-mediated cardioprotection, we will discuss technical issues related to studying protein O-GlcNAcylation in biological systems. The reader should gain an understanding of what protein O-GlcNAcylation is, and, that its roles in the acute and chronic disease settings appear distinct.
Keywords: Myocardial ischemia, Glucose, Diabetes mellitus, Mitochondria
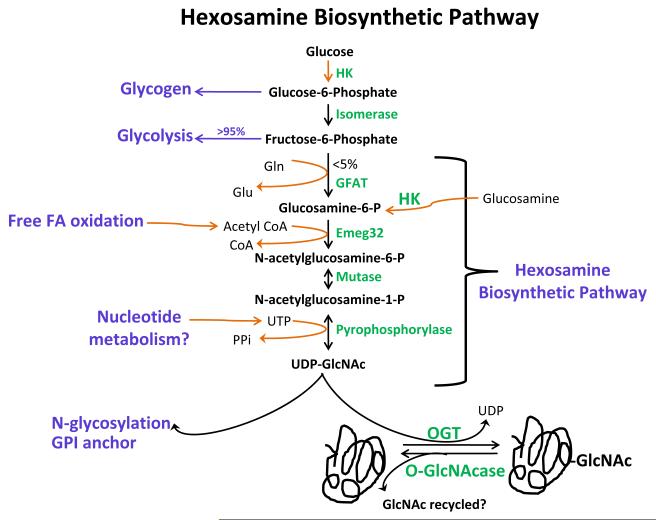
Hexosamine Biosynthetic Pathway
Much has been written about glycolysis, beta-oxidation, and the other major metabolic pathways in cells. Yet, there are several under-investigated accessory glycolytic pathways, whose importance in the cardiovascular system is now beginning to be appreciated. Eukaryotic glycosylation represents a highly varied and complex collection of biological pathways, which are too broad for serious discussion here. This review will focus on one unique form of glycosylation and the reader should refer to definitive sources1 for information on other forms of glycosylation. The hexosamine biosynthetic pathway (HBP) exemplifies one such accessory pathway for glucose metabolism. Based on evidence from cell lines2, the HBP consumes a small fraction of glucose and involves a series of enzyme-catalyzed reactions ending with the formation of uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc). This pathway (see Figure 1) begins with the rate limiting conversion of fructose-6-phosphate to glucosamine-6-phosphate by L-glutamine: fructose-6-phosphate amidotransferase (GFAT) using glutamine. The next critical reaction involves the conversion of gluocosamine-6-phosphate to N-acetylglucosamine-6-phosphate by the glucosamine-6-phosphate acetyltransferase (Emeg32) using acetyl-coenzyme A (CoA). Once formed, UDP-GlcNAc provides the glycoside precursor for glycoproteins, glycolipids, proteoglycans, and more germane to this review, serves as the nucleotide sugar for the post-translational glycosylation of nuclear, cytoplasmic and mitochondrial proteins known as O-GlcNAc. In general, little is known about the relative flux through HBP in the heart or vasculature.
Figure 1. Hexosamine Biosynthetic Pathway.
Phosphorylated glucose enters either the glycogen synthetic pathway or is further converted to fructose-6-phosphate by glucose-6-phosphate isomerase. The majority of fructose-6-phosphate is channeled to glycolysis. Less than 5% of glucose uptake is ultimately channeled to a unique accessory pathway for glucose metabolism, the hexosamine biosynthetic pathway (HBP). This pathway begins with the rate-limiting enzyme, L-glutamine:D-fructose-6-phosphate amidotransferase (GFAT), followed by acetylation of gluocsamine-6-phosphate by glucosamine-6-phosphate acetyltransferase (Emeg32) to N-acetylglucosamine-6-phosphate (GlcNAc-6-P). Next are two reversible reactions: the conversion of GlcNAc-6-P to GlcNAc-1-P by phosphate-acetylglucosamine mutase and then the formation uridine diphosphate-GlcNAc (UDP-GlcNAc) by UDP-GlcNAc pyrophosphorylase. This high-energy molecule serves as the monosaccharide donor for the post-translational modification by O-GlcNAc transferase (OGT). O-GlcNAcase (OGA) removes O-GlcNAc modification from proteins.
The diverse nature of the precursors of UDP-GlcNAc links HBP to several metabolic pathways. Once glucose enters the cell it is phosphorylated to glucose-6-phosphate, metabolized to fructose-6-phosphate and a fraction is diverted to the HBP2. Glutamine, which enters the HBP at the rate-limiting step, is a highly abundant nonessential amino acid in muscle cells and potentially links HBP to amino acid metabolism. Acetyl-CoA, a ubiquitous metabolic intermediate, links HBP to lipolysis/lipogenesis, glucose oxidation, and amino acid catabolism. Finally, the HBP requires ATP at the final step, i.e. the conversion of N-acetylglucosamine-1-phosphate to UDP-GlcNAc by UDP-GlcNAc pyrophosphorylase. Because the precursors of UDP-GlcNAc are nutrient derived and potentially from other metabolic pathways, UDP-GlcNAc, and hence the O-GlcNAc post-translational modification, might act as a nutrient/metabolic sensor.
Little is known about the regulation of HBP flux in the heart. Eukaryotic GFAT is highly conserved and regulated transcriptionally3 and post-translationally by cAMP-dependent protein kinase4, 5 and by UDP-GlcNAc feedback inhibition6. GFAT exists in two isoforms, GFAT1 (highly expressed in the pancreas, placenta and testis) and GFAT2 (highly expressed in the heart and CNS). Emeg32 is critical for maintaining the proper intracellular concentration of UDP-GlcNAc7, thus, it may indirectly regulate O-GlcNAcylation. Thus, investigations in this area will undoubtedly provide important insights into cardiovascular disease.
N-glycosylation vs. O-GlcNAcylation vs. Phosphorylation
O-GlcNAc is a post-translational modification of nuclear, cytoplasmic and mitochondrial proteins first described in 1984 by Torres and Hart8. In their study, they attempted to probe for glycans on surface proteins of lymphocytes using β-D-1-4-galactosylaminyl transferase8. Most of the proteins labeled were intracellular proteins and the labels were incorporated on single GlcNAc residues rather than complex polysaccharide associated with cell surface proteins8. O-GlcNAcylation is in many ways distinct from ‘classical’ protein glycosylation. First, O-GlcNAc modified proteins are found mostly within the nucleus, cytoplasm, or mitochondria contrary to N-glycosylation, which predominates in cell surfaces, the lumen of membranous organelles, the endoplasmic reticulum, and Golgi apparatus9, 10. Second, O-GlcNAc is not elongated into complex structures or further modified with the exception of a nuclear pore protein, unlike the extraordinarily complex array of glycans found on extracellular glycoproteins8, 11, 12. Third, O-GlcNAc rapidly cycles on and off proteins on a time scale similar to that of phosphorylation/dephosphorylation but unlike extracellular complex glycans, which are essentially static13-16. Fourth, there is yet no obvious consensus sequence for the addition of O-GlcNAc to proteins while N-glycosylation has Asn-X-Ser/Thr sequence (where X could be any amino acid other than proline or aspartic acid). Fifth, GlcNAc is added to proteins through an O-linkage on the hydroxyl group of Ser/Thr, while in N-glycosylation the monosaccharides are added through an N-linkage on the amide group of Asn.
O-GlcNAcylation is one of the most common post-translational modifications 17 and is similar to protein phosphorylation in that: Both O-GlcNAcylation and phosphorylation post-translational modifications are found on serine and threonine residues18, 19; both modifications are dynamically added and removed from proteins in response to cellular signals20-22; both alter the functions and/or associations of the modified protein. O-GlcNAc differs from protein phosphorylation in that only two enzymes catalyze the addition and removal of O-GlcNAc from proteins, while over 600 genetically distinct kinases and phosphatases regulate the addition and removal of phosphorylation in mammalian cells. Even though many phosphorylation sites are also known glycosylation sites20, 21, the view that O-GlcNAc and phosphorylation exist in a “yin-yang” 18, or simply reciprocal relationship, likely represents an overly simplistic model.
O-GlcNAc and O-phosphate site-mapping studies suggest that there are several types of dynamic interplay between O-GlcNAc and O-phosphate. There is evidence of competitive occupancy at the same site, for example that which occurs in the transcription factor c-Myc22-24, estrogen receptor-β25, 26, oncoprotein SV-40 large T-antigen, and endothelial nitric oxide synthase27; that is, a site is either O-GlcNAc modified, phosphorylated, or unmodified. In alternative occupancy occurring at adjacent sites, such as that observed in the tumor suppressor p53 28 and synapsin I 29, glycosylation can inhibit phosphorylation at adjacent sites by steric hindrance or modulation of protein structure. Other highly complex interactions also likely exist and do not fall into either of the former categories30, 21. Furthermore, the interplay between O-GlcNAc and O-phosphate is also underscored by the recent finding that OGT transiently forms complexes containing the catalytic subunit of protein phosphatase 1c (PP1c) 31, hence in some contexts there may exist a single enzyme complex for the addition of GlcNAc and removal of phosphate.
Enzymatic Regulation of O-GlcNAcylation
Modulation of protein O-GlcNAcylation is achieved by the concerted action of two highly evolutionarily conserved enzymes, a uridine diphospho-N-acetylglucosamine: peptide β-N-acetylglucosaminyl transferase (O-GlcNAc transferase; aka OGT) and O-β-N-acetylglucosaminidase (O-GlcNAcase; aka OGA, GCA, or mgea5). O-GlcNAc transferase (OGT) is a soluble, ubiquitously expressed, and highly conserved enzyme in all multi-cellular eukaryotic organisms involved with the addition of a single β-N-acetylglucosamine (GlcNAc) moiety via an O-linkage to serine/threonine amino residues nuclear, cytoplasmic and mitochondrial proteins32-35. OGT is expressed in all tissue types examined and most abundant in the glucose-sensing cells of the pancreas and in the brain. OGT is primarily located in the nucleus and has an optimum pH of ~632, 33. OGT is encoded by a single copy X-linked gene in mammals while plants have two OGT homologs, spy and secret agent32, 33, 36, 37. Even though OGT is coded by a single gene in mammals, alternative splicing of OGT mRNA leads to three isoforms: nucleocytoplasmic OGT (ncOGT), mitochondrial OGT (mOGT), and short OGT (sOGT)32, 33, 38. These isoforms share an identical C-terminal catalytic domain but have distinct N-terminal domains contributing to their differential localization and unique targeting sequences38-40. Structurally, OGT contains an N-terminal tetratricopeptide repeat (TPR), a linker region and C-terminal catalytic domains32, 33. TPR domain consists of a 34 amino acid repeat varying from 3-12 involved in inter-subunit interaction, protein-protein interaction, subcellular targeting, substrate recognition, cell cycle regulation, and transcriptional control33, 41-46. The linker region is the least conserved sequence of OGT. The catalytic domain of OGT is thought to have a UDP-GlcNAc binding site and is involved with the glycosylation of proteins47. Post-translational modification of OGT by tyrosine phosphorylation and O-GlcNAc modification, UDP-GlcNAc concentration, and protein-protein interaction are thought to regulate OGT activity 32, 33, 48, 49.
Recently, insulin signaling was shown to regulate OGT50, 51. In neuro-2a neuroblastoma cells, OGT mRNA and protein expression are regulated in an AMP-activated protein kinase-dependent manner, whereas OGT enzymatic activity is regulated in a p38 MAPK-dependent manner48. Moreover, activated p38 has been shown to interact with OGT and recruit it to specific substrates, such as neurofilament H during glucose deprivation48. Tissue specific OGT mutation causes disturbance in somatic cell function52, while conventional OGT deletion is embryonic-lethal36, hence O-GlcNAc is important for cellular viability.
O-β-N-acetylglucosaminidase (O-GlcNAcase; aka OGA, GCA, mgea5) is a soluble, highly conserved enzyme, expressed in all eukaryotic organisms involved with the removal of O-GlcNAc modification from proteins53. O-GlcNAcase is primarily located in the cytoplasm with an optimum pH of 5.5-7 and coded for by a single gene. Two splice variants of O-GlcNAcase have been reported in rats both lacking O-GlcNAcase activity but retained HAT activity. The spliced variant detected in Goto-Kakizaki rats (~90kDa) lacks exon 8 while the spliced variant in Sprague-Dawley (~84kDa) lacks both exons 8 and 9. Structurally, O-GlcNAcase is a 917 amino acid protein with an N-terminal hexosaminidase and a C-terminal histone acetyltransferase domain (HAT) 53-57. The N-terminal domain is similar to hyaluronidase and was originally identified as meningioma expressed antigen 553, 55-57. While there may be some activity against hyaluron in vitro, the preferred substrate for O-GlcNAcase is O-GlcNAc53, 58. The C-terminal HAT domain can acetylate free histones and nucleosomal histone proteins54. It is of interest to note that caspase 3 can cleave O-GlcNAcase into HAT and hexosaminidase domains with no change in the activity of each domain 58. Protein-protein interaction and phosphorylation are also thought to regulate O-GlcNAcase activity53, 58, though the data in this regard are limited. Interestingly, Hanover and colleagues59 have described a short form of OGA that seemingly lacks the HAT domain.
Cell Cycle
Cellular growth, division, and maturation are ordered processes and are tightly controlled by a number of different extracellular events and genetic programs. To this end, Slawson et al. showed convincing evidence that O-glycosylation was modulated during the cell cycle, being lowest at M phase and highest at G1/S and G2/M60. Most recently, it was demonstrated that OGT and O-GlcNAcase interact transiently with the mitotic kinase Aurora B and the protein phosphatase 1 61. The strongest support for a functional link between O-GlcNAc and cell cycle is the observation that several proteins are modified by O-GlcNAc in a cell cycle dependent manner, including c-Myc62, keratins20, YY163, and vimentin61. Also supporting this notion, OGT knockout cells became growth arrested52. Interestingly, altering extracellular glucosamine levels has been implicated in growth arrest in some cancer cells64, further strengthening the observation that O-GlcNAc cycling is important for regulation of the cell cycle.
Transcriptional Regulation
Numerous proteins are responsible for the correct control and maintenance of transcription in the eukaryotic nucleus. Chromatin remodeling (stimulated by the activity of histone acetyltransferase) in response to stimuli, permits transcriptional machinery to initiate mRNA synthesis. Post-translational modification of key proteins has distinct roles in controlling this process. Teleologically, it should not be surprising that O-GlcNAc signaling can influence transcription because OGT is known to associate with histone deacetylase complexes44. O-GlcNAcase also contains a domain with reported histone acetyltransferases (HAT) activity in aa583-917 and shares high homology with HAT in residues 772-89954. Mutation in aspartic acid and phenylalanine residues ablates activity and correlates with evidence suggesting that O-GlcNAcase is capable of acetylating either nucleosomal histone proteins or free core histones54.
Experimental evidence supports O-GlcNAcylation as an important post-translational modification directly regulating transcription. In fact, several transcription factors have been identified to be regulated by O-GlcNAc-modification65-68. O-GlcNAcylation can either suppress or enhance transcription, depending on the promoter involved and other associated co-activator/repressor proteins. For example, OGT can mediate transcriptional repression after being recruited to promoter regions by association with the transcriptional corepressor mSin3A44. Others have shown that the transcription factor STAT5A alters gene activation by preferentially binding to the co-activator of transcription, CREB-binding protein, when O-GlcNAc modified69.
O-GlcNAc modification of Sp1 has multiple effects on the function of Sp1 as a transcription factor68. Augmented O-GlcNAc modification of Sp1 drives the transcription of plasminogen activator and extracellular matrix proteins, which have an important role in diabetic cardiovascular disease, while reduction of Sp1 O-GlcNAcylation increased Sp1 proteasomal susceptibility70. Several post-translational modifications are necessary to control its activity, among these are eight O-GlcNAc modified sites71. The O-GlcNAcylation of Sp1 seems to have an exquisite logic regarding the modification site of the protein. O-GlcNAcylation of the DNA-binding domain of the C-terminus augments its activity72, 73. Similarly, inhibition of O-GlcNAcase increased Sp1 activity, while overexpression of O-GlcNAcase, RNAi against OGT, or a dominant negative form of OGT reduced the activity of Sp174. Conversely, if the O-GlcNAc modification occurs in sites located in the N-terminus, the result is an inhibition of its transactivation potential72, inhibiting protein-protein interaction. The 11 TPR repeats of OGT (AA1-485) are the residues essential for Sp1 transcriptional repression and protein-protein interaction between OGT and mSin3A repressor 44. Interestingly, insulin elevates nuclear O-GlcNAcylation of Sp175.
The ability of OGT to promote O-GlcNAc modification on RNA polymerase II on the same residues as phosphorylation66, provides a clue regarding how the O-GlcNAc modification can influence cellular transcriptional status. More specifically, O-GlcNAcylation of RNA polymerase II occurs in the C-terminus of the enzyme, which induces a conformational change, blocks phosphorylation on these residues, and potentially regulates gene expression66. The O-GlcNAcylation of RNA polymerase II is facilitated by a recruitment of OGT to transcriptional complexes by the OGT interacting protein OIP-10676. Conversely, in vitro experiments have revealed that a single phosphate residue on the C-terminus of RNA polymerase II blocks the activity of OGT on the enzyme30.
Transcriptional repression by OGT does not apply for all transcriptions factors. For example, the O-GlcNAc modification on FOXO1 promotes promoter activation of some gluconeogenic genes77, 78. Similarly, O-GlcNAcylation of the transcriptional coactivator CRTC2 induces nuclear translocation and also contributes for gluconeogenic gene transcription79. CRTC2 is a coactivator for the cAMP response element binding protein (CREB) that has been reported to be O-GlcNAc modified, producing transcriptional repression in vitro67. Another interesting example is the transcription factor YY-1, which is incapable of binding to DNA when in complex with the retinoblastoma protein (pRb). O-GlcNAcylation of YY-1 relieves the DNA binding inhibition by releasing the protein from a complex with the pRb protein and, consequently, activates transcription63. As in many complex systems, questions still remain unanswered. For example, how does O-GlcNAc accomplish the task of activating some transcription factors and inhibiting others? What has to be determined is how O-GlcNAc ‘selects’ which genes must be turned off or on.
Involvement in Diabetes and Insulin Signaling
Diabetes remains a primary risk factor for the development of heart disease. Numerous reports have implicated alterations in O-GlcNAc signaling in diabetic pathophysiology. From simple approaches like exposing cells to glucosamine or hyperglycemia, or, in a more advanced system such as using diet-induced or genetic animal models, data support the notion that O-GlcNAc may contribute to diabetes80, 81. Most recently, studies have described potential mechanisms relating high glucose78, 79 and the enzyme OGT51 to insulin resistance (a hallmark of type II diabetes). Montiminy’s group found that the transducer of regulated cyclic adenosine monophosphate response element–binding protein 2 (TORC2 or CRTC2) is a substrate for OGT and is O-GlcNAcylated at Ser70 and Ser171, which are known phosphorylation sites79. Phosphorylation of CRTC2 prevents its nuclear translocation via interaction with the chaperone protein 14:3:382. The O-glycosylation of CRTC2 impairs its phosphorylation and releases it from the complex with 14:3:3 protein79 (Figure 2). In addition to this effect, the O-GlcNAc modification of CRTC2 promotes activation of a conserved cyclic adenosine 3′-5′ monophosphate (cAMP) response element (CRE) on the glucose-6-phosphatase (G6Pase) promoter79, which is required for G6Pase transcription 83 in response to glucose. So, it appears that a balance between O-GlcNAc and phosphorylation must exist in order to avoid disturbances in insulin signaling.
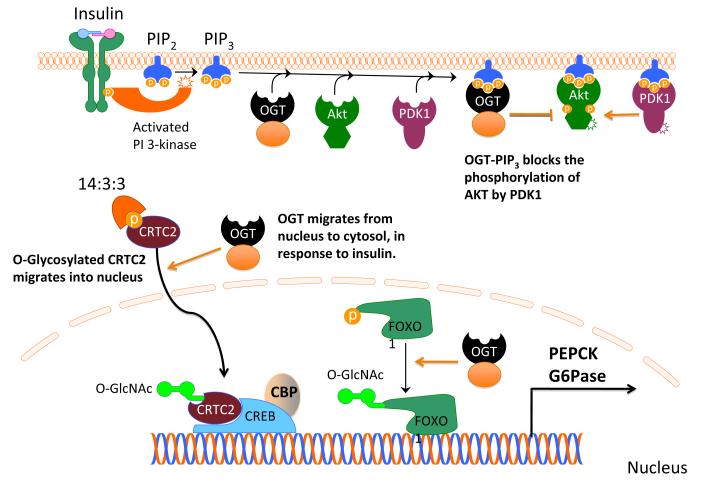
Figure 2. O-GlcNAc Affects Insulin Signaling.
Insulin binds to tyrosine kinase receptor and rapidly activates intracellular signaling. PI3K gives rise to PIP3, which serves to anchor PDK1 and AKT on the membrane. According to Yang et al51, PIP3 also recruits OGT to the plasma membrane where OGT attenuates insulin signaling by O-glycosylation of Thr 308, consequently inhibiting phosphorylation of Akt at the same residue. Excessive activation of OGT could disturb insulin signaling. Hyperglycemia may also stimulate the O-GlcNAcylation of CRTC2 and its migration into the nucleus, where this coactivator binds to CREB:CBP and stimulates transcription of gluconeogenic genes (PEPCK and G6Pase)79. The transcription factor FOXO1 is also O-GlcNAcylated under similar conditions and stimulates gluconeogenic gene transcription77, 166.
CRTC2 is not the only O-GlcNAc modified protein that augments expression of gluconeogenic genes after exposing cells to glucose. Housley and co-workers demonstrated that the transcription factor FOXO1 is also O-GlcNAc modified in diabetes, resulting in increased expression of gluconeogenic genes PEPCK and G6Pase. These authors also found that O-GlcNAc elevated expression of several antioxidant genes in association with elevated glucose production by hepatocytes 77, 78.
Recently, an innovative hypothesis regarding insulin signaling has emerged (see Figure 2). Yang et al., showed that insulin induces nuclear to cytoplasmic translocation of OGT, where OGT binds the lipid phosphatidylinositol-3,4,5-trisphosphate at the plasma membrane51. This interaction is not constitutively active, but may underlie an important mechanism in insulin signaling. Such dynamic trafficking of OGT results in altered phosphorylation of key insulin signaling molecules and in attenuation of insulin signaling. Based on the fact that OGT shares some limited, regional homology with protein phosphatase 5, which exhibits affinity for lipids, they hypothesized the interaction of OGT with lipids. Moreover, the authors identified the regulatory domain, necessary to bind lipids, in the C-terminus of OGT as being rich in lysine residues (K981, K982, K986, K989). The N-terminus of OGT may contribute by making OGT specific to phosphatidylinositol-3,4,5-trisphosphate compared to others lipids of the same class51. Sustained activity of OGT by nutrient excess, for example, impairs insulin signaling51. Moreover, augmenting O-GlcNAc levels via OGT overexpression, or inhibiting O-GlcNAcase (via PUGNAc) also recapitulates the phenomenon of insulin resistance51, 79, 84-86.
Others have specifically investigated the potential contribution of O-GlcNAcylation due to the pathogenesis of diabetes/hyperglycemia. In the intact animal, Hu et al.,87 were first able to link cardiac dysfunction in streptozotocin-induced diabetic mice to excessive O-GlcNAcylation. Work from McClain et al.,81 showed that overexpressing OGT induces insulin resistance in myocytes. OGT upregulation has also been reported in other diabetic mouse models81. Unfortunately, limited clinical insights exist regarding O-GlcNAc signaling. However, insights from some small studies relate cases of human diabetes to impairments in O-GlcNAc signaling. Namely, one study examining type II diabetes in Mexican-Americans found reduced O-GlcNAcase expression with the progression of the disease88. It is then plausible that the O-GlcNAc network rearrangements (with increased OGT levels or decreased O-GlcNAcase, or both) are the major contributors to the development and progression of the disease. More recently, Hart’s laboratory has demonstrated the concept of using blood samples for diagnoses in human patients with diabetes89. So, if O-GlcNAc contributes to the pathogenesis of diabetes, is there a potential mechanism? Work from Dillmann’s group suggests that hyperglycemia reduces the levels of the sarcoplasmic reticulum Ca-ATPase 2A (SERCA2A), which was correlated with prolonged Ca2+ transients and reversed by O-GlcNAcase90. Indeed, more work is needed to determine the potential mechanisms.
Vascular dysfunction represents an important aspect of insulin resistance and diabetes. Limited data exists in patients, but Federici et al found that O-GlcNAcylation was elevated in carotid plaques from patients with diabetes compared to patients without 91. Interestingly, some groups have identified eNOS to be an important target of O-GlcNAcylation in this process27, 91, 92. O-GlcNAcylation modification of eNOS apparently inhibits its ability to generate NO, promote vasodilation, and limit platelet aggregation, and, contributes to mitochondrial oxidative stress92. Although eNOS may directly be modified by O-GlcNAc, its impaired activation may also be due to impaired Akt phosphorylation at serine 1177, which is apparently sensitive to GFAT inhibition27. Many questions remain regarding the interplay among diabetes, O-GlcNAc, and cardiovascular function but current evidence supports the notion that chronic elevations in O-GlcNAc participate in the etiology and the pathogenesis of diabetes. However, the next section of this review will also demonstrate the context of the O-GlcNAc signal, particularly its duration, determines its role in pathophysiology.
Role of O-GlcNAc as an Alarm/Stress Signal
Physiological or chemical stress normally induces signal transduction events that involve activation and/or production of molecules and proteins that diminish the effects of deleterious signaling pathways93, 94. Protein phosphorylation is typically the mechanism associated with these signal transduction pathways but in 2004, Zachara et al. showed that O-GlcNAc might be a stress-induced signal. This was a pivotal study for those interested in basic mechanisms of cellular survival. In their study, when multiple cell lines were subjected to diverse stressors (heat shock, ethanol, UV, hypoxia, reductive, oxidative and osmotic stress), there was a rapid and global increase in _O_-GlcNAc levels95. Moreover, diminished O-GlcNAc levels via OGT knockout (in MEF cells) or OGT knockdown (in Cos-7 and Neuro-2A cells) sensitized cells to stress, while augmented O-GlcNAc levels via O-GlcNAcase inhibition (in Neuro-2A cells) or OGT overexpression (in Cos-7 cells) augmented stress tolerance. Since then, several studies have reinforced the idea that O-GlcNAc may mediate stress-induced signal transduction pathways in various systems.
O-GlcNAc is an Acute Stress Signal in the Heart
Does protein O-GlcNAcylation change in the acutely stressed heart? Several groups have shown that O-GlcNAc signaling is altered in vitro when cardiac myocytes are subjected to oxidative, hypoxic, and ER stress, and in vivo following acute myocardial ischemic and trauma-hemorrhagic shock. In a bid to characterize changes in the O-GlcNAc profile following short-term induction of oxidative 10, 96-113 stress, Jones et al. showed that O-GlcNAc levels increased early upon induction of oxidative stress and decreased by 45 minutes109. Such decrement in O-GlcNAc levels corresponded to exacerbated mitochondrial dysfunction and cellular injury. How O-GlcNAc levels change during hypoxia-reoxygenation still remains unclear. However, data from Champattanachai et al. showed that _O_-GlcNAc levels rise following four hours hypoxia and early reoxygenation113, which was confirmed in a follow-up study by the same group112. Whether such findings are consistent for all durations of hypoxia and reoxygenation remains to be seen. Finally, Ngoh et al recently showed that pharmacologic induction of ER stress (with tunicamycin or brefeldin A) in NRCMs augmented O-GlcNAc signaling103. Work in isolated, perfused rat hearts in Chatham’s laboratory revealed that simulated ischemia alone augments O-GlcNAc levels during the reflow phase105. A follow-up study from the same group also showed that low-flow global ischemia augmented both UDP-HexNAc and O-GlcNAc levels. The increase in O-GlcNAc levels occurred early in the low-flow phase and then declined during reflow110. Whether this is true for the intact myocardium remains to be tested. The differential response of O-GlcNAc levels to different models of isolated heart ischemia and in vivo myocardial ischemia may provide insights into the regulation of _O_-GlcNAc signaling in the hypoxic myocardium.
Severe injury such as trauma-hemorrhagic shock induces stress hormones leading to a hypermetabolic state. An early response to severe injury is systemic hyperglycemia and enhanced peripheral glucose uptake114. Even though the effects of hyperglycemia in trauma are still debatable, Mizock et al. showed that stress-induced hyperglycemia, or the provision of additional glucose, could be beneficial by providing an adequate supply of glucose necessary for energy production in critical organs114. Considering the protective effect of augmented _O_-GlcNAc signaling shown by Zachara et al.95, it is plausible that the increase in glucose uptake occurring during trauma-hemorrhage may boost flux through the HBP and consequently augment _O_-GlcNAc levels. Indeed, recent reports by Chatham’s group showed that trauma-hemorrhage models reduced O-GlcNAc signaling in rats96-98.
Inducing brief non-lethal episodes of ischemia and reperfusion to the heart prior to an episode of sustained lethal myocardial ischemia has the capacity to dramatically reduce myocardial injury. This phenomenon, termed ischemic preconditioning (IPC)115, is a transient, self-defense mechanism present in the heart and many other organs, including the kidney, liver, and brain. The ability of ischemic preconditioning to reduce myocardial infarct size is significant and reproducible, and, serves as the gold standard for studies of cardioprotection116. Intense investigation of the mechanisms responsible for the protective effects of preconditioning has revealed numerous potential mediators and downstream effectors of preconditioning, but cause and effect relationships have not been fully delineated. However, several groups have successfully demonstrated that ischemic preconditioning enhances glucose uptake 117-119. Since the monosaccharide donor for O-GlcNAcylation of proteins, i.e. UDP-GlcNAc, is derived from an accessory pathway for glucose metabolism (HBP), it is possible that the increase in glucose uptake occurring during preconditioning may boost flux through the HBP and consequently augment _O_-GlcNAc levels. Indeed, a recent report showed that either early or delayed ischemic preconditioning can augment cardiac O-GlcNAc levels in vivo109. Although it was not determined whether the changes in O-GlcNAcylation of proteins observed in preconditioning contributes to its protective effects, pharmacologic augmentation of O-GlcNAc levels is sufficient to reduce infarct size in vivo109. It would be interesting to determine whether ischemic preconditioning relies upon O-GlcNAc signaling for cardioprotection, and also determine if preconditioning of other organs like the brain and liver elevates O-GlcNAc signaling. Clearly, O-GlcNAc represents an acute stress signal in the heart. The next question is: Does nutrient/pharmacologic/genetic alteration of O-GlcNAc signaling influence cell survival?
Hexosamine Biosynthetic Pathway Flux in Cardiomyocyte Survival
This section of the review will focus on approaches to alter O-GlcNAc signaling via manipulating HBP flux/activity. The rate-limiting step of the HBP requires glutamine, a non-essential amino acid abundant in muscle tissues, to form glucosamine-6-phosphate. Hence, glutamine contributes to the formation of UDP-GlcNAc, thereby driving the O-GlcNAc modification of proteins. In isolated perfused hearts, augmentation of flux through the HBP with glutamine or prior to hypoxia-reoxygenation increased O-GlcNAc levels reduced cardiac damage and preserved post-hypoxic contractile function during the reflow phase107. Conversely, perfusion of the hypoxic isolated heart with azaserine, blocked the HBP-mediated increase in O-GlcNAc levels, diminished functional recovery, and exacerbated post-hypoxic tissue injury105-107. Though the cardioprotective effect of glutamine had been reported by others prior to this study120, the mechanism was not attributed to alterations in flux through HBP or O-GlcNAc signaling. However, results from an in vivo porcine model of myocardial ischemia-reperfusion injury did not demonstrate protection121.
Glucosamine contributes to the formation of UDP-GlcNAc, thereby driving the O-GlcNAc modification of proteins. Using an approach similar to the aforementioned glutamine studies, Chatham’s group perfused isolated rat hearts with glucosamine105, 107, 110, which likely enters the HBP downstream of GFAT and depends on phosphorylation by hexokinase. Glucosamine treatment reduced injury resulting from calcium paradox and hypoxia-reoxygenation105, 107, 110, 111, 122. In neonatal cardiac myocytes, Champattanachai et al. showed that augmentation of O-GlcNAc levels with high glucose or glucosamine improved post-hypoxic cellular viability and attenuated necrosis and apoptosis113. Conversely, euglycemic cardiomyocytes or hyperglycemic cardiomyocytes treated with azaserine (i.e., a GFAT inhibitor) were more sensitive to hypoxic stress113. Interestingly, in vivo augmentation of O-GlcNAc levels using glucosamine after severe injury such as hemorrhagic shock has been shown to improve cardiac function and peripheral organ perfusion in rats. Taken together, these results provide evidence that the protective effect of glucosamine is associated with augmented O-GlcNAc signaling and can be translated to the in vivo environment. Clearly, approaches to boost hexosamine flux promote cardiomyocyte survival.
OGT in Cardiomyocyte Survival
Additional support for cytoprotection associated with global augmentation of O-GlcNAc levels can be found in studies using genetic and molecular approaches to evaluate the enzymes controlling the presence of O-GlcNAc on proteins. Studies involving pharmacologic inhibition of O-GlcNAc transferase (OGT) have been limited, likely because the few inhibitors described have not been well characterized and may exhibit some level of toxicity. The most popular OGT inhibitor, alloxan, is a uracil and UDP-GlcNAc analog and may be an irreversible inhibitor of OGT123. Nevertheless, use of such compounds has yielded consistent results. In one study, alloxan not only blocked the glucosamine-mediated increase in O-GlcNAc levels but also inhibited angiotensin II-induced increase in intracellular Ca2+ in NRCMs104. Although results from these studies are cautiously interpreted due to the high concentration of alloxan used, its toxicity, its lack of specificity124, and because of the recent revelation that alloxan may also inhibit O-GlcNAcase125, they provide important insight into the necessity of OGT signaling in the context of elevated hexosamine biosynthesis. “Compound 4” and “compound 5”, were recently described to be potent OGT inhibitors126, 127. Ngoh et al showed that both compounds 4 and 5 (referred to as TT04 and TT40 in their paper) reduced O-GlcNAc levels and exacerbated post-hypoxic cardiomyocyte injury128. Moreover, inhibition of OGT also exaggerated the post-hypoxic collapse of mitochondrial membrane potential128.
Genetic manipulation of OGT has been shown to alter cardiomyocyte survival post-hypoxia. Ngoh et al showed that adenoviral-mediated OGT overexpression (AdOGT) significantly elevated OGT protein expression, augmented O-GlcNAc levels, and reduced post-hypoxic cardiac myocyte death, while OGT knockdown (via short interfering RNA) or knockout (via cre-lox recombination) reduced O-GlcNAc levels and exacerbated post-hypoxic cell death128. In addition, post-hypoxic mitochondrial membrane potential was also better preserved in the AdOGT group compared with the post-hypoxic control virus (AdGFP). This pro-survival role of OGT was supported by findings from Champattanachai et al. showing that OGT overexpression attenuated H2O2-induced loss of mitochondrial membrane potential and hypoxia-induced apoptosis while OGT knockdown (via siRNA) sensitized NRCMs to H2O2-induced loss of mitochondrial membrane potential and hypoxia-induced apoptosis 112. Thus, OGT seems essential in the constitutive, as well as inducible, abilities of the cell to withstand lethal stressors. In other words, OGT promotes cell survival during acute cardiomyocyte stress.
O-GlcNAcase in Cardiomyocyte Survival
Unlike OGT, there are several pharmacologic inhibitors of O-GlcNAcase, including streptozotocin129, PUGNAc130, 1,2-dideoxy-2-methyl-D-glucopyranoso[2,1-_d_]-2-thiazoline(NButGT)131, GlcNAcstatin132, and Thiamet-G133. PUGNAc, a GlcNAc analog, is the most widely studied inhibitor of O-GlcNAcase and prevents the binding of O-GlcNAcase to GlcNAc. Thus, PUGNAc prevents the removal of O-GlcNAc leading to a rapid increase in O-GlcNAc levels. Even though PUGNAc lacks the cytotoxic effects of STZ134, it potentially inhibits other lysosomal hydrolases and shows limited specificity for O-GlcNAcase over β-hexosaminidase131.
In NRCMs, augmenting O-GlcNAc levels using PUGNAc attenuated post-hypoxic113, 135 and oxidative stress-induced109 injury and inhibited both post-hypoxic135 and H2O2-induced109 mitochondrial depolarization. Similar findings were observed in isolated perfused hearts where PUGNAc administration early in reperfusion improved cardiac functional recovery, reduced troponin release, and attenuated calpain-mediated proteolysis of α-fodrin and Ca2+/calmodulin-dependent protein kinase II compared to untreated control106, 107. The cytoprotective effects of PUGNAc seen in NRCMs and isolated perfused hearts, can be replicated in an in vivo setting. Jones et al. showed in an in vivo murine model of myocardial ischemia-reperfusion injury that PUGNAc reduced infarct size following acute myocardial ischemia reperfusion109.
NAG-thiazoline131 inhibits O-GlcNAcase and has 1500-fold greater specificity for O-GlcNAcase over β-hexosaminidase than PUGNAc, but has not been as widely studied as PUGNAc and is not widely available. Recently, Champattanachai et al showed that NButGT can attenuate cardiac myocyte death following hypoxia and oxidative stress112. Few studies have addressed the role of O-GlcNAcase inhibition in cellular injury beyond the use of PUGNAc or NButGT. As with inhibitors used in the OGT work above, concern remains regarding the off-target effects of the putative inhibitors of O-GlcNAcase. Thus, the use of other approaches, such as RNA interference or adenoviruses, could assuage concerns of some of the off-target effects of the aforementioned pharmacologic approaches. Indeed, Ngoh et al recently showed that siRNA knockdown of O-GlcNAcase augmented O-GlcNAc levels, preserved post-hypoxic cardiomyocyte membrane potential, and mitigated cellular injury while overexpression of O-GlcNAcase reduced O-GlcNAc levels, sensitized NRCMs to loss of mitochondrial membrane potential, and exacerbated cellular injury135. In short, O-GlcNAcase activity antagonizes cell survival during acute cardiomyocyte stress.
Mechanisms of O-GlcNAc Signaling in Cytoprotection
While the specific mechanisms underlying the cytoprotection associated with O-GlcNAc signaling remain to be determined, several putative mechanisms have been advanced to explain augmented stress tolerance. O-GlcNAc signaling likely involves numerous intracellular targets, which may contribute to varying extents during myocardial preservation. Progress in the area of target protein identification has been restricted because of the limitations of the tools necessary for the identification of O-GlcNAc targets. Zachara et al showed that increased O-GlcNAc signaling activated transcription of heat shock proteins HSP40 and HSP7095. Because the cardioprotective effects of glucosamine observed in isolated perfused hearts occurred early105, 110, there may be mechanisms other than de novo protein synthesis contributing to O-GlcNAc-mediated cytoprotection. Recent studies from the Jones laboratory revealed that modulating O-GlcNAc levels may alter O-GlcNAc modification on (at least) the mitochondrial voltage-dependent anion channel (VDAC), which may represent a unique mechanism of cytoprotection109, 128, 135, 136. Activation of mPTP formation is a critical step in mitochondrial mediated death pathway, and, although the molecular identity of the mitochondrial permeability transition pore (mPTP) remains debatable, VDAC has been widely recognized as a putative component, or at least a modulator of mPTP. In these studies, treatment of mice with PUGNAc (an O-GlcNAcase inhibitor), increased O-GlcNAc modification of VDAC and produced resistance of isolated, adult cardiac mitochondria to calcium-induced mitochondrial swelling109. Conversely, treating mice with Compound 4 (a putative OGT inhibitor) reduced O-GlcNAc modification of VDAC and sensitized isolated, adult cardiac mitochondria to mPTP formation128. Augmented O-GlcNAc levels via OGT overexpression or O-GlcNAcase inhibition (with PUGNAc or siRNA) preserved post-hypoxic mitochondrial membrane potential128, 135. Moreover, boosting HBP flux (with glucosamine), OGT overexpression, and O-GlcNAcase inhibition (with NButGT) attenuate H2O2-induced loss of mitochondrial membrane potential and cytochrome c release in NRCMs109, 112, 136. O-GlcNAc levels affect post-hypoxic mitochondrial Ca2+ overload in NRCMs. Overexpression of O-GlcNAcase exacerbated hypoxia-induced Ca2+ overload while inhibition of O-GlcNAcase mitigated hypoxia-induced Ca2+ overload. Several studies have implicated Ca2+ overload as a key contributor to mitochondrial permeability transition leading to ischemia-reperfusion injury and inhibiting the rise in mitochondrial [Ca2+] has been shown to confer cardioprotection following acute myocardial ischemia137, 138. Therefore, it is possible that blocking mitochondrial Ca2+ overload may be an upstream action of O-GlcNAc signaling to prevent mPTP formation in addition to direct effects on mPTP components (Figure 3). Finally, augmented O-GlcNAc levels have been shown to increase mitochondrial Bcl-2 in NRCMs subjected to hypoxia-reoxygenation. Since Bcl-2 is thought to inhibit mPTP formation by interacting with VDAC, it is possible that augmented O-GlcNAc levels would activate Bcl-2 translocation to the mitochondria, increasing the interaction of Bcl-2 with VDAC and subsequently blocking mPTP formation and the release of death factor from the mitochondria. Thus, there is a potential mechanistic link among a likely target of O-GlcNAc signaling, mitochondrial preservation, and cell viability.
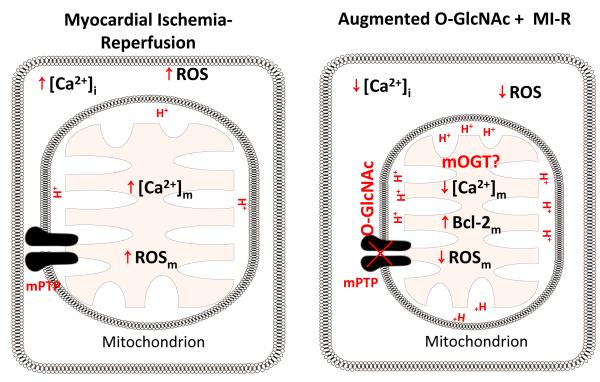
Figure 3. Mitochondria and O-GlcNAc Signaling.
Myocardial ischemia induces mitochondrial Ca2+ overload and ROS generation, with subsequent mitochondrial permeability transition pore formation (mPTP). Formation of mPTP causes loss of mitochondrial trans-inner membrane potential, mitochondrial swelling, rupture of mitochondrial membrane, and cytochrome c release. Augmentation of O-GlcNAc levels prior to myocardial ischemia attenuates ischemia-induced Ca2+ overload, ROS generation, and subsequent mPTP formation. Augmented O-GlcNAc signaling also mitigates mPTP formation by possibly augmenting O-GlcNAcylation of VDAC and/or BCl-2 interaction with VDAC. This is an example of one potential mechanism; there are likely several. Moreover, augmented O-GlcNAc levels diminish mPTP-mediated mitochondrial swelling, loss of mitochondrial membrane potential, and cytochrome c release.
Using glucosamine to boost O-GlcNAc levels, others have shown activation of p38 MAPK and reduce calpain proteolytic activity, reduced ischemic contracture, and attenuated reperfusion induced arrhythmias in isolated perfused hearts107, 110. Such effects could be related to alterations in calcium handling 87, 90, 104, 105, 135 and/or heat shock protein activation95, 109. Considering the evidence for O-GlcNAc-mediated regulation of the ubiquitin-proteasome system70, 139-141, modulation of UPS activity could represent yet another target in the portfolio of O-GlcNAc mediated cytoprotection. Indeed, just as the targets of O-GlcNAc modification are numerous, so too are the potential mechanisms responsible for cytoprotection. Regardless of the mechanisms (and there are likely multiple targets), the salient feature is that acute, global changes in cellular O-GlcNAcylation reflect a pro-adaptive stress response.
O-GlcNAc signaling and Vascular Injury/Inflammation
Arterial hypertension is a multifactorial condition considered a major risk factor for cardiovascular disease. Hypertension is characterized by abnormal vascular reactivity, impaired endothelium-dependent relaxation, and enhanced sensitivity to vasoconstrictors. Several proteins involved in vascular function have been identified to be O-GlcNAc modified27, 91, such as endothelial nitric oxide synthase (eNOS) and protein kinase B (PKB/Akt). Even though it is well established that O-GlcNAc is critical for cellular function, very few studies have addressed the vascular effects of O-GlcNAcylation. Recently, Lima et al. showed that the aorta and mesentery of deoxycorticosterone acetate-salt hypertensive rats have augmented O-GlcNAc levels compared to control142. Such elevated O-GlcNAc signaling was associated with increased reactivity to constrictor stimuli, phenylephrine, and impaired endothelium-dependent vasodilatation to acetylcholine. Whether the change in O-GlcNAc signaling observed with DOCA hypertension is true for all forms of hypertension remains to be tested, though this same group143 has continued to extend their findings.
Inflammation contributes to the pathogenesis of numerous cardiovascular diseases. Even though our understanding of O-GlcNAc signaling in most of cardiovascular pathophysiology remains limited, several recent studies indicate its potential impact extends beyond the cardiomyocyte. Oparil’s group tested the hypothesis that in vivo arterial injury may be affected by alterations in O-GlcNAc signaling 99. Using ovariectomized rats, balloon injury of the carotid artery produced the expected inflammation and vascular pathology in this model. However, treatment with either glucosamine (enhances HBP flux) or PUGNAc (which inhibits O-GlcNAcase and increases O-GlcNAc levels) reduced leukocyte infiltration, inhibited TNF-α-stimulated chemokine and adhesion molecule (ICAM-1 and VCAM-1) expression, IκB-α phosphorylation and NF-κB activation. These data indicate anti-inflammatory effects of augmented O-GlcNAc signaling in their model. Conversely, Tostes’ group has found a potential role for O-GlcNAcylation in the pathophysiology of hypertension142-144, implying a complex interplay in the vasculature.
Severe injury such as trauma-hemorrhagic shock has been shown to alter O-GlcNAc signaling and that enhanced O-GlcNAc signaling improved functional recovery in the heart following hemorrhagic shock96-98. In series of in vivo studies from Chatham’s group, glucosamine administration during resuscitation significantly attenuated hemorrhagic shock induced increase in circulating tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) levels, ICAM expression, IκB-α phosphorylation, NF-κB expression and NF-κB DNA-binding activity in rat heart97, 98. The same group also confirmed this finding with an O-GlcNAcase inhibitor (PUGNAc). In addition, intravenous administration of PUGNAc 30 minutes after the onset of resuscitation following trauma-hemorrhage in rats showed attenuated circulating tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) levels supporting the protective effect of O-GlcNAc signaling on stress-mediated inflammation96.
Technical limitations/suggestions
Since its discovery over two decades ago, several analytical procedures have been evaluated to identify O-GlcNAc modified proteins 145. Because of its substoichiometric concentration, labile nature, lack of charge, and small mass, identification of O-GlcNAc modification has been difficult. The initial tool employed enzymatic labeling of terminal O-GlcNAc residues with radioactive uridine diphospho-galactose (UDP[3H]Gal) using galactosyltransferase8, 146, 147. Because O-linkage of GlcNAc to a protein is resistant to peptide/N-glycosidase F (PNGase F), nonspecifically UDP[3H]Gal tagged N-linked oligosaccharides are cleaved by PNGase F treatment. The main limitation of this technique is that O-GlcNAc is not very accessible to galactosyltransferase, thereby limiting its utility in many instances.
Others have also used succinylated wheat germ agglutinin (sWGA)48, 49, 148-150 to identify O-GlcNAc-modified proteins. sWGA binds to any terminal GlcNAc residue; hence this technique is not particularly selective. The specificity of this technique may be improved using PNGase F to remove non-specific N-linked modifications bound to sWGA. Another disadvantage of this method is that sWGA is less sensitive and only proteins with multiple O-GlcNAc residues are readily detected. Sensitivity may be improved by using sWGA-conjugated Sepharose column to isolate and enrich O-GlcNAc–modified proteins from cell extracts.
The development of monoclonal antibodies that react with O-GlcNAc in the context of protein structure has significantly increased the efficiency of identifying O-GlcNAc–modified proteins151-155. CTD 110.6 was raised against an O-GlcNAc–modified peptide from the large subunit RNA polymerase II CTD155, while RL2 antibody was raised against O-GlcNAc-modified peptide from nuclear pore proteins151-154. Despite their broad immunoreactivity to the O-GlcNAc modification, they are somewhat restrictive in their target specificity and may require more than one O-GlcNAc site, especially for low molecular weight proteins. Recently, new antibodies have been described, which may improve target identification156. To further confirm the specificity of antibody based approaches (Figure 4), O-GlcNAc immunoblots should be incubated with exogenous GlcNAc and O-GlcNAc antibody. Moreover, it would also be useful to demonstrate a loss of signal in parallel aliquots of protein sample to demonstrate that the ‘O-GlcNAc immunoreactivity’ is sensitive to O-GlcNAcase (think of using a phosphatase to confirm a specific phosphorylation signal).
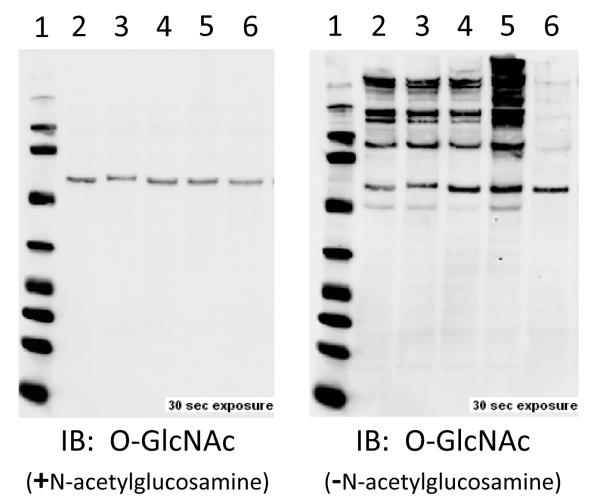
Figure 4. Common Control Measures for O-GlcNAc Immunoblots.
O-GlcNAc immunoblots show multiple bands because it is a post-translational modification of numerous proteins. The blot on the left is the result of a CTD110.6 antibody co-incubated with N-acetylglucosamine, which competes for binding with the antibody. The blot on the right is the same membrane and primary antibody (CTD110.6), without GlcNAc. In addition, the lysate loaded in lane 6 is the result of a parallel aliquot of lane 5 incubated with O-GlcNAcase (in vitro), showing the loss of immunopositivity and validating the signal is O-GlcNAc. Such simple measures can confirm the fidelity of the O-GlcNAc signal via westerns.
Several chemical approaches have also been developed to analyze O-GlcNAc residues 58, 146, 157-163. Initial attempts at O-GlcNAc site mapping were time consuming and complicated by numerous HPLC peptide purifications and manual Edman degradation reactions146, 157, 158. Moreover, the low stoichiometry of the O-GlcNAc modification required a higher starting concentration of purified protein. Because of this, a combination of alkaline β-elimination, collision-induced dissociation (CID), and electrospray ionization mass spectrometry have been employed159 by some experts. Even though alkaline β-elimination reduced the CID energy needed to ionize and fragment the peptide for sequencing by reducing the size of the glycopeptide and CID fragment while preserving O-GlcNAc modification, it causes significant peptide degradation. Another more recent approach, mild β-elimination followed by Michael addition with dithiothreitol (BEMAD)160, tags the α,β-unsaturated carbonyl (the product of β-elimination) with a nucleophilic tag stabilizing the O-linkage during collision-induced dissociation. Tagging allowed for site identification by LC-MS/MS, making BEMAD useful for mass spectrometry160. Not only does the BEMAD method allow for simultaneous study of O-GlcNAc and O-phosphate quantitatively, it also allows the enrichment of either post-translational modification in the study of normal versus diseased states.
Recently, Bertozzi’s group161 reported that OGT and O-GlcNAcase could tolerate analogs of their natural substrates. OGT incorporated can incorporate an azide modified GlcNAc (GlcNAz) into protein targets. Once labeled, these GlcNAz-modified proteins can be covalently derivatized with various biochemical probes at the site of protein glycosylation using Staudinger ligation. This strategy could identify O-GlcNAc-modified proteins, as well as map protein target sites that bear O-GlcNAc modification. Because UDP-GlcNAc is incorporated into several classes of glycoconjugates, specificity must be demonstrated with properly controlled experiments when cells are labeled metabolically. Similar, more specific and improved techniques, tagging-via-substrate162 and Click-chemistry163 have been described. These techniques are highly sensitive and especially useful for lower molecular weight proteins. Despite the development of such techniques, the lack of a recognizable consensus motif somewhat complicates the analyses of O-GlcNAc function and limits predictive capabilities. Currently, it appears that amino acids modified by O-GlcNAc often are surrounded by serine/threonine residues, with a proline often three amino acids to the N-terminal side of the modification 164, 17, 165. However, there are numerous exceptions to such guidelines. Should a clear consensus sequence be established, this field would experience even more growth than its present rate.
Conclusions
Most aspects of cardiovascular biology remain untapped for the potential involvement of O-GlcNAc signaling. The current status of O-GlcNAc in cardiac biology represents an exciting time for discovery. Indeed, multiple phenomena will likely be identified as regulated by O-GlcNAc signaling in the immediate future. Moreover, our limited understanding of diabetes and its impact on metabolism in the cardiovascular system will remain important areas of investigation. Based on current evidence, it appears that O-GlcNAc signaling participates in the pathophysiology of diabetes. Although significant advances in O-GlcNAc proteomics have occurred in the last five years, a long road of technological innovation and dissemination remains.
For the uninitiated, many questions likely remain, which may relate to consensus sequence (not clearly identified), the promoters for OGT/O-GlcNAcase (not reported), and the biophysical impact of O-GlcNAcylation on proteins (no uniform answer). The current literature suggests that O-GlcNAcylation is a metabolic sensor, but this review emphasizes an emerging role for O-GlcNAcylation as a robust stress response. Of course, such a possibility is not mutually exclusive with the current ‘metabolic sensor’ function of O-GlcNAc. Although readers (and reviewers) want to know exactly what the targets of O-GlcNAcylation are in each specific context, it is the authors’ opinion that absence of such information should not be the sole barrier to publication or funding. After all, we have collectively accepted targeted mutagenesis studies of Ser/Thr residues to be definitive evidence of the importance of specific kinase targets. Were we inadvertently precluding the possibility of O-GlcNAcylation? Should we collectively re-evaluate some of our conclusions regarding phosphorylation of Ser/Thr in this new context?
What is clear is that in addition to its purported functions as a metabolic sensor, O-GlcNAcylation of proteins apparently can also reflect cellular stress in the cardiovascular system.
Acknowledgements
We are grateful to the members of the Jones Laboratory for technical support.
Sources of Funding This laboratory has been supported by grants from the NIH-NHLBI (R01 HL083320 and R01 HL094419), American Heart Association National Center (0535270N), NIH-NCRR (P20 RR024489), and Kentucky Science and Engineering Foundation grant (KSEF-1677-RDE-011) to SPJ. GAN was an American Heart Association Predoctoral Fellow (0715493B). HTF is an American Heart Association Postdoctoral Fellow (0825643D). AZ is supported by an ARRA Supplement from the NIH.
Non-standard Abbreviations and Acronyms
HBP
hexosamine biosynthetic pathway
UDP-GlcNAc
uridine diphospho-β-N-acetylglucosamine
GFAT
glutamine:fructose amidotransferase
Emeg32
glucosamine-6-phosphate acetyltransferase
O-GlcNAc
β-O-linked N-acetylglucosamine
OGT
uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetylglucosaminyltransferase
O-GlcNAcase
β-N-acetylglucosaminidase
PGC-1α
peroxisome proliferator-activated receptor-gamma coactivator-1α
NRCMs
neonatal rat cardiac myocytes
DON
6-diazo-5-oxo-L-norleucine
PUGNAc
O-(2-acetamido-2-deoxy-d-glycopyranosylidene)amino-_N_-phenylcarbamate
Ad OGT
adenoviral delivered OGT
Ad OGA
adenoviral delivered O-GlcNAcase
Ad GFP
adenoviral delivered green fluorescent protein
GlcN
glucosamine
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of glycobiology. 2009:784. [PubMed] [Google Scholar]
- 2.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 3.Paterson AJ, Kudlow JE. Regulation of glutamine:Fructose-6-phosphate amidotransferase gene transcription by epidermal growth factor and glucose. Endocrinology. 1995;136:2809–2816. doi: 10.1210/endo.136.7.7789306. [DOI] [PubMed] [Google Scholar]
- 4.Chang Q, Su K, Baker JR, Yang X, Paterson AJ, Kudlow JE. Phosphorylation of human glutamine:Fructose-6-phosphate amidotransferase by camp-dependent protein kinase at serine 205 blocks the enzyme activity. J. Biol. Chem. 2000;275:21981–21987. doi: 10.1074/jbc.M001049200. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Riesland L, Paterson AJ, Kudlow JE. Phosphorylation of mouse glutamine-fructose-6-phosphate amidotransferase 2 (gfat2) by camp-dependent protein kinase increases the enzyme activity. J Biol Chem. 2004;279:29988–29993. doi: 10.1074/jbc.M401547200. [DOI] [PubMed] [Google Scholar]
- 6.Graack HR, Cinque U, Kress H. Functional regulation of glutamine:Fructose-6-phosphate aminotransferase 1 (gfat1) of drosophila melanogaster in a udp-n-acetylglucosamine and camp-dependent manner. Biochem J. 2001;360:401–412. doi: 10.1042/0264-6021:3600401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehmelt G, Wakeham A, Elia A, Sasaki T, Plyte S, Potter J, Yang Y, Tsang E, Ruland J, Iscove NN, Dennis JW, Mak TW. Decreased udp-glcnac levels abrogate proliferation control in emeg32-deficient cells. EMBO J. 2000;19:5092–5104. doi: 10.1093/emboj/19.19.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres CR, Hart GW. Topography and polypeptide distribution of terminal n-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for o-linked glcnac. J. Biol. Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 9.Hart GW. Glycosylation. Curr Opin Cell Biol. 1992;4:1017–1023. doi: 10.1016/0955-0674(92)90134-x. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Oyeleye MO, Dillmann WH. Increased enzymatic o-glcnacylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem. 2009;284:547–555. doi: 10.1074/jbc.M808518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heese-Peck A, Cole RN, Borkhsenious ON, Hart GW, Raikhel NV. Plant nuclear pore complex proteins are modified by novel oligosaccharides with terminal n-acetylglucosamine. Plant Cell. 1995;7:1459–1471. doi: 10.1105/tpc.7.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heese-Peck A, Raikhel NV. A glycoprotein modified with terminal n-acetylglucosamine and localized at the nuclear rim shows sequence similarity to aldose-1-epimerases. Plant Cell. 1998;10:599–612. doi: 10.1105/tpc.10.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ku NO, Omary MB. Expression, glycosylation, and phosphorylation of human keratins 8 and 18 in insect cells. Exp Cell Res. 1994;211:24–35. doi: 10.1006/excr.1994.1054. [DOI] [PubMed] [Google Scholar]
- 14.Roquemore EP, Chevrier MR, Cotter RJ, Hart GW. Dynamic o-glcnacylation of the small heat shock protein alpha b-crystallin. Biochemistry. 1996;35:3578–3586. doi: 10.1021/bi951918j. [DOI] [PubMed] [Google Scholar]
- 15.Dong DL, Hart GW. Purification and characterization of an o-glcnac selective n-acetyl-beta-d-glucosaminidase from rat spleen cytosol. J. Biol. Chem. 1994;269:19321–19330. [PubMed] [Google Scholar]
- 16.Favreau C, Worman HJ, Wozniak RW, Frappier T, Courvalin JC. Cell cycle-dependent phosphorylation of nucleoporins and nuclear pore membrane protein gp210. Biochemistry. 1996;35:8035–8044. doi: 10.1021/bi9600660. [DOI] [PubMed] [Google Scholar]
- 17.Hart GW, Housley MP, Slawson C. Cycling of o-linked [beta]-n-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 18.Hart GW, Greis KD, Dong LY, Blomberg MA, Chou TY, Jiang MS, Roquemore EP, Snow DM, Kreppel LK, Cole RN, et al. O-linked n-acetylglucosamine: The “Yin-yang” Of ser/thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv Exp Med Biol. 1995;376:115–123. [PubMed] [Google Scholar]
- 19.Hart GW, Kreppel LK, Comer FI, Arnold CS, Snow DM, Ye Z, Cheng X, DellaManna D, Caine DS, Earles BJ, Akimoto Y, Cole RN, Hayes BK. O-glcnacylation of key nuclear and cytoskeletal proteins: Reciprocity with o-phosphorylation and putative roles in protein multimerization. Glycobiology. 1996;6:711–716. doi: 10.1093/glycob/6.7.711. [DOI] [PubMed] [Google Scholar]
- 20.Chou CF, Omary MB. Mitotic arrest-associated enhancement of o-linked glycosylation and phosphorylation of human keratins 8 and 18. J Biol Chem. 1993;268:4465–4472. [PubMed] [Google Scholar]
- 21.Chou CF, Smith AJ, Omary MB. Characterization and dynamics of o-linked glycosylation of human cytokeratin 8 and 18. J Biol Chem. 1992;267:3901–3906. [PubMed] [Google Scholar]
- 22.Kamemura K, Hart GW. Dynamic interplay between o-glycosylation and o-phosphorylation of nucleocytoplasmic proteins: A new paradigm for metabolic control of signal transduction and transcription. Progress in nucleic acid research and molecular biology. 2003;73:107–136. doi: 10.1016/s0079-6603(03)01004-3. [DOI] [PubMed] [Google Scholar]
- 23.Chou TY, Dang CV, Hart GW. Glycosylation of the c-myc transactivation domain. Proc Natl Acad Sci U S A. 1995;92:4417–4421. doi: 10.1073/pnas.92.10.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou TY, Hart GW, Dang CV. C-myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem. 1995;270:18961–18965. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- 25.Cheng X, Cole RN, Zaia J, Hart GW. Alternative o-glycosylation/o-phosphorylation of the murine estrogen receptor beta. Biochemistry. 2000;39:11609–11620. doi: 10.1021/bi000755i. [DOI] [PubMed] [Google Scholar]
- 26.Cheng X, Hart GW. Glycosylation of the murine estrogen receptor-alpha. J Steroid Biochem Mol Biol. 2000;75:147–158. doi: 10.1016/s0960-0760(00)00167-9. [DOI] [PubMed] [Google Scholar]
- 27.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW. Modification of p53 with o-linked n-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–1083. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 29.Cole RN, Hart GW. Glycosylation sites flank phosphorylation sites on synapsin i: O-linked n-acetylglucosamine residues are localized within domains mediating synapsin i interactions. J Neurochem. 1999;73:418–428. doi: 10.1046/j.1471-4159.1999.0730418.x. [DOI] [PubMed] [Google Scholar]
- 30.Comer FI, Hart GW. Reciprocity between o-glcnac and o-phosphate on the carboxyl terminal domain of rna polymerase ii. Biochemistry. 2001;40:7845–7852. doi: 10.1021/bi0027480. [DOI] [PubMed] [Google Scholar]
- 31.Wells L, Kreppel LK, Comer FI, Wadzinski BE, Hart GW. O-glcnac transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J Biol Chem. 2004;279:38466–38470. doi: 10.1074/jbc.M406481200. [DOI] [PubMed] [Google Scholar]
- 32.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique o-glcnac transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 33.Lubas WA, Hanover JA. Functional expression of o-linked glcnac transferase. Domain structure and substrate specificity. J Biol Chem. 2000;275:10983–10988. doi: 10.1074/jbc.275.15.10983. [DOI] [PubMed] [Google Scholar]
- 34.Haltiwanger RS, Blomberg MA, Hart GW. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-n-acetylglucosamine:Polypeptide beta-n-acetylglucosaminyltransferase. J Biol Chem. 1992;267:9005–9013. [PubMed] [Google Scholar]
- 35.Haltiwanger RS, Holt GD, Hart GW. Enzymatic addition of o-glcnac to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-n-acetylglucosamine:Peptide beta-n-acetylglucosaminyltransferase. J Biol Chem. 1990;265:2563–2568. [PubMed] [Google Scholar]
- 36.Shafi R, Iyer SP, Ellies LG, O’Donnell N, Marek KW, Chui D, Hart GW, Marth JD. The o-glcnac transferase gene resides on the x chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A. 2000;97:5735–5739. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartweck LM, Scott CL, Olszewski NE. Two o-linked n-acetylglucosamine transferase genes of arabidopsis thaliana l. Heynh. Have overlapping functions necessary for gamete and seed development. Genetics. 2002;161:1279–1291. doi: 10.1093/genetics/161.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolte D, Muller U. Human o-glcnac transferase (ogt): Genomic structure, analysis of splice variants, fine mapping in xq13.1. Mamm Genome. 2002;13:62–64. doi: 10.1007/s00335-001-2108-9. [DOI] [PubMed] [Google Scholar]
- 39.Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA. Mitochondrial and nucleocytoplasmic targeting of o-linked glcnac transferase. J Cell Sci. 2003;116:647–654. doi: 10.1242/jcs.00246. [DOI] [PubMed] [Google Scholar]
- 40.Hanover JA, Yu S, Lubas WB, Shin S-H, Ragano-Caracciola M, Kochran J, Love DC. Mitochondrial and nucleocytoplasmic isoforms of o-linked glcnac transferase encoded by a single mammalian gene. Archives of Biochemistry and Biophysics. 2003;409:287–297. doi: 10.1016/s0003-9861(02)00578-7. [DOI] [PubMed] [Google Scholar]
- 41.Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E. The superhelical tpr-repeat domain of o-linked glcnac transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol. 2004;11:1001–1007. doi: 10.1038/nsmb833. [DOI] [PubMed] [Google Scholar]
- 42.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear o-glcnac transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–32022. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 43.Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL, Pollard S, Stephenson FA. Identification, molecular cloning, and characterization of a novel gabaa receptor-associated protein, grif-1. J Biol Chem. 2002;277:30079–30090. doi: 10.1074/jbc.M200438200. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Zhang F, Kudlow JE. Recruitment of o-glcnac transferase to promoters by corepressor msin3a: Coupling protein o-glcnacylation to transcriptional repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- 45.Iyer SP, Hart GW. Roles of the tetratricopeptide repeat domain in o-glcnac transferase targeting and protein substrate specificity. J Biol Chem. 2003;278:24608–24616. doi: 10.1074/jbc.M300036200. [DOI] [PubMed] [Google Scholar]
- 46.Iyer SP, Hart GW. Dynamic nuclear and cytoplasmic glycosylation: Enzymes of o-glcnac cycling. Biochemistry. 2003;42:2493–2499. doi: 10.1021/bi020685a. [DOI] [PubMed] [Google Scholar]
- 47.Wrabl JO, Grishin NV. Homology between o-linked glcnac transferases and proteins of the glycogen phosphorylase superfamily. J Mol Biol. 2001;314:365–374. doi: 10.1006/jmbi.2001.5151. [DOI] [PubMed] [Google Scholar]
- 48.Cheung WD, Hart GW. Amp-activated protein kinase and p38 mapk activate o-glcnacylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283:13009–13020. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung WD, Sakabe K, Housley MP, Dias WB, Hart GW. O-linked beta-n-acetylglucosaminyltransferase substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J Biol Chem. 2008;283:33935–33941. doi: 10.1074/jbc.M806199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whelan SA, Lane MD, Hart GW. Regulation of the o-linked beta-n-acetylglucosamine transferase by insulin signaling. J Biol Chem. 2008;283:21411–21417. doi: 10.1074/jbc.M800677200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links o-glcnac transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 52.O’Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent x-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24:1680–1690. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic o-glycosylation of nuclear and cytosolic proteins: Cloning and characterization of a neutral, cytosolic beta-n-acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 54.Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE. Characterization of the histone acetyltransferase (hat) domain of a bifunctional protein with activable o-glcnacase and hat activities. J. Biol. Chem. 2004;279:53665–53673. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- 55.Farook VS, Bogardus C, Prochazka M. Analysis of mgea5 on 10q24.1-q24.3 encoding the beta-o-linked n-acetylglucosaminidase as a candidate gene for type 2 diabetes mellitus in pima indians. Mol Genet Metab. 2002;77:189–193. doi: 10.1016/s1096-7192(02)00127-0. [DOI] [PubMed] [Google Scholar]
- 56.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of mgea5, a cytoplasmic hyaluronidase and a beta-n-acetylglucosaminidase. Biochem Biophys Res Commun. 2001;283:634–640. doi: 10.1006/bbrc.2001.4815. [DOI] [PubMed] [Google Scholar]
- 57.Heckel D, Comtesse N, Brass N, Blin N, Zang KD, Meese E. Novel immunogenic antigen homologous to hyaluronidase in meningioma. Hum Mol Genet. 1998;7:1859–1872. doi: 10.1093/hmg/7.12.1859. [DOI] [PubMed] [Google Scholar]
- 58.Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW. Dynamic o-glycosylation of nuclear and cytosolic proteins: Further characterization of the nucleocytoplasmic beta-n-acetylglucosaminidase, o-glcnacase. J Biol Chem. 2002;277:1755–1761. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- 59.Kim EJ, Kang DO, Love DC, Hanover JA. Enzymatic characterization of o-glcnacase isoforms using a fluorogenic glcnac substrate. Carbohydr Res. 2006;341:971–982. doi: 10.1016/j.carres.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in o-linked beta-n-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005;280:32944–32956. doi: 10.1074/jbc.M503396200. [DOI] [PubMed] [Google Scholar]
- 61.Slawson C, Lakshmanan T, Knapp S, Hart GW. A mitotic glcnacylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol Biol Cell. 2008;19:4130–4140. doi: 10.1091/mbc.E07-11-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between o-glycosylation and o-phosphorylation of nucleocytoplasmic proteins: Alternative glycosylation/phosphorylation of thr-58, a known mutational hot spot of c-myc in lymphomas, is regulated by mitogens. J Biol Chem. 2002;277:19229–19235. doi: 10.1074/jbc.M201729200. [DOI] [PubMed] [Google Scholar]
- 63.Hiromura M, Choi CH, Sabourin NA, Jones H, Bachvarov D, Usheva A. Yy1 is regulated by o-linked n-acetylglucosaminylation (o-glcnacylation) J Biol Chem. 2003;278:14046–14052. doi: 10.1074/jbc.M300789200. [DOI] [PubMed] [Google Scholar]
- 64.Bekesi JG, Winzler RJ. Inhibitory effects of d-glucosamine on the growth of walker 256 carcinosarcoma and on protein, rna, and DNA synthesis. Cancer Res. 1970;30:2905–2912. [PubMed] [Google Scholar]
- 65.Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of n-acetylglucosamine to sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci U S A. 2001;98:6611–6616. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly WG, Dahmus ME, Hart GW. Rna polymerase ii is a glycoprotein. Modification of the cooh-terminal domain by o-glcnac. J Biol Chem. 1993;268:10416–10424. [PubMed] [Google Scholar]
- 67.Lamarre-Vincent N, Hsieh-Wilson LC. Dynamic glycosylation of the transcription factor creb: A potential role in gene regulation. J Am Chem Soc. 2003;125:6612–6613. doi: 10.1021/ja028200t. [DOI] [PubMed] [Google Scholar]
- 68.Jackson SP, Tjian R. O-glycosylation of eukaryotic transcription factors: Implications for mechanisms of transcriptional regulation. Cell. 1988;55:125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- 69.Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, Groner B. The coactivator of transcription creb-binding protein interacts preferentially with the glycosylated form of stat5. J Biol Chem. 2004;279:3563–3572. doi: 10.1074/jbc.M306449200. [DOI] [PubMed] [Google Scholar]
- 70.Han I, Kudlow JE. Reduced o glycosylation of sp1 is associated with increased proteasome susceptibility. Mol Cell Biol. 1997;17:2550–2558. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wierstra I. Sp1: Emerging roles--beyond constitutive activation of tata-less housekeeping genes. Biochem Biophys Res Commun. 2008;372:1–13. doi: 10.1016/j.bbrc.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 72.Kudlow JE. Post-translational modification by o-glcnac: Another way to change protein function. J Cell Biochem. 2006;98:1062–1075. doi: 10.1002/jcb.20926. [DOI] [PubMed] [Google Scholar]
- 73.Chung SS, Kim JH, Park HS, Choi HH, Lee KW, Cho YM, Lee HK, Park KS. Activation of ppargamma negatively regulates o-glcnacylation of sp1. Biochem Biophys Res Commun. 2008;372:713–718. doi: 10.1016/j.bbrc.2008.05.096. [DOI] [PubMed] [Google Scholar]
- 74.Goldberg HJ, Whiteside CI, Hart GW, Fantus IG. Posttranslational, reversible o-glycosylation is stimulated by high glucose and mediates plasminogen activator inhibitor-1 gene expression and sp1 transcriptional activity in glomerular mesangial cells. Endocrinology. 2006;147:222–231. doi: 10.1210/en.2005-0523. [DOI] [PubMed] [Google Scholar]
- 75.Majumdar G, Harrington A, Hungerford J, Martinez-Hernandez A, Gerling IC, Raghow R, Solomon S. Insulin dynamically regulates calmodulin gene expression by sequential o-glycosylation and phosphorylation of sp1 and its subcellular compartmentalization in liver cells. J Biol Chem. 2006;281:3642–3650. doi: 10.1074/jbc.M511223200. [DOI] [PubMed] [Google Scholar]
- 76.Iyer SP, Akimoto Y, Hart GW. Identification and cloning of a novel family of coiled-coil domain proteins that interact with o-glcnac transferase. J Biol Chem. 2003;278:5399–5409. doi: 10.1074/jbc.M209384200. [DOI] [PubMed] [Google Scholar]
- 77.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-glcnac regulates foxo activation in response to glucose. J Biol Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, Hart GW. A pgc-1alpha:O-glcnac transferase complex regulates foxo transcription factor activity in response to glucose. J Biol Chem. 2008 doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the creb coactivator crtc2. Science. 2008;319:1402–1405. doi: 10.1126/science.1151363. [DOI] [PubMed] [Google Scholar]
- 80.Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated by o-linked n-acetylglucosamine. J Biol Chem. 2003;278:10022–10027. doi: 10.1074/jbc.M207787200. [DOI] [PubMed] [Google Scholar]
- 81.McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. PNAS. 2002;99:10695–10699. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, Okamoto M, Montminy M. The creb coactivator torc2 functions as a calcium- and camp-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Pedersen TA, Bereshchenko O, Garcia-Silva S, Ermakova O, Kurz E, Mandrup S, Porse BT, Nerlov C. Distinct c/ebpalpha motifs regulate lipogenic and gluconeogenic gene expression in vivo. EMBO J. 2007;26:1081–1093. doi: 10.1038/sj.emboj.7601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McClain DA. Hexosamines as mediators of nutrient sensing and regulation in diabetes. J Diabetes Complications. 2002;16:72–80. doi: 10.1016/s1056-8727(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 85.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by o-glcnac results in insulin resistance associated with defects in akt activation in 3t3-l1 adipocytes. Proc Natl Acad Sci U S A. 2002;99:5313–5318. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arias EB, Kim J, Cartee GD. Prolonged incubation in pugnac results in increased protein o-linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes. 2004;53:921–930. doi: 10.2337/diabetes.53.4.921. [DOI] [PubMed] [Google Scholar]
- 87.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of o-glcnacase improves contractile function in the diabetic heart. Circ Res. 2005;96:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 88.Lehman DM, Fu DJ, Freeman AB, Hunt KJ, Leach RJ, Johnson-Pais T, Hamlington J, Dyer TD, Arya R, Abboud H, Goring HH, Duggirala R, Blangero J, Konrad RJ, Stern MP. A single nucleotide polymorphism in mgea5 encoding o-glcnac-selective n-acetyl-beta-d glucosaminidase is associated with type 2 diabetes in mexican americans. Diabetes. 2005;54:1214–1221. doi: 10.2337/diabetes.54.4.1214. [DOI] [PubMed] [Google Scholar]
- 89.Park K, Saudek CD, Hart GW. Increased expression of {beta}-n-acetylglucosamindase (o-glcnacase) in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes. 2010 doi: 10.2337/db09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear o-glcnacylation. J. Biol. Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 91.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by o-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 92.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiang JG, Tsokos GC. Heat shock protein 70 kda: Molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 94.Kregel KC. Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- 95.Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic o-glcnac modification of nucleocytoplasmic proteins in response to stress: A survival response in mammalian cells. J Biol Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 96.Zou L, Yang S, Hu S, Chaudry IH, Marchase RB, Chatham JC. The protective effects of pugnac on cardiac function after trauma-hemorrhage are mediated via increased protein o-glcnac levels. Shock. 2007;27:402–408. doi: 10.1097/01.shk.0000245031.31859.29. [DOI] [PubMed] [Google Scholar]
- 97.Zou L, Yang S, Champattanachai V, Hu S, Chaudry IH, Marchase RB, Chatham JC. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein o-glcnacylation and attenuation of nf-{kappa}b signaling. Am J Physiol Heart Circ Physiol. 2009;296:H515–523. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang S, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock. 2006;25:600–607. doi: 10.1097/01.shk.0000209563.07693.db. [DOI] [PubMed] [Google Scholar]
- 99.Xing D, Feng W, Not LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, Oparil S. Increased protein o-glcnac modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol. 2008 doi: 10.1152/ajpheart.01259.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Not LG, Marchase RB, Fulop N, Brocks CA, Chatham JC. Glucosamine administration improves survival rate after severe hemorrhagic shock combined with trauma in rats. Shock. 2007;28:345–352. doi: 10.1097/shk.0b013e3180487ebb. [DOI] [PubMed] [Google Scholar]
- 101.Not LG, Brocks CA, Vamhidy L, Marchase RB, Chatham JC. Increased o-linked beta-n-acetylglucosamine levels on proteins improves survival, reduces inflammation and organ damage 24 hours after trauma-hemorrhage in rats. Crit Care Med. 2010;38:562–571. doi: 10.1097/CCM.0b013e3181cb10b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. Journal of Molecular and Cellular Cardiology. 2008;45:313–325. doi: 10.1016/j.yjmcc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-glcnac signaling attenuates er stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol. 2009;297:H1711–1719. doi: 10.1152/ajpheart.00553.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagy T, Champattanachai V, Marchase RB, Chatham JC. Glucosamine inhibits angiotensin ii-induced cytoplasmic ca2+ elevation in neonatal cardiomyocytes via protein-associated o-linked n-acetylglucosamine. Am J Physiol Cell Physiol. 2006;290:C57–65. doi: 10.1152/ajpcell.00263.2005. [DOI] [PubMed] [Google Scholar]
- 105.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein o-glcnac levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–312. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein o-glcnac levels. J Mol Cell Cardiol. 2007;42:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu J, Marchase RB, Chatham JC. Increased o-glcnac levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am J Physiol Heart Circ Physiol. 2007;293:H1391–1399. doi: 10.1152/ajpheart.00285.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, Chen YF, Darley-Usmar V, Oparil S, Chatham JC. Protein o-glcnacylation: A new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol. 2009;296:H13–28. doi: 10.1152/ajpheart.01056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by n-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 110.Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat hearts associated with increased o-linked n-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol. 2007;292:H2227–2236. doi: 10.1152/ajpheart.01091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fulop N, Marchase RB, Chatham JC. Role of protein o-linked n-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein o-glcnac and increased mitochondrial bcl-2. Am J Physiol Cell Physiol. 2008;294:C1509–1520. doi: 10.1152/ajpcell.00456.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated o-glcnac. Am J Physiol Cell Physiol. 2007;292:C178–187. doi: 10.1152/ajpcell.00162.2006. [DOI] [PubMed] [Google Scholar]
- 114.Mizock BA. Alterations in fuel metabolism in critical illness: Hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533–551. doi: 10.1053/beem.2001.0168. [DOI] [PubMed] [Google Scholar]
- 115.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 116.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. Journal of Molecular and Cellular Cardiology. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 117.Weiss RG, de Albuquerque CP, Vandegaer K, Chacko VP, Gerstenblith G. Attenuated glycogenolysis reduces glycolytic catabolite accumulation during ischemia in preconditioned rat hearts. Circ Res. 1996;79:435–446. doi: 10.1161/01.res.79.3.435. [DOI] [PubMed] [Google Scholar]
- 118.Tong H, Chen W, London RE, Murphy E, Steenbergen C. Preconditioning enhanced glucose uptake is mediated by p38 map kinase not by phosphatidylinositol 3-kinase. J. Biol. Chem. 2000;275:11981–11986. doi: 10.1074/jbc.275.16.11981. [DOI] [PubMed] [Google Scholar]
- 119.Janier MF, Vanoverschelde JL, Bergmann SR. Ischemic preconditioning stimulates anaerobic glycolysis in the isolated rabbit heart. Am J Physiol. 1994;267:H1353–1360. doi: 10.1152/ajpheart.1994.267.4.H1353. [DOI] [PubMed] [Google Scholar]
- 120.Khogali SE, Harper AA, Lyall JA, Rennie MJ. Effects of l-glutamine on post-ischaemic cardiac function: Protection and rescue. J Mol Cell Cardiol. 1998;30:819–827. doi: 10.1006/jmcc.1998.0647. [DOI] [PubMed] [Google Scholar]
- 121.Kristensen J, Maeng M, Mortensen UM, Berg J, Rehling M, Nielsen TT. Lack of cardioprotection from metabolic support with glutamine or glutamate in a porcine coronary occlusion model. Scand Cardiovasc J. 2005;39:115–120. doi: 10.1080/14017430510009078. [DOI] [PubMed] [Google Scholar]
- 122.Chatham JC, Not LG, Fulop N, Marchase RB. Hexosamine biosynthesis and protein o-glycosylation: The first line of defense against stress, ischemia, and trauma. Shock. 2008;29:431–440. doi: 10.1097/shk.0b013e3181598bad. [DOI] [PubMed] [Google Scholar]
- 123.Konrad RJ, Zhang F, Hale JE, Knierman MD, Becker GW, Kudlow JE. Alloxan is an inhibitor of the enzyme o-linked n-acetylglucosamine transferase. Biochem Biophys Res Commun. 2002;293:207–212. doi: 10.1016/S0006-291X(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 124.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 125.Lee TN, Alborn WE, Knierman MD, Konrad RJ. Alloxan is an inhibitor of o-glcnac-selective n-acetyl-[beta]-d-glucosaminidase. Biochem Biophys Res Commun. 2006;350:1038–1043. doi: 10.1016/j.bbrc.2006.09.155. [DOI] [PubMed] [Google Scholar]
- 126.Gross BJ, Kraybill BC, Walker S. Discovery of o-glcnac transferase inhibitors. Journal of the American Chemical Society. 2005;127:14588–14589. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- 127.Gross BJ, Swoboda JG, Walker S. A strategy to discover inhibitors of o-linked glycosylation. J Am Chem Soc. 2008;130:440–441. doi: 10.1021/ja078125s. [DOI] [PubMed] [Google Scholar]
- 128.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol. 2008;45:313–325. doi: 10.1016/j.yjmcc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roos MD, Xie W, Su K, Clark JA, Yang X, Chin E, Paterson AJ, Kudlow JE. Streptozotocin, an analog of n-acetylglucosamine, blocks the removal of o-glcnac from intracellular proteins. Proc Assoc Am Physicians. 1998;110:422–432. [PubMed] [Google Scholar]
- 130.Horsch M, Hoesch L, Vasella A, Rast DM. N-acetylglucosaminono-1,5-lactone oxime and the corresponding (phenylcarbamoyl)oxime. Novel and potent inhibitors of beta-n-acetylglucosaminidase. Eur J Biochem. 1991;197:815–818. doi: 10.1111/j.1432-1033.1991.tb15976.x. [DOI] [PubMed] [Google Scholar]
- 131.Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. O-glcnacase uses substrate-assisted catalysis: Kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem. 2005;280:25313–25322. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- 132.Dorfmueller HC, Borodkin VS, Schimpl M, Shepherd SM, Shpiro NA, van Aalten DM. Glcnacstatin: A picomolar, selective o-glcnacase inhibitor that modulates intracellular o-glcnacylation levels. J Am Chem Soc. 2006;128:16484–16485. doi: 10.1021/ja066743n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ. A potent mechanism-inspired o-glcnacase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 134.Haltiwanger RS, Grove K, Philipsberg GA. Modulation of o-linked n-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide o-glcnac-beta-n-acetylglucosaminidase inhibitor o-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-n-phenylcarbamate. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 135.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res. 2009;104:41–49. doi: 10.1161/CIRCRESAHA.108.189431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ngoh GA, Jones SP. New insights into metabolic signaling and cell survival: The role of beta-o-linkage of n-acetylglucosamine. J Pharmacol Exp Ther. 2008;327:602–609. doi: 10.1124/jpet.108.143263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Murata M, Akao M, O’Rourke B, Marban E. Mitochondrial atp-sensitive potassium channels attenuate matrix ca2+ overload during simulated ischemia and reperfusion: Possible mechanism of cardioprotection. Circ Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- 138.Griffiths EJ, Ocampo CJ, Savage JS, Rutter GA, Hansford RG, Stern MD, Silverman HS. Mitochondrial calcium transporting pathways during hypoxia and reoxygenation in single rat cardiomyocytes. Cardiovasc Res. 1998;39:423–433. doi: 10.1016/s0008-6363(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 139.Su K, Roos MD, Yang X, Han I, Paterson AJ, Kudlow JE. An n-terminal region of sp1 targets its proteasome-dependent degradation in vitro. J Biol Chem. 1999;274:15194–15202. doi: 10.1074/jbc.274.21.15194. [DOI] [PubMed] [Google Scholar]
- 140.Su K, Yang X, Roos MD, Paterson AJ, Kudlow JE. Human sug1/p45 is involved in the proteasome-dependent degradation of sp1. Biochem J. 2000;348(Pt 2):281–289. [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. O-glcnac modification is an endogenous inhibitor of the proteasome. Cell. 2003;115:715–725. doi: 10.1016/s0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]
- 142.Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein o-glcnacylation. Hypertension. 2009;53:166–174. doi: 10.1161/HYPERTENSIONAHA.108.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Saleh MA, Pollock DM, Fortes ZB, Carvalho MH, Ergul A, Webb RC, Tostes RC. O-glcnacylation contributes to augmented vascular reactivity induced by endothelin 1. Hypertension. 2010;55:180–188. doi: 10.1161/HYPERTENSIONAHA.109.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lima VV, Rigsby CS, Hardy DM, Webb RC, Tostes RC. O-glcnacylation: A novel post-translational mechanism to alter vascular cellular signaling in health and disease: Focus on hypertension. J Am Soc Hypertens. 2009;3:374–387. doi: 10.1016/j.jash.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zachara NE. Detecting the “O-glcnac-ome”; detection, purification, and analysis of o-glcnac modified proteins. Methods Mol Biol. 2009;534:1–30. doi: 10.1007/978-1-59745-022-5_19. [DOI] [PubMed] [Google Scholar]
- 146.Roquemore EP, Dell A, Morris HR, Panico M, Reason AJ, Savoy LA, Wistow GJ, Zigler JS, Jr., Earles BJ, Hart GW. Vertebrate lens alpha-crystallins are modified by o-linked n-acetylglucosamine. J Biol Chem. 1992;267:555–563. [PubMed] [Google Scholar]
- 147.Hanover JA, Cohen CK, Willingham MC, Park MK. O-linked n-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987;262:9887–9894. [PubMed] [Google Scholar]
- 148.Taylor RP, Geisler TS, Chambers JH, McClain DA. Up-regulation of o-glcnac transferase with glucose deprivation in hepg2 cells is mediated by decreased hexosamine pathway flux. J Biol Chem. 2009;284:3425–3432. doi: 10.1074/jbc.M803198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Taylor RP, Parker GJ, Hazel MW, Soesanto Y, Fuller W, Yazzie MJ, McClain DA. Glucose deprivation stimulates o-glcnac modification of proteins through up-regulation of o-linked n-acetylglucosaminyltransferase. J Biol Chem. 2008;283:6050–6057. doi: 10.1074/jbc.M707328200. [DOI] [PubMed] [Google Scholar]
- 150.Yamamoto K, Tsuji T, Matsumoto I, Osawa T. Structural requirements for the binding of oligosaccharides and glycopeptides to immobilized wheat germ agglutinin. Biochemistry. 1981;20:5894–5899. doi: 10.1021/bi00523a037. [DOI] [PubMed] [Google Scholar]
- 151.Snow CM, Senior A, Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. The Journal of Cell Biology. 1987;104:1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park MK, D’Onofrio M, Willingham MC, Hanover JA. A monoclonal antibody against a family of nuclear pore proteins (nucleoporins): O-linked n-acetylglucosamine is part of the immunodeterminant. Proc Natl Acad Sci U S A. 1987;84:6462–6466. doi: 10.1073/pnas.84.18.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Holt GD, Haltiwanger RS, Torres CR, Hart GW. Erythrocytes contain cytoplasmic glycoproteins. O-linked glcnac on band 4.1. Journal of Biological Chemistry. 1987;262:14847–14850. [PubMed] [Google Scholar]
- 154.Holt GD, Snow CM, Senior A. Haltiwanger RS, Gerace L, Hart GW. Nuclear pore complex glycoproteins contain cytoplasmically disposed o-linked n-acetylglucosamine. J Cell Biol. 1987;104:1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Comer FI, Vosseller K, Wells L, Accavitti MA, Hart GW. Characterization of a mouse monoclonal antibody specific for o-linked n-acetylglucosamine. Anal Biochem. 2001;293:169–177. doi: 10.1006/abio.2001.5132. [DOI] [PubMed] [Google Scholar]
- 156.Teo CF, Ingale S, Wolfert MA, Elsayed GA, Not LG, Chatham JC, Wells L, Boons GJ. Glycopeptide-specific monoclonal antibodies suggest new roles for o-glcnac. Nat Chem Biol. 2010;6:338–343. doi: 10.1038/nchembio.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reason AJ, Morris HR, Panico M, Marais R, Treisman RH, Haltiwanger RS, Hart GW, Kelly WG, Dell A. Localization of o-glcnac modification on the serum response transcription factor. J Biol Chem. 1992;267:16911–16921. [PubMed] [Google Scholar]
- 158.Reason AJ, Blench IP, Haltiwanger RS, Hart GW, Morris HR, Panico M, Dell A. High-sensitivity fab-ms strategies for o-glcnac characterization. Glycobiology. 1991;1:585–594. doi: 10.1093/glycob/1.6.585. [DOI] [PubMed] [Google Scholar]
- 159.Greis KD, Hayes BK, Comer FI, Kirk M, Barnes S, Lowary TL, Hart GW. Selective detection and site-analysis of o-glcnac-modified glycopeptides by beta-elimination and tandem electrospray mass spectrometry. Anal Biochem. 1996;234:38–49. doi: 10.1006/abio.1996.0047. [DOI] [PubMed] [Google Scholar]
- 160.Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW. Mapping sites of o-glcnac modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteomics. 2002;1:791–804. doi: 10.1074/mcp.m200048-mcp200. [DOI] [PubMed] [Google Scholar]
- 161.Vocadlo DJ, Hang HC, Kim EJ, Hanover JA, Bertozzi CR. A chemical approach for identifying o-glcnac-modified proteins in cells. Proc Natl Acad Sci U S A. 2003;100:9116–9121. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nandi A, Sprung R, Barma DK, Zhao Y, Kim SC, Falck JR. Global identification of o-glcnac-modified proteins. Anal Chem. 2006;78:452–458. doi: 10.1021/ac051207j. [DOI] [PubMed] [Google Scholar]
- 163.Gurcel C, Vercoutter-Edouart AS, Fonbonne C, Mortuaire M, Salvador A, Michalski JC, Lemoine J. Identification of new o-glcnac modified proteins using a click-chemistry-based tagging. Anal Bioanal Chem. 2008;390:2089–2097. doi: 10.1007/s00216-008-1950-y. [DOI] [PubMed] [Google Scholar]
- 164.Chalkley RJ, Thalhammer A, Schoepfer R, Burlingame AL. Identification of protein o-glcnacylation sites using electron transfer dissociation mass spectrometry on native peptides. Proceedings of the National Academy of Sciences. 2009;106:8894–8899. doi: 10.1073/pnas.0900288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, Lynn AJ, Snedecor JO, Guan S, Medzihradszky KF, Maltby DA, Schoepfer R, Burlingame AL. O-linked n-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol Cell Proteomics. 2006;5:923–934. doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 166.Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, Hart GW. A pgc-1alpha-o-glcnac transferase complex regulates foxo transcription factor activity in response to glucose. J Biol Chem. 2009;284:5148–5157. doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]