Relationship between Vitamin D, Parathyroid Hormone, and Bone Health (original) (raw)
Vitamin D insufficiency as it relates to bone health occurs at a serum 25OHD level < 20 ng/ml (50 nmol/liter).
Abstract
Context:
There is a controversy regarding the definition of vitamin D insufficiency as it relates to bone health.
Objective:
The objective of the study was to examine the evidence for a threshold value of serum 25-hydroxyvitamin D (25OHD) that defines vitamin D insufficiency as it relates to bone health.
Design and Participants:
This was a cross-sectional analysis of baseline data in 488 elderly Caucasian women, mean age 71 yr, combined with a literature review of 70 studies on the relationship of serum PTH to serum 25OHD.
Setting:
The study was conducted in independent-living women in the midwest United States.
Main Outcome Measure:
The relationship between serum 25OHD, serum PTH, and serum osteocalcin and 24-h urine N-telopeptides was evaluated.
Results:
Serum PTH was inversely correlated with serum 25OHD (r = −0.256, P < 0.0005), but no threshold as defined by suppression of serum PTH was found within the serum 25OHD range 6–60 ng/ml (15–150 nmol/liter). However, in contrast, there was a threshold for bone markers, serum osteocalcin and urine N-telopeptides, that increased only below a serum 25OHD of approximately 18 ng/ml (45 nmol/liter). Calcium absorption was not correlated with serum PTH and serum 25OHD, and no threshold was found. A literature review of 70 studies generally showed a threshold for serum PTH with increasing serum 25OHD, but there was no consistency in the threshold level of serum 25OHD that varied from 10 to 50 ng/ml (25–125 nmol/liter).
Conclusions:
Vitamin D insufficiency should be defined as serum 25OHD less than 20 ng/ml (50 nmol/liter) as it relates to bone.
Serum 25-hydroxyvitamin D (25OHD) is considered to be the best indicator of overall vitamin D status of an individual. Severe vitamin D deficiency [serum 25OHD < 10 ng/ml (25 nmol/liter)] is associated with malabsorption of calcium, secondary hyperparathyroidism, leading to increased bone resorption, accelerated cortical bone loss, and increased fractures. Very low levels of serum 25OHD can also cause osteomalacia.
The definition of vitamin D insufficiency is less clear-cut. The World Health Organization originally defined it as a serum 25OHD less than 20 ng/ml (50 nmol/liter) (1). There have been many publications describing the relationship of serum PTH to serum 25OHD, and some but not all papers defined a level of serum 25OHD at which serum PTH levels decreased and reached a plateau. This threshold has been used to define vitamin D insufficiency. Recent influential reviews stated that a serum 25OHD level of 30 ng/ml (75 nmol/liter) should be used as the defining level for vitamin D insufficiency because in the studies analyzed in those reviews, serum PTH showed a plateau at serum 25OHD of approximately 30 ng/ml (75 nmol/liter) (2, 3). Clinical chemistry laboratories now define vitamin D insufficiency as a serum 25OHD level less than 30 ng/ml (75 nmol/liter) and normal values as greater than 30 ng/ml (75 nmol/liter) on their report forms and have adopted this view unquestioningly. This has led to an epidemic of vitamin D insufficiency, and it has been suggested that 1 billion people worldwide have vitamin D deficiency or insufficiency (3).
A critical question, however, is what levels of serum PTH are harmful to bone because it was assumed that elevated serum PTH was related to bone loss (3). Independent of vitamin D metabolism, there is an age-related decrease in calcium absorption that probably contributes to secondary hyperparathyroidism (4).
We examined the relationship between serum PTH and serum 25OHD in our own data for evidence of a threshold or plateau and also examined the relationship between bone markers and serum 25OHD. A literature review of studies relating serum PTH and serum 25OHD was performed to determine whether serum PTH showed a plateau or was maximally suppressed in relation to serum 25OHD and whether we could find supporting evidence for a threshold value of serum 25OHD of 30 ng/ml (75 nmol/liter) (3).
Materials and Methods
Literature review
We did an extensive search on MEDLINE using terms vitamin D (title/abstract) or 25-hydroxyvitamin D (title/abstract) or cholecalciferol (title/abstract) and parathyroid hormone (title/abstract) or PTH (title/abstract). This search retrieved 4092 studies that were conducted from January 1988 to June 2010. We then screened abstracts that assessed the relationship between serum 25OHD and serum PTH and stated a level of serum 25OHD at which serum PTH plateaus and/or is maximally suppressed either by statistical methods or by interpretation from graphical depictions. There were a total of 70 studies that showed a relationship between serum 25OHD and PTH (5–57, 59–66, 82, 83). Seven of these studies were counted twice: one study mentioned two thresholds based on different statistical models (6), three studies found different thresholds based on participants' age (17, 62, 82), one found different thresholds based on race (23), and two studies found different thresholds based on gender (28, 48).
Study subjects
Information on our study subjects was derived from the baseline data of a 3-yr intervention trial [Sites Testing Osteoporosis Prevention or Intervention (STOP IT)] in elderly women, described previously in detail (58). They were 488 healthy independent women living at home. Excluded were women who had any disease or took medication that affected calcium, vitamin D metabolism, and bone.
Dietary intake of calcium and vitamin D was collected from 7-d food diaries.
Biochemical measurements
Serum 25OHD was measured by a competitive binding assay after chromatographic separation and purification on Sep-Pak cartridges (Waters Associates, Milford, MA). The limit of detection was 5 ng/ml, and the interassay variation was 5%. Serum PTH was measured as the intact molecule 1–84 by immunometric assay (Nichols Institute, San Juan Capistrano, CA). The limit of detection was 1 pg/ml, and the interassay coefficient of variation was 3.5%. Serum osteocalcin was measured with RIA (INCSTAR Corp., Stillwater, MN); the limit for the assay was 0.78 ng/ml, and the interassay coefficient of variation was 4.5%. Twenty-four hour urine N-telopeptides (NTx/Cr) were measured by an ELISA (INCSTAR). Calcium absorption was measured fasting; 100 mg elemental calcium was mixed with 5 μCi Ca45 in 200 ml distilled water. Blood samples were collected at 1, 2, and 3 h for estimation of Ca45, and calcium absorption was expressed as the percentage absorbed per liter after 2 h.
Statistical methods
All baseline demographic and clinical variables were continuous and presented as mean and sd, and all bivariate associations were evaluated by Pearson's correlation (r). Four multivariate linear regression analyses were conducted to assess the association between serum 25OHD and serum PTH, serum osteocalcin, NTx/Cr, and calcium absorption after adjusting for age, body mass index (BMI), or weight, calcium intake, and serum creatinine or glomerular filtration rate (GFR). An additional covariate 1,25-dihydroxyvitamin D [1,25(OH)2D] was added to the calcium absorption analysis. The decision to use BMI vs. body weight and serum creatinine vs. GFR was determined by the largest bivariate association with the outcome variable of interest. Calcium absorption was corrected for body size using a 15% of total body weight (67), BMI was used for serum osteocalcin and urine NTx/Cr and total body weight used for serum PTH. Serum creatinine was used for calcium absorption, serum PTH, and urine NTx/Cr, whereas GFR was used for serum osteocalcin. The unique effect of serum 25OHD after adjustment for covariates is presented by the semipartial correlation (sr) and the squared sr (sr2). The _sr_2 is interpreted similarly to model _R_2 except that instead of overall variance explained, _sr_2 indicates the amount of variance in the outcome that is explained uniquely by serum 25OHD.
For each outcome variable, subjects with missing data and those with outlying (univariate or multivariate) data were identified and removed before analysis. Because the sample size was large, only clearly disconnected data points with z greater than 3.29 were considered univariate outliers. Data were considered multivariate outliers with Mahalanobis distance at P < 0.001. Furthermore, square root transformations were performed on serum PTH, and natural log transformations were performed on vitamin D intake and urine NTx/Cr (uNTx/Cr) to normalize the distribution and reduce the influence of outliers.
To assess whether a threshold within serum 25OHD exists, nonlinear and piecewise linear regression were used. These analyses were conducted only for outcomes significantly related to serum 25OHD, as indicated by multivariate linear regression analyses described above. For all piecewise analysis, a single threshold was hypothesized. The search for the threshold began using the constrained nonlinear regression procedure with a serum 25OHD threshold of 20 ng/ml and starting values (i.e. the intercept and slopes) based on the results of the bivariate linear regression. Because the convergence of nonlinear regression is based heavily on the particular starting values, a series of piecewise linear regression analyses followed using threshold values 10 ng/ml above and below (at 0.5 increments) the converged serum 25OHD threshold value. The optimal threshold value was chosen based on adjusted _R_2, the F statistic, model se, and the t value and associated P value for the threshold variable (68). Overall model fit for all regression analyses (both linear and piecewise linear) was assessed by the distribution of residuals and added-variable plots.
Finally, the interaction effect between calcium intake and 25OHD on serum PTH was assessed using a linear regression analysis. Unless indicated otherwise, two-tailed P < 0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics (version 18.0.2; SPSS Inc., Chicago, IL).
Results
Literature review
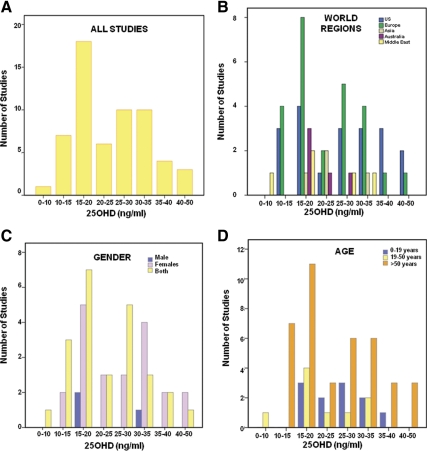
There was a large variability in the reported level of serum 25OHD at which serum PTH reached a plateau or was maximally suppressed (Fig. 1A): 10 ng/ml or less in one study (5), 10–15 ng/ml in seven studies (6–11, 62), 15–20 ng/ml in 18 studies (12–27, 48, 82), 20–25 ng/ml in six studies (28–33), 25–30 ng/ml in 10 studies (6, 34–39, 41–43), 30–35 ng/ml in 10 studies (17, 23, 44–50, 83), 35–40 ng/ml in four studies (51–54), 40–50 ng/ml in three studies (55–57), no plateau in eight studies, (28, 40, 59–64), and no relationship at all between serum 25OHD and serum PTH in three studies (65, 66, 82).
Fig. 1.
Various levels of serum 25OHD (nanograms per milliliter) at which serum PTH (picograms per milliliter) plateaus and/or is maximally suppressed. A, Data here are presented from 59 studies (not shown, eight studies in which serum PTH continually decreased with increasing serum 25OHD and three studies that found no relationship between serum 25OHD and serum PTH). B–D, Location, age, and gender of study populations included in these studies.
Subgroup analysis
Subgroup analysis based on geographical location revealed that most of the studies were conducted in the United States and Europe (Fig. 1B) with some from the Middle East, Australia, and Asia. Subgroup analysis based on age and gender of study population is shown in Fig. 1, C and D, respectively. A majority of studies included both men and women or women alone, and three studies included men alone. Most studies (65%) had data on older populations (>50 yr age).
Study subjects
The baseline characteristics of our own study subjects are shown in Table 1. The mean age of subjects was 71.4 yr (range 65–78 yr).
Table 1.
Baseline characteristics of study population
| Variables | Mean | sd |
|---|---|---|
| n = 488 | ||
| Age (yr) | 71.4 | 3.6 |
| BMI | 26.8 | 4.7 |
| Blood urea nitrogen (mg/dl) | 15.3 | 4.3 |
| Serum creatinine (mg/dl) | 0.87 | 0.16 |
| Serum calcium (mg/dl) | 9.3 | 0.3 |
| Serum PTH (pg/ml) | 37.2 | 14.8 |
| Serum 25OHD (ng/ml) | 31.3 | 10.5 |
| Serum 1,25(OH)2D (pg/ml) | 34.4 | 8.0 |
| Serum osteocalcin (ng/ml) | 3.8 | 1.3 |
| 24-h urine NTX (nmol BCE/mmol Cr) | 50.8 | 26.7 |
| Calcium absorption (% actual dose/liter) | 2.6 | 0.7 |
| GFR (mL/min per 1.73 m2) | 71.8 | 19.3 |
| Vitamin D intake (μg/d) | 3.5 | 2.0 |
| Calcium intake (mg/d) | 739 | 310 |
Correlations between serum PTH, serum osteocalcin, 24 h urine NTx/Cr, and serum 25OHD
The results of the multivariate linear regression analysis are presented in Table 2. After adjusting for baseline demographic and clinical variables, serum 25OHD was inversely associated with serum PTH (sr = −0.256, P = 0.0066; sr2 = 0.066), serum osteocalcin (sr = −0.108, P = 0.015; sr2 = 0.012), and urine NTx/Cr (r = −0.120, P = 0.007; sr2 = 0.014).
Table 2.
Multivariate linear regression results
| Slope | se | P | 95% CI for slope | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Square root-transformed serum PTH (n = 478)a | |||||
| Serum 25OHD (ng/ml) | −0.0311 | 0.0050 | <0.0005 | −0.0410 | −0.0212 |
| Age (yr) | 0.0009 | 0.0128 | 0.9420 | −0.0242 | 0.0261 |
| Weight (kg) | 0.0168 | 0.0038 | <0.0005 | 0.0094 | 0.0242 |
| Calcium intake (mg/d) | 0.0003 | 0.0002 | 0.0820 | −0.0006 | 0.0000 |
| Serum creatinine (mg/dl) | 1.0733 | 0.2922 | 0.0003 | 0.4992 | 1.6475 |
| Constant | 4.9945 | 0.9964 | |||
| Serum osteocalcin (n = 475)b | |||||
| Serum 25OHD (ng/ml) | −0.0146 | 0.0060 | 0.0153 | −0.0263 | −0.0028 |
| Age (yr) | −0.0026 | 0.0154 | 0.8867 | −0.0328 | 0.0276 |
| BMI (kg/m2) | −0.0691 | 0.0125 | <0.00005 | −0.0938 | −0.0445 |
| Calcium intake (mg/d) | 0.00002 | 0.0002 | 0.9263 | −0.0004 | 0.0004 |
| GFR (ml/min per 1.73 m2) | −0.0053 | 0.0029 | 0.0715 | −0.0110 | 0.0005 |
| Constant | 6.6426 | 1.2092 | |||
| Natural log-transformed uNTx/Cr (n = 475)c | |||||
| Serum 25OHD (ng/ml) | −0.0062 | 0.0023 | 0.0065 | −0.0106 | −0.0017 |
| Age (yr) | 0.0002 | 0.0058 | 0.9697 | −0.0111 | 0.0115 |
| BMI (kg/m2) | −0.0220 | 0.0047 | <0.0005 | −0.0312 | −0.0127 |
| Calcium intake (mg/d) | −0.0001 | 0.0001 | 0.3139 | −0.0002 | 0.0001 |
| Serum creatinine (mg/dl) | −0.4620 | 0.1309 | 0.0005 | −0.7193 | −0.2048 |
| Constant | 5.0372 | 0.4439 | |||
| Calcium absorption (n = 470)d | |||||
| Serum 25OHD (ng/ml) | 0.0036 | 0.0031 | 0.2472 | −0.0025 | 0.0096 |
| Age (yr) | −0.0214 | 0.0078 | 0.0066 | −0.0367 | −0.0060 |
| 15% body weight (kg) | −0.0667 | 0.0154 | <0.0005 | −0.0970 | −0.0364 |
| Calcium intake (mg/d) | −0.0002 | 0.0001 | 0.0227 | −0.0004 | 0.00003 |
| Serum creatinine (mg/dl) | −0.1276 | 0.1811 | 0.4814 | −0.4835 | 0.2283 |
| Serum 125OH2D (pg/ml) | 0.0194 | 0.0038 | <0.0005 | 0.0120 | 0.0268 |
| Constant | 4.2719 | 0.6368 |
In addition, increasing body weight or BMI was associated with an increase in serum PTH and a decrease in urine NTx/Cr and serum osteocalcin and calcium absorption (all P < 0.0005). Serum creatinine correlated with increased serum PTH and decreased urine NTx/Cr (both P < 0.0005).
Identification of serum 25OHD threshold for serum PTH, bone markers, and role of calcium intake
The derivations of regression equations are provided below.
Piecewise linear regression equations
Generic form is: Ŷ = β0 + β1(25OHD) + β2(25OHD − threshold)dummy for threshold.
Serum osteocalcin (Fig. 2C)
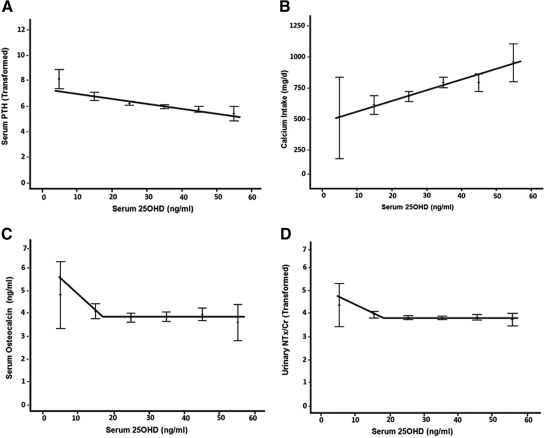
Fig. 2.
Relationship between serum 25OHD, serum PTH, bone markers, and calcium intake. A, Serum PTH was inversely correlated with serum 25OHD (r = −0.338, P < 0.0005) was continually decreasing with increasing serum 25OHD. Nonlinear regression failed to identify a threshold for serum PTH. Calcium intake was significantly positively correlated with serum 25OHD (r = 0.295, P < 0.0005; B). For serum osteocalcin, nonlinear regression converged on a threshold of 17.034 ng/ml (95% CI 12.61–21.46). Piecewise linear regression indicated an optimal threshold of 17 ng/ml (42.4 nmol/liter; P = 0.007; adjusted _R_2 = 0.016). Before this threshold value, statistically significant decreases in serum osteocalcin were indicated with increases in serum 25OHD; however, this effect plateaued after threshold (C). For uNTx/Cr, nonlinear regression converged on a threshold of 18.0 ng/ml (95% CI 14.347–21.653), with an optimal threshold of 17.5 ng/ml (43.7 nmol/liter; P = 0.0002; adjusted _R_2 = 0.036). Similarly, a plateau effect was evidenced after the threshold (D).
Predicted serum osteocalcin = 6.163 − 0.141(25OHD) + 0.141(25OHD − 17) (dummy), where the dummy is 1 if 25OHD is 17 or greater and the dummy is 0 if 25OHD is less than 17.
However, as you can see in Fig. 2C, the intercept is dependent on whether the individual has serum 25OHD before or after the threshold (i.e. intercept before is 6.163 and intercept after is 3.765). Thus, to provide the reader with this type of information, the exact same equation as above is written as: predicted serum osteocalcin = [6.163 − (0.141)(17) (dummy)] − 0.141(25OHD) + (0.141)(17) (dummy), where the dummy is 1 if 25OHD is 17 or greater and the dummy is 0 if 25OHD is less than 17.
Natural log uNTx/Cr (Fig. 2D)
Predicted LN uNTx/Cr = 4.989 − 0.068 (25OHD) + 0.068 (25OHD − 17.5) (dummy), where the dummy is 1 if 25OHD is 17.5 or greater and the dummy is 0 if 25OHD is less than 17.5.
Similar to above, the intercept is dependent on whether the individual has serum 25OHD before or after the threshold; thus, the predicted LN uNTx/Cr = [4.989 − (.068)(17.5) (dummy)] − 0.068 (25OHD) + (.068)(17.5) (dummy), where the dummy is 1 if 25OHD is 17.5 or greater and the dummy is 0 if 25OHD is less than 17.5.
Statistically significant thresholds (i.e. change in slope before and after threshold) were indicated for bone markers at serum 25OHD levels less than 18 ng/ml (45 nmol/liter). For serum osteocalcin, nonlinear regression converged on a threshold of 17.034 ng/ml [95% confidence interval (CI) 12.61–21.46]. Piecewise linear regression indicated an optimal threshold of 17 ng/ml (42.4 nmol/liter; P = 0.007; adjusted _R_2 = 0.016). Before this threshold value, statistically significant decreases in serum osteocalcin were indicated with increases in serum 25OHD; however, this effect plateaued after threshold (Fig. 2C). For natural log-transformed uNTx/Cr, nonlinear regression converged on a threshold of 18 ng/ml (95% CI 14.347–21.653), with an optimal threshold of 17.5 ng/ml (43.7 nmol/liter; P = 0.0002; adjusted _R_2 = 0.036). Similarly, a plateau effect was evidenced after the threshold (Fig. 2D). Finally, nonlinear regression failed to identify a threshold for square root-transformed serum PTH (Fig. 2A). Slopes, ses, and 95% CIs of the piecewise regression analyses are provided in Table 3.
Table 3.
Piecewise linear regression resultsa
| Slope | se | P | 95% CI for slope | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Serum osteocalcin (n = 475) | |||||
| Slope change at threshold | 0.141 | 0.052 | 0.0070 | 0.039 | 0.242 |
| Before threshold (serum 25OHD <17) | −0.141 | 0.049 | 0.0040 | −0.237 | −0.045 |
| After threshold (serum 25OHD ≥17) | 0.000 | 0.007 | 0.9290 | −0.013 | 0.120 |
| Intercept before threshold | 6.163 | 0.792 | |||
| Natural log uNTx/Cr (n = 475) | |||||
| Slope change at threshold | 0.068 | 0.018 | 0.0002 | 0.033 | 0.103 |
| Before threshold (serum 25OHD <17.5) | −0.068 | 0.017 | 0.00006 | −0.101 | −0.035 |
| After threshold (serum 25OHD ≥17.5) | 0.000 | 0.003 | 0.8980 | 0.005 | −0.104 |
| Intercept before threshold | 4.989 | 0.277 |
There was a significant correlation between serum PTH and calcium intake (r = −0.181, P < 0.0005). Serum 25OHD was positively correlated with total calcium intake (r = 0.303, P < 0.0005; Fig. 2B) and vitamin D intake (r = 0.289, P < 0.0005_)._ Thus, the subjects with a higher total calcium intake had higher serum 25OHD levels. However, the results of a linear regression analysis indicated that after controlling for main effects, the interaction between the calcium intake and serum 25OHD had no significant (P = 0.116) effect on serum PTH.
Serum 25OHD, 1,25(OH)2D, and calcium absorption
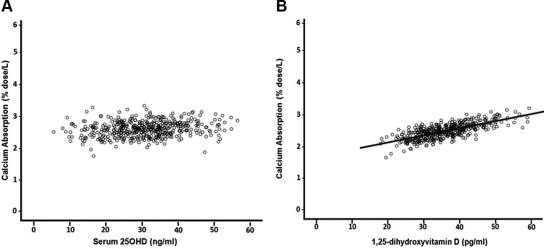
The results of the multivariate linear regression analyses are presented in Table 2. There was no significant association between serum 25OHD and calcium absorption for both unadjusted and adjusted models (r = 0.068, P = 0.071; sr = 0.050, P = 0.247, respectively; Fig. 3A shows adjusted model) and thus no threshold. There was a positive correlation between calcium absorption and serum 1,25(OH)2D for both unadjusted and adjusted models (r = 0.266; P < 0.0005 and sr = 0.224; P < 0.0005; sr2 = 0.050, respectively; adjusted model Fig. 3B). An increase in age and body weight was associated with decreased calcium absorption (P = 0.0066 and P < 0.0005, respectively).
Fig. 3.
Relationship between calcium absorption and vitamin D metabolites. A, Calcium absorption was not associated with serum 25OHD after adjustment for age, body weight, calcium intake, serum 1,25(OH)2D, and serum creatinine (r = 0.05, P = 0.247). B, Calcium absorption was significantly correlated positively with serum 1,25(OH)2D after adjustment for age, body weight, calcium intake, serum 25OHD, and serum creatinine (r = 0.224, P < 0.0005).
The correlation between calcium absorption and serum PTH was not statistically significant (r = 0.046, P = 0.324).
Discussion
In our own study of 488 postmenopausal Caucasian women, we found that serum PTH decreased continuously as serum 25OHD increased from 6 to 60 ng/ml (15–150 nmol/liter) without evidence of a plateau, whereas the bone markers serum osteocalcin and uNTx/Cr initially decreased between a serum 25OHD of 6–18 ng/ml (15–45 nmol/liter) but then plateaued between serum 25OHD of 18 to 60 ng/ml (45–150 nmol/liter).
We did not find any significant effect of the interaction of calcium intake and serum 25OHD on serum PTH. This might be explained by the relatively few number of subjects (n = 69) with serum 25OHD levels less than 20 ng/ml. However, the few studies that looked at the interaction between calcium intake and serum 25OHD in terms of serum PTH suppression found that at low levels of serum 25OHD (<20 ng/ml), calcium intake was associated with serum PTH (13, 69–71). In a study from Iceland, subjects with low serum 25OHD less than 10 ng/ml (25 nmol/liter) had significantly higher serum PTH associated with calcium intake less than 800 mg/d but above a serum 25OHD of 18 ng/ml (45 nmol/liter), calcium intake had no effect on serum PTH (13). In a study of elderly subjects (85 yr), vitamin D 400 IU/d increased serum 25OHD from 19 to 25 ng/ml (47.4–62.4 nmol/liter) but failed to produce a significant change in serum PTH and serum osteocalcin; however, the mean calcium intake was low (450 mg/d) (69). In a study from Australia of postmenopausal women, 1000 IU/d of vitamin D3 and 1000 mg/d calcium significantly increased serum 25OHD from 18.5 to 23 ng/ml (46.2–57.4 nmol/liter) and significantly suppressed serum PTH and serum C-terminal telopeptides (70). Neither calcium nor vitamin D alone suppressed serum PTH, although calcium alone but not vitamin D alone significantly suppressed serum C-terminal telopeptides.
In a placebo-controlled study of healthy subjects, aged 55 yr, with baseline serum 25OHD level of 26.8 ng/ml (67 nmol/liter), calcium 1200 mg/d alone significantly reduced the bone turnover markers serum C-terminal telopeptides of type 1 collagen and serum amino-terminal propeptide of type I procollagen compared with placebo (71), whereas vitamin D alone (4000 IU/d) had no significant effect on markers; there was no significant effect of calcium or vitamin D on serum PTH. In a study of healthy females aged 47 yr from England and a mean calcium intake of 570 mg/d, vitamin D3 800 IU/d increased serum 25OHD from 29 to 39 ng/ml (72.4–97.3 nmol/liter) with no change in serum PTH and bone markers, but serum PTH decreased significantly only in the lowest quartile of serum 25OHD (<24 ng/ml (60 nmol/liter; n = 18) (36).
In a 4-yr randomized, placebo-controlled trial of elderly women aged 75 yr and baseline serum 25OHD of 23.6 ng/ml (59 nmol/liter), treatment with 750 mg/d of calcium or 15 μg of 25OH vitamin D3 significantly reduced serum PTH. Calcium intervention was more effective than 25-hydroxyvitamin D3 with the effect of latter seen only at lower calcium intake (<716 mg/d) but not at higher calcium intake (>716 mg/d) (72). Also, bone markers serum osteocalcin and uNTx/Cr did not decrease in any groups; on the contrary, bone markers increased in the vitamin D and placebo groups and stayed the same in the calcium group.
Many other studies in which serum PTH declined significantly after vitamin D and calcium intervention started with low baseline serum 25OHD levels less than 20 ng/ml (50 nmol/liter) (73–75).
Calcium absorption was not associated with serum PTH or serum 25OHD but was associated significantly with serum 1,25(OH)2D. Another recent study showed no correlation between calcium absorption and serum 25OHD levels [mean serum 25OHD 21 ng/ml (52.4 nmol/liter)] (76). In contrast, Heaney (77) suggested that calcium absorption increased with increasing serum 25OHD and did not reach a plateau until 32 ng/ml (80 nmol/liter); these results were derived from a heterogeneous group of five studies, three of which did not directly measure calcium absorption. One study showed that calcium absorption was positively correlated with serum 25OHD only when serum 25OHD level was less than 4 ng/ml (10 nmol/liter), and absorption did not increase with higher serum 25OHD (78). So in studies in which serum 25OHD was greater than 5 ng/ml (12.5 nmol/liter), a threshold effect might have been missed.
It has been suggested that because serum PTH is still elevated at a serum 25OHD of 30 ng/ml (75 nmol/liter), it is an indicator of secondary hyperparathyroidism and because PTH resorbs bone, it must be an adverse finding (2, 3), but many of the studies did not measure bone resorption, bone loss, or fractures. In our study increased bone resorption was associated only with serum 25OHD less than 18 ng/ml (45 nmol/liter). There are other bone studies that confirm this threshold. A nested case-control study from the Women's Health Initiative found that the risk of hip fractures was significantly greater in women with mean serum 25OHD 19 ng/ml or less (47.5 nmol/liter) compared with the group with mean serum 25OHD 28.3 ng/ml or greater (70.7 nmol/liter) [odds ratio 1.71, 95% confidence limits (CL) 1.05–2.79] (79). In another case-cohort study, 436 men with incident nonspine fractures vs. 1608 controls, the group in the lowest quartile of serum 25OHD less than 20 ng/ml (50 nmol/liter) had more hip fractures compared with men in the top quartile of serum 25OHD (≥28 ng/ml) (70 nmol/liter) [hazard ratio (HR) 2.36, 95% CL 1.08–5.15] (80). In the National Health and Nutrition Examination Survey III study consisting of 1917 white men and women 65 yr of age or older, the risk of hip fracture was significantly lower in the group with serum 25OHD 25 ng/ml or greater (62.5 nmol/liter) than those with serum 25OHD less than 25 ng/ml (62.5 nmol/liter) [relative risk 0.64, 95% CL 0.46–0.89] (81). In another recent study of fractures in 1194 men with a median follow-up of 11 yr, it was found that serum 25OHD levels of less than 16 ng/ml (40 nmol/liter) were associated with an increased risk of fracture (HR 1.65, 95% CL 1.09–2.49) (82). In another Swedish study involving 986 ambulatory women, aged 75 yr, the HR for sustaining a fracture was 2.04 (95% CL 1.04–4.04) in the group with serum 25OHD less than 20 ng/ml (50 nmol/liter) (84).
In a longitudinal study of bone density from the Osteoporotic Fractures in Men study, the rate of bone loss in the hip was higher in the group with serum 25OHD levels less than 20 ng/ml (50 nmol/liter) compared with greater than 20 ng/ml (50 nmol/liter) (85). A bone biopsy study in Germany performed in 675 autopsies of men and women showed that about 96.5% of osteomalacia cases occurred at a serum 25OHD level of less than 20 ng/ml (50 nmol/liter), and almost 99% occurred at serum 25OHD levels of less than 25 ng/ml (62.4 nmol/liter) (86).
The accumulation of these findings support the concept that a serum 25OHD greater than 20 ng/ml (50 nmol/liter) is adequate for bone health, and there are no bone data supporting the need for a higher serum 25OHD of 30 ng/ml (75 nmol/liter).
It is not clear what is the clinical significance of higher serum PTH at serum 25OHD levels greater than 20 ng/ml (50 nmol/liter) when bone markers are not increased. Age-related decreases in calcium absorption and renal function and low calcium intake are some of the explanations. Several other factors affect serum PTH levels including race (23), gender (48), weight (20), serum leptin (45), and serum SHBG (8). These factors may play a role in the range of serum 25OHD levels at which serum PTH is maximally suppressed.
We saw a continuous decline in serum PTH over the normal range of serum 25OHD up to 60 ng/ml (150 nmol/liter), and these are levels that none would suggest represents vitamin D insufficiency. Possibly the decreasing serum PTH is a sign of the pharmacological effect of higher serum 25OHD or 1,25(OH)2D acting on the vitamin D response element in the PTH gene.
With regard to the literature review, there are some limitations in defining a threshold serum 25OHD from various studies. There are different methods for measurement of serum 25OHD and serum PTH among the 70 papers. The study populations are variable in terms of age, race, location, diet, gender, and culture, and these factors may influence the relationship between serum 25OHD and serum PTH. The role of calcium intake might be important in suppressing serum PTH, particularly at low serum 25OHD levels, as discussed above. Most of the studies did not analyze the effect of calcium intake while assessing the relationship between serum PTH and serum 25OHD. Twenty-four studies showed a threshold level for serum PTH at a serum 25OHD between 10 and 20 ng/ml (25–50 nmol/liter), and six studies showed a threshold between 20 and 25 ng/ml (50–62.4 nmol/liter). Thirty studies showed a threshold level of serum 25OHD of less than 25 ng/ml (62.4 nmol/liter) in terms of serum PTH suppression. Several studies including our own failed to find any threshold level of serum 25OHD at which serum PTH plateaus. What is very clear is that of the 70 studies we reviewed, a serum 25OHD threshold varying between 10 and 50 ng/ml (25–150 nmol/liter) was found.
In summary, the practice of defining vitamin D insufficiency as a serum 25OHD less than 30 ng/ml (75 nmol/liter) (3) based on serum PTH suppression is not supported by the literature review. Taking into account our own results on bone markers, the five large hip fracture studies and the bone loss data from Osteoporotic Fractures in Men, the totality of the data show that vitamin D insufficiency as it relates to bone occurs at a serum 25OHD less than 20 ng/ml (50 nmol/liter). Whether this threshold is higher for diseases other than bone remains to be established by clinical trials.
Acknowledgments
This work was supported by National Institutes of Health Grants AG28168, UO1-AG10373, and RO1-AG10358.
This paper was presented in abstract form at the 92nd Annual Meeting of The Endocrine Society, San Diego, CA, 2010.
Disclosure Summary: A.J.S., R.W.W., and X.F. have nothing to disclose. J.C.G. receives consulting fees and honoraria from Wyeth and Roche, and he has also served on the advisory council and as a spokesperson for Wyeth and Roche.
Footnotes
Abbreviations:
BMI
Body mass index
CI
confidence interval
CL
confidence limits
GFR
glomerular filtration rate
HR
hazard ratio
NTx/Cr
urine N-telopeptide
1,25(OH)2D
1,25-dihydroxyvitamin D
25OHD
25-hydroxyvitamin D
sr
semipartial correlation
sr2
squared sr
uNTx/Cr
urine NTx/Cr.
References
- 1.World Health Organization Scientific Group on the Prevention and Management of Osteoporosis 2003. Prevention and management of osteoporosis: report of a WHO scientific group. Geneva: World Health Organization [Google Scholar]
- 2.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. 2005. Estimates of optimal vitamin D status. Osteoporos Int 16:713–716 [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. 2007. Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- 4.Ebeling PR, Sandgren ME, DiMagno EP, Lane AW, DeLuca HF, Riggs BL. 1992. Evidence of an age-related decrease in intestinal responsiveness to vitamin D: relationship between serum 1,25-dihydroxyvitamin D3 and intestinal vitamin D receptor concentrations in normal women. J Clin Endocrinol Metab 75:176–182 [DOI] [PubMed] [Google Scholar]
- 5.Gannagé-Yared MH, Chemali R, Yaacoub N, Halaby G. 2000. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res 15:1856–1862 [DOI] [PubMed] [Google Scholar]
- 6.Durazo-Arvizu RA, Dawson-Hughes B, Sempos CT, Yetley EA, Looker AC, Cao G, Harris SS, Burt VL, Carriquiry AL, Picciano MF. 2010. Three-phase model harmonizes estimates of the maximal suppression of parathyroid hormone by 25-hydroxyvitamin D in persons 65 years of age and older. J Nutr 140:595–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahota O, Mundey MK, San P, Godber IM, Lawson N, Hosking DJ. 2004. The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone 35:312–319 [DOI] [PubMed] [Google Scholar]
- 8.Ooms ME, Lips P, Roos JC, van der Vijgh WJ, Popp-Snijders C, Bezemer PD, Bouter LM. 1995. Vitamin D status and sex hormone binding globulin: determinants of bone turnover and bone mineral density in elderly women. J Bone Miner Res 10:1177–1184 [DOI] [PubMed] [Google Scholar]
- 9.Gloth FM, 3rd, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. 1995. Vitamin D deficiency in homebound elderly persons. JAMA 274:1683–1686 [DOI] [PubMed] [Google Scholar]
- 10.Souberbielle JC, Cormier C, Kindermans C, Gao P, Cantor T, Forette F, Baulieu EE. 2001. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J Clin Endocrinol Metab 86:3086–3090 [DOI] [PubMed] [Google Scholar]
- 11.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. 1998. Hypovitaminosis D in medical inpatients. N Engl J Med 338:777–783 [DOI] [PubMed] [Google Scholar]
- 12.Levis S, Gomez A, Jimenez C, Veras L, Ma F, Lai S, Hollis B, Roos BA. 2005. Vitamin d deficiency and seasonal variation in an adult south Florida population. J Clin Endocrinol Metab 90:1557–1562 [DOI] [PubMed] [Google Scholar]
- 13.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. 2005. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 294:2336–2341 [DOI] [PubMed] [Google Scholar]
- 14.Seamans KM, Hill TR, Wallace JM, Horigan G, Lucey AJ, Barnes MS, Taylor N, Bonham MP, Muldowney S, Duffy EM, Strain JJ, Kiely M, Cashman KD. 2010. Cholecalciferol supplementation throughout winter does not affect markers of bone turnover in healthy young and elderly adults. J Nutr 140:454–460 [DOI] [PubMed] [Google Scholar]
- 15.Bacon CJ, Woo J, Lau EM, Lam CW, Gamble GD, Reid IR. 2010. Effects of 25-hydroxyvitamin D level and its change on parathyroid hormone in premenopausal Chinese women. Osteoporos Int 21:1935–1941 [DOI] [PubMed] [Google Scholar]
- 16.Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. 2009. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int 20:1407–1415 [DOI] [PubMed] [Google Scholar]
- 17.Arabi A, Baddoura R, El-Rassi R, El-Hajj Fuleihan G. 2010. Age but not gender modulates the relationship between PTH and vitamin D. Bone 47:408–412 [DOI] [PubMed] [Google Scholar]
- 18.Ardawi MS, Qari MH, Rouzi AA, Maimani AA, Raddadi RM. 30 April 2010. Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy saudi pre- and postmenopausal women. Osteoporos Int 10.1007/s00198-010-1249-7 [DOI] [PubMed] [Google Scholar]
- 19.Docio S, Riancho JA, Pérez A, Olmos JM, Amado JA, González-Macías J. 1998. Seasonal deficiency of vitamin D in children: a potential target for osteoporosis-preventing strategies? J Bone Miner Res 13:544–548 [DOI] [PubMed] [Google Scholar]
- 20.Need AG, O'Loughlin PD, Morris HA, Horowitz M, Nordin BE. 2004. The effects of age and other variables on serum parathyroid hormone in postmenopausal women attending an osteoporosis center. J Clin Endocrinol Metab 89:1646–1649 [DOI] [PubMed] [Google Scholar]
- 21.Outila TA, Kärkkäinen MU, Lamberg-Allardt CJ. 2001. Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr 74:206–210 [DOI] [PubMed] [Google Scholar]
- 22.Kauppinen-Mäkelin R, Tähtelä R, Löyttyniemi E, Kärkkäinen J, Välimaki MJ. 2001. A high prevalence of hypovitaminosis D in Finnish medical in- and outpatients. J Intern Med 249:559–563 [DOI] [PubMed] [Google Scholar]
- 23.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. 2000. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab 85:4125–4130 [DOI] [PubMed] [Google Scholar]
- 24.Cheng S, Tylavsky F, Kröger H, Kärkkäinen M, Lyytikäinen A, Koistinen A, Mahonen A, Alen M, Halleen J, Väänänen K, Lamberg-Allardt C. 2003. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr 78:485–492 [DOI] [PubMed] [Google Scholar]
- 25.Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK. 2006. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr 84:602–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Need AG, Horowitz M, Morris HA, Nordin BC. 2000. Vitamin D status: effects on parathyroid hormone and 1, 25-dihydroxyvitamin D in postmenopausal women. Am J Clin Nutr 71:1577–1581 [DOI] [PubMed] [Google Scholar]
- 27.Malabanan A, Veronikis IE, Holick MF. 1998. Redefining vitamin D insufficiency. Lancet 351:805–806 [DOI] [PubMed] [Google Scholar]
- 28.Hill TR, Cotter AA, Mitchell S, Boreham CA, Dubitzky W, Murray L, Strain JJ, Flynn A, Robson PJ, Wallace JM, Kiely M, Cashman KD. 2010. Vitamin D status and parathyroid hormone relationship in adolescents and its association with bone health parameters: analysis of the northern Ireland young heart's project. Osteoporos Int 21:695–700 [DOI] [PubMed] [Google Scholar]
- 29.Green TJ, Skeaff CM, Rockell JE, Venn BJ, Lambert A, Todd J, Khor GL, Loh SP, Muslimatun S, Agustina R, Whiting SJ. 2008. Vitamin D status and its association with parathyroid hormone concentrations in women of child-bearing age living in Jakarta and Kuala Lumpur. Eur J Clin Nutr 62:373–378 [DOI] [PubMed] [Google Scholar]
- 30.Trivedi DP, Doll R, Khaw KT. 2003. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, Saluja B, Ganie MA, Singh S. 2005. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr 82:477–482 [DOI] [PubMed] [Google Scholar]
- 32.Haden ST, Fuleihan GE, Angell JE, Cotran NM, LeBoff MS. 1999. Calcidiol and PTH levels in women attending an osteoporosis program. Calcif Tissue Int 64:275–279 [DOI] [PubMed] [Google Scholar]
- 33.Rockell JE, Skeaff CM, Venn BJ, Williams SM, Green TJ. 2008. Vitamin D insufficiency in New Zealanders during the winter is associated with higher parathyroid hormone concentrations: implications for bone health? NZ Med J 121:75–84 [PubMed] [Google Scholar]
- 34.Razzaghy-Azar M, Shakiba M. 2010. Assessment of vitamin D status in healthy children and adolescents living in Tehran and its relation to iPTH, gender, weight and height. Ann Hum Biol 37:692–701 [DOI] [PubMed] [Google Scholar]
- 35.Jesudason D, Need AG, Horowitz M, O'Loughlin PD, Morris HA, Nordin BE. 2002. Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone 31:626–630 [DOI] [PubMed] [Google Scholar]
- 36.Patel R, Collins D, Bullock S, Swaminathan R, Blake GM, Fogelman I. 2001. The effect of season and vitamin D supplementation on bone mineral density in healthy women: a double-masked crossover study. Osteoporos Int 12:319–325 [DOI] [PubMed] [Google Scholar]
- 37.Melin AL, Wilske J, Ringertz H, Sääf M. 1999. Vitamin D status, parathyroid function and femoral bone density in an elderly Swedish population living at home. Aging (Milano) 11:200–207 [PubMed] [Google Scholar]
- 38.Rajakumar K, Fernstrom JD, Janosky JE, Greenspan SL. 2005. Vitamin D insufficiency in preadolescent African-American children. Clin Pediatr (Phila) 44:683–692 [DOI] [PubMed] [Google Scholar]
- 39.Zeghoud F, Vervel C, Guillozo H, Walrant-Debray O, Boutignon H, Garabédian M. 1997. Subclinical vitamin D deficiency in neonates: definition and response to vitamin D supplements. Am J Clin Nutr 65:771–778 [DOI] [PubMed] [Google Scholar]
- 40.Vieth R, Ladak Y, Walfish PG. 2003. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab 88:185–191 [DOI] [PubMed] [Google Scholar]
- 41.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. 2005. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224 [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Alonso C, Naves-Diaz ML, Fernandez-Martin JL, Diaz-Lopez JB, Fernandez-Coto MT, Cannata-Andia JB. 2003. Vitamin D status and secondary hyperparathyroidism: the importance of 25-hydroxyvitamin D cut-off levels. Kidney Int Suppl S44–S48 [DOI] [PubMed] [Google Scholar]
- 43.Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, Delmas PD, van der Vijgh WJ. 1988. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab 67:644–650 [DOI] [PubMed] [Google Scholar]
- 44.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, Ragi-Eis S, Chandler J. 2006. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med 260:245–254 [DOI] [PubMed] [Google Scholar]
- 45.Maetani M, Maskarinec G, Franke AA, Cooney RV. 2009. Association of leptin, 25-hydroxyvitamin D, and parathyroid hormone in women. Nutr Cancer 61:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillemant J, Taupin P, Le HT, Taright N, Allemandou A, Pérès G, Guillemant S. 1999. Vitamin D status during puberty in French healthy male adolescents. Osteoporos Int 10:222–225 [DOI] [PubMed] [Google Scholar]
- 47.Soontrapa S, Soontrapa S, Pongchaiyakul C, Somboonporn C, Somboonporn W, Chailurkit LO. 2001. Prevalence of hypovitaminosis D in elderly women living in urban area of Khon Kaen Province, Thailand. J Med Assoc Thai 84(Suppl 2):S534–S541 [PubMed] [Google Scholar]
- 48.Lamberg-Allardt CJ, Outila TA, Kärkkainen MU, Rita HJ, Valsta LM. 2001. Vitamin D deficiency and bone health in healthy adults in Finland: could this be a concern in other parts of Europe? J Bone Miner Res 16:2066–2073 [DOI] [PubMed] [Google Scholar]
- 49.Isaia G, Giorgino R, Rini GB, Bevilacqua M, Maugeri D, Adami S. 2003. Prevalence of hypovitaminosis D in elderly women in Italy: clinical consequences and risk factors. Osteoporos Int 14:577–582 [DOI] [PubMed] [Google Scholar]
- 50.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. 1997. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443 [DOI] [PubMed] [Google Scholar]
- 51.McKenna MJ, Freaney R. 1998. Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Osteoporos Int 8(Suppl 2):S3–S6 [DOI] [PubMed] [Google Scholar]
- 52.Harkness L, Cromer B. 2005. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int 16:109–113 [DOI] [PubMed] [Google Scholar]
- 53.Gallagher JC, Kinyamu HK, Fowler SE, Dawson-Hughes B, Dalsky GP, Sherman SS. 1998. Calciotropic hormones and bone markers in the elderly. J Bone Miner Res 13:475–482 [DOI] [PubMed] [Google Scholar]
- 54.Krall EA, Sahyoun N, Tannenbaum S, Dallal GE, Dawson-Hughes B. 1989. Effect of vitamin D intake on seasonal variations in parathyroid hormone secretion in postmenopausal women. N Engl J Med 321:1777–1783 [DOI] [PubMed] [Google Scholar]
- 55.Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. 2010. Serum levels of vitamin D, PTH and calcium and breast cancer risk-a prospective nested case-control study. Int J Cancer 127:2159–2168 [DOI] [PubMed] [Google Scholar]
- 56.Kinyamu HK, Gallagher JC, Rafferty KA, Balhorn KE. 1998. Dietary calcium and vitamin D intake in elderly women: effect on serum parathyroid hormone and vitamin D metabolites. Am J Clin Nutr 67:342–348 [DOI] [PubMed] [Google Scholar]
- 57.Dawson-Hughes B, Harris SS, Dallal GE. 1997. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr 65:67–71 [DOI] [PubMed] [Google Scholar]
- 58.Gallagher JC, Fowler SE, Detter JR, Sherman SS. 2001. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab 86:3618–3628 [DOI] [PubMed] [Google Scholar]
- 59.Benjamin A, Moriakova A, Akhter N, Rao D, Xie H, Kukreja S, Barengolts E. 2009. Determinants of 25-hydroxyvitamin D levels in African-American and Caucasian male veterans. Osteoporos Int 20:1795–1803 [DOI] [PubMed] [Google Scholar]
- 60.Ho-Pham LT, Nguyen ND, Lai TQ, Eisman JA, Nguyen TV. 23 April 2010. Vitamin D status and parathyroid hormone in a urban population in Vietnam. Osteoporos Int 10.1007/s00198-010-1207-4 [DOI] [PubMed] [Google Scholar]
- 61.Reginster JY, Frederick I, Deroisy R, Dewe W, Taquet AN, Albert A, Collette J, Pirenne H, Zheng SX, Gosset C. 1998. Parathyroid hormone plasma concentrations in response to low 25-OH vitamin D circulating levels increases with age in elderly women. Osteoporos Int 8:390–392 [DOI] [PubMed] [Google Scholar]
- 62.Bates CJ, Carter GD, Mishra GD, O'Shea D, Jones J, Prentice A. 2003. In a population study, can parathyroid hormone aid the definition of adequate vitamin D status? A study of people aged 65 years and over from the British National Diet and Nutrition Survey. Osteoporos Int 14:152–159 [DOI] [PubMed] [Google Scholar]
- 63.El-Hajj Fuleihan G, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, Tannous R. 2001. Hypovitaminosis D in healthy schoolchildren. Pediatrics 107: E53. [DOI] [PubMed] [Google Scholar]
- 64.Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. 2009. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab 94:1244–1250 [DOI] [PubMed] [Google Scholar]
- 65.Cerdá Gabaroi D, Peris P, Monegal A, Albaladejo C, Martinez MA, Muxi A, Martínez de Osaba MJ, Surís X, Guañabens N. 2010. Search for hidden secondary causes in postmenopausal women with osteoporosis. Menopause 17:135–139 [DOI] [PubMed] [Google Scholar]
- 66.Rucker D, Allan JA, Fick GH, Hanley DA. 2002. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ 166:1517–1524 [PMC free article] [PubMed] [Google Scholar]
- 67.Nordin BE, Morris HA, Wishart JM, Scopacasa F, Horowitz M, Need AG, Clifton PM. 1998. Modification and validation of a single-isotope radiocalcium absorption test. J Nucl Med 39:108–113 [PubMed] [Google Scholar]
- 68.Marsh LC, Cormier DR. 2001. Spline regression models. Sage University Paper series on quantitative applications in the social sciences, series no. 07-137. Thousand Oaks, CA: Sage Publications, Inc [Google Scholar]
- 69.Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. 2002. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res 17:709–715 [DOI] [PubMed] [Google Scholar]
- 70.Thomas SD, Need AG, Nordin BE. 2010. Suppression of C-terminal telopeptide in hypovitaminosis D requires calcium as well as vitamin D. Calcif Tissue Int 86:367–374 [DOI] [PubMed] [Google Scholar]
- 71.Aloia J, Bojadzievski T, Yusupov E, Shahzad G, Pollack S, Mikhail M, Yeh J. 2010. The relative influence of calcium intake and vitamin D status on serum parathyroid hormone and bone turnover biomarkers in a double-blind, placebo-controlled parallel group, longitudinal factorial design. J Clin Endocrinol Metab 95:3216–3224 [DOI] [PubMed] [Google Scholar]
- 72.Peacock M, Liu G, Carey M, McClintock R, Ambrosius W, Hui S, Johnston CC. 2000. Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J Clin Endocrinol Metab 85:3011–3019 [DOI] [PubMed] [Google Scholar]
- 73.Grados F, Brazier M, Kamel S, Mathieu M, Hurtebize N, Maamer M, Garabédian M, Sebert JL, Fardellone P. 2003. Prediction of bone mass density variation by bone remodeling markers in postmenopausal women with vitamin D insufficiency treated with calcium and vitamin D supplementation. J Clin Endocrinol Metab 88:5175–5179 [DOI] [PubMed] [Google Scholar]
- 74.Larsen ER, Mosekilde L, Foldspang A. 2004. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res 19:370–378 [DOI] [PubMed] [Google Scholar]
- 75.Krieg MA, Jacquet AF, Bremgartner M, Cuttelod S, Thiébaud D, Burckhardt P. 1999. Effect of supplementation with vitamin D3 and calcium on quantitative ultrasound of bone in elderly institutionalized women: a longitudinal study. Osteoporos Int 9:483–488 [DOI] [PubMed] [Google Scholar]
- 76.Aloia JF, Chen DG, Yeh JK, Chen H. 2010. Serum vitamin D metabolites and intestinal calcium absorption efficiency in women. Am J Clin Nutr 92:835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heaney RP. 2005. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol 97:13–19 [DOI] [PubMed] [Google Scholar]
- 78.Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. 2008. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res 23:1859–1863 [DOI] [PubMed] [Google Scholar]
- 79.Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR. 2008. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med 149:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cauley JA, Parimi N, Ensrud KE, Bauer DC, Cawthon PM, Cummings SR, Hoffman AR, Shikany JM, Barrett-Connor E, Orwoll E; for the Osteoporotic Fractures in Men (MrOS) Research Group 2010. Serum 25 hydroxyvitamin D and the risk of hip and non-spine fractures in older men. J Bone Miner Res 25:545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Looker AC, Mussolino ME. 2008. Serum 25-hydroxyvitamin D and hip fracture risk in older U.S. white adults. J Bone Miner Res 23:143–150 [DOI] [PubMed] [Google Scholar]
- 82.Melhus H, Snellman G, Gedeborg R, Byberg L, Berglund L, Mallmin H, Hellman P, Blomhoff R, Hagstrom E, Arnlov J, Michaelsson K. 2010. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab 95:2637–2645 [DOI] [PubMed] [Google Scholar]
- 83.Lappe JM, Davies KM, Travers-Gustafson D, Heaney RP. 2006. Vitamin D status in a rural postmenopausal female population. J Am Coll Nutr 25:395–402 [DOI] [PubMed] [Google Scholar]
- 84.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. 2005. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA study of elderly women. Osteoporos Int 16:1425–1431 [DOI] [PubMed] [Google Scholar]
- 85.Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, Fink HA, Barrett-Connor E, Zmuda JM, Shikany JM, Orwoll ES. 2009. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab 94:2773–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, Püschel K, Amling M. 2010. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 25:305–312 [DOI] [PubMed] [Google Scholar]